Publisher's Note: There is a Blood Commentary on this article in this issue.
Key Points
Two-year survivors of autologous BMT in childhood have a substantially increased risk of late mortality compared with the general population.
There has been a significant decline in all-cause mortality in children undergoing autologous BMT over the past 3 decades.
Abstract
Autologous blood or marrow transplantation (BMT) is a curative option for several types of childhood cancer. However, there is little information regarding the risk of late mortality. We examined all-cause mortality, relapse-related mortality (RRM), and nonrelapse-related mortality (NRM) in 2-year survivors of autologous BMT performed before age 22 between 1980 and 2010 at 1 of 2 US transplant centers. Vital status information was collected using medical records, National Death Index, and Accurint databases. Overall survival was calculated using Kaplan-Meier techniques. Cumulative incidence of mortality used competing risk methods. Standardized mortality ratio (SMR) was calculated using age-, sex-, and calendar-specific mortality rates from Centers for Disease Control and Prevention. Cox regression analysis was used to determine predictors of all-cause late mortality. Among the 345 2-year survivors, 103 deaths were observed, yielding an overall survival of 70.3% 15 years post-BMT. The leading causes of death included primary disease (50.0%), subsequent neoplasm (21.4%), and infection (18.2%). Overall, the cohort was at a 22-fold increased risk of late mortality (SMR, 21.8; 95% CI, 17.9-26.3), compared with the general population. Mortality rates remained elevated among the 10-year survivors (SMR, 20.6; 95% CI, 9.9-37.2) but approached those of the general population ≥15 years post-BMT. The 10-year cumulative incidence of RRM (14.3%) exceeded that of NRM (10.4%). The 10-year cumulative mortality rate declined over time (<1990, 35.1%; 1990-1999, 25.6%; 2000-2010, 21.8%; P = .05). In conclusion, childhood autologous BMT recipients have an increased risk of late mortality, compared with the general population. The late mortality rates have declined over the past 3 decades.
Visual Abstract
Introduction
Autologous blood or marrow transplantation (BMT) is used with curative intent for several types of childhood cancer. Studies of long-term survival in adult autologous BMT recipients, as well as in mixed cohorts of adults and children, have shown higher late mortality rates than in the general population, with the leading causes of late death being relapse or progressive disease and subsequent neoplasms (SNs).1-3 Because the primary cancer types treated with autologous BMT differ between children and adults, cohorts restricted to children are required to fully understand the life-time risks of cause-specific mortality among childhood autologous BMT recipients. Furthermore, the risk of cardiovascular disease and SNs likely varies by age at transplantation and length of follow-up, necessitating comprehensive assessment of the life-time risk of cause-specific late mortality. Finally, changes in transplant practice over the past 3 decades could have possibly influenced late mortality risk. However, there is little information regarding the long-term outcome after autologous BMT performed in childhood and whether mortality rates in this population have changed over the past 3 decades.4 We address the existing gaps in knowledge by examining all-cause mortality, relapse-related mortality (RRM), and nonrelapse-related mortality (NRM) in children treated with autologous BMT between 1980 and 2010, permitting assessment of change in late mortality rates over these 3 decades.
Methods
The Blood or Marrow Transplant Survivor Study-2 is a collaborative effort between City of Hope and University of Minnesota, examining the long-term outcome of individuals who have survived 2 or more years after BMT performed at 1 of the 2 participating institutions. To be included in this analysis, the patients had to have received autologous BMT between 1980 and 2010 at age 21 years or younger, and survived for at least 2 years after transplantation, regardless of disease status. Patients who received a second BMT were excluded.
Information on primary diagnosis, conditioning regimen, stem cell source, disease status at transplantation, and demographic characteristics was obtained on all eligible cases from the institutional transplant databases. National Death Index Plus5 and/or medical records provided information regarding the date and cause of death through 31 December 2015. Additional information from Accurint databases6 was used to extend the vital status information through 31 December 2016. All patients were assigned a primary and, if present, a secondary cause of death, independently by 2 separate investigators (A.S.H. and J.W.). In the event of discrepant assignments, a third investigator (S.B.) provided adjudication. In the analyses of RRM and NRM, all patients with primary disease as a primary or secondary cause of death were assigned relapse-related cause of death. The Human Subjects Committee at the participating institutions approved the Blood or Marrow Transplant Survivor Study-2 protocol. Informed consent was obtained in accordance with the Declaration of Helsinki.
Statistical analysis
Kaplan-Meier techniques were used to describe overall survival conditional on surviving 2+ years from autologous BMT, as well as all-cause late mortality for patients transplanted over 3 eras: <1990, 1990 to 1999, and 2000 to 2010. Cumulative incidence of RRM and NRM were calculated using competing risk methods. Standardized mortality ratio (SMR), a ratio of observed to expected number of deaths, was used to compare the mortality experienced by this cohort to the age- (5-year interval), sex-, and calendar-specific mortality of the US general population. Person-years at risk were computed from the date 2 years after autologous BMT to either the date of death or date of censoring (31 December 2016), whichever occurred first. The expected number of deaths was calculated by multiplying the number of person-years in each defined stratum by the corresponding US mortality rates, obtained from Centers for Disease Control and Prevention.7 Ninety-five percent confidence interval (CI) of the SMR was calculated using the Poisson regression method described by Vandenbroucke.8 Absolute excess risk (AER) (ie, the survivors’ additional risk for death) was defined as the difference between the observed and expected number of deaths, per 1000 person-years of follow-up.
