Abstract
Diabetic retinopathy (DR) and glaucoma are emerging causes of blindness and visual impairment in India and the world. Both diseases do not have any early warning symptoms, and once the symptoms appear, the diseases are reasonably advanced. Because of the long-standing nature of the diseases, one cannot adopt the cataract detection and treatment model so successfully developed in India. It requires an altogether different approach for screening and related infrastructure including human capital development. The solutions developed to reduce the burden of DR/glaucoma should be customized to urban, semi-urban, and rural areas. Greater advocacy, improving the health-seeking behavior, development of infrastructure and skilled personnel appropriate for the points of care, and an emphasis in comprehensive eye care are some of the solutions.
Keywords: Community care, diabetic retinopathy, glaucoma
The two emerging causes of blindness and visual impairment are diabetic retinopathy (DR) and glaucoma. The year 2010 global burden of disease estimated that DR and glaucoma, respectively, caused 1% and 8% of blindness and 1% and 2% of visual impairment.[1,2] A meta-analysis of 35 studies (1980–2008) indicated that the overall prevalence of any DR in diabetics was 34.6%, proliferative DR was 6.96%, diabetic macular edema was 6.81%, and vision-threatening DR was 10.2%. An Indian study in 11 cities spread over 9 states has shown that nearly 45% of people with diabetes mellitus had vision loss when they first presented to an eye care facility.[3]
A meta-analysis of 50 published population-based studies has shown that the global prevalence of glaucoma in people in the age group of 40–80 is 3.54%.[4] This report also states that the 64.3 million people with glaucoma detected in 2013 is likely to increase to 76.0 million in the year 2020 and to 111.8 million people in the year 2040. The Indian report had estimated that 11.2 million people lived with glaucoma in the year 2009, including 6.48 million people with primary open-angle glaucoma (POAG) and 2.54 million people with primary angle-closure glaucoma (PACG).[5]
The rising numbers of people with DR and glaucoma present several challenges in awareness creation, screening, service delivery, and capacity building in the community. The present communication is based on the panel discussion done with the objective of having consensus and recommendations for implementing holistic community care solutions for DR and glaucoma.
Methods
A panel discussion on the care of DR and glaucoma at the community level was done under the vision 2020 India program. This involved four panelists with experience in community care, including care for glaucoma and retina. The panelists were asked questions that covered the entire range of care at the community level, related infrastructure, and human resources pertaining to DR and glaucoma. The panelists were sent these questions through E-mail before the conference, and discussion points were written during the meeting. All responses were further verified through E-mail communication and summarized. We have presented opinions of panelists for each question in a summarized consensus statement. The goal of this article is to provide practical solutions in addressing the challenges in the management of community care models for DR and glaucoma.
Community care for diabetic retinopathy
What is the goal of community care in diabetic retinopathy?
The goal of community care for DR is to provide a comprehensive, preventive, cost-effective, easily accessible, and sustainable diabetes eye care with inbuilt components of awareness creation, service delivery, and capacity building. Since diabetes is a chronic disease and screening activity is an ongoing repetitive exercise, the point of care must be located close to the rural community for better compliance.
The aim should be for a minimum 80% coverage of eligible population with diabetes mellitus (DM) who needs DR screening. The British Diabetic Association (Diabetes UK) has established standards for any DR screening program of at least 80% sensitivity and specificity.[6] It is expected to have low specificity with many “not-required-for-treatment” referrals in the program in the beginning but is likely to improve over a period of time.
Whom should it reach?
We live in near epidemics of diabetes. We need a strategy of high yield of sight-threatening DR and wide reach. A high yield is possible with a targeted DR screening. Targeted screening aims to screen known diabetics, high-risk occupations with sedentary activity, associated risk factors such as abdominal obesity, and comorbid conditions such as nephropathy, neuropathy, and ischemic heart disease. A regular DR screening program in collaboration with nephrology clinics (nephropathy and retinopathy are both microvascular complications of DM and go hand in hand) is very rewarding. The model should have wide reach covering rural/urban areas/slums as well as special groups such as transgenders/HIV.
What are the recruitment strategies for diabetic retinopathy?
