Abstract
The increasing miniaturization and affordability of sensors and circuitry has led to the current level of innovation in the area of wearable and microsensor solutions for health monitoring. This facilitates the development of solutions that can be used to measure complex health outcomes in nonspecialist and remote settings. In this article, we review a number of innovations related to brain monitoring including portable and wearable solutions to directly measure brain electrical activity, and solutions measuring aspects related to brain function such as sleep patterns, gait, cognition, voice acoustics, and gaze analysis. Despite the need for more scientific validation work, we conclude that there is enough understanding of how to implement these approaches as exploratory tools that may provide additional valuable insights due to the rich and frequent data they produce, to justify their inclusion in clinical study protocols.
Brain function is highly complex. As Emerson M. Pugh stated: “If our brains were simple enough for us to understand them, we'd be so simple that we couldn't.”1 Consequently, a wide variety of tools exist to assess different components and aspects of brain activities. These include tools that directly measure brain functioning, such as via electroencephalography (EEG), and those measuring aspects related to brain function such as sleep patterns, gait, cognition, and gaze analysis.
In this article we focus on the emerging area of remote‐monitoring sensors, wearable devices, and mHealth (mobile health—the use of mobile devices, such as smartphones and tablet computers, in medical care) applications, and how these might be leveraged in large‐scale clinical trials and patient monitoring beyond marketing approval.
WEARABLE AND MOBILE APPLICATIONS
A sensor is a device or device component that detects and measures physical or chemical information from a surrounding physical environment, and translates this into an electrical output signal.2 Microsensors are miniature sensors that have electrical and mechanical operation components, also termed microelectromechanical systems (MEMS). These are usually produced by integrated circuit manufacturing from silicon or similar materials. A wearable device contains one or more sensors that are integrated into clothing or other accessories that can be worn on the body,3 such as on a wrist band, belt, headband, adhesive patch, contact lens, or glasses. In the context of brain monitoring, a wearable device may be, for example, a forehead headband containing sensors able to measure EEG signals associated with the frontal cortex. The use of reliable, high‐performance microsensors in the area of medicine is of growing importance for patient health monitoring,4 personal wellness, and clinical research.
The miniaturization of sensors and circuitry has led to the current proliferation in wearable devices. The ability to manufacture smaller, faster, and smarter processors and sensors has generated a huge growth in the availability of affordable consumer devices associated with the personal health and wellness market. The global connected health and wellness devices market was estimated to be $123.2 billion in 2015 and is expected to reach $612.0 billion by 2024.5
In the consumer wellness arena, we already see wearables and mHealth applications directed towards the monitoring and maintenance of brain health. Aspects of brain health include the way we think, feel, play, work, recall information, and sleep. Ensuring that we continually exercise our brains is thought to improve these aspects of brain health, although recent research indicates this activity may be less important than other aspects such as exercise, healthy eating, and positive social interactions.6 Brain‐training applications, delivered on mobile and cloud‐based web platforms, are aimed at increasing and maintaining aspects of cognitive function. Most use game and puzzle play to test, measure, and help to improve and maintain focus, processing speed, memory, attention, and problem‐solving skills. Popular examples include Fit Brains Trainer (Rosetta Stone, Arlington, VA), Elevate Brain Training (Elevate, San Francisco, CA), and Lumosity (Lumos Labs, San Francisco, CA). Importantly, the NeuroNation brain‐training application (NeuroNation, Berlin, Germany) is reimbursed by German health insurance. Some clinical research studies have shown positive improvements of executive function associated with brain training, for example, in reducing the cognitive deficits associated with chemotherapy in breast cancer survivors.7
Also popular in the consumer market are applications enabling brain training through neurofeedback. Using wearable headsets that enable EEG measurement of brain activity, it is possible to provide immediate feedback to users in the pursuit of brain health, in particular the combat or control of certain states or emotions. During neurofeedback, EEG signals received are summarized and presented to the user in a simple, visual format. For example, when training to improve attention, visual feedback may illustrate the degree of EEG activity associated with both efficient active thinking (beta brain wave activity) and slow and inefficient brain wave activity. In normal circumstances, we have no way of differentiating between aspects of our brain wave activity. However, providing immediate feedback through neurofeedback applications provides this immediate insight and enables the subject, over time, to learn how to modify their brain activity, and hence modify their brain wave patterns to consciously adjust the pattern of activity presented on screen. The ultimate aim is to enable the user to continue to recognize and modify brain activity for periods of time when the device is not worn. This kind of consumer application is commercially available for areas of brain wave training including the management of stress and anxiety and improving focus and concentration.8
APPLICATION TO CLINICAL TRIALS
It is feasible that the same technology could be leveraged for the study and measurement of treatment interventions in clinical trials. However, in our highly regulated industry, the validity of outcomes data collected using wearable and remote devices, and mHealth applications, must be subject to appropriate levels of rigorous examination. This has recently been comprehensively examined by the Critical Path Institute's Electronic Patient‐Reported Outcome (ePRO) Consortium who have put forward recommendations for the evidence needed to support the selection of a wearable device or remote sensor for use in clinical trials to support labeling claims.2 We provide a summary of these recommendations later in this article.
The focus of this article is on the use of wearable devices and mobile applications that measure electrical brain activity directly, and that measure aspects related to brain function such as sleep patterns, cognition, gaze analysis, and speech acoustical analysis. In most cases, the solutions we consider are emerging technologies that show merit for use in clinical trials, at least in the provision of exploratory endpoints and data that support other study endpoints measuring the same concepts of interest. We summarize the main technologies considered in Table 1, with an indication of whether there is a sufficient body of validation work and other evidence to support their use in clinical trials.
Table 1.
Remote‐monitoring devices and their applicability for use in clinical trials
| Device type | Examples of relevant devicesa | Measurement(s) | Usability | Strength of recommendation for clinical trial useb |
|---|---|---|---|---|
| Portable EEG headband | MUSE (InteraXon Inc., Toronto, Canada) | Event‐related brain potential | HIGH: regular use for short intervals of time daily | B |
| Emotiv EPOC (Emotiv Inc., Sydney, Australia) | ||||
| Wrist‐worn actigraphy device | Actiwatch 2 (Philips Respironics, Murrysville, PA) | Sleep quality, quantity and circadian rhythms | HIGH: regular use for multiple days | A |
| Motionwatch 8 (CamNtech, Cambridge, UK) | ||||
| Peripheral arterial tone wrist/hand‐worn device | WatchPAT (Itamar Medical, Caesarea, Israel) | Sleep architecture | HIGH: regular use for short intervals of time daily | B |
| Forehead worn sleep monitor | Sleep Profiler (Advanced Brain Monitoring, Carlsbad, CA) | EEG, EOG, EMG, ECG | MEDIUM: Use of periods of one or two consecutive nights at intervals throughout study | A |
| Non‐contact sleep sensor | Beddit 3 Sleep monitoring system (Apple, Cupertino, CA) | Sleep quality and quantity and circadian rhythms | HIGH: regular use for multiple days | C |
| S + sleep sensor (ResMed, San Diego, CA) | ||||
| ES contact‐free sensor (EarlySense, Ramat Gan, Israel) | ||||
| Wearable gait monitor | Kinesis Health Technologies (Dublin, Ireland) | Detailed gait measurements | HIGH: regular use for short in‐clinic performance tests | B |
| McRoberts (The Hague, The Netherlands) | HIGH: regular use for multiple days | |||
| APDM Wearable Technologies (Portland, OR) | HIGH: regular use for short in‐clinic performance tests | |||
| MC10 (Boston, MA) | HIGH: regular use for multiple days | |||
| Gait monitoring insole | F‐Scan™ system (Tekscan Inc., South Boston, MA) | Detailed gait measurements | MEDIUM: use for short in‐clinic performance tests | C |
| Moticon insole (Moticon GmbH, Munich, Germany) | HIGH: regular use for multiple days | |||
| Cognitive function | Project EVO (Akili Interactive Labs, Boston, MA) | Multitasking | HIGH: regular use for at‐home tests | B |
| Mobile eye tracking solutions | SensoMotoric Instruments (SMI, Teltow, Germany) | Eye tracking endpoints | HIGH: regular use for at‐home tests | B |
| Tobii (Stockholm, Sweden) | ||||
| Right Eye (Bethesda, MD) | ||||
| GazeCapture (MIT, Boston, MA) | HIGH: regular use for at‐home tests | C |
The authors selected a sample from the ever growing portfolio of available devices to illustrate the level of confidence for different concepts. Grade B or C does not disqualify the device, but is only indicating that a rather new concept is used, still requiring further validation.
