Abstract
This work provides a perspective on the qualification and verification of physiologically based pharmacokinetic (PBPK) platforms/models intended for regulatory submission based on the collective experience of the Simcyp Consortium members. Examples of regulatory submission of PBPK analyses across various intended applications are presented and discussed. European Medicines Agency (EMA) and US Food and Drug Administration (FDA) recent draft guidelines regarding PBPK analyses and reporting are encouraging, and to advance the use and acceptability of PBPK analyses, more clarity and flexibility are warranted.
WHY IS PBPK MODEL QUALIFICATION NEEDED?
In the last decade, PBPK modeling and simulation has earned its rank in the model‐informed drug development paradigm. Applications of PBPK modeling can impact various stages of drug development, ranging from early compound selection for first in human (FIH) trials to dosing recommendations in product labeling.1 The rise of PBPK applications in drug development and the increasing number of submissions to regulatory agencies2, 3, 4 have recently prompted the FDA and EMA to issue draft PBPK guidelines for industry.5, 6 In the EMA draft guideline, special emphasis has been given to “qualification” of platform and reporting of PBPK modeling and simulation, while the FDA draft guidance focuses on the format and content of reporting PBPK analyses for regulatory submissions.
Given the importance of PBPK modeling and simulation in the drug development process, 34 PBPK modeling scientists representing 25 companies in the Simcyp Consortium7 and professor Malcom Rowland have collaborated to develop this perspective review. This consortium has been the engine driving research and development as well as applications of PBPK using the Simcyp platform for nearly 16 years. The collective technical and drug development experiences in PBPK modeling and simulation has been accumulated over the years by the Simcyp Consortium members, who meet annually to share experiences in PBPK applications and discuss the strategic and scientific direction of the PBPK platform. Thus, the perspective of this group is well positioned to provide a “guideline” or recommendations on how to handle PBPK qualification procedures that are intended for regulatory submission and decision‐making. The aim of this work is to provide a consortium perspective on 1) process of qualifying PBPK platforms; 2) steps for verification of the drug models; 3) extension of platform qualification for various intended uses; 4) examples of PBPK qualification procedures for regulatory submissions; 5) reporting analysis plan templates; and 6) highlights of remaining challenges and future opportunities.
In general, a software platform is an operating environment that is used to write (in the case of coding a model), compile (in the case of building a model in software or platform), and run applications. In the case of a PBPK model, the platform includes three key components: computational framework, physiological framework of the system, and drug properties. The computational component includes the program code, model structure, mathematical equations, as well as a runtime engine for executing applications. The physiological framework comprises system‐dependent parameters that describe the physiology of human or preclinical species. These system parameters are population‐specific and account for population variability and correlation between parameters. A PBPK platform may also contain a database of virtual populations such as healthy volunteers of different ethnicities, or populations with organ impairment. The drug model component of the PBPK platform comprises drug‐dependent parameters, and will vary depending on the question to be addressed by the PBPK modeling. In addition, within the platform a selection of appropriate mechanistic models describing absorption, distribution, metabolism, and elimination can be applied to the drug model.
Recently, definitions of the various terminology used in modeling practices, including “qualification” and “verification” of models, were described by Rostami‐Hodjegan.8 While qualification generally refers to a set of prerequisites that ensure “permission” to handle the intended use, verification, on the other, hand focuses on the predictive performance of the model. The documentation needed to support the qualification and verification of a PBPK platform should cover all three components of the platform. The software qualification is intended to ensure that the software does what it is intended to do from a computational perspective.9 Qualification of the system‐dependent components involves documentation of the physiological framework, the equations used to describe the system, as well as the physiological parameters feeding it. The drug model verification documents consistency between the input parameters and underlying mechanisms and assumptions within the related physiological system and the ability of the model to successfully simulate sets of observed data, sometimes following several iterations of a learn and confirm process. Figure 1 summarizes the overall framework of a PBPK analysis package intended for regulatory submission.
Figure 1.
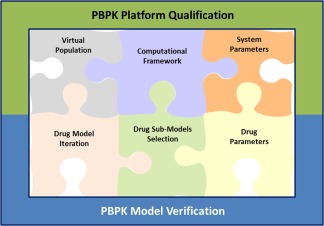
General components of a PBPK analysis package for submission to regulatory health authorities. Green frame represents the PBPK platform components that undergo qualification; blue frame represents the PBPK components that undergo verification. Model iteration is considered a verification step when new data emerge (i.e., clinical observations) and new learnings are applied to the drug model. The model iteration is an essential step towards verification of the parameters and assumptions that were originally implemented, including newly generated data to confirm prior assumptions and optimize parameters where necessary, a process that is generally accepted as good modeling practice across various areas of modeling and simulation.
The intended use of PBPK modeling and simulations in supporting regulatory submissions may cover a wide range of applications in the areas of drug–drug interactions (DDI), and extrapolation across different populations such as pediatric PK analysis and absorption modeling. The EMA draft guideline on PBPK is currently focused on DDI and pediatric PK analyses. However, there is scope for extending the guidelines to other areas of applications such as mechanistic absorption modeling,10 hepatic or renal impairment,11 multiple dose prediction from single‐dose data, and support justifications for proposed commercial products with regard to change in formulation.12
In this review we suggest a framework for PBPK platform qualification, drug model verification, and reporting procedures, along with practical guidance to address the technical and strategic aspects of PBPK analyses for regulatory submissions.
PLATFORM QUALIFICATION
A PBPK platform is an integrated software environment that allows building and running PBPK models that may or may not provide compound or population‐specific databases. From a software perspective, a platform includes various components such as graphical user interface (GUI), data structures, collections of various models, computational engine, as well as interfaces for presenting the simulation results. The PBPK models within a platform are developed to handle specific tasks based on certain assumptions. For example, a minimal PBPK model is developed to predict the drug plasma concentration and some of the relevant PK parameters such as area under the curve (AUC), maximum concentration (Cmax), time to achieve maximum concentration (Tmax), and clearance values. As long as the general model assumptions are valid and the correct drug and system parameters are used, the model can be used to simulate any drug regardless of how the drug is eliminated or what enzymes are involved. An example of such a model is the liver well‐stirred model,13 which is a common element of PBPK models used for a wide range of compounds where hepatic transporters are not involved, and has generally served the community well.
Similarly, the kinetics of competitive inhibition for a given enzyme are generally determined using the following equation:14
where Vm is the maximal metabolism rate, Km the Michaelis constant, S the unbound substrate concentration, I the unbound inhibitor concentration at the inhibition site, and KI the inhibitor constant. This equation is derived using first principles and has also been the basis of regulatory guidelines on DDI over the last two decades and is widely used for competitive inhibition.
Therefore, when models are developed based on generally acceptable scientific principles and the assumptions are reasonable, these models are considered qualified for the intended use irrespective of the enzymes they are applied to (i.e., CYP3A4 vs. CYP2C8).
Computational framework
Design qualification (DQ)
DQ is the documented collection of activities that define the processes of design, implementation, functional, and operational specifications and intended purpose of an instrument or a software platform. These processes are often part of a comprehensive quality assurance (QA) framework. DQ may be performed by the instrument manufacturer or the user. The supplier is generally responsible for robust design and maintaining documentation describing how the platform is developed and data in databases are analyzed and populated. Nonetheless, the user should ensure that the platform and relevant databases are suitable for their intended application and may evaluate whether the supplier has adopted a quality system that provides reliable software and databases. As an example, the QA system used to develop the Simcyp platform is described in Jamei et al.7
Installation qualification
When a user receives a software package and installs it onto their computer, it may have different settings from the machine used to develop the software. Therefore, it is necessary to ensure correct installation of the platform and confirm that results can be robustly reproduced on the users' computers. To this end, specific test simulations should be carried out to verify that the platform uses the same input file and generates similar output files as provided by the platform producer. These procedures ensure that the results are reproducible and no unintended problems are introduced while installing the platform (more information in Supplementary Text 1).
System parameters
A PBPK model is a mathematical model consisting of tissue and organ compartments, which are connected by circulating arterial and venous blood systems. Each compartment is defined by tissue‐specific volume and blood flow rate. These compartments generally include, but are not restricted to, adipose tissue, bone, brain, gut, heart, kidney, liver, lung, muscle, pancreas, skin, and spleen. Basic PBPK models assume perfusion rate‐limited distribution in all tissue compartments, whereas permeability rate‐limited distribution with active transport processes can be incorporated for specific organs, depending on the software. In addition, the gut compartment, which typically consists of the lumen and enterocytes for unabsorbed and absorbed drugs, respectively, is more complex to incorporate drug absorption processes. The gut compartment is generally divided further into subcompartments serially arranged corresponding to the different regions of the gastrointestinal tract such as duodenum, jejunum, and ileum. Each subcompartment requires specific system parameters such as tissue volume, surface area, fluid dynamics, pH, gastric emptying time, and intestinal transit time. Furthermore, some tissue/organ compartments, mainly gut, liver, and kidney, require physiological system parameters of drug‐metabolizing enzymes/transporters, such as their abundances or activities including genotypes and synthesis/degradation rates. There are also other important system parameters such as plasma protein and haematocrit concentrations, amounts of microsomal protein/hepatocytes per liver, and glomerular filtration rate of the kidney. These system parameters depend on specific population demographics such as age, gender, genotype, and disease state, and may exhibit different variability and covariance in each population. Therefore, the system parameters should be defined in specific virtual populations of interest such as healthy adult subjects of certain ethnicities, patients with impairment of organ function (e.g., liver and kidney), pediatric and geriatric patients, and cancer patients.
Drug parameters
Information pertaining to particular compounds (built‐in compound files in PBPK platforms) should be accompanied by documentation detailing the intended use and performance verification for the compound files. In general, the verification process of a compound file whether provided as part of the PBPK platform or newly developed is the same and is discussed in more detail in the “Drug model verification” section of this article. Information on the source of the parameters used in the model should be provided (i.e., whether they are derived solely from in vitro data or from clinical studies). The mechanistic components included in a compound file will vary from compound to compound and is dependent also on the intended use of the compound file. For example, if a compound file is developed for an inhibitor of a CYP isozyme that is not involved in its own metabolism and is primarily cleared by metabolism, then it may be sufficient to have a model that accurately describes the concentration of the compound at the site of inhibition with appropriate information on its potency (Ki) as an inhibitor of the CYP isozyme in question. In contrast, when developing a compound file for a substrate of a particular CYP isozyme it is necessary to ensure that in addition to describing the plasma concentration vs. time profile of the compound accurately, the fraction of the systemic clearance occurring via the isozyme in question is also accurately described. Parameters obtained by estimation techniques should be clearly identified and the data used in the fitting procedure described. Although the ideal scenario is to have separate sets of data for model building and model verification, in many cases there are insufficient data in the public domain to achieve this. When this arises this limitation of the model should be acknowledged. It is also helpful to list mechanisms not considered in the model. For instance, if a compound file is developed to enable the file to be used as a CYP 3A4 inhibitor and the compound is also known to inhibit other transporters or enzymes, but these are not considered in the model, this should be stated. If the compound file is developed as a potential DDI victim, it is useful to show performance verification in both the uninhibited and inhibited states. Likewise, for a perpetrator of DDI it is useful to show the performance of the compound model after single and multiple dosing, as well as showing the ability of the compound model to recover published drug interactions.
Sensitivity analysis
During the course of developing a specific compound or population model, there can be uncertainty in the true value of some of the parameters. This may be due, for example, to the absence of a specific parameter or unreliability of the in vitro data. In these cases, it is useful to assess the impact of uncertainty in those specific parameters or specific modeling assumptions may have on the simulation outcome. This is commonly done through a sensitivity analysis where the selected parameters are changed within a given reasonable range and a selected set of endpoints are investigated. Identifying whether an input parameter has a significant impact on the outcome of a simulation is highly valuable, as it assists with making decisions on which in vitro assays should be performed at what stage of drug development and how much resource should be invested in obtaining a particular parameter for a given compound.15, 16
Use of clinical data to refine/improve models
As part of the PBPK model development best practices, the predictive performance of compound models is evaluated by assessing the model's ability to recover clinically observed data. The observed data may also be used to refine and/or improve the model performance. In such cases the model is fit to the data to estimate a few of the model parameters to improve the model's predictive performance.17
There may be combinations of parameter values in a PBPK model that equally well fit or describe the observed data, potentially introducing identifiability issues. The problem may be reduced or avoided through rational selection of parameters to be fitted based on in‐depth knowledge of the compounds' physicochemical properties and elimination pathways, as well as the intended use of the model. Generally, sensitivity analysis is recommended to be undertaken prior to selecting the model parameters for fitting, as this helps to decide which parameters should, or can, be fitted.
