Abstract
Background
Recently, although studies have investigated the role of NSAIDs in the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis (PEP), selection of the ideal drug, the time and route of its administration for the appropriate population remain controversial.
Methods
A systematic search was done in sources including PubMed, Embase, Web of Science, the Cochrane Library Central, and ClinicalTrials.gov from from August 1, 1990 to August 1, 2017. Randomized controlled trials comparing the prophylactic use of NSAIDs versus a placebo were included. Statistical analysis was performed using the RevMan 5.3 software to assess the outcomes.
Results
A total of 21 randomized controlled trials were included in the meta-analysis. Our study showed that NSAIDs significantly reduced the incidence of PEP (RR, 0.61, 95%CI,0.52–0.72; p < 0.00001). The analysis showed that indomethacin administration post-ERCP (RR, 0.47; 95% CI, 0.31–0.70; p = 0.0002) appeared to be more effective in preventing PEP than indomethacin administration pre-ERCP (RR, 0.59; 95% CI, 0.45–0.79; P = 0.0003), but there was no significant difference between the high-risk and average-risk population(p = 0.13). In the diclofenac group, it was noted that administration of diclofenac pre-ERCP (RR, 0.32; 95% CI, 0.16–0.63; p = 0.001) was more effective than that in post-ERCP (RR, 0.65; 95% CI, 0.27–1.599; p = 0.35). The relative risk of PEP was 0.63 (95% CI, 0.27–1.50; p = 0.30) in high-risk patients and 0.41 (95% CI, 0.17–0.98; p = 0.02) in average-risk patients. With regard to the route of administration, PEP decreased significantly only in patients receiving the drug rectally (RR, 0.53; 95% CI, 0.44–0.63; p < 0.00001), but not for those who received intramuscularly (RR, 0.74; 95% CI, 0.47–1.17; p = 0.20), intravenously (RR, 0.97; 95% CI, 0.51–1.83; p = 0.93), and orally (RR = 0.88; 95% CI, 0.55–0.1.43; p = 0.62).
Conclusions
Rectal administration of NSAIDs (both indomethacin and diclofenac) was effective in preventing PEP in unselected patients. A single dose of indomethacin after ERCP might be effective in preventing PEP in both high-risk and average-risk patients. However, diclofenac administered rectally before ERCP might be protective against PEP in high-risk patients compared to a placebo. However, more high quality head-to-head RCTs are required.
Keywords: NSAIDs, Indomethacin, Diclofenac, ERCP, Pancreatitis, Meta-analysis
Background
With the continuous improvement of endoscopic instruments and technical means, endoscopic retrograde cholangiopancreatography (ERCP) has gained more attention from clinicians for the diagnosis and treatment of biliary and pancreatic diseases. ERCP via its minimally invasive benefit in the diagnosis of biliary and pancreatic diseases is challenged by a higher potential for serious complications than any other standard endoscopic technique. Frequent complications after ERCP include pancreatitis, postoperative bleeding, and gastrointestinal perforation. The most frequent of these is pancreatitis. Due to the different group characteristics and modes of operation, the incidence of post-ERCP pancreatitis (PEP) is not the same; some studies have reported it to be about 2–4%, while others have reported that it could increase to 8–20% in some high-risk patients, [1]. Most patients with PEP have a mild onset and are cured after minimal treatment. However, some patients develop severe pancreatitis and eventually die. The reported mortality rate is approximately 0.2–0.6% [2, 3]. Thus, more effective prevention methods are still needed. To date, many drugs and endoscopic methods have been investigated to prevent the occurrence of PEP [4–6]. Prophylactic pancreatic duct stenting is considered to effectively prevent PEP in some studies, but its clinical use is limited due to the need for higher technical capacity and cost. Drug classes that have been studied to prevent PEP include nifedipine, nitroglycerin, steroids, protease inhibitors, and somatostatin [7–12]. NSAIDs have been shown to decrease the incidence of PEP. Early studies of randomized controlled trials (RCTs) explored the administration of indomethacin or diclofenac rectally for the prevention of PEP. Subsequent meta-analysis and RCT studies confirm the role of NSAIDs in preventing PEP. Given these previous studies, the European Society of Gastrointestinal Endoscopy (ESGE) and the Japanese Society of Hepato-Biliary-Pancreatic Surgery guidelines recommend routine administration of indomethacin to prevent PEP [13, 14]. However, some recent high-quality RCT studies have shown that NSAIDs may not play a role in reducing the incidence of PEP [15–17]. In different RCT studies, the drug type, route of administration, time of the administration, and study population are not the same, with a lack of a targeted head in the clinical research. At the same time, recent meta-analysis indicated different conclusions about the specific time and route of administration of NSAIDs to prevent PEP. A survey from 29 countries showed a skeptical attitude towards NSAIDs in preventing PEP by a significant percentage of clinicians because of the lack of convincing evidence [18]. Therefore, in order to further clarify the role of NSAIDs in the prevention of PEP, determine the specific drug type, time and route of administration, and its application to the appropriate population, a more systematic, comprehensive, and rigorous evaluation is necessary. In the current study, we collected high-quality RCTs to provide a reliable evidence base for clinical trials of NSAIDs in the prevention of PEP.
Methods
Search strategy
Two authors (YX.C.and B.W.) independently conducted a comprehensive search in sources including PubMed, Embase, Web of Science, Cochrane Library Central, and ClinicalTrials.gov from August 1, 1990 to August 1, 2017. English search terms included albeit were not limited to the following: nonsteroidal anti-inflammatory drugs, NSAIDs, diclofenac, indomethacin, post-endoscopic retrograde cholangiopancreatography pancreatitis, post-ERCP pancreatitis, pancreatitis, endoscopic retrograde cholangiopancreatography, and ERCP. The search was limited initially to publications of human RCTs. The references of the articles identified after an initial search were also manually reviewed.
Inclusion and exclusion criteria
The following inclusive selection criteria were applied: (1) An RCT must compare the incidence of PEP with NSAID and placebo administration or no treatment. (2) The participants must have had a clinical diagnosis of PEP. (3) Studies must report the drug type, route of administration, time of administration, and incidence of PEP in each arm.
We excluded those that (1) were non-RCTs, retrospective studies, review articles, case reports, abstract, editorials, and letters to the editor, (2) published by the same author or agency repeatedly, and (3) had insufficient data on outcome measures of PEP.
Data extraction
In order to ensure the homogeneity of the extracted data, two authors (YM.W and WB.D) independently extracted the original data in the literature onto a standardized form: the first author, year of publication, country, sample size, types of NSAIDs, drug dose, time of administration, route of administration, and study population. If necessary, we contact the author of the study to obtain the study data. Conflicts in data abstraction were resolved by a consensus, and by referring to the original article.
Risk of bias asscessment
The authors independently assessed the quality of the literature in accordance with the Cochrane Collaboration Handbook [19]. The scoring system included the following criteria: random sequence generation, allocation concealment, blinding of participants and personnel, blinding the result assessment, incomplete data of the results, selective reporting, and other sources of bias.
Study quality was also assessed with the Jadad scale of randomized controlled trials (RCTs) [20]. Two reviewers (YX.L. and B.W.) independently assessed the quality of the included studies and discrepancies were resolved by discussion in plenum.
Statistical analysis
All statistical analyses were performed using the Review Manager (RevMan) version 5.3 software (Cochrane Informatics and Knowledge Management Department). Risk ratios (RR) with a 95% confidence interval (CI) were used for dichotomous outcomes. Studies with an I2 of 25 to 50% were considered to have low heterogeneity; studies with an I2 of 50 to 75% were considered to have moderate heterogeneity; and studies with an I2 > 75% were considered to have high heterogeneity.Random effect modelling was applied if the I2 > 50%. If not, fixed effect models were constructed. The publication bias was evaluated by χ2 test and funnel plots. The heterogeneity among studies was evaluated by χ2 test. A 2-tailed P value of < 0.05 was considered to be statistically significant. We also assessed the potential for publication bias through a visual inspection of a funnel plot asymmetry. The meta-analysis was conducted according to the PRISMA statement.
Results
Selected study and characteristics of the trials
Based on our search criteria, we identified 650 papers from the respective search engines, of which 460 duplicate articles were excluded. The remaining 190 studies were retrieved for their titles and abstracts, leaving 24 articles that appeared to meet our selection criteria. From these articles, three were excluded because they were retrospective studies. Finally, 21 RCTs [15–17, 21–38] with 6134 participants were included in the meta-analysis. A detailed flowchart of the selection process is shown in Fig. 1.
