Abstract
The ability to harness a patient’s immune system to target malignant cells is now transforming the treatment of many cancers, including hematologic malignancies. The adoptive transfer of T cells selected for tumor reactivity or engineered with natural or synthetic receptors has emerged as an effective modality, even for patients with tumors that are refractory to conventional therapies. The most notable example of adoptive cell therapy is with T cells engineered to express synthetic chimeric antigen receptors (CARs) that reprogram their specificity to target CD19. CAR T cells have shown remarkable antitumor activity in patients with refractory B-cell malignancies. Ongoing research is focused on understanding the mechanisms of incomplete tumor elimination, reducing toxicities, preventing antigen escape, and identifying suitable targets and strategies based on established and emerging principles of synthetic biology for extending this approach to other hematologic malignancies. This review will discuss the current status, challenges, and potential future applications of CAR T-cell therapy in hematologic malignancies.
Introduction
The observation that tumor regression can be mediated by adoptive transfer of major histocompatibility complex (MHC)–restricted αβ T cells that recognize tumor-associated self-antigens, viral antigens, minor histocompatibility antigens, or neoantigens has fueled interest in developing adoptive cell therapy (ACT) for cancer.1-8 However, the technical challenges of isolating and expanding T cells of defined specificity and MHC restriction from each patient have heretofore limited ACT to proof-of-principle studies. These obstacles have been overcome by the ability to modify T cells by transferring genes encoding synthetic chimeric antigen receptors (CARs) that redirect specificity to a cell-surface molecule in a non–MHC-restricted fashion. Genetically engineered cellular medicines have remarkable potency, and the use of CAR T cells that target CD19 recently received US Food and Drug Administration (FDA) approval for treating advanced acute lymphoblastic leukemia (ALL) and non-Hodgkin lymphoma (NHL).9,10
CAR design and T-cell engineering
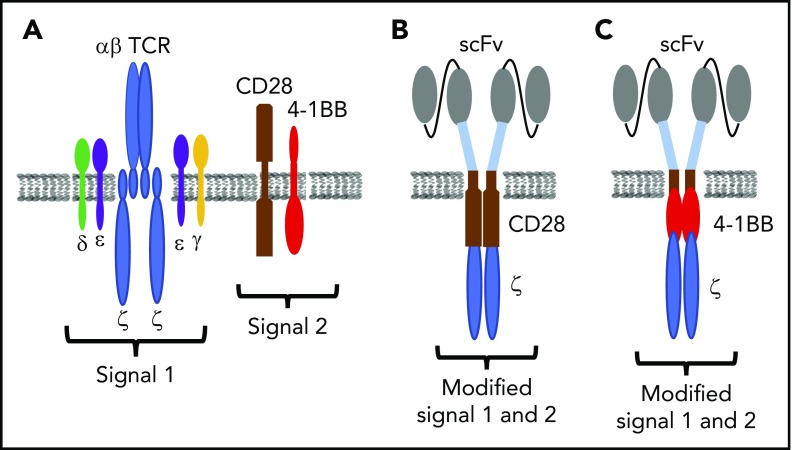
CARs are expressed from a fusion gene that encodes a single-chain variable fragment (scFv) of a monoclonal antibody as a targeting domain, linked via spacer and transmembrane sequences to a signaling module. The first generation of CARs was designed with only the CD3ζ chain to mimic natural T-cell receptor (TCR) signaling.11 T cells expressing such CARs lysed target cells but proliferated poorly and proved ineffective in clinical trials.12-14 Second- and third-generation CARs incorporated ≥1 intracellular signaling domains from costimulatory molecules such as CD28, 4-1BB, or OX40 in tandem with CD3ζ. Consistent with the role of costimulation in T-cell activation, these CARs markedly augmented T-cell cytokine production and proliferation.15-18 However, it should be noted that in current CAR designs, signal 1 is only provided by the CD3ζ chain, and costimulation delivered through a CAR differs structurally and temporally from that provided by natural ligand/receptor interactions (Figure 1). The effect of these differences in signaling on T-cell fate and function remains to be fully elucidated. CARs that contain 4-1BB/CD3ζ or CD28/CD3ζ signaling domains have been evaluated in clinical trials, but different gene delivery vectors, T-cell product composition, lymphodepletion regimens, and patient characteristics have precluded definitive comparison of the efficacy, toxicity, persistence, and function of T cells expressing these constructs.
Figure 1.
CAR design. (A) Schematic of TCR and costimulatory molecule expression on T cells. (B-C) Synthetic single-chain receptors designed to deliver modified signal 1 and signal 2 in an scFv/CD28/CD3ζ (B) or scFv/4-1BB/CD3ζ format (C).
The CAR transgene is usually introduced randomly into the cellular genome by γ retroviral or lentiviral transduction and transcribed off of viral or mammalian promoters in the construct. Targeted integration of the CAR into defined genomic loci under control of endogenous promoter elements has been achieved in preclinical studies and can provide more homogeneous expression of the CAR and improve therapeutic efficacy.19 Most commonly, unselected T cells are transduced after activation with mitogenic antibodies and expanded briefly in interleukin-2 (IL-2) alone or with other γ chain cytokines before reinfusion. Because T cells consist of CD4 and CD8 subsets, each of which are subdivided into naïve (TN), central and effector memory (TCM/TEM), tissue resident memory, and effector subsets, this approach can lead to considerable heterogeneity in the CAR T-cell product. Preclinical models and data from clinical trials suggest that transducing less differentiated T cells or having greater numbers of TN or TCM in the product results in superior persistence and function in vivo.20-22 Therefore, some groups have selected defined subsets or virus-specific memory T cells for genetic modification or employed culture conditions that promote the outgrowth of TN or TCM phenotype cells.23-25 Introducing CARs into virus-specific T cells has theoretical advantages because cell persistence could be maintained by physiologic antigen recognition, but a potential caveat is that simultaneous TCR and CAR engagement may promote T-cell exhaustion and apoptosis.26,27 Gene editing is also being used to delete the TCR and other endogenous genes to prepare allogeneic off-the-shelf CAR T cells that at least transiently engraft across MHC barriers in severely immunocompromised hosts. This approach was used successfully to target CD19 but was associated with mild graft-versus-host disease mediated by residual unedited TCR+ cells in the product.28 Natural killer (NK) cells and NK T cells can be modified to express CARs, and these cell types may also be suited for off-the-shelf administration.29,30 Thus, manipulating cell product composition is a fertile area of research to improve potency and reduce toxicity, and future applications of synthetic biology are likely to include engineering cell subsets that confer advantages in particular clinical settings.
