Abstract
Alpha‐1‐antitrypsin deficiency (A1ATD) due to homozygosity for the Z allele (ZZ) is an established risk factor for cirrhosis, but the liver disease risk in heterozygous Z allele carriers (MZ) is controversial. The aim of the present study was to determine the prevalence of the MZ genotype among patients with cirrhosis and the associated risk of decompensation and liver transplantation/mortality. An unselected cohort of 561 patients with cirrhosis and 248 deceased liver donors were genotyped for the A1ATD risk alleles Z and S using a validated allelic discrimination assay. Clinical and biochemical parameters were assessed in 488 genotype MM and 52 MZ patients at baseline when cirrhosis was diagnosed and at the last contact, before liver transplantation or death, as study endpoints. MZ prevalence was 2.8% among liver donors, 5.8%, 9.1%, 10.9%, and 19.0% in patients with cirrhosis and Model for End‐Stage Liver Disease–sodium (MELD‐Na) ≤10, 11‐20, 21‐30, and >30, respectively. Among liver transplant recipients, MZ prevalence was 9.7%. MS prevalence was not different between donors, patients with cirrhosis, or transplant recipients. At the end of follow‐up, MELD‐Na scores were higher among heterozygous Z risk allele carriers (16 versus 19; P = 0.03). Decompensation of cirrhosis with ascites or encephalopathy was significantly more frequent in patients with MZ than in MM patients. In the subgroup with transferrin (Tf) saturation >50% or Tf <180 mg/dL, MZ patients had a significantly higher risk of liver transplantation or death than MM patients. In conclusion, the genotype MZ is a genetic risk factor for more advanced cirrhosis and decompensation. MZ patients with cirrhosis and hypotransferrinemia or increased Tf saturation are at higher risk of death and liver transplantation. Liver Transplantation 24 744–751 2018 AASLD.
Abbreviations
- A1AT
alpha‐1‐antitrypsin
- A1ATD
alpha‐1‐antitrypsin deficiency
- ALT
alanine transaminase
- BMI
body mass index
- CI
confidence interval
- CRP
C‐reactive protein
- ER
endoplasmic reticulum
- INR
international normalized ratio
- IQR
interquartile range
- MELD
Model for End‐Stage Liver Disease
- MELD‐Na
Model for End‐Stage Liver Disease–sodium
- NAFLD
nonalcoholic fatty liver disease
- PI
protease inhibitor
- SERPINA1
serpin peptidase inhibitor A1
- Tf
transferrin
Alpha‐1‐antitrypsin deficiency (A1ATD)–associated liver disease is caused by the aggregation and intracellular accumulation of the mutant protein in hepatocytes. The most relevant hepatic risk allele for A1ATD is the serpin peptidase inhibitor A1 (SERPINA1) Z allele. A substitution of a glutamic acid to lysine in position 342 of the serine protease inhibitor (PI) alpha‐1‐antitrypsin (A1AT) causes irreversible misfolding and intracellular protein polymerization.1
Clinical consequences of A1AT accumulation in hepatocytes include cholestatic hepatitis in newborns and development of cirrhosis, which can affect patients at all ages.2 Homozygosity for the Z allele is an established risk factor, but it is unclear why only a minority of homozygous individuals ever develop clinically significant liver disease.3 The risk conferred by heterozygosity for the Z allele is controversial, and genotype MZ is not a generally accepted risk factor. The S allele is another common A1ATD risk allele, which does not cause abnormal protein folding and is not associated with an increased liver disease risk, unless in compound heterozygosity with the Z allele (SZ).4
In A1ATD‐associated lung disease, the severity of emphysema is inversely correlated to the concentration of A1AT in plasma.5, 6 The plasma concentration of the acute phase protein A1AT is only in part determined by the SERPINA1 genotype and is highly variable in MZ patients. This complicates studies on the liver disease risk mediated by the MZ genotype because not all patients have low A1AT plasma concentrations.7 A1ATD is defined as A1AT in plasma <80 mg/dL, which has a high positive predictive value for PI*Z or PI*S in patients with normal liver function.8 However, normal plasma A1AT concentrations exclude neither Z or S allele carrier status nor the presence of Z protein accumulation in the liver.7, 8
Hepatocellular damage correlates with the amount of Z protein accumulation within the cell, which cannot be assessed noninvasively.9 Data from epidemiological studies suggest that A1ATD due to heterozygous SERPINA1 mutations is not associated with an increased cirrhosis risk.4, 10 However, studies in patients with additional risk factors for chronic liver disease such as hepatitis C virus infection, cystic fibrosis, or hemochromatosis show that Z allele carriers are more likely to progress to or present with advanced cirrhosis.11, 12, 13, 14
To overcome the limitation that the concentration of A1AT in plasma has poor sensitivity and specificity for diagnosing heterozygous risk alleles, especially in the context of advanced liver disease, we genotyped an unselected group of patients with liver cirrhosis, liver transplant recipients, and liver donors for the SERPINA1 S‐ and Z‐risk alleles and compared allele frequencies among groups. To determine the liver disease risk mediated by the MZ genotype, clinical and biochemical parameters of liver function and Model for End‐Stage Liver Disease–sodium (MELD‐Na) scores were ascertained at baseline when the diagnosis of cirrhosis was made and at follow‐up before death or liver transplantation.
