Abstract
Hepatocyte growth factor (HGF)/c‐Met pathway dysregulation is a mechanism for epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs). Ficlatuzumab (AV‐299; SCH 900105), a humanized IgG1κ HGF inhibitory monoclonal antibody, prevents HGF/c‐Met pathway ligand–mediated activation. This phase 1b study assessed the safety/tolerability, pharmacokinetics/pharmacodynamics, and antitumor activity of ficlatuzumab plus gefitinib in Asian patients with previously treated advanced non–small cell lung cancer (NSCLC). Patients received intravenous ficlatuzumab either 10 mg/kg (cohort 1; n = 3) or 20 mg/kg (cohort 2; n = 12) every 2 weeks plus oral gefitinib 250 mg daily. Patients tolerated the drug combination well. Four treatment‐related grade 3/4 adverse events were reported in 3 patients (cohort 2). Pharmacokinetic profiles for ficlatuzumab and gefitinib were consistent with prior single‐agent trials. Partial responses were achieved in 5 patients (4 confirmed), all in cohort 2; objective response rate (ORR) was 33% (duration, 1.9–6.4 months). Responding patients had no prior EGFR TKI treatment, 2 without an EGFR mutation. Four additional patients