QUALIFICATION OF VIRTUAL POPULATIONS
With regard to the qualification of populations used in PBPK modeling and simulation, the recently issued draft guidelines from both the EMA and FDA primarily focus on the healthy volunteer population, with the EMA draft guideline providing additional guidance on model applications using a pediatric virtual population. However, pharmacokinetic alterations in other special populations such as geriatric, obese, hepatic, or renal impairment and pregnancy have been reported and a PBPK‐guided dosing recommendation strategy for such special populations is currently lacking. In a recent analysis on initial approval of new molecular entities (NMEs), it was noted that ∼50–80% of NMEs lacked clear dosing recommendations for severe renal and hepatic impairment, with another 15–30% NMEs lacking such recommendations for mild renal and hepatic impairment.18 Among special populations, the pediatric population has been reasonably well studied, although primarily with CYP3A substrates.19 Potential strategies to develop, qualify, and verify other virtual special populations are described below.
Population qualification in PBPK models involves development of the system‐dependent parameters, followed by prospective prediction of PK in the population of interest (verification). The development of any virtual population in PBPK platforms involves evaluating the ability of the platform to construct virtual individuals with anatomical, physiological, genetic, and biological values similar to the observed values, i.e., does a specific population generate age, body weight, body height, and tissue volumes similar to the observed data? In the case of establishing a “healthy volunteer” population, population‐related data are generally assembled from public health databases such as NHANES (USA), NISRA (UK), and Statistics Bureau (Japan). Different ethnic populations (Caucasians, Chinese, Japanese, and African, etc.) may be developed either as completely new populations or by adapting a previously built population for relevant demographics, physiological parameters, and incorporation of genetic polymorphisms of metabolizing enzymes and transporters. Comparison of observed interethnic PK differences of the model drug with PK predicted by PBPK modeling may be considered an essential component of verification of an ethnic population.
In the case of special populations, changes in system parameters of the healthy volunteer population that describe the population of interest such as pediatric, pregnancy, renal/hepatic impairment, geriatrics, and obesity are made based upon either mechanistic evidence or fitting of the model parameters to observed clinical data.20, 21, 22, 23, 24, 25 For example, to establish a pediatric population, ontogeny changes in enzymes and transporters need to be explored by both quantification of the enzyme amount and evaluation of observed in vivo clearance of probe substrates.26 Another example is the establishment of an “oncology” population, where modeling of observed data in cancer patients led to identification of “albumin binding” and “alpha‐acid glycoprotein binding” as key descriptors of a cancer population.27 Finally, population verification is accomplished by successful recovery of observed PK data of a drug or group of drugs in the special population model. Upon this verification step, other drugs can be tested using the virtual special population in a prospective manner to make appropriate dosing recommendations. A generic scheme for qualification of special populations is outlined in Figure 2.
Figure 2.
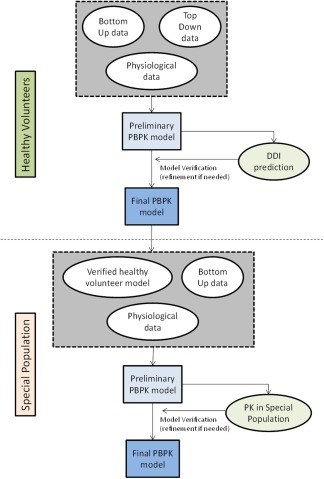
Steps for qualification of virtual populations using PBPK modeling and simulation.
DRUG MODEL VERIFICATION
Development and Verification of drug models
PBPK model development is an iterative process that may involve multiple cycles of “predict, learn, confirm.” Usually, a base model is first developed using experimentally determined or in silico predicted physicochemical and in vitro drug absorption, distribution, metabolism, and excretion (ADME) parameters. The predictions from the base model are then compared with the observed clinical PK data (e.g., PK from single ascending dose (SAD) or multiple ascending dose (MAD) studies) where a selection of model parameters may be adjusted to improve the drug model predictive performance based on sound physiological and scientific evidence. The predictive performance of the refined model will then be confirmed by comparing the prediction with the additional clinical PK data such as results from a DDI study that were not used in previous steps. Since the complexity of the model increases as additional mechanisms are identified from new in vitro or clinical data, at any given stage during the drug model development process, a compound model can be considered verified for a particular use or application if the predictive performance for that particular use or application is assessed to be satisfactory based on commonly accepted criteria (see below for criteria). For example, a drug may initially have been only identified as an inhibitor of CYP3A, but later found to be an inhibitor of a drug transporter as well. As long as the compound PBPK model is able to satisfactorily demonstrate its predictive performance of a DDI with a sensitive CYP3A substrate, the model is considered verified for its predictive performance for a DDI via CYP3A inhibition, although its predictive performance may have not yet been verified for a drug transporter inhibition. An exception would be if multiple mechanisms affect the same enzyme or transporter, or enzyme–transporter interplay. For example, if a drug inhibits and induces CYP3A4, the model needs to be verified for both mechanisms prior to declaring that the model is verified for CYP3A‐related applications.
It is important to distinguish the studies used for initial model development and refinement (the training dataset) (see section on approaches to PBPK models), from the dataset from studies used for model verification. The design of a clinical study and the use of data to support model verification should be based on the purpose of the application. If a substrate PBPK model is to be applied for DDI predictions with moderate and/or weak inhibitors, it is preferable to evaluate the predictive performance against the observed DDI with a strong inhibitor of the same enzyme.
For both model refinement and verification, Cmax, Tmax, CLpo (and CL and V if i.v. data are available), and AUC are some key parameters that may be used for comparison between predicted and observed values. Visual inspection of overlays of predicted and observed PK profiles is also performed. When evaluating the accuracy and acceptability of predictions, a commonly applied criteria is for values to be within 2‐fold of the observed values. However, results from one controlled clinical study may not be representative of the larger population, especially for drugs that exhibit high variability in PK or if the sample size was small in such studies. As a result, the 2‐fold criterion may be unreasonable for such drugs or studies. Instead, Abduljalil et al.29 proposed to evaluate the success of model predictions taking into account study sample size and the observed variance of the parameter of interest. Separately, the predictive performance of DDI simulations is usually evaluated by comparing the geometric mean ratios of Cmax and AUC in the presence or absence of an interaction, and their respective 90% CI. Guest et al.30 proposed that the predictive performance for DDI be based on the observed AUC ratios, instead of the 2‐fold rule, which tends to introduce bias at lower interaction levels. We encourage PBPK modelers to consider incorporating the above approaches when evaluating the accuracy of model predictions. However, depending on the intended use, therapeutic area, safety, and efficacy factors, the acceptable performance may be adjusted accordingly.
Role of sensitivity analysis in drug model verification
In addition to verification with observed data, sensitivity analysis of drug parameters that have high impact on prediction results and/or have high uncertainty is another important step in attaining confidence in the drug model. Uncertainty in a PBPK model is introduced by two main sources: 1) the underlying biology or mechanisms, and 2) drug‐related parameters. As discussed elsewhere in this article, the model assumptions and their potential impact on the predictions should be justified and explained based on available scientific evidence.
Additionally, there can be significant variation and thus uncertainty in the experimentally determined values of drug parameters. Further, some parameter values may be derived entirely from in silico predictions or parameter estimations from observed data, and should be evaluated using sensitivity analysis. However, the parameters and their ranges should be based on what is known about the mechanism and should not be chosen arbitrarily. The scientific rationale should be made clear. A global sensitivity analysis for every parameter that can influence the simulation outcome is unlikely to be informative, since many parameters may impact the outcome within these complex models. For instance, changing tissue blood flow and organ sizes will change the PK profile of a drug, but it is not clear how such an analysis would be informative within the same intended population without scientific justification.
Parameter identifiability
A PBPK model consists of many parameters, some of which may have unknown values either due to technical difficulties in measurement techniques or because they may have never been measured before. The unknown parameters are usually estimated through fitting of the model to the observed data from well‐defined study sets using a known specific dosing regimen.17, 31 A controlled input specifies data with observations from confident well‐defined study sets, e.g., at a known specific dose. However, estimation of model parameters through this approach can have limitations when the number of unknown parameters is large relative to the information contained in the available data. Various sets of parameter values can result in an equally good fit to the data in a way that individual parameters cannot be uniquely identified. In this case, the model loses mechanistic meaning and applicability and is said to be “unidentifiable.” As a result, extrapolation to populations outside the studied conditions is unjustified or may lead to false conclusions.17, 31
Various mathematical identifiability analysis approaches have been previously described in the literature.32, 33
Proposed approaches to deal with identifiability issues when fitting a PBPK model include measuring some of the unknown physiological parameter values, if possible, reduction in the number of parameters (by grouping several unidentifiable parameters into a single identifiable parameter), redefining parameters (reparameterization), or generating data that could be used in calibration with a different in vivo dataset.31, 32, 34, 35 Several statistical approaches can also be used to declare a PBPK model as identifiable.36 Overall, structural identifiability is not an issue for PBPK models when the model structure and parameter values are justified mechanistically and the PK properties are verified against observed data.37
APPROACHES TO BUILDING PBPK MODELS
PBPK models may be initiated based on in vitro understanding of drug‐related ADME mechanisms (i.e., “bottom‐up” approach). Alternatively, PBPK model development may be based on observed clinical data (i.e., “top‐down” approach). While both of these approaches have their advantages, it is becoming apparent that a verified bottom‐up or an integrative “middle‐out” approach may provide enhanced flexibility of PBPK models by applying the “predict, learn, confirm, apply paradigm” and allow a priori decision‐making. A summary of the various PBPK modeling approaches and corresponding data availability and applications is presented in Table 1.
Table 1.
Summary of PBPK modeling approaches and their applications
| Modeling approach | Data availability | Examples of modeling scenarios | General applications |
|---|---|---|---|
| Bottom‐up | Physiochemical properties and blood binding (LogP, pKa, fup, B/P) | Projection of human drug distribution | Provide mechanistic understanding |
| In vitro permeability and pharmaceutics information | Projection of human PK parameters and FIH dose | ||
| In vitro metabolism and transporters substrate data | Enzyme/transporter DDI projection (victim and perpetrator) | ||
| In vitro metabolism and transporters perpetrator data | |||
| In vivo ADME information in preclinical species | |||
| Top‐down | Clinical concentration‐time profiles from single or multiple ascending doses with summary of PK parameters | Development of model and identify parameters and their intersubject variability as well as identifying covariates | Support clinical trial decisions |
| Middle‐out | Physiochemical properties and in vitro ADME data may be available, but key in vitro quantitative or mechanistic data may be lacking | Refined predictions of DDI (perpetrator or victim) | Provide mechanistic understanding and support clinical trial decisions |
| Clinical concentration‐time profiles after single and multiple ascending doses with summary of PK parameters | Special populations (pediatrics, organ impairment), | ||
| May have clinical DDI data available as a victim and/or perpetrator for key CL pathway(s) | Formulation optimization or selection; in silico bioequivalence | ||
| In vivo human ADME or mass‐balance data |
“Bottom‐up” approach
A “bottom‐up” approach involves modeling of the mechanisms that define ADME processes and the related concentration profiles. This approach relies on high‐quality in vitro and preclinical data and may be verified later in drug development as clinical data become available (Table 1). The application of “bottom‐up” models depends heavily on the quality of the initial data, as well as the availability of verified in vitro–in vivo extrapolation (IVIVE) factors and scalars. Moreover, at early stages of drug development, a degree of uncertainty is associated with scalars for extrapolation of in vitro data to in vivo settings, especially where transporters are involved. As a result, early bottom‐up models will require verification with in vivo clinical data, and in some cases calibration of parameters through a “middle‐out” approach in order to be considered in regulatory decision‐making.
“Top‐down” approach
A “top‐down” approach often involves fitting of the model parameters to clinically observed plasma concentration–time profile and/or urine data of a drug following administration of single intravenous (i.v.) dose, single or multiple oral ascending doses, DDI scenarios, or exposure across multiple formulations. This approach is commonly used in population PK (PopPK) data analysis where statistical approaches are applied. In top‐down approaches the main objectives are to build a model that describes observed data, estimate parameters means and their intersubject variability, and to identify significant covariates of PK parameters. There are various statistical and visual approaches to assess the model goodness of fit. Usually, the optimized models are capable of interpolating data but extrapolation to outside the data space used to fit the model is challenging.