Patients and Methods
For this single‐center retrospective study, informed consent for genetic testing, biobanking, and survival analysis was obtained from all patients included. This study was approved a priori by the ethics committee of the Medical University of Innsbruck (study number UN4218/AN3478).
PATIENT SELECTION AND DATA ACQUISITION
An electronic medical record search of documents from the Gastroenterology and Hepatology Department of the University Hospital of Innsbruck was carried out. Patients referred for genotyping of SERPINA1 to the Laboratory of Hepatology, Medical University of Innsbruck, Austria, between January 1, 2000 and December 31, 2016 (n = 2117) were analyzed. Patients with cirrhosis were identified by an electronic medical record search of documents of the University Hospital of Innsbruck for the diagnosis of cirrhosis. A total of 16,931 documents were identified. These documents referred to 596 individual patients with SERPINA1 genotype MM or MZ. A manual search in these documents was carried out to confirm the correct diagnosis of cirrhosis based on clinical, laboratory, radiological, and/or histological findings (545 patients, 51 were excluded because cirrhosis could not be confirmed) and to obtain additional clinical data (etiology of liver disease and hepatic decompensation). Patients with ZZ (n = 3) and SZ (n = 2) were also excluded. DNA samples from 248 SERPINA1 genotyped liver transplant donors and 21 MS patients with cirrhosis were used as a control group for comparison of genotype frequency.
Time of death was obtained from social insurance institutions. Baseline and follow‐up biochemical parameters were determined by routine laboratory tests and were extracted from the electronic medical records. Baseline laboratory parameters collected at the time of first cirrhosis diagnosis were recorded for each patient. The study endpoint was defined as last patient contact, liver transplantation, or death. Laboratory parameters at the last possible follow‐up visit were recorded. For liver transplant recipients, the last blood test prior to liver transplantation was used. For survival analysis, the time from the diagnosis of cirrhosis to liver transplantation or death as a combined endpoint was assessed. Genotyping was carried out using an internally validated TaqMan allelic discrimination assay (TaqMan SNP Genotyping assay for reference SNP ID number rs17580 and rs28929474, ThermoFisher Scientific, Vienna, Austria).
STATISTICAL ANALYSIS
Quantitative data were expressed as means ± standard deviation if normally distributed or for non‐normally distributed variables as medians with first and third quartiles. Normality of distribution was determined by the Kolmogorov‐Smirnov test. Frequencies are reported as absolute numbers or percentages as indicated. Quantitative variables were compared using the paired or unpaired Student t test or the nonparametric Mann‐Whitney U or Wilcoxon signed‐rank test as appropriate. Contingency tables were tested for significance using the Fisher's exact or χ2 tests. A P value <0.05 was considered as statistically significant. Receiver operating characteristic calculations were performed, and the Youden index was applied to calculate the optimal threshold. Binary logistic regression analysis was used to estimate the probability of the association between explanatory variables with SERPINA1 genotype. Survival was graphed using the Kaplan‐Meier method, and groups were compared with the log‐rank test. To assess prognostic significance of specific parameters in a cumulative incidence analysis, the Gray's test was used. To identify predictors of mortality, a Cox proportional hazards model was used. Correlation was carried out using the Spearman's rank correlation coefficient. The Benjamini‐Hochberg method was used with an accepted false discovery rate of <0.05 to correct for multiple testing. All statistical analyses were performed using SPSS Statistics, version 22 (IBM, Chicago, IL); MedCalc, version 17 (MedCalc Software, Ostend, Belgium); and R, version 3.4.1 (R Project for Statistical Computing, Vienna, Austria).
