Abstract
Quantitative risk assessment (QRA) for food allergens has made considerable progress in recent years, yet acceptability of its outcomes remains stymied because of the limited extent to which it has been possible to incorporate severity as a variable. Reaction severity, particularly following accidental exposure, depends on multiple factors, related to the allergen, the host and any treatments, which might be administered. Some of these factors are plausibly still unknown. Quantitative risk assessment shows that limiting exposure through control of dose reduces the rates of reactions in allergic populations, but its impact on the relative frequency of severe reactions at different doses is unclear. Food challenge studies suggest that the relationship between dose of allergenic food and reaction severity is complex even under relatively controlled conditions. Because of these complexities, epidemiological studies provide very limited insight into this aspect of the dose‐response relationship. Emerging data from single‐dose challenges suggest that graded food challenges may overestimate the rate of severe reactions. It may be necessary to generate new data (such as those from single‐dose challenges) to reliably identify the effect of dose on severity for use in QRA. Success will reduce uncertainty in the susceptible population and improve consumer choice.
Keywords: allergenic foods, eliciting dose, precautionary allergen labelling, risk assessment, severity
Abbreviations
- DBPCFC
double‐blind, placebo‐controlled food challenge
- ED
eliciting dose
- IgE
Immunoglobulin E
- ISO
International Organization for Standardization
- MED
minimum eliciting dose
- PAL
precautionary allergen labelling
- QRA
quantitative risk assessment
- sIgE
specific immunoglobulin E
- VITAL
Voluntary Incidental Trace Allergen Labelling
- WHO‐IPCS
World Health Organization‐International Programme for Chemical Safety
1. INTRODUCTION
The unintended presence of food allergens, for instance due to cross‐contamination, is recognized as a food safety risk and has resulted in the increasing use of precautionary allergen labelling (PAL) (eg, “may contain X”). While the deliberate inclusion of 14 major common allergens in food products is regulated through EU legislation,1 unintended allergen presence is still handled only tangentially, particularly as regards food safety legislation. Thus, food may be considered “unsafe” if the information provided about it is inaccurate or misleading, or if it is injurious to health, for example due to the “particular health sensitivities of a specific category of consumers”.2
What constitutes “injurious to health” to the allergic population is not explicitly defined or quantified in legislation. For allergens, the nature of any resulting reaction (of which severity is a critical component) would seem a priori to be an important consideration. The Food Allergen Labelling and Consumer Protection Act (2004) (FALCPA) in the USA more explicitly enshrines the concept of an “allergic response that causes a risk to human health,” which implies that some reactions do not pose such a risk.
Approaches around the world differ in the assessment, management and communication of the potential risk of unintended allergen presence. Some authorities take a zero tolerance approach, where any detectable allergen must be declared, while others use quantitative benchmarks to inform such decisions. Much progress has been made in characterizing the population distribution of minimum eliciting doses (MEDs) triggering reactions in allergic individuals, for many regulated food allergens. This has led to the concept of reference doses to inform action levels or thresholds for allergen management and, specifically, the need for PAL—ideally derived through the use of quantitative risk assessment (QRA) models. For the first time, these enable a measured estimate of the likelihood that a specific exposure or dose will elicit a reaction. However, a number of evidence gaps remain such as the lack of data on the relationship between dose (amount) of allergen eaten and reaction severity. This is a critical issue because severe reactions are of great concern, both from the public health and the individual perspectives.3
In this paper, we discuss how dose affects reaction severity with a particular focus on Paracelsus's toxicological paradigm “the dose makes the poison”4: specifically “does the proportion of severe reactions increase with dose?” We also examine if the derivation of reference doses can be improved by including severity as a variable, and whether this would enhance their value in risk assessment and risk management.
2. RISK AND RISK ASSESSMENT
Risk is defined as “[exposure to] the possibility of loss, injury, or other adverse or unwelcome circumstance; a chance or situation involving such a possibility” (Oxford English Dictionary). Risk is ubiquitous in all aspects of life, and many entities, including the International Organization for Standardization (ISO) and the WHO‐International Programme for Chemical Safety (WHO‐IPCS), have produced definitions specific to their own activities. All of these definitions have in common the concept of risk as probability, associated with uncertainty about the outcome. The concept of risk is wide‐ranging, and any discussion must therefore carefully define the risk concept at issue to avoid confusion and ambiguity.
2.1. Risk and health outcomes
The risk to allergic consumers associated with allergenic foods is the probability and nature of an adverse event (ie, an allergic reaction) following exposure. This concept of risk clearly includes not only the probability that an effect will be experienced, but also a consideration of what that effect might be, that is, severity. In this context, the most appropriate operational definition of risk is that proposed by WHO‐IPCS in Environmental Health Criteria 2405 as “a function of the probability of an adverse health effect and the severity of that effect, consequential to a hazard(s) in food.”
Assessment of the risk associated with allergenic foods, as defined above, has been extensively discussed.6, 7 In the approach described, the hazard is characterized through modelling the population dose distribution of MEDs obtained through double‐blind, placebo‐controlled food challenges (DBPCFCs).8, 9 The generation of these data from human studies is a strength, but the methodology used for DBPCFC (where challenges are stopped at objective symptoms) limits our ability to characterize the effect of dose on reaction severity. Better knowledge about the severity component and how it varies with dose would provide great benefit, reducing uncertainty among the susceptible population and giving them more choice through the reduced need for PAL.
