Abstract
Objective
To compare magnetic resonance images (MRIs) of the sacroiliac (SI) joints of healthy subjects and individuals with known mechanical strain acting upon the SI joints to those of patients with axial spondyloarthritis (SpA) and patients with chronic back pain.
Methods
Three readers who had received standardized training and were blinded with regard to study group randomly scored MRIs of the SI joints of 172 subjects, including 47 healthy individuals without current or past back pain, 47 axial SpA patients from the Spondyloarthritis Caught Early (SPACE) cohort (with a previous MRI confirmed positive for sacroiliitis), 47 controls with chronic back pain (irrespective of MRI results) from the SPACE cohort, 7 women with postpartum back pain, and 24 frequent runners. MRIs were scored according to the Assessment of SpondyloArthritis international Society (ASAS) definition and Spondyloarthritis Research Consortium of Canada (SPARCC) index.
Results
Of the 47 healthy volunteers, 11 (23.4%) had an MRI positive for sacroiliitis, compared to 43 (91.5%) of 47 axial SpA patients and 3 (6.4%) of 47 patients with chronic back pain. Three (12.5%) of the 24 runners and 4 (57.1%) of the 7 women with postpartum back pain had a positive MRI. Using a SPARCC cutoff of ≥2 for positivity, 12 (25.5%) of 47 healthy volunteers, 46 (97.9%) of 47 positive axial SpA patients, 5 (10.6%) of 47 controls with chronic back pain, 4 (16.7%) of 24 runners, and 4 (57.1%) of 7 women with postpartum back pain had positive MRIs. Deep bone marrow edema (BME) lesions were not found in healthy volunteers, patients with chronic back pain, or runners, but were found in 42 (89.4%) of 47 positive axial SpA patients and in 1 (14.3%) of 7 women with postpartum back pain.
Conclusion
A substantial proportion of healthy individuals without current or past back pain has an MRI positive for sacroiliitis according to the ASAS definition. Deep (extensive) BME lesions are almost exclusively found in axial SpA patients.
Axial spondyloarthritis (SpA) is among the most prevalent forms of chronic inflammatory arthritis 1, 2. In recent decades, magnetic resonance imaging (MRI) has been increasingly used to visualize inflammation in the sacroiliac (SI) joints. Inflammation on MRI facilitates the early identification of patients with axial SpA since it precedes structural damage on radiography 3, 4. Of all patients with axial SpA, 20–42% have active sacroiliitis on MRI 5, 6, 7, 8, but signs of presumed sacroiliitis may also occur in individuals without axial SpA. One study by Weber et al showed that of 59 healthy volunteers, up to 22% had signs of sacroiliitis on MRI 9. Arnbak and colleagues showed that of 1,020 unselected patients with chronic back pain, 21% had sacroiliitis on MRI according to the Assessment of SpondyloArthritis international Society (ASAS) definition 10. The specificity of MRI of the SI joints for SpA‐specific sacroiliitis is not well known and physicians may rely too much on a positive finding 11.
In order to obtain insight into the prevalence and extent of SI joint inflammation in healthy individuals, and in those with known mechanical strain acting upon the SI joints, we compared MRIs of the SI joints in 1) healthy individuals without any signs of current or past back pain, 2) patients with classic axial SpA with a documented positive MRI of the SI joints after central reading, 3) patients with chronic back pain, 4) frequent runners, and 5) women with postpartum back pain.
The primary hypothesis was that the difference between axial SpA and non–axial SpA with regard to the presence of sacroiliitis on MRI was quantitative (extent) rather than qualitative (present versus absent).
Patients and methods
Selection of the study participants. The present study included participants from the Maasstad MRI project and the Spondyloarthritis Caught Early (SPACE) cohort. The Maasstad MRI project included healthy participants, frequent runners, and women with postpartum back pain 12. Healthy participants were employees of Maasstad Hospital and Erasmus University Medical Center (Rotterdam, The Netherlands). Male and female participants between 18 and 45 years of age were included. Runners who were running at least 5 km twice per week were recruited via an athletics club. Healthy participants and runners with any form of current or past acute or chronic back pain and those with contraindication to MRI were excluded. Women with postpartum back pain that presented ≤3 months after pregnancy were included in the Maasstad MRI project and additionally selected from the SPACE cohort.
