Abstract
Background
Non‐melanoma skin cancer (NMSC) and actinic keratosis (AK) are very common among fair‐skinned individuals. A disease continuum from AK to squamous cell carcinoma (SCC) has been frequently postulated. AK and NMSC may influence quality of life (QL) of patients, and it can be suspected that disease progression entails a QL reduction. The purpose of this study was to document QL in patients with NMSC and AK using the health‐outcome questionnaire EQ‐5D‐5L.
Methods
The study was designed as a non‐interventional, prospective, cross‐sectional study. Patients with AK, SCC, basal cell carcinoma (BCC) or multiple diagnoses were enrolled in this study in 29 dermatological centres across Germany. Patients were asked to complete the EQ‐5D‐5L (compromising EQ Index and EQ VAS), and the dermatologists provided diagnosis, disease history and treatment data.
Results
A total of 1184 patients were enrolled and diagnosed as follows: 73% AK, 49% BCC and 17% SCC. 66% had a single diagnosis, 28% two different diagnoses and 6% three different diagnoses. QL was strongly associated with patients’ diagnosis. Patients with a single AK diagnosis had significantly higher mean EQ VAS (78) than patients with BCC (74), SCC (72), and BCC plus SCC (69), P < 0.050. When the effects of disease progression were calculated, patients with AK plus SCC reported significantly less mean EQ VAS (71) than patients with a single AK diagnosis (78), P < 0.011.
Conclusions
While rarely being imminently life‐threatening, NMSC and AK have an impact on QL as quantified by the EQ‐5D‐5L. This impact is associated with diagnosis (AK vs. NMSC) and clinical progression (AK vs. AK plus SCC). Both lead to a clear decline in QL. This shows that disease progression is perceived and judged as detrimental by patients and that AK and NMSC should be diligently treated to preserve and restore QL.
Introduction
Non‐melanoma skin cancer (NMSC) is a collective term that describes several forms of cutaneous neoplasia that do not stem from melanocytes. Among these neoplasia, squamous cell carcinoma (SCC) and basal cell carcinoma (BCC) are the most common forms that are considered the most common malignancies in the Western world.1 Thus, these cancers represent a major healthcare problem due to the large group of affected patients. In Germany, the incidence was 119 of 100 000 for women and 145 of 100 000 for men in a recent screening period, which makes NMSC 6.5 times more common than malignant melanoma.2 Among the affected patients, 53% are male, and the age‐standardised incidence is as high as 108.2 per 100 000 (European standard) and thus in the incidence range of prostate cancer.3 In women, the incidence rate is 77.8 per 100 000 (European standard), which ranks between breast cancer (123.8 per 100 000) and colorectal cancer (38.0 per 100 000).3 Identified risk factors for the development of NMSC are age (strong increase in male patients over 60), fair skin phototype (Fitzpatrick I and II) and (cumulative) UV‐exposure.
A further risk factor, particularly for SCC, is the presence of actinic keratosis (AK). These epidermal neoplastic lesions have been described as epidermal carcinoma‐in‐situ.4, 5 A large cohort analysis revealed that 65% of all primary SCCs occurred in lesions previously clinically diagnosed as AKs.6 Thus, it is reasonable to include AK in the wider scope of NMSC, whenever prognostic or epidemiological considerations are made. AK is, just as both SCC and BCC, an ultraviolet‐light‐induced lesion of the skin that may progress to invasive carcinoma.7, 8 It is the most common lesion with malignant potential. AK is mostly seen in Caucasians on skin areas of long‐term sun exposure.9 Epidemiological data show a high occurrence rate of AK, which is even higher in regions with higher ultraviolet exposure. For the United States, the prevalence ranges from 11% to 26%,9 while in Australia, it ranges from 40% to 60%.10 In Europe, a prevalence of 15% in men and 6% in women has been documented.11 Over the age of 70 years, 34% of men and 18% of women were found to have AK.11 While this represents a massive disease burden on society, NMSC and AK tend to involve lower morbidity and mortality than malignant melanoma12 and can be treated or managed successfully in many cases. Still, treatment of BCC and SCC routinely involves surgery and –given the predominant occurrence of these cancers in sun‐exposed areas such as the face and scalp –surgical measures can be gruelling, stressful and cosmetically unfavourable for the patients.13
The numerous therapeutic options available for the treatment of AK14 are regarded as a valuable approach to SCC prevention.15 There is reason to assume that NMSC goes along with a reduction in health‐related quality of life (QL), and various studies have tried to quantify this reduction with various patient‐reported outcome measures (PROMs).13 Still, no robust data set is available for German‐speaking countries. So far, some groups have used standard dermatological instruments such as DLQI16, 17, 18 or Skindex‐16,19, 20 others developed specialised instruments for patients with NMSC21 and AK22, 23 and validated them in different populations.24, 25 While such an approach may be useful for assessing the disease burden of individual NMSC subtypes, AK and NMSC represent a clinical continuum. It is thus worthwhile investigating, how QL is affected in a cross‐sectional selection of patients with different stages of epidermal neoplasia in the sense of disease progression. Additionally, in their recent structured review on PROMs, authors from the United Kingdom expressed the need to analyse NMSC QL using a common standardised instrument and recommended the implementation of EQ‐5D in this disease spectrum.26, 27 The EQ Index in particular represents a very robust outcome measure28 that can also be implemented in the calculation of quality‐adjusted life years (QALYs) in future health economic evaluations of NMSC treatments.29
This study included patients with NMSC and AK and was designed to elucidate whether the proposed disease continuum is also accompanied by impairment in QL.
Methods
Study design
This prospective, cross‐sectional, German‐wide, multicentre study was carried out in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the University of Regensburg (Institutional Review Board Number 15‐160‐0036). Between October 2015 and February 2016, 1194 consecutive patients with NMSC and AK treated at 29 local medical practices or hospitals were recruited. Inclusion criteria were as follows: age ≥18 years, current diagnosis of NMSC (BCC or SCC) and/or AK, and informed consent. Ten patients did not meet the inclusion criteria and were excluded from the analyses, which resulted in a total number of 1184 patients.