Figure 1 illustrates semiquantitatively the risks associated with allergic reactions, characterized as type (as symptom severity) and relative frequency of outcomes following allergen exposure, ranging from no symptoms to life‐threatening symptoms and finally to death, a rare but unpredictable outcome. As discussed below, the perception of severity can differ significantly among stakeholders including allergic individuals, their parents/carers, and healthcare professionals, even for the same reactions.
Figure 1.

Hierarchy of risks faced by people susceptible to food allergy
2.2. Dose control and management of cross‐contamination risk
A quantitative risk assessment process provides an estimate of the probability that a specific population will experience allergic reactions under defined conditions of exposure. The challenge of defining management thresholds for a population where there is high variation in individuals' allergen thresholds has been described elsewhere.6 However, risk managers from national food safety authorities and the food industry need to interpret the outcome of the risk assessment by reference to a defined concentration of allergen, such as the action levels derived through the Voluntary Incidental Trace Allergen Labelling (VITAL) system,10 to determine exposure and risk at a population level. Consumption data add a further variable that should reflect a normal food intake. Once the management threshold level (action level) is determined for the specific food product, with all associated uncertainties, it should be compared to the actual levels determined by analytical methods to establish whether or not a product is “safe” for allergic individuals.
Summary Section 2
Risk assessment in food allergy includes both the probability of experiencing an allergic reaction and the effect on health reflected in reaction severity;
Incorporating reaction severity into risk assessment and management could reduce uncertainty in the susceptible population and offer more consumer choice with less need for PAL.
3. SEVERITY (SCORING) AND STAKEHOLDER VIEWS
Severity is a relative term, which can be qualitatively categorized. A moderate reaction is more “severe” than a mild reaction, but is less “severe” than a severe reaction.3 International organizations have put forward definitions of anaphylaxis. EAACI defines anaphylaxis as a “severe, potentially life‐threatening systemic hypersensitivity reaction”,11 and similarly, NIAID defines food‐induced anaphylaxis as: “a serious allergic reaction that is rapid in onset and may cause death”.12 Some researchers have attempted to quantify severity by reference to the number and nature of symptoms and have tried to account for influences other than dose of allergen13 in a way that would allow objective comparison of carefully documented reactions. However, this approach has been confounded by the variable documentation of formal, guideline‐based or research‐focused food challenges.14, 15
3.1. Perception of severity
Severity is a highly subjective term which stakeholders use and interpret in different ways. Some symptoms may be visually severe (such as rash, facial swelling), without involving respiratory or cardiovascular compromise. Others (eg impaired cognition, fluctuating consciousness and subtle abnormalities in cardiac output) are potentially life‐threatening, but may not appear significant to nonhealthcare professionals or laypersons. Indeed, nonexpert clinicians in ambulatory settings, lacking familiarity with the diversity of generalized allergic reactions, may also over‐ or underestimate reaction severity.
Operationally, and for the purposes of deciding on acceptability, it may be easier to define a nonsevere reaction: Such a reaction would be self‐limiting without treatment, would not interfere with daily life activities and would be of short duration. This definition overlaps with a suggested definition of “an allergic response that poses a risk to human health” made in a US FDA public consultation on thresholds.
Other perspectives on severity go beyond clinical symptoms and their significance. Time off work, disruption of scheduled activity, and direct and indirect economic loss may be judged more severe consequences of an allergic reaction. Process failures in the food chain may have “severe” reputational, economic or legal consequences for companies perceived to be at fault, irrespective of clinical impact.
Most allergists consider the fact that they do not usually see their patients in the throes of an allergic reaction as a barrier to the optimal use of severity data. Retrospective assessment of severity can be difficult, but an allergy‐focused clinical history looking for reports or (even better) contemporaneous documentation of airway or cardiovascular compromise (the quintessential features of “severe” reactions) are the most useful clinical assessments.
3.2. Acceptable risk and severity
Risk assessment provides a quantitative risk estimate, but ultimately its purpose is to help define the acceptability or tolerability of a specified risk, of which severity is a critical component.
During a workshop organized by ILSI Europe's Food Allergy Task Force in 2016, representatives of different stakeholder groups, including those from the regulatory community, considered the use of reference doses. All participants accepted that there was a degree of risk associated with current approaches (and that such risk is largely accepted). It was thought that using the ED10 (dose needed to elicit objective symptoms in 10% of the allergic population) as likely to result in an unacceptable rate of severe reactions in more sensitive allergic individuals. Further characterization of the nature of symptoms experienced by such individuals reacting to an ED05 or ED01 was needed, to establish its wider acceptability. Consumers might be prepared to tolerate mild allergic symptoms if they were confident that such symptoms would be self‐limiting. Participants noted that exposure to allergens in amounts lower than the proposed ED01/05 level is unlikely to elicit severe reactions, a position supported by the results of a single‐dose challenge study in peanut‐allergic individuals.16
3.3. Communication of risks
Better information and education of patients and healthcare professionals about the consequences of exposure to defined low amounts may help them understand the risks of using an ED01/05 level for allergen risk management and make it more acceptable. Healthcare professionals stressed the importance of a correct and proper diagnosis of food allergy and proposed that knowledge of an individual's MED, obtained from open food challenges or even from a single‐dose challenge, could also be valuable, even with its limitations. Individuals, including people with food allergies, differ in their acceptance of risk, ranging from risk‐averse to risk‐taking and even risk‐seeking.17 There is an urgent need to demystify food‐induced allergic reactions and provide education that not all reactions (or even the majority) are anaphylaxis or life‐threatening. Better communication with other stakeholders involved in assessing, managing or communicating allergen risks, and providing practical guidance on allergen avoidance, was identified as a general need. Importantly, any strategy utilizing reference doses must acknowledge the need to provide appropriate support and education to those who may react to levels of allergen exposure below the action levels.