We selected positive and negative controls from the SPACE cohort. The SPACE cohort is an ongoing, prospective, longitudinal, multicenter cohort that started in 2009 and has been described in detail elsewhere 13. In short, the SPACE cohort includes patients ages ≥16 years with chronic back pain (with a duration of ≥3 months but ≤2 years) with an onset before the age of 45 years. We used baseline data unless specified otherwise. Positive SPACE controls were defined as patients with axial SpA (who were diagnosed by a rheumatologist and fulfilled the ASAS criteria for axial SpA [3]) with an MRI of the SI joints that was previously scored as positive for sacroiliitis according to the ASAS definition by at least 2 of 3 central readers. Negative SPACE controls were defined as patients with chronic back pain (who were neither diagnosed as having axial SpA nor fulfilled the ASAS criteria for axial SpA), irrespective of their MRI findings. Healthy controls were matched with positive and negative SPACE controls for age and sex.
The local medical ethics committees of the participating sites approved the study, and all participants gave their written informed consent.
MRI. MRI was performed on 1.5T systems. At least 12 slices of coronal oblique T1‐weighted turbo spin‐echo and short tau inversion recovery sequences of the SI joints were acquired. The slice thickness was 4 mm.
Scoring. Three readers (JdW, MdH, and RL) who had received standardized training and were blinded with regard to subject group independently scored all MRIs in random order. The readers were instructed to quantify MRIs as positive or negative by judging for the presence or absence of bone marrow edema (BME) according to the ASAS definition that was recently updated by the ASAS MRI working group 14. As this definition describes, the focus was on scoring only lesions that were considered “highly suggestive of axial SpA” as positive. In addition, the readers quantified the extent of BME according to the Spondyloarthritis Research Consortium of Canada (SPARCC) scoring system 15. For SPARCC scoring, 6 consecutive coronal slices were selected and each SI joint was divided into 4 quadrants: the upper and lower ilium and upper and lower sacrum. Each quadrant was assigned a score of 1 for the presence of BME or 0 for the absence of BME. Each coronal slice per SI joint was also scored for the presence of “intense” lesions (high signal as bright as or brighter than that of veins or intervertebral discs) or “deep” lesions (a homogeneous, unequivocal increase in signal extending ≥1 cm from the articular surface). The maximum SPARCC score for all 6 slices was 48 for BME, 12 for intensity, and 12 for depth, resulting in a maximum total score of 72 15. To determine the distribution of BME anatomically in the SI joints, we used the SPARCC distribution of the 4 quadrants, divided into anterior and posterior slices. BME was considered to occur in a particular region if it was concordantly recorded at that region by at least 2 of the 3 readers.
In the analysis, an MRI was considered positive if at least 2 of 3 readers agreed it met the ASAS MRI working group criteria for defining sacroiliitis by MRI 14. SPARCC scores are presented as the mean total SPARCC scores from all 3 readers. We chose SPARCC score cutoff values of ≥2 and ≥5 to discriminate between low and high SPARCC scores.
Statistical analysis. Categorical data are presented as the number (percent), and continuous data are presented as the mean ± SD or as the median (interquartile range) as appropriate. The chi‐square test and Mann‐Whitney U test were used for comparing categorical and continuous data, respectively. Interreader agreement on positive/negative MRI of the SI joints was investigated using Cohen's kappa coefficient and interpreted according to the standards of Landis and Koch 16. Interreader agreement on SPARCC scores was evaluated using intraclass correlation coefficients (ICCs). We performed all analyses in SPSS version 24.0.
Results
Study participants. In total, we evaluated 141 MRIs of the SI joints of 47 healthy participants from the Maasstad MRI project, matched with 47 patients with axial SpA with MRIs positive for sacroiliitis and 47 controls with chronic back pain irrespective of MRI outcome from the SPACE cohort. We also included MRIs of the SI joints of 24 runners from the Maasstad MRI project and 7 women with postpartum back pain (4 from the Maasstad MRI project and 3 from the SPACE cohort). The baseline characteristics of the study population are shown in Table 1.