A: Ready for use to derive trial endpoints based on validation evidence reported and/or existing use. B: Recommended for exploratory use and supportive data, more validation data and experience needed. C: Approach has merit but more research and evaluation is needed to make solid recommendations for use in clinical research.
CONSIDERATIONS FOR EVIDENCE TO SUPPORT DEVICE USE AND ENDPOINT DEVELOPMENT
In clinical trials, a study endpoint is defined as a characteristic or variable that reflects how a patient feels, functions, or survives.9 An endpoint description includes information defining how and when they are measured, how they are calculated, rules for missing data, and how they are analyzed. In the absence of formal regulatory guidance, the Critical Path Institute's ePRO Consortium reported consensus recommendations on the evidence required to support wearable device selection and endpoints derived from wearables data.2
When using any sensor or device to measure health outcomes and endpoints in clinical research, it is important to demonstrate the reliability and validity of outcome data collected, the ability of the outcome measures to reflect one or more concepts of interest as defined by the clinical trial objectives, and to demonstrate the suitability and interpretability of endpoint measures derived from these data. Some of this evidence may be available through market clearance/certification processes, but it is not a requirement for devices to be market cleared or certified when used in clinical research.
Reliability
Intra‐ and interdevice reliability should be demonstrated by assessment of test–retest reliability using the same and different units of the same device. Typically, this will be assessed using the intraclass correlation coefficient. To ensure reliability is maintained, device manufacturers must be able to demonstrate that devices are produced in adherence to a quality system to ensure equivalence of devices between batches and with the reliability data provided.
Concurrent validity
Concurrent validity is important to demonstrate that the approach is truly measuring what is intended. This is typically performed by comparing results to a gold standard methodology that is regarded as an accurate measure of the concept of interest.
Content validity
It is important to demonstrate that the endpoint(s) derived are considered important to patients and a relevant outcome within the disease/treatment studied. In the case that outcomes are not already well understood, this content validity can be obtained through qualitative data collection in patients or other reporters such as physicians or other caregivers.
Ability to detect change
Outcome measures and derived endpoints should, when used in a clinical trial, be seen to be sensitive enough to detect change when a change exists. This is normally demonstrated by controlled studies involving an intervention that is understood to create a change in the outcome of interest.
Endpoint interpretability
For an endpoint to be suitable for use in a clinical drug submission, it is important to understand meaningful change. In other words, it should be understood what degree of change in the endpoint can be interpreted as clinically relevant to the patient. This may be represented by the minimal important difference (MID) or minimally clinically important difference (MCID), or the minimal individual change that distinguishes a responder from a nonresponder. There are well‐established methodologies used to estimate MCID and responder definition (see Ref. 10, for example).
In the remaining sections of this article, we review some of the validation evidence supporting the use of emerging wearable and remote monitoring technologies that aim to directly or indirectly assess aspects of brain activity.
ELECTROENCEPHALOGRAPHY (EEG) AND EVOKED POTENTIALS (EP)
The first scientist to record human EEG data was Hans Berger in 1924.11 Since then, its fundamental principle has not changed. Electrodes placed typically on the scalp measure voltage fluctuations resulting from ionic current flows within the neurons of the brain as a result of brain activity. Signals are filtered and computerized analysis helps with the visualization of the recordings, known as quantitative EEG (qEEG) or EEG‐mapping (Figure 1).
Figure 1.
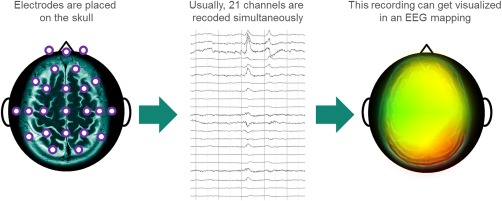
The principle of EEG‐mapping.
EEG mapping has found some application in clinical research to explore the pattern of brain activity resulting from psychotropic drugs and mental disorders,12 which have typical signatures in qEEG, but this has not been accepted as a surrogate endpoint in central nervous system (CNS) clinical drug development. The “pharmaco‐EEG,” i.e., the analysis of different EEG patterns under the influence of psychotropic pharmaceuticals, still has some place in the determination of cerebral bioavailability utilizing time‐ and dose‐efficacy relations, as well as the evaluation of bioequipotency of different formulations of compounds.13, 14, 15
One further potential application of qEEG is the analysis of different EEG patterns under different external stimuli and sensations such as pain. Since pain is typically assessed based on subjective patient self‐report, any pain study is exposed to a high level of placebo response16 and qEEG may provide an objective pain measurement.
Wearable devices to measure EEG and EP data
Wearable devices that measure EEG brain activity are worn predominantly around the forehead. Signals collected by a series of dry electrodes are filtered and interpreted by firmware within the device, to provide a continuous EEG signal trace. In health and wellness, mobile EEG applications are typically associated with two main development areas. The first uses measured brain activity to enable the user to control a product to produce a physical action or enable communication. A good example is the “Mind Speller” application, developed by researchers at the Catholic University of Leuven and IMEC, Belgium.17 The second main area, particularly for consumer applications, is development of brain‐training applications using neurofeedback as described above.
There are a number of good examples of portable EEG headband devices (Figure 2) suitable for consumer product development of smartphone and PC applications by leveraging their established software development kits. Examples include MUSE (InteraXon, Toronto, Canada), Emotiv EPOC (Emotiv, Sydney, Australia), and ZenZone (NeuroSky, San Jose, CA). The MUSE device, for example, comprises a headband worn across the forehead that contains seven sensors positioned across the forehead and behind each ear. Newer developments also include in‐ear EEG recording using several electrodes positioned in the outer ear canal and on the concha by means of an ear piece. This provides the possibility of continuous recording for longer periods of time due to good user acceptance, comfort, and discrete positioning.18 Although not initially intended for clinical research, most devices on the market also offer software development kits that allow researchers to access the raw signal data for research purposes.
Figure 2.

The EMOTIV EPOC EEG device. Image reproduced with permission of Emotiv Inc, Sydney, Australia.
Providing a highly portable solution that can be used to collect frequent data from patients in remote settings, such as the home, and continuous mobile monitoring promises the translation of neuroscientific knowledge into clinical and daily life applications.19 However, the reliability, accuracy, and precision of EEG trace data collected in this way must be examined to ensure that they are fit for use to provide exploratory data in clinical trials and regulatory submissions. One key difference is in the number of electrodes and the way they attach to the skin. A conventional clinic‐based EEG is typically recorded using 21 electrodes positioned across the scalp. This enables electrical activity to be detected across much of the brain regions (Figure 1). However, a forehead headband device, for example, relies predominantly on measurement from the frontal cortex and on a well‐fitting device to ensure electrodes are held in contact correctly and do not detach or generate electrical disturbances that may affect the EEG measurement through movement. Device firmware, however, may be able to identify and filter out some electrical disturbances detected.