The recruitment strategies for DR ideally should consist of both vertical and horizontal linkages. Unlike an isolated vertical model like cataract program, appropriate horizontal linkages with all health-care personnel who come in contact with people with DM are vital. These health personnel include the physicians, diabetologists, village-based registered medical practitioners (RMPs), pharmacists, Accredited Social Health Activists, self-help group women, and Anganwadi workers. A recruitment strategies for DR customized to the locality (such as a Dandora village-based announcement and a mobile-based app in rural and urban India, respectively)[7,8] and linkages with nongovernmental organizations (Lions, Rotary, Inner Wheel, etc.) are the effective steps.[7] An innovative model of recruiting people with diabetes through schoolchildren has been tried effectively in a district-wide DR screening program.[9]
What are the innovative models of diabetic retinopathy screening?
Technology-based (fundus camera) screening model led by physician (urban) and RMP (rural) with network of eye care technicians is ideal in terms of geographic reach and cost effectivity. This physician-led model should have vertical linkage with ophthalmologists trained in DR care and management. The opportunity cost is less in physician-led model that effectively differentiates between sight-threatening and nonsight-threatening DR; the former cases must be referred to the retina specialist and the latter cases benefit from good control of health parameters and lifestyle changes.
What are the infrastructure requirements of community care?
The photography-based screening model is ideal as it is objective and could have an inbuilt audit evaluation tools to test the efficiency.[10] With the modern user-friendly cameras, it is relatively easy to train semi-skilled people to take fundus photograph of the posterior pole and distinguish grossly between a normal and abnormal fundus photo. The DR community screening could also be delivered from a one-stop mobile van equipped with point-of-care technology to test the eye (vision, refraction, intraocular pressure, and fundus photograph) and perform the essential biochemical tests (hemoglobin A1c, lipid profile, and microalbuminuria). It could be further improved with additional facilities to screen all potential micro- and macro-vascular complications of DM. However, with each increment of facility, there is proportionate rise in cost of care. Electronic storage of all data with unique ID, transmitting the data for safe storage, and tracking the patients for service delivery and/or longitudinal screening will be the most ideal for a chronic disease such as DM and DR. Participation of local ophthalmologists help create an effective yet sustainable mobile DR screening model.[11]
What are the customized awareness and advocacy strategies for diabetic retinopathy?
Awareness creation must be continuous, sustainable, and widespread. World Sight Day and World Diabetes Day are two good opportunities for providing momentum to an ongoing awareness campaigns. Considering the burden of DM, we wonder if the screening guidelines could be part of curriculum, from school to medical college.
What are the innovative models for facilitating modifiable risk factor reduction?
Control of blood pressure is known to decrease microvascular complications,[12] and the presence of hypertension is associated with higher incidence of diabetic macular edema.[13] Incentivizing the tests for the vital health indices connected to DM and lifestyle changes could help people improve the health seeking behavior. These could include from conditional cash transfer for seeking care to nudging with celebrity ambassadorship.
Service delivery models for diabetic retinopathy
The mainstays of treatment for DR are retinal lasers, intravitreal antivascular endothelial growth factor injection, and vitreoretinal surgery. Many patients will not require vitreoretinal surgery if retinal lasers and intravitreal injections are given at the right stage of the disease and at required quantity. The decision for these treatments depends on the stage of retinopathy identified by retinal examinations by ophthalmoscopy, fluorescein angiography, and optical coherence tomography (OCT). Thus, it is necessary to familiarize the ophthalmologists in these investigation procedures and treatment processes. The necessary equipment could be placed in a fixed or shared mobile platform.
What are the key performance indicators for diabetic retinopathy?