Lessons from targeting B-cell malignancies with CD19 CAR T cells
B-cell tumors are ideal to target with CAR T cells because they express lineage-specific molecules such as CD19, CD20, and CD22 that are not expressed on other tissues. CD19 CAR T cells have proven to be highly effective for treating several types of B-cell malignancies and recently received FDA approval for children and young adults with relapsed or chemotherapy-refractory (R/R) ALL and for adults with R/R NHL.9,10 The results of clinical trials targeting CD19 have been instructive for understanding the limitations of this new modality and the challenges for extending this approach to other targets and tumor types.
ALL
Chemotherapy is highly effective in childhood ALL, but the outcome for adult patients and the subset of children who relapse remains poor.31,32 Several single-institution studies have evaluated treatment of R/R ALL with CD19 CAR T cells containing either a 4-1BB/CD3ζ or CD28/CD3ζ signaling domain and demonstrated complete remission (CR) rates of 70% to 93%25,33-36 (Table 1). The treatment regimen included lymphodepleting chemotherapy, usually consisting of cyclophosphamide (Cy) alone or Cy and fludarabine (Cy/Flu), before the IV administration of CAR T cells. Lymphodepletion reduces endogenous lymphocyte numbers, thereby increasing availability of homeostatic cytokines that promote the survival of transferred T cells.37,38 The necessity for lymphodepletion and the optimal regimen have not been systematically studied; however, the CR rate and duration of CAR T-cell persistence were superior with Cy/Flu compared with Cy alone.25,36 CR rate is not affected by tumor burden; however, patients with high numbers of leukemic blasts have greater toxicity, which in one study could be mitigated by infusing a lower CAR T-cell dose without compromising efficacy.36
Table 1.
Phase 1/2 trials of autologous CD19-targeted CAR T cells for patients with R/R ALL
| Reference | No. of patients treated | CAR construct | CAR T-cell dose or dose range | CR rate, %/MRD− CR, % |
|---|---|---|---|---|
| 33 | 16 (adults) | CD19scFv*/CD28/CD3ζ | 3 × 106/kg | 88/75 |
| 34 | 30 (25 CAYA; 5 adults) | CD19scFv/4-1BB/CD3ζ | 0.76 × 106/kg to 20.6 × 106/kg | 90/78.5 |
| 35 | 21 (CAYA) | CD19scFv/CD28/CD3ζ | 2.8 × 104/kg to 2 × 106/kg | 70/60 |
| 36 | 30 (adults) | CD19scFv/4-1BB/CD3ζ | 2 × 105/kg to 2 × 107/kg (CD4:CD8, 1:1) | 97/93 |
| 25 | 43 (CAYA) | CD19scFv/4-1BB/CD3ζ | 0.5 × 106/kg to 1 × 107/kg (CD4:CD8, 1:1) | 93/93 |
| 9† | 68 (CAYA) | CD19scFv/4-1BB/CD3ζ | 0.2 × 106/kg to 5.4 × 106/kg | 83/83 |
CAYA, children and young adults; MRD, minimal residual disease.
CD19 scFv is derived from the murine monoclonal antibody (mAb) SJ25-C1; CD19 scFv in the other studies is from the murine mAb FMC-63.
Multicenter trial.
The ELIANA multicenter pivotal trial of 4-1BB/CD3ζ CAR T cells for relapsed ALL in children and young adults enrolled 88 patients, and 68 received CAR T cells.9 The CR rate in treated patients was 83%, and the relapse-free probability at 12 months among responders was 64%. These results provided the basis for the first FDA-approved T-cell therapy and a major breakthrough in the ACT field.
NHL
CD19 CAR T cells have also been effective for treating patients with R/R B-cell NHL, including patients who relapsed after myeloablative autologous hematopoietic stem-cell transplantation. Case reports and single-center trials with CD28/CD3ζ or 4-1BB/CD3ζ CAR T cells demonstrated antitumor activity in follicular lymphoma, transformed follicular lymphoma, diffuse large B-cell lymphoma, and primary mediastinal B-cell lymphoma, and outcomes compared favorably with those of alternative salvage regimens21,39-43 (Table 2). A multicenter phase 2 trial, which led to FDA approval, administered CD28/CD3ζ CAR T cells to 101 patients with R/R NHL and reported objective response and CR rates of 82% and 54%, respectively, and an overall survival at 18 months of 52%.10
Table 2.