Table 1.
Characteristics of the healthy participants, patients with axial SpA with a previous MRI positive for sacroiliitis (positive controls), patients with chronic back pain (negative controls), runners, and women with postpartum back paina
| Healthy participants (n = 47) | Patients with axial SpA (n = 47) | Controls with chronic back pain (n = 47) | Runners (n = 24) | Women with postpartum back pain (n = 7) | |
|---|---|---|---|---|---|
| Men | 21 (44.7) | 21 (44.7) | 21 (44.7) | 12 (50.0) | 0 (0) |
| Age, mean ± SD years | 30.9 ± 6.5 | 31.5 ± 6.9 | 30.7 ± 6.5 | 34.3 ± 7.7 | 30.1 ± 7.2 |
| Axial SpA diagnosis | 0 (0) | 47 (100) | 0 (0) | 0 (0) | 0 (0) |
| HLA–B27 positive | NA | 35 (74.5) | 0 (0) | NA | NA |
| Duration of back pain, mean ± SD months | NA | 13.4 ± 7.4 | 12.8 ± 7.8 | NA | NA |
| Inflammatory back pain | NA | 35 (74.5) | 26 (55.3) | NA | NA |
| Alternating buttock pain | NA | 40 (85.1) | 20 (42.6) | NA | NA |
| ASAS axial SpA criteria | NA | 47 (100) | NA | NA | NA |
| Modified New York criteria | NA | 13 (29.5) | 0 (0) | NA | NA |
Except where indicated otherwise, values are the number (%). SpA = spondyloarthritis; MRI = magnetic resonance imaging; NA = not applicable; ASAS = Assessment of SpondyloArthritis international Society.
Scoring agreement. The 3 readers agreed moderately well on the absence or presence of BME (absolute agreement within the 3 reader pairs 75%, 76%, and 80%, respectively; Cohen's κ = 0.48, 0.50, and 0.59, respectively). Readers correlated far better on absolute SPARCC scores (ICCs of 0.82, 0.82, and 0.96, respectively).
Scoring results. Scoring according to the ASAS definition. Figure 1 shows the proportion of subjects with MRIs positive for sacroiliitis according to the ASAS definition in the different groups. Of the 47 healthy participants, 11 (23.4%) had a positive MRI. Of the 47 positive controls with axial SpA, 43 (91.5%) had a positive MRI, and of the 47 controls with chronic back pain, only 3 (6.4%) had a positive MRI. Of the 24 runners, 3 (12.5%) had a positive MRI, and of the 7 women with postpartum back pain, 4 (57.1%) had a positive MRI.
Figure 1.
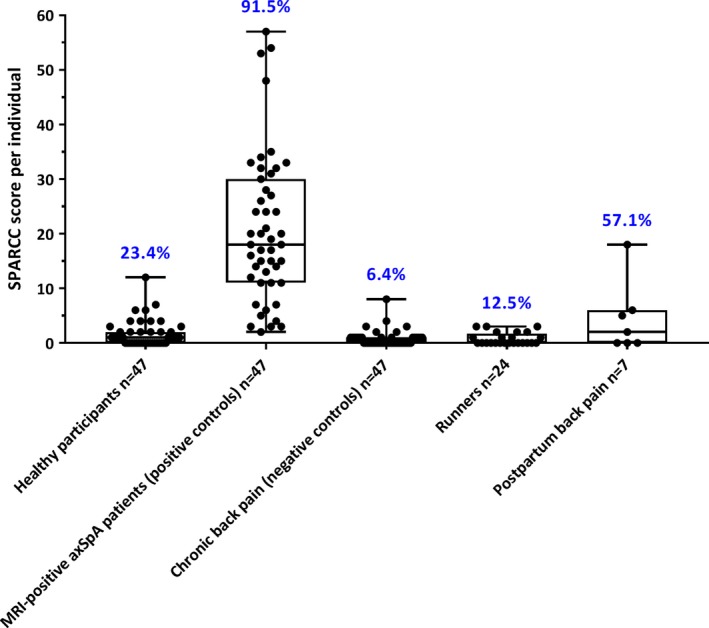
Sacroiliitis on magnetic resonance images (MRIs) of the sacroiliac joints in the study population. Data are shown as box plots. Each box represents the 25th to 75th percentiles. Lines inside the boxes represent the median. Lines outside the boxes represent the minimum and maximum values. Each circle represents a single subject. Values shown above the box plots are the percentage of subjects with an MRI indicating sacroiliitis according to the Assessment of SpondyloArthritis international Society definition. For positive controls and negative controls, “positive” and “negative” refer to the final diagnosis (axial spondyloarthritis [axSpA] or no axial SpA) after vigorous diagnostic evaluation, and not to the MRI result per se. SPARCC = Spondyloarthritis Research Consortium of Canada.