Documentation and procedure
Patients filled in the EQ‐5D‐5L (EuroQol 5 dimensions 5 levels) to report their degree of QL. The EQ‐5D‐5L is a reliable and valid tool used in patient and general population groups in various countries.30, 31 The questionnaire consists of a descriptive system including five dimensions (mobility, self‐care, usual activities, pain and anxiety), each rated on five levels (no problem, slight problems, moderate problems, severe problems and extreme problems), and a visual analogue scale (VAS) assessing the current health status on a scale ranging from 0 (worst) to 100 (best).32 The developers of the EQ‐5D‐5L emphasise the need to use both parts of the questionnaire.32 According to the guidelines of the EuroQol Group,32 the five dimensions were transformed into individual health status profiles (range = 11111 [best] to 55555 [worst]) and then converted into German‐specific EQ Indices (range = −0.21 [worst] to 1.00 [best]) based on time trade‐off (TTO) valuation technique. Each possible health status profile will be weighted differently based on country‐specific preference values of the general population. Country‐specific value sets make the EQ Indices comparable across countries and allow for detecting differences due to social–cultural values and economic systems.30, 31, 33 This one‐dimensional utility index can be used to calculate QALYs in subsequent health economic analyses.
Patients were asked to provide information regarding basic demographic variables (age, sex, marital status and number of children, educational level and current professional activity). Educational level was classified as low, medium or high according to the International Standard Classification of Education (ISCED).34
Clinicians in charge of treating patients provided information on diagnosis, number of lesions, treatment period (in months) and current and past treatments. The treatments were recorded as follows: physical (excision, excision with histographic control of resection margin, curettage and/or electrodesiccation, laser therapy, radiotherapy and cryosurgery), drug (5% 5‐FU, imiquimod, 0.5% 5‐FU/10% salicylic acid‐lacquer, 3% diclofenac‐sodium/hyaluronic acid gel and ingenol mebutate), photodynamic therapy as well as watch and wait.
Statistical analyses
Descriptive analyses (frequencies [n], percentages [%], mean [m], standard deviation [SD], 95% confidence interval [95% CI], median [med] and quartiles [Q1/Q3]) were used to describe sociodemographic, clinical and QL data. Normal distribution of continuous variables was examined with the Shapiro–Wilk test.
Spearman's rank correlation was used to assess the correlation between EQ Index and EQ VAS. To examine whether a proposed disease continuum is also accompanied by impairment in QL (EQ Index and EQ VAS), two‐way ANCOVAs (analyses of covariance) were conducted. Age and sex were included in the model as both factors were consistently found to be related with QL (e.g.35). Two different ANCOVA models were used to examine QL in patients with different diagnosis. In the first model, patients were categorised according their diagnosis as follows: (i) AK (single diagnosis of AK), (ii) BCC (including single BCC diagnosis as well as combined diagnoses of AK plus BCC), (iii) SCC (including single SCC diagnosis as well as combined diagnoses of AK plus SCC and (iv) BCC plus SCC (including combined diagnoses of BCC plus SCC as well as combined diagnoses of AK plus BCC plus SCC). To examine estimates from cross‐sectional data whether disease progression results in higher QL impairment, a second model was used with the following patient groups: (i) single diagnosis of AK, (ii) single diagnosis of SCC, and (iii) combined diagnoses of AK plus SCC. To account for the non‐normal distribution of the QL data, calculations were repeated using RANCOVAs (analyses of covariance‐based ranks of QL data),36 which yielded comparable results (results not reported, but available upon request).
The programme IBM SPSS Statistics 24 was used for all statistical analyses. The significance level was set at P (two‐sided) ≤ 0.050.
Results
Patient characteristics
Sociodemographic data (Table 1). The median age was 74 years (Q1/Q3 = 66/79 years, range = 32 to 95 years). The majority of the patients were male (61%), had a low educational level (64%), and were retired (73%).
Table 1.
Sociodemographic data
| N = 1184 | Med | Q1/Q3 | Range |
|---|---|---|---|
| Age a | 74 | 66/79 | 32–95 |
| n | % | ||
| Sex | |||
| Male | 726 | 61.3 | |
| Female | 433 | 36.6 | |
| Missing value | 25 | 2.1 | |
| Marital status | |||
| Married/living in permanent relationship | 813 | 69.2 | |
| Single/divorced/widowed | 354 | 29.9 | |
| Missing value | 11 | 0.9 | |
| Children | |||
| No | 481 | 59.4 | |
| Yes (med = 2, Q1/Q3 = 2/2) | 703 | 40.6 | 1–14 |
| Educational level | |||
| Low | 760 | 64.2 | |
| Medium | 140 | 11.8 | |
| High | 235 | 19.8 | |
| Missing value | 49 | 4.1 | |
| Professional activity | |||
| Student/trainee/job seeker | 4 | 0.4 | |
| Employer | 116 | 9.8 | |
| Worker | 19 | 1.6 | |
| Civil servant | 28 | 2.4 | |
| Freelancer | 64 | 5.4 | |
| Housewife/househusband | 50 | 4.2 | |
| Pensioner | 863 | 72.9 | |
| Missing value | 40 | 3.3 | |
Missing values for n = 21 (1.8%).
Clinical data
The diagnoses were documented as follows (multiple answers were permissible): 73% AK, 49% BCC and 17% SCC. 66% had a single diagnosis, 28% two different diagnoses and 6% three different diagnoses. The majority of the lesions were located on the head (86%). Table 2 presents detailed clinical data. The median treatment period was 49 months (Q1/Q3 = 16/101 months, range = 0 to 308 months, n = 1105). Table 3 presents treatments broken down by diagnoses.