Only a small quantity of research has been reported to validate the reliability of these systems for event‐related brain potential (ERP) research.20, 21, 22, 23, 24, 25, 26, 27, 28 Research to date, however, has shown the promise of these techniques. A study using the Emotiv EPOC headband solution compared continuous EEG measurements using the portable headband and a research‐grade EEG device (Neuroscan v. 4.3), using an EEG electrode cap (EasyCap, Herrsching, Germany) fitted with 14 Ag‐AgCl electrodes.21 The Emotive EPOC EEG Headset samples the EEG signal at a rate of 120 samples per second from 14 different electrodes, and its firmware filters out artifacts outside the bandwidth range of 0.2–45 Hz. The authors reported very good concordance between the systems for the P1, N1, P2, N2, and P3 ERP peaks in 19 6–12‐year‐old children under passive and active listening conditions. While the proportion of accepted measurement epochs was lower using the headset in comparison to the Neuroscan device, likely due to the differences in stability of electrode placement, there were sufficient acceptable epochs measured with the Emotiv headband to provide reliable ERPs. This study showed greater association between headband and full EEG system measurements, in comparison to an earlier study that showed only moderate associations when measured in adults.20
A validation study using the MUSE technology showed that the portable headband was able to accurately quantify the N200, P300, and reward positivity ERP components in two experimental paradigms.27 These tests were conducted rapidly (taking less than 10 minutes to perform), illustrating the utility of low‐cost portable EEG systems in the conduct of field and clinical research.
Artifact and noise filtering is an important aspect of obtaining reliable data from portable EEG headsets. The causes of biological artifacts include eyelid and eye movement, pulse artifacts due to electrode placement close to blood vessel, frontal scalp muscle activity, head or body movements, and sweat/skin artifacts caused by changes in skin potential.26 Many of these are easily differentiated from the EEG and can be removed by filtering, although eyelid and eye movement artifacts are more difficult but can be controlled by fixed gaze or subtracting eye artifacts by measurement using electrodes on or near the eyebrows. This was illustrated by one study using the MUSE and Neurosky headbands, which were unable to detect blinking signals.26 Common sources of technical artifacts include electrostatic and electromagnetic interference.
We consider the use of portable headset and earpiece devices for the measurement of EEG data remotely and/or continuously as an emerging opportunity where more research is needed to demonstrate the reliability and validity of data collected in this way (Table 1). Particular opportunity areas are discussed below.
Pain measurement
EEG data has been successfully used to provide objective measurement of pain.29 Increased alpha and theta power at spontaneous EEG and low amplitudes of ERP during various stimuli seem to be clinical characteristics of individuals with chronic pain.30 PainQx (New York, NY) uses research‐grade and portable EEG to assess neural brain activity and uses proprietary algorithms to interpret and describe the patient's pain state. This objective measurement of pain may be useful alongside traditional self‐assessment scales and patient‐reported outcomes instruments, and may be useful in evaluating the real‐time effects of analgesic and narcotic drugs.
Using EEG‐mapping, PainQx provides quantitative measures of activity in different regions of the brain that are involved in the sensation and perception of pain, termed the “Pain Matrix,” filtering out areas not related to the sensation and perception of pain. Areas of interest are isolated, identified, correlated, and weighted to produce an objective measurement of a patient's pain state. This approach seems to allow a differentiation between high and low pain condition in chronic pain—similar to the use of heart rate in acute pain. Future research will show whether it may also help to more reliably identify responders than current subjective pain scales.
Alzheimer's disease
ERPs may also provide objective information about processes in the brain. In a typical ERP protocol, a stimulus sequence of frequent (standard) and infrequent (target) tones are played, and an unexpected (distractor) tone is included occasionally. The subject is instructed to respond on hearing the infrequent target tone. This protocol generates a waveform that consists of a series of ERP components that produce positive and negative deflections in the ERP waveform. This provides a direct measure of cortical synaptic activity, indexing sensory and cognitive processes. The P300 component (a positive deflection ∼300 ms after the target tone) reflects attention and working memory and has been shown to be useful in detecting clinically relevant changes in cognitive function in Alzheimer's disease patients.31 ERPs provide a real‐time physiological measure of fundamental cognitive processes, which can be used in proof‐of‐concept studies, but also is not yet accepted as a surrogate endpoint in pivotal trials.
Traumatic brain injury/contusion
Another field currently lacking a sufficiently easy to use and objective measure of disease stage and outcome is mild brain contusion, a traumatic event occurring frequently during sporting activities such as American football, rugby, and soccer. Today, the diagnosis and management of contusion is performed using subjective tools and self‐reports of symptoms limiting the clinical impact of these tests. However, companies such as Cerora (Bethlehem, PA) have developed biosensors, mainly based on EEG methodology, which when used together with traditional cognitive tests empower researchers and clinicians to make better and more informed decisions about disease stage and outcomes.32, 33 However, no data yet exist which would support this concept, thus the specificity and sensitivity of the approach remains uncertain.
Epilepsy
Mobile EEG may facilitate long‐term monitoring in an outpatient or home environment. If usage for reasonably long periods can be enabled through unobtrusive wearable devices, this long‐term monitoring ability may be particularly interesting in the study of epilepsy, where seizure incidence is unpredictable and needs to be recorded outside the clinic environment. This kind of continuous monitoring data may be valuable in the detection and characterization of seizures, the recognition of subtle seizures that may otherwise go unrecognized by the patient, or determining seizure incidence in the event that a patient presents with seizure‐like symptoms. Due to the importance of monitoring and characterizing seizure incidence in clinical trials to assess new interventions, the ability to generate objective data through continuous monitoring will be of great value. Long‐term EEG measurement in an ambulatory setting has already shown promise in the assessment of patients who are difficult to diagnose or manage following evaluation of routine in‐clinic EEG assessments. Examples include: differentiating between nocturnal epilepsy and other sleep disorders such as abnormal movements during sleep, and accurate characterization of seizure and frequency.34 Currently, more work is needed to understand if this is a reliable, valid, and practically feasible approach.
PORTABLE SLEEP ASSESSMENT TECHNOLOGIES
We spend a third of our lives asleep; it is an essential element of all of our lives. It is well established that there is a bimodal relationship between sleep disturbance and clinical disease both in terms of cause and effect. While there is still significant debate as to the definition, diagnosis, and measurement of sleep, perhaps the simplest definition of sleep focuses on the behavioral definition that describes sleep as “a reversible behavioral state of perceptual disengagement from and unresponsiveness to the environment.”35
Measuring aspects of sleep and its effect can be important in clinical drug development where improvements or worsening in sleep may be observed as a direct or indirect response to treatment. Different objective measurements of aspects of sleep may be important, depending on the concept of interest for the clinical investigation. Measures of sleep architecture and sleep continuity may be beneficial in the monitoring of brain health related to neurodegenerative disorders. Sleep spindles (short bursts of high‐frequency brain activity) during nonrapid eye movement sleep have been shown to relate to cognitive decline in Parkinson's disease, and reduced slow wave sleep is associated with Alzheimer's disease.36
Sleep outcome measures/parameters
Sleep architecture refers to the basic structural organization of normal sleep. Normal adult sleep consists of alternating periods of rapid eye movement (REM) and non‐REM (N‐REM) sleep, with N‐REM sleep accounting for 75–80% of the sleep period. Sleep consists of four episodes or stages of N‐REM sleep followed by REM sleep, the N‐REM and REM sleep cycles being progressively longer throughout the course of the full night's sleep cycle.
In addition to sleep architecture, sleep quality, sleep quantity, circadian rhythmicity, sleep consolidation, regularity, and napping are also important factors in assessing sleep and wake patterns. The following outcome measures are commonly estimated to assess sleep quality and quantity: sleep onset latency, wake after sleep onset, sleep efficiency, number of awakenings, and total sleep time.
Depending on the sleep parameter under investigation, there are different technologies and methodologies that can be employed. An important consideration in technology selection is the recommendations from the International Classification of Sleep Assessment.37 These are generally adopted by the biopharmaceutical industry in the conduct of clinical development programs, and have particular importance where sleep data will be used as a primary or secondary endpoint in a regulatory new drug application.