SPARCC scores. Figure 1 shows the individual and mean SPARCC scores for the different patient categories. The mean ± SD SPARCC score was 1.7 ± 2.4 for the 47 healthy participants, 20.9 ± 13.7 for the 47 positive axial SpA controls, and 0.8 ± 1.4 for the 47 non‐SpA back pain controls. The mean ± SD SPARCC score was 0.8 ± 1.1 for the runners and 4.5 ± 6.3 for the 7 women with postpartum back pain.
At a SPARCC cutoff score of ≥2, 12 (25.5%) of the 47 healthy volunteers, 46 (97.9%) of the 47 positive axial SpA controls, 5 (10.6%) of the 47 non‐SpA back pain controls, 4 (16.7%) of the 24 runners, and 4 (57.1%) of the 7 women with postpartum back pain were considered to have sacroiliitis on MRI. At a SPARCC cutoff score of ≥5, 4 (8.5%) of the 47 healthy volunteers, 41 (87.2%) of the 47 positive patients with axial SpA, 1 (2.1%) of the 47 patients with chronic back pain, 0 (0%) of the 24 runners, and 2 (28.6%) of the 7 women with postpartum back pain were considered to have sacroiliitis on MRI.
Deep lesions (a homogeneous, unequivocal increase in signal ≥1 cm from the articular surface) were almost exclusively found in positive controls with axial SpA (42 [89.4%] of the 47 had deep lesions). Such lesions were not found in the healthy volunteers, controls with chronic back pain, or runners and were found in only 1 (14.3%) of the 7 women with postpartum back pain.
Anatomic location of BME. We compared the location of BME in MRIs considered positive for sacroiliitis to those considered negative for sacroiliitis (according to the ASAS definition) (Table 2). In positive MRIs, BME was found most frequently in the posterior lower ilium (in 53 [82.8%] of 64 MRIs). In negative MRIs, BME was found most frequently in the anterior lower ilium (in 18 [16.7%] of 108 MRIs). Overall, BME was most frequently found in the posterior lower ilium (in 69 [40.1%] of 172 MRIs), and least frequently found in the anterior lower sacrum (in 40 [23.3%] of 172 MRIs).
Table 2.