Fig. 1.
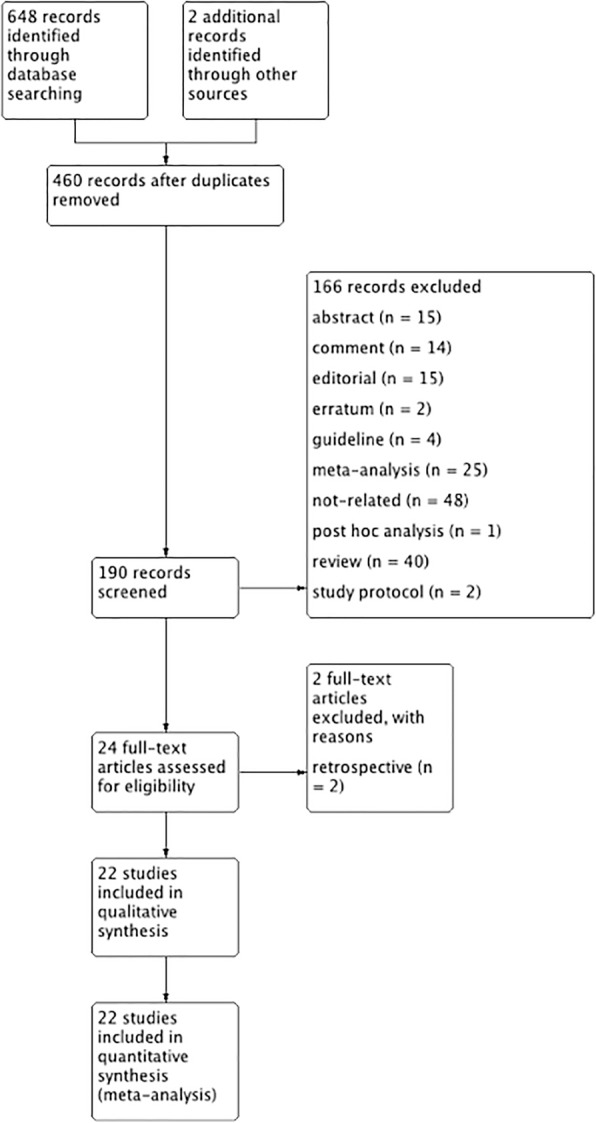
Flow diagram of the published articles evaluated for inclusion in this meta-analysis
The 6134 patients were divided equally into 3082 in the treatment and 3052 into control groups, respectively. Sample sizes ranged from 100 to 665, and the incidence rate of PEP varied from 2.24–16.43%. Diclofenac was used in eight studies [16, 21, 27, 29, 30, 32, 34, 38], indomethacin in 9 studies [15, 17, 22, 24-26, 28, 31, 33, 38], while valdecoxib [23], ketoprofen [37], naproxen [35], and celecoxib [36] were used in one study, respectively. NSAIDs were administered rectally in 14 studies [15–17, 22, 24–31, 33, 35], orally in two studies [36, 38], intravenously in two studies [23, 37], intramuscularly in two studies [21, 32], and intramuscularly and rectally in one study [34]. NSAIDs were administered pre-ERCP in 13 studies [17, 21, 23, 24, 26, 28, 30, 31, 33–38], post-ERCP in six studies [16, 22, 25, 27, 29, 32], during ERCP in one study [15], and pre-ERCP combined with post-ERCP in one study [38]. Six studies evaluated only patients at high-risk for developing PEP [16, 22, 25, 27, 29, 38], whereas 15 studies evaluated patients at average-risk for developing PEP [15, 17, 21, 23, 24, 26–28, 30–36]. The main characteristics of the studies included in this meta-analysis are shown in Table 1.
Table 1.
Characteristics of studies included in the systematic review
| Sample size | Jadad score | ||||
|---|---|---|---|---|---|
| Author Year | Setting | NSAIDs(n) | Placebo(n) | NSAIDs intervention | |
| Abu-Safieh [21] et al. 2014 | Single center | 89 | 93 | 75 mg diclofenac intramuscular before ERCP | 3 |
| Andrade-Dávila [22] et al. 2015 | Single center | 82 | 84 | 100 mg indomethacin rectal immediately after ERCP | 3 |
| Bhatia [23] et al. 2011 | Single center | 121 | 126 | 20 mg valdecoxib intravenous at the start of ERCP. | 3 |
| Cheon [38] et al. 2007 | Single center | 105 | 102 | 50 mg diclofenac 30–90 min oral before ERCP and 4–6 h after | 5 |
| Döbrönte [24] et al. 2012 | Single center | 130 | 98 | 100 mg indomethacin rectal 10 min before ERCP | 2 |
| Döbrönte [17] et al. 2014 | Multicenter | 347 | 318 | 100 mg indomethacin rectal 10–15 min before sedo-analgesic premedication | 2 |
| Elmunzer [25] et al. 2012 | Single center | 295 | 307 | 100 mg indomethacin rectal immediately after ERCP | 5 |
| Hosseini [26] et al. 2016 | Single center | 100 | 105 | 100 mg of indomethacin rectal two hours before the ERCP procedure | 3 |
| Kato [36] et al. 2017 | Single center | 85 | 85 | 400 mg celecoxib oral 1 h before ERCP | 2 |
| Khoshbaten [27] et al. 2008 | Single center | 50 | 50 | 100 mg diclofenac rectal after ERCP within 1 h | 4 |
| LevenickV [15] et al. 2015 | Single center | 223 | 226 | 2 * 50 mg indomethacin rectal during ERCP | 5 |
| Lua [16] et al. 2015 | Single center | 69 | 75 | 100 mg diclofenac rectal immediately after ERCP | 2 |
| Mansour-GhanaeiV [35] et al. 2016 | Multicenter | 162 | 162 | 500 mg naproxen rectal immediately before ERCP. | 3 |
| Montaño LozaV [28] et al. 2007 | Single center | 75 | 75 | 100 mg indomethacin rectal two hours before the procedure | 1 |
| Murray [29] et al. 2003 | Single center | 110 | 110 | 100 mg indomethacin rectal 2 h before ERCP | 5 |
| Otsuka [30] et al. 2012 | Multicenter | 51 | 53 | 50 mg (25 mg, if body weight < 50 kg) diclofenac rectal 30 min before ERCP | 2 |
| Park [32] et al. 2015 | Single center | 173 | 170 | 90 mg diclofenac intramuscular immediately after ERCP | 5 |
| Patai [31] et al. 2015 | Single center | 270 | 269 | 100 mg indomethacin rectal within 1 h before ERCP | 5 |
| Quadros Ono’frio [37] et al. 2017 | Single center | 224 | 223 | 100 mg ketoprofen intravenous during 20 min, immediately before the procedure, | 2 |
| Sotoudehmanesh [33] et al. 2007 | Single center | 221 | 221 | 100 mg indomethacin rectal mmediately before ERCP | 4 |
| UÇAR [34] et al. (1) 2016 | Single center | 50 | 50 | 100 mg diclofenac sodium rectal 30–90 min before the procedure. | 2 |
| UÇAR [34] et al. (2) 2016 | Single center | 50 | 50 | 75 mg diclofenac sodium IM 30–90 min before the procedure. | 2 |
Methodological quality and risk of bias
Methodological quality of included studies was evaluated by two investigators (YX.C. and B.W.) using the Cochrane Collaboration tool for assessing the risk of bias. Each trial was given an overall summary assessment of low, unclear, or high risk of bias. Discrepancies in the quality assessment were discussed and resolved by two reviewers (YM.X. and WB.D.). Figure 2 presents an overview of the methodological quality of the studies included in the review.
Fig. 2.