Results
In this cohort of 540 patients with cirrhosis, heterozygous Z allele carriers and patients with SERPINA1 genotype MM had comparable liver function at baseline and a significantly higher median MELD‐Na and Model for End‐Stage Liver Disease (MELD) score at the end of follow‐up (Fig. 1A,B; Supporting Table 1). Age and underlying etiology of liver disease were not significantly different between the group of MM and MZ patients (Tables 1 and 2). Patient stratification by MELD‐Na quartiles showed a gradually increasing prevalence of risk allele carriers (MZ) in subgroups with higher MELD‐Na scores (Fig. 1C). When individual markers of liver function were compared, albumin and fibrinogen were significantly lower and international normalized ratio (INR) was significantly higher in the MZ group (Table 2). These findings show that across etiologies MZ is associated with more advanced liver disease at the end of follow‐up.
Figure 1.
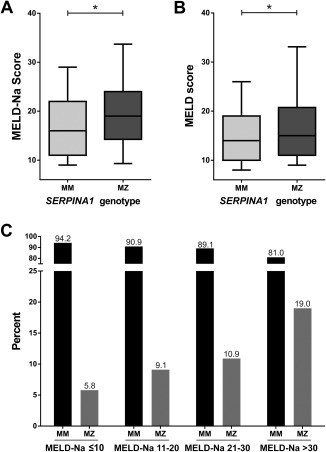
(A) MELD‐Na and (B) MELD scores of patients with MM or MZ SERPINA1 genotype at the time of follow‐up. (C) Comparison of SERPINA1 genotype frequencies in patients grouped according to MELD‐Na score quartiles. The overall degree of association between increasing MELD‐Na score and MZ prevalence was statistically significant (P = 0.01, 1‐sided Mantel‐Haenszel test for linear‐by‐linear association).
Table 1.
Etiologies of Patients With the MM or MZ Genotype
| n | MM | n | MZ | |
|---|---|---|---|---|
| Alcoholic liver disease | 122 | 25% | 10 | 23% |
| NAFLD/cryptogenic | 144 | 30% | 11 | 26% |
| Viral | 138 | 28% | 14 | 33% |
| Other | 84 | 17% | 8 | 18% |
NOTE: Nine patients with the clinical diagnosis A1ATD were excluded from the analysis.
Table 2.
Patient Demographics and Follow‐Up Biochemical Characteristics in Patients With SERPINA1 MM and MZ Genotype
| n | MM | n | MZ | P Value | |
|---|---|---|---|---|---|
| Age, years | 488 | 59.0 (52.3‐65.5) | 52 | 59.2 (52.6‐64.5) | 0.95 |
| BMI, kg/m2 | 305 | 25.5 (22.5‐28.6) | 30 | 28.1 (24.6‐30.8) | 0.03 |
| MELD score | 488 | 14 (10‐19) | 52 | 15 (11‐21) | 0.047 |
| MELD‐Na score | 488 | 16 (11‐22) | 52 | 19 (14‐24) | 0.03 |
| A1AT, mg/dL | 378 | 170 (146‐197) | 39 | 100 (81‐118) | <0.001 |
| Iron, μmol/L | 443 | 17.2 (10.6‐26.1) | 44 | 20.4 (12.9‐27.3) | 0.20 |
| Ferritin, μg/L | 443 | 196.0 (62.0‐510.0) | 44 | 280.5 (82‐686.8) | 0.13 |
| Tf, mg/dL | 447 | 214.0 (151.0‐267.0) | 48 | 199.0 (151.0‐244.3) | 0.31 |