Cox regression analysis was used to determine predictors of all-cause late mortality. The evaluated risk factors included sex, race/ethnicity, age at autologous BMT, stem cell source, primary disease, conditioning regimen, disease status at transplantation (standard risk or high risk of relapse at transplantation) and transplant era. Patients who underwent autologous BMT in first or second complete remission after acute leukemia or lymphoma were considered standard risk for relapse at transplantation; all other patients were considered at high risk. All analyses were performed with SAS software version 9.4 (SAS Institute Inc., Cary, NC).
Results
Patient characteristics
The demographic and clinical characteristics of the cohort overall and by primary diagnosis are shown in Table 1. In this cohort of 345 individuals who had survived 2 or more years after autologous BMT performed in childhood, 212 (61.4%) were males, and the median age at transplantation was 16.0 years (range, 0.6-21.9 years). The most common primary diagnoses were HL (26%), neuroblastoma (23%), AML (13%), ALL (13%), and NHL (9%). The distribution of the cohort over the 3 treatment eras was <1990 (21%), 1990 to 1999 (37%), and 2000 to 2010 (41%). Bone marrow was the source of stem cells in 47% of the patients, and 53% had received PBSCs. TBI was used as part of the conditioning regimen in 36% of the patients, and cyclophosphamide was used in 56% and melphalan in 39% of patients.
Table 1.
Demographic and clinical characteristics of 345 2-year survivors of autologous BMT in childhood
| Variables | Entire cohort | Primary diagnosis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALL | AML | HL | NHL | Neuroblastoma | Other malignant disease* | |||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| Number of patients | 345 | 100.0 | 43 | 12.5 | 45 | 13.0 | 90 | 26.1 | 31 | 9.0 | 79 | 22.9 | 57 | 16.5 |
| Sex | ||||||||||||||
| Male | 212 | 61.4 | 34 | 79.1 | 24 | 53.3 | 52 | 57.8 | 22 | 71.0 | 49 | 62.0 | 31 | 54.4 |
| Female | 133 | 38.6 | 9 | 20.9 | 21 | 46.7 | 38 | 42.2 | 9 | 29.0 | 30 | 38.0 | 26 | 45.6 |
| Race/ethnicity | ||||||||||||||
| Non-Hispanic white | 264 | 76.5 | 36 | 83.7 | 35 | 77.8 | 59 | 65.6 | 25 | 80.6 | 64 | 81.0 | 45 | 78.9 |
| Hispanic | 44 | 12.8 | 3 | 7.0 | 5 | 11.1 | 18 | 20.0 | 1 | 3.2 | 9 | 11.4 | 8 | 14.0 |
| Non-Hispanic black | 20 | 5.8 | 3 | 7.0 | 4 | 8.9 | 7 | 7.8 | 3 | 9.7 | 2 | 2.5 | 1 | 1.8 |
| Other | 10 | 2.9 | 1 | 2.3 | 1 | 2.2 | 4 | 4.4 | 1 | 3.2 | 2 | 2.5 | 1 | 1.8 |
| Unknown | 7 | 2.0 | 0 | 0.0 | 0 | 0.0 | 2 | 2.2 | 1 | 3.2 | 2 | 2.5 | 2 | 3.5 |
| Age at BMT, y | ||||||||||||||
| 0-4 | 97 | 28.1 | 13 | 30.2 | 11 | 24.4 | 0 | 0.0 | 1 | 3.2 | 59 | 74.7 | 13 | 22.8 |
| 5-9 | 57 | 16.5 | 15 | 34.9 | 6 | 13.3 | 3 | 3.3 | 6 | 19.4 | 14 | 17.7 | 13 | 22.8 |
| 10-14 | 55 | 15.9 | 6 | 14.0 | 10 | 22.2 | 15 | 16.7 | 4 | 12.9 | 3 | 3.8 | 17 | 29.8 |
| 15-21 | 136 | 39.4 | 9 | 20.9 | 18 | 40.0 | 72 | 80.0 | 20 | 64.5 | 3 | 3.8 | 14 | 24.6 |
| Year of BMT | ||||||||||||||
| <1990 | 74 | 21.4 | 34 | 79.1 | 12 | 26.7 | 11 | 12.2 | 9 | 29.0 | 7 | 8.9 | 1 | 1.8 |
| 1990-1999 | 129 | 37.4 | 9 | 20.9 | 25 | 55.6 | 32 | 35.6 | 11 | 35.5 | 31 | 39.2 | 21 | 36.8 |
| 2000-2010 | 142 | 41.2 | 0 | 0.0 | 8 | 17.8 | 47 | 52.2 | 11 | 35.5 | 41 | 51.9 | 35 | 61.4 |
| Source of stem cells | ||||||||||||||
| Bone marrow | 163 | 47.2 | 41 | 95.3 | 32 | 71.1 | 30 | 33.3 | 15 | 48.4 | 32 | 40.5 | 13 | 22.8 |
| PBSCs | 181 | 52.5 | 2 | 4.7 | 13 | 28.9 | 60 | 66.7 | 16 | 51.6 | 47 | 59.5 | 43 | 75.4 |
| Cord blood | 1 | 0.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.8 |
| Conditioning regimen | ||||||||||||||
| TBI | 124 | 35.9 | 42 | 97.7 | 21 | 46.7 | 19 | 21.1 | 27 | 87.1 | 14 | 17.7 | 1 | 1.7 |
| Etoposide | 204 | 59.1 | 2 | 4.7 | 17 | 37.8 | 88 | 97.8 | 18 | 58.1 | 71 | 89.9 | 8 | 14.0 |
| Cyclophosphamide | 192 | 55.7 | 32 | 74.4 | 40 | 88.9 | 80 | 88.9 | 29 | 93.5 | 6 | 7.6 | 5 | 8.8 |
| Melphalan | 133 | 38.6 | 0 | 0.0 | 0 | 0.0 | 10 | 11.1 | 2 | 6.5 | 72 | 91.1 | 49 | 86.0 |