Polysomnography
Polysomnography (PSG) has been considered the gold standard technology for measuring sleep architecture. PSG is a simultaneous multiparametric assessment that is carried out overnight and consists of the following physiological parameters: EEG, electrooculography (EOG), and surface electromyography (EMG). It can also include measurement of pulse oximetry, respiratory effort, and core temperature. Because of its complexity and the quantity of instrumentation needed, PSG has until recently been restricted to specialized sleep laboratories and requires skilled sleep laboratory technicians for analysis and interpretation.
Despite being considered a gold standard, it is not without limitations for use in clinical development, in particular the cost and the need to access heavily subscribed specialist laboratory services. A major issue is the “first night affect,” where subjects sleeping in an abnormal environment may affect the sleep parameters measured. In addition, the manual assessment of data can result in high interrater variability in assessments.
To overcome the cost and specialist services needed with PSG, much attention has been given to alternative approaches and technology to facilitate the assessment of sleep in nonclinical settings. We review the key technologies below.
Remote sleep assessment: Actigraphy
Actigraphy is the use of an accelerometer to measure gross motor movements and is based on the simple premise that when there is no activity the probability is that the individual is asleep, and when there is movement the probability is that the individual is awake38 (Figure 3). The use of accelerometers to identify sleep and rest patterns dates back to the 1970s.39 Since its conception, a number of devices have been developed with sophisticated algorithms that filter ambient noise and have been validated against PSG in their ability to reliably assess certain sleep parameters, leading to its use as a tool to assess sleep quality and quantity outside of the clinical laboratory setting.40 The low‐burden nature of the technology, its suitability as a means of capturing real‐life sleep and activity data over weeks and months has meant that this technology is widely used both by the research community and in drug development to assess changes in sleep and activity patterns.
Figure 3.
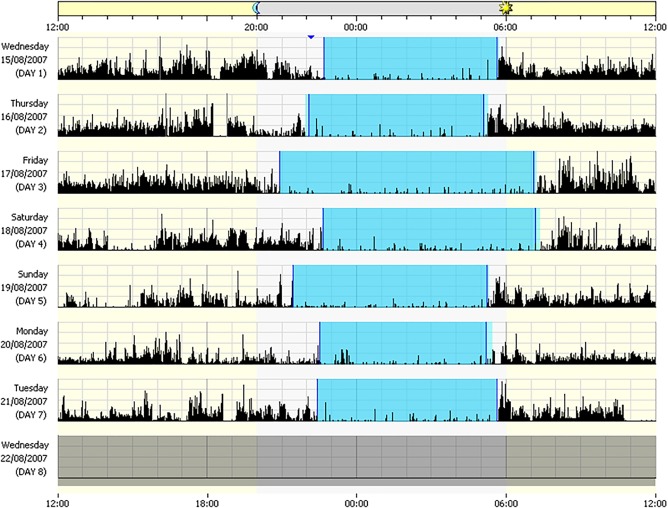
An actogram showing changes in daily activity and sleep patterns. Black bars represent periods of activity. Shaded blue areas represent resting/sleep periods. Image reproduced with permission of Philips Respironics, Murrysville, PA.
Actigraphy has been demonstrated to provide good sleep parameter estimates to assess sleep quality, quantity, and circadian rhythms,41 but it is not a validated technology for assessing sleep architecture (REM and N‐REM Sleep). Actigraphy‐determined sleep is highly correlated with PSG outcomes in normal adults (>90%),42 but less so for other populations such as insomniacs, where the correlation can be as low as 50% for some of the parameters such as sleep onset latency.41, 43 Different devices and their associated firmware and software have different sensitivities to immobility and movement. Sensitivity to immobility allows the device to identify periods of sleep. Sensitivity to mobility allows the device to identify periods of wakefulness. In general, actigraphy devices are very good predictors of sleep, but less sensitive to wakefulness, and as a result can overestimate total sleep time. When selecting a device for use in a clinical trial, it is important to ensure that the device has been validated in the population being studied.
Actigraphy with additional physiological channels
More recently, some newer actigraphy devices also incorporate the capability to measure additional physiological parameters such as heart rate, respiration rate, galvanic skin response, skin temperature, and pulse oximetry. This has enabled the assessment of sleep architecture in addition to sleep quality and quantity. One example among this class of devices is the WatchPAT (Itamar Medical, Caesarea, Israel) which measures peripheral arterial tone (PAT), along with actigraphy and other measures. While developed for home‐based assessment of sleep apnea,44, 45, 46 there is emerging research suggesting that good agreement between PAT and polysomnography data, and that specific PAT patterns can be observed during different sleep stages, which enables the recognition of REM and N‐REM sleep, including detection of lighter stages from deeper, slow wave sleep.46, 47
Ambulatory PSG and EEG
While it is possible to conduct more extensive testing in the home environment using ambulatory PSG that can monitor and record a number of aspects including EEG, EOG, EMG, ECG, pulse rate, air flow, respiratory movement/effort, and oxygen saturation, its use can be expensive and may require specialist teams to manage and implement. A practical alternative device, Sleep Profiler (Advanced Brain Monitoring, Carlsbad, CA), is worn on the forehead overnight and measures EEG, EOG, EMG, ECG, pulse rate, head position, head movement, and snoring incidence using a variety of sensors held in place with a headband (Figure 4). Studies have successfully demonstrated the concurrent validity of sleep biomarkers recorded using data from only one or two nights in comparison to PSG,36 indicating the validity of the approach for use in clinical or research applications. This enables the monitoring of sleep architecture and continuity for multiple nights at home, or for continuous periods in an ICU. Automated sleep staging algorithms associated with the use of Sleep Profiler have been shown to provide reliable results in comparison to manual ratings.36, 48, 49
Figure 4.

Sleep Profiler EEG device to assess sleep architecture and continuity. Image reproduced with permission of Advanced Brain Monitoring, Carlsbad, CA.
Noncontact sensor technology
A new class of sleep assessment tools have recently emerged that claim to measure sleep parameters without the requirement to be worn.
The Beddit 3 Sleep monitoring system (Apple, Cupertino, CA) uses a flexible piezoelectric film sensor that is placed beneath the bed sheet. This is able to measure the forces caused by the body on the bed to detect tiny movements that can be interpreted to estimate pulse (heart pumping), breathing effort (thorax extension), and overall body movement. Validation work comparing heart rate estimates to ECG50 and breathing effort to the respiration effort signal in PSG51 show some promise but more work is needed. The Beddit system uses a BlueTooth connection with a mobile phone hub to transmit the data to a central server and apply algorithms to estimate heart rate, respiratory variation, activity, sleep stages, and stress reactions. A validation study of a similar piezoelectric sensor device, the ES contact‐free sensor (EarlySense, Ramat Gan, Israel) showed good reliability of total sleep time estimates compared to PSG.52
A second approach, the S + sleep sensor (ResMed, San Diego, CA), uses a bedside sleep monitor to detect ultralow‐power radiofrequency waves to monitor the movements of the subject in bed, such as the expansion and relaxation of the chest during respiration, and overall body movements such as positional changes, arm twitches, and shrugs. The device uses novel proprietary algorithms to identify sleep stages (wake (W), light sleep (N1, N2 sleep), deep sleep (N3 sleep), REM or N‐REM). Robust validation work is needed for this approach to be of use in clinical research (Table 1).
GAIT ASSESSMENT
Assessment of gait provides significant insights into the progression and treatment of neurodegenerative diseases. For example, the predictive value of gait abnormalities as a risk factor for the development of dementia has been identified,53 and more recently, evidence from clinical practice and epidemiological studies show that gait and cognition are interrelated.54 Similar associations have been identified in Parkinson's disease, where an association between global cognitive function and pace‐related measures of gait, turning, and postural sway has been identified.55
In‐clinic assessment of temporal and spatial gait parameters can be achieved using performance tests conducted on pressure pad systems such as GaitRITE (CIR Systems, Franklin, NJ) and Zeno Walkway (ProtoKinetics, Havertown, PA), or using 3D motion capture solutions such as the Vicon marker‐based camera system (Vicon Motion Systems, Oxford, UK). These solutions provide robust gait parameter estimates, but often require conduct at specialist centers, which may limit the scale at which they can be used in clinical trials.