Anatomic distribution of bone marrow edema in the SI joint quadrants on MRIa
| Upper ilium | Lower ilium | Upper sacrum | Lower sacrum | |||||
|---|---|---|---|---|---|---|---|---|
| Anterior | Posterior | Anterior | Posterior | Anterior | Posterior | Anterior | Posterior | |
| Positive MRI of the SI joints (n = 64) | 44 (68.8) | 34 (53.1) | 48 (75.0) | 53 (82.8) | 39 (60.9) | 37 (57.8) | 37 (57.8) | 43 (67.2) |
| Negative MRI of the SI joints (n = 108) | 4 (3.7) | 7 (6.5) | 18 (16.7) | 16 (14.8) | 5 (4.6) | 7 (6.5) | 3 (2.8) | 4 (3.7) |
| Healthy participants (n = 47) | 2 (4.3) | 2 (4.3) | 10 (21.3) | 18 (38.3) | 4 (8.5) | 2 (4.3) | 3 (6.4) | 6 (12.8) |
| Patients with axial SpA (n = 47) | 39 (83.0) | 34 (72.3) | 41 (87.2) | 41 (87.2) | 33 (70.2) | 36 (76.6) | 33 (70.2) | 35 (74.5) |
| Controls with chronic back pain (n = 47) | 2 (4.3) | 4 (8.5) | 11 (23.4) | 5 (10.6) | 3 (6.4) | 3 (6.4) | 1 (2.1) | 2 (4.3) |
| Runners (n = 24) | 4 (16.7) | 1 (4.2) | 1 (4.2) | 2 (8.3) | 2 (8.3) | 1 (4.2) | 1 (4.2) | 2 (8.3) |
| Women with postpartum back pain (n = 7) | 1 (14.3) | 0 (0) | 3 (43.0) | 3 (43.0) | 2 (28.6) | 2 (28.6) | 2 (28.6) | 2 (28.6) |
Magnetic resonance images (MRIs) that indicated sacroiliitis according to the Assessment of SpondyloArthritis international Society (ASAS) definition were considered positive. Those that did not meet the ASAS definition were considered negative. Values are the number (%). SI = sacroiliac; SpA = spondyloarthritis.
Discussion
The results of our study suggest that 1) one‐fourth of asymptomatic healthy individuals and more than half of women presenting with postpartum back pain who do not have axial SpA may have an MRI positive for sacroiliitis according to the ASAS definition, meaning that their MRI lesions are considered highly suggestive of axial SpA by trained readers, 2) frequent runners do not seem to have more lesions than healthy asymptomatic individuals, 3) high SPARCC scores (≥5) rarely occur in healthy individuals, patients with chronic back pain without axial SpA, or runners, 4) deep (extensive) lesions are highly specific for axial SpA–associated sacroiliitis, and 5) BME lesions in healthy participants are preferably but not exclusively located in the lower iliac bone.
Our finding of positive MRIs in healthy individuals is consistent with the findings of Weber and Maksymowych, who showed that 25% of healthy participants have BME lesions on MRIs of the SI joints 17. Our findings of positive MRIs in women with postpartum back pain are also consistent with a previous study showing that 60% of women with postpartum back pain have BME lesions on MRI of the SI joints 18. Differently than we hypothesized, our data suggest that the proportion of runners with BME lesions on MRI of the SI joints is not higher than the proportion of healthy participants with BME lesions on MRI of the SI joints. However, little demographic or lifestyle data were collected, and our group of healthy participants might have included runners. Further research should correct for possible confounding factors such as body mass index and general health status.
In this study, BME lesions in MRIs scored positive for sacroiliitis and in the MRIs of healthy participants were most frequently found in the posterior lower ilium, which is consistent with data from Weber et al showing a similar preferential location in amateur and professional athletes 19. In patients with axial SpA, lesions occur throughout the SI joints. BME was seen in the anterior lower ilium in 16.7% of the MRIs in which lesions were not highly specific for SpA.
The percentage of patients with chronic back pain with BME lesions was lower than the percentage of healthy volunteers with BME lesions (6.4% versus 23.4%, respectively). This is an artifact caused by channeling patients with an MRI negative for sacroiliitis toward the (non–axial SpA) chronic back pain group, a rational diagnostic approach since a clinical diagnosis of axial SpA (or the exclusion thereof) relies on clinical signs and laboratory findings as well as imaging results (MRI). Similarly, the 91.5% positive MRI rate in the axial SpA group is the consequence of channeling. Prior MRI studies have been used to classify these patients as having axial SpA. As such, the low prevalence of MRI positivity found in the chronic back pain group—and the high prevalence found in the axial SpA group—adds to the credibility of our findings (internal validity).
One of the most important reasons to perform this study is that misclassification of MRIs of the SI joints as positive is a real threat, which may lead to a spuriously high number of patients being diagnosed as having axial SpA. Evidence of such a spurious mechanism has been reported by Arnbak et al 10 and Van Hoeven et al 20, showing that nearly 25% of patients with chronic back pain could be classified as having axial SpA when MRI of the SI joints was the leading factor in the diagnostic consideration. Since we and others have demonstrated that MRIs that are highly suggestive of axial SpA may be seen frequently in unaffected individuals, relying too much on a positive MRI finding will result in overdiagnosis, and consequently in overtreatment, of these patients who may have nonspecific chronic back pain rather than axial SpA.