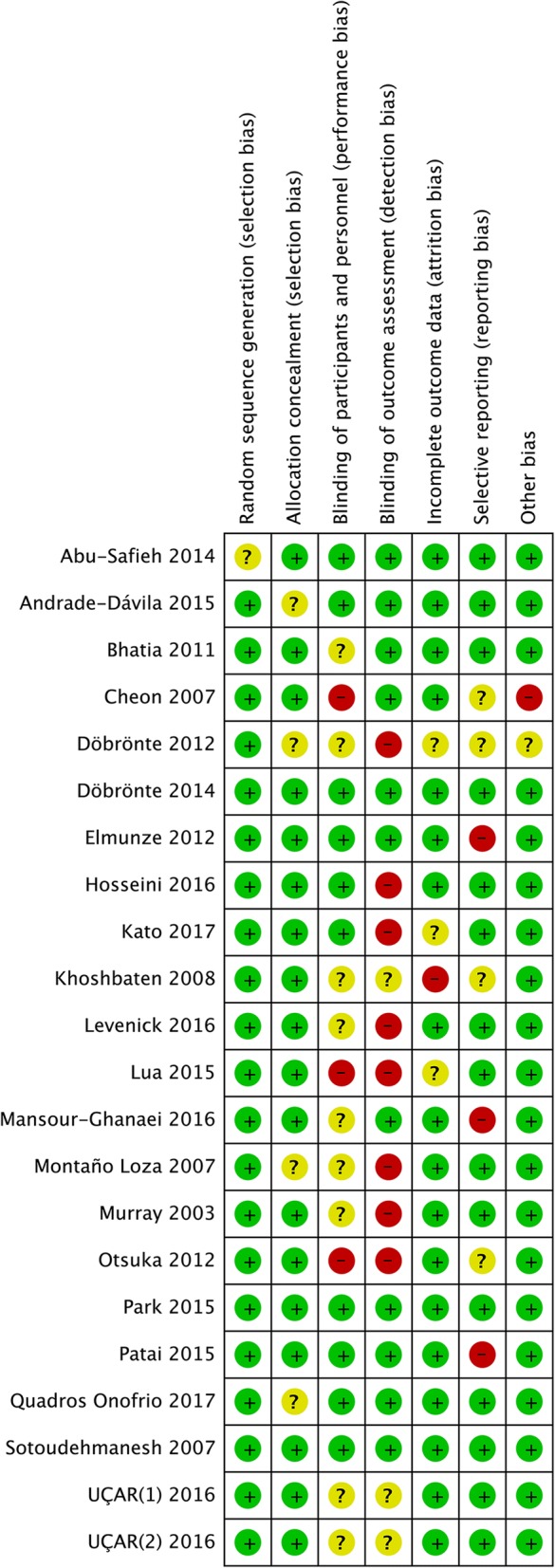
Consensus risk of bias assessment of the included studies. Green, low risk; yellow, unclear; red, high risk
Sensitivity analysis
The influence of a single study on the overall meta-analysis estimate was investigated by omitting one study at a time, and the omission of any study made no significant difference, indicating that our results were statistically reliable.
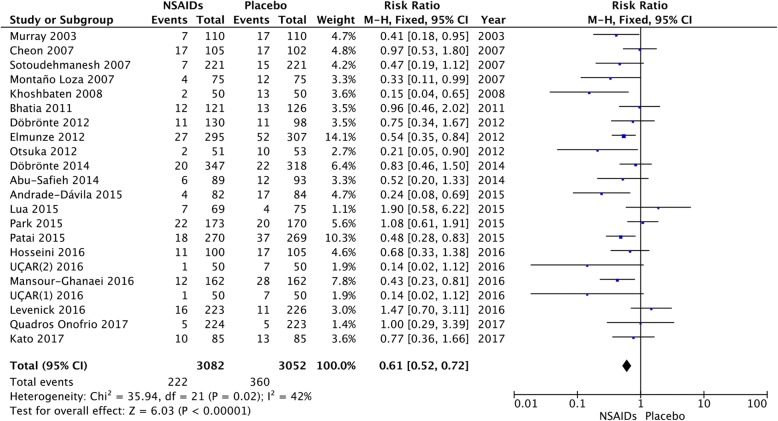
Risk reduction of PEP
We accepted the anthor’s classification stratification of the original studies because the border between studies performed on average-risk or high-risk patients was not well defined. Figure 3 shows the risk of PEP among all the included studies. Because the heterogeneity among these studies was not significant (I2 = 42%), we calculated the pooled estimates using the fixed-effects model. The RR of PEP was decreased by NSAIDs to 0.59 (95% CI, 0.51–0.68; P < 0.001).
Fig. 3.
Forest plot of the meta-analysis comparing NSAIDs and placebo for incidence of PEP
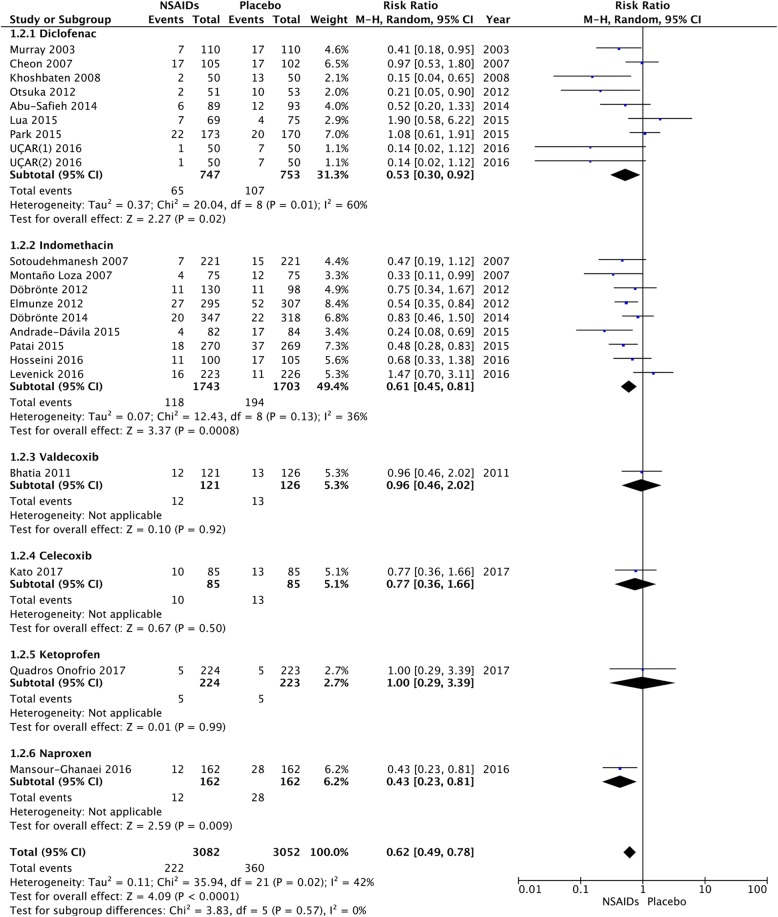
Type of NSAIDs
We calculated the pooled estimates using the fixed-effects model (I2 = 42%). Compared with a placebo, the RR of PEP significantly decreased to 0.61 (95% CI, 0.45–0.82; P = 0.0009) in the diclofenac group, to 0.60 (95% CI, 0.48–0.74; P < 0.00001) in the indomethacin group, and to 0.43 (95% CI, 0.23–0.81; P = 0.009) in the naproxen group. The results with the different types of NSAIDs did not differ significantly (P = 0.55) (Fig. 4).
Fig. 4.