Table 2.
Clinical data
| n | % | |
|---|---|---|
| Diagnosis (multiple answers) | ||
| Actinic keratosis | 869 | 73.4 |
| Basal cell carcinoma | 578 | 48.8 |
| Superficial | 282 | 23.6 |
| Nodular | 231 | 19.5 |
| Other | 82 | 6.9 |
| Missing value | 64 | 5.4 |
| Squamous cell carcinoma | 204 | 17.2 |
| Number of diagnosis | ||
| 1 | 785 | 66.3 |
| 2 | 331 | 28.0 |
| 3 | 68 | 5.7 |
| Number of lesions | ||
| Actinic keratosis | 869 | |
| 1–3 | 329 | 37.9 |
| 4–6 | 232 | 26.7 |
| >6/field | 271 | 31.2 |
| Missing value | 37 | 4.3 |
| Basal cell carcinoma | 578 | |
| 1–3 | 429 | 74.2 |
| 4–6 | 34 | 5.9 |
| >6 | 28 | 4.8 |
| Missing value | 87 | 15.1 |
| Squamous cell carcinoma | 204 | |
| 1–3 | 171 | 83.8 |
| 4–6 | 9 | 4.4 |
| >6 | 4 | 2.0 |
| Missing value | 20 | 9.8 |
| Localisation of lesions (multiple answers) | ||
| Head | 1014 | 85.6 |
| Trunk | 180 | 15.2 |
| Extremities | 218 | 18.4 |
| Missing value | 42 | 3.5 |
| Actinic keratosis | 869 | |
| Head | 780 | 89.8 |
| Trunk | 40 | 4.6 |
| Extremities | 93 | 10.7 |
| Missing value | 53 | 6.1 |
| Basal cell carcinoma | 578 | |
| Head | 405 | 70.1 |
| Trunk | 146 | 25.3 |
| Extremities | 111 | 19.2 |
| Missing value | 19 | 3.3 |
| Squamous cell carcinoma | 204 | |
| Head | 155 | 76.0 |
| Trunk | 9 | 4.4 |
| Extremities | 44 | 21.6 |
| Missing value | 10 | 4.9 |
Table 3.
Treatment types (multiple answers)
| All | Actinic keratosis | Basal cell carcinoma | Squamous cell carcinoma | |||||
|---|---|---|---|---|---|---|---|---|
| n = 1184 | n = 869 | n = 578 | n = 204 | |||||
| Current treatment | n | % | n | % | n | % | n | % |
| No/no information | 114 | 9.6 | 88 | 10.1 | 188 | 32.5 | 92 | 45.1 |
| Yes | 1070 | 90.4 | 781 | 89.9 | 390 | 67.5 | 112 | 54.9 |
| Physical therapy | 661 | 55.8 | 351 | 40.4 | 329 | 56.9 | 92 | 45.1 |
| Excision | 246 | 20.8 | 49 | 5.6 | 179 | 31.0 | 50 | 24.5 |
| Excision with histographic control of resection margin | 188 | 15.9 | 16 | 1.8 | 149 | 25.8 | 42 | 20.6 |
| Curettage | 84 | 7.1 | 78 | 9.0 | 12 | 2.1 | 1 | 0.5 |
| Laser | 53 | 4.5 | 50 | 5.8 | 3 | 0.5 | 0 | 0 |
| Radiotherapy | 2 | 0.2 | 2 | 0.2 | 0 | 0 | 0 | 0 |
| Cryosurgery | 226 | 19.1 | 219 | 25.2 | 13 | 2.2 | 1 | 0.5 |
| Drug therapy | 251 | 21.2 | 245 | 28.2 | 5 | 0.9 | 2 | 1.0 |
| 5‐FU | 61 | 5.2 | 60 | 6.9 | 0 | 0 | 1 | 0.5 |
| Imiquimod | 29 | 2.4 | 26 | 3.0 | 3 | 0.5 | 0 | 0 |
| 5‐FU/salicylic acid‐lacquer | 49 | 4.1 | 48 | 5.5 | 1 | 0.2 | 0 | 0 |
| Diclofenac‐sodium/hyaluronic acid gel | 114 | 9.6 | 111 | 12.8 | 2 | 0.3 | 1 | 0.5 |
| Ingenol mebutate | 27 | 2.3 | 27 | 3.1 | 0 | 0 | 0 | 0 |
| Photodynamic therapy | 342 | 28.9 | 315 | 36.2 | 41 | 7.1 | 2 | 1.0 |
| Watch and wait | 101 | 8.5 | 54 | 6.2 | 42 | 7.3 | 18 | 8.8 |
| Past treatment | ||||||||
| No/no information | 389 | 32.9 | 231 | 26.6 | 269 | 46.5 | 88 | 43.1 |
| Yes | 795 | 67.1 | 638 | 73.4 | 309 | 53.5 | 116 | 56.9 |
| Physical therapy | 599 | 50.6 | 397 | 45.7 | 288 | 49.8 | 112 | 54.9 |
| Excision | 257 | 21.7 | 99 | 11.4 | 154 | 26.6 | 54 | 26.5 |
| Excision with histographic control of resection margin | 183 | 15.5 | 26 | 3.0 | 136 | 23.5 | 58 | 28.4 |
| Curettage | 140 | 11.8 | 125 | 14.4 | 20 | 3.5 | 7 | 3.4 |
| Laser | 63 | 5.3 | 58 | 6.7 | 4 | 0.7 | 1 | 0.5 |
| Radiotherapy | 5 | 0.4 | 2 | 0.2 | 0 | 0 | 3 | 1.5 |
| Cryosurgery | 246 | 20.8 | 226 | 26.0 | 26 | 4.5 | 10 | 4.9 |
| Drug therapy | 298 | 25.2 | 283 | 32.6 | 14 | 2.4 | 3 | 1.5 |
| 5‐FU | 78 | 6.6 | 76 | 8.7 | 1 | 0.2 | 1 | 0.5 |
| Imiquimod | 81 | 6.8 | 70 | 8.1 | 11 | 1.9 | 1 | 0.5 |
| 5‐FU/salicyclic acid‐lacquer | 52 | 4.4 | 52 | 6.0 | 0 | 0 | 0 | 0 |
| diclofenac‐sodium/hyaluronic acid gel | 201 | 17.0 | 196 | 22.6 | 4 | 0.7 | 2 | 1.0 |
| Ingenol mebutate | 47 | 4.0 | 46 | 5.3 | 2 | 0.3 | 1 | 0.5 |
| Photodynamic therapy | 274 | 23.1 | 255 | 29.3 | 33 | 5.7 | 6 | 2.9 |
| Watch and wait | 53 | 4.5 | 43 | 4.9 | 14 | 2.4 | 1 | 0.5 |
QL data
Four hundred and forty‐three (38%) of the patients reported no problems in any of the five dimensions of the EQ‐5D‐5L (health status profile = 11111). Table 4 presents the distribution of the EQ‐5D‐5L dimensions by levels. The median EQ Index was 0.91 (Q1/Q3 = 0.83/1.00, m = 0.87, SD = 0.18, range = −0.21 to 1.00, n = 1162) and the median EQ VAS was 90 (Q1/Q3 = 65/90, m = 75, SD = 19, range = 3 to 100, n = 1175). There exists a strong and positive association between EQ Index and EQ VAS (r s(1154) = 0.65, P < 0.001).