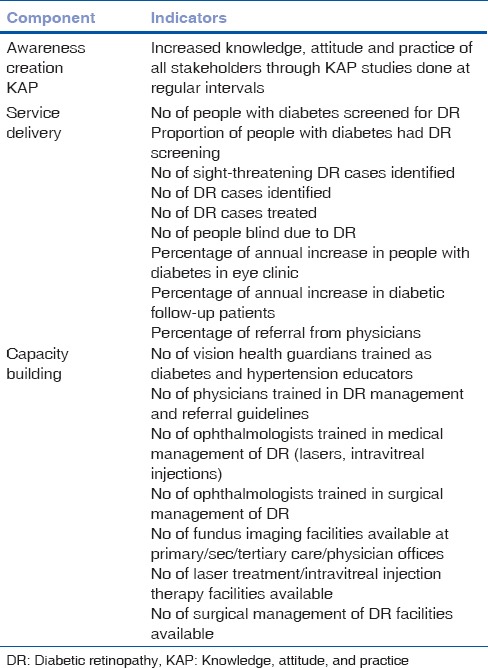
The indicators belong to three interdependent important dimensions of DR: (1) awareness creation, (2) service delivery, and (3) capacity building [Table 1].
Table 1.
Key performance indicators in diabetic retinopathy
What is the future scope of community care for diabetic retinopathy?
This depends on efficient, cost-effective, and sustainable technique and technology. An artificial learning neural networks and cloud-based automated grading are the new expanding horizons.[14,15] Political will and adequate health financing would play a significant role.
Community care for glaucoma
What are the goals of community care for glaucoma?
The simplistic goal of community eye care in glaucoma is to diagnose and treat. Mass community screening is unlikely to be cost effective, and in addition, there is a lack of trained personnel and required infrastructure. An opportunistic screening is a good alternative. This could be eased with a comprehensive eye examination of all people above the age group of 40 reporting to the clinic. This includes measurement of presenting and corrected visual acuity, slit-lamp examination (including Van Herick test and intraocular pressure [IOP] measurement), gonioscopy (if required), and dilated stereoscopic evaluation of the optic disc and retina. A standard oblique flashlight test in the detection of narrow angles has reported to have 76.3% sensitivity, and 80.7% specificity is still not good for community screening.[16] A good screening is one that achieves 85% sensitivity and 95%–98% specificity.[17]
Whom should it reach?
In glaucoma screening, a comprehensive eye examination for anyone attending the clinic is a good starting point. Studies have shown that increasing age, people residing in urban areas, people with diabetes, those with high myopia, and people with a positive family history of glaucoma have higher risk for POAG.[18,19] Similarly, risk factors for PACG include advanced age, female gender, those with family history of glaucoma, short stature, people with narrow palpebral aperture, and people with high hyperopia.[20] The target coverage must be more intensive with increasing age since the prevalence of POAG shows a significant rise with age. Studies have shown a POAG prevalence of 3.45% for those over 40 years, 5.11% for those over 50 years, and 7.50% for those over 60 years.[21]
What are the innovative models of screening for glaucoma?
It would be ideal if one could define what should be done for glaucoma detection at each level of care, i.e., primary, secondary, and tertiary levels, so that one does not miss anyone who is screened/examined at each level. Technology-based (fundus camera) screening model led by a physician (urban) and a RMP (rural) with network of vision technician/optometrist is ideal with adequate reach and appropriate opportunity cost. A simple doable way of increasing the glaucoma yield would be to screen all cataract patients brought from the community for cataract surgery. The increasing use of phone-based fundus camera for posterior pole photography is an innovation in right direction. An automated grading of the disc-cup ratio will be a cost-effective strategy.
What are the infrastructure requirements of community care for glaucoma?
Glaucoma detection at the primary level would essentially need a slit lamp, tonometer, 90 D/78 D lenses, or a direct ophthalmoscope. A nonmydriatic camera for posterior segment evaluation will be an added advantage, and when coupled with Internet, a tele-consultation would be a distinct possibility. It is always possible to place the equipment in a mobile vehicle, similar to DR detection. A visual field analyzer is required at secondary level of care; OCT and ultrasound biometry are required at tertiary level of care.
What are the customized awareness and advocacy for glaucoma?
Unlike diabetes and DR, there is little awareness about glaucoma. Different strategies for increasing awareness have been described. These include developing culture and region-specific posters and awareness materials and advocacy through celebrities/public figures urging people for an annual eye examination and through neighborhood assembly where the stable glaucoma patients speak up their experience. Awareness can also be raised by conducting rallies and events on occasions such as World Sight Day and Glaucoma Awareness Week. All these awareness programs should emphasize on annual eye examination, for all above 40 years of age, those with family history of glaucoma as well as those with diabetes, hypertension, and other lifestyle diseases.
