Abstract
Immune therapies are fast becoming paradigm-changing treatment options for patients with hematologic cancers. The field has grown exponentially as it expands to nonmalignant blood diseases. This Perspective article introduces the review series describing some of the latest advances in this field and highlighting some of the current obstacles and new opportunities for the future. Specifically, the series provides in-depth discussion on a selection of emerging immunotherapies now available to patients for hematologic diseases, including cancer vaccines, chimeric antigen receptor T cells, and immunotherapies to regulate inflammation in nonmalignant blood disorders.
Catherine M. Bollard, MBChB, MD, and Leslie S. Kean, MD, PhD, share highlights of this review series.
Cancer immunotherapies, which rely on harnessing a patient’s immune system to kill malignant cells, are booming as a treatment option for patients with hematologic malignancies. The field has grown exponentially since Science designated cancer immunotherapy, including checkpoint inhibitors and chimeric antigen receptor T cells (CARTs), as “breakthrough therapy” of the year for 2013.1 At the time, some feared that Science risked hyping an approach that had shown efficacy in only a tiny fraction of patients. However, this proved unwarranted, because hundreds of patients have now been enrolled in immunotherapy trials. Phase 1 and 2 clinical trials have led to the approval of several new immunotherapies by the US Food and Drug Administration (FDA) (reviewed in the October 2017 “Focus on immunotherapy” special issue from ASH Clinical News [available at www.ashclinicalnews.org/special-issues]). Even more exciting, in 2017, the FDA approved the first 2 CD19 CART products as live drugs.2,3 Currently, not all medical centers have access to these cell-based treatments because they are still restricted to centers able to manage the life-threatening toxicities associated with CART cell therapy, such as the cytokine release syndrome and neurotoxicity. However, the possibilities seem limitless, and it appears likely that they will soon be broadly used.2 Furthermore, several other products are currently under review by the FDA, and other promising new therapies are being developed.
Why such enthusiasm, and how do these therapies work? Immunotherapies have now shown efficacy in hundreds of patients with hematological malignancies refractory to conventional therapies, including some targeted therapies. The efficacy is in part related to the mechanism of action, which is different from the previous classes of treatments patients have generally received.4
Immunotherapies use the patients’ own immune cells to kill cancer cells in a more targeted way as compared with chemotherapies that generally kill fast proliferating cells, including tumor cells as well as healthy bystander cells, such as normal hematopoietic stem cells, intestinal, skin and hair stem cells, sperm, and eggs. Similarly, immune-based therapies can provide highly tumor-specific cytolytic activity while limiting normal tissue damage. The principle of action is simple: the immune killer cell, often a cytotoxic T cell (CTL), will kill cancer cells, infected cells (particularly with viruses), or cells that are damaged in other ways. CTLs recognize a specific epitope through a T-cell receptor (TCR) and class I major histocompatibility recognition or via a surface antigen on the tumor (eg, CAR-engineered T cells). Upon recognition of their epitope/antigen, degranulation occurs, with release of granules containing cytolytic molecules such as granzymes and perforin. Moreover, other immune cells such as natural killer (NK) cells, invariant NK T cells, γδ T cells, and immune cells engineered with natural or synthetic receptors also have antitumor potency.
Checkpoint molecules such as cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed death-1 (PD-1) and its ligand PDL-1, work by putting the brakes on CTLs, preventing them3 from attacking tumor cells. To overcome this potent tumor-induced immune evasion mechanism, checkpoint inhibitors block these molecules, thereby unleashing the immune response.
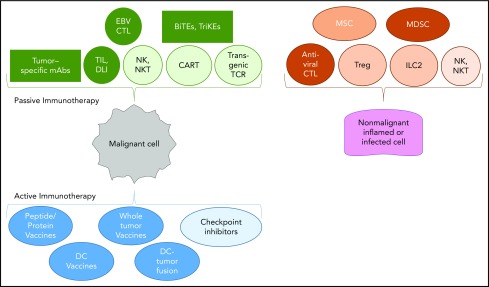
The invited reviews in the review series focus on 4 key areas of interest in the field. These were selected from an increasing panoply of immune-based therapies now available to patients, including nonengineered T cells, transgenic TCR-modified T cells, antibody-based treatments, NK cells, bi- and tri-specific T-cell engagers, and checkpoint inhibitors (Figure 1).
Figure 1.