Table 4.
Description of the EQ‐5D‐5L
| n = 1162a | Mobility | Self‐care | Usual activities | Pain | Anxiety | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| No problem | 759 | 65.3 | 966 | 83.1 | 834 | 71.8 | 590 | 50.8 | 819 | 70.5 |
| Slight problems | 215 | 18.5 | 107 | 9.2 | 201 | 17.3 | 341 | 29.3 | 228 | 19.6 |
| Moderate problems | 112 | 9.6 | 58 | 5.0 | 76 | 6.5 | 151 | 13.3 | 76 | 6.5 |
| Severe problems | 67 | 5.8 | 24 | 2.1 | 41 | 3.5 | 59 | 5.1 | 35 | 3.0 |
| Extreme problems | 9 | 0.8 | 7 | 0.6 | 10 | 0.9 | 18 | 1.5 | 4 | 0.3 |
Missing values for n = 22 (1.9%).
Association between quality of life and diagnosis
Model 1
To assess QL differences between patients with different NMSC diagnoses and AK, model 1 made the following comparisons: AK vs. BCC vs. SCC vs. BCC plus SCC.
Using the EQ Index as a dependent variable, model 1 was statistically significant (F (8/1134) = 18.322, P < 0.001). Main effects were found for diagnosis (F (3/1134) = 5.687, P = 0.001), sex (F (1/1134) = 13.985, P < 0.001) and age (F (1/1134) = 102.743, P < 0.001). Patients with BCC plus SCC reported significantly less QL than patients with AK (P = 0.002, 9%) (Fig. 1a). Men (m = 0.88, 95% CI = 0.86/0.90) had a significantly higher mean EQ Index than women (m = 0.82, 95% CI = 0.80/0.85, P < 0.001). There was no interaction effect between diagnosis and sex (F (3/1134) = 2.469, P = 0.061).
Figure 1.
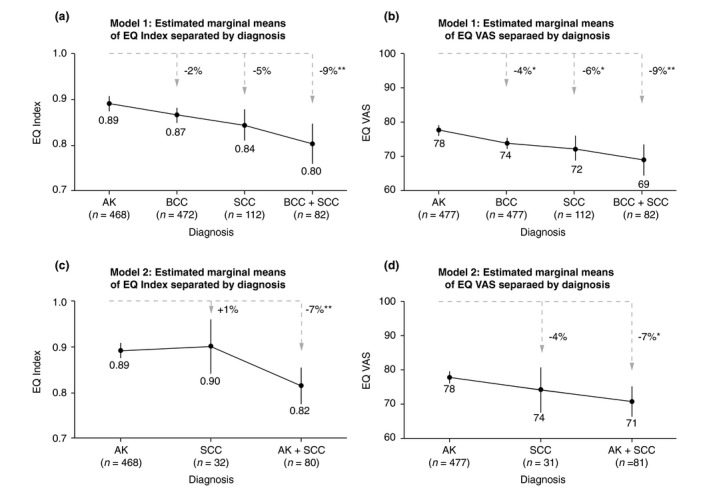
Quality of life differences (adjusted for age) between diagnoses. QL was measured by EQ Index and EQ VAS of the EQ‐5D‐5L. ANCOVA model 1 compares QL between patients with AK and NMSC diagnoses; Model 2 compares in detail QL differences between patients with AK and SCC to estimate whether disease progression results in higher QL impairment. All corrected ANCOVA models were significant: 1a F (8/1134) = 18.322, P < 0.001, 1b F (8/1148) = 14.402, P < 0.001, 1c F (6/580) = 11.931, P < 0.001, and 1d F (6/589) = 7.227, P < 0.001. Based on the estimated marginal means of QL (adjusted for age) of patients with AK, QL decrease (in percentage) for patients with NMSC diagnoses is presented along with significant levels: * P ≤ 0.050, ** P ≤ 0.010, *** P ≤ 0.001.
Using EQ VAS as a dependent variable, model 1 was also statistically significant (F (8/1148) = 14.402, P < 0.001). Main effects were found for diagnosis (F (3/1134) = 6.496, P < 0.001) and age (F (1/1148) = 74.510, P < 0.001). Patients with a single AK diagnosis had significantly higher mean EQ VAS than patients with BCC (P = 0.013, 4%), SCC (P = 0.040, 6%) and BCC plus SCC (P = 0.004, 9%) (Fig. 1b). There was no main effect for sex (F (1/1148) = 0.377, P = 0.539), and no interaction effect between diagnosis and sex (F (3/1148) = 0.047, P = 0.986).