The only means to avoid overdiagnosis of axial SpA as a diagnostician is to act based on thorough knowledge of the clinical syndrome of axial SpA and MRI abnormalities thereof, to ask for diagnostic MRI of the SI joints only in patients in whom there is a moderately high suspicion of axial SpA (e.g., based on clinical algorithms) 21, and to always consider alternative (more likely) diagnoses before making a diagnosis of axial SpA. A highly specific and sensitive gold standard for axial SpA is lacking (a positive MRI finding can definitely not serve as a gold standard) and the diagnosis of axial SpA relies on skillful pattern recognition rather than on diagnostic imaging. Obviously, the ASAS criteria for axial SpA are not meant to differentiate subjects with no back pain from patients with axial SpA in clinical practice. But the purpose of this study was not to validate the ASAS criteria; this study was undertaken to test the specificity of MRI of the SI joints (and not to test the diagnostic value of MRI of the SI joints). A sound starting point then is to compare 2 extreme groups. Therefore, in this study we included patients with chronic back pain who do not meet the ASAS classification criteria as a negative control group, and have contrasted them to patients with axial SpA formerly judged to have positive MRI findings who do meet the ASAS classification criteria as a positive control group.
Our finding that deep (extensive) lesions appeared almost exclusively in axial SpA controls has not been observed before and needs confirmation in another cohort of patients with axial SpA. This finding may help to create a stricter definition of a positive finding on MRI of the SI joints indicating axial SpA, so that overdiagnosis of axial SpA (and associated health care expenses) will be limited.
Our study has important strengths. The three readers who had received standardized training were completely blinded with regard to all of the clinical information and information regarding the hospital of recruitment during the scoring process. The study set was a deliberate mix of previously positive and previously negative control MRIs, derived from the SPACE cohort, in order to constrain bias by reader expectation. These controls were matched for sex and age. Most importantly, though, the experienced readers were instructed (and agreed) to only score according to ASAS guidelines, which indicates that only MRI findings that are highly suggestive of axial SpA should be considered positive, and MRIs of the SI joints with solitary white spots, which may count in the SPARCC scoring system, should be considered negative. The not more than moderate interreader agreement obtained among these experienced and trained readers testifies to the inherent difficulty of interpreting the ASAS definition that starts with “lesions highly suggestive of axial SpA.” Since patients with axial SpA have far more extensive lesions than non‐SpA patients or healthy individuals, and this extent is reflected by high SPARCC scores, interreader agreements based on continuous SPARCC scores were far better. But this finding will not help to avoid misdiagnosis since a diagnosis is based on a binomial choice (positive or negative) rather than on a SPARCC score.
A final strength of this study is that it combined several groups of individuals with and individuals without symptoms in one setting. It allows us to speculate that runners may not show higher rates of MRI positivity than asymptomatic individuals, but that postpartum patients with back pain potentially do.
Our study also has limitations. First, the clinical and demographic information on healthy participants, runners, and women with postpartum back pain was rather limited. Individuals who we considered asymptomatic could still have had SpA‐related symptoms that would have been revealed if more data had been obtained according to a study protocol (such as the SPACE protocol). It is unlikely that the 25% of MRIs that were classified as positive were related to undiagnosed or early axial SpA, since individuals with any sign of current or past back pain were excluded. Second, the groups of runners and women with postpartum back pain were rather small, due to reasons of convenience, and therefore conclusions should be drawn with caution. Third, this study focused exclusively on inflammatory lesions. To date, however, it is unclear if potential refining of SpA‐specific inflammatory lesions or alternatively combining information on inflammation and structural changes may help in the diagnosis of axial SpA 14. Fourth, the arbitrary threshold of back pain starting within 3 months after delivery in the group of women with postpartum back pain was not supported by the literature. However, it was our aim to include only women in whom incident back pain was most likely related to delivery. Fifth, in this study, different MRI scanners were used, which in theory may violate the blinding of the readers and thus contribute to a biased result, but several MRI scanners were used across subgroups, and the same type of MRI scanner was used in different subgroups, so we conclude that such a form of expectation bias is highly unlikely.