| Busulfan | 75 | 21.7 | 1 | 2.3 | 26 | 57.8 | 0 | 0.0 | 0 | 0.0 | 6 | 7.6 | 42 | 73.7 |
| Carmustine | 76 | 22.0 | 0 | 0.0 | 0 | 0.0 | 69 | 76.7 | 4 | 12.9 | 2 | 2.5 | 1 | 1.8 |
| Carboplatin | 71 | 20.6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 61 | 77.2 | 10 | 17.5 |
| Other chemotherapy | 143 | 41.5 | 40 | 93.0 | 25 | 55.6 | 8 | 8.9 | 6 | 19.4 | 18 | 22.8 | 46 | 80.7 |
| Other radiotherapy | 3 | 0.9 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 3.2 | 1 | 1.3 | 1 | 1.8 |
| Disease status at BMT | ||||||||||||||
| Standard risk of relapse† | 133 | 38.6 | 32 | 74.4 | 44 | 97.8 | 35 | 38.9 | 22 | 71.0 | 0 | 0.0 | 0 | 0.0 |
| High risk of relapse | 212 | 61.4 | 11 | 25.6 | 1 | 2.2 | 55 | 61.1 | 9 | 29.0 | 79 | 100.0 | 57 | 100.0 |
| Number of deaths | 103 | 29.9 | 21 | 48.8 | 6 | 13.3 | 23 | 25.6 | 8 | 25.8 | 25 | 31.6 | 20 | 35.1 |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma; PBSC, peripheral blood stem cells; TBI, total body irradiation.
Includes 25 Ewing sarcomas, 11 Wilms tumor, 9 central nervous system (CNS) tumors, 4 desmoplastic small round cell tumors, 6 soft tissue sarcoma, 1 ovarian tumor, and 1 hepatoblastoma.
Standard risk: first or second complete remission after acute leukemia or lymphoma; all others high risk.
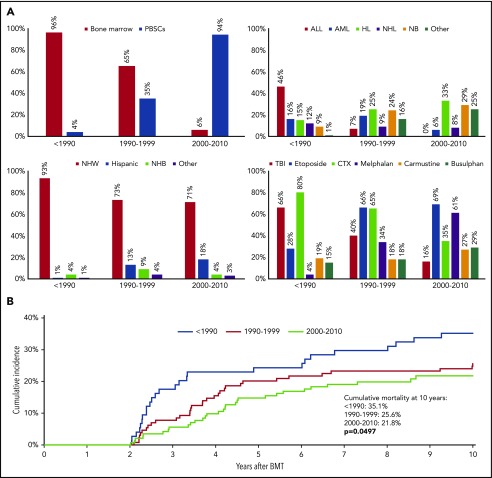
The demographic and clinical characteristics of the 2-or-more-year survivor cohort by treatment era, are shown in supplemental Table 1 (available on the Blood Web site); key differences are summarized in Figure 1A. The use of bone marrow as stem cell source has been replaced by PBSCs. Patients with ALL are no longer treated with autologous BMT, and the use of autologous BMT for AML peaked in the 1990s and declined thereafter. In contrast, autologous BMT for HL, NHL, and neuroblastoma has increased over the past 3 decades. TBI-containing conditioning, as well as the use of cyclophosphamide, has declined over time; in contrast, the use of melphalan and busulfan has increased, likely reflecting the change in prevalence of the underlying diseases.
Figure 1.
Treatment characteristics and cumulative mortality in 345 2-year survivors of autologous BMT in childhood, by treatment period. (A) Demographic and treatment characteristics of 2-year survivors of autologous BMT in childhood, by treatment era. CTX, cyclophosphamide; NB, neuroblastoma; NHB, non-Hispanic black; NHW, non-Hispanic whites. (B) Cumulative mortality in 2-year survivors of autologous BMT in childhood, by treatment era.
Overall survival
After a median follow-up of 14.2 years (range, 2.0-35.3 years), 103 deaths (29.9%) were observed among the 2-or-more-year survivors. Median age at death was 19.9 years (range, 4.1-36.7 years). Conditional on surviving the first 2 years, the overall survival rate at 5, 10, and 15 years after transplantation was 81.2%, 73.8%, and 70.3%, respectively. The overall survival rates when ALL and AML were excluded from the cohort were comparable (not shown). As shown in Table 2, the overall 5-year survival rate conditional on surviving 5, 10, and 15 years for the entire cohort, was 90.9%, 95.3%, and 99.3%, respectively. Table 2 also shows the overall 5-year survival rates by primary diagnosis.
Table 2.
Overall conditional survival in 2-year survivors after autologous BMT in childhood, by primary diagnosis
| Overall survival at each additional 5 y after BMT, y | Entire cohort | Primary diagnosis | ||||||
|---|---|---|---|---|---|---|---|---|
| ALL | AML | HL | NHL | Neuroblastoma | Other malignant disease* | |||
| N | % | % | % | % | % | % | % | |
| 2+ | 280 | 81.2 | 67.4 | 95.6 | 86.7 | 90.3 | 78.5 | 71.9 |
| 5+ | 207 | 90.9 | 89.7 | 95.4 | 88.7 | 96.3 | 89.4 | 88.4 |
| 10+ | 162 | 95.3 | 96.2 | 97.5 | 95.3 | 82.0 | 97.6 | 100.0 |
| 15+ | 196 | 99.3 | 100.0 | 100.0 | 96.6 | 100.0 | 100.0 | 100.0 |
Includes 25 Ewing sarcomas, 11 Wilms tumor, 9 CNS tumors, 4 desmoplastic small round cell tumors, 6 soft tissue sarcoma, 1 ovarian tumor, 1 hepatoblastoma, and 1 multiple myeloma.