| Tf saturation % | 443 | 36.0 (19.0‐69.0) | 44 | 42.0 (20.0‐82.5) | 0.16 |
| Creatinine, mg/dL | 488 | 0.9 (0.7‐1.1) | 52 | 0.92 (0.8‐1.2) | 0.70 |
| Bilirubin, mg/dL | 488 | 2.0 (1.1‐3.9) | 52 | 2.71 (1.4‐5.1) | 0.03 |
| INR | 488 | 1.3 (1.2‐1.6) | 52 | 1.5 (1.3‐1.8) | 0.004 |
| Fibrinogen, mg/dL | 466 | 231.0 (166.8‐319.3) | 51 | 164.0 (96.0‐243.0) | <0.001 |
| Albumin, mg/dL | 486 | 3452.0 (3003.8‐3930.0) | 52 | 3090.0 (2762.5‐3730.0) | 0.02 |
| Platelets, G/L | 488 | 81.0 (53.0‐123.0) | 52 | 72.5 (53.0‐130.0) | 0.88 |
| Sodium, mmol/L | 488 | 137.0 (134.0‐140.0) | 52 | 136.5 (132.0‐139.8) | 0.06 |
| CRP, mg/dL | 488 | 0.6 (0.2‐1.5) | 52 | 0.8 (0.3‐1.6) | 0.28 |
| ALT, U/L | 488 | 33.0 (22‐51.8) | 52 | 33.0 (23‐58.3) | 0.69 |
| Time diagnosis to follow‐up years | 488 | 2.4 (1.0‐5.4) | 52 | 2.0 (0.8‐4.6) | 0.16 |
| Death or transplantation after 1 year (numbers at risk), n | 488 | 80 (375) | 52 | 11 (37) | 0.43 |
| Death or transplantation after 3 years (numbers at risk), n | 488 | 189 (218) | 52 | 25 (20) | 0.27 |
NOTE: Data are given as median (IQR), unless otherwise noted.
When patients were grouped by transplant status, the prevalence of MZ was highest among liver transplant recipients. Heterozygosity for the Z allele was less prevalent in nontransplanted patients with cirrhosis than in liver transplant patients, but still significantly more common than in a control group of liver donors, representing a control group from the same geographical region (8.8% versus 3%; P = 0.01). This difference was not found for the S allele, which had a similar prevalence among the 3 groups (Fig. 2). Taken together, these findings demonstrate that the Z but not the S allele is more prevalent among patients with end‐stage liver disease and liver transplant recipients than in liver donors.
Figure 2.
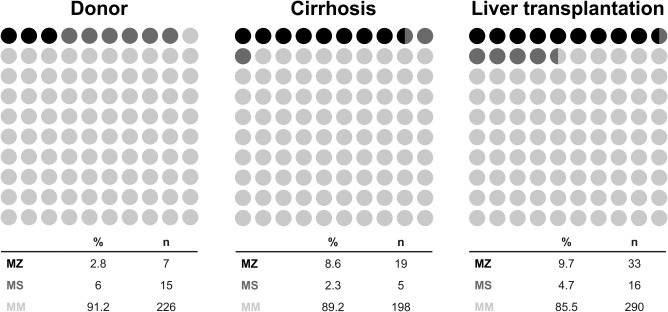
Comparison of SERPINA1 genotype frequencies in patients with liver cirrhosis, liver transplant recipients, and controls (healthy liver donors).
In search for specific decompensating events in association with PI*MZ, the prevalence of ascites, hepatic encephalopathy, variceal bleeding, and hepatocellular carcinoma was compared between groups of patients with cirrhosis and different SERPINA1 genotypes. This analysis revealed that at the end of follow‐up, hepatic encephalopathy and ascites were significantly more common in MZ patients. No statistically significant differences in variceal bleeding or HCC were observed (Table 3).
Table 3.