Selected phase 1/2 trials of autologous CD19-targeted CAR T cells for patients with R/R NHL
| Reference | No. of patients treated | CAR construct | CAR T-cell dose or dose range | ORR, %/CR rate, % |
|---|---|---|---|---|
| 21 | DLBCL (13), TFL (4), FL (2), PMBL (2), MCL (1) | CD19scFv/CD28/CD3ζ | 1 × 106/kg to 6 × 106/kg | 73/55 |
| 41 | DLBCL (2), TFL (2), PCNSL (1) | CD19scFv/CD28/CD3ζ and CD19scFv/CD3ζ | 2 × 107/m2 to 2 ×108/m2 | 0/0 |
| 42 | DLBCL (11), TFL (10), FL (5), MCL (4) | CD19scFv/4-1BB/CD3ζ | 2 × 105/kg to 2 × 107/kg (CD4:CD8, 1:1) | 63/33 |
| 43 | DLBCL (14), FL (14) | CD19scFv/4-1BB/CD3ζ | 2 × 105/kg to 2 × 107/kg | 64/57 |
| 10 | DLBCL (77), PMBCL (8), TFL (16) | CD19scFv/CD28/CD3ζ | 2 × 106/kg | 82/54 |
DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; MCL, mantle cell lymphoma; ORR, overall response rate; PCNSL, primary central nervous system lymphoma; PMBL, primary mediastinal B-cell lymphoma; TFL – transformed follicular lymphoma.
CLL
The investigation of CD19 CAR T cells in chemotherapy-refractory chronic lymphocytic leukemia (CLL) progressed slower than in ALL and NHL because of the efficacy of novel agents such as ibrutinib, idelalisib, and venetoclax.44-47 An early trial reported CR in 2 of 3 patients with R/R CLL after treatment with 4-1BB/CD3ζ CAR T cells.48 In a follow-up report, 8 of 14 patients in this trial responded to CAR T cells, including 4 patients who achieved a minimal residual disease–negative CR by deep sequencing for the clonal immunoglobulin H rearrangement. Turtle et al49 reported 24 patients treated with 4-1BB/CD3ζ CAR T cells, including 22 patients who had progressed on ibrutinib or were ibrutinib intolerant. Overall response and CR rates by International Workshop on Chronic Lymphocytic Leukemia criteria were 74% and 21%, respectively. Fifty-eight percent of patients who had deep sequencing of bone marrow samples after therapy had no malignant immunoglobulin H sequences detected. These data demonstrate that CAR T cells have efficacy and perhaps curative potential in patients with CLL for whom chemoimmunotherapy, ibrutinib, and venetoclax have failed.49 Defining a role for CAR T cells earlier in CLL therapy will require mitigating toxicities of CAR T cells.
Toxicities of CD19 CAR T cells
Infusion of CD19 CAR T cells is a disruptive approach that is changing treatment paradigms for B-cell malignancies, but unique toxicities such as cytokine release syndrome (CRS) and neurotoxicity occur frequently.50 The clinical features and pathogenesis of these complications continue to be the subjects of intense research to assist in defining effective prevention and treatment strategies.51-53
CRS
The onset of CRS coincides with activation and proliferation of CAR T cells in vivo and typically occurs within the first few days after T-cell infusion. CRS is characterized by a spectrum of symptoms that are mild in a majority of patients but can be severe, with high fevers, hypotension, tachycardia, capillary leak syndrome, respiratory compromise, and coagulopathy. Serum cytokines including interferon-γ, IL-6, IL-8, monocyte chemoattractant protein-1 (MCP-1), and macrophage inflammatory protein 1b and acute phase reactants such as C-reactive protein and ferritin are commonly elevated after the infusion of CAR T cells, indicative of a marked systemic inflammatory response.25,33-36,42,49,53 Rarely, patients will develop fulminant hemophagocytic lymphohistiocytosis.54 Risk factors for CRS include high tumor burden, higher numbers of CD19+ cells in the bone marrow, pretreatment thrombocytopenia, and higher CAR T-cell dose.53 CRS is currently treated by the administration of tocilizumab to block systemic effects of IL-6 and dexamethasone to inhibit cytokine production by activated CAR T and other immune cells. Studies of the pathogenesis of CRS are necessary to identify new approaches to intercede that do not inhibit antitumor effects of CAR T cells.
Neurotoxicity
Neurologic symptoms including headache, delirium, aphasia, focal neurologic deficits, seizures, and loss of consciousness are frequently observed in patients treated with CD19 CAR T cells, concurrently with or after CRS. Symptoms usually resolve over time, although rare patients have developed fatal cerebral hemorrhage or edema.50,52 Risk factors for neurotoxicity include high tumor burden or CAR T-cell dose, CRS, and preexisting neurologic abnormalities.52 Neurotoxicity is associated with endothelial activation, disseminated intravascular coagulation, and increased blood-brain barrier permeability.52 A predictive algorithm based on the presence of fever and high serum IL-6 and MCP-1 concentrations has been developed to identify patients within the first 36 hours after CAR T-cell infusion who are at high risk of subsequent severe neurotoxicity and might be candidates for testing novel strategies to prevent neurotoxicity.52
B-cell aplasia
The elimination of normal B cells is an expected on-target adverse effect of targeting CD19. Transient or prolonged loss of normal B cells is well tolerated, and B cells recover eventually in most patients when the number of CAR T cells declines. Vector designs that include a mechanism to accelerate elimination of CAR T cells have been developed, including coexpression of a cell-surface truncated epidermal growth factor receptor downstream of a T2A ribosomal skip element that can be targeted by cetuximab. In preclinical models, cetuximab administration eliminated CD19 CAR T cells and restored B-cell numbers without leukemia relapse.55 This approach could be applied to patients who achieve durable remission of their malignancy and have persistent B-cell aplasia.