Relative mortality
Table 3 summarizes the relative mortality in this population, using age-, sex-, and calendar-specific rates from the general US population. Overall, the cohort was at a 21.8-fold higher risk of late mortality (95% CI, 17.9-26.3), compared with the general population. When ALL and AML were excluded, the SMR of the cohort increased slightly (SMR = 25.7; 95% CI, 20.3-31.9). The SMR was significantly increased in all groups of primary diagnoses: ALL (SMR = 24.0), AML (SMR = 6.8), HL (SMR = 15.2), NHL (SMR = 13.0), and neuroblastoma (SMR = 52.6). Mortality rates continued to remain higher than the general population among those who had survived at least 10 years after transplantation (SMR = 20.6; 95% CI, 9.9-37.2) but were comparable to those of the general population among the 15-year survivors (SMR = 1.3; 95% CI, 0.5-2.8). The AER of the entire cohort was 19.4 all-cause deaths per 1000 person-years (95% CI, 15.5-23.4). Exclusion of ALL and AML from the cohort did not change the AER of the cohort appreciably (AER = 21.8; 95% CI, 16.7-26.9).
Table 3.
SMR and AER for all-cause late mortality among 2-year survivors of autologous BMT in childhood
| Variable | Entire cohort, | Number of all-cause deaths | Rate of all-cause death per 1000 person-years | ||||||
|---|---|---|---|---|---|---|---|---|---|
| no. of patients | Observed | Expected | SMR | 95% CI | Observed | Expected | AER | 95% CI | |
| All patients | 345 | 103 | 4.7 | 21.8 | 17.9-26.3 | 20.4 | 0.9 | 19.4 | 15.5-23.4 |
| Sex | |||||||||
| Male | 212 | 53 | 3.9 | 13.8 | 10.4-17.8 | 15.7 | 1.1 | 14.6 | 10.4-18.8 |
| Female | 133 | 50 | 0.9 | 57.9 | 43.3-75.4 | 29.6 | 0.5 | 29.0 | 20.9-37.2 |
| Age at BMT, y | |||||||||
| 0-4 | 97 | 28 | 0.8 | 36.4 | 24.5-51.6 | 19.6 | 0.5 | 19.1 | 11.8-26.3 |
| 5-9 | 57 | 20 | 0.7 | 29.2 | 18.2-43.9 | 20.9 | 0.7 | 20.2 | 11.0-29.3 |
| 10-14 | 55 | 19 | 0.7 | 26.2 | 16.1-39.7 | 24.7 | 0.9 | 23.8 | 12.7-34.9 |
| 15-21 | 136 | 36 | 2.5 | 14.2 | 10.1-19.4 | 18.9 | 1.3 | 17.6 | 11.4-23.7 |
| Year of BMT | |||||||||
| <1990 | 74 | 33 | 1.8 | 18.2 | 12.7-25.2 | 22.8 | 1.3 | 21.5 | 13.8-29.3 |
| 1990-1999 | 129 | 37 | 1.9 | 19.7 | 14.2-26.8 | 16.8 | 0.9 | 15.9 | 10.5-21.3 |
| 2000-2010 | 142 | 33 | 1.0 | 32.0 | 22.3-44.2 | 23.5 | 0.7 | 22.7 | 14.7-30.7 |
| Source of stem cells | |||||||||
| Bone marrow | 163 | 53 | 3.1 | 17.0 | 14.1-24.6 | 17.4 | 1.0 | 16.4 | 11.7-21.4 |
| PBSCs | 181 | 50 | 1.6 | 31.6 | 22.0-39.9 | 25.0 | 0.8 | 24.2 | 17.3-31.1 |
| Primary diagnosis | |||||||||
| ALL | 43 | 21 | 0.9 | 24.0 | 15.2-35.8 | 26.8 | 1.1 | 25.7 | 14.2-37.2 |
| AML | 45 | 6 | 0.9 | 6.8 | 2.7-13.8 | 6.5 | 1.0 | 5.5 | 0.3-10.7 |
| HL | 90 | 23 | 1.5 | 15.2 | 9.8-22.2 | 18.9 | 1.3 | 17.7 | 9.9-25.4 |
| NHL | 31 | 8 | 0.6 | 13.0 | 6.0-24.3 | 15.9 | 1.2 | 14.7 | 3.7-25.7 |
| Neuroblastoma | 79 | 25 | 0.5 | 52.6 | 24.6-76.0 | 24.2 | 0.5 | 23.8 | 14.3-33.3 |
| Other malignant disease* | 57 | 20 | 0.4 | 56.4 | 35.1-84.8 | 33.2 | 0.6 | 32.6 | 18.1-47.2 |
| Disease status at HCT | |||||||||
| Standard risk of relapse† | 133 | 39 | 2.5 | 15.4 | 11.1-20.8 | 17.1 | 1.1 | 16.0 | 10.6-21.4 |
| High risk of relapse | 212 | 64 | 2.2 | 29.2 | 22.6-37.0 | 23.0 | 0.8 | 22.2 | 16.6-27.9 |
| Overall survival after HCT, y | |||||||||
| 2-5 | 65 | 65 | 0.1 | 660.8 | 512.9-834.7 | 319.5 | 0.5 | 319.0 | 241.3-396.7 |
| 6-9 | 73 | 24 | 0.3 | 78.4 | 51.1-114.1 | 41.9 | 0.5 | 41.4 | 24.6-58.1 |
| 10-14 | 45 | 9 | 0.4 | 20.6 | 9.9-37.2 | 15.9 | 0.8 | 15.2 | 4.8-25.5 |
| 15+ | 162 | 5 | 3.9 | 1.3 | 0.5-2.8 | 1.3 | 1.0 | 0.3 | −0.9-1.5 |
Includes 25 Ewing sarcomas, 11 Wilms tumor, 9 CNS tumors, 4 desmoplastic small round cell tumors, 6 soft tissue sarcoma, 1 ovarian tumor, 1 hepatoblastoma, and 1 multiple myeloma.