Panoply of immunotherapies for malignant and nonmalignant hematological diseases. Passive immunotherapies consist of molecules from ex vivo activated cells that, following infusion or adoptive transfer, compensate for deficient immune functions. Active immunotherapies stimulate the patient’s immune system via a vaccine or inhibit an important checkpoint of T-cell activation. Antiviral CTLs, EBV (Epstein-Barr virus), CMV (cytomegalovirus), adenovirus, HHV6 (human herpesvirus 6), cytotoxic T cells; BiTEs and TriKEs, bi- and tri-specific T-cell engagers; DC, dendritic cells; DLI, donor lymphocyte injection following allogeneic hematopoietic cell transplantation; EBV CTL, nonengineered Epstein-Barr virus–specific cytotoxic T lymphocyte used to treat EBV+ lymphomas; ILC2, innate lymphocyte 2; mAbs, monoclonal antibodies; MDSCs, myeloid-derived suppressive cells; MSCs, mesenchymal stem cells; NKT, natural killer T cells; TIL, tumor-infiltrating T cells that have been extracted from the autologous tumor and expanded in IL-2; Treg, regulatory T cell.
CARTs, from the FDA-approved CD19 CARTs to the new generation of CARTs with new targets
The initial CART studies for hematological malignancies were developed targeting CD20 and then CD19.1,2 Currently, CD19, CD20, CD22, or now dual B targeting CARTs are available to treat acute lymphoblastic leukemia, non-Hodgkin lymphoma, and chronic lymphocytic leukemia.3-5 Development of CARTs for other hematological malignancies has been more challenging due to the target antigens being expressed on vital normal cells such as CD7 being expressed on T-leukemic cells but also normal T cells or CD33 and CD123 being expressed on acute myeloid leukemia cells but also all other myeloid cells, leading potentially to a full myeloablation requiring hematopoietic stem cell transplantation.6 However, more CART studies have moved to the clinic (eg, CD5 CART)7 and are currently in the clinic for patients with T-cell lymphoma (NCT #03081910), and alternative approaches and other targets are currently being developed and are likely to be available soon. CD30 CARTs are currently being evaluated clinically for the treatment of Hodgkin lymphoma and anaplastic large-cell lymphoma.8,9 Multiple myeloma can be treated with NY-ESO-1 TCR engineered T cells, or B-cell maturation antigen CARTs.10,11
Despite amazing disease responses, tumor-induced immune escape due to antigen loss is still a concern with CART therapy, and we do not know yet the impact of CARTs as a first-line treatment regimen. Thus, it is crucial to propose randomized trials to understand whether CARTs will be able to replace standard of care, which for acute lymphoblastic leukemia is a combination of high-dose chemotherapy that typically lasts 2 years or, in the case of relapsed/refractory disease, allogeneic hematopoietic stem cell transplantation. Other important questions have to be answered in the next decade. Among them is whether the CART treatment costs will be sustainable. In addition, the processing of CARTs is still relatively labor intensive because each product requires patient’s autologous T cells. Therefore, there is a push to create “off-the-shelf” T-cell therapy products.
CART toxicities
Besides the expected on-target effects, there are 2 major toxicities that still limit the access of CD19-CART treatments to specialized centers. The cytokine release syndrome mainly driven by interleukin-6 (IL-6) generally occurs in the first few days after infusion and corresponds to T-cell activation and proliferation in a lymphodepleted individual. Blockade of IL-6 with the anti–IL-6 receptor monoclonal antibody tocilizumab reduces the symptoms without inhibiting the antitumoral effects.12 More problematic and currently without a definitive medical intervention is the neurotoxicity induced by CARTs, the biology of which is less clear but involves endothelial activation and T-cell infiltration of both CARTs and non-CARTs in the brain, resulting in panencephalitis.13,14 Preemptive use of biomarkers (before 36 hours postinfusion), such as IL-6, monocyte chemoattractant protein, IL-1RA, etc, will allow for risk stratification and facilitate management and symptomatic treatment.
Vaccines
In the context of antitumor therapies, the term “vaccine” is used for methods that enhance the presentation of tumor-associated epitopes to the patient’s immune system with the goal of activating the immune system against these epitopes to treat existing malignancies. Well-studied tumor-associated antigens in hematological malignancies are Wilms tumor 1, preferentially expressed antigen in melanoma, survivin, and proteinase-3 (particularly PR1, which is HLA-A2 restricted) in acute myeloid leukemia along with CD138 and B-cell maturation antigen in myeloma. Several types of vaccines are possible depending on the source of antigens: (1) classical vaccine with known peptides or proteins derived from tumor-associated antigens, (2) personalized vaccines based on patient neoantigens that can be identified by a combination of deep sequencing of the patient’s tumor whole genome and computational algorithms, (3) vaccines with whole tumor cells as a source of antigen, (4) vaccines aided by ex vivo generated dendritic cells, which are professional antigen-presenting cells, (5) vaccines achieved by dendritic cell/tumor fusion in acute myeloid leukemia, and (6) vaccines with whole tumor cells genetically modified to secrete an immune stimulatory cytokine, granulocyte-macrophage colony-stimulating factor, and then irradiated (GVAX).