Alternative approaches, measuring gait parameters remotely in free‐living settings, may provide a richer and more informative picture of gait in comparison to a clinic performance snapshot.56
Newer approaches that facilitate the collection of robust, objective, and sensitive measurements of a variety of gait parameters include the capability to measure both in‐clinic or outside the clinic setting. The rich data that these provide may enable establishment of new and more responsive gait endpoints. Kinesis Health Technologies (Dublin, Ireland), McRoberts (The Hague, The Netherlands), APDM Wearable Technologies (Portland, OR), and MC10 (Boston, MA) offer easy‐to‐use in‐clinic and remote systems for objective assessment and estimation of gait parameters such as cadence, gait speed, double support, lateral step variability, foot strike angle, toe off angle, stance, step duration, stride length, swing velocity, and toe out angle. Most employ multiply located sensors along with algorithms to interpret simultaneous signals to provide gait parameters and assessments.
Promising areas of innovation include the embedding of pressure sensors and accelerometers into footwear insoles such as the F‐Scan system (Tekscan, South Boston, MA) and Moticon's insole (Moticon, Munich, Germany) (Figure 5). These approaches, in addition to other e‐textile applications, may provide a patient‐centric approach to enable the collection of gait assessment data in a frictionless manner in free‐living settings. Studies using the Moticon sensor‐instrumented insole solution, for example, have demonstrated good validation and reliability of gait parameters collected.57, 58 In addition, the use of the 3D depth cameras associated with motion‐based gaming platforms and other applications to track 3D joint coordinates and movements, such as Microsoft Kinect (Redmond, WA) and Intel RealSense (Santa Clara, CA) have shown promise in measuring gait parameters based on simple in‐clinic performance tests, reducing reliance on specialist centers.59, 60
Figure 5.
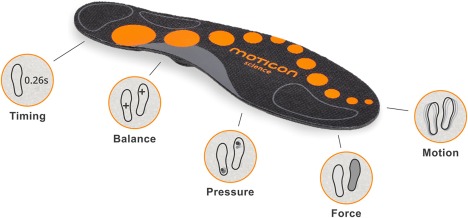
Moticon insole system for gait analysis. Image reproduced with permission of Moticon GmbH, Munich, Germany.
One limitation of the collection of rich free‐living gait assessment data is the understanding of the context associated with the data. Changes in external factors such as terrain, footwear, clothing, the size and shape of living space, ease of access to outside spaces, and the weather all may potentially impact remote gait assessment.
COGNITIVE FUNCTION TESTING
Cognition is the ability to perceive and react, process, and understand, store, and retrieve information, make decisions, and produce appropriate responses.61 Many mental disorders are associated with disrupted cognitive function such as dysfunctions of attention, concentration, and memory. Cognitive function testing can help to identify which brain mechanisms are involved in the symptoms, and can help determine, and assess the impact of, treatment. Cognitive function in clinical trials is traditionally measured in laboratory conditions using a battery of computerized tests, for example, using the CDR (Bracket, Arlington, VA) 62 and CANTAB (Cambridge Cognition, Cambridge, UK)63 systems.
Nonlaboratory measurement of cognition
While a huge body of work exists validating the use of the main cognitive function testing platforms in the study of many CNS and other conditions, including the collection of large banks of normative data to provide reference, for practical reasons laboratory tests are typically limited to smaller studies and infrequent assessment. More recently, approaches to testing have been developed that enable more frequent assessment in nonlaboratory settings and in larger groups of patients. This offers the possibility of continued testing in clinical trials and postmarket evaluations.
Some approaches to the measurement of reaction times, memory, and problem‐solving have been executed through the context of video game play, leveraging smartphone and tablet technology. Project:Evo, for example, is a game application developed by Akili Interactive Labs (Boston, MA) that can be used to measure and improve interference processing, a key component of executive function. The premise of the game, which operates on mobile phones and tablets, is to provide an environment where a player's ability to process out distractions during the focused conduct of a specific action can be assessed and measured. It is intended to be an engaging alternative to conventional cognitive test batteries.64 This game platform is currently being tested in a variety of global clinical studies in multiple patient populations, including attention deficit hyperactivity disorder (ADHD), autism, depression, and traumatic brain injury. Pfizer (New York, NY), for example, has reported that outcomes measures derived from gameplay on the platform were able to distinguish between amyloid‐positive older healthy subjects vs. an age‐matched comparison group of amyloid‐negative subjects; suggesting the game may be valuable as a noninvasive biomarker for Alzheimer's disease screening and tracking.65 Shire (Dublin, Ireland) is also funding investigations on the use of the game in ADHD clinical trials.
Smartphones offer great potential to present visual cognitive function tests in a remote setting. Initial work has shown the approach can provide reliable estimates of aspects of cognitive function and good concurrent validity when compared to gold standard approaches.66 The Apple ResearchKit provides a platform to develop a variety of patient performance tests and assessments, including a number of tests of cognition such as the paced visual serial addition test, a spatial memory test, and a simple reaction time test.67 While some basic validation work has been reported on the use of these tests,68 more work is required to demonstrate reliable and valid measurement.
Wearable devices, in particular smart watches, may also offer the ability to frequently deliver and measure simple tests of cognition in a remote setting. Cambridge Cognition has expanded their laboratory‐based testing solutions to include remote testing via a wearable device. A preliminary study of a two‐back symbol memory test delivered on the Microsoft Band 2 provided data that supports the feasibility of cognitive assessment on wearable devices.69
EYE TRACKING
Eye‐tracking measures provide valuable, noninvasive insights into brain function and cognition. For example, gaze analysis is useful in assessing attention and cognitive strategies; pupil dilation is modulated by noradrenaline and is related to arousal and mental activity; and blink rate is modulated by dopamine, which is related to learning and goal‐oriented behavior.70
Pupillometry has increasing value as a marker of physiological state. Pupil diameter fluctuations have been shown to track rapid changes in adrenergic and cholinergic activity in the cortex in animal models,71 and to provide a measure of task disinterest in studies of mental fatigue and task disassociation.72
Spontaneous blink rate can provide an indirect measure of dopamine activity in the CNS,73 and reductions in blink rate are observed with the administration of dopamine receptor antagonists.74 Studies on infants have shown that eye movement can be used as an early diagnostic tool for autism diagnosis; with children on the autistic spectrum favoring focusing on images of geometric design rather than those of human faces.75
Measuring fixation stability and saccadic movements (rapid movements of the eyes that abruptly change the point of fixation) have been shown to have clinical significance for a number of CNS disorders such as Huntington's disease, progressive supranuclear palsy, and Parkinson's disease.76 In the early stages of dementia, saccadic eye movement recording may help discriminate between Lewy body dementia and Alzheimer's disease.77
Eye tracking is gaining increasing interest as a potential biomarker for brain injury. Studies assessing treatments in traumatic brain injury are difficult to conduct for various reasons, including heterogeneity in the study population due to the lack of accurate diagnosis and classification schemes, and the lack of robust objective outcome measures. One of the reasons for the failure of the ProTECT III and SyNAPSe clinical trials of progesterone treatment of acute traumatic brain injury was cited as a lack of adequate biomarkers for brain injury.78 Eye tracking, however, has been proposed as an objective biomarker for brain injury and concussion.79
Portable eye tracking technology
Eye tracking is still the preserve of a small number of specialist companies that supply validated eye‐tracking technology; SensoMotoric Instruments (SMI, Teltow, Germany; now owned by Apple) and Tobii (Stockholm, Sweden) are considered leaders in this space. Both offer lab‐based camera systems (Figure 6) and more portable solutions by incorporating their technology into glasses and virtual reality headsets. Right Eye (Bethesda, MD) is a newer eye tracking provider with a cloud‐based solution, and has been shown to provide reliable estimates of some eye‐tracking parameters.80
Figure 6.