Finally, labelling is an area where much needs to be done to assure better understanding and therefore protection of allergic consumers. Consumers and healthcare professionals alike are confused as to the meaning and limitations of PAL. Information communicated through allergen labelling needs to be as simple as possible, and supported by education and advice.
Summary Section 3
Different stakeholders have different perspectives on what constitutes a severe allergic reaction to foods;
There is no universally accepted system for scoring the severity of food‐allergic reactions, but most clinicians would consider reactions involving airway or cardiovascular compromise as severe;
Despite the interpretative difficulties of differences in perception and context, a consensus may be possible on what constitutes a nonsevere reaction: namely, of short duration, self‐limiting and with no or limited impact on daily life activities;
Severity is important in relation to acceptable risk, as the notion of which allergic symptoms are acceptable is dependent on the severity of such symptoms.
4. FACTORS INFLUENCING THE SEVERITY OF ALLERGIC REACTIONS
Fatal reactions are rare but also unpredictable. Turner et al3 recently reviewed our ability (or inability) to reliably predict severity. Several factors, acting together, are likely to contribute to the outcome of a reaction and its severity (Figure 2):
Figure 2.

The “Swiss cheese” model. Adapted from reference28
Allergen‐related factors: The nature and circumstances of allergen exposure are likely to impact upon reaction severity. Peanut and tree nuts, seafood and cow's milk are the most common causes of fatal food‐induced anaphylaxis in the UK, the USA and Australia.18, 19, 20 In contrast, soya appears less likely to trigger severe reactions.18 The food matrix in which the allergen is presented can affect severity: high fat (eg chocolate) and heavily spiced foods may affect the kinetics of allergen bioavailability, potentially delaying symptom onset, minimizing initial mild oral symptoms and by confounding “early warning signs” failing to limit the amount of allergen consumed.21, 22 Heat‐processing can alter the structure of proteins and therefore their recognition by specific immunoglobulin E (sIgE) and, ultimately, the nature of any reaction.23 The relationship between dose (exposure) and reaction severity is less clear (see next section), although some (but not all) data suggest that severe reactions to very low doses are uncommon.24
Host factors: These include factors which might affect the ability of an allergen to stimulate a host effector cell response (ie an allergic reaction) as well as factors which may modulate the response. Perhaps the most studied factor is the level of allergen‐specific IgE, which, however, shows a poor correlation with reaction severity (either historical, or that occurring at in‐hospital food challenge), as do “components” or IgE against specific epitopes.3 “Extrinsic” factors (also referred to as cofactors or augmentation factors) such as exercise, stress, medication and alcohol can impact upon severity, as reviewed elsewhere.3, 25, 26 Some individuals are also able to compensate physiologically for an allergic reaction, to the extent of recovering spontaneously from food‐induced anaphylaxis.27 All these complicate evaluation of the relationship between dose and severity.
Factors affecting reaction outcome: Severity, and the subsequent outcome, depend not only on the nature of the reaction, but any subsequent intervention to control the reaction. Delays in seeking medical attention and/or administration of adrenaline (epinephrine) are common factors reported in fatal anaphylaxis. Many severe reactions can have a good outcome if appropriately treated with epinephrine. Contact or inhaled exposures are less likely to trigger severe reactions. If dose affects the rate of symptom progression, then a higher dose might limit the time available to administer rescue medication. The ultimate outcome of an allergic reaction expressed as a severity score will thus require great care to integrate into a QRA. Smith et al28 proposed the “Swiss cheese” model to illustrate how these factors might interact to result in reactions of different severity. Essentially, this approach postulates that a severe reaction results from alignment of a whole set of circumstances, of which ingested dose is only one factor, as illustrated in Figure 2.
Summary Section 4
Reaction severity is determined by multiple factors, related to the allergen, the host and any treatments which might be administered;
These factors integrate in a multiplicity of ways to produce a reaction of a certain severity, as illustrated by the “Swiss cheese” model.
5. CURRENT KNOWLEDGE ON THE EFFECT OF DOSE ON SEVERITY OF FOOD‐ALLERGIC REACTIONS
Protection from severe reactions constitutes an important focus of ensuring the safety of food‐allergic consumers. In practice, this means avoidance of the offending allergen, but how absolute that avoidance must be remains a critical question. The interest in the relationship between the level of exposure (dose) and the severity of any resulting allergic reaction stems from the observation that of all the parameters that may influence reaction severity, only dose can be managed by the food industry.