Decompensating Events in Patients With Cirrhosis Stratified by SERPINA1 MM and MZ Genotype
| n | MM | n | MZ | P | |
|---|---|---|---|---|---|
| No ascites | 144 | 30.2% | 9 | 18.0% | 0.046 |
| Ascites | 333 | 69.8% | 41 | 82.0% | |
| No encephalopathy | 251 | 52.4% | 19 | 38.8% | 0.047 |
| Encephalopathy | 228 | 47.6% | 30 | 61.2% | |
| No variceal bleeding | 328 | 78.8% | 38 | 82.6% | 0.35 |
| Variceal bleeding | 88 | 21.2% | 8 | 17.4% | |
| No hepatocellular carcinoma | 329 | 67.4% | 40 | 76.9% | 0.11 |
| Hepatocellular carcinoma | 159 | 32.6% | 12 | 23.1% |
Next, the diagnostic utility of A1AT concentration in serum was assessed for the identification of MZ patients at baseline. As shown in Fig. 3, mean serum A1AT concentration was significantly lower in the MZ patient group when compared with MM patients (108.0 versus 174.0 mg/dL; P < 0.001). Although A1AT is negatively correlated with MELD‐Na score (ρ = –0.13; P = 0.009; Fig. 4), binary logistic regression analysis showed that the plasma concentration of A1AT is a MELD‐Na independent predictor of SERPINA1 genotype (P < 0.001). The optimal diagnostic test performance was achieved with a cutoff of 137 mg/dL A1AT concentration (Supporting Fig. 1). An A1AT concentration ≤137 mg/dL had a sensitivity of 84% (95% confidence interval [CI], 70.9%‐92.8%) and a specificity of 86.9% (95% CI, 83.4%‐89.9%) for the diagnosis of risk allele carrier status. The corresponding negative predictive value is 97.7%, and the positive predictive value is 43.2%.
Figure 3.
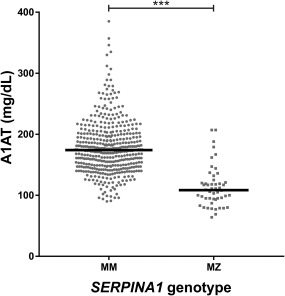
A1AT plasma concentrations of patients with SERPINA1 genotype MM and MZ at the time of diagnosis of cirrhosis.
Figure 4.
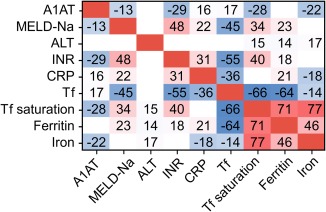
Correlation matrix among A1AT plasma concentration, markers of liver disease, inflammation, and iron metabolism. Tile colors code for direction and magnitude of the correlation. The first 2 decimal digits of ρ are displayed. Only significant correlations are shown.
In search for laboratory parameters associated with A1AT, a significant negative correlation with MELD‐Na and INR as surrogates of liver function was confirmed. In addition, A1AT and C‐reactive protein (CRP) as an indicator of inflammation were positively correlated. A1AT is negatively correlated with transferrin (Tf) saturation and positively with Tf (ρ = –0.28, P < 0.001; ρ = 0.17, P < 0.001; Fig. 4), but no significant correlation with the acute‐phase reactant ferritin was found.
To study the impact of the MZ genotype on survival, a Kaplan‐Meier curve analysis was carried out. As shown in Fig. 5A, the proportion of patients surviving without liver transplantation was lower in the group of Z allele carriers at all time points, when compared with MM patients. However, this difference did not reach statistical significance (P = 0.07). To further stratify survival analysis for established risk factors for liver transplantation and death, patient subgroups with MELD‐Na score ≥15, Tf saturation ≥50%, or Tf ≤ 180 mg/dL were analyzed independently. As shown in Fig. 5B‐D, MZ was associated with increased risk of liver transplantation/mortality as compared with MM when stratified for each risk factor. Significant differences were noted in patients with a Tf saturation ≥50% and in patients with Tf ≤ 180 mg/dL. Multivariate Cox regression analysis further showed that in the subgroup of patients with high Tf saturation, the SERPINA1 genotype was a MELD‐Na independent predictor of transplantation/survival (hazard ratio, 1.32; 95% CI, 1.03‐1.70; P = 0.03; Supporting Table 2).
Figure 5.