Model 2
The second model examined the effects of disease progression in more detail by comparing three groups of patients: single diagnosis of AK, single diagnosis of SCC and combined diagnosis of AK plus SCC.
Using the EQ Index as a dependent variable, model 2 was statistically significant (F (6/580) = 11.931, P < 0.001). Main effects were found for diagnosis (F (2/580) = 6.356, P = 0.002) and age (F (1/580) = 46.134, P < 0.001). Patients with AK plus SCC reported significantly less QL than patients with AK (P = 0.002, 7%) (Fig. 1c). There was no main effect for sex (F (1/580) = 0.929, P = 0.335), and no interaction between diagnosis and sex (F (2/1134) = 1.339, P = 0.263).
Using the EQ VAS as a dependent variable, model 2 was statistically significant (F (6/589) = 7.227, P < 0.001). Main effects were found for diagnosis (F (2/589) = 4.482, P = 0.012) and age (F (1/589) = 26.691, P < 0.001). Patients with AK plus SCC reported significantly less QL than patients with AK (P = 0.011, 7%) (Fig. 1d). There was no main effect for sex (F (1/589) = 0.025, P = 0.874), and no interaction between diagnosis and sex (F (2/589) = 0.081, P = 0.922).
Table 5 presents medians and quartiles of QL values (not adjusted for age) separated for the fixed factors of both models.
Table 5.
Unadjusted descriptive statistics of QL measures broken down by diagnosis and sex
| Groups | EQ INDEX | EQ VAS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Male | Female | All | Male | Female | ||||||||
| n | Med Q1/Q3 | n | Med Q1/Q3 | n | Med Q1/Q3 | n | Med Q1/Q3 | n | Med Q1/Q3 | n | Med Q1/Q3 | ||
| ANCOVA model 1 | AK | 468 | 1.00 (0.83/1.00) | 308 | 1.00 (0.83/1.00) | 160 | 0.92 (0.83/1.00) | 477 | 80 (70/90) | 312 | 80 (70/90) | 165 | 80 (70/92) |
| BCC | 472 | 0.91 (0.83/1.00) | 268 | 0.91 (0.83/1.00) | 204 | 0.91 (0.81/1.00) | 477 | 80 (65/90) | 272 | 80 (65/90) | 205 | 80 (65/90) | |
| SCC | 112 | 0.91 (0.72/1.00) | 73 | 0.91 (0.78/1.00) | 39 | 0.86 (0.68/1.00) | 112 | 75 (51/90) | 72 | 77 (55/90) | 40 | 72 (50/86) | |
| BCC + SCC | 82 | 0.89 (0.72/1.00) | 62 | 0.91 (0.75/1.00) | 20 | 0.81 (0.54/0.98) | 82 | 70 (50/82) | 61 | 70 (50/81) | 21 | 70 (55/88) | |
| ANCOVA model 2 | AK | 468 | 1.00 (0.83/1.00) | 308 | 1.00 (0.83/1.00) | 160 | 0.92 (0.83/1.00) | 477 | 80 (70/90) | 312 | 80 (70/90) | 165 | 80 (70/92) |
| SCC | 32 | 0.91 (0.84/1.00) | 20 | 0.91 (0.83/1.00) | 12 | 0.91 (0.87/1.00) | 31 | 80 (60/90) | 18 | 80 (58/90) | 13 | 80 (55/90) | |
| AK+SCC | 80 | 0.91 (0.70/1.00) | 53 | 0.91 (0.74/1.00) | 27 | 0.74 (0.55/0.92) | 81 | 75 (50/90) | 54 | 75 (54/90) | 27 | 70 (50/85) | |
Discussion
Only in recent years have PROMs received increasing recognition in the assessment of the burden of benign and malignant cutaneous tumours.37 NMSC in all their different manifestations affect a substantial proportion of the general public, especially in the second half of life.8, 38 Mortality with these conditions is generally low, except for invasive SCC, which has a disease‐specific death risk of 2%.39 Still, the different forms of NMSC may impact patient well‐being by being cosmetically unfavourable or even disfiguring and by showing locally destructive growth patterns. In addition to that, there is a continuum from AK to invasive disease (SCC).7, 8, 40, 41 Thus, part of the perceived burden may be patient insecurity about their prognosis and its progression. However, in the absence of comparable data and representative studies, it is difficult to determine how far a diagnosis of NMSC and/or AK might influence the life of patients. Thus, we have designed such a study as a non‐interventional, prospective, cross‐sectional study in the context of healthcare research in patients with NMSC and AK in Germany. The EQ‐5D‐5L questionnaire was chosen for its robustness and cross‐cultural applicability and its further use for pharmacoeconomic analyses. The population‐based EQ Index is used for these pharmacoeconomic analyses, whereas the EQ VAS represents patient–individual self‐assessment.30 In the present study, the EQ‐5D‐5L proved its sensitivity to discern between groups of patients with different levels of disease progression. Nevertheless, some absolute values obtained in the present study population (e.g. med = 0.91 for the EQ Index, med = 90 for the EQ VAS, perfect health status profile of 11 111 in 38% of the patients) indicate that the EQ‐5D‐5L is still prone to ceiling effects.