Standard risk: first or second complete remission after acute leukemia or lymphoma; all others high risk.
Specific causes of death
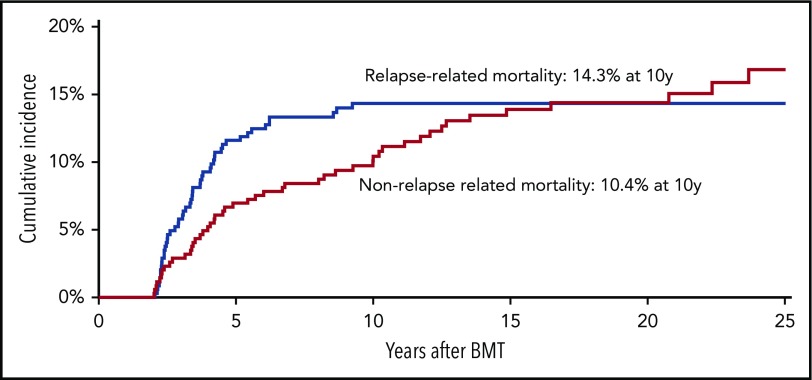
The leading causes of death included primary disease (50.0%), SNs (21.4%), and infection (18.4%) (supplemental Table 2). All 49 deaths because of primary disease occurred within the first 2 to 9 years after transplantation. Primary disease was the cause of death for 75%, 43%, 30%, 17%, and 0% of all deaths in patients with neuroblastoma, ALL, HL, AML, and NHL, respectively. Of the 18 patients who died because of infection, only 1 had primary disease registered as a secondary cause of death. In addition, of those who died because of infection, 12 died during the first 2- to 5-year period post-BMT and 4 after 5 to 9 years. The most common primary diagnoses among those who died because of infection were HL (N = 5), neuroblastoma (N = 4), ALL (N = 4), and NHL (N = 3). Specific SNs associated with SN-related deaths were leukemia (n = 6), brain tumors (n = 4), lymphoma (n = 3), breast cancer (1), and other miscellaneous SNs (n = 7). The 10-year cumulative incidence of RRM (14.3%) exceeded that of NRM (10.4%) (Figure 2). However, although the incidence of RRM plateaued at 10 years, that of NRM continued to climb, crossing the RRM incidence at 15 years from BMT.
Figure 2.
Cumulative RRM and NRM in 345 patients undergoing autologous BMT in childhood and surviving ≥2 years.
Predictors of late mortality
Table 4 summarizes the results from the multiple regression analysis of all-cause late mortality for the entire cohort. The hazard of all-cause late mortality was lower among those transplanted for AML (HR = 0.36; 95% CI, 0.13-1.01) but was higher among those transplanted for neuroblastoma (HR = 2.36; 95% CI, 0.98-5.71) when compared with patients transplanted for HL. All-cause late mortality was significantly higher among females (HR = 1.82; 95% CI, 1.19-2.78) when compared with males. Finally, all-cause mortality declined over the 3 decades. No significant difference in all-cause mortality was observed by race/ethnicity, age at transplantation, stem cell source, disease status at transplantation, or conditioning regimen.
Table 4.
Hazard of all-cause late mortality among 2-year survivors of autologous BMT in childhood, for the entire cohort and lymphoma and neuroblastoma survivors
| Variables | Multivariable analysis, entire cohort | Multivariable analysis, HL + NHL | Multivariable analysis, neuroblastoma | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HRadj | 95% CI | P | HRadj | 95% CI | P | HRadj | 95% CI | P | |
| Sex | |||||||||
| Male | 1.00 | 1.00 | 1.00 | ||||||
| Female | 1.82 | 1.19-2.78 | .006 | 1.69 | 0.76-3.85 | .2 | 3.33 | 1.35-8.33 | .008 |
| Race/ethnicity | |||||||||
| Non-Hispanic white | 1.00 | 1.00 | 1.00 | ||||||
| Hispanic | 1.08 | 0.59-1.99 | .8 | 1.85 | 0.67-5.10 | .2 | 0.77 | 0.21-2.76 | .7 |
| Non-Hispanic black | 1.37 | 0.58-3.26 | .5 | 1.89 | 0.47-7.71 | .4 | — | ||
| Other | 0.38 | 0.05-2.76 | .3 | — | 9.66 | 0.89-105.0 | .06 | ||
| Unknown | 0.51 | 0.08-3.69 | .5 | — | — | ||||
| Age at BMT* | 1.03 | 0.98-1.07 | .3 | 0.96 | 0.89-1.04 | .3 | 1.16 | 1.08-1.25 | <.001 |
| Year of BMT | |||||||||
| <1990 | 1.00 | 1.00 | 1.00 | ||||||
| 1990-1999 | 0.47 | 0.26-0.86 | .01 | 0.82 | 0.30-2.24 | .7 | 0.24 | 0.05-1.32 | .1 |
| 2000-2010 | 0.33 | 0.15-0.73 | .006 | 0.15 | 0.04-0.61 | .007 | 1.15 | 0.17-7.62 | .9 |
| Source of stem cells | |||||||||
| Bone marrow | 1.00 | 1.00 | 1.00 | ||||||
| PBSCs/cord blood | 1.29 | 0.72 2.30 | .4 | 1.12 | 0.44-2.85 | .9 | 0.95 | 0.14-6.60 | 1.0 |
| Primary diagnosis | |||||||||
| HL | 1.00 | 1.00 | |||||||
| NHL | 1.15 | 0.44-3.01 | .8 | 1.39 | 0.43-4.49 | .6 | |||
| ALL | 2.40 | 0.91-6.32 | .08 | ||||||
| AML | 0.36 | 0.13-1.01 | .05 | ||||||
| Neuroblastoma | 2.36 | 0.98-5.71 | .06 | ||||||
| Other malignant disease† | 2.29 | 1.06-4.92 | .03 | ||||||
| Disease status at BMT | |||||||||
| Standard risk of relapse‡ | 1.00 | 1.00 | |||||||
| High risk of relapse | 1.49 | 0.82-2.74 | .2 | 1.53 | 0.68-3.46 | .3 | |||
| Conditioning regimen | |||||||||
| TBI | |||||||||
| No | 1.00 | 1.00 | 1.00 | ||||||
| Yes | 0.58 | 0.29-1.16 | .1 | 0.34 | 0.11-1.03 | .06 | 1.39 | 0.23-8.63 | .7 |
HRadj, hazard ratio, adjusted for all given variables in table.