What are the innovative models for facilitating modifiable risk factor reduction for glaucoma?
The only modifiable systemic risk factor with some evidence is the presence of hypertension and diabetes mellitus.[22,23] It is also shown that the prevalence of glaucoma is higher in urban population than rural population.[20,21] This raises an important question about lifestyle habits. Although there is no direct evidence about these lifestyle habits, it is always good to change habits and adopt healthy lifestyle, which can also prevent many other diseases.
Service delivery models for glaucoma?
The service delivery depends on the point of care. At the primary care level, it should be early detection and referral, at secondary care level, it should be medical care, and at the tertiary level, it should be both medical and surgical treatment. An early detection in primary level is possible when the allied ophthalmic personnel (vision technician) are trained to using some of the devices mentioned earlier. In our experience, with basic training, a vision technician could detect 30%–35% of glaucoma.[24] The secondary level care for glaucoma will include diagnosis and prescription of antiglaucoma medications. Keeping in view of the prevalence of PACG in Indian population, the availability of YAG laser is essential and training in peripheral iridotomy is mandatory.
Affordability and compliance are equally important issues. Unfortunately, the newer (and more effective) antiglaucoma medications are relatively expensive. A government subsidy and/or insurance cover will help reduce the financial burden. Glaucoma patients need long-term follow-ups. While the long-term benefit is from proper advocacy to improve the health-seeking behavior and establishing fixed eye care facility as close as possible to people, one could consider taking the point of care close to the people as a short-term solution.
Low vision and rehabilitation should be reserved for those who have their quality of life affected with glaucoma. This should be at all levels appropriate for the point of care.
What are the key performance indicators for glaucoma?
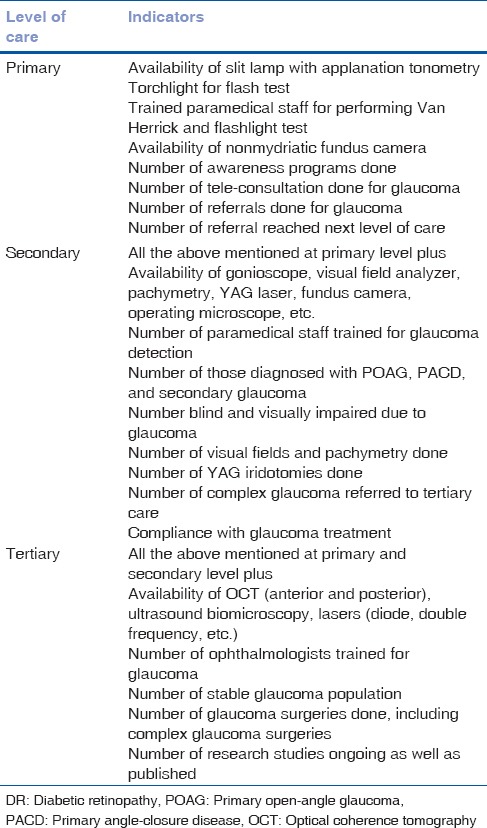
Indicators can be defined for each component of health system for primary, secondary, and tertiary care. These include infrastructure, equipment, trained eye health personnel in service delivery, and sustainability [Table 2].
Table 2.
Key performance indicators in glaucoma
What is the future scope of community care?
This depends on efficient, cost-effective, and sustainable technique and technology. We anticipate that community care for glaucoma would be integrated with other noncommunicable eye diseases. A machine learning model for detection of glaucoma at an early state is an exciting future possibility.[25]
Conclusion
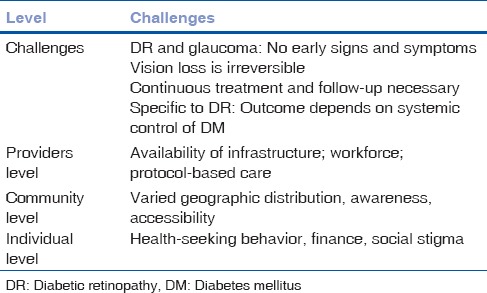
The challenges in community care in DR and glaucoma are many. While the diseases themselves pose some challenges, there are also challenges at all levels of care provider, community, and individual [Table 3].