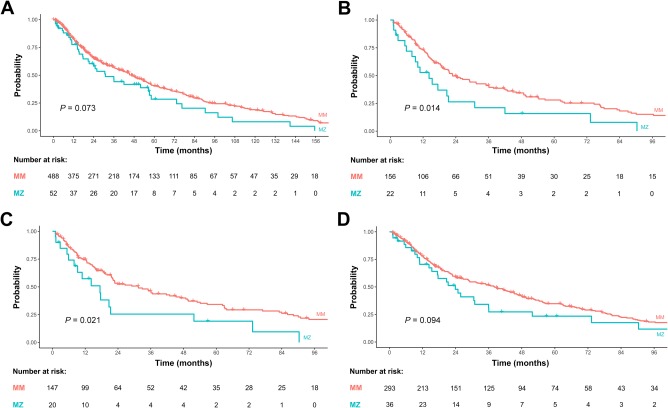
Kaplan‐Meier analysis: (A) transplant‐free survival in all patients stratified by SERPINA1 genotype. Transplant‐free survival in the subgroup of patients with (B) Tf saturation ≥ 50%, (C) with Tf of ≤180 mg/dL, and (D) with MELD‐Na ≥15 at baseline.
To assess the effect of the SERPINA1 genotype on each outcome of the combined endpoint transplantation/mortality separately, a competitive risk analysis was carried out. The majority of patients underwent liver transplantation, and only a small proportion of patients died. As a result, for the entire cohort and all subgroups analyzed (patients with Tf saturation ≥50%, Tf of ≤180 mg/dL or MELD‐Na ≥15 at baseline), the likelihood for receiving a donor organ was higher in patients with the MZ genotype at all time points, whereas mortality did not differ between subgroups (Supporting Fig. 2).
Discussion
In this cohort of patients with cirrhosis, we found that MZ patients have significantly higher MELD‐Na scores and impaired hepatic synthetic function without differences in kidney function. These findings support the conclusion that the MZ genotype is a risk factor for more advanced disease in patients with cirrhosis.
Hepatocellular accumulation of misfolded A1AT Z protein polymers is an established risk factor for the development of cirrhosis in adults homozygous for the Z allele.15, 16 In contrast, the risk conferred by the MZ genotype is controversial.11, 17 It has been proposed that endoplasmic reticulum (ER)–stress response and ER‐associated degradation can protect affected cells from potential damage and remove the mutant protein. When only a single allele is affected as in MZ patients, these protective mechanisms are more likely to fully compensate for the genetic defect.16, 18 In accordance with this hypothesis, one can speculate that additional hepatocellular stressors such as alcohol, fatty liver, or chronic hepatitis virus infection are more likely to overcharge the cellular ER‐stress response in MZ patients and cause cell death.
Findings from our cohort also demonstrate that higher MELD‐Na scores and more advanced liver disease are reflected in a higher prevalence of patients with the MZ genotype, but not the genotype MS, among patients with liver cirrhosis and liver transplant recipients when compared with liver donors. The prevalence of MZ in the donor group matched the expected frequency of the general Austrian population (2.9%).19
The higher liver transplantation rate among MZ patients was also paralleled by more frequent hepatic decompensation with ascites and hepatic encephalopathy but not due to variceal hemorrhage or occurrence of hepatocellular carcinoma. Epidemiological studies suggest the ZZ patients have an increased risk for the development of hepatocellular carcinoma.15 Obvious differences between previous studies and the present cohort are that liver disease in MZ patients may not just be a mild variant of ZZ A1ATD and the increased risk reported in patients with the A1AT phenotype PI*ZZ could be accounted for by the increased prevalence of cirrhosis in these patients. PI*ZZ therefore is a risk factor for HCC when compared with a control group without cirrhosis, but patients with established cirrhosis heterozygous for the Z allele do not have a higher HCC risk than patients with cirrhosis and SERPINA1 genotype MM.
The risk for reaching the combined endpoint death or liver transplantation was not significantly different for patients with cirrhosis with genotype MZ and MM. In contrast, in the subgroup of patients with low Tf (≤180 mg/dL) or elevated Tf saturation (≥50%), MZ patients had significantly worse outcomes compared with MM controls. This observation further supports previous studies showing an association between A1ATD and iron metabolism.20, 21, 22, 23 The functional link between iron and A1AT in cirrhosis is also reflected in the results of our correlation analysis. Impaired hepatic synthetic function is expected to reduce the concentration of A1AT and Tf, whereas inflammation is expected to increase A1AT but reduce Tf. The observed negative correlation between A1AT and Tf suggests that in cirrhosis, inflammation rather than liver function determines the functional interaction of A1AT and serum iron parameters.