A volunteer wearing an EEG cap in front to the Tobii Pro Spectrum eye tracking platform. Image reproduced with permission of Acuity ETS Ltd., Reading, UK.
Researchers at the Massachusetts Institute of Technology, the University of Georgia, and the Max Planck Institute for Informatics have developed a mobile application, GazeCapture, operating on Apple mobile devices, which displays a sequence of dots that the user is able to track and fixate. The mobile device's front‐facing camera simultaneously captures eye movements during the performance task. The associated machine‐learning algorithms have been shown to be capable of robustly predicting gaze with low errors on both smartphone and tablet devices,81 and this approach enables the collection of rich eye‐tracking performance test data remotely in large‐scale studies.
The increasing use of eye tracking to assess consumer behavior, and its use in gaming platforms and Virtual Reality, means significant continued investment in the technology solutions servicing this area. This is likely to result in increasing capabilities and mobile‐enablement of eye‐tracking technology, which will benefit applications for clinical research.
VOICE ACOUSTICAL ANALYSIS
An area of ongoing research involves the capture and acoustical analysis of the speech patterns of patients. Studies of voice acoustical analysis of patients with extremely early‐stage Parkinson's disease suggested that voice acoustical changes can be good predictors of early onset of the disease.82 In depression, certain voice acoustical parameters such as speaking rate and pitch variability have been shown to correlate well with conventional measures of disease severity such as the Hamilton Depression Rating Scale.83 A methodology study comparing the acoustical measures made from recordings obtained using state‐of‐the‐art laboratory recording equipment and simultaneous recording over the telephone using an Interactive Voice Response system indicated that the data obtained by both methods were highly comparable, enabling the possibility of large‐scale longitudinal testing from home settings.84 More recently, phonation tests for Parkinson's disease patients have been developed in clinical research mobile apps using both Apple ResearchKit67 and on the Android platform.85, 86, 87 This opens the possibility of using such inexpensive techniques in large‐scale clinical trials.
CONCLUSION
The rapid developments in the wearable device and remote sensor market are driven predominantly by the miniaturization of sensors and circuitry and the improvements in functionality and processing power of mobile devices such as smartphones and tablet computers. In the area of health, and specifically in brain monitoring, as described in this article, these developments are leading to opportunities to develop and leverage innovative devices to make measurements normally confined to the clinic. Rapid technological developments can be seen in the area of portable EEG monitoring, and in other areas related to brain function including sleep assessment, gait analysis, cognitive function testing, eye tracking, and voice analysis.
Aside from the measurement of sleep parameters by wrist actigraphy, which already provides well‐accepted endpoints for clinical trials, the technologies described in this article are emerging and the subject of continued research providing validation and utility evidence. It is acknowledged that more research is needed to better understand the reliability and validity of these emerging technologies, and to generate the required understanding of new endpoints derived from their data. Despite this, there is enough understanding of how to implement these approaches as exploratory tools, which may provide additional valuable insights due to the rich and frequent data they produce, to justify their inclusion in clinical study protocols.
FUNDING
No funding was received for this work.
CONFLICT OF INTEREST
The authors declare no competing interests for this work.
References
- 1. Pugh, G.E. The Biological Origin of Human Values. Abingdon‐on‐Thames, UK: Routledge & Kegan Paul; 1978. [Google Scholar]
- 2. Byrom, B. et al Selection of and evidentiary considerations for wearable devices and their measurements for use in regulatory decision making: recommendations from the ePRO Consortium. Value Health (in press). [DOI] [PubMed] [Google Scholar]
- 3. Wright, R. & Keith, L. Wearable technology: If the tech fits, wear it. J. Electron. Resources Med. Libraries 11, 204–216 (2014). [Google Scholar]
- 4. Tsoukalas, D. , Chatzandroulis, S. & Goustouridis, D. Capacitive microsensors for biomedical applications In: Encyclopedia of Medical Devices and Instrumentation (ed. Webster J.G.) 1–12. Hoboken, NJ: John Wiley & Sons; 2006. [Google Scholar]
- 5.Grand View Research. Connected health and wellness devices market report. August 2016.
- 6. Souders, D.J. , Boot, W.R. , Blocker, K. , Vitale, T. & Roque, N.A. , Charness, N. Evidence for narrow transfer after short‐term cognitive training in older adults. Front. Aging Neurosci. 9, 41–50 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kesler, S . et al Cognitive training for improving executive function in chemotherapy‐treated breast cancer survivors. Clin. Breast Cancer 13, 299–306 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Byrom, B . Brain monitoring devices in clinical trials. App. Clin. Trials <http://www.appliedclinicaltrialsonline.com/brain-monitoring-devices-clinical-trials> (2015). Accessed 28 November 2017. [Google Scholar]
- 9. Biomarkers Definitions Working Group . Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 69, 89–95 (2001). [DOI] [PubMed] [Google Scholar]
- 10. Food and Drug Administration . Guidance for Industry: Patient‐Reported Outcome Measures—Use in Medical Product Development to Support Labeling Claims. <http://www.fda.gov/downloads/Drugs/.../Guidances/UCM193282.pdf> (2009). Accessed 14 November 2017.
- 11. Collura, T.F. History and evolution of electroencephalographic instruments and techniques. J. Clin. Neurophysiol. 10, 476–504 (1993). [DOI] [PubMed] [Google Scholar]
- 12. Saletu, B. Pharmaco‐EEG profiles of typical and atypical antidepressants. Adv. Biochem. Psychopharmacol. 32, 257–268 (1982). [PubMed] [Google Scholar]
- 13. Saletu, B. , Anderer, P. , Kinsperger, K. & Grünberger, J. Topographic brain mapping of EEG in neuropsychopharmacology. Part II. Clinical applications (pharmaco EEG imaging). Methods Find. Exp. Clin. Pharmacol. 9, 385–408 (1987). [PubMed] [Google Scholar]
- 14. Jobert, M. et al Guidelines for the recording and evaluation of pharmaco‐EEG data in man: the International Pharmaco‐EEG Society (IPEG). Neuropsychobiology 66, 201–220 (2012). [DOI] [PubMed] [Google Scholar]
- 15. Jobert, M . et al Guidelines for the recording and evaluation of pharmaco‐sleep studies in man: the International Pharmaco‐EEG Society (IPEG). Neuropsychobiology 67, 127–167 (2013). [DOI] [PubMed] [Google Scholar]
- 16. Waber, R.L. , Shiv, B. , Carmon, Z. & Ariely, D. Commercial features of placebo and therapeutic efficacy. JAMA 299, 1016–1017 (2008). [DOI] [PubMed] [Google Scholar]
- 17. Katholic University of Leuven . Campus Insight Magazine. <http://www.kuleuven.be/ci/28/28.pdf> (May 2010). Accessed 28 November 2017.