Table 3.
Challenges in planning a community care of DR and glaucoma
The cost of care, from detection to treatment to follow-up in DR and glaucoma, is high. Hence, this has to be distributed at different levels of care in such a manner that the continuous screening is done at primary level, medical and follow-up care is done at secondary level, and the surgical treatment is done at the tertiary level. An integrated model of primary and secondary eye care is effective in rural population of certain states in India.[26] The bigger concerns are development and deployment of skilled eye care personnel. A situational analysis done in 11 cities spread over 9 states has reported country's unpreparedness to handle the emerging burden of DR.[27] Two factors were identified: (1) lack of focus on building sustainable synergies within and outside the health sector and (2) poor convergence between the national health programs.[28] What is true for DR care is equally true for glaucoma care.
Financial support and sponsorship
This study was financially supported by Hyderabad Eye Research Foundation, Hyderabad.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614–8. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 2.Anjana RM, Deepa M, Pradeepa R, Mahanta J, Narain K, Das HK, et al. Prevalence of diabetes and prediabetes in 15 states of india: Results from the ICMR-INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 2017;5:585–96. doi: 10.1016/S2213-8587(17)30174-2. [DOI] [PubMed] [Google Scholar]
- 3.Shukla R, Gudlavalleti MV, Bandyopadhyay S, Anchala R, Gudlavalleti AS, Jotheeswaran AT, et al. Perception of care and barriers to treatment in individuals with diabetic retinopathy in india: 11-city 9-state study. Indian J Endocrinol Metab. 2016;20:S33–41. doi: 10.4103/2230-8210.179772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chopra V, Varma R, Francis BA, Wu J, Torres M, Azen SP, et al. Type 2 diabetes mellitus and the risk of open-angle glaucoma the los angeles latino eye study. Ophthalmology. 2008;115:227–320. doi: 10.1016/j.ophtha.2007.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George R, Ve RS, Vijaya L. Glaucoma in India: Estimated burden of disease. J Glaucoma. 2010;19:391–7. doi: 10.1097/IJG.0b013e3181c4ac5b. [DOI] [PubMed] [Google Scholar]
- 6.Squirrell DM, Talbot JF. Screening for diabetic retinopathy. J R Soc Med. 2003;96:273–6. doi: 10.1258/jrsm.96.6.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rani PK, Raman R, Agarwal S, Paul PG, Uthra S, Margabandhu G, et al. Diabetic retinopathy screening model for rural population: Awareness and screening methodology. Rural Remote Health. 2005;5:350. [PubMed] [Google Scholar]
- 8.Joiner KL, Nam S, Whittemore R. Lifestyle interventions based on the diabetes prevention program delivered via eHealth: A systematic review and meta-analysis. Prev Med. 2017;100:194–207. doi: 10.1016/j.ypmed.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sethu Sheeladevi JS, Pujari S, Rani PK. Impact of a district-wide diabetes prevention programme involving health education for children and the community. Health Educ J. 2014;73:363–9. [Google Scholar]
- 10.Das T, Raman R, Ramasamy K, Rani PK. Telemedicine in diabetic retinopathy: Current status and future directions. Middle East Afr J Ophthalmol. 2015;22:174–8. doi: 10.4103/0974-9233.154391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murthy KR, Murthy PR, Kapur A, Owens DR. Mobile diabetes eye care: Experience in developing countries. Diabetes Res Clin Pract. 2012;97:343–9. doi: 10.1016/j.diabres.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 12.UK prospective diabetes study group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–13. [PMC free article] [PubMed] [Google Scholar]