Mechanisms of tumor escape from CAR T cells
Despite the high initial response rate with CD19 CAR T cells in ALL, relapses occur in a significant fraction of patients.9,25,34-36 Relapse with CD19+ leukemia cells can be the result of short in vivo persistence of CAR T cells, either from intrinsic deficiencies of the T-cell product or an immune response to the CAR scFv.36 In cases where CAR T cells persist, relapse with leukemia blasts that express little or no surface CD19 molecules can occur. Some CD19− relapses result from the outgrowth of leukemia cells that express a splice variant of CD19 that lacks the epitope targeted by the scFv.56
The overall response rate to CD19 CAR T cells in NHL is 70% to 80%, but the CR rate is much lower than that observed in ALL.10,42,43 As with ALL, poor CAR T-cell proliferation and in vivo persistence correlate with incomplete response, and CD19 loss variants have been observed. Additional mechanisms for incomplete tumor eradication are likely operative in NHL. Some tumors express PD-L1 and/or possess cells in the tumor microenvironment that can inhibit the proliferation and effector function of CAR T cells.57,58 Targeting these pathways with combination therapies may improve outcome. Alternative strategies to improve efficacy include selecting more effective T-cell subsets during CAR T-cell production and engineering CAR T cells to provide additional costimulatory signals such as 4-1BBL or secrete cytokines such as IL-12 that alter the tumor microenvironment.59,60 Ongoing studies to interrogate the mechanisms of incomplete responses to CAR T cells are needed to inform combination strategies that improve responses.
Alternative target antigens in B-cell malignancies
CD19 is an ideal target for CAR T cells because it is expressed uniformly at high site density on B-cell malignancies and is not shed as a soluble molecule. However, targeting a single antigen in cancer is fraught with the potential for antigen loss variants to emerge. There are alternative cell-surface molecules on B-cell tumors that have been targeted with CAR T cells, including surface immunoglobulin light chain, CD20, and CD22. A phase 1 trial evaluated CD28/CD3ζ immunoglobulin κ light chain–specific CAR T cells in 16 patients with NHL, CLL, or multiple myeloma and reported CR in 2 patients with NHL and partial responses or stable disease in 6 other patients.61 CD20 has been targeted with a third-generation CD28/4-1BB/CD3ζ CAR, and transient responses were observed in 3 of 4 patients, despite use of a suboptimal nonviral method for T-cell transduction.62 4-1BB/CD3ζ CD22-specific CAR T cells were also used to treat 21 patients with R/R ALL.63 CD19 CAR T cells had failed in 15 of these patients, and 9 had leukemic blasts that were CD19− or dim. CD22 CAR T cells expanded in the blood of treated patients to peak levels that were similar to those reported for CD19 CAR T cells. Overall, 12 (57%) of 21 patients achieved CR, including 11 of 15 patients treated at CAR T-cell doses of >1 × 106/kg.63 Three patients remained in CR for >6 months, but 7 patients relapsed, with tumor cells that had diminished surface expression of CD22. The antitumor effects of CAR T cells specific for CD19, immunoglobulin light chain, CD20, and CD22 individually suggest that targeting multiple molecules simultaneously might increase the frequency of patients achieving durable remissions without adding to toxicity.
Multivalent targeting could be accomplished by transducing separate aliquots of T cells with CARs specific for different target molecules, by transducing 1 aliquot of T cells to express >1 CAR, by using separate viral vectors or a single multicistronic construct64 or by designing vectors that encode a bispecific or multispecific CAR in a single-chain format.65,66 Formulating separate T-cell products increases the cost of manufacturing, and introducing >1 CAR into T cells has the potential to alter signaling in unforeseen ways. Bispecific CARs for CD19/CD20, CD19/CD123, and CD19/CD22 have been designed and are effective in preclinical models.63,65,66 However, achieving the optimal stoichiometry of antigen binding on tumor cells can be challenging. Another approach is to transduce T cells with a universal CAR that recognizes a short peptide tag that can be incorporated into scFvs or full-length antibodies that could serve as a targeting module that binds to and sensitizes tumor cells for recognition by CAR T cells.67,68 This strategy may require repeated infusion of tagged scFvs or antibodies to ensure complete tumor destruction, but it has the potential advantage of limiting serious toxicities because of the shorter half-life of the targeting module.67,68
CAR T cells for other hematologic malignancies
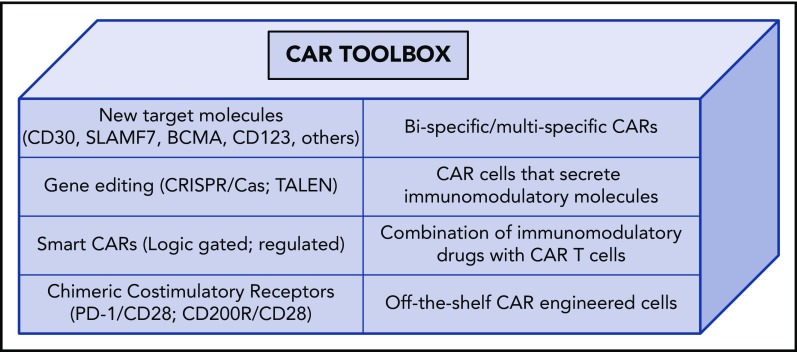
New strategies that employ synthetic biology for altering cell specificity and function are rapidly evolving and will be necessary to meet the challenges in extending CAR T cells to other hematologic cancers where target antigens are not always readily identified. Novel CAR designs, gene editing, chimeric chemokine receptors, chimeric costimulatory receptors, and logic-gated and multispecific receptors represent just a few of the tools being developed to augment efficacy and broaden the utility of ACT in cancer, autoimmunity, and transplantation69-78 (Figure 2). A majority of these strategies have only been evaluated in preclinical models but have promise for clinical applications.
Figure 2.
CAR toolbox. Toolbox of applications in synthetic biology that may extend and enhance the efficacy and safety of ACT.