So far, very few studies exist that report utilities for NMSC. However, patient groups in these studies were small (n = 8 to 41)19, 42, 43 and the used tools were heterogeneous (for a review see13). In the present study, the average, age‐adjusted EQ Index was 0.87 for patients with BCC (n = 472) and 0.84 for patients with SCC (n = 112). Especially for AK, data on utilities are sparse. Pharmacoeconomic calculations (e.g.29, 44, 45) using either standard gamble or TTO methodology resulted in utilities of 0.99 and 0.98.46, 47 However, these two studies included a total number of 25 patients and were not specific for AK but for a broad variety of dermatological conditions. A standardised method using EQ‐5D‐5L was recently published by a research group from Denmark and Sweden.48 In total, 312 patients with AK were included in this study. The reported outcomes were as follows: EQ Index = 0.88 and EQ VAS = 79. These utilities are in line with those found in the present study: age‐adjusted mean EQ Index = 0.89 (n = 468), age‐adjusted mean EQ VAS = 78 (n = 477). Although the Swedish and Danish authors reported a decrease in utilities by 4% when severe actinic damage or previous SCC was present, this difference failed to reach statistical significance.48 Moreover, the authors did not include patients with SCC, BCC and multiple diagnoses and could thus not assess the potential impact of disease progression.48 On the other hand, the key findings of the present study can be used to approximate the impact of potential disease progression on QL by comparing patients with relatively benign AK to those having developed SCC and multiple diagnoses.
In addition, the present study has the benefit that several forms of NMSC and multiple diagnoses were assessed along with AK although a larger subgroup size might have been desirable, especially for the smallest subgroup of n = 31 patients with SCC only. A further limitation of the present study is its cross‐sectional design, which does not allow for longitudinal QL reassessment. The data are restricted to Germany, but the country‐specific EQ Index allows comparisons across countries. QL reference values for several European countries including Germany are available.49, 50
ANCOVAs significantly showed that QL is correlated with age and differs between diagnoses. Older patients reported significantly lower QL than younger patients. The analyses showed a clear reduction in QL, when patients with tumour diagnoses (SCC, BCC, or SCC plus BCC) are compared to those with AK only (4–9%). More importantly, the comparison of patients with AK to those with AK plus SCC demonstrates a significant decline in QL (7%). This may serve as initial evidence that progression from AK to SCC is accompanied by a decrease in QL. This is not automatically evident, because progression to SCC does not immediately mean gross functional impairment, but patients still perceive it as meaningful and detrimental. Therefore, dermatologists are advised to pay full attention to AK and initiate treatments with highest clearance probabilities as early as possible. Treatment of AK should be performed with both, the intention to prevent SCC and to preserve and restore QL in patients. To observe these effects in more detail, a longitudinal study is warranted, following up patients with AK progressing to SCC over time. Moreover, further analyses are planned within the present data set focusing on the predictors of QL, such as lesion site (especially sites of high cosmetic importance), number of lesions and treatment types. Another important research question for the future is a thorough comparison between NMSC/AK patients with reference data from the general population and from other patient groups.51, 52, 53, 54
In conclusion, we analysed QL data from 1184 patients with NMSC and AK, which makes this study one of the largest reported PROM studies in the field of dermatology. Our results suggest that disease progression from AK to SCC is associated with a significant reduction in QL. This finding should be interpreted as a valid reason to treat AK with due diligence and to acknowledge the fact that NMSC, albeit seldom life‐threatening, has considerable impact on patients’ health and well‐being.
Acknowledgements
The authors wish to thank all German dermatologists who contributed to the data collection: Urte Hammann, Johannes Glutsch, Gertraud Krähn‐Senftleben, Dirk Pappai, Jens‐Joachim Brücher, Harald Brüning, Rolf Dominicus, Kai‐Jochen Friedrich, Eva‐Maria Sahre, Elena Tasler‐Salloum, Rolf Ostendorf, Cord Brütt, Ruth Weissberg, Stephan Wortmann, Rolf‐Günther Fleischer, Michael Ardabili, Erwin Kempf, Madeleine Schunter, Holger Petering, Dagmar Ludolph‐Hauser, Bernd Salzer, Beate Maria Schmid, Annekatrin Becker, Dagmar Richter‐Hinz and Uwe Reinhold.
The authors are also indebted to the excellent organisational support by Inga Engels‐Kunz. We are grateful to Monika Schöll for her linguistic advice.
Conflicts of interest disclosure
KM, CT, UH, JG, GK‐S and MK had no conflict of interest. HL is the general manager of and BN employed by Biofrontera Pharma GmbH. WGP‐D, KS and RMS have been paid as scientific consultants by Biofrontera Pharma GmbH.
Funding sources
The study was funded by Biofrontera Pharma GmbH, Germany, a company that manufactures and distributes a medical product for the treatment of actinic keratosis and basal cell carcinoma.
References
- 1. Lomas A, Leonardi‐Bee J, Bath‐Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol 2012; 166: 1069–1080. [DOI] [PubMed] [Google Scholar]
- 2. Eisemann N, Waldmann A, Geller AC et al Non‐melanoma skin cancer incidence and impact of skin cancer screening on incidence. J Invest Dermatol 2014; 134: 43–50. [DOI] [PubMed] [Google Scholar]
- 3. Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft DK, AWMF) . S3‐Leitlinie Prävention von Hautkrebs: Kurzversion 1.1 [AWMF‐Registernummer: 032/0520L] 2014. Available at: http://www.awmf.org/uploads/tx_szleitlinien/032-052OLk_Pravention_von_Hautkrebs_2014-04.pdf [last accessed 20 July 2017].