- 13.Das T, Wallang B, Semwal P, Basu S, Padhi TR, Ali MH, et al. Changing clinical presentation, current knowledge-attitude-practice, and current vision related quality of life in self-reported type 2 diabetes patients with retinopathy in Eastern India: The LVPEI eye and diabetes study. J Ophthalmol. 2016;2016:3423814. doi: 10.1155/2016/3423814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srinivasan S, Shetty S, Natarajan V, Sharma T, Raman R. Development and validation of a diabetic retinopathy referral algorithm based on single-field fundus photography. PLoS One. 2016;11:e0163108. doi: 10.1371/journal.pone.0163108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brady CJ, Villanti AC, Pearson JL, Kirchner TR, Gupta OP, Shah CP, et al. Rapid grading of fundus photographs for diabetic retinopathy using crowdsourcing. J Med Internet Res. 2014;16:e233. doi: 10.2196/jmir.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He M, Huang W, Friedman DS, Wu C, Zheng Y, Foster PJ, et al. Slit lamp-simulated oblique flashlight test in the detection of narrow angles in Chinese eyes: The liwan eye study. Invest Ophthalmol Vis Sci. 2007;48:5459–63. doi: 10.1167/iovs.07-0670. [DOI] [PubMed] [Google Scholar]
- 17.Stamper RL. Glaucoma screening. J Glaucoma. 1998;7:149–50. [PubMed] [Google Scholar]
- 18.Vijaya L, George R, Baskaran M, Arvind H, Raju P, Ramesh SV, et al. Prevalence of primary open-angle glaucoma in an urban South Indian population and comparison with a rural population. The chennai glaucoma study. Ophthalmology. 2008;115:648–540. doi: 10.1016/j.ophtha.2007.04.062. [DOI] [PubMed] [Google Scholar]
- 19.Garudadri C, Senthil S, Khanna RC, Sannapaneni K, Rao HB. Prevalence and risk factors for primary glaucomas in adult urban and rural populations in the Andhra Pradesh eye disease study. Ophthalmology. 2010;117:1352–9. doi: 10.1016/j.ophtha.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Vijaya L, George R, Arvind H, Baskaran M, Ve Ramesh S, Raju P, et al. Prevalence of primary angle-closure disease in an urban South Indian population and comparison with a rural population. The Chennai glaucoma study. Ophthalmology. 2008;115:655–600. doi: 10.1016/j.ophtha.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 21.Nangia V, Jonas JB, Matin A, Bhojwani K, Sinha A, Kulkarni M, et al. Prevalence and associated factors of glaucoma in rural central India. The central India eye and medical study. PLoS One. 2013;8:e76434. doi: 10.1371/journal.pone.0076434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan TCW, Bala C, Siu A, Wan F, White A. Risk factors for rapid glaucoma disease progression. Am J Ophthalmol. 2017;180:151–7. doi: 10.1016/j.ajo.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Jung Y, Han K, Park HL, Park CK. Type 2 diabetes mellitus and risk of open-angle glaucoma development in Koreans: An 11-year nationwide propensity-score-matched study. Diabetes Metab. 2017 doi: 10.1016/j.diabet.2017.09.007. pii: S1262-3636(17) 30519-0. [DOI] [PubMed] [Google Scholar]
- 24.Suram V, Addepalli UK, Krishnaiah S, Kovai V, Khanna RC. Accuracy of vision technicians in screening ocular pathology at rural vision centres of Southern India. Clin Exp Optom. 2016;99:183–7. doi: 10.1111/cxo.12345. [DOI] [PubMed] [Google Scholar]
- 25.Kim SJ, Cho KJ, Oh S. Development of machine learning models for diagnosis of glaucoma. PLoS One. 2017;12:e0177726. doi: 10.1371/journal.pone.0177726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao GN, Khanna RC, Athota SM, Rajshekar V, Rani PK. Integrated model of primary and secondary eye care for underserved rural areas: The L V prasad eye institute experience. Indian J Ophthalmol. 2012;60:396–400. doi: 10.4103/0301-4738.100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilbert CE, Babu RG, Gudlavalleti AS, Anchala R, Shukla R, Ballabh PH, et al. Eye care infrastructure and human resources for managing diabetic retinopathy in India: The India 11-city 9-state study. Indian J Endocrinol Metab. 2016;20:S3–10. doi: 10.4103/2230-8210.179768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaiha SM, Shukla R, Gilbert CE, Anchala R, Gudlavalleti MV. Is India's policy framework geared for effective action on avoidable blindness from diabetes? Indian J Endocrinol Metab. 2016;20:S42–50. doi: 10.4103/2230-8210.179773. [DOI] [PMC free article] [PubMed] [Google Scholar]