T-cell malignancies
The development of CAR T cells for T-cell malignancies is more challenging than for B-cell malignancies, because candidate target antigens are also expressed on normal T cells. Thus, to avoid fratricide, it is necessary to combine gene editing to abrogate expression of the target molecule on T cells that are engineered to express the CAR. CD7 is expressed in T-cell leukemia, and CRISPR/Cas9-mediated editing has been used to delete CD7 in primary T cells before transduction with a CD7-specific CAR.79 Edited CD7-specific CAR T cells are functional and mediate antitumor activity in preclinical models. A limitation of this approach not yet adequately addressed in the preclinical models is the expected elimination of normal CD7+ T and NK cells and their progenitors, which would compromise host immunity. Persisting CAR T cells could potentially provide some immune function through their endogenous TCR; however, repertoire diversity would be constrained, and CAR expression may adversely affect TCR signaling.26
Rather than targeting a lineage-specific molecule on all T cells, an innovative alternative is to employ a CAR specific for only 1 TCR β chain constant region (TCRBC), analogous to targeting either κ or λ immunoglobulin light chains in B-cell malignancies. Two highly homologous genes (Cβ1 and Cβ2) encode TCRBCs, and they are expressed in a mutually exclusive manner. The clonal nature of T-cell malignancies means that the tumor will express either TCRBC1 or TCRBC2, whereas the normal T-cell compartment contains a significant quantity of T cells expressing either TCRBC. Thus, if a CAR targeted only the TCRBC expressed by the malignant T cells, this would not damage the normal T cells that express the alternative TCRBC. By screening anti-TCR antibodies, Maciocia et al80 identified an scFv that selectively bound to TCRBC1, with specificity determined by 2 adjacent amino acid residues that differ between TCRBC1 and TCRBC2. Only TCRBC2+ CAR T cells grew out from cultures transduced with a CD28/OX40/CD3ζ CAR constructed from this scFv, and these CAR T cells specifically recognized TCRBC1+ T-cell leukemia and lymphoma cells in vitro and in xenograft models in immunodeficient mice.80 This approach holds promise for targeted immunotherapy of the subset of T-cell malignancies that express TCRBC1 without compromising the entire T-cell compartment.
HL and ALCL
CD30 (TNFRSF8) is a member of the tumor necrosis factor receptor superfamily expressed at high levels in Hodgkin lymphoma (HL) and anaplastic large-cell lymphoma (ALCL) and on activated T and B cells. Clinical trials of an antibody-drug conjugate (brentuximab) targeting CD30 have shown antitumor activity in HL without toxicity to normal T or B cells, suggesting CD30 is a candidate target for CAR T cells. Wang et al81 described results of 18 patients with HL treated with autologous T cells engineered to express a 4-1BB/CD3ζ CD30 CAR. Treatment was well tolerated, and 7 of 18 patients achieved a partial response. Ramos et al82 treated 7 patients with HL and 2 patients with ALCL with T cells expressing a CD28/CD3ζ CD30 CAR. No toxicity was observed, and 3 of the 9 patients achieved CR, with 2 remaining in CR >24 months. These studies demonstrate that targeting CD30 with CAR T cells is safe and can have antitumor activity in CD30+ malignancies. As with B-cell NHL, analysis of resistance mechanisms is essential to design strategies that would improve outcomes.
Multiple myeloma
Multiple myeloma responds initially to immunomodulatory agents, proteasome inhibitors, and monoclonal antibodies, but relapse is inevitable, and curative therapy remains elusive. ACT is an attractive approach, but administration of myeloma-infiltrating lymphocytes or NY-ESO-1 TCR-engineered T cells after autologous stem-cell transplantation did not show definitive evidence of antitumor efficacy.83,84 An early effort to treat myeloma with CAR T cells targeting CD19 resulted in a prolonged remission after autologous stem-cell transplantation in a single case report.85 However, the rarity of CD19 expression on myeloma and the uncertain mechanism for eradication of antigen-negative tumor cells suggest the need to define better target molecules.85 CD38 and SLAMF7 have been successfully targeted with monoclonal antibodies,86-88 and CARs specific for these molecules showed activity in preclinical models.89,90 However, CD38 is expressed on other hematopoietic cells and some nonhematopoietic cells, and SLAMF7 is expressed on activated T cells, raising concern that CAR T cells targeting these molecules may be toxic in the clinic.
The tumor necrosis factor receptor family member BCMA, which is a receptor for BAFF and APRIL, is at present the most attractive for CAR T cells in myeloma. BCMA is expressed on both plasma cells and myeloma cells but is absent on normal tissues.91-93 Seckinger et al94 reported surface BCMA expression in 100% of untreated and relapsed patients with myeloma.94 A potential limitation of targeting BCMA is that it is cleaved by γ secretase in myeloma cells, leading to variation in surface expression and increased levels of soluble BCMA that can inhibit CAR recognition.95 Despite this limitation, BCMA CARs have demonstrated significant clinical efficacy in initial clinical trials. Kochenderfer et al96 treated 12 patients using unselected T cells engineered with a CD28/CD3ζ BCMA CAR. A very good partial response was observed in 2 patients, partial response in 1 patient, and stringent CR in 1 patient.96 Antitumor effects seemed to correlate with CAR T-cell dose, although too few patients were treated at each dose level for a definitive conclusion. Toxicity was modest, with 2 patients at the highest CAR T-cell dose experiencing CRS.96 Two phase 1 trials of BCMA CAR T cells in R/R myeloma were reported at the 2017 American Society of Clinical Oncology Annual Meeting.97,98 Although follow-up was short, significant antitumor activity was observed in both studies, with a subset of treated patients achieving CR. These results demonstrate that targeting BCMA with CAR T cells has substantial activity in myeloma, although longer follow-up is needed to determine the durability of responses and to identify resistance mechanisms that prevent complete tumor elimination in patients with partial responses. Recent preclinical studies also suggest that CAR T-cell efficacy is enhanced in combination with lenalidomide.99 Thus, studies combining CAR T cells with upfront therapies in myeloma are warranted.