Forest plot of the subgroup meta-analysis of the incidence of PEP with different types of NSAIDs
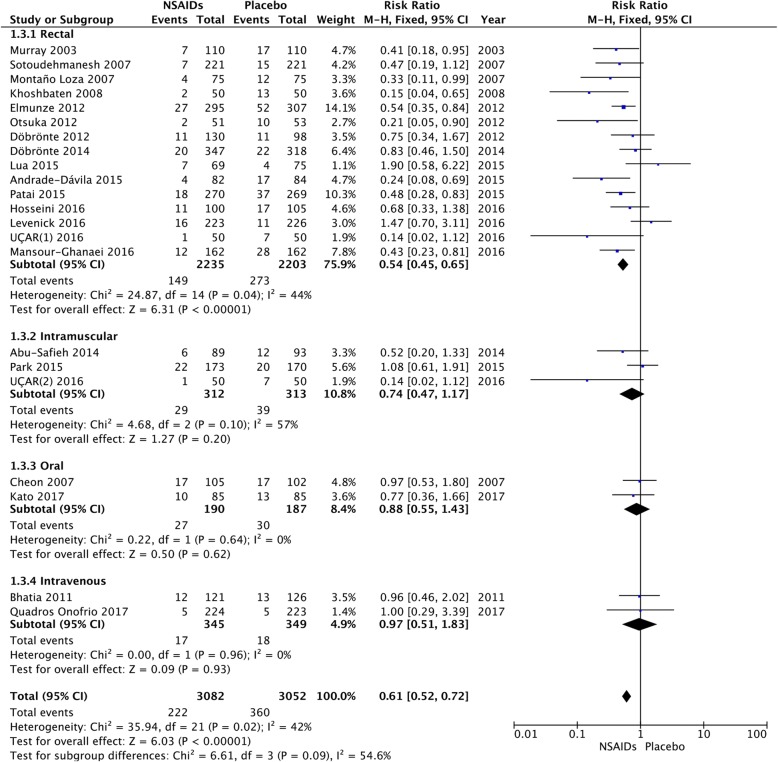
Route of administration
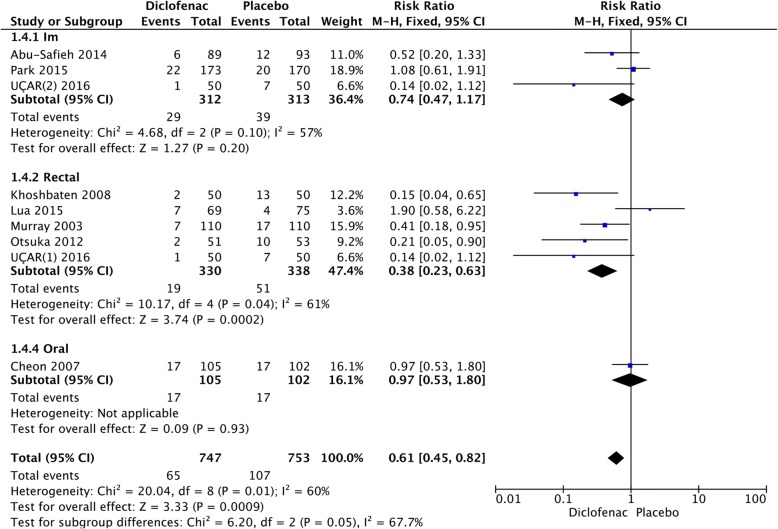
We calculated the pooled estimates using the fixed-effects model (I2 = 42%). The route of administration of the NSAIDs was divided into four: rectal, intramuscular, intravenous, and oral. PEP decreased significantly only in patients who received the drug rectally (RR = 0.54; 95% CI, 0.45–0.65; P < 0.00001), but did not for those who received the drug intramuscularly (RR = 0.74; 95% CI, 0.47–1.17; P = 0.20), intravenously (RR = 0.97; 95% CI, 0.51–1.83; P = 0.93), and orally (RR = 0.88; 95% CI, 0.55–0.1.43; P = 0.62) (Fig. 5). On comparing the incidence of PEP in the different routes of administration, rectal administration was the only effective route in the diclofenac group (RR = 0.38; 95% CI, 0.23–0.63; P = 0.0002) (Fig. 6).
Fig. 5.
Forest plot of the subgroup meta-analysis of the incidence of PEP based on the route of administration
Fig. 6.
Forest plot of the subgroup meta-analysis of the incidence of PEP based on the route of administration in the diclofenac group
Time of administration
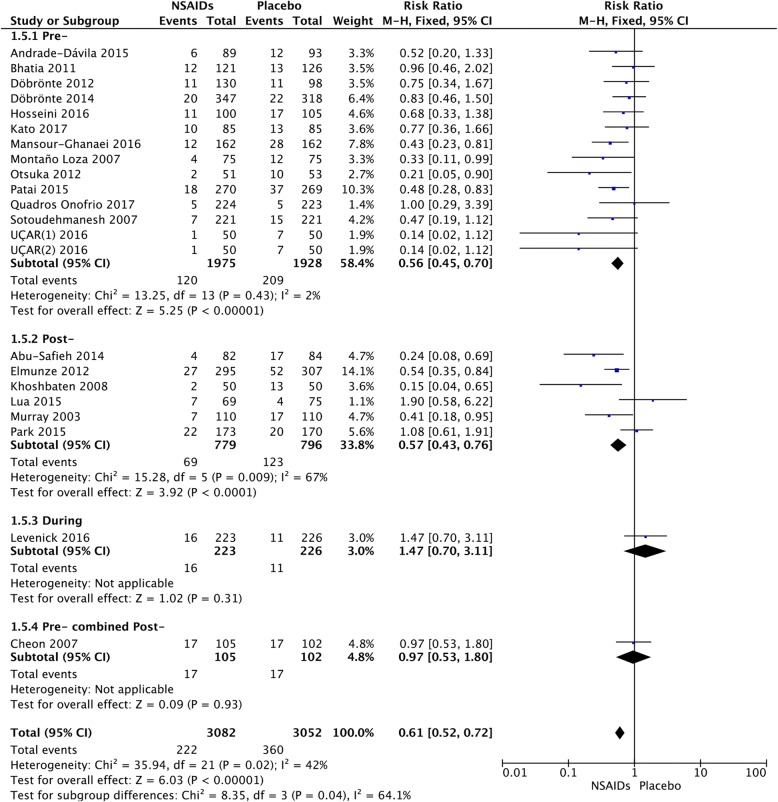
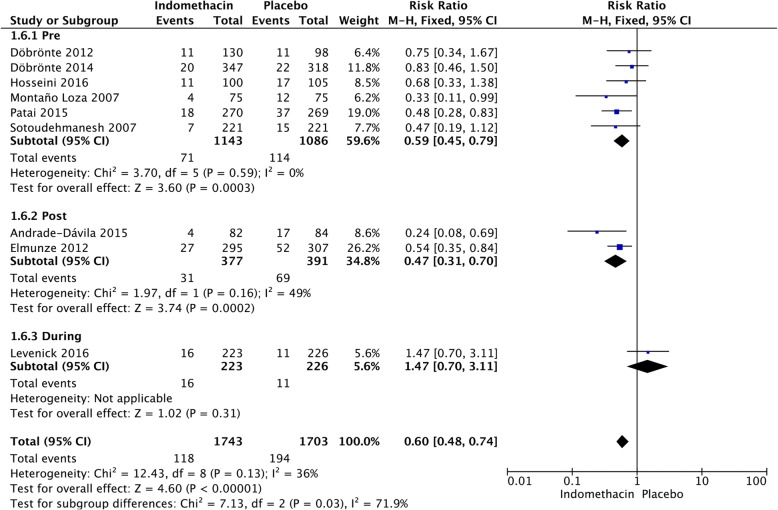
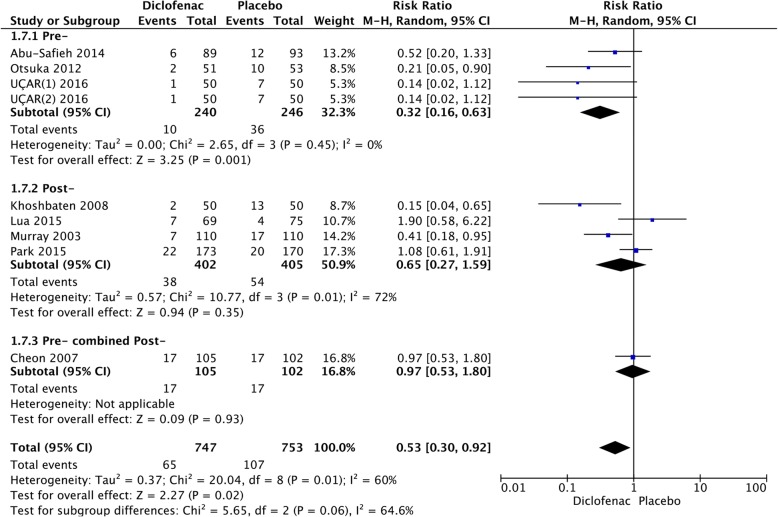
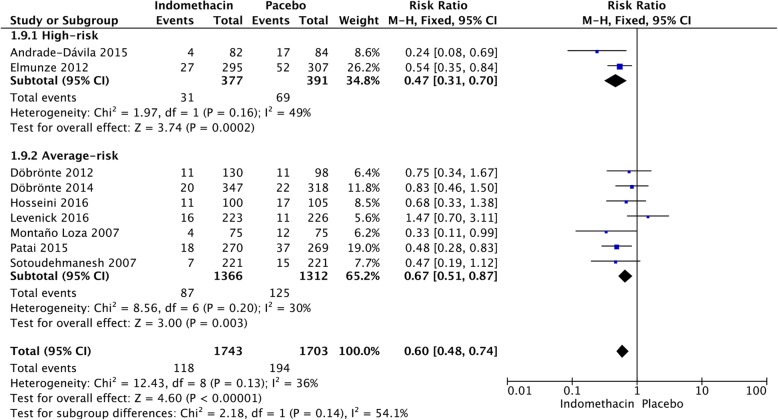
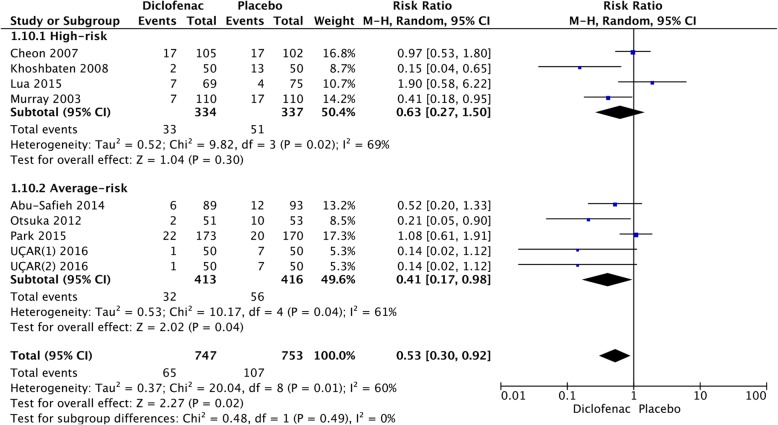
We calculated the pooled estimates using the fixed-effects model (I2 = 42%). The efficacy of NSAIDs was compared according to the time of administration, presented in Fig. 7. After stratifying the subgroups by time of administration, NSAIDs administered pre-ERCP (RR = 0.56; 95% CI, 0.45–0.70; P < 0.00001) were more effective than those administered post-ERCP (RR = 0.57; 95% CI, 0.43–0.76; P < 0.0001) (Fig. 7). The analysis showed that indomethacin administered post-ERCP (RR = 0.47; 95% CI, 0.31–0.70; P = 0.0002) appeared to be more effective in preventing PEP than those administered pre-ERCP (RR = 0.59; 95% CI, 0.45–0.79; P = 0.0003) (Fig. 8). In the diclofenac group, it was noted that the drug administered pre-ERCP (RR = 0.32; 95% CI, 0.16–0.63; P = 0.001) was more effective in preventing PEP than those administered post-ERCP (RR = 0.65; 95% CI, 0.27–1.599; P = 0.35) (Fig. 9).
Fig. 7.
Forest plot of the subgroup meta-analysis of the incidence of PEP based on the time of administration
Fig. 8.
Forest plot of the subgroup meta-analysis of the incidence of PEP based on the time of administration in the indomethacin group
Fig. 9.
Forest plot of the subgroup meta-analysis of the incidence of PEP based on the time of administration in the diclofenac group