Finally, readers agreed only moderately on the presence or absence of BME. Interreader agreement is dependent on several factors, including the quality of the images, the presence of a unique protocol, and the level of training of the readers. This study included patients and individuals from different sources, scanned with different machines, by different technicians, using slightly different protocols, as in common clinical practice. This level of variability has an impact on measurement error. While readers were trained in a standardized manner, the variability in the quality of the images likely resulted in kappa values that were slightly lower than values obtained in, for example, randomized controlled trials or cohort studies conducted under a protocol. Given these limitations and considering the high level of interreader agreement on SPARCC scores, the interreader agreement in this study is still very acceptable.
We conclude that a substantial proportion of healthy and asymptomatic individuals, runners, and women with postpartum back pain may have positive findings on MRI of the SI joints that are highly suggestive, but not reflective, of axial SpA. Patients with axial SpA have more extensive lesions (reflected by SPARCC scores ≥5 and the presence of deep lesions) than healthy, asymptomatic individuals.
Author contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. de Winter had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
de Winter, van Hoeven, Baeten, van der Heijde, Weel, Landewé.
Acquisition of data
de Winter, de Hooge, de Jong, van Hoeven, de Koning, Berg, Ramonda, Baeten, van der Heijde, Weel.
Analysis and interpretation of data
de Winter, de Hooge, van de Sande, Baeten, van der Heijde, Landewé.
Additional disclosures
Author Baeten is an employee of UCB.
Acknowledgments
We acknowledge Maureen Leeuw, research nurse (Academic Medical Center) and Arno Roeterink, MD (Maasstad Hospital) for their help with the scoring of MRIs.
Drs. Weel and Landewé contributed equally to this work.
References
- 1. Reveille JD, Witter JP, Weisman MH. Prevalence of axial spondylarthritis in the United States: estimates from a cross‐sectional survey. Arthritis Care Res (Hoboken) 2012;64:905–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dougados M, Baeten D. Spondyloarthritis. Lancet 2011;377:2127–37. [DOI] [PubMed] [Google Scholar]
- 3. Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. [DOI] [PubMed] [Google Scholar]
- 4. Dougados M, Sepriano A, Molto A, van Lunteren M, Ramiro S, de Hooge M, et al. Sacroiliac radiographic progression in recent onset axial spondyloarthritis: the 5‐year data of the DESIR cohort. Ann Rheum Dis 2017;76:1823–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ciurea A, Scherer A, Exer P, Bernhard JJ, Dudler J, Beyeler B, et al. Tumor necrosis factor α inhibition in radiographic and nonradiographic axial spondyloarthritis: results from a large observational cohort. Arthritis Rheum 2013;65:3096–106. [DOI] [PubMed] [Google Scholar]
- 6. Blachier M, Canouï‐Poitrine F, Dougados M, Lethuaut A, Fautrel B, Ferkal S, et al. Factors associated with radiographic lesions in early axial spondyloarthritis: results from the DESIR cohort. Rheumatology (Oxford) 2013;52:1686–93. [DOI] [PubMed] [Google Scholar]
- 7. Heuft‐Dorenbosch L, Weijers R, Landewé R, van der Linden S, van der Heijde D. Magnetic resonance imaging changes of sacroiliac joints in patients with recent‐onset inflammatory back pain: inter‐reader reliability and prevalence of abnormalities. Arthritis Res Ther 2006;8:R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van den Berg R, Lenczner G, Thévenin F, Claudepierre P, Feydy A, Reijnierse M, et al. Classification of axial SpA based on positive imaging (radiographs and/or MRI of the sacroiliac joints) by local rheumatologists or radiologists versus central trained readers in the DESIR cohort. Ann Rheum Dis 2015;74:2016–21. [DOI] [PubMed] [Google Scholar]