Notwithstanding the potential importance of this, data describing this relationship are scarce and often inadequate, particularly outside controlled challenge studies. The available data can be found in epidemiological studies of populations, clinical case reports, and studies using graded food challenges and are summarized in Table 1. A recently introduced research tool is the single‐dose food challenge,29 originally designed to validate reference doses derived from dose distribution models.
Table 1.
Selected studies assessing a potential relationship between dose and severity
| Reference | Type of study | Statistical methods | Dose and outcome | Comments |
|---|---|---|---|---|
| 13 | Epidemiological, community‐based and oral food challenge | Spearman's rank correlation | Weak association only | Does not support the role of dose in reaction severity |
| 30 | Case series of fatalities |
No formal statistical analysis Frequencies and percentages reported |
No information on dose | Study design cannot inform on role of dose in severity |
| 31 | Case series of fatalities | No formal statistical analysis Frequencies and percentages reported | No information on dose | Study design cannot inform on role of dose in severity |
| 33 | Case report | None | Not measured but probably low dose | Study type cannot inform on role of dose in severity |
| 38 | Oral food challenge | Logistic regression | Severe reactions at every dose | Suggests no role of dose in severity, but starting dose high relative to reference doses |
| 39 | Oral food challenge | Dose distribution modelling | Higher MEDs associated with more severe reactions | Seen only for peanut (not milk, egg or soy) but excluded all mild reactors |
5.1. Epidemiological and clinical case report data
The effect of dose on reaction severity has proved difficult to study. Reasons include a lack of (precise) information about the presence, quantity and bioavailability of the allergen ingested (precisely the variable under study), as well as the relative contribution of factors other than dose to the outcome. A community‐based study where authors estimated the ingested dose13 showed only a modest contribution to reaction severity. Case series of (near) deaths have identified factors associated with severe outcomes of accidental food‐allergic reactions in the community.30, 31 These include adolescence/young adulthood, concomitant asthma, peanut or tree nut or cow's milk ingestion and delayed treatment with adrenaline (epinephrine). However, given the low incidence of fatal and near‐fatal reactions to foods,32 the vast majority of patients with these “high risk” characteristics will probably never develop truly life‐threatening reactions when exposed. It is difficult to determine the amounts consumed in fatal/severe reactions: many severe reactions occur after relatively large doses of allergenic food, but exceptions exist.33, 34 Current data are thus inadequate to describe the relationship between dose and severity, although while the levels of exposure under these circumstances may be small in absolute terms, they are typically many orders of magnitude greater than any reference doses proposed for allergen management.
5.2. Oral food challenge data
The “gold standard” for diagnosis of food allergy is a graded food challenge; data generated by these procedures (relating to ED) have been analysed to discern the relationship between severity and dose. However, results have proved inconclusive.3, 13 The principal reason why challenge studies continue to be used in this way is that exposure (ie dose of allergen) can be precisely defined. Further advantages include direct, contemporaneous observations of reactions, control of cofactors and (severity‐modifying) medications. Despite these benefits, this approach has important drawbacks. First, these studies are principally aimed at identifying thresholds of reactivity (MEDs) and the challenge is, with very few exceptions, stopped at the first objective sign of a reaction. Other precautions taken to minimize the risk and limit reaction severity include (in some studies) the exclusion of patients with recent severe reactions or severe and/or unstable comorbidities; conducting challenges under baseline conditions; and prompt and optimal treatment of reactions. It has been suggested that giving incremental doses in succession may affect the impact of subsequent doses, by inducing short‐term oral tolerance; this would underestimate the effect of any given dose, relative to the same dose given in isolation.35 Conversely, reactions may be triggered by the cumulative dose, rather than that given immediately prior to reaction: confounding any attempt to relate severity to dose.36 Finally, even reactions seen at food challenge are open to considerable inter‐ and intra‐observer variability.37
Analyses examining the relationship between MED and reaction severity report that relatively severe reactions occur unpredictably and at any dose.37, 38 In order to quantify the effect of ED on reaction severity, Pettersson et al (manuscript in preparation) analysed data arising from 734 positive challenges at a single centre, using multiple regression analysis to build a prediction model for challenge reaction severity. This analysis showed that MED could only predict 4.4% of the variance of reaction severity (and all known factors together, only 23.5% of total severity variance).
Zhu et al39 retrospectively classified the severity of MEDs from graded food challenges performed at several centres and modelled their distribution in the study population. For peanut, higher MEDs were associated with more severe reactions, but no clear relationship was discerned for milk, egg or soy. Importantly, Zhu et al were unable to obtain and therefore include MEDs associated with mild symptoms in their analysis: these constituted over 40% of the MEDs in the source studies.39
Not all data from food challenges suggest that severe reactions occur at any dose. Ballmer‐Weber et al24 using a challenge protocol where the initial dose was 0.003 mg protein, observed a clear absence of severe symptoms to peanut, hazelnut, celery, fish and shrimp at lower doses, and more frequent severe symptoms as doses increased. This is in contrast to Rolinck‐Werninghaus et al38 who, using an initial dose of 3‐5 mg protein (approximately an ED10 for the allergens tested), reported severe reactions at all doses. The higher starting dose may have obscured the relationship between dose and severity.