The prognostic implication of the Z allele renders diagnosis of this risk factor important. In contrast to previous studies, we have used genotyping instead of isoelectric focusing for the diagnosis of the SERPINA genotype in unselected patients with cirrhosis. Although median A1AT concentration was significantly lower in MZ patients, the positive predictive value was only 43%. This highlights that quantitative analysis of A1AT concentration in plasma is insufficient to screen for the risk allele. Accordingly, Cox regression analysis did not show that A1AT concentration is a MELD‐Na–independent significant predictor of clinically relevant endpoints. In contrast, MZ is an independent risk factor for liver disease severity, liver transplantation and death in patients with cirrhosis, and elevated Tf saturation.
According to current guidelines, only patients with unexplained chronic liver disease should be tested. Our results indicate that in all patients with cirrhosis, regardless of the underlying etiology Z allele carriers are at higher risk.24 Considering the low positive predictive value of A1AT serum concentration, for accurate risk assessment and prognostication genotyping for the Z allele should be considered the first‐line test in all patients with cirrhosis. Currently, early liver transplantation is the only causal treatment for A1ATD, but targeted therapies are under development, which include chemical chaperones, autophagy stimulating agents, and silencing of A1AT production in the liver (reviewed in Ref. 16).
In conclusion, SERPINA1 genotyping for the Z allele when cirrhosis is diagnosed can assist risk assessment. PI*MZ patients with low Tf concentration or elevated Tf saturation are especially more likely to develop encephalopathy or ascites.
Supporting information
Additional supporting information may be found in the online version of this article.
Supporting Information Figure 1
Supporting Information Figure 2
Supporting Information 1
Stefan Schneeberger consults for Astrellas and Chiesi; is on the speaker's bureau for Astellas, Genzyme, and MSD; and receives grants/contracts (unrestricted) from Sandoz and Chiesi.
The study was funded by the Medical University of Innsbruck. Benedikt Schaefer received a grant from the Verein zur Foerderung der Wissenschaft in Gastroenterologie and Hepatologie. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1. Greene CM, Marciniak SJ, Teckman J, Ferrarotti I, Brantly ML, Lomas DA, et al. α1‐antitrypsin deficiency. Nat Rev Dis Primers 2016;2:16051. [DOI] [PubMed] [Google Scholar]
- 2. Chu AS, Chopra KB, Perlmutter DH. Is severe progressive liver disease caused by alpha‐1‐antitrypsin deficiency more common in children or adults? Liver Transpl 2016;22:886‐894. [DOI] [PubMed] [Google Scholar]
- 3. Teckman JH, Rosenthal P, Abel R, Bass LM, Michail S, Murray KF, et al.; for Childhood Liver Disease Research Network (ChiLDReN) . Baseline analysis of a young alpha‐1‐antitrypsin deficiency liver disease cohort reveals frequent portal hypertension. J Pediatr Gastroenterol Nutr 2015;61:94‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nelson DR, Teckman J, Di Bisceglie AM, Brenner DA. Diagnosis and management of patients with alpha1‐antitrypsin (A1AT) deficiency. Clin Gastroenterol Hepatol 2012;10:575‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DeMeo DL, Silverman EK. Alpha1‐antitrypsin deficiency. 2: genetic aspects of alpha(1)‐antitrypsin deficiency: phenotypes and genetic modifiers of emphysema risk. Thorax 2004;59:259‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chorostowska‐Wynimko J. Disease modification in emphysema related to alpha‐1 antitrypsin deficiency. COPD 2016;13:807‐815. [DOI] [PubMed] [Google Scholar]
- 7. Kok KF, Willems HL, Drenth JP. The cut‐off value of 100 mg/dl is insufficient to detect heterozygous alpha‐1 antitrypsin‐deficient liver disease patients. Liver Int 2010;30:491‐492. [DOI] [PubMed] [Google Scholar]