AML
Acute myeloid leukemia (AML) is more common than ALL in adults, and patients age >60 years respond poorly to conventional therapy. The success of CAR T cells in ALL has encouraged efforts to apply this approach in AML; however, identifying suitable targets for CAR T cells has been problematic. Lewis Y antigen (LeY), CD33, CD123, and CLL1 are being studied as targets for CAR T cells, but they have the disadvantage of having heterogeneous expression on leukemic cells or being expressed on normal hematopoietic progenitors.100 A clinical trial targeting LeY with CAR T cells demonstrated limited antitumor activity, and studies targeting CD123 are ongoing. A transcriptomic and proteomic analysis of AML identified 3 pairs of molecules that might be amenable to combinatorial CAR targeting to minimize on-target, off-tumor reactivity and maximize recognition of leukemia.101 CARs incorporating scFvs that bind to HLA/peptide complexes such as HLA-A2/WT1 are also being investigated.102 Further study of the specificity and sensitivity of these reagents is required, but they represent alternatives to targeting broadly expressed AML surface molecules.
Future perspectives
The genetic modification of T cells to target cancer represents a disruptive new approach to therapy that is now approved for advanced B-cell malignancies. Longer follow-up will determine what fraction of patients achieve durable remissions with current approaches, and ongoing studies of primary and adaptive resistance mechanisms that prevent complete tumor eradication should provide direction for improving outcomes. The cost and complexity of manufacturing CAR T cells are significant barriers to general use and, until solved, will complicate the evaluation of CAR T cells earlier in therapeutic regimens, which could reduce the morbidities of repeated cycles of multiagent chemotherapy. Future studies comparing CAR T cells with standard therapies will be imperative in B-cell malignancies to determine potential benefits and disadvantages of cell-based therapy. Significant challenges remain in applying this approach beyond B-cell malignancies, but it is important to realize that the field is in its infancy. Fundamental advances in T-cell biology, synthetic receptor design, and signaling have the potential to overcome current obstacles and provide the next generation of genetically engineered cellular therapies.
Acknowledgments
This work was supported by the National Institutes of Health, National Cancer Institute grants CA136551, CA114536, and CA18029 (S.R.R.), the Netherlands Association for Scientific Research Rubicon Award (M.J.P.), and the Bezos Family Immunotherapy Initiative (A.I.S.).
Authorship
Contribution: All authors reviewed relevant literature and wrote the paper.
Conflict-of-interest disclosure: S.R.R. has stock or other ownership in Juno Therapeutics, has a consulting or advisory role for Juno Therapeutics, Cell Medica, and Adaptive Biotechnologies, and receives research funding from Juno Therapeutics. S.R.R. also holds patents on methods and composition of cell therapy. The remaining authors declare no competing financial interests.
Correspondence: Stanley R. Riddell, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Seattle, WA 98109; e-mail: sriddell@fhcrc.org.
References
- 1.Bollard CM, Gottschalk S, Torrano V, et al. . Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J Clin Oncol. 2014;32(8):798-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bollard CM, Rooney CM, Heslop HE. T-cell therapy in the treatment of post-transplant lymphoproliferative disease. Nat Rev Clin Oncol. 2012;9(9):510-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu YC, Parker LL, Lu T, et al. . Treatment of patients with metastatic cancer using a major histocompatibility complex class II-restricted T-cell receptor targeting the cancer germline antigen MAGE-A3. J Clin Oncol. 2017;35(29):3322-3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robbins PF, Kassim SH, Tran TL, et al. . A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res. 2015;21(5):1019-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348(6230):62-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevanović S, Pasetto A, Helman SR, et al. . Landscape of immunogenic tumor antigens in successful immunotherapy of virally induced epithelial cancer. Science. 2017;356(6334):200-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran E, Turcotte S, Gros A, et al. . Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344(6184):641-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warren EH, Fujii N, Akatsuka Y, et al. . Therapy of relapsed leukemia after allogeneic hematopoietic cell transplantation with T cells specific for minor histocompatibility antigens. Blood. 2010;115(19):3869-3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maude SL, Laetsch TW, Buechner J, et al. . Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neelapu SS, Locke FL, Bartlett NL, et al. . Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci USA. 1993;90(2):720-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brocker T. Chimeric Fv-zeta or Fv-epsilon receptors are not sufficient to induce activation or cytokine production in peripheral T cells. Blood. 2000;96(5):1999-2001. [PubMed] [Google Scholar]
- 13.Hombach A, Wieczarkowiecz A, Marquardt T, et al. . Tumor-specific T cell activation by recombinant immunoreceptors: CD3 zeta signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3 zeta signaling receptor molecule. J Immunol. 2001;167(11):6123-6131. [DOI] [PubMed] [Google Scholar]
- 14.Jensen MC, Popplewell L, Cooper LJ, et al. . Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant. 2010;16(9):1245-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maher J, Brentjens RJ, Gunset G, Rivière I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol. 2002;20(1):70-75.11753365 [Google Scholar]
- 16.Imai C, Mihara K, Andreansky M, et al. . Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18(4):676-684. [DOI] [PubMed] [Google Scholar]
- 17.Kowolik CM, Topp MS, Gonzalez S, et al. . CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006;66(22):10995-11004. [DOI] [PubMed] [Google Scholar]
- 18.Finney HM, Akbar AN, Lawson AD. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J Immunol. 2004;172(1):104-113. [DOI] [PubMed] [Google Scholar]