“Middle‐out” approach
The “middle‐out” approach is a combination of bottom‐up and top‐down approaches. In this approach the initial model relies on a foundation of high‐quality physicochemical, in vitro, preclinical, and mass‐balance data in combination with other in silico or built‐in PBPK prediction of drug distribution parameters. The model predictive performance and some of key parameters, such as the fraction metabolized (fm) by CYP3A4, can be optimized to recover the observed DDI with inhibitors.38 The refinement of model parameters using clinical data may be performed by either sensitivity analysis or using more powerful parameter‐estimation algorithms, including sparse data methods such as nonlinear mixed effects and Bayesian maximum‐likelihood procedures (for more information on parameter optimization, refer to Platform qualification section). The parameter(s) to be refined in a middle‐out PBPK model are usually uncertain due to experimental challenges, measurement with low confidence, or lack of data (such as kinetic parameters and ontogeny profiles of transporters, etc.) and can be sourced from a semimechanistic PopPK model.
The model predictive performance should be assessed using external verification datasets from independent clinical studies to verify the model. After verification, the refined model can be used to address clinically relevant questions, such as requesting a waiver for dedicated clinical trials, or to extrapolate PK from adults and younger children to neonates and infants to examine the existing dosing algorithm in adults.39, 40
INTENDED USE OF PBPK MODELING AND SIMULATION
A framework for modeling and simulation (M&S) in regulatory reviews was proposed by Manolis et al.,41 where the degree of regulatory scrutiny, level of documentation, and the need for early dialog is proportional to the impact level of the M&S exercise in regulatory decision‐making. In this section, we elaborate on the criteria and description of the different impact levels for PBPK modeling at the level of regulatory submission.
The impact that a PBPK analysis would have can be broadly categorized as being high, medium, or low, depending on the development stage and the questions being addressed. The majority of PBPK analyses share common themes that can be used to provide general guidance of the three categories.
High regulatory impact PBPK analysis
As stated in the recent EMA draft guideline on the qualification and reporting of PBPK M&S, high‐impact PBPK analyses are those in which results of trial simulations have been used in lieu of clinical studies to inform drug labels or as a basis to request waivers for clinical studies.6 The application of PBPK M&S in this manner has the potential to affect various sections of the drug label including drug interactions, contraindications, dosage and administration, and indications and use. Some examples of United States Package Inserts (USPIs) that used PBPK M&S to inform these sections are summarized in Tables 2, 3. High‐impact PBPK M&S analysis consists of models that make reasonable assumptions, have appropriately identified parameters with values that have either been experimentally determined or estimated to be reasonable for the purposes, and the model has been verified for its intended use against clinical data. Criteria are suggested below to qualify a high‐impact PBPK model, along with some examples and extrapolations to medium‐ and low‐impact PBPK analyses. Based on the collective experience within the Consortium, characteristics of high impact PBPK analysis comprise the following:
A qualified PBPK platform is used to build the drug model.
Drug model is built and verified for a specific mechanism and specified for the intended use (e.g., for a victim DDI prediction the percentage cleared by metabolism and fm for specific enzymes need to be known and verified, whereas in a perpetrator DDI prediction the inhibition parameters need to be known and verified).
Input drug parameters can be experimentally measured from in vitro systems, predicted from in silico tools, or estimated from available clinical or in vivo data as described in the “middle‐out” approach section above.
Drug model building is guided using a training set of clinical data.
Drug model verification for the intended use is performed using an independent set of clinical data.
Sensitivity analyses of uncertain parameters are performed and evaluated.
An evaluation of the risk to patients is made based on the outcome of PBPK predictions, including an evaluation of the level of uncertainty.
The PBPK analyses results in recommendations and appropriate use in product labels and/or regulatory decision‐making.
Table 2.
Examples of DDI PBPK analyses and their impact on drug development and regulatory decision
| Drug | Key theme (impact level) and question(s) | Victim/perpetrator? | Brief description | Internal impact | Qualification dataset | FDA/EMA response |
|---|---|---|---|---|---|---|
|
Dasabuvir (marketed) Arya et al., 201715 Shebley et al., 201716 |
DDI (high) Request to add contraindication with a non‐studied concomitant medication, and update safety labeling in the USPI |
Victim: Sensitive CYP2C8 substrate ‐ DDI potential with a CYP2C8 inhibitor | Dasabuvir is a component of Viekira Pak and is a CYP2C8 sensitive substrate, with potential for QTc prolongation at high multiples of therapeutic concentrations. Based on a publication from a DDI study between repaglinide (CYP2C8 substrate) and clopidogrel (CYP2C8 inhibitor), FDA requested a contraindication and update to the QT safety labeling of Viekira Pak containing dasabuvir when coadministered with clopidogrel. | PBPK modeling was conducted to account for all dasabuvir and clopidogrel mechanisms of disposition and DDI, simulations were used as a rebuttal. Intended use was to waive additional clinical study, contraindication and update to the safety labeling of a recently marketed drug. |
Clinical PK (SAD/MAD), DDIs, absolute bioavailability. Literature data for qualification of additional perpetrator PBPK models (i.e., clopidogrel and its glucuronide metabolite PK models and in vitro parameters). |
FDA: Accepted. FDA commentary on usefulness of model verification and reporting was published along with sponsor manuscript. EMA: Not submitted by the sponsor. |
|
Lesinurad (marketed) Not published |
DDI and genetic polymorphism effect (high) Lesinurad is cleared mainly via CYP2C9 metabolism and renal excretion. The polymorphic nature of CYP2C9 and renal impairment may increase the exposure of lesinurad |
Victim: CYP2C9 substrate, renal clearance | EMA asked for implications of CYP2C9 DDI risks and pharmacogenomics in patients with renal impairment | Study waiver |
PK in renal impairment population and polymorphism information within Simcyp used for PBPK simulations. Label Information: Lesinurad exposure is increased when coadministered with inhibitors of CYP2C9 and in CYP2C9 poor metabolizers. Patients taking moderate inhibitors of CYP2C9 and patients known to be CYP2C9 poor metabolizers should be treated with caution |
FDA: Accepted. EMA: Accepted. |
|
Sonidegib (marketed) Einolf et al., 201760 |
DDI (high) 1) Could the ketoconazole and rifampin clinical DDI study in healthy volunteers (HV) be bridged to cancer patients at the lower marketed dose of sonidegib? 2) Recommend dose modification with moderate and strong perpetrators of CYP3A (dose adjustment, acute or chronic dosing) |
Victim: CYP3A4 substrate | Model development: Absorption (first order model), Distribution (full PBPK), Elimination (retrograde calculation of fmCYP3A 0.75), Population (Healthy Volunteers); Data set for model building: PK in HV and cancer patients; Data set for model verification: DDI with ketoconazole and rifampin in HV; Model application: Effect of strong and moderate CYP3A perpetrators on the PK of sonidegib in cancer patients after 1) a single dose of sonidegib 2) steady‐state sonidegib with acute dosing of perpetrator (14 days) or 3) chronic dosing of both sonidegib and perpetrator (steady‐state). | Modeling used for label negotiations. No additional DDI study necessary at the lower marketed dose of 200 mg. No moderate CYP3A perpetrator clinical DDI studies requested. Modeling used to understand impact of dosing perpetrator after acute or chronic sonidegib dosing. | PK model building: 1) Single dose (200 mg) HV(1 trial); 2) Single dose (800 mg) HV (3 trials), cancer patients (1 trial); 3) Multiple dose 200 and 800 mg patients (1 trial); DDI victim model building: model based upon human ADME and enzyme reaction phenotyping; Model verification: ketoconazole and rifampin trial (using 800 mg sonidegib dose). |
FDA: Accepted. EMA: Accepted. |
|
Eliglustat (marketed) 28; Manuscript in preparation |
DDI (high) Support dose recommendations for eliglustat alone and during concomitant administration with CYP2D6 and/or CYP3A inhibitors for CYP2D6 EMs, IMs, and PMs. |
Victim: CYP2D6 and CYP3A substrate; mechanism‐based inhibitor of CYP2D6 | Model development: first order absorption, enzyme kinetics metabolism for elimination with additional renal clearance, and a dynamic model incorporating competitive and MBI of CYP2D6; Model verification: clinical studies for eliglustat alone and coadministration of a potent CYP2D6 inhibitor (paroxetine) or a potent CYP3A inhibitor (ketoconazole); Model application: simulations with different drug combinations (strong and/or moderate CYP2D6 and/or CYP3A inhibitors), dose regimens, and CYP2D6 phenotypes to provide appropriate dosing guidance | Study waiver: only two studies with three DDI scenarios with strong inhibitor(s) addressed with clinical studies to inform drug labeling, additional twelve DDI scenarios addressed with simulation results |
Model built and verified using multiple dose PK data for drug alone, and additional DDI studies. Simcyp built‐in library models for inhibitors with minor modifications (paroxetine, terbinafen, ketoconazole, fluconazole). BCS I drug, P‐gp substrate, low PPB (<90%). |
FDA: Accepted. EMA: Accepted. |
|
Ribociclib (marketed) Manuscript in preparation |
DDI (high) Recommend dose modification with strong CYP3A inhibitors. Ribociclib CYP3A DDI potential as perpetrator |
Perpetrator: MBI of CYP3A and CYP3A4 substrate | The DDI of perpetrators following single and multiple dose of ribociclib was simulated and a dose reduction to 200 mg and 400 mg when co‐administered with strong CYP3A inhibitors was justified, if the strong inhibitor cannot be avoided. DDI impact of ribociclib on the sensitive CYP3A substrate midazolam at 400 mg ribociclib was clinically explored. No change in ribociclib absorption was predicted when changing gastric pH to simulate the impact of PPIs, which was confirmed by population PK (PopPK) and non‐compartmental analysis (NCA) approaches. | Ritonavir study performed at 400 mg single dose of ribociclib. Other doses and multiple administration, impacting fmCYP3A4 due to auto‐inhibition, were simulated. DDI impact of moderate inhibitors was solely based on simulations. Midazolam DDI was investigated at one ribociclib dose, DDI was predicted for all other doses. No PPI study was performed. | SAD/MAD, ritonavir DDI, Midazolam DDI. |
FDA: Accepted. EMA: Accepted. |
|
Olaparib (marketed) US FDA Clinical Pharmacology Review61 |
DDI (high) Magnitude of DDI as a victim of CYP3A inhibition and perpetrator of CYP3A inhibition/induction, and P‐gp inhibition |
Victim and perpetrator: CYP3A4 substrate and a mechanism‐based inhibitor and weak inducer CYP3A | Simulations conducted to evaluate magnitude of DDI with CYP3A inhibitors and as a perpetrator of CYP3A inhibition and induction and P‐gp inhibition. | Study waiver. EMA requested 3 clinical studies to address (1) Olaparib as a perpetrator of CYP3A inhibition & induction, (2) Olaparib as a perpetrator of P‐gp inhibition and (3) Olaparib as a victim drug with moderate CYP3A inhibitors |
Simcyp compound library files. For P‐gp DDI, verification was performed with verapamil as a known P‐gp inhibitor. Ki was assumed to be IC50/2. Induction EC50/Emax was not calibrated within Simcyp. Predictions of Olaparib as a victim drug with CYP3A inducers (moderate and weak) were accepted by FDA but not by EMA. |
FDA: Accepted. EMA: Accepted. Model results for RI and TDI were accepted but not for CYP induction. |