- 8. Bornhorst JA, Greene DN, Ashwood ER, Grenache DG. α1‐Antitrypsin phenotypes and associated serum protein concentrations in a large clinical population. Chest 2013;143:1000‐1008. [DOI] [PubMed] [Google Scholar]
- 9. Lindblad D, Blomenkamp K, Teckman J. Alpha‐1‐antitrypsin mutant Z protein content in individual hepatocytes correlates with cell death in a mouse model. Hepatology 2007;46:1228‐1235. [DOI] [PubMed] [Google Scholar]
- 10. Sveger T. The natural history of liver disease in alpha 1‐antitrypsin deficient children. Acta Paediatr Scand 1988;77:847‐851. [DOI] [PubMed] [Google Scholar]
- 11. Regev A, Guaqueta C, Molina EG, Conrad A, Mishra V, Brantly ML, et al. Does the heterozygous state of alpha‐1 antitrypsin deficiency have a role in chronic liver diseases? interim results of a large case‐control study. J Pediatr Gastroenterol Nutr 2006;43(suppl 1):S30‐S35. [DOI] [PubMed] [Google Scholar]
- 12. Graziadei IW, Joseph JJ, Wiesner RH, Therneau TM, Batts KP, Porayko MK. Increased risk of chronic liver failure in adults with heterozygous alpha1‐antitrypsin deficiency. Hepatology 1998;28:1058‐1063. [DOI] [PubMed] [Google Scholar]
- 13. Eigenbrodt ML, McCashland TM, Dy RM, Clark J, Galati J. Heterozygous alpha 1‐antitrypsin phenotypes in patients with end stage liver disease. Am J Gastroenterol 1997;92:602‐607. [PubMed] [Google Scholar]
- 14. Bartlett JR, Friedman KJ, Ling SC, Pace RG, Bell SC, Bourke B, et al.; for Gene Modifier Study Group . Genetic modifiers of liver disease in cystic fibrosis. JAMA 2009;302:1076‐1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eriksson S, Carlson J, Velez R. Risk of cirrhosis and primary liver cancer in alpha 1‐antitrypsin deficiency. N Engl J Med 1986;314:736‐739. [DOI] [PubMed] [Google Scholar]
- 16. Lomas DA, Hurst JR, Gooptu B. Update on alpha‐1 antitrypsin deficiency: new therapies. J Hepatol 2016;65:413‐424. [DOI] [PubMed] [Google Scholar]
- 17. Cacciottolo TM, Gelson WT, Maguire G, Davies SE, Griffiths WJ. Pi*Z heterozygous alpha‐1 antitrypsin states accelerate parenchymal but not biliary cirrhosis. Eur J Gastroenterol Hepatol 2014;26:412‐417. [DOI] [PubMed] [Google Scholar]
- 18. Carrell RW, Lomas DA. Alpha1‐antitrypsin deficiency‐‐a model for conformational diseases. N Engl J Med 2002;346:45‐53. [DOI] [PubMed] [Google Scholar]
- 19. de Serres FJ, Blanco I. Prevalence of α1‐antitrypsin deficiency alleles PI*S and PI*Z worldwide and effective screening for each of the five phenotypic classes PI*MS, PI*MZ, PI*SS, PI*SZ, and PI*ZZ: a comprehensive review. Ther Adv Respir Dis 2012;6:277‐295. [DOI] [PubMed] [Google Scholar]
- 20. Rabinovitz M, Gavaler JS, Kelly RH, Van Thiel DH. Association between heterozygous alpha 1‐antitrypsin deficiency and genetic hemochromatosis. Hepatology 1992;16:145‐148. [DOI] [PubMed] [Google Scholar]
- 21. Kaserbacher R, Propst T, Propst A, Graziadei I, Judmaier G, Vogel W. Association between heterozygous alpha 1‐antitrypsin deficiency and genetic hemochromatosis. Hepatology 1993;18:707‐708. [PubMed] [Google Scholar]
- 22. Elzouki AN, Hultcrantz R, Stål P, Befrits R, Eriksson S. Increased PiZ gene frequency for alpha 1 antitrypsin in patients with genetic haemochromatosis. Gut 1995;36:922‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schaefer B, Haschka D, Finkenstedt A, Petersen BS, Theurl I, Henninger B, et al. Impaired hepcidin expression in alpha‐1‐antitrypsin deficiency associated with iron overload and progressive liver disease. Hum Mol Genet 2015;24:6254‐6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sandhaus RA, Turino G, Brantly ML, Campos M, Cross CE, Goodman K, et al. The diagnosis and management of alpha‐1 antitrypsin deficiency in the adult. Chronic Obstr Pulm Dis 2016;3:668‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article.
Supporting Information Figure 1
Supporting Information Figure 2
Supporting Information 1