A recent re‐analysis of challenge data by Wainstein & Turner,40 in which dose escalation continued despite the occurrence of mild symptoms, reported three patterns of reaction: those whose first symptom was anaphylaxis, a group with milder symptoms progressing to anaphylaxis as the dose increased or treatment was delayed, and finally a group who did not progress to anaphylaxis irrespective of dose (see Figure 3). This suggests that a relationship between dose and severity will not be readily apparent in individuals who react with anaphylaxis as the initial objective symptom, depending on starting dose. At a group or population level, the relationship between dose and reaction severity may thus be obscured by the heterogeneity of the tested allergic population.
Figure 3.
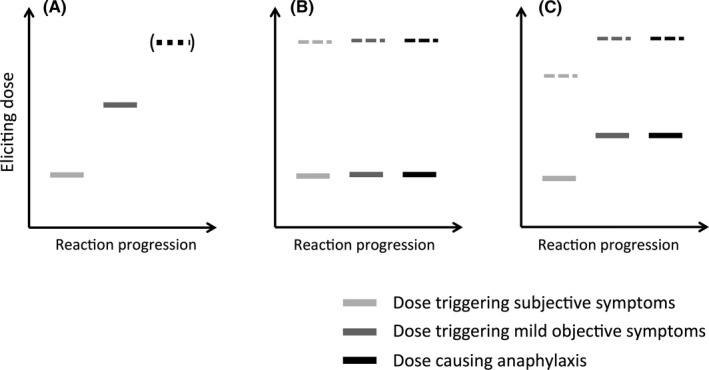
Different patterns of clinical reactivity are seen at food challenge. Many individuals will experience initially subjective symptoms, with objective symptoms appearing with further doses (A). Anaphylaxis will only develop if the food challenge continues. Others will experience anaphylaxis as their first objective symptom: either at a dose of allergen exposure with no preceding subjective symptoms (B), or with prior subjective symptoms (C). Note that anaphylaxis can occur at all levels of exposure (both at low levels of allergen exposure, represented by the solid bars, and higher doses indicated by dotted lines). Reproduced (with permission) from reference40
5.3. Single‐dose challenges
Zurzolo et al29 introduced single‐dose challenges to validate the EDs calculated from population dose‐distribution curves: a single dose (corresponding to a specific ED value, for example ED05 for the food in question, derived from a dose distribution curve) is given to unselected individuals with the relevant allergy and the occurrence and characteristics of any reactions recorded. In the only study published to date, the ED05 for peanut was validated (1.5 mg peanut protein), with 8 of 378 individuals meeting predetermined criteria for a positive, objective reaction. No severe reactions occurred.16 Additional “single‐dose” data can be found by studying historical food challenge studies, where much higher starting doses were used. Two such studies (where initial doses were >200 mg protein) had a high proportion (up to 30%) of severe symptoms among first dose reactors.41, 42 These imply that a quantifiable relationship does exist between severity and dose. However, the circumstances leading to relatively severe reactions at relatively low doses (3‐5 mg protein) in regular (multiple dose) food challenges remain unclear,38 and further data from single‐dose challenges are needed before definitive conclusions can be drawn. Again it must be stressed that the doses in the Rolinck‐Werninghaus study38 are at least 1.5 orders of magnitude higher than any amounts to which industry is seeking to manage the allergens tested.
Summary Section 5
The only modifiable parameter, which may be controlled by public health measures for food allergy, is exposure to the allergen, that is dose;
While limiting exposure is known to decrease the rates of reactions in allergic populations, the impact of this on the relative frequency of severe reactions at different doses is unclear;
Reaction severity following accidental exposure depends on a number of factors and variables, some of which are plausibly still unknown. As a result, epidemiological studies provide very limited insight into the dose‐response relationship;
Food challenge studies suggest that the relationship between dose of allergenic food and reaction severity is complex and difficult to describe. Double‐blind, placebo‐controlled food challenges may overestimate the severity of reactions at any given dose, possibly because of cumulation of doses;
Emerging data from single‐dose challenges suggest that graded food challenges may overestimate the rate of severe reactions.
6. SEVERITY, DOSE AND QUANTITATIVE RISK ASSESSMENT
Quantitative risk assessment models for food‐allergic reactions have been developed with probabilistic, Bayesian interfaces to estimate the likelihood of eliciting a reaction to defined amounts of allergen.43, 44 Quantitative risk assessment requires quantitative description of any variable (including severity) and the associated variability and uncertainty, which is currently hindered by the lack of an agreed severity scoring scheme. Once operational, the QRA would predict not only the number of reactions for any given dose (as currently), but also the number of reactions at any given degree of severity within the scoring scheme, which would define any specified MED value. In risk management terms, this could translate as a (management) threshold dose for severe reactions, which could be set, for instance, as 1/10th of the threshold dose for severe reactions.