|
Trametinib (marketed) Chen et al., 201561 |
DDI (high) Requested to provide clinical studies to investigate the inhibition of intestinal BCRP. In vitro BCRP inhibition data flagged the potential risk of in vivo DDI according to the EMA regulatory guidelines. |
Perpetrator: Weak BCRP inhibitor | In vitro Trametinib is a weak BCRP inhibitor, however based upon the EMA DDI guidance criteria the in vivo risk in the gut could not be excluded using in vitro data alone. Predicted intestinal concentrations were simulated using GastroPlus. Complete inhibition was predicted for the first 40 minutes post dose and partial inhibition was predicted up to 1.6 hours post dose and restricted to the duodenum and jejunum. Recommendation was to limit the co‐administration of sensitive BCRP substrates to 2 hours post trametnib administration | Previously constructed GastroPlus Model of trametinib was developed for other applications, therefore minimal work was required to construct the model in response to the agency. Absorption was simulated and the outputs of the model (predicted concentrations vs. time) along the intestinal track were used as input in the DDI prediction guidelines, internal static modeling as well as cross referencing data in the Washington database to inform concomitant medications at risk. No clinical BCRP DDI study was conducted |
In vitro BCRP inhibition data. Sponsor was requested to further discuss the interaction potential between trametinib and drugs mainly absorbed in the duodenum and jejunum. Outcome: Using the University of Washington database a list of BCRP substrates absorbed within 1‐2 hours after oral administration was constructed. This list was further refined to exclude those substrates in which the DDI mechanism was known, leaving behind a list of substrates that may potentially be affected by BCRP inhibition. |
FDA: Not submitted by the sponsor. EMA: Accepted. |
|
Eribulin (marketed) EMA Assessment Report 62 |
DDI (high) What is the risk of CYP3A‐mediated DDI with eribulin? |
Perpetrator: Moderate CYP3A inhibitor | In response to request from regulators for a clinical DDI trial with midazolam, a PBPK model was developed and simulation results were provided to support a minimal DDI risk. Simulations were performed for clinically relevant doses as well at supra therapeutic doses | Eribulin is an oncology drug, a clinical DDI trial would have to be conducted in patients causing delay in the development program. | Clinical PK data Simulation was in healthy volunteers. |
FDA: Not submitted by the sponsor. EMA: Accepted. Requested additional sensitivity analysis on the values of Ki. The analysis was performed and provided to the EMA. Results were accepted and DDI trial with midazolam was waived. |
|
Clopidogrel (marketed) Djebli et al., 201563 |
DDI, genetic polymorphisms and sequential metabolism (high) Compound as a victim; DDI (different CYPs involved) and impact of CYP2C19 genetic polymorphism on clopidogrel and secondary active metabolite |
Victim: CYP2C19 substrate | To predict simultaneously the PK of the parent drug and its primary and secondary metabolites in populations with genetically different activity for CYP2C19 | More accurate prediction of DDI and impact of the different CYPs involved in the 2 metabolic steps of clopidogrel (clopidogrel to 2‐oxo‐clopidogrel and 2‐oxo‐clopidogrel to clopi‐H4 active metabolite) |
Bespoke models built with the specific secondary metabolite module in Simcyp. Clinical studies used with CYP2C19 PM, EM, IM, and UM and DDI study for model refinement and qualification; qualification of the CYP2C19 IM and UM virtual populations. |
FDA: Not submitted by the sponsor. EMA: Accepted. Agency requested simulations to evaluate the impact of DDI and genetic polymorphisms on the PK of clopidogrel and its active secondary metabolite. |
|
Crizotinib (marketed) Mao et al., 201348 Yamazaki et al., 201549 |
DDI (medium ‐ high) What is the risk of CYP3A‐mediated crizotinib DDIs with moderate CYP3A inducer? |
Perpetrator: Mechanism‐based inhibitor of CYP3A and CYP3A4 substrate | Crizotinib is a CYP3A substrate (fm ~0.8) and a moderate time‐dependent inactivator (increased midazolam exposure, AUCR ~4). | Company planned to revise labels, eg, no dose modification with concomitant moderate CYP3A inducers |
Clinical DDI results with midazolam, ketoconazole, rifampin, single‐ and multiple‐dose PK data. Simcyp compound file of efavirenz was developed and verified based on data in literature. |
FDA: Accepted. Support of dosing recommendations for concomitant medications that are moderate CYP3A inducers in FDA Briefing Document, March 15, 2017. EMA: Not accepted. IVIVE for induction is not well established due to the complexity in induction mechanism. |
|
Panobinostat (marketed) Einolf et al., 201764 |
DDI (high) Effect of gastric pH modifying agents on PK of panobinostat |
Victim: pH‐dependent solubility, lower at higher pH | Panobinostat is a drug with a pH dependent solubility profile. Compounds that can increase the pH may decrease the solubility of panobinostat | PBPK simulations suggested no interaction with pH modulators |
Clinical PK. No effect of elevated pH on the PK of panobinostat was expected based on PBPK simulations. |
FDA: Accepted. Results added to USPI. EMA: No response. |
|
Naloxegol (marketed) Zhou et al., 201665 |
DDI (medium) Naloxegol Simulations as a victim of CYP3A and P‐gp DDIs |
Victim: CYP3A and P‐gp substrate | Dose adjustment when dosed with CYP3A modulators and P‐gp inhibitors | Study waiver | Simcyp compound library files. No Naloxegol P‐gp kinetic parameters available, assumed to be same as digoxin. |
FDA: Accepted. EMA: Accepted. |
|
Osimertinib (marketed) US FDA66 |
DDI (high) PBPK Simulations were performed as a victim of CYP3A and to inform dose adjustment with strong/moderate/weak CYP3A inducers |
Victim: CYP3A substrate | Dose adjustment when dosed with CYP3A inducers | Clinical study waiver | Simcyp compound library files, Dexamethasone (company internally developed compound file), rosuvastatin clinical DDI study available. |
FDA: Accepted. EMA: Not accepted. |
|
Lenvatinib (NDA submission) Luzon et al., 20163 |
DDI (high) What is the risk of CYP2C8‐mediated DDI with lenvatinib (Lenvima) |
Perpetrator: CYP2C8 inhibitor | Lenvatinib was shown to be an inhibitor of CYP2C8 in vitro. PBPK simulations were performed to evaluate the DDI risk with repaglinide, and demonstrated it was negligible | Company decided to ask for waiver of clinical DDI trial with CYP2C8 substrate (repaglinide) based on the PBPK modeling results | Clinical PK data. |
FDA: No Comments. EMA: Accepted. Waived a clinical DDI trial based on simulations. |
|
Cobimetinib (NDA submission) Budha et al., 201638 |
DDI (high) What is the effect of perpetrators on the exposure of cobimetinib? |
Victim: CYP3A4 substrate | PBPK model verified using DDI data with itraconazole. DDI with moderate and mild inhibitors and strong and moderate inducers predicted using the PBPK model | Corresponding DDI studies waived |
Clinical PK, and DDI study with itraconazole fm CYP3A4 estimated with sensitivity analysis to reproduce itraconazole DDI study result. |
FDA: Accepted (dose modification with moderate inhibitor and avoid use with strong/moderate inducer; simulations results included in USPI). Predicted DDI outcome in oncology patient population and at the steady state after multiple dose. EMA: Accepted (caution with moderate inhibitor and avoid use with strong/moderate inducer). Request to perform sensitivity analysis for additional parameters, including Fg. Request to qualify the model with more DDI data (qualification dataset was not submitted). |
|
Perampanel (NDA submission) Gidal et al., 201746 |
DDI (high) Explore reasons for lack of clinically significant DDI with ketoconazole, in spite of drug being 100% CYP3A substrate. Reverse EMA's major objection to file |
Victim: CYP3A4 substrate | Perampanel has been shown to be eliminated exclusively by CYP3A metabolism. However, a clinical DDI study with ketoconazole showed only a ~25% increase in perampanel AUC. Regulators saw this as evidence of lack of understanding of the elimination pathways of perampanel, and requested repetition of the DDI trial and further studies. PBPK modelling supported original understanding of a poorly designed DDI trial. PBPK analyses provided further support that there were no feasible trials to be conducted. | Changes in labeling with warning that in spite of the low DDI shown in the DDI trial with ketoconazole, a higher effect cannot be excluded therefore care should be taken when co‐administering perampanel with CYP3A inhibitors. | Clinical PK, DDI studies. |
FDA: No comment from FDA regarding the PBPK analysis EMA: Accepted. Additional sensitivity analyses were requested. Major objection was removed. |
|
Perampanel (NDA submission) Gidal et al., 201746 |
DDI (high) Explore the changes in PK profile of perampanel (Fycompa) after discontinuation of co‐administered CYP3A inducing anti‐epileptic drugs; explore potential dose titration strategies to maintain plasma concentrations at the same levels as during co‐administration |
Victim: CYP3A4 substrate | Fycompa is currently approved for combination with other anti‐epileptic drugs, many of which are CYP3A inducers. Perampanel is a CYP3A substrate, and therefore its plasma concentrations are expected to be affected by removal of co‐administered CYP3A inducers when converting to monotherapy. PBPK simulations were used to explore many different scenarios and suggest potential down titration options. | Simulations supported the regulatory filing | Clinical PK, DDI studies. |
FDA: No comment from FDA regarding the PBPK analysis. EMA: Accepted. Additional sensitivity analyses were requested. PBPK modeling was accepted and Major Objection was removed. Submission was approved. |
|
NCE (NDA submission) Not published |
DDI (medium) NCE was shown to be a substrate of UGT. What is the predicted DDI with UGT inducers (oxcarbazepine, phenobarbital, and phenytoin) |
Victim: UGT substrate | NCE PBPK model was built and verified. Simulations were conducted with inducers of UGT that have not been studied clinically. Simcyp compound files were used for carbamazepine and phenytoin. Sponsor used internally generated in vitro data for UGT induction parameters (EC50/Emax). | Support labeling recommendations | NCE model was verified using PK data results from two clinically‐studied inducers, efavirenz and carbamazepine. |
FDA: Not accepted. Insufficient data to determine appropriate dosing recommendations. EMA: Accepted. (based on modeled inducers being of similar potency to those clinically studied). |
|
Lenvatinib (NDA submission) Luzon et al., 20163 |
DDI (high) What is the risk of CYP3A‐mediated DDI lenvatinib |
Perpetrator: CYP3A4 inhibitor | Lenvatinib was shown to be an inhibitor of CYP3A in vitro. PBPK simulations were performed to evaluate the DDI risk with midazolam and demonstrated it was negligible (predicted mean AUCR ∼ 1.19). These results were included in the submission with a request for a waiver of the clinical DDI trial | Company decided to ask for waiver of clinical DDI trial with CYP3A substrate (midazolam) based on the PBPK modeling results |
PK predictions were verified against phase I PK data An analysis showed that despite potential for CYP3A inhibition in the intestine, this was unlikely to happen since inhibitory concentrations would not be reached due to solubility limitation. |
FDA: No comments on PBPK simulations. EMA: Accepted. Model predictions for CYP2C8 DDI but not for CYP3A DDI risk. Intestinal DDI predictions were not as well accepted as liver DDI predictions. Maintained the request for the clinical DDI study. |
|
NCE (NDA submission) Not published |
DDI (high) NCE is an inhibitor of CYP3A4. A DDI study was conducted with midazolam, however the final clinical dose and formulation were not used in the study. |
Perpetrator: CY3A inhibitor | PBPK simulations were conducted to predict the effect with the registration dose and formulation. | Support labeling recommendations | Model was verified using clinical DDI data from the original midazolam study. |
FDA: Accepted. EMA: Accepted. Requested additional sensitivity analysis on the in vitro CYP3A4 IC50. |
Table 3.