Average-risk versus high-risk
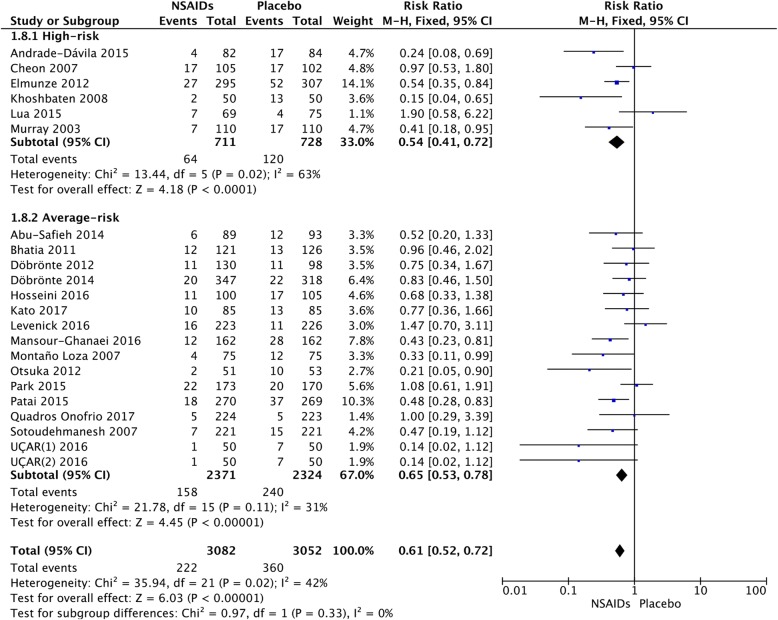
After stratification, according to different risk populations, it was noted that NSAIDs were effective in both high-risk (RR = 0.54; 95% CI, 0.41–0.72; P < 0.0001) and average-risk patients (RR = 0.61; 95% CI, 0.51–0.72; P < 0.00001) (Fig. 10). There was no significant difference between the two groups (P = 0.52). Indomethacin was associated with a decrease in the incidence of PEP in the high-risk (RR = 0.47; 95% CI, 0.31–0.70; P = 0.0002) and average-risk population (RR = 0.67; 95% CI, 0.51–0.87; P = 0.003) (Fig. 11). There was no significant difference between the two groups (P = 0.13). Figure 12 shows the risk of PEP among the diclofenac group that included high-risk and average-risk patients for PEP. The estimated pooled relative risks of PEP were 0.63 (95% CI, 0.27–1.50; P = 0.30) in high-risk patients and 0.41 (95% CI, 0.17–0.98; P = 0.02) in average-risk patients (Fig. 12).
Fig. 10.
Forest plot of the subgroup meta-analysis of the incidence of PEP by risk
Fig. 11.
Forest plot of the subgroup meta-analysis of the incidence of PEP by risk in the indomethacin group
Fig. 12.
Forest plot of the subgroup meta-analysis of the incidence of PEP by risk in the diclofenac group
Publication Bias
A funnel plot analysis was conducted to examine the publication bias. The graphic funnel plot of the 21 studies seemed to be symmetrical, which means that a publication bias is unlikely in this meta-analysis (Fig. 13).
Fig. 13.
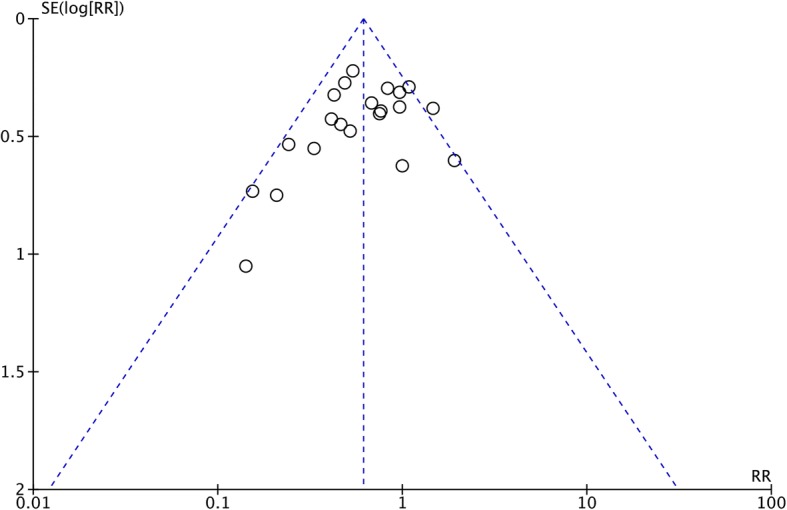
Funnel plot of standard error by log relative risk
Discussion
Our meta-analysis showed that the rectal administration of NSAIDs might be the most effective in decreasing the incidence of PEP. Further subgroup analysis showed that indomethacin and diclofenac were able to reduce the incidence of PEP significantly compared with the placebo control. However, no differences were observed between the two drug groups. Rectal was the only effective route of administration. While indomethacin could reduce the incidence of PEP regardless of patients having average or high risk. In contrast, diclofenac could only reduce the incidence of PEP in average-risk patients. In the subgroup analysis of the time of administration, we found that while indomethacin was able to reduce the incidence of PEP when administered before or after ERCP, it seemed to be more effective when administered after ERCP. Meta-analysis showed that pre-ERCP administration of diclofenac could reduce the occurrence of PEP; however, it was unable to do the same when administered post-ERCP.