While current probabilistic models do not include severity as a variable, data are available that could already inform the severity variables for risk assessment models. The proportion of severe reactions at a given dose may be estimated for a number of foods from studies, which include severity information (eg16, 24, 38, 41, 42). Any estimate for proportion of severe reactions would need to be calculated for each allergen (where sufficient data are available). Additional variables that modify severity (ie cofactors) could ultimately be added to the QRA framework as more data become available.
Summary Section 6
Probabilistic risk models may be improved if a quantitative expression of severity could be extracted from clinical data;
It may be necessary to generate new data (such as those from single‐dose challenges) in order to reliably identify the effect of dose on severity for use in QRA.
7. CONCLUSIONS AND RECOMMENDATIONS
Quantitative analyses of data from controlled food challenges have provided the basis for deriving benchmarks for allergen management. New experimental approaches to the validation of these benchmarks, such as the single‐dose challenge, appear promising insofar as they can also provide data on the characteristics of reactions at a single dose, including reaction severity. However, such data are yet to be integrated into and contribute to the outputs of current models, despite the value this would add from the perspective of public health and risk assessment.
Concepts of risk vary among stakeholders, and different stakeholders perceive severity in very different ways. Factors other than dose may influence severity, both through their intrinsic importance but also because they might dominate or mask the effect of dose. The expert group concluded that these factors, whether related to the allergen, the host or their effect on reaction outcome, play a major role and often obscure the effect of dose.
Overall, the expert group concluded that data available on the relationship between dose and severity are currently of insufficient quality to be incorporated operationally into dose distribution modelling approaches which describe the relationship between dose of allergen and the proportion of the allergic population likely to react. Consequently, the current focus should remain with efforts to base benchmarks for allergen management (reference doses) on the latter relationship, although the incorporation of severity parameters should continue to be explored. The group noted that if the principal public health goal is to minimize severe reactions, then reference doses based on current data incorporate a “safety factor,” albeit one that cannot currently be quantified. Additional work to understand the impact of dose and other factors which determine severity will be of value in order to better protect the allergic population and ensure that measures taken to manage allergens are both effective and proportionate. In the context of setting safe limits for allergen management, for example for application of PAL, single‐dose challenges can provide valuable information about the characteristics of a reaction that might follow consumption of a product or meal on a single occasion which unintentionally contained an allergen (eg by cross‐contact) in an small amount not exceeding in total the dose tested.
Developing a shared understanding among stakeholders of severity, and its implications for allergen risk assessment, may be as important to the latter's more general acceptance as refining the underlying science. Communicating the issues discussed and conclusions reached in this paper to healthcare professionals and people with food allergies will be critical to developing this understanding, and the expert group recognized the associated difficulties. Further research into conveying risk messages through labelling is also needed to ensure better understanding and therefore protection of allergic consumers.
CONFLICTS OF INTEREST
All authors have completed the ICMJE Form for Disclosure of Potential Conflicts of Interest.
AUTHOR CONTRIBUTIONS
All authors contributed to the conception and conduct of the research described. AD and RC wrote the paper. AD had primary responsibility for final content. All authors critically read, commented on and approved the final manuscript.
ACKNOWLEDGMENTS
The authors wish to thank Professor Katie Allen (Murdoch Children's Research Institute, AU) for critically reviewing the draft manuscript. Furthermore, the authors warmly thank all participants of the workshop on “How Far Can We Control the Severity of Food Allergic Reactions by Controlling Exposure to Allergenic Foods?” organized by ILSI Europe on 15‐16 September 2016 in Brussels, Belgium.
Dubois AEJ, Turner PJ, Hourihane J, et al. How does dose impact on the severity of food‐induced allergic reactions, and can this improve risk assessment for allergenic foods? Allergy. 2018;73:1383–1392. https://doi.org/10.1111/all.13405
Funding information
This work was conducted by an expert group of the European branch of the International Life Sciences Institute, ILSI Europe. This publication was coordinated by the Food Allergy Task Force. Industry members of this task force are listed on the ILSI Europe website at http://ilsi.eu/task-forces/food-safety/food-allergy/. Experts are not paid for the time spent on this work; however, the nonindustry members within the expert group were offered support for travel and accommodation costs from the Food Allergy Task Force to attend meetings to discuss the manuscript and a small compensatory sum (honorarium) with the option to decline. The expert group carried out the work, that is collecting/analysing data/information and writing the scientific paper separate to other activities of the task force. The research reported is the result of a scientific evaluation in line with ILSI Europe's framework to provide a precompetitive setting for public‐private partnership. ILSI Europe facilitated scientific meetings and coordinated the overall project management and administrative tasks relating to the completion of this work. For further information about ILSI Europe, please email info@ilsieurope.be or call +3227710014. The opinions expressed herein and the conclusions of this publication are those of the authors and do not necessarily represent the views of ILSI Europe nor those of its member companies or any regulatory authority.
René W. R. Crevel: At time of writing. New affiliation since August 2017: René Crevel Consulting Ltd, UK.