Examples of non‐DDI PBPK analyses and their impact on drug development and regulatory decisions
| Drug | Key theme (impact level) and question(s) | Brief description | Internal impact | Qualification dataset | FDA/EMA response |
|---|---|---|---|---|---|
|
Lesinurad (marketed) Pepin et al., 201667 |
Absorption: specifications for dissolution and particle size (high) Using an validated in silico model to support proposed specifications for dissolution and particle size without having to do a in vivo relative BA or bioequivalence study |
In silico PBPK model (GastroPlus) using 3 compartment PK model based on IV and PO clinical data with a mechanistic absorption model based on in vitro dissolution data fitting particle size distribution. | Waiver granted for a clinical relative bioequivelance study |
IV and PO clinical data. Validated using clinical data from a batch that was bioinequivalent. Several methods to input in vitro dissolution data into the model were evaluated. |
FDA: Accepted. PBPK model accepted in support of proposed control strategy without the need for a relative BA study. EMA: Not submitted by the sponsor. |
|
Canagliflozin (marketed) Tistaert et al., 2015) 68 |
Absorption (high) Differences in particle size between different API batches manufactured in a different will not lead to differences in oral bioavailability and no relative bioavailability study is needed |
During formulation development the granulation and milling processes were slightly changed. Non‐particle‐engineered to particle‐engineered. Bottom‐up approach predicted physchem and measured solubilities; particle size distribution combined with compartmental PK based on clinical data. | PBPK modeling was used to assess particle size sensitivity of canaglifozin bioavailability, without the need to perform a relative BA study | PK of canagliflozin across 3 dose levels of the non‐particle size engineered tablets. And a nonclinical bioavailability study in beagle dogs. |
FDA: Accepted. Requested additional information upon reviewing the data package: (1) Physchem property data; including intrinsic dissolution profile comparison; (2) GastroPlus model and simulation details, including description, assumption and validation for the model. Also include the scenarios, parameters and interpretations for the simulation; (3) the data for each trial used in the cross‐study comparison. EMA: Not submitted by the sponsor. |
|
Ribociclib (marketed) Samant et al., 201757 |
Absorption (high) Impact of PPIs on ribociclib absorption |
No change in absorption was predicted when changing stomach pH to simulate the impact of PPIs, which was confirmed by clinical data that were evaluated using PopPK and NCA approaches. | No PPI study was performed. | SAD/MAD, ritonavir DDI, midazolam DDI |
FDA: Accepted. (using PopPK and PBPK approach) EMA: Not accepted. Agency accepted approach based on PopPK and NCA but not PBPK. |
|
Eribulin (NDA submission) Not published |
Pediatric (Low–moderate) What is the starting dose of eribulin in children |
A PBPK model was developed for eribulin and used to perform simulations with the Simcyp pediatric population. Model predicted that the starting dose in 6 – 12 year old patients should be half of the therapeutic doses in adults. CL characteristic CYP3A metabolism, but mainly biliary excretion (which was converted into HLM CLint with the retrograde calculator) | PBPK confirmed results from traditional population‐based scaling approaches to set the starting dose for the pediatric program |
Clinical PK data. Results of the first pediatric trial showed that the model predicted the clearance of 12 – 18 year old patients very well. Clearance of 6 – 12 year old was slightly over predicted, but within acceptable range. |
FDA: No comment. Starting dose was accepted. EMA: No comment. Starting dose was accepted. |
|
Blinatumomab (NDA submission) Xu et al., 201556 |
PD of drug‐mediated drug interaction (high) Is transient cytokine elevation resulting from the immunotherapy Blinatumomab likely to result in clinically meaningful DDIs? |
Blinatumomab immunotherapy mediates transient cytokine elevation. Cytokine elevations may affect CYP activities. A PBPK model was established to evaluate the effect of transient cytokine elevation on CYP activities. Transient cytokine elevation observed during blinatumomab treatment has a low DDI potential. | No DDI study was planned or performed. | The predictability of the PBPK model was first verified by predicting transient CYP suppression in human hepatocytes after incubation with cytokine cocktail for 2 days. Additional model verification was applied to chronic CYP suppression observed in rheumatoid arthritis patients (published data). |
FDA: Applicant's PBPK predictions are not recommended to be included in the drug label. However, a DDI study was not required. EMA: Agency supported the same drug label language with regards to drug interactions as in the USPI. |
|
Quetiapine (late development) Johnson et al., 201469 |
Pediatrics (medium) Bridging formulations. Quetiapine XR and Quetiapine formulations and extrapolating from adult to pediatric |
Could we set a dose for the XR formulation in children without performing a trial based on existing preclinical and clinical exposure data? | Inform dose selection in children | Internal compound file |
FDA: Accepted. EMA: Not submitted by the sponsor. |
|
Deflazacort (late development) US FDA Clinical Pharmacology Review 44 |
Pediatrics (medium) Effect of CYP3A4 perpetrators in pediatric population |
PBPK model built in adult population with DDIs CYP3A4 verified with clinical data. Pediatric PK data showed no change in PK compared to adults | Dose adjustments with CYP3A4 perpetrators in line with adult adjustments |
DDI CYP3A4 in adult population. A case could be made to support same dose adjustments in pediatrics as in adults. |
FDA: Accepted. EMA: No response. |
Examples of high‐impact PBPK analyses are summarized in Table 2. While it is true that a majority of examples consist of predictions of enzymatic drug interactions with perpetrator or victim drugs, advances in our understanding of biology and physiology and the maturation of PBPK M&S tools have allowed for broader applications. For example, the eliglustat (Cerdelga) USPI provides information on drug interactions in a previously unstudied and limited pharmacogenetic subpopulation (i.e., CYP2D6 poor metabolizers (PMs) and intermediate metabolizers (IMs)) and using PBPK M&S. The USPI of panobinostat (Farydak), a drug with a pH‐dependent solubility profile, provides guidance around the use with gastric pH‐modifying agents without prior clinical DDI data with a pH‐modifying agent. The willingness of regulators to accept the results of PBPK M&S analyses in place of clinical study data, especially in a previously unstudied area or subpopulation, speaks to the development of confidence in the application of PBPK M&S analyses in place of clinical study data to inform drug labels.
Medium regulatory impact PBPK analysis
It appears that medium‐impact PBPK analysis may not (yet) require the same level of verification as the high‐impact PBPK analysis. Such analysis may include an area of science for which knowledge is still evolving and may also require further maturation of the PBPK tools.
The use of PBPK analysis for prediction of transporter‐based drug interactions is evolving. As our knowledge around the role and impact of drug transporters on the PK of substrate drugs increases, the application of PBPK to predict transporter‐based drug interactions increases as highlighted in recent literature.42 The examples discussed in Pan et al. highlight the rising application of PBPK M&S to understand enzyme–transporter interplay and the impact of transporters on the PK of the drugs that may benefit from subsequent clinical studies.
Other examples of medium‐impact PBPK M&S could be to inform clinical development plans. These include, using PBPK to predict drug interactions early on so as to either plan for or to rule out the need for clinical DDI studies, inform the study design (i.e., appropriate perpetrator, dose adjustment of substrate and perpetrator, duration of study, etc.) and can serve as an important basis for discussion with regulators.
Another area that may qualify as medium impact is the application of PBPK models in the pediatric population. For some processes, such as maturation of drug‐metabolizing enzymes, age‐related data are available and applied in pediatric models.23, 43 A recent example of a medium‐impact application was deflazacort (Emflaza), for which a PBPK model was used in a regulatory submission to support dose adjustments when coadministered with CYP3A4 perpetrators in pediatric populations. The model was built in an adult population and DDIs were verified with clinical trial data. Further verification in pediatric subjects was performed in age ranges of 4–11 and 12–16 years of age and, as predicted, no changes in PK compared to adults were observed.44
Low regulatory impact PBPK analysis
Low‐impact PBPK analyses are those applications for which generally there are no clinical data to verify model assumptions/parameters and may include, for example, prediction of human PK prior to FIH study to inform selection of PK sampling timepoints, dose escalation steps, or other design‐related decisions.
An example of an area lacking clinical data for PBPK modeling is in the conduct of simulations for pregnant women. While PBPK modeling is being used to explore this scenario, clinical data are lacking to verify pregnancy predictions. A recent example to illustrate this is the use of PBPK simulations to evaluate potential changes in exposures to perampanel throughout pregnancy, and the possible need to adjust doses.45 However, if explorative predictions are being used to design a clinical trial, this could become a medium‐impact PBPK analysis.
Examples of PBPK submissions to regulatory health authorities
Examples of PBPK models being used to support submissions to regulatory agencies over the past few years have been summarized (Tables 2, 3). As highlighted in previous publications, the majority (71%) of these PBPK‐based submissions were related to DDI cases, while non‐DDI cases represent a smaller (29%) portion of the submissions.
These case studies cover a broad range of themes, including but not limited to: DDIs, pH‐dependent interaction with proton pump inhibitors (PPIs), and special populations across various phases of drug development. Each example is presented with a brief description of the background, key regulatory questions being addressed, and a summary of the qualification dataset and regulatory dialog. Both successful and unsuccessful case studies from a sponsor or regulatory acceptance perspectives are presented in order to note trends across the regulatory community.
The examples were compiled via an informal survey among the Simcyp Consortium members. Participants were asked to report cases where PBPK models were used to address key questions regarding DDI‐ and non‐DDI‐related issues. Case studies in which PBPK analyses were used in the submission were described, with information on the modeling approach (e.g., bottom up / middle out / top down) and use of clinical data for model qualification. Additionally, participants were asked to report any feedback they received from the FDA and/or the EMA in response to the PBPK submission.
There were 20 examples provided for DDI‐related issues, including 13 for drugs already on the market, seven during new drug application submissions, or at the end of phase II development. The key DDI issues for all compounds were considered to have a medium to high impact on the submission. There were eight examples provided for drugs already on the market or in late development for which PBPK models were used to address issues related to special populations (pediatrics, polymorphisms, or race) or changes in absorption due to formulation (i.e., dissolution, particle size).
Figure 3 summarizes the rates of acceptance or nonacceptance of PBPK analyses by regulatory health authorities. The majority of submissions (≥80%) were accepted by both the FDA and EMA with minor rates of rejection.
Figure 3.
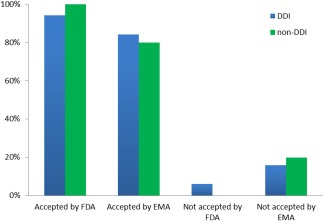
Rates of acceptance of PBPK analyses by the FDA or EMA among DDI and non‐DDI related submissions.
DDI‐related cases accepted by the FDA and/or EMA
There were 13 submissions that were accepted by both the FDA and EMA and seven cases in which one or the other regulatory body approved the submission based on the PBPK DDI prediction. In these cases, the drugs involved were mainly affecting CYP3A as a victim and/or perpetrator, although DDI predictions for drugs that were substrates of CYP2C8 and CYP2C9 or inhibitors of CYP2C9 were also included. Specific key questions for these drugs focused on whether nonstudied concomitant medication could be contraindicated to update the safety labeling accordingly, making dose adjustments when concomitant therapies included CYP inducers or inhibitors, and the effect of CYP2C9 polymorphisms and renal impairment on the clearance of a victim drug. For example, eliglustat (Cerdelga) is a case in which the USPI was based on two clinical DDI studies with three concomitant medication scenarios (i.e., the effect of the strong CYP2D6 inhibitor, paroxetine, on eliglustat pharmacokinetics in CYP2D6 extensive metabolizers (EMs) and IMs, and the effect of the strong CYP3A inhibitor, ketoconazole, in EMs). The remaining 12 scenarios were all based on PBPK simulated results.
Examples of DDI‐related cases requiring follow‐up
Objections raised from the EMA in five cases related to sensitivity analyses and once these were performed, the objection was removed and the results of the DDI simulations accepted. Of these cases, three were submitted to the FDA and the results were also accepted without follow‐up.
An example of these cases is perampanel (Fycompa). Perampanel is a low hepatic extraction ratio drug which is rapidly and completely absorbed over the clinically relevant oral dosing range of 2–12 mg/day. CYP3A is primarily responsible for its elimination; however, its elimination is very slow, with a mean elimination t1/2 of ∼105 h. The oral clearance of perampanel is expected to be significantly affected by concomitant administration of drugs that either inhibit or induce CYP3A metabolism. Therefore, a clinical DDI study with ketoconazole 200 mg QD was conducted using a standard study design. Somewhat unexpectedly, the results indicated a mean increase in perampanel AUC of only ∼20%.46 During EMA review of the regulatory submission in 2012, this result was interpreted as evidence indicating that the elimination pathway of perampanel was likely not primarily via CYP3A, and thus had not been adequately characterized. This resulted in subsequent requests for additional in vitro studies and follow‐up clinical DDI studies to better understand perampanel's elimination and potential DDI risk. To explore and gain a more complete understanding of the effects of ketoconazole on perampanel PK, a PBPK model was developed in Simcyp v. 11. A middle‐out approach was used, where the mean CL/F of perampanel from various clinical studies was back‐converted to a CYP3A intrinsic clearance using the retrograde calculator, and all other parameters were modeled by bottom‐up IVIVE. Prediction performance of the model was successfully verified against perampanel PK from both single and multiple‐ascending doses. In addition, it was also verified by adequately predicting the DDI with the CYP3A inducer, carbamazepine. DDI simulations were performed using the same study design of the clinical study with ketoconazole predicted AUC ratios and were in good agreement with the mean observed AUC ratio.30 Further exploration of the simulation outputs and additional DDI simulations with ketoconazole 200 mg b.i.d. and itraconazole (CYP3A inhibitor with a longer t1/2) led to the conclusion that the lack of a higher DDI effect was an artifact of the standard clinical DDI study design, such that the coadministration of perampanel and ketoconazole was of too short a duration for a drug with almost complete absorption and very slow elimination. A series of additional simulations were performed, using study designs that either extended perampanel sampling up to 60 days postdose, or utilized supratherapeutic doses of ketoconazole. These simulations were submitted to the EMA and showed that higher AUC ratios could be achieved, but only under nonfeasible clinical trial conditions (i.e., supratherapeutic doses of ketoconazole).