Immunotherapies to regulate inflammation in nonmalignant hematological diseases
Interestingly, immune cells can also be used as a therapeutic tool to regulate the unwanted aberrant immune reaction in nonmalignant diseases as, for example, in graft-versus-host disease. Several subsets of immune cells have been explored with regulatory T cells, and mesenchymal stem cells have already been used in the clinic for several years. The primary end point was successfully achieved in a recent phase 3 cell therapy trial for acute graft-versus-host disease (NCT #02336230).15
Review series articles
We are therefore pleased to introduce the following series of reviews describing the latest advances in these areas and highlighting some of the current obstacles and new opportunities for the near future:
Alexander I. Salter, Margot J. Pont, and Stanley R. Riddell, “Chimeric antigen receptor–modified T cells: CD19 and the road beyond”
Leslie S. Kean, “Defining success with cellular therapeutics: the current landscape for clinical end point and toxicity analysis”
David Avigan and Jacalyn Rosenblatt, “Vaccine therapy in hematologic malignancies”
Bruce R. Blazar, Kelli P. A. MacDonald, and Geoffrey R. Hill, “Immune regulatory cell infusion for graft-versus-host disease prevention and therapy”
We believe the review series on Emerging Immunotherapies for Hematologic Diseases will be of value to a large Blood audience because discoveries in this field have been accelerating over recent years, and they have changed the treatment landscape for almost all hematological diseases from malignant to benign.
Authorship
Contribution: S.P., S.Z.P., and C.M.B. conceived and wrote the paper.
Conflict-of-interest disclosure: S.P. is an inventor on a patent on “Methods of detection of graft-versus-host disease” (13/573766). C.M.B. serves on the Scientific Advisory Boards for Cellectis, Torque, and Neximmune. S.Z.P. declares no competing financial interests.
Correspondence: Catherine M. Bollard, Center for Cancer and Immunology Research, Children’s National Health System, The George Washington University, 111 Michigan Ave, NW, Washington, DC 20010; e-mail: cbollard@cnmc.org.
REFERENCES
- 1.Till BG, Jensen MC, Wang J, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112(6):2261-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kochenderfer JN, Yu Z, Frasheri D, Restifo NP, Rosenberg SA. Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood. 2010;116(19):3875-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turtle CJ, Hay KA, Hanafi LA, et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol. 2017;35(26):3010-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gill S, Tasian SK, Ruella M, et al. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood. 2014;123(15):2343-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mamonkin M, Rouce RH, Tashiro H, Brenner MK. A T-cell-directed chimeric antigen receptor for the selective treatment of T-cell malignancies. Blood. 2015;126(8):983-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang CM, Wu ZQ, Wang Y, et al. Autologous T cells expressing CD30 chimeric antigen receptors for relapsed or refractory hodgkin lymphoma: an open-label phase I trial. Clin Cancer Res. 2017;23(5):1156-1166. [DOI] [PubMed] [Google Scholar]
- 9.Ramos CA, Ballard B, Zhang H, et al. Clinical and immunological responses after CD30-specific chimeric antigen receptor-redirected lymphocytes. J Clin Invest. 2017;127(9):3462-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rapoport AP, Stadtmauer EA, Binder-Scholl GK, et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med. 2015;21(8):914-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali SA, Shi V, Maric I, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128(13):1688-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127(26):3321-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gust J, Hay KA, Hanafi LA, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7(12):1404-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taraseviciute A, Tkachev V, Ponce R, et al. Chimeric antigen receptor T cell-mediated neurotoxicity in non-human primates [published online ahead of print 21 March 2018]. Cancer Discov. doi:10.1158/2159-8290.CD-17-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaudhury SNE, Mahadeo KM, Prockop SE, et al. A phase 3 single-arm, prospective study of remestemcel-L, ex-vivo cultured adult human mesenchymal stromal cells, for the treatment of steroid refractory acute gvhd in pediatric patients. BMT Tandem Meetings; Salt Lake City, UT; 2018. [Google Scholar]