- 18. Looney, D.P. et al The in‐the‐ear recording concept user‐centered and wearable brain monitoring. IEEE Pulse 3, 32–42 (2012). [DOI] [PubMed] [Google Scholar]
- 19. Bleichner, M.G. et al Exploring miniaturized EEG electrodes for brain‐computer interfaces. An EEG you do not see? Physiol. Rep. 3, 1–9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Badcock, N.A. , Mousikou, P. , Mahajan, Y. , de Lissa, P. , Thie, J. & McArthur, G. Validation of the Emotiv EPOC EEG gaming system for measuring research quality auditory ERPs. PeerJ 1, e38 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Badcock, N.A. et al Validation of the Emotiv EPOC EEG system for research quality auditory event‐related potentials in children. PeerJ 3, e907 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Debener, S. , Minow, F. , Emkes, R. , Gandras, K. & de Vos, M. How about taking a low‐cost, small, and wireless EEG for a walk?, Psychophysiology 49, 1617–1621 (2012). [DOI] [PubMed] [Google Scholar]
- 23. Duvinage, M. , Castermans, T. , Petieau, M. , Hoellinger, T. , Cheron, G. & Dutoit, T. Performance of the Emotiv Epoc headset for P300‐based applications. Biomed. Eng. Online 12, 56–70 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wascher, E. , Heppner, H. & Hoffmann, S. Towards the measurement of event‐related EEG activity in real‐life working environments. Int. J. Psychophysiol. 91, 3–9 (2014). [DOI] [PubMed] [Google Scholar]
- 25. Gramann, K. , Ferris, D.P. , Gwin, J. & Makeiq, S. Imaging natural cognition in action. Int. J. Psychophysiol. 91, 22–29 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maskeliunas, R. , Damasevicius, R. , Martisius, I. & Vasiljevas, M. Consumer‐grade EEG devices: are they usable for control tasks? PeerJ 4, e1746 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Krigolson, O.E. , Williams, C.C. , Norton, A. , Hassall, C.D. & Colino, F.L. Choosing MUSE: Validation of a low‐cost, portable EEG system for ERP research. Front. Neurosci. 11, 109–118 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kuziek, J.W. , Shienh, A. & Mathewson, K.E. Transitioning EEG experiments away from the laboratory using a Raspberry Pi 2. J. Neurosci. Methods 277, 75–82 (2017). [DOI] [PubMed] [Google Scholar]
- 29. Prichep, L.S. , John, E.R. , Howard, B. , Merkin, H. & Hiesiger, E.M. Evaluation of the pain matrix using EEG source localization: a feasibility study. Pain Med. 12, 1241–1248 (2011). [DOI] [PubMed] [Google Scholar]
- 30. dos Santos Pinheiro, E.S. et al Electroencephalographic patterns in chronic pain: a systematic review of the literature. PLoS ONE 11: e0149085 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Drago, V . et al Disease tracking markers for Alzheimer's disease at the prodromal (MCI) stage. J. Alzheimers Dis. 26, 159–199 (2011). [DOI] [PubMed] [Google Scholar]
- 32. Simon, A.J. & Devilbiss, D.M. Sports concussion and mild traumatic brain injury assessment is enhanced with portable, non‐invasive biosensor arrays. Med. Sci. Sports Exerc. 47, 11 (2015). [Google Scholar]
- 33. Simon, A.J. & Devilbiss, D.M. multivariate models of biosensor data to actively assess sports concussion and mild traumatic brain injury. Neurology 86, 011 (2016) [Google Scholar]
- 34. Smith, S.M.J. EEG in the diagnosis, classification and management of patients with epilepsy. J. Neurol. Neurosurg. Psychiatry 76, ii2–ii7 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carskadon, M.A. & Dement, W.C. Monitoring and staging human sleep In: Principles and Practice of Sleep Medicine, 5th ed. (eds. Kryger M.H., Roth T., & Dement W.C.) 16–26. St. Louis, MO: Elsevier Saunders; 2011. [Google Scholar]
- 36. Levendowski, D. , Ferini‐Strambi, L. , Gamaldo, C. , Cetel, M. , Rosenberg, R. & Westbrook, P.R. The accuracy, night‐to‐night variability, and stability of frontopolar sleep electroencephalography biomarkers. Clin. Sleep Med. 13, 791–803 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Institute of Medicine (US) Committee on Sleep Medicine and Research . Sleep physiology In: Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem (eds. Colten H.R. &. Altevogt B.M.) 33–54. Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- 38. Schenck, C.H. , Mahowald, M.W. & Sack, R.L. Assessment and management of insomnia. JAMA 289, 2475–2479 (2003). [DOI] [PubMed] [Google Scholar]
- 39. Kripke, D.F. , Mullaney, D.J. , Messin, S. & Wyborney, V.G. Wrist actigraphic measures of sleep and rhythms. Electroencephalogr. Clin. Neurophysiol. 44, 674–676 (1978). [DOI] [PubMed] [Google Scholar]
- 40. Morgenthaler, T . et al Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep 30, 519–529 (2007). [DOI] [PubMed] [Google Scholar]
- 41. Martin, J.L. & Hakim, A.D. Wrist actigraphy. Chest 139, 1514–1527 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ancoli‐Israel, S. , Cole, R. , Alessi, C. , Chamber, M. , Moorcroft, W. & Pollak, C.P. The role of actigraphy in the study of sleep and circadian rhythms. Sleep 26, 342–392 (2003). [DOI] [PubMed] [Google Scholar]
- 43. Sivertsen, B . et al A comparison of actigraphy and polysomnography in older adults treated for chronic primary insomnia. Sleep 29, 1353–1358 (2006). [DOI] [PubMed] [Google Scholar]
- 44. Yalamanchali, S. , Farajian, V. , Hamilton, C. , Pott, T.R. , Samuelson, C.G. & Friedman, M. Diagnosis of obstructive sleep apnea by peripheral arterial tonometry: meta‐analysis. JAMA Otolaryngol. Head Neck Surg. 139, 1343–1350 (2013). [DOI] [PubMed] [Google Scholar]
- 45. Pinto, J.A. , Mello de Goday, L.B. , Ribeiro, R.C. , Mizoguchi, E.I. , Hirsch, L.A.M. & Gomes, L.M. Accuracy of peripheral arterial tonometry in the diagnosis of obstructive sleep apnea. Braz. J. Otorhinolaryngol. 81, 473–478 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pepin, J.L. , Tamisier, R. , Borel, J.C. , Baquet, J.P. & Levy, P. A critical review of peripheral arterial tone and pulse transit time as indirect diagnostic methods for detecting sleep disordered breathing and characterizing sleep structure. Curr. Opin. Pulm. Med. 15, 550–558 (2009). [DOI] [PubMed] [Google Scholar]
- 47. Herscovici, S. , Pe'er, A. , Papyan, S. & Lavie, P. Detecting REM sleep from the finger: an automatic REM sleep algorithm based on peripheral arterial tone (PAT) and actigraphy. Physiol. Meas. 28, 129–140 (2007). [DOI] [PubMed] [Google Scholar]
- 48. Stepnowsky, C. , Levendowski, D. , Popovic, D. , Ayappa, I. & Rapoport, D.M. Scoring accuracy of automated sleep staging from a bipolar electroocular recording compared to manual scoring by multiple raters. Sleep Med. 14, 1199–1207 (2013). [DOI] [PubMed] [Google Scholar]
- 49. Levendowski, D.J. , Popovic, D. , Berka, C. & Westbrook, P.R. Retrospective cross‐validation of automated sleep staging using electroocular recording in patients with and without sleep disordered breathing. Int. Arch. Med. 5, 21–29 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Paalasmaa, J. , Toivonen, H. & Partinen, M. Adaptive heartbeat modeling for beat‐to‐beat heart rate measurement in ballistocardiograms. IEEE J. Biomed. Health Inform. 19, 1945–1952 (2015). [DOI] [PubMed] [Google Scholar]
- 51. Paalasmaa, J. , Leppäkorpi, L. & Partinen, M. Quantifying respiratory variation with force sensor measurements. 33rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (2011). [DOI] [PubMed]
- 52. Tal, A. , Shinar, Z. , Shaki, D. , Codish, S. & Goldbart, A. Validation of contact‐free sleep monitoring device with comparison to polysomnography. J. Clin. Sleep Med. 13, 517–522 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Beauchet, O. , Allali, G. , Launay, C. , Herrmann, F.R. & Annweiler, C. Gait variability at fast‐pace walking speed: a biomarker of mild cognitive impairment?, J. Nutr. Health Aging 17, 235–239 (2013). [DOI] [PubMed] [Google Scholar]
- 54. Verghese, .J , Lipton, R.B. , Hall, C.B. , Kuslansky, G. , Katz, M.J. & Buschke, H. Abnormality of gait as a predictor of non‐Alzheimer's dementia. N. Engl. J. Med. 347, 1761–1768 (2002). [DOI] [PubMed] [Google Scholar]
- 55. Montero‐Odasso, M. , Verghese, J. , Beauchet, O. & Hausdorff, J.M. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J. Am. Geriatr. Soc. 60, 2127–2136 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pal, G. , O'Keefe, J. , Robertson‐Dick, E. , Bernard, B. , Anderson, S. & Hall, D. Global cognitive function and processing speed are associated with gait and balance dysfunction in Parkinson's disease. J. NeuroEng. Rehabil. 13, 94–101 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Braun, B.J. et al Validation and reliability testing of a new, fully integrated gait analysis insole. J. Foot Ankle Res. 8, 54–60 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Oerbekke, M.S. , Stukstette, M.J. , Schutte, K. , de Bie, R.A. , Pisters M.F. & Vanwanseele, B. Concurrent validity and reliability of wireless instrumented insoles measuring postural balance and temporal gait parameters. Gait Posture 51, 116–124 (2017). [DOI] [PubMed] [Google Scholar]
- 59. Eltoukhy, M. , Kuenze, C. , Jeonghoon, O. , Jacopetti, M. , Wooten, S. & Signorile, J. Microsoft Kinect can distinguish differences in over‐ground gait between older persons with and without Parkinson's disease. Med. Eng. Phys. 44, 1–7 (2017). [DOI] [PubMed] [Google Scholar]
- 60. Breedon, P. , Byrom, B. , Siena, L. , Muehlhausen, W. Enhancing the measurement of clinical outcomes using Microsoft Kinect. International Conference on Interactive Technologies and Games (iTAG) 2016. IEEE Xplore. <http://ieeexplore.ieee.org/document/7782516> (2016). Accessed 20 November 2017. [Google Scholar]
- 61. Cambridge Cognition . What is Cognition? <http://www.cambridgecognition.com/blog/entry/what-is-cognition> (2015). Accessed 20 November 2017.