- 4. Ackerman AB. Solar keratosis is squamous cell carcinoma. Arch Dermatol 2003; 139: 1216–1217. [DOI] [PubMed] [Google Scholar]
- 5. Roewert‐Huber J, Stockfleth E, Kerl H. Pathology and pathobiology of actinic (solar) keratosis ‐ an update. Br J Dermatol 2007; 157(Suppl 2): 18–20. [DOI] [PubMed] [Google Scholar]
- 6. Criscione VD, Weinstock MA, Naylor MF, Luque C, Eide MJ, Bingham SF. Actinic keratoses: Natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer 2009; 115: 2523–2530. [DOI] [PubMed] [Google Scholar]
- 7. Glogau RG. The risk of progression to invasive disease. J Am Acad Dermatol 2000; 42: 23–24. [DOI] [PubMed] [Google Scholar]
- 8. Werner RN, Sammain A, Erdmann R, Hartmann V, Stockfleth E, Nast A. The natural history of actinic keratosis: a systematic review. Br J Dermatol 2013; 169: 502–518. [DOI] [PubMed] [Google Scholar]
- 9. Salasche SJ. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol 2000; 42: 4–7. [DOI] [PubMed] [Google Scholar]
- 10. Frost CA, Green AC. Epidemiology of solar keratoses. Br J Dermatol 1994; 131: 455–464. [DOI] [PubMed] [Google Scholar]
- 11. Memon AA, Tomenson JA, Bothwell J, Friedmann PS. Prevalence of solar damage and actinic keratosis in a Merseyside population. Br J Dermatol 2000; 142: 1154–1159. [DOI] [PubMed] [Google Scholar]
- 12. Lewis KG, Weinstock MA. Nonmelanoma skin cancer mortality (1988–2000): the Rhode Island follow‐back study. Arch Dermatol 2004; 140: 837–842. [DOI] [PubMed] [Google Scholar]
- 13. Gaulin C, Sebaratnam DF, Fernandez‐Penas P. Quality of life in non‐melanoma skin cancer. Australas J Dermatol 2015; 56: 70–76. [DOI] [PubMed] [Google Scholar]
- 14. Werner RN, Stockfleth E, Connolly SM et al Evidence‐ and consensus‐based (S3) Guidelines for the Treatment of Actinic Keratosis ‐ International League of Dermatological Societies in cooperation with the European Dermatology Forum – Short version. J Eur Acad Dermatol Venereol 2015; 29: 2069–2079. [DOI] [PubMed] [Google Scholar]
- 15. Berman B, Cohen DE, Amini S. What is the role of field‐directed therapy in the treatment of actinic keratosis? Part 1: overview and investigational topical agents. Cutis 2012; 89: 241–250. [PubMed] [Google Scholar]
- 16. Blackford S, Roberts D, Salek MS, Finlay A. Basal cell carcinomas cause little handicap. Qual Life Res 1996; 5: 191–194. [DOI] [PubMed] [Google Scholar]
- 17. Rhee JS, Matthews BA, Neuburg M, Smith TL, Burzynski M, Nattinger AB. Skin cancer and quality of life: assessment with the Dermatology Life Quality Index. Dermatol Surg 2004; 30: 525–529. [DOI] [PubMed] [Google Scholar]
- 18. Steinbauer J, Koller M, Kohl E, Karrer S, Landthaler M, Szeimies RM. Quality of life in health care of non‐melanoma skin cancer – results of a pilot study. J Dtsch Dermatol Ges 2011; 9: 129–135. [DOI] [PubMed] [Google Scholar]
- 19. Chen T, Bertenthal D, Sahay A, Sen S, Chren MM. Predictors of skin‐related quality of life after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. Arch Dermatol 2007; 143: 1386–1392. [DOI] [PubMed] [Google Scholar]
- 20. Chren MM, Sahay AP, Bertenthal DS, Sen S, Landefeld CS. Quality‐of‐life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol 2007; 127: 1351–1357. [DOI] [PubMed] [Google Scholar]
- 21. Rhee JS, Matthews BA, Neuburg M, Logan BR, Burzynski M, Nattinger AB. The skin cancer index: clinical responsiveness and predictors of quality of life. Laryngoscope 2007; 117: 399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Esmann S, Vinding GR, Christensen KB, Jemec GB. Assessing the influence of actinic keratosis on patients’ quality of life: the AKQoL questionnaire. Br J Dermatol 2013; 168: 277–283. [DOI] [PubMed] [Google Scholar]
- 23. Esmann S. Patients’ perspectives on actinic keratosis. Curr Probl Dermatol 2015; 46: 8–13. [DOI] [PubMed] [Google Scholar]
- 24. Miller IM, Vinding G, Zarchi K, Esmann S, Murrell DF, Jemec GB. Differences in disease‐specific quality of life in patients with actinic keratosis in Australia and Denmark. Acta Dermatovenerol Croat 2016; 24: 25–28. [PubMed] [Google Scholar]
- 25. Longo Imedio I, Serra‐Guillen C. Adaptation and validation of the Spanish version of the Actinic Keratosis Quality of Life questionnaire. Actas Dermosifiliogr 2016; 107: 474–481. [DOI] [PubMed] [Google Scholar]
- 26. Gibbons E, Casanas I, Comabella C, Fitzpatrick R. A structured review of patient‐reported outcome measures for patients with skin cancer, 2013. Br J Dermatol 2013; 168: 1176–1186. [DOI] [PubMed] [Google Scholar]
- 27. Black N. Patient‐reported outcome measures in skin cancer. Br J Dermatol 2013; 168: 1151. [DOI] [PubMed] [Google Scholar]
- 28. Herdman M, Gudex C, Lloyd A et al Development and preliminary testing of the new five‐level version of EQ‐5D (EQ‐5D‐5L). Qual Life Res 2011; 20: 1727–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tolley K, Kemmett D, Thybo S, Nasr R, Smethurst H. A cost‐utility analysis of ingenol mebutate gel for the treatment of actinic keratosis: a Scottish perspective. Eur J Health Econ 2016; 17: 287–304. [DOI] [PubMed] [Google Scholar]
- 30. EuroQol . EQ‐5D 2017. Available at: https://euroqol.org/ [last accessed 20 July 2017].
- 31. Versteegh M, Vermeulen K, Evers S, de Wit GA, Prenger R, Stolk E. Dutch tariff for the five‐level version of EQ‐5D. Value Health 2016; 19: 343–352. [DOI] [PubMed] [Google Scholar]
- 32. vanReenen M , Janssen B. EQ‐5D‐5L User Guide: EuroQol Research Foundation; 2015. https://euroqol.org/wp-content/uploads/2016/09/EQ-5D-5L_UserGuide_2015.pdf. [last accessed 20 July 2017].