There have been other research studies on the prevention of PEP administration of NSAIDs recently [23, 36, 37]. Most of the previous studies have confirmed the role of diclofenac and indomethacin in reducing the incidence of PEP. However, unlike previous studies, a recent large-sample retrospective study reported that diclofenac did not reduce the incidence of PEP in low-risk patients compared with the control group [39]. Additionally, in a recent RCT study, Levenick et al. also found that indomethacin did not reduce the incidence of PEP [15]. To the best of our knowledge, to date, no large-scale RCT studies have directly compared indomethacin and diclofenac with the aim of investigating the differences between the two drugs regarding the possibility of preventing PEP. However, only high quality head- to-head RCTs are required in the future. After inclusion of the latest RCT studies, our meta-analysis showed that the incidence of PEP in the indomethacin and diclofenac groups was significantly lower than that in the control group, but there was no significant difference between the two groups alone. No study has compared rectal indomethacin and diclofenac in a head-to-head trial to see if there is any difference in the efficacy between these 2 agents. Previous meta-analysis showed that diclofenac was more effective than other NSAIDs in reducing the incidence of PEP [40, 41]. However, one of the studies described only the rectal route of administration [40], and the other included the study included the rectal 、intravous、intramuscular and oral administration [41]. In contrast, the present study included only comprehensive and recent RCT studies. The influence of a single study on the overall meta-analysis estimate was investigated by omitting one study at a time, and this omission did not have a significant impact on the analysis. However, in all meta-analyses, including the present meta-analysis, the sample of the diclofenac group was relatively small, and the route of administration was different, which could make the results confusing to some extent. Some scholars have suggested that, although there is a certain similarity in the chemical structure, indomethacin is the most potent among all NSAIDs in inhibiting the degree of inflammatory response [42] . Makela et al. found that indomethacin is a better PLA2 inhibitor compared with diclofenac [42]. Then, indomethacin would theoretically be better for PEP prevention than diclofenac. However, there is no published study directly comparing the effect of different NSAIDs by rectal administration. Therefore, the difference of indomethacin and diclofenac in the prevention of PEP still needed further study.
Previous studies of different RCTs were different with regard to the time of administration. The meta-analysis included preoperative, intraoperative, postoperative, and preoperative combined with postoperative. The results showed that NSAIDs administered pre-ERCP may be more effective than those administered post-ERCP. This result is similar to the conclusion of a meta-analysis in 2017 that incorporates 16 studies [40]. However, there are different voices on the ideal time of administration [43–46]. Analysis of the causes may be related to the study population, types of NSAIDs, route of administration, and sample of studies included. This meta-analysis analyzes the time of administration of indomethacin and diclofenac, respectively for the first time. Further studies showed that incidence of PEP was lower in the group administered with indomethacin preoperatively and postoperatively than that in the control group and indomethacin appears to be more effective when administered after ERCP. A large multicenter study in 2016 showed better results in preoperative administration by comparing postoperative administration in unselected patients and postoperative administration in high-risk patients, but this study was inadequate for high-risk patients after surgery. Interestingly, in this meta-analysis, the two studies of indomethacin administration involved all high-risk patients. Whether this phenomenon will have an impact on the outcome still warrants more research. Wan et al.. showed that preoperative administration of indomethacin was better than postoperative administration [47], but in this study, the authors combined the intraoperative administration of Levenick et al. and postoperative administration [15]. The studies of Inamdar et al. and Sethi et al. showed no difference between the preoperative and postoperative groups [45, 46], but the above studies included eight and seven studies, respectively. The subgroup analysis of diclofenac showed that preoperative diclofenac administration could reduce the incidence of PEP effectively, while the incidence of PEP in the postoperative and control groups did not reveal any difference. The study of Patai et al.. showed that there was no differences between before and closely after ERCP in the use of diclofenac or indomethacin [46]. This meta-analysis has not analyzes the time of administration of indomethacin and diclofenac, respectively. However, there is still little research on diclofenac, and further studies are needed to determine the optimal dosing time of diclofenac or indomathacin. Thus, more targeted investigation is needed for determining a specific opportunistic time.
NSAID administration in the prevention of PEP of a specific population is also controversial. The definition of risk of ERCP were varied in the included studies. However, we accepted the original author’s classifications. In this study, we found no difference in the incidence of PEP between average-risk and high-risk patients. A similar conclusion has been drawn in the studies of Shen et al... [48] and Patai et al [46]. This conclusion provides the basis for the recommendations of the ESGE and Japanese Society of Hepato-Biliary-Pancreatic Surgery in its guidelines for the prevention of PEP. Further analysis showed that the effect of indomethacin on the prevention of PEP was not different between average-risk and high-risk patients. In the meta-analysis of Inamdar et al., the authors suggest that indomethacin is effective in high-risk patients and may not be effective for average-risk patients, but only eight studies were included in the analysis, and the authors believe that the study of Patai et al [31]. was for high-risk populations [49]. Elmunzer et al. showed that indomethacin was more effective in high-risk patients, but 82.3% of patients with sphincter of Oddi Dysfunction(SOD) were likely to have an effect in this study [50]. The subgroup meta-analysis of diclofenac group showed that the incidence of PEP had no difference between the high-risk and control groups but was significantly lower in the average-risk group compared with that in the control group. In the previous meta-analysis, there is currently no subgroup analysis of the population of administered with diclofenac. The difference between indomethacin and diclofenac may be related to their differences in inhibition of phospholipase A 2 [42]. However, in the current RCT and meta-analyses, the definition of PEP risk factors and inclusion criteria are different. Therefore, the above conclusions may be very questionable.
NSAIDs currently have four common routes of administration, including intravenous, intramuscular, oral, and rectal. Of the 21 studies included, 15 studies used the rectal route. Interestingly, so far, a non-rectal administration study concluded that NSAIDs did not reduce the incidence of PEP. Analysis of the causes may be related to the pharmacokinetics of NSAIDs. Rectal administration maximizes drug bioavailability and faster absorption, rapid concentration of drugs, and early suppression of inflammatory responses in pancreatitis. But more clear mechanisms need to be further studied.
However, this study had the following limitations: (1) As the quality of the literature was different, this may have caused some heterogeneity in our study and influenced the conclusion. The heterogeneous definitions of PEP, risk of PEP and indications for ERCP may add potential bias to the results. Also, the heterogeneous of patient (benign or malignant) may cause bias. The criteria of severity for PEP were different in previous studies. (2) This was a meta-analysis at the study level, and confounding factors at the patient level could not be properly assessed and incorporated into the analysis. Therefore, an additional multicenter and large sample of high-quality RCTs is needed to compare the effects of the different routes, dosage, and time of administration on the incidence of PEP.
Conclusions
Although there are some limitations in this study, we believe that the rectal administration of indomethacin or diclofenac can effectively reduce the incidence of PEP based on our meta-analysis of 21 RCT studies. However, different drug types, their specific time and route of administration, and appropriate population should be considered. More high quality head-to-head RCTs are required.
Acknowledgments
Funding
The cost of this meta-analysis in the design of this study and collection of data is supported by the scientific and technological research project of JinHua, China (No: 2015–3-085).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- CI
Confidence interval
- ERCP
Endoscopic retrograde cholangiopancreatography
- NSAIDs
Nonsteroidal anti-inflammatory drugs
- PEP
Post-ERCP pancreatitis
- RCTs
Randomized controlled trials
- RR
Relative Risk
- SOD
Sphincter of Oddi dysfunction
Authors’ contributions
YXL designed the research, analyzed the data and wrote the manuscript; YXC, BW, YMX and WBD collected literatures and conducted the analysis of pooled data; YXL conceived and designed the research, and revised the manuscript as corresponding author. All authors have read and approved the final version of this manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yunxiao Lyu, Email: lvyunxiao1986@gmail.com.
Yunxiao Cheng, Email: chengyunxiao1962@gmail.com.
Bin Wang, Email: wangbin.china@163.com.
Yueming Xu, Email: xuyaoming1962@gmail.com.
Weibing Du, Email: duweibin1961@gmail.com.