- 62. Wesnes, K.A. , Simpson, P.M. & Christmas, L. The assessment of human information processing abilities in psychopharmacology In: Human Psychopharmacology, Measures and Methods (eds. Hindmarch I. &. Stonier P.D.) 79–92. Hoboken, NJ: Wiley‐Blackwell; 1987. [Google Scholar]
- 63. Cambridge Cognition . CANTAB® [Cognitive assessment software]. <http://www.cantab.com> (2017). Accessed 28 November 2017.
- 64. Byrom, B . Clinical trials Re‐spec: The role of games and gamification in clinical trials. International Conference on Interactive Technologies and Games (iTAG) 2015. IEEE Xplore. <http://ieeexplore.ieee.org/document/7399486> (2015). Accessed 20 November 2017. [Google Scholar]
- 65. Goodman, J . et al A randomized, double‐blind, placebo‐controlled trial to study difference in cognitive learning associated with repeated self‐administration of remote computer tablet‐based application assessing dual‐task performance based on amyloid status in healthy elderly volunteers. J. Prev. Alzheimers. Dis. 3, 280–281 (2016). [Google Scholar]
- 66. Brouillette, R.M. et al Feasibility, reliability, and validity of a smartphone based application for the assessment of cognitive function in the elderly. PLoS One 8, e65925 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Apple Inc . Apple ResearchKit. <http://www.apple.com/uk/researchkit> (2017). Accessed 28 November 2017.
- 68. Byrom, B . et al Applicability of Apple Research Kit to deliver cognitive testing in clinical trials: results of a pilot study. Presented at ISPOR 22nd Annual International Meeting, Boston, MA, 20–24 May 2017. <http://www.ispor.org/ScientificPresentationsDatabase/Presentation/71452> Accessed 28 November 2017.
- 69. Cormack, F.K. , Taptikllis, N.T. , Barnett, J.H. , King, J. & Fenhert, B. High‐frequency monitoring of cognition, mood and behaviour using commercially available wearable devices. Alzheimers Dement. 12, 159 (2016). [Google Scholar]
- 70. Eckstein, M.K. , Guerra‐Carrillo, B. , Miller Singley, A.T. & Bunge, S.A. Beyond eye gaze: What else can eye tracking reveal about cognition and cognitive development? Dev. Cogn. Neurosci. 25, 69–91 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Reimer, J. et al Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nat. Commun. 7, 13289–13295 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hopstaken, J.F. , van der Linden, D. , Bakker, A.B. & Kompier, M.A.J. A multifaceted investigation of the link between mental fatigue and task disengagement. Psychophysiology 52, 305–315 (2015). [DOI] [PubMed] [Google Scholar]
- 73. Jongkees, B.J. & Colzato, L.S. Spontaneous eye blink rate as predictor ofdopamine‐related cognitive function—a review. Neurosci. Biobehav. Rev. 71, 58–82 (2016). [DOI] [PubMed] [Google Scholar]
- 74. Karson, C.N. , Berman, K.F. , Donnelly, E.F. , Mandelson, W.B. , Kleinman, J.E. & Wyatt, R.J. Speaking, thinking, and blinking. Psychiatry Res. 5, 243–246 (1981). [DOI] [PubMed] [Google Scholar]
- 75. Pierce, K. , Conant, D. , Hazin, R. , Stoner, R. & Desmond, J. Preference for geometric patterns early in life as a risk factor for autism. Arch. Gen. Psychiatry 68, 101–109 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Termsarasab, P. , Thammongkolchai, T. , Rucker, J.C. & Frucht, S.J. The diagnostic value of saccades in movement disorder patients: a practical guide and review. J. Clin. Mov. Disord. 2, 14–23 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mosimann, U.P. , Müri, R.M. , Burn, D.J. , Felblinger, J. , O'Brien, J.T. & McKeith, I.G. Saccadic eye movement changes in Parkinson's disease dementia and dementia with Lewy bodies. Brain 128, 1267–1276 (2005). [DOI] [PubMed] [Google Scholar]
- 78. Schwamm, L.H. Progesterone for traumatic brain injury — resisting the sirens' song. N. Engl. J. Med. 371, 2522–2523 (2014). [DOI] [PubMed] [Google Scholar]
- 79. Samadani, U. Will eye tracking change the way we diagnose and classify concussion and structural brain injury. Concussion 1, 1–3 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Murray, N.P. , Hunfalvay, M. & Bolte, T. The reliability, validity, and normative data of interpupillary distance and pupil diameter using EyeTracking technology. Trans. Vis. Sci. Tech. 6, 2–13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Krafka, K . et al Eye tracking for everyone. IEEE Conference on Computer Vision and Pattern Recognition (CVPR), 2016.<http://ieeexplore.ieee.org/document/7780608/> (2016). Accessed 28 November 2017.
- 82. Harel, B. , Cannizzaro, M. & Snyder, P.J. Variability in fundamental frequency during speech in prodromal and incipient Parkinson's disease: a longitudinal case study. Brain Cogn. 56, 24–29 (2004). [DOI] [PubMed] [Google Scholar]
- 83. Cannizzaro, M. , Harel, B. , Reilly, N. , Chappell, P. & Snyder, P.J. Voice acoustical measurement of the severity of major depression. Brain Cogn. 56, 30–35 (2004). [DOI] [PubMed] [Google Scholar]
- 84. Cannizzaro, M.S. , Reilly, N. , Mundt, J.C. & Snyder, P.J. Remote capture of human voice acoustical data by telephone: A methods study. Clin. Linguist. Phon. 19, 649–658 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Roche . Roche app measures Parkinson's disease fluctuations. <http://www.roche.com/media/store/roche_stories/roche-stories-2015-08-10.htm> (2017). Accessed 28 November 2017.
- 86. Roche . Smart biomarkers and innovative disease‐modifying therapies for Parkinson's disease. <http://www.nature.com/nature/outlook/parkinsons-disease/pdf/roche.pdf> (2017). Accessed 28 November 2017.
- 87. Gravitz, L. Technology: Monitoring gets personal. Nature 538, S8–S10 (2016). [DOI] [PubMed] [Google Scholar]