Age included in the model as a continuous variable.
Includes 25 Ewing sarcomas, 11 Wilms tumor, 9 CNS tumors, 4 desmoplastic small round cell tumors, 6 soft tissue sarcoma, 1 ovarian tumor, 1 hepatoblastoma, and 1 multiple myeloma.
Standard risk: first or second complete remission after acute leukemia or lymphoma; all others high risk.
Late mortality by treatment era
The all-cause 10-year cumulative mortality rate declined over the 3 eras (<1990, 35.1%; 1990-1999, 25.6%; 2000-2010, 21.8%; P = .05) (Figure 1B). Adjusting for demographic and clinical variables, as well as conditioning regimen, the HR of all-cause mortality declined in the more recent treatment eras (<1990 [referent group]; 1990-1999 [HR = 0.47; 95% CI, 0.26-0.86; P = .01]; 2000-2010 [HR = 0.33; 95% CI, 0.15-0.73; P = .006; Ptrend ≤ .0001) (Table 4). The HR of all-cause mortality by treatment era for the more prevalent primary cancers is summarized in Table 4. Among lymphoma patients, the all-cause mortality declined over time, with a HR of 0.15 (95% CI, 0.04-0.61) among those transplanted 2000 to 2010, compared with those transplanted <1990. There was no difference in the hazard of all-cause mortality over time among neuroblastoma patients.
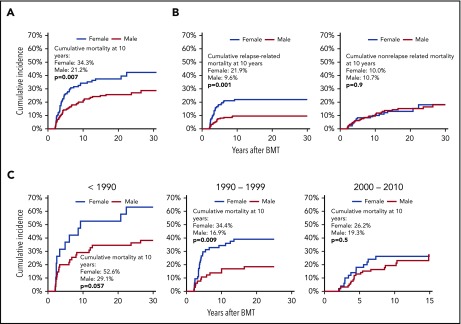
Late mortality by sex
The cumulative incidence of all-cause mortality among female survivors exceeded that among male survivors (Figure 3A); this relation was seen throughout follow-up (P = .007). The SMR was higher among females than males (SMR = 57.9; 95% CI, 43.3-75.4 vs SMR = 13.8; 95% CI, 10.4-17.8; P ≤ .0001), as was the AER (AER = 29.0; 95% CI, 20.9-37.2 vs AER = 14.6; 95% CI, 10.4-18.8) (Table 3). In the multivariable regression analysis, after adjusting for demographic and clinical variables, as well as conditioning regimen, the hazard of all-cause mortality was higher among females than males (HR = 1.82; 95% CI, 1.19-2.78) (Table 4). The HR of all-cause mortality was higher among females than males among the neuroblastoma patients (HR = 3.33; 95% CI, 1.35-8.33) (Table 4). The HR of all-cause mortality was 1.7-fold higher in female lymphoma patients when compared with male lymphoma patients, but this difference did not reach statistical significance (P = .2). Figure 3C shows the cumulative incidence of all-cause mortality by sex over the 3 treatment eras. The cumulative incidence of all-cause late mortality of females exceeded that of males among those treated before 1990 and 1990 to 1999, whereas the difference decreased in the most recent transplant era, with a nonsignificant difference at 10 years after transplantation.
Figure 3.
Late mortality in 345 2-year survivors of autologous BMT in childhood, by sex. (A) Cumulative mortality in 2-year survivors of autologous BMT in childhood, by sex. (B) Cumulative relapse-related (left panel) and nonrelapse related (right panel) mortality in 2-year survivors of autologous BMT in childhood, by sex. (C) Cumulative all-cause mortality in 2-year survivors of autologous BMT in childhood, by sex and treatment era.