References
- 1.Freeman ML, Guda NM. Prevention of post-ERCP pancreatitis: a comprehensive review. Gastrointest Endosc. 2004;59(7):845–864. doi: 10.1016/S0016-5107(04)00353-0. [DOI] [PubMed] [Google Scholar]
- 2.Freeman ML, DiSario JA, Nelson DB, Fennerty MB, Lee JG, Bjorkman DJ, Overby CS, Aas J, Ryan ME, Bochna GS, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc. 2001;54(4):425–434. doi: 10.1067/mge.2001.117550. [DOI] [PubMed] [Google Scholar]
- 3.Barthet M, Lesavre N, Desjeux A, Gasmi M, Berthezene P, Berdah S, Viviand X, Grimaud JC. Complications of endoscopic sphincterotomy: results from a single tertiary referral center. Endoscopy. 2002;34(12):991–997. doi: 10.1055/s-2002-35834. [DOI] [PubMed] [Google Scholar]
- 4.Freeman ML. Pancreatic stents for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis. Clin Gastroenterol Hepatol. 2007;5(11):1354–1365. doi: 10.1016/j.cgh.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Fazel A, Quadri A, Catalano MF, Meyerson SM, Geenen JE. Does a pancreatic duct stent prevent post-ERCP pancreatitis? A prospective randomized study. Gastrointest Endosc. 2003;57(3):291–294. doi: 10.1067/mge.2003.124. [DOI] [PubMed] [Google Scholar]
- 6.Singh P, Das A, Isenberg G, Wong RC, Sivak MV, Jr, Agrawal D, Chak A. Does prophylactic pancreatic stent placement reduce the risk of post-ERCP acute pancreatitis? A meta-analysis of controlled trials. Gastrointest Endosc. 2004;60(4):544–550. doi: 10.1016/S0016-5107(04)02013-9. [DOI] [PubMed] [Google Scholar]
- 7.Prat F, Amaris J, Ducot B, Bocquentin M, Fritsch J, Choury AD, Pelletier G, Buffet C. Nifedipine for prevention of post-ERCP pancreatitis: a prospective, double-blind randomized study. Gastrointest Endosc. 2002;56(2):202–208. doi: 10.1016/S0016-5107(02)70178-8. [DOI] [PubMed] [Google Scholar]
- 8.Moreto M, Zaballa M, Casado I, Merino O, Rueda M, Ramirez K, Urcelay R, Baranda A. Transdermal glyceryl trinitrate for prevention of post-ERCP pancreatitis: a randomized double-blind trial. Gastrointest Endosc. 2003;57(1):1–7. doi: 10.1067/mge.2003.29. [DOI] [PubMed] [Google Scholar]
- 9.Budzynska A, Marek T, Nowak A, Kaczor R, Nowakowska-Dulawa E. A prospective, randomized, placebo-controlled trial of prednisone and allopurinol in the prevention of ERCP-induced pancreatitis. Endoscopy. 2001;33(9):766–772. doi: 10.1055/s-2001-16520. [DOI] [PubMed] [Google Scholar]
- 10.Dumot JA, Conwell DL, Zuccaro G, Jr, Vargo JJ, Shay SS, Easley KA, Ponsky JL. A randomized, double blind study of interleukin 10 for the prevention of ERCP-induced pancreatitis. Am J Gastroenterol. 2001;96(7):2098–2102. doi: 10.1111/j.1572-0241.2001.04092.x. [DOI] [PubMed] [Google Scholar]
- 11.Cavallini G, Frulloni L. Antiproteasic agents in the prevention of post-ERCP pancreatitis: rationale for use and clinical results. Jop. 2003;4(1):75–82. [PubMed] [Google Scholar]
- 12.Arvanitidis D, Anagnostopoulos GK, Giannopoulos D, Pantes A, Agaritsi R, Margantinis G, Tsiakos S, Sakorafas G, Kostopoulos P. Can somatostatin prevent post-ERCP pancreatitis? Results of a randomized controlled trial. J Gastroenterol Hepatol. 2004;19(3):278–282. doi: 10.1111/j.1440-1746.2003.03297.x. [DOI] [PubMed] [Google Scholar]
- 13.Dumonceau JM, Andriulli A, Elmunzer BJ, Mariani A, Meister T, Deviere J, Marek T, Baron TH, Hassan C, Testoni PA, et al. Prophylaxis of post-ERCP pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) guideline - updated June 2014. Endoscopy. 2014;46(9):799–815. doi: 10.1055/s-0034-1377875. [DOI] [PubMed] [Google Scholar]
- 14.Yokoe M, Takada T, Mayumi T, Yoshida M, Isaji S, Wada K, Itoi T, Sata N, Gabata T, Igarashi H, et al. Japanese guidelines for the management of acute pancreatitis: Japanese guidelines 2015. J Hepatobiliary Pancreat Sci. 2015;22(6):405–432. doi: 10.1002/jhbp.259. [DOI] [PubMed] [Google Scholar]
- 15.Levenick JM, Gordon SR, Fadden LL, Levy LC, Rockacy MJ, Hyder SM, Lacy BE, Bensen SP, Parr DD, Gardner TB. Rectal indomethacin does not prevent post-ERCP pancreatitis in consecutive patients. Gastroenterology. 2016;150(4):911–917. doi: 10.1053/j.gastro.2015.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lua GW, Muthukaruppan R, Menon J. Can rectal diclofenac prevent post endoscopic retrograde cholangiopancreatography pancreatitis? Dig Dis Sci. 2015;60(10):3118–3123. doi: 10.1007/s10620-015-3609-9. [DOI] [PubMed] [Google Scholar]
- 17.Dobronte Z, Szepes Z, Izbeki F, Gervain J, Lakatos L, Pecsi G, Ihasz M, Lakner L, Toldy E, Czako L. Is rectal indomethacin effective in preventing of post-endoscopic retrograde cholangiopancreatography pancreatitis? World J Gastroenterol. 2014;20(29):10151–10157. doi: 10.3748/wjg.v20.i29.10151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanna MS, Portal AJ, Dhanda AD, Przemioslo R. UK wide survey on the prevention of post-ERCP pancreatitis. Frontline Gastroenterol. 2014;5(2):103–110. doi: 10.1136/flgastro-2013-100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 21.Abu-Safieh Y, Altiti R, Lobadeh M. Diclofenac vs. placebo in a randomized double blind controlled trial, in post ERCP pancreatitis. Am J Clin Med Res. 2014;2(2):43–46. doi: 10.12691/ajcmr-2-2-1. [DOI] [Google Scholar]
- 22.Andrade-Davila VF, Chavez-Tostado M, Davalos-Cobian C, Garcia-Correa J, Montano-Loza A, Fuentes-Orozco C, Macias-Amezcua MD, Garcia-Renteria J, Rendon-Felix J, Cortes-Lares JA, et al. Rectal indomethacin versus placebo to reduce the incidence of pancreatitis after endoscopic retrograde cholangiopancreatography: results of a controlled clinical trial. BMC Gastroenterol. 2015;15:85. doi: 10.1186/s12876-015-0314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhatia V, Ahuja V, Acharya SK, Garg PK. A randomized controlled trial of valdecoxib and glyceryl trinitrate for the prevention of post-ERCP pancreatitis. J Clin Gastroenterol. 2011;45(2):170–176. doi: 10.1097/MCG.0b013e3181eb600e. [DOI] [PubMed] [Google Scholar]
- 24.Dobronte Z, Toldy E, Mark L, Sarang K, Lakner L. Effects of rectal indomethacin in the prevention of post-ERCP acute pancreatitis. Orv Hetil. 2012;153(25):990–996. doi: 10.1556/OH.2012.29403. [DOI] [PubMed] [Google Scholar]
- 25.Elmunzer BJ, Scheiman JM, Lehman GA, Chak A, Mosler P, Higgins PD, Hayward RA, Romagnuolo J, Elta GH, Sherman S, et al. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med. 2012;366(15):1414–1422. doi: 10.1056/NEJMoa1111103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hosseini M, Shalchiantabrizi P, Yektaroudy K, Dadgarmoghaddam M, Salari M. Prophylactic effect of rectal indomethacin administration, with and without intravenous hydration, on development of endoscopic retrograde cholangiopancreatography pancreatitis episodes: a randomized clinical trial. Arch Iran Med. 2016;19(8):538–543. [PubMed] [Google Scholar]
- 27.Khoshbaten M, Khorram H, Madad L, Ehsani Ardakani MJ, Farzin H, Zali MR. Role of diclofenac in reducing post-endoscopic retrograde cholangiopancreatography pancreatitis. J Gastroenterol Hepatol. 2008;23(7 Pt 2):e11–e16. doi: 10.1111/j.1440-1746.2007.05096.x. [DOI] [PubMed] [Google Scholar]