Edited by: Bodo Niggemann
REFERENCES
- 1. The European Parliament and the Council of the European Union . Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers. 2011. https://doi.org/10.3000/19770677.l_2011.304.eng
- 2. The European Parliament and the Council of the European Union . Regulation (EC) No 178/2002 of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. 2002. doi:2004R0726 ‐ v.7 of 05.06.2013
- 3. Turner PJ, Baumert JL, Beyer K, et al. Can we identify patients at risk of life‐threatening allergic reactions to food? Allergy. 2016;71:1241‐1255. [DOI] [PubMed] [Google Scholar]
- 4. Kroes R, Galli C, Munro I, et al. Threshold of toxicological concern for chemical substances present in the diet: a practical tool for assessing the need for toxicity testing. Food Chem Toxicol. 2000;38:255‐312. [DOI] [PubMed] [Google Scholar]
- 5. Food and Agriculture Organization of the United Nations, World Health Organization . Principles and methods for the risk assessment of chemicals in food. 2009.
- 6. Crevel RWR, Baumert JL, Baka A, et al. Development and evolution of risk assessment for food allergens. Food Chem Toxicol. 2014;67:262‐276. [DOI] [PubMed] [Google Scholar]
- 7. Eastmond DA, Hartwig A, Anderson D, et al. Mutagenicity testing for chemical risk assessment: update of the WHO/IPCS Harmonized Scheme. Mutagenesis. 2009;24:341‐349. [DOI] [PubMed] [Google Scholar]
- 8. Crevel RWR, Briggs D, Hefle SL, Knulst AC, Taylor SL. Hazard characterisation in food allergen risk assessment: the application of statistical approaches and the use of clinical data. Food Chem Toxicol. 2007;45:691‐701. [DOI] [PubMed] [Google Scholar]
- 9. Taylor SL, Crevel RWR, Sheffield D, Kabourek J, Baumert J. Threshold dose for peanut: risk characterization based upon published results from challenges of peanut‐allergic individuals. Food Chem Toxicol. 2009;47:1198‐1204. [DOI] [PubMed] [Google Scholar]
- 10. Taylor SL, Baumert JL, Kruizinga AG, et al. Establishment of reference doses for residues of allergenic foods: report of the VITAL expert panel. Food Chem Toxicol. 2014;63:9‐17. [DOI] [PubMed] [Google Scholar]
- 11. Muraro A, Roberts G, Worm M, et al. Anaphylaxis: guidelines from the European academy of allergy and clinical immunology. Allergy. 2014;69:1026‐1045. [DOI] [PubMed] [Google Scholar]
- 12. Boyce JA, Assa'ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID‐sponsored expert panel report. Nutr Res. 2011;31:61‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hourihane JO, Grimshaw KEC, Lewis SA, et al. Does severity of low‐dose, double‐blind, placebo‐controlled food challenges reflect severity of allergic reactions to peanut in the community? Clin Exp Allergy. 2005;35:1227‐1233. [DOI] [PubMed] [Google Scholar]
- 14. McBride D, Keil T, Grabenhenrich L, et al. The EuroPrevall birth cohort study on food allergy: baseline characteristics of 12,000 newborns and their families from nine European countries. Pediatr Allergy Immunol. 2012;23:230‐239. [DOI] [PubMed] [Google Scholar]
- 15. Sampson HA, Van Wijk RG, Bindslev‐Jensen C, et al. Standardizing double‐blind, placebo‐controlled oral food challenges: American Academy of Allergy, Asthma & Immunology‐European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J Allergy Clin Immunol. 2012;130:1260‐1274. [DOI] [PubMed] [Google Scholar]
- 16. Hourihane JO, Allen KJ, Shreffler WG, et al. Peanut Allergen Threshold Study (PATS): novel single‐dose oral food challenge study to validate eliciting doses in children with peanut allergy. J Allergy Clin Immunol. 2017;139:1583‐1590. [DOI] [PubMed] [Google Scholar]
- 17. Barnett J, Muncer K, Leftwich J, et al. Using'may contain'labelling to inform food choice: a qualitative study of nut allergic consumers. BMC Public Health. 2011;11:734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Turner PJ, Gowland MH, Sharma V, et al. Increase in anaphylaxis‐related hospitalizations but no increase in fatalities: an analysis of United Kingdom national anaphylaxis data, 1992‐2012. J Allergy Clin Immunol. 2015;135:956‐963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ma L, Danoff TM, Borish L. Case fatality and population mortality associated with anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133:1075‐1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mullins RJ, Wainstein BK, Barnes EH, Liew WK, Campbell DE. Increases in anaphylaxis fatalities in Australia from 1997 to 2013. Clin Exp Allergy. 2016;46:1099‐1110. [DOI] [PubMed] [Google Scholar]
- 21. van Odijk J, Ahlstedt S, Bengtsson U, Borres MP, Hulthen L. Double‐blind placebo‐controlled challenges for peanut allergy the efficiency of blinding procedures and the allergenic activity of peanut availability in the recipes. Allergy. 2005;60:602‐605. [DOI] [PubMed] [Google Scholar]