- 33. Sakthong P, Charoenvisuthiwongs R, Shabunthom R. A comparison of EQ‐5D index scores using the UK, US, and Japan preference weights in a Thai sample with type 2 diabetes. Health Qual Life Outcomes 2008; 6: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Development OfEC‐oa . Classifying educational programmes: manual for ISCED‐97 implementation in OECD countries 1999. Available at: http://www.oecd.org/education/skills-beyond-school/1962350.pdf. [last accessed 20 July 2017]
- 35. McCaffrey N, Kaambwa B, Currow DC, Ratcliffe J. Health‐related quality of life measured using the EQ‐5D‐5L: South Australian population norms. Health Qual Life Outcomes 2016; 14: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Akritas MG, Arnold SF, Brunner E. Nonparametric hypotheses and rank statistics for unbalanced factorial designs. J Am Stat Assoc 1997; 92: 258–265. [Google Scholar]
- 37. Waalboer‐Spuij R, Nijsten TE. A review on quality of life in keratinocyte carcinoma patients. G Ital Dermatol Venereol 2013; 148: 249–254. [PubMed] [Google Scholar]
- 38. Rudolph C, Schnoor M, Eisemann N, Katalinic A. Incidence trends of nonmelanoma skin cancer in Germany from 1998 to 2010. J Dtsch Dermatol Ges 2015; 13: 788–797. [DOI] [PubMed] [Google Scholar]
- 39. Schmults CD, Karia PS, Carter JB, Han J, Qureshi AA. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: a 10‐year, single‐institution cohort study. JAMA Dermatol 2013; 149: 541–547. [DOI] [PubMed] [Google Scholar]
- 40. Fernandez‐Figueras MT, Carrato C, Saenz X et al Actinic keratosis with atypical basal cells (AK I) is the most common lesion associated with invasive squamous cell carcinoma of the skin. J Eur Acad Dermatol Venereol 2015; 29: 991–997. [DOI] [PubMed] [Google Scholar]
- 41. Fernandez Figueras MT. From actinic keratosis to squamous cell carcinoma: pathophysiology revisited. J Eur Acad Dermatol Venereol 2017; 31(Suppl 2): 5–7. [DOI] [PubMed] [Google Scholar]
- 42. Lear W, Akeroyd JE, Mittmann N, Murray C. Measurement of utility in nonmelanoma skin cancer. J Cutan Med Surg 2008; 12: 102–106. [DOI] [PubMed] [Google Scholar]
- 43. Seidler AM, Bramlette TB, Washington CV, Szeto H, Chen SC. Mohs versus traditional surgical excision for facial and auricular nonmelanoma skin cancer: an analysis of cost‐effectiveness. Dermatol Surg 2009; 35: 1776–1787. [DOI] [PubMed] [Google Scholar]
- 44. Wilson EC. Cost effectiveness of imiquimod 5% cream compared with methyl aminolevulinate‐based photodynamic therapy in the treatment of non‐hyperkeratotic, non‐hypertrophic actinic (solar) keratoses: a decision tree model. Pharmacoeconomics 2010; 28: 1055–1064. [DOI] [PubMed] [Google Scholar]
- 45. Soini EJ, Hallinen T, Sokka AL, Saarinen K. Cost‐utility of first‐line actinic keratosis treatments in Finland. Adv Ther 2015; 32: 455–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Littenberg B, Partilo S, Licata A, Kattan MW. Paper Standard Gamble: the reliability of a paper questionnaire to assess utility. Med Decis Making 2003; 23: 480–488. [DOI] [PubMed] [Google Scholar]
- 47. Chen SC, Bayoumi AM, Soon SL et al A catalog of dermatology utilities: a measure of the burden of skin diseases. J Investig Dermatol Symp Proc 2004; 9: 160–168. [DOI] [PubMed] [Google Scholar]
- 48. Tennvall GR, Norlin JM, Malmberg I, Erlendsson AM, Haedersdal M. Health related quality of life in patients with actinic keratosis–an observational study of patients treated in dermatology specialist care in Denmark. Health Qual Life Outcomes 2015; 13: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Greiner W, Weijnen T, Nieuwenhuizen M et al A single European currency for EQ‐5D health states. Results from a six‐country study. Eur J Health Econ 2003; 4: 222–231. [DOI] [PubMed] [Google Scholar]
- 50. Hinz A, Kohlmann T, Stobel‐Richter Y, Zenger M, Brahler E. The quality of life questionnaire EQ‐5D‐5L: psychometric properties and normative values for the general German population. Qual Life Res 2014; 23: 443–447. [DOI] [PubMed] [Google Scholar]
- 51. Szende A, Janssen B. Cross‐Country Analysis of EQ‐5D Data In Szende A, Janssen B, Cabases J, eds. Self‐Reported Population Health: An International Perspective based on EQ‐5D. Springer Netherlands, Dordrecht, 2014: 31–36. [Google Scholar]
- 52. Huber MB, Reitmeir P, Vogelmann M, Leidl R. EQ‐5D‐5L in the general German population: comparison and evaluation of three yearly cross‐section surveys. Int J Environ Res Public Health 2016; 13: 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sullivan PW, Mulani PM, Fishman M, Sleep D. Quality of life findings from a multicenter, multinational, observational study of patients with metastatic hormone‐refractory prostate cancer. Qual Life Res 2007; 16: 571–575. [DOI] [PubMed] [Google Scholar]
- 54. Wallwiener M, Simoes E, Sokolov AN, Brucker SY, Fasching PA, Graf J. Health‐related quality of life in metastatic and adjuvant breast cancer patients. Geburtshilfe Frauenheilkd 2016; 76: 1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]