- 28.Montano Loza A, Rodriguez Lomeli X, Garcia Correa JE, Davalos Cobian C, Cervantes Guevara G, Medrano Munoz F, Fuentes Orozco C, Gonzalez Ojeda A. Effect of the administration of rectal indomethacin on amylase serum levels after endoscopic retrograde cholangiopancreatography, and its impact on the development of secondary pancreatitis episodes. Rev Esp Enferm Dig. 2007;99(6):330–336. doi: 10.4321/S1130-01082007000600005. [DOI] [PubMed] [Google Scholar]
- 29.Murray B, Carter R, Imrie C, Evans S, O'Suilleabhain C. Diclofenac reduces the incidence of acute pancreatitis after endoscopic retrograde cholangiopancreatography. Gastroenterology. 2003;124(7):1786–1791. doi: 10.1016/S0016-5085(03)00384-6. [DOI] [PubMed] [Google Scholar]
- 30.Otsuka T, Kawazoe S, Nakashita S, Kamachi S, Oeda S, Sumida C, Akiyama T, Ario K, Fujimoto M, Tabuchi M, et al. Low-dose rectal diclofenac for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: a randomized controlled trial. J Gastroenterol. 2012;47(8):912–917. doi: 10.1007/s00535-012-0554-7. [DOI] [PubMed] [Google Scholar]
- 31.Patai A, Patai AV, Solymosi N, Tulassay Z, Herszenyi L. Prevention of acute pancreatitis following endoscopic retrograde cholangiopancreatography. Orv Hetil. 2015;156(18):715–719. doi: 10.1556/OH.2015.30143. [DOI] [PubMed] [Google Scholar]
- 32.Park SW, Chung MJ, Oh TG, Park JY, Bang S, Park SW, Song SY. Intramuscular diclofenac for the prevention of post-ERCP pancreatitis: a randomized trial. Endoscopy. 2015;47(1):33–39. doi: 10.1055/s-0034-1390743. [DOI] [PubMed] [Google Scholar]
- 33.Sotoudehmanesh R, Khatibian M, Kolahdoozan S, Ainechi S, Malboosbaf R, Nouraie M. Indomethacin may reduce the incidence and severity of acute pancreatitis after ERCP. Am J Gastroenterol. 2007;102(5):978–983. doi: 10.1111/j.1572-0241.2007.01165.x. [DOI] [PubMed] [Google Scholar]
- 34.Ucar R, Biyik M, Ucar E, Polat I, Cifci S, Ataseven H, Demir A. Rectal or intramuscular diclofenac reduces the incidence of pancreatitis afterendoscopic retrograde cholangiopancreatography. Turk J Med Sci. 2016;46(4):1059–1063. doi: 10.3906/sag-1502-104. [DOI] [PubMed] [Google Scholar]
- 35.Mansour-Ghanaei F, Joukar F, Taherzadeh Z, Sokhanvar H, Hasandokht T. Suppository naproxen reduces incidence and severity of post-endoscopic retrograde cholangiopancreatography pancreatitis: randomized controlled trial. World J Gastroenterol. 2016;22(21):5114–5121. doi: 10.3748/wjg.v22.i21.5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kato K, Shiba M, Kakiya Y, Maruyama H, Ominami M, Fukunaga S, Sugimori S, Nagami Y, Watanabe T, Tominaga K, et al. Celecoxib oral Administration for Prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: a randomized prospective trial. Pancreas. 2017;46(7):880–886. doi: 10.1097/MPA.0000000000000852. [DOI] [PubMed] [Google Scholar]
- 37.de Quadros Onofrio F, Lima JCP, Watte G, Lehmen RL, Oba D, Camargo G, Dos Santos CEO. Prophylaxis of pancreatitis with intravenous ketoprofen in a consecutive population of ERCP patients: a randomized double-blind placebo-controlled trial. Surg Endosc. 2017;31(5):2317–2324. doi: 10.1007/s00464-016-5234-x. [DOI] [PubMed] [Google Scholar]
- 38.Cheon YK, Cho KB, Watkins JL, McHenry L, Fogel EL, Sherman S, Schmidt S, Lazzell-Pannell L, Lehman GA. Efficacy of diclofenac in the prevention of post-ERCP pancreatitis in predominantly high-risk patients: a randomized double-blind prospective trial. Gastrointest Endosc. 2007;66(6):1126–1132. doi: 10.1016/j.gie.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Rainio M, Lindstrom O, Udd M, Louhimo J, Kylanpaa L. Diclofenac does not reduce the risk of post-endoscopic retrograde cholangiopancreatography pancreatitis in low-risk units. J Gastrointest Surg. 2017;21(8):1270–1277. doi: 10.1007/s11605-017-3412-3. [DOI] [PubMed] [Google Scholar]
- 40.Hou YC, Hu Q, Huang J, Fang JY, Xiong H. Efficacy and safety of rectal nonsteroidal anti-inflammatory drugs for prophylaxis against post-ERCP pancreatitis: a systematic review and meta-analysis. Sci Rep. 2017;7:46650. doi: 10.1038/srep46650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rustagi T, Njei B. Factors affecting the efficacy of nonsteroidal anti-inflammatory drugs in preventing post-endoscopic retrograde cholangiopancreatography pancreatitis: a systematic review and meta-analysis. Pancreas. 2015;44(6):859–867. doi: 10.1097/MPA.0000000000000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makela A, Kuusi T, Schroder T. Inhibition of serum phospholipase-A2 in acute pancreatitis by pharmacological agents in vitro. Scand J Clin Lab Invest. 1997;57(5):401–407. doi: 10.3109/00365519709084587. [DOI] [PubMed] [Google Scholar]
- 43.Inamdar S, Berzin TM, Berkowitz J, Sejpal DV, Sawhney MS, Chutanni R, Pleskow DK, Trindade AJ. Decompensated cirrhosis may be a risk factor for adverse events in endoscopic retrograde cholangiopancreatography. Liver int. 2016;36(10):1457–1463. doi: 10.1111/liv.13100. [DOI] [PubMed] [Google Scholar]
- 44.Yang C, Zhao Y, Li W, Zhu S, Yang H, Zhang Y, Liu X, Peng N, Fan P, Jin X. Rectal nonsteroidal anti-inflammatory drugs administration is effective for the prevention of post-ERCP pancreatitis: an updated meta-analysis of randomized controlled trials. Pancreatology. 2017;17(5):681-688. [DOI] [PubMed]
- 45.Li L, Han Z, Yuan H, Zhang G, Jia Y, He C. Nonsteroidal anti-inflammatory drugs reduce the incidence of post-endoscopic retrograde cholangiopancreatography pancreatitis: a meta-analysis. J Hepatobiliary Pancreat Sci. 2017;24(9):520-529. [DOI] [PubMed]
- 46.Patai A, Solymosi N, Mohacsi L, Patai AV. Indomethacin and diclofenac in the prevention of post-ERCP pancreatitis: a systematic review and meta-analysis of prospective controlled trials. Gastrointest Endosc. 2017;85(6):1144–1156. doi: 10.1016/j.gie.2017.01.033. [DOI] [PubMed] [Google Scholar]
- 47.Wan J, Ren Y, Zhu Z, Xia L, Lu N. How to select patients and timing for rectal indomethacin to prevent post-ERCP pancreatitis: a systematic review and meta-analysis. BMC Gastroenterol. 2017;17(1):43. doi: 10.1186/s12876-017-0599-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen C, Shi Y, Liang T, Su P. Rectal NSAIDs in the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis in unselected patients: systematic review and meta-analysis. Dig Endosc. 2017;29(3):281–290. doi: 10.1111/den.12816. [DOI] [PubMed] [Google Scholar]
- 49.Inamdar S, Han D, Passi M, Sejpal DV, Trindade AJ. Rectal indomethacin is protective against post-ERCP pancreatitis in high-risk patients but not average-risk patients: a systematic review and meta-analysis. Gastrointest Endosc. 2017;85(1):67–75. doi: 10.1016/j.gie.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 50.Elmunzer BJ, Waljee AK. Can rectal NSAIDs replace prophylactic pancreatic stent placement for the prevention of post-ERCP pancreatitis? Gastroenterology. 2014;146(1):313–315. doi: 10.1053/j.gastro.2013.11.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.