Examples of DDI‐related cases not accepted by the FDA and/or EMA
There were four cases (Table 2) in which the PBPK simulation results were not accepted by either the FDA or EMA. While lenvatinib was only submitted to the EMA, crizotinib, osimertinib, and oxcarbazepine were submitted to both regulatory agencies and both gave opposing feedback.
Lenvatinib was shown to be an inhibitor of CYP3A in vitro. PBPK simulations were performed to evaluate the DDI risk with midazolam and demonstrated it was negligible (predicted mean fold‐increase in AUC (AUCR) was ∼1.19). These results were included in the submission with a request for a waiver of the clinical DDI trial. In addition to PBPK modeling, an analysis was provided showing that, in spite of the potential for CYP3A inhibition in the intestine, this was unlikely to happen since the solubility of lenvatininb did not allow for concentrations that would significantly inhibit the enzyme. Results from a formal clinical DDI trial with midazolam (Eisai internal data on file) confirmed predictions by the model. In spite of accepting the predictions of the model for a CYP2C8 DDI, the EMA did not accept the predictions for CYP3A DDI risk, since experience with intestinal DDI predictions were not as well developed as the liver DDI predictions, and therefore the EMA maintained the request for the clinical DDI study. Crizotinib (Xalkori) is an orally available small molecule that has been approved globally for the treatment of patients with ALK‐ or ROS1‐positive metastatic nonsmall‐cell lung cancer.47 It is a CYP3A substrate and time‐dependent inhibitor.48, 49 Accordingly, a clinical DDI study of crizotinib (as the interacting drug) with midazolam was conducted in cancer patients who received a single oral dose of midazolam (2 mg) before and after 28‐day multiple oral administration of the crizotinib recommended dose of 250 mg twice daily. The AUCR for midazolam was 3.7, suggesting crizotinib was a moderate CYP3A inhibitor. Crizotinib PBPK models, which were developed and verified in the Simcyp Simulator, based on a middle‐out approach, reasonably predicted midazolam AUCR.48 Moreover, crizotinib single‐dose DDI studies (as the substrate drug) with ketoconazole (200 mg twice daily) and rifampin (600 mg once daily) were conducted in healthy volunteers, resulting in crizotinib AUCRs of 3.2 and 0.18, respectively. PBPK models previously developed and verified reasonably predicted crizotinib AUCRs with these interacting drugs.49 Based on these results, a crizotinib DDI with the moderate CYP3A inducer, efavirenz, was predicted using PBPK modeling following the development and verification of efavirenz PBPK models.50, 51, 52 First, the PBPK model predicted efavirenz exposures in single‐ and multiple‐dose studies and DDI studies with the CYP3A4 substrate drug, maraviroc, adequately matched clinically observed results. Next, a DDI with efavirenz was predicted to result in an ∼30% decrease in crizotinib oral exposure. These modeling results were submitted to the FDA and EMA for crizotinib dosing recommendation with moderate CYP3A inducers. While the FDA accepted the results to support dosing recommendations for comedications that are moderate CYP3A inducers,53 the EMA argued that the IVIVE for induction is not well established due to the complexity of this mechanism.
The argument against the use of PBPK to evaluate CYP (or UGT) induction does not appear to be scientifically justified and is confusing, especially since a concerted effort by the Innovation and Quality Consortium has shown the validity of such an approach for CYP3A induction across multiple drugs.54 Based on the crizotinib example presented here, the available system‐related information on CYP expression and turnover in PBPK platforms, and the cited literature, it is scientifically justifiable to apply PBPK modeling and simulation for the evaluation of CYP (and arguably UGT) induction by drugs as victims or perpetrators. Wagner et al. reported a number of cases submitted to the FDA involving reasonable prediction of induction using PBPK modeling.55
Osimertinib was in late development and is a CYP3A4 substrate. The sponsor requested a waiver for DDI studies involving interactions with CYP3A4 inducers with different potencies in order to adjust dosing accordingly. The Simcyp Simulator was used to simulate the DDIs of osimertinib as a victim drug with CYP3A inducers (moderate and weak); however, the simulation results were not accepted by the EMA to justify the requested waiver.
A fourth example of a drug submission that received different feedback from the FDA and EMA was for an NCE. This NCE is a UGT substrate; therefore, in order to support labeling recommendations, a PBPK model was built to simulate DDIs with UGT inducers that have not been studied clinically. The qualification of the model was based on PK data obtained from two clinically studied inducers, efavirenz and carbamazepine. The EMA did accept the submission based on the PBPK simulations; however, the FDA did not accept the results as they considered the data to be insufficient to determine appropriate dosing recommendations.
Non‐DDI related cases
Of the eight cases involving non‐DDI predictions using PBPK models, one case (blinatumomab) represented a drug interaction mediated by the pharmacodynamics of the investigational drug. This case is of interest because it relates to a drug interaction mediated by therapeutic proteins by downregulation of CYPs as a result of cytokine release.56 Currently, there is limited information on clinical DDIs related to cytokine elevation, as well as relevant PBPK models to predict them.
Of the non‐DDI cases presented in the survey, two examples described how predictions by PBPK models of starting doses for pediatric patients could be based on adult doses, which were accepted by the FDA and EMA. One of these cases, quetiapine, also included bridging of formulations. PBPK models were extended to include simulations of different dosing regimens for different race groups, including countries in Asia, which are known to be affected by race–genotype interactions. PBPK modeling was used to understand if prospective genotyping of patients was needed and whether CYP2C19 PM patients should be excluded from the trial. Simulations suggested only a small impact of CYP2C19 genetic polymorphism, due to the moderate contribution of CYP2C19 and also due to much smaller CYP2C19 expression in Asians vs. Westerners. Thus, even though the frequency of CYP2C19 PMs is higher, the overall impact was not considered clinically relevant, leading to an all‐comer trial with retrospective analysis of CYP2C19 PMs. In another case, a middle‐out approach was used to simulate 12 different dosing regimens for CYP2D6 polymorphic patients based on three clinical DDI scenarios with strong inhibitors. Clinical data with strong inhibitors were also used to refine and verify the model. The goal of waiving further clinical DDI trials was accepted by the FDA and the EMA.
There were three cases in which PBPK models were used to simulate the absorption of drugs, two using GastroPlus and one using Simcyp (Table 3). PBPK results predicting no effect of different stomach pH values on ribociclib bioavailability complemented the clinical observations for the lack of effect of PPIs on ribociclib exposure as evaluated via NCA and PopPK approaches.57 The FDA reviewed and accepted the PBPK and PopPK approaches. These examples demonstrate the validity and appropriateness of using PBPK to address non‐DDI issues and sponsors and regulators are encouraged to develop and accept such approaches in support of drug development and submissions.
REPORTING FORMAT OF PBPK SUBMISSIONS
PBPK analyses may produce lengthy output files that summarize the input parameters, simulation results, and sensitivity analyses results. The FDA has issued draft guidance on how to report PBPK analyses. In addition to the FDA's draft guidance, we describe suggested elements of the PBPK reports that are intended for regulatory submission (Table 4). In addition, a suggested PBPK analysis plan (Supplementary Text 2), quality control checklist (Supplementary Text 3), and a PBPK report template are provided (Supplementary Text 4).
Table 4.
Suggested sections of a PBPK modeling report intended for regulatory submission
| Title | Purpose | Audience | Important elements of content |
|---|---|---|---|
| Executive summary (synopsis) |
Summary of modeling process Key results and conclusions |
All readers |
• Objective(s) of the modeling process • High‐level methodology • Key results • Main conclusions • Suggestion: limit to 1‐2 pages |
| Introduction (background) | Background information to clarify the purpose and the context of the analysis | All readers |
• Background information to place the modeling work in the context of the development program • Background information on the modeled drug (e.g, ADME properties) |
| Objective(s) | Statement of the analysis objective | All readers | Precise objectives that answer important development question(s) |
| Materials and methods | A detailed documentation of methods used in the modeling process | Technical | See detailed description of the subsections below. |
| Overview of modeling strategy | An overview to describe the adopted strategy from model building to model application | All readers | • For example, a graphical workflow illustrating the sequence of the modeling steps. |
| Model assumptions | Description and discussion/justification of the main assumptions in the drug model | Technical |
A summary of the main assumptions, such as: • ADME related assumptions, for example, Perfusion limited or permeability limited distribution is needed or assumed; any assumptions on transporters involved; assumptions on the involved metabolizing enzymes and/or percent of enzyme contributions/fraction metabolized, etc. • Population related assumptions, for example, assumed changes in any system‐specific parameters, assumed enzyme ontogeny profiles when scaling to children (especially if the ontogeny profile is not included in the built‐in database) |
| Modeling parameters | A summary of the main model parameters | Technical |
• System‐Specific Parameters • Drug‐Specific Parameters |
| System‐specific parameters | Description of the system‐specific parameters incorporated in the model | Technical |
• Highlight any system‐specific data that were modified/added by the modeler to the software built‐in database • If specialized PBPK modeling software tools are used, list or provide references to the most relevant system‐parameters |
| Drug specific parameters | Summary of the drug‐specific parameters utilized in the model | Technical |
• Table summarizing all drug specific parameters that were utilized in the model such as the drug physicochemical properties, fraction unbound, blood to plasma ratio, intrinsic clearance and metabolic pathways information, permeability, solubility, etc. • If any parameters were optimized/fitted, a description/discussion should be provided |
| Parameter estimation | An optional subsection devoted to explain and discuss parameter estimation procedures, if applicable | Technical | |
| Drug model structure | Short description of the individual components of the final PBPK model | Technical |
• A summary of the sub‐models that constitute the final PBPK model, for example: • Absorption: First order vs. ACAT vs. ADAM • Distribution: minimal vs. full PBPK; method used to calculate tissue partition coefficients • Elimination: method used to represent drug clearance (in vivo measure vs. organ clearance vs. enzyme kinetics) |
| Pharmacokinetic/clinical data | Description of the clinical data used in model development or evaluation | All readers | If pharmacokinetic/clinical data were used, a summary of the main information regarding the number of clinical studies and brief description of their design, dose route. |
| Simulation design (conditions) | Description of simulation conditions for the model development, verification) evaluation), and application | All readers |
A description of simulation conditions, which usually includes information such as: • Characteristics of the virtual population (defining demographics, diseased or healthy status, adult vs. pediatric vs. geriatric, number of virtual subjects/trials, etc.) • Dosing information (dose, route of administration, formulation, fed/fasting condition) • Simulation duration |
| Model evaluation and qualification Criteria | A detailed description of how model results will be evaluated with the acceptance criteria. | All readers |
A detailed description of how model results will be evaluated with the acceptance criteria and the strategy of model qualification depending on the available observed data. This usually includes one or more elements such as: • Visual predictive checks (VPC) • Comparison of key PK parameters • Numerical metrics |
| Sensitivity analysis | A component devoted to investigate how changes in the model key input parameters can influence the simulation outputs | Technical | |
| Software tools | List software tools used in the model development and evaluation process with the corresponding version. | All readers |
Summary of all software tools used in the model development and evaluation process with the corresponding version(s): • PBPK modeling software • Tools for data evaluation or visualization • Tools for data digitizing, if applicable |
| Results | Description of the obtained results | Technical/All readers |
• Description of the evaluation/qualification results • Description of model application results |
| Model evaluation/qualification | One or more sub‐sections on the model evaluation/qualification results | All readers |
• Graphical and tabular displays • if possible provide Forest plots showing the AUC and Cmax ratios |
| Model application | One or more subsections on the model application results | All readers | • Graphical and tabular displays |
| Discussion and conclusion |
• Explanation of the relevance of the results • Present main findings • Discuss model limitations |
All readers |
• Place results in technical and clinical context • Discuss clinical relevance of the analysis • Highlight any model limitations • Present conclusions as a bullet point list |
| Appendices |
• Modeling Analysis Plan, if applicable • Quality Control documentation, if applicable • List of Model Data Files, if applicable |
SUMMARY AND CONCLUSION
This review provides a perspective on the qualification and verification of PBPK platforms and models that are intended for submission to regulatory health authorities based on the collective experience of Simcyp Consortium members. Examples of PBPK analyses that were submitted to health authorities across various intended applications are summarized (Tables 2, 3), and suggestions regarding the components of a PBPK analysis to be submitted to health authorities are also provided (Supplementary Texts 2, 3 and 4). Although the EMA and FDA have recently issued draft guideline/guidance regarding PBPK modeling and simulation analyses, more clarity is needed regarding the process of qualification of PBPK platforms, and importantly, more flexibility is warranted to advance the use and acceptability of PBPK analyses that are intended for regulatory decision‐making. This could be accomplished, for example, by having more flexibility around the criteria for platform qualification and drug model verification without restricting the use of PBPK to certain scenarios (i.e., CYP3A DDIs).