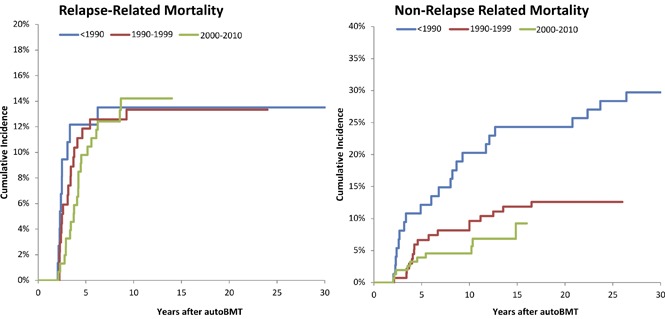
The 10-year cumulative incidence of RRM was higher among females than males (females, 21.9%; males, 9.6%; P = .001), whereas that of NRM was similar among the sexes (females, 10.0%; males, 10.7%; P = .9) (Figure 3B). The proportion of deaths because of primary disease was higher among females as compared with males across all primary diagnoses. The cumulative incidence by gender and treatment era for RRM was similar to that of all-cause late mortality, with a cumulative incidence of RRM of females exceeding that of males among those transplanted 1990 and 1990 to 1999, and a noticeably decreased difference in the most recent transplant era, with a nonsignificant difference at 10 years post-BMT (supplemental Figure 1).
Discussion
Findings from this study of late mortality after autologous BMT performed in childhood, the largest of its kind to date, show that the late mortality rates of childhood autologous BMT recipients for ALL, AML, HL, NHL, and neuroblastoma are significantly higher than those of the general population. Relapse is the leading cause of death during the first 10 years after transplantation. Importantly, there has been a significant decline in all-cause mortality in children undergoing autologous BMT over the past 3 decades.
The present study demonstrates that patients who survive 2 or more years after autologous BMT performed before the age of 22 years have an overall survival of 81% and 74%, 5 and 10 years after transplantation, respectively. These numbers are slightly higher than the corresponding overall survival rates of 76% and 71%, respectively, among 2-year survivors of autologous BMT in childhood reported by Schecher et al.4 The difference in distribution of primary diagnoses in the 2 cohorts may contribute to the discrepancy in survival rates, where the study by Schechter et al included a higher proportion of patients with neuroblastoma and CNS tumors and a lower proportion with ALL and lymphomas.
The current study also demonstrates that this cohort was at a 22-fold increased risk of late mortality compared with the general population. The SMR of the 251 2-year survivors of autologous BMT reported by Schechter et al was 733.4 In the latter cohort, 39% had a primary diagnosis of neuroblastoma, and 40% of these suffered a late death. In the current study, patients with neuroblastoma (23% of the entire cohort) had the lowest overall 5- and 10-year survival rate apart from ALL survivors (results not shown) and the highest SMR. The lack of relative mortality rates by childhood cancer type in the study by Schechter et al prevent further comparisons regarding the potential influence of differences in distribution of primary diagnoses on the mortality rates. Notably, the relative mortality in the present cohort changed only minimally when ALL and AML, cancer types not treated with autologous BMT anymore, were excluded from the analyses.
In the current report, the cumulative mortality among females exceeded that of males throughout follow-up, and the SMR and the AER were higher among females. Likewise, the multivariable analysis showed that females had almost twice the risk of all-cause late mortality compared with males. Our results are in contrast to those reported by Schechter et al, in which male survivors were 1.8 times more likely to die than females.4 In concordance with the present study, the SMR for females was almost twice that of males in the study by Ashton et al, although that study was restricted to adult autologous BMT recipients.1
Our study shows that the increased risk of late mortality among females compared with males was mainly driven by an increased risk of RRM. The proportion of deaths because of primary disease was higher among females as compared with males across all primary diagnoses. Furthermore, the cumulative incidence of RRM in females treated before 2000 exceeded that of males, whereas the sex difference decreased markedly during the most recent treatment period, with a nonsignificant difference at 10 years post-BMT. The reason for the higher incidence of RRM among females from the earlier eras is unclear; we were unable to explore this in greater detail because of the relatively small number.
The present study demonstrates that relapse of the primary disease is the leading cause of death during the first 10 years after transplantation and causes half of all late deaths. In the study by Schechter et al, 57 of the 64 patients who suffered a late death after autologous BMT died as a result of relapse.4 Also, in cohorts including adult autologous BMT recipients, relapse is the leading cause of death.1-3 In contrast to the present study, in which all late deaths because of relapse occurred within the first decade after transplantation, relapse remained the most frequent cause of death 10 or more years after autologous BMT in a cohort of adult autologous recipients, although the proportion of deaths from nonrelapse causes increased over time.1 Our findings, supported by the findings of others, show that prevention of disease recurrence remains one of the greatest challenges for autologous BMT recipients. In our study, infection was a major cause of death, even without a primary disease concurrently leading to death. The large majority of deaths because of infection among the 2-year survivors occurred during the first 5 years post-BMT. Thus, a high degree of suspicion of an infection, as well as an aggressive management plan for infections, is important, in addition to strategies for preventing deaths from relapse such as transplantation earlier in the disease course and in those who are in complete remission, modification of preparative regimens and maintenance therapies, as well as monitoring and early intervention for recurrent or residual disease. Moreover, the rates of nonrelapse deaths because of the emergence of SNs (leukemia, brain tumors, lymphoma), as well as cardiac and pulmonary diseases, add substantially to the long-term health issues faced by survivors of autologous BMT. Therefore, the results of the current report have important implications for the long-term care after autologous BMT performed in childhood, showing the need for life-long follow-up with risk-based surveillance for early detection of complications. In addition to emphasizing this, the findings of the present study of the role of infection for late mortality should be incorporated in long-term follow-up guidelines after autologous BMT performed in childhood.9
The results from our study need to be interpreted with some caution. Our study is based on causes of death registered on death certificates. Because the cause(s) of death on the death certificates are not always perfect, some degree of misclassification is inherent in our study.3,10,11 Striving to overcome this bias, 3 coauthors reviewed the causes of death recorded on the death certificates. Although treatment era can be used as a surrogate for changes in treatment protocols and supportive care over time, we were unable to fully assess mortality risk associated with particular types of cancer treatment or changes in supportive care practices, because of the lack of this information. Although observing an encouraging decline in all-cause late mortality over time, we were not able to dissect this further by cause-specific mortality because of the relatively small numbers. In addition, the number of cases was only large enough to examine lymphomas and neuroblastoma separately in the multivariable analysis. Furthermore, when interpreting the findings of the present study, it is important to keep in mind that it is restricted to 2-year survivors, why a large share of patients undergoing autologous BMT for tumors of CNS, bone and soft tissue are likely not included in the present cohort, owing to the high mortality rates because of recurrent disease in the first 2 years post-BMT in these patients.12,13 These limitations notwithstanding, the current investigation describes the late mortality experience of a large cohort of patients undergoing autologous BMT in childhood followed for a median of 14 years. In addition, vital status was ascertained using a variety of resources such as the National Death Index Plus, medical records, and the Accurint database, ensuring complete and comprehensive follow-up of all patients in the cohort. Furthermore, detailed clinical data, including conditioning regimen, were available for all patients. Finally, inclusion of patients transplanted between 1980 and 2010 allowed us to evaluate trends in late mortality for patients transplanted over a period of 3 decades.