- 9. Weber U, Lambert RG, Østergaard M, Hodler J, Pedersen SJ, Maksymowych WP. The diagnostic utility of magnetic resonance imaging in spondylarthritis: an international multicenter evaluation of one hundred eighty‐seven subjects. Arthritis Rheum 2010;62:3048–58. [DOI] [PubMed] [Google Scholar]
- 10. Arnbak B, Grethe Jurik A, Hørslev‐Petersen K, Hendricks O, Hermansen LT, Loft AG, et al. Associations between spondyloarthritis features and magnetic resonance imaging findings: a cross‐sectional analysis of 1,020 patients with persistent low back pain. Arthritis Rheumatol 2016;68:892–900. [DOI] [PubMed] [Google Scholar]
- 11. Deodhar A. Sacroiliac joint magnetic resonance imaging in the diagnosis of axial spondyloarthritis: “a tiny bit of white on two consecutive slices” may be objective, but not specific [editorial]. Arthritis Rheumatol 2016;68:775–8. [DOI] [PubMed] [Google Scholar]
- 12. Van Hoeven L, Luime JJ, de Buck PD, Hazes JM, Weel AE. Bone marrow edema and structural lesions in the sacroiliac joint in a large cohort of patients with axial spondyloarthritis, chronic low back pain and healthy controls [abstract]. Arthritis Rheum 2013;65 Suppl:S1239. [Google Scholar]
- 13. Van den Berg R, de Hooge M, van Gaalen F, Reijnierse M, Huizinga T, van der Heijde D. Percentage of patients with spondyloarthritis in patients referred because of chronic back pain and performance of classification criteria: experience from the Spondyloarthritis Caught Early (SPACE) cohort. Rheumatology (Oxford) 2013;52:1492–9. [DOI] [PubMed] [Google Scholar]
- 14. Lambert RG, Bakker PA, van der Heijde D, Weber U, Rudwaleit M, Hermann KG, et al. Defining active sacroiliitis on MRI for classification of axial spondyloarthritis: update by the ASAS MRI working group. Ann Rheum Dis 2016;75:1958–63. [DOI] [PubMed] [Google Scholar]
- 15. Maksymowych WP, Inman RD, Salonen D, Dhillon SS, Krishnananthan R, Stone M, et al. Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of spinal inflammation in ankylosing spondylitis. Arthritis Rheum 2005;53:502–9. [DOI] [PubMed] [Google Scholar]
- 16. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74. [PubMed] [Google Scholar]
- 17. Weber U, Maksymowych WP. Sensitivity and specificity of magnetic resonance imaging for axial spondyloarthritis. Am J Med Sci 2011;341:272–7. [DOI] [PubMed] [Google Scholar]
- 18. Eshed I, Miloh‐Raz H, Dulitzki M, Lidar Z, Aharoni D, Liberman B, et al. Peripartum changes of the sacroiliac joints on MRI: increasing mechanical load correlating with signs of edema and inflammation kindling spondyloarthropathy in the genetically prone. Clin Rheumatol 2015;34:1419–26. [DOI] [PubMed] [Google Scholar]
- 19. Weber U, Jurik AG, Zejden A, Larsen E, Jørgensen SH, Rufibach K, et al. Bone marrow oedema in sacroiliac joints of young athletes shows most frequently in the posterior inferior ilium. Ann Rheum Dis 2017;76 Suppl 2:101. [Google Scholar]
- 20. Van Hoeven L, Luime J, Han H, Vergouwe Y, Weel A. Identifying axial spondyloarthritis in Dutch primary care patients, ages 20–45 years, with chronic low back pain. Arthritis Care Res (Hoboken) 2014;66:446–53. [DOI] [PubMed] [Google Scholar]
- 21. Van den Berg R, de Hooge M, Rudwaleit M, Sieper J, van Gaalen F, Reijnierse M, et al. ASAS modification of the Berlin algorithm for diagnosing axial spondyloarthritis: results from the SPondyloArthritis Caught Early (SPACE)‐cohort and from the Assessment of SpondyloArthritis international Society (ASAS)‐cohort. Ann Rheum Dis 2013;72:1646–53. [DOI] [PubMed] [Google Scholar]