The intended use of PBPK modeling and simulation for regulatory submissions should clearly state the objectives of the analysis. For example, answering a regulatory question about the potential DDI of an NME under untested clinical scenarios using PBPK should be framed in the context of dosing recommendations and/or labeling. In this case, the intended use is to provide model‐based evidence for making certain dosing or labeling claims when clinical data are unavailable. However, additional clinical data may emerge following the initial PBPK submission, and it is imperative to revisit the model and apply the new data if another intended use of the PBPK model is considered. Conversely, a sponsor may decide to submit a PBPK package that includes verification steps of the platform to make DDI claims for a dosage regimen (intended use #1) and later on decide to file another submission for a different dosage regimen (intended use #2). In this case, however it may appear that there are two separate intended uses of the PBPK submissions; requalification of the PBPK platform in the second submission may not be required.
The predictive performance of PBPK models is commonly assessed using a comparative approach whereby the accuracy of a particular simulated scenario is represented as a ratio of predicted or simulated parameter vs. observed parameter. A more challenging question to answer or get agreement on is what value of predicted/observed ratio would constitute a successful prediction? A number of factors need to be considered in answering this question, including the intended purpose of the simulation, the therapeutic index of the drug or drugs in question, or steepness of the exposure–response relationship. The answer to each of these questions will influence what may be considered an acceptable prediction.
A few technical challenges that can hamper the qualification of a PBPK platform are summarized in Table 5. Some of these are related to qualification, parameter identifiability, and sensitivity analysis, and are discussed in Supplementary Text 1.
Table 5.
Summary of remaining technical challenges and knowledge gaps for applying PBPK modeling and simulation in drug development
| Area of PBPK application | Specific purpose | PK characterization | Knowledge gaps |
|---|---|---|---|
| DDI involving enzymes | Drug as victim |
For enzymes expressed in multiple sites (liver, intestine, kidney), difficult to assess in vivo contribution of each site to metabolism. For example, in the absence of i.v. PK data for poorly soluble compounds, Fg cannot be estimated. When multiple enzymes metabolize a compound, mass balance studies may not differentiate between the enzymes. The requirement of in vivo DDI studies for each enzyme is difficult to justify. |
Abundance data for non‐ CYP mechanisms Sufficient clinical datasets for qualification of non‐CYP3A mechanisms |
| Drug as perpetrator | The selection of an appropriate range for sensitivity analysis to cover the uncertainty in the in vitro data (Ki, KI, kinact, EC50 and Emax) is key to ensuring a realistic assessment of DDI. |
Sufficient clinical datasets for qualification of non‐CYP3A mechanisms IVIVE for non‐CYP mechanisms |
|
| DDI involving transporters | Drug as victim |
Similar to those presented above for ‘Drug as victim’ of enzyme inhibition. In addition, it is difficult to determine the relative contribution of transporter in vivo vs. enzyme to elimination of the drug. |
IVIVE and scaling factors |
| Drug as perpetrator | Similar to those presented above for ‘Drug as perpetrator’ of enzyme inhibition. | Sufficient clinical datasets for qualification | |
| Extrapolation from healthy to other populations |
Pediatrics, Elderly, Obese Ethnic bridging/ pharmacogenetics Organ impairment |
Mechanistic understanding of PK in base population may be challenged by lack of i.v. data. When multiple enzymes/transporters are involved in the elimination of drug, it is difficult to determine the relative contribution of each protein to elimination of the drug. |
Knowledge of any additional pathways or compensatory mechanisms, not observed in base population. Difficult to verify in vivo relevance of enzyme and transporter abundance, ISEFs and pharmacogenetic data for different enzymes. |
| Absorption/ formulation related | Food‐drug interactions | Quantitative assessment of fraction absorbed and contributing mechanisms non‐identifiable, in the absence of i.v. data for poorly soluble compounds, when Fg and Fh cannot be estimated. | IVIVE and IVIVC for BCS II and IV drugs |
Overall, the EMA and FDA draft guidelines on PBPK modeling and simulation analyses provide a significant milestone for this field and pave the way for a broader application of PBPK modeling and simulation in drug development. This perspective aims at informing and influencing any updated regulatory guidelines to further support the use of model‐informed drug development approaches in the registration and approval of new drug therapies.
FUNDING
No funding was received for this work.
CONFLICT OF INTEREST
Masoud Jamei and Iain Gardner are employees of Simcyp Limited (a Certara Company). Malcolm Rowland is the chairman of Simcyp Limited (a Certara Company) Scientific Advisory Board. The other authors have no conflicts of interest to declare.
Supporting information
Supplementary Text 1: Installation qualification, challenges and future opportunities.
Supplementary Text 2: PBPK analysis plan.
Supplementary Text 3: PBPK modelling quality control guidance document.
Supplementary Text 4: Report template for PBPK modeling and analyses: recommended by the Simcyp consortium
ACKNOWLEDGMENTS
We are grateful for review and constructive input from Professor Amin Rostami‐Hodjegan, Professor of Systems Pharmacology at the University of Manchester, UK, and Senior Vice President of Research & Development and Chief Scientific Officer at Certara. We also thank Dr. Klaus Jensen for participating in early discussions, Dr. Allison Kitten for assistance in formatting the article, and Miss Eleanor Savill and Miss Rosalie Bower for their assistance with collecting the references and preparation of the article.
References
- 1. Jones, H.M. et al Physiologically based pharmacokinetic modeling in drug discovery and development: a pharmaceutical industry perspective. Clin. Pharmacol. Ther. 97, 247–262 (2015). [DOI] [PubMed] [Google Scholar]
- 2. Wagner, C. et al Application of physiologically based pharmacokinetic (PBPK) modeling to support dose selection: report of an FDA public workshop on PBPK. CPT Pharmacometrics Syst. Pharmacol. 4, 226–230 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Luzon, E. , Blake, K. , Cole, S. , Nordmark, A. , Versantvoort, C. & Berglund, E.G. Physiologically based pharmacokinetic modeling in regulatory decision‐making at the European Medicines Agency. Clin. Pharmacol. Ther. DOI: 10.1002/cpt.1539 (2016) [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 4. Sato, M. et al Quantitative modeling and simulation in PMDA: a Japanese regulatory perspective. CPT Pharmacometrics Syst. Pharmacol. 6, 413–415 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. United States Food and Drug Administration. Guidance for Industry: physiologically Based Pharmacokinetic Analyses—Format and Content. December, 2016. <https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM531207.pdf>.
- 6. European Medicines Agency . Guideline on the qualification and reporting of physiologically based pharmacokinetic (PBPK) modelling and simulation (2016). <http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/07/WC500211315.pdf>.
- 7. Jamei, M. et al The Simcyp population based simulator: architecture, implementation, and quality assurance. In Silico Pharmacol. 1, 9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rostami‐Hodjegan, A . Reverse translation in PBPK and QSP: going backwards in order to go forward with confidence. Clin. Pharmacol. Ther. (2017) [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Friedrich, C.M. A model qualification method for mechanistic physiological QSP models to support model‐informed drug development. CPT Pharmacometrics Syst. Pharmacol. 5, 43–53 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lennernas, H. et al In vivo predictive dissolution (IPD) and biopharmaceutical modeling and simulation: future use of modern approaches and methodologies in a regulatory context. Mol. Pharm. 14, 1307–1314 (2017). [DOI] [PubMed] [Google Scholar]
- 11. Marsousi, N. , Desmeules, J.A. , Rudaz, S. & Daali, Y. Usefulness of PBPK modeling in incorporation of clinical conditions in personalized medicine. J. Pharm. Sci., DOI: 10.1016/j.xphs.2017.1004.1035 (2017) [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 12. Chow, E.C. , Talattof, A. , Tsakalozou, E. , Fan, J. , Zhao, L. & Zhang, X. Using physiologically based pharmacokinetic (PBPK) modeling to evaluate the impact of pharmaceutical excipients on oral drug absorption: sensitivity analyses. AAPS J. 18, 1500–1511 (2016). [DOI] [PubMed] [Google Scholar]
- 13. Pang, K.S. & Rowland, M. Hepatic clearance of drugs. II. Experimental evidence for acceptance of the “well‐stirred” model over the “parallel tube” model using lidocaine in the perfused rat liver in situ preparation. J. Pharmacokinet. Biopharm. 5, 655–680 (1977). [DOI] [PubMed] [Google Scholar]
- 14. Gillette, J.R. Factors affecting drug metabolism. Ann. N. Y. Acad. Sci. 179, 43–66 (1971). [DOI] [PubMed] [Google Scholar]
- 15. Arya, V. , Zhao, P. , Reynolds, K.S. , Mishra, P. & Younis, I.R. Utilizing PBPK modeling to evaluate the potential of a significant drug‐drug interaction between clopidogrel and dasabuvir: a scientific perspective. Clin. Pharmacol. Ther., DOI: 10.1002/cpt.1699 (2017) [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16. Shebley, M. , Fu, W. , Badri, P. , Bow, D. & Fischer, V. Physiologically based pharmacokinetic modeling suggests limited drug‐drug interaction between clopidogrel and dasabuvir. Clin. Pharmacol. Ther., DOI: 10.1002/cpt.1689 (2017) [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tsamandouras, N. , Rostami‐Hodjegan, A. & Aarons, L. Combining the ‘bottom up’ and ‘top down’ approaches in pharmacokinetic modelling: fitting PBPK models to observed clinical data. Br. J. Clin. Pharmacol. 79, 48–55 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jadhav, P.R. et al A proposal for scientific framework enabling specific population drug dosing recommendations. J. Clin. Pharmacol. 55, 1073–1078 (2015). [DOI] [PubMed] [Google Scholar]
- 19. Rioux, N. & Waters, N.J. Physiologically based pharmacokinetic modeling in pediatric oncology drug development. Drug Metab. Dispos. 44, 934–943 (2016). [DOI] [PubMed] [Google Scholar]
- 20. Johnson, T.N. , Boussery, K. , Rowland‐Yeo, K. , Tucker, G.T. & Rostami‐Hodjegan, A. A semi‐mechanistic model to predict the effects of liver cirrhosis on drug clearance. Clin. Pharmacokinet. 49, 189–206 (2010). [DOI] [PubMed] [Google Scholar]
- 21. Ghobadi, C. et al Application of a systems approach to the bottom‐up assessment of pharmacokinetics in obese patients: expected variations in clearance. Clin. Pharmacokinet. 50, 809–822 (2011). [DOI] [PubMed] [Google Scholar]
- 22. Rowland Yeo, K. , Aarabi, M. , Jamei, M. & Rostami‐Hodjegan, A. Modeling and predicting drug pharmacokinetics in patients with renal impairment. Expert Review Clin. Pharmacol. 4, 261–274 (2011). [DOI] [PubMed] [Google Scholar]
- 23. Abduljalil, K. , Furness, P. , Johnson, T.N. , Rostami‐Hodjegan, A. & Soltani, H. Anatomical, physiological and metabolic changes with gestational age during normal pregnancy. Clin. Pharmacokinet. 51, 365–396 (2012). [DOI] [PubMed] [Google Scholar]
- 24. Sayama, H. , Takubo, H. , Komura, H. , Kogayu, M. & Iwaki, M. Application of a physiologically based pharmacokinetic model informed by a top‐down approach for the prediction of pharmacokinetics in chronic kidney disease patients. AAPS J. 16, 1018–1028 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou, W. et al Predictive performance of physiologically based pharmacokinetic and population pharmacokinetic modeling of renally cleared drugs in children. CPT Pharmacometrics Syst. Pharmacol. 5, 475–483 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johnson, T.N. , Rostami‐Hodjegan, A. & Tucker, G.T. Prediction of the clearance of eleven drugs and associated variability in neonates, infants and children. Clin. Pharmacokinet. 45, 931–956 (2006). [DOI] [PubMed] [Google Scholar]
- 27. Cheeti, S. , Budha, N.R. , Rajan, S. , Dresser, M.J. & Jin, J.Y. A physiologically based pharmacokinetic (PBPK) approach to evaluate pharmacokinetics in patients with cancer. Biopharm. Drug Dispos. 34, 141–154 (2013). [DOI] [PubMed] [Google Scholar]
- 28. United States Food and Drug Administration . Clinical Pharmacology Biopharmaceutics Review(s) for Cerdelga (2014). <https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205494Orig1s000ClinPharmR.pdf>.