- 19.Eyquem J, Mansilla-Soto J, Giavridis T, et al. . Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543(7643):113-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118(1):294-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kochenderfer JN, Somerville RPT, Lu T, et al. . Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J Clin Oncol. 2017;35(16):1803-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klebanoff CA, Crompton JG, Leonardi AJ, et al. . Inhibition of AKT signaling uncouples T cell differentiation from expansion for receptor-engineered adoptive immunotherapy. JCI Insight. 2017;2(23):95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sommermeyer D, Hudecek M, Kosasih PL, et al. . Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia. 2016;30(2):492-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cruz CR, Micklethwaite KP, Savoldo B, et al. . Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood. 2013;122(17):2965-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardner RA, Finney O, Annesley C, et al. . Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129(25):3322-3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y, Kohler ME, Chien CD, et al. . TCR engagement negatively affects CD8 but not CD4 CAR T cell expansion and leukemic clearance. Sci Transl Med. 2017;9(417):eaag1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghosh A, Smith M, James SE, et al. . Donor CD19 CAR T cells exert potent graft-versus-lymphoma activity with diminished graft-versus-host activity. Nat Med. 2017;23(2):242-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qasim W, Zhan H, Samarasinghe S, et al. . Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med. 2017;9(374):eaaj2013. [DOI] [PubMed] [Google Scholar]
- 29.Liu E, Tong Y, Dotti G, et al. . Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia. 2018;32(2):520-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian G, Courtney AN, Jena B, et al. . CD62L+ NKT cells have prolonged persistence and antitumor activity in vivo. J Clin Invest. 2016;126(6):2341-2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pui CH, Yang JJ, Hunger SP, et al. . Childhood acute lymphoblastic leukemia: progress through collaboration. J Clin Oncol. 2015;33(27):2938-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ronson A, Tvito A, Rowe JM. Treatment of relapsed/refractory acute lymphoblastic leukemia in adults. Curr Oncol Rep. 2016;18(6):39. [DOI] [PubMed] [Google Scholar]
- 33.Davila ML, Riviere I, Wang X, et al. . Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6(224):224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maude SL, Frey N, Shaw PA, et al. . Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. . T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turtle CJ, Hanafi LA, Berger C, et al. . CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982;155(4):1063-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gattinoni L, Finkelstein SE, Klebanoff CA, et al. . Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202(7):907-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kochenderfer JN, Somerville RPT, Lu T, et al. . Long-duration complete remissions of diffuse large B cell lymphoma after anti-CD19 chimeric antigen receptor T cell therapy. Mol Ther. 2017;25(10):2245-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kochenderfer JN, Wilson WH, Janik JE, et al. . Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116(20):4099-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savoldo B, Ramos CA, Liu E, et al. . CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121(5):1822-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turtle CJ, Hanafi LA, Berger C, et al. . Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med. 2016;8(355):355ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schuster SJ, Svoboda J, Chong EA, et al. . Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377(26):2545-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burger JA, Tedeschi A, Barr PM, et al. ; RESONATE-2 Investigators. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Byrd JC, Harrington B, O’Brien S, et al. . Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):323-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones JA, Mato AR, Wierda WG, et al. . Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: an interim analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol. 2018;19(1):65-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts AW, Davids MS, Pagel JM, et al. . Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):311-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalos M, Levine BL, Porter DL, et al. . T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95):95ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turtle CJ, Hay KA, Hanafi LA, et al. . Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol. 2017;35(26):3010-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neelapu SS, Tummala S, Kebriaei P, et al. . Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee DW, Gardner R, Porter DL, et al. . Current concepts in the diagnosis and management of cytokine release syndrome [published correction appears in Blood. 2015;126(8):1048]. Blood. 2014;124(2):188-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gust J, Hay KA, Hanafi LA, et al. . Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7(12):1404-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hay KA, Hanafi LA, Li D, et al. . Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130(21):2295-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014;20(2):119-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paszkiewicz PJ, Fräßle SP, Srivastava S, et al. . Targeted antibody-mediated depletion of murine CD19 CAR T cells permanently reverses B cell aplasia. J Clin Invest. 2016;126(11):4262-4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sotillo E, Barrett DM, Black KL, et al. . Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5(12):1282-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Azzaoui I, Uhel F, Rossille D, et al. . T-cell defect in diffuse large B-cell lymphomas involves expansion of myeloid-derived suppressor cells. Blood. 2016;128(8):1081-1092. [DOI] [PubMed] [Google Scholar]
- 58.Xu-Monette ZY, Zhou J, Young KH. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood. 2018;131(1):68-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stephan MT, Ponomarev V, Brentjens RJ, et al. . T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nat Med. 2007;13(12):1440-1449. [DOI] [PubMed] [Google Scholar]
- 60.Pegram HJ, Lee JC, Hayman EG, et al. . Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119(18):4133-4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramos CA, Savoldo B, Torrano V, et al. . Clinical responses with T lymphocytes targeting malignancy-associated κ light chains. J Clin Invest. 2016;126(7):2588-2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Till BG, Jensen MC, Wang J, et al. . CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood. 2012;119(17):3940-3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fry TJ, Shah NN, Orentas RJ, et al. . CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen KH, Wada M, Pinz KG, et al. . A compound chimeric antigen receptor strategy for targeting multiple myeloma. Leukemia. 2018;32(2):402-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zah E, Lin MY, Silva-Benedict A, Jensen MC, Chen YY. T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunol Res. 2016;4(6):498-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ruella M, Barrett DM, Kenderian SS, et al. . Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J Clin Invest. 2016;126(10):3814-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cartellieri M, Feldmann A, Koristka S, et al. . Switching CAR T cells on and off: a novel modular platform for retargeting of T cells to AML blasts. Blood Cancer J. 2016;6(8):e458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rodgers DT, Mazagova M, Hampton EN, et al. . Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies. Proc Natl Acad Sci USA. 2016;113(4):E459-E468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brown CE, Alizadeh D, Starr R, et al. . Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375(26):2561-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chmielewski M, Kopecky C, Hombach AA, Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 2011;71(17):5697-5706. [DOI] [PubMed] [Google Scholar]