- 22. Grimshaw KEC, King RM, Nordlee JA, Hefle SL, Warner JO, Hourihane JOB. Presentation of allergen in different food preparations affects the nature of the allergic reaction–a case series. Clin Exp Allergy. 2003;33:1581‐1585. [DOI] [PubMed] [Google Scholar]
- 23. Nowak‐Wegrzyn A, Fiocchi A. Rare, medium, or well done? The effect of heating and food matrix on food protein allergenicity. Curr Opin Allergy Clin Immunol. 2009;9:234‐237. [DOI] [PubMed] [Google Scholar]
- 24. Ballmer‐Weber BK, Fernandez‐Rivas M, Beyer K, et al. How much is too much? Threshold dose distributions for 5 food allergens. J Allergy Clin Immunol. 2015;135:964‐971. [DOI] [PubMed] [Google Scholar]
- 25. Niggemann B, Beyer K. Factors augmenting allergic reactions. Allergy. 2014;69:1582‐1587. [DOI] [PubMed] [Google Scholar]
- 26. Wolbing F, Biedermann T. Anaphylaxis: opportunities of stratified medicine for diagnosis and risk assessment. Allergy. 2013;68:1499‐1508. [DOI] [PubMed] [Google Scholar]
- 27. Noimark L, Wales J, Du Toit G, et al. The use of adrenaline autoinjectors by children and teenagers. Clin Exp Allergy. 2012;42:284‐292. [DOI] [PubMed] [Google Scholar]
- 28. Smith PK, Hourihane JO, Lieberman P. Risk multipliers for severe food anaphylaxis. World Allergy Organ J. 2015;8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zurzolo GA, Allen KJ, Taylor SL, et al. Peanut Allergen Threshold Study (PATS): validation of eliciting doses using a novel single‐dose challenge protocol. Allergy Asthma Clin Immunol. 2013;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bock SA, Muñoz‐Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107:191‐193. [DOI] [PubMed] [Google Scholar]
- 31. Pumphrey RSH, Gowland MH. Further fatal allergic reactions to food in the United Kingdom, 1999‐2006. J Allergy Clin Immunol. 2007;119:1018‐1019. [DOI] [PubMed] [Google Scholar]
- 32. Umasunthar T, Leonardi‐Bee J, Hodes M, et al. Incidence of fatal food anaphylaxis in people with food allergy: a systematic review and meta‐analysis. Clin Exp Allergy. 2013;43:1333‐1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Steensma DP. The kiss of death: a severe allergic reaction to a shellfish induced by a good‐night kiss. Mayo Clin Proc. 2003;78:221‐222. [DOI] [PubMed] [Google Scholar]
- 34. Maloney JM, Chapman MD, Sicherer SH. Peanut allergen exposure through saliva: assessment and interventions to reduce exposure. J Allergy Clin Immunol. 2006;118:719‐724. [DOI] [PubMed] [Google Scholar]
- 35. Niggemann B, Lange L, Finger A, Ziegert M, Müller V, Beyer K. Accurate oral food challenge requires a cumulative dose on a subsequent day. J Allergy Clin Immunol. 2012;130:261‐263. [DOI] [PubMed] [Google Scholar]
- 36. Blumchen K, Beder A, Beschorner J, et al. Modified oral food challenge used with sensitization biomarkers provides more real‐life clinical thresholds for peanut allergy. J Allergy Clin Immunol. 2014;134:390‐398. [DOI] [PubMed] [Google Scholar]
- 37. van Erp FC, Knulst AC, Meijer Y, Gabriele C, van der Ent CK. Standardized food challenges are subject to variability in interpretation of clinical symptoms. Clin Transl Allergy. 2014;4:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rolinck‐Werninghaus C, Niggemann B, Grabenhenrich L, Wahn U, Beyer K. Outcome of oral food challenges in children in relation to symptom‐eliciting allergen dose and allergen‐specific IgE. Allergy. 2012;67:951‐957. [DOI] [PubMed] [Google Scholar]
- 39. Zhu J, Pouillot R, Kwegyir‐Afful EK, Luccioli S, Gendel SM. A retrospective analysis of allergic reaction severities and minimal eliciting doses for peanut, milk, egg, and soy oral food challenges. Food Chem Toxicol. 2015;80:92‐100. [DOI] [PubMed] [Google Scholar]
- 40. Turner PJ, Wainstein BK. Crossing the threshold: can outcome data from food challenges be used to predict risk of anaphylaxis in the community? Allergy. 2017;72:9‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Perry TT, Matsui EC, Conover‐Walker MK, Wood RA. Risk of oral food challenges. J Allergy Clin Immunol. 2004;114:1164‐1168. [DOI] [PubMed] [Google Scholar]
- 42. Sicherer SH, Morrow EH, Sampson HA. Dose‐response in double‐blind, placebo‐controlled oral food challenges in children with atopic dermatitis. J Allergy Clin Immunol. 2000;105:582‐586. [DOI] [PubMed] [Google Scholar]
- 43. Spanjersberg MQI, Kruizinga AG, Rennen MAJ, Houben GF. Risk assessment and food allergy: the probabilistic model applied to allergens. Food Chem Toxicol. 2007;45:49‐54. [DOI] [PubMed] [Google Scholar]
- 44. Rimbaud L, Heraud F, La Vieille S, Leblanc J‐C, Crepet A. Quantitative risk assessment relating to adventitious presence of allergens in food: a probabilistic model applied to peanut in chocolate. Risk Anal. 2010;30:7‐19. [DOI] [PubMed] [Google Scholar]