- 29. Abduljalil, K. , Cain, T. , Humphries, H. & Rostami‐Hodjegan, A. Deciding on success criteria for predictability of pharmacokinetic parameters from in vitro studies: an analysis based on in vivo observations. Drug Metab. Dispos. 42, 1478–1484 (2014). [DOI] [PubMed] [Google Scholar]
- 30. Guest, E.J. , Aarons, L. , Houston, J.B. , Rostami‐Hodjegan, A. & Galetin, A. Critique of the two‐fold measure of prediction success for ratios: application for the assessment of drug‐drug interactions. Drug Metab. Dispos. 39, 170–173 (2011). [DOI] [PubMed] [Google Scholar]
- 31. Slob, W. , Janssen, P.H. & van den Hof, J.M. Structural identifiability of PBPK models: practical consequences for modeling strategies and study designs. Crit. Rev. Toxicol. 27, 261–272 (1997). [DOI] [PubMed] [Google Scholar]
- 32. Yates, J.W. Structural identifiability of physiologically based pharmacokinetic models. J. Pharmacokinet. Pharmacodyn. 33, 421–439 (2006). [DOI] [PubMed] [Google Scholar]
- 33. Godfrey, K.R. , Jones, R.P. & Brown, R.F. Identifiable pharmacokinetic models: the role of extra inputs and measurements. J. Pharmacokinet. Biopharm. 8, 633–648 (1980). [DOI] [PubMed] [Google Scholar]
- 34. Cheung, S.Y. , Yates, J.W. & Aarons, L. The design and analysis of parallel experiments to produce structurally identifiable models. J. Pharmacokinet. Pharmacodyn. 40, 93–100 (2013). [DOI] [PubMed] [Google Scholar]
- 35. Cheung, S.Y. , Majid, O. , Yates, J.W. & Aarons, L. Structural identifiability analysis and reparameterisation (parameter reduction) of a cardiovascular feedback model. Eur. J. Pharm. Sci. 46, 259–271 (2012). [DOI] [PubMed] [Google Scholar]
- 36. Garcia, R.I. , Ibrahim, J.G. , Wambaugh, J.F. , Kenyon, E.M. & Setzer, R.W. Identifiability of PBPK models with applications to dimethylarsinic acid exposure. J. Pharmacokinet. Pharmacodyn. 42, 591–609 (2015). [DOI] [PubMed] [Google Scholar]
- 37. Agoram, B. Evaluating systems pharmacology models is different from evaluating standard pharmacokinetic‐pharmacodynamic models. CPT Pharmacometrics Syst. Pharmacol. 3, e101 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Budha, N.R. et al Evaluation of cytochrome P450 3A4‐mediated drug‐drug interaction potential for cobimetinib using physiologically based pharmacokinetic modeling and simulation. Clin. Pharmacokinet. 55, 1435–1445 (2016). [DOI] [PubMed] [Google Scholar]
- 39. Lukacova, V. , Goelzer, P. , Reddy, M. , Greig, G. , Reigner, B. & Parrott, N. A physiologically based pharmacokinetic model for ganciclovir and its prodrug valganciclovir in adults and children. AAPS J. 18, 1453–1463 (2016). [DOI] [PubMed] [Google Scholar]
- 40. Jorga, K. et al Bottom‐up meets top‐down: complementary physiologically based pharmacokinetic and population pharmacokinetic modeling for regulatory approval of a dosing algorithm of valganciclovir in very young children. Clin. Pharmacol. Ther. 100, 761–769 (2016). [DOI] [PubMed] [Google Scholar]
- 41. Manolis, E. , Rohou, S. , Hemmings, R. , Salmonson, T. , Karlsson, M. & Milligan, P.A. The role of modeling and simulation in development and registration of medicinal products: output from the EFPIA/EMA modeling and simulation workshop. CPT Pharmacometrics Syst. Pharmacol. 2, e31 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pan, Y. et al The application of physiologically based pharmacokinetic modeling to predict the role of drug transporters: scientific and regulatory perspectives. J. Clin. Pharmacol. 56 Suppl 7, S122–131 (2016). [DOI] [PubMed] [Google Scholar]
- 43. Salem, F. , Johnson, T.N. , Abduljalil, K. , Tucker, G.T. & Rostami‐Hodjegan, A. A re‐evaluation and validation of ontogeny functions for cytochrome P450 1A2 and 3A4 based on in vivo data. Clin. Pharmacokinet. 53, 625–636 (2014). [DOI] [PubMed] [Google Scholar]
- 44. United States Food and Drug Administration, CDER . Deflazacort clinical pharmacology review (2016). <https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208684,208685Orig1s000ClinPharmR.pdf>.
- 45. Schuck, E. , Rege, B. , Laurenza, A. , Williams, B. , Ferry, J. & Hussein, Z. Predictions of the pharmacokinetic profile of perampanel during pregnancy using physiologically based pharmacokinetic (PBPK) modeling (P3.237). Neurology. 88, P3.237 (2017). [Google Scholar]
- 46. Gidal, B.E. et al Effect of enzyme inhibition on perampanel pharmacokinetics: why study design matters. Epilepsy Res. 134, 41–48 (2017). [DOI] [PubMed] [Google Scholar]
- 47. Xalkori (Crizotinib) [US prescribing information]. New York: Pfizer Labs, 2017. [Google Scholar]
- 48. Mao, J. , Johnson, T.R. , Shen, Z. & Yamazaki, S. Prediction of crizotinib‐midazolam interaction using the Simcyp population‐based simulator: comparison of CYP3A time‐dependent inhibition between human liver microsomes versus hepatocytes. Drug Metab. Dispos. 41, 343–352 (2013). [DOI] [PubMed] [Google Scholar]
- 49. Yamazaki, S. , Johnson, T.R. & Smith, B.J. Prediction of drug‐drug interactions with crizotinib as the CYP3A substrate using a physiologically based pharmacokinetic model. Drug Metab. Dispos. 43, 1417–1429 (2015). [DOI] [PubMed] [Google Scholar]
- 50. Hyland, R. , Dickins, M. , Collins, C. , Jones, H. & Jones, B. Maraviroc: in vitro assessment of drug‐drug interaction potential. Br. J. Clin. Pharmacol. 66, 498–507 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Abel, S. , Jenkins, T.M. , Whitlock, L.A. , Ridgway, C.E. & Muirhead, G.J. Effects of CYP3A4 inducers with and without CYP3A4 inhibitors on the pharmacokinetics of maraviroc in healthy volunteers. Br. J. Clin. Pharmacol. 65 Suppl 1, 38–46 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rekic, D. , Roshammar, D. , Mukonzo, J. & Ashton, M. In silico prediction of efavirenz and rifampicin drug‐drug interaction considering weight and CYP2B6 phenotype. Br. J. Clin. Pharmacol. 71, 536–543 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. United States Food and Drug Administration Briefing Document . Pharmaceutical Science and Clinical Pharmacology Advisory Committee Meeting: strategies, approaches, and challenges in model‐informed drug development (MIDD). March 15, 2017. <https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AdvisoryCommitteeforPharmaceuticalScienceandClinicalPharmacology/UCM544838.pdf> (2017).
- 54. Einolf, H.J. et al Evaluation of various static and dynamic modeling methods to predict clinical CYP3A induction using in vitro CYP3A4 mRNA induction data. Clin. Pharmacol. Ther. 95, 179–188 (2014). [DOI] [PubMed] [Google Scholar]
- 55. Wagner, C. , Pan, Y. , Hsu, V. , Sinha, V. & Zhao, P. Predicting the effect of CYP3A inducers on the pharmacokinetics of substrate drugs using physiologically based pharmacokinetic (PBPK) modeling: an analysis of PBPK submissions to the US FDA. Clin. Pharmacokinet. 55, 475–483 (2016). [DOI] [PubMed] [Google Scholar]
- 56. Xu, Y. , Hijazi, Y. , Wolf, A. , Wu, B. , Sun, Y.N. & Zhu, M. Physiologically based pharmacokinetic model to assess the influence of blinatumomab‐mediated cytokine elevations on cytochrome P450 enzyme activity. CPT Pharmacometrics Syst. Pharmacol. 4, 507–515 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Samant, T. et al Ribociclib bioavailability is not affected by gastric pH changes or food intake: in silico and clinical evaluations. Clin. Pharmacol. Ther. doi: 10.1002/cpt.940. [Epub ahead of print] (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. United States Food and Drug Administration . Pregnancy, Lactation, and Reproductive Potential: labeling for Human Prescription Drug and Biological Products—Content and Format. Guidance for Industry. <https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM425398.pdf> (Silver Spring, MD: FDA; 2014).
- 59. Einolf, H.J. , Zhou, J. , Won, C. , Wang, L. & Rebello, S. A physiologically‐based pharmacokinetic modeling approach to predict drug‐drug interactions of sonidegib (LDE225) with perpetrators of CYP3A in cancer patients. Drug Metab. Dispos. 45, 361–374 (2017). [DOI] [PubMed] [Google Scholar]
- 60. United States Food and Drug Administration . Clinical Pharmacology Biopharmaceutics Review(s) for Olaparib (2014). <https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/206162Orig1s000ClinPharmR.pdf>.
- 61. Chen, L.‐F. et al Evaluation of potential drug interactions for trametinib, a novel MEK inhibitor for anticancer therapy [abstract]. <http://abstracts.aaps.org/Verify/AAPS2015/PosterSubmissions/W4293.pdf> (2015).
- 62. European Medicines Agency, Eribulin Assessment Report (2014). <http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/002084/WC500170478.pdf>.
- 63. Djebli, N. , Fabre, D. , Boulenc, X. , Fabre, G. , Sultan, E. & Hurbin, F. Physiologically based pharmacokinetic modeling for sequential metabolism: effect of CYP2C19 genetic polymorphism on clopidogrel and clopidogrel active metabolite pharmacokinetics. Drug Metab. Dispos. 43, 510–522 (2015). [DOI] [PubMed] [Google Scholar]
- 64. Einolf, H.J. et al Physiologically‐based pharmacokinetic model predictions of panobinostat (LBH589) as a victim and perpetrator of drug‐drug interactions. Drug Metab. Dispos., DOI: 10.1124/dmd.1117.076851 (2017) [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 65. Zhou, D. , Bui, K. , Sostek, M. & Al‐Huniti, N. Simulation and prediction of the drug‐drug interaction potential of naloxegol by physiologically based pharmacokinetic modeling. CPT Pharmacometrics Syst. Pharmacol. 5, 250–257 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. United States Food and Drug Administration . Osimertinib Clinical Pharmacology Review (2014). <https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/208065Orig1s000ClinPharmR.pdf>.
- 67. Pepin, X.J. , Flanagan, T.R. , Holt, D.J. , Eidelman, A. , Treacy, D. & Rowlings, C.E. Justification of drug product dissolution rate and drug substance particle size specifications based on absorption PBPK modeling for lesinurad immediate release tablets. Mol. Pharm. 13, 3256–3269 (2016). [DOI] [PubMed] [Google Scholar]
- 68. Tistaert, C . Using PBPK modeling to simulate the disposition of canagliflozin [presentation]. AAPS 2015, Florida. <https://zerista.s3.amazonaws.com/item_files/b892/attachments/58875/original/3464.pdf> (2015).
- 69. Johnson, T.N. , Zhou, D. & Bui, K.H. Development of physiologically based pharmacokinetic model to evaluate the relative systemic exposure to quetiapine after administration of IR and XR formulations to adults, children and adolescents. Biopharm. Drug Dispos. 35, 341–352 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text 1: Installation qualification, challenges and future opportunities.
Supplementary Text 2: PBPK analysis plan.
Supplementary Text 3: PBPK modelling quality control guidance document.
Supplementary Text 4: Report template for PBPK modeling and analyses: recommended by the Simcyp consortium