- 71.Craddock JA, Lu A, Bear A, et al. . Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J Immunother. 2010;33(8):780-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med. 2013;5(215):215ra172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grada Z, Hegde M, Byrd T, et al. . TanCAR: a novel bispecific chimeric antigen receptor for cancer immunotherapy. Mol Ther Nucleic Acids. 2013;2:e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Juillerat A, Marechal A, Filhol JM, et al. . Design of chimeric antigen receptors with integrated controllable transient functions. Sci Rep. 2016;6:18950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu X, Ranganathan R, Jiang S, et al. . A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors. Cancer Res. 2016;76(6):1578-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roybal KT, Rupp LJ, Morsut L, et al. . Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell. 2016;164(4):770-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ellebrecht CT, Bhoj VG, Nace A, et al. . Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. 2016;353(6295):179-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu L, Sommermeyer D, Cabanov A, Kosasih P, Hill T, Riddell SR. Inclusion of Strep-tag II in design of antigen receptors for T-cell immunotherapy. Nat Biotechnol. 2016;34(4):430-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gomes-Silva D, Srinivasan M, Sharma S, et al. . CD7-edited T cells expressing a CD7-specific CAR for the therapy of T-cell malignancies. Blood. 2017;130(3):285-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maciocia PM, Wawrzyniecka PA, Philip B, et al. . Targeting the T cell receptor β-chain constant region for immunotherapy of T cell malignancies. Nat Med. 2017;23(12):1416-1423. [DOI] [PubMed] [Google Scholar]
- 81.Wang CM, Wu ZQ, Wang Y, et al. . Autologous T cells expressing CD30 chimeric antigen receptors for relapsed or refractory Hodgkin lymphoma: an open-label phase I trial. Clin Cancer Res. 2017;23(5):1156-1166. [DOI] [PubMed] [Google Scholar]
- 82.Ramos CA, Ballard B, Zhang H, et al. . Clinical and immunological responses after CD30-specific chimeric antigen receptor-redirected lymphocytes. J Clin Invest. 2017;127(9):3462-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Noonan KA, Huff CA, Davis J, et al. . Adoptive transfer of activated marrow-infiltrating lymphocytes induces measurable antitumor immunity in the bone marrow in multiple myeloma. Sci Transl Med. 2015;7(288):288ra78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rapoport AP, Stadtmauer EA, Binder-Scholl GK, et al. . NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med. 2015;21(8):914-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Garfall AL, Maus MV, Hwang WT, et al. . Chimeric antigen receptor T cells against CD19 for multiple myeloma. N Engl J Med. 2015;373(11):1040-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dimopoulos MA, Oriol A, Nahi H, et al. ; POLLUX Investigators. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319-1331. [DOI] [PubMed] [Google Scholar]
- 87.Palumbo A, Chanan-Khan A, Weisel K, et al. ; CASTOR Investigators. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754-766. [DOI] [PubMed] [Google Scholar]
- 88.Lonial S, Dimopoulos M, Palumbo A, et al. ; ELOQUENT-2 Investigators. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373(7):621-631. [DOI] [PubMed] [Google Scholar]
- 89.Gogishvili T, Danhof S, Prommersberger S, et al. . SLAMF7-CAR T cells eliminate myeloma and confer selective fratricide of SLAMF7+ normal lymphocytes. Blood. 2017;130(26):2838-2847. [DOI] [PubMed] [Google Scholar]
- 90.Drent E, Groen RW, Noort WA, et al. . Pre-clinical evaluation of CD38 chimeric antigen receptor engineered T cells for the treatment of multiple myeloma. Haematologica. 2016;101(5):616-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Novak AJ, Darce JR, Arendt BK, et al. . Expression of BCMA, TACI, and BAFF-R in multiple myeloma: a mechanism for growth and survival. Blood. 2004;103(2):689-694. [DOI] [PubMed] [Google Scholar]
- 92.Tai YT, Acharya C, An G, et al. . APRIL and BCMA promote human multiple myeloma growth and immunosuppression in the bone marrow microenvironment. Blood. 2016;127(25):3225-3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carpenter RO, Evbuomwan MO, Pittaluga S, et al. . B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res. 2013;19(8):2048-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Seckinger A, Delgado JA, Moser S, et al. . Target expression, generation, preclinical activity, and pharmacokinetics of the BCMA-T cell bispecific antibody EM801 for multiple myeloma treatment. Cancer Cell. 2017;31(3):396-410. [DOI] [PubMed] [Google Scholar]
- 95.Laurent SA, Hoffmann FS, Kuhn PH, et al. . γ-Secretase directly sheds the survival receptor BCMA from plasma cells. Nat Commun. 2015;6:7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ali SA, Shi V, Maric I, et al. . T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128(13):1688-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Berdeja JG, Lin Y, Raje NS, et al. . First-in-human multicenter study of bb2121 anti-BCMA CAR T-cell therapy for relapsed/refractory multiple myeloma: updated results [abstract]. J Clin Oncol. 2017;35(15 suppl). Abstract 3010. [Google Scholar]
- 98.Fan X, Zhao W, Liu J, et al. . Durable remissions with BCMA-specific chimeric antigen receptor (CAR)-modified T cells in patients with refractory/relapsed multiple myeloma [abstract]. J Clin Oncol. 2017;35(18 suppl). Abstract LBA3001. [Google Scholar]
- 99.Otáhal P, Průková D, Král V, et al. . Lenalidomide enhances antitumor functions of chimeric antigen receptor modified T cells. OncoImmunology. 2015;5(4):e1115940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tashiro H, Sauer T, Shum T, et al. . Treatment of acute myeloid leukemia with T cells expressing chimeric antigen receptors directed to C-type lectin-like molecule 1. Mol Ther. 2017;25(9):2202-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Perna F, Berman SH, Soni RK, et al. . Integrating proteomics and transcriptomics for systematic combinatorial chimeric antigen receptor therapy of AML. Cancer Cell. 2017;32(4):506-519.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rafiq S, Purdon TJ, Daniyan AF, et al. . Optimized T-cell receptor-mimic chimeric antigen receptor T cells directed toward the intracellular Wilms tumor 1 antigen. Leukemia. 2017;31(8):1788-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]