In summary, this study demonstrates that 2-year survivors of autologous BMT performed in childhood have a substantially increased risk of late mortality compared with the general population, with females being at a particularly increased risk. Relapse is the leading cause of death; however, while RRM plateaus with time, NRM becomes the major cause of death after 10 years from transplantation. Nonetheless, there has been a significant decline in all-cause mortality in children undergoing autologous BMT over the past 3 decades.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
This work was supported in parts by grants from the National Cancer Institute, National Institutes of Health (R01 CA078938), the Leukemia Lymphoma Society (R6502-16), and the Swedish Childhood Cancer Foundation (TJ2016-0014).
Footnotes
Presented in part at the 59th annual meeting of the American Society of Hematology, Atlanta, GA, 10 December 2017.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.S.H., Y.C., J.W., R.B., D.S., J.F.W., J.R., S.J.F., D.J.W., S.H.A., M.A., and S.B. contributed to study design; A.S.H., J.W., K.B., L.F., L.H., M.K., E.N., M.P., S.H.A., M.A., and S.B. contributed to data collection; A.S.H., Y.C., R.B., S.H.A., M.A., and S.B. contributed to analyses and interpretation of results; A.S.H. and S.B. wrote the manuscript; and all authors critically revised the manuscript for important intellectual content and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Smita Bhatia, Institute for Cancer Outcomes and Survivorship, Department of Pediatrics, School of Medicine, University of Alabama at Birmingham, 1600 7th Ave South, Lowder 500, Birmingham, AL 35233; e-mail: sbhatia@peds.uab.edu.
References
- 1.Ashton LJ, Le Marsney RE, Dodds AJ, et al. A population-based cohort study of late mortality in adult autologous hematopoietic stem cell transplant recipients in Australia. Biol Blood Marrow Transplant. 2014;20(7):937-945. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia S, Robison LL, Francisco L, et al. Late mortality in survivors of autologous hematopoietic-cell transplantation: report from the Bone Marrow Transplant Survivor Study. Blood. 2005;105(11):4215-4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin PJ, Counts GW Jr, Appelbaum FR, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol. 2010;28(6):1011-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schechter T, Pole JD, Darmawikarta D, et al. Late mortality after hematopoietic SCT for a childhood malignancy. Bone Marrow Transplant. 2013;48(10):1291-1295. [DOI] [PubMed] [Google Scholar]
- 5.National Death Index. Vol. 2018. https://www.cdc.gov/nchs/ndi/index.htm. Accessed 19 January 2018.
- 6.Accurint databases. Vol. 2018. http://www.accurint.com. Accessed 19 January 2018.
- 7.Centers for Disease Control and Prevention. https://wonder.cdc.gov/mortSQL.html. Accessed 19 January 2018.
- 8.Vandenbroucke JP. A shortcut method for calculating the 95 per cent confidence interval of the standardized mortality ratio. Am J Epidemiol. 1982;115(2):303-304. [Google Scholar]
- 9.Chow EJ, Anderson L, Baker KS, et al. Late effects surveillance recommendations among survivors of childhood hematopoietic cell transplantation: a children’s oncology group report. Biol Blood Marrow Transplant. 2016;22(5):782-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Möller TR, Garwicz S, Barlow L, et al. ; Nordic Society for Pediatric Hematology and Oncology. Decreasing late mortality among five-year survivors of cancer in childhood and adolescence: a population-based study in the Nordic countries. J Clin Oncol. 2001;19(13):3173-3181. [DOI] [PubMed] [Google Scholar]
- 11.Smith Sehdev AE, Hutchins GM. Problems with proper completion and accuracy of the cause-of-death statement. Arch Intern Med. 2001;161(2):277-284. [DOI] [PubMed] [Google Scholar]
- 12.Marachelian A, Butturini A, Finlay J. Myeloablative chemotherapy with autologous hematopoietic progenitor cell rescue for childhood central nervous system tumors. Bone Marrow Transplant. 2008;41(2):167-172. [DOI] [PubMed] [Google Scholar]
- 13.Gardner SL, Carreras J, Boudreau C, et al. Myeloablative therapy with autologous stem cell rescue for patients with Ewing sarcoma. Bone Marrow Transplant. 2008;41(10):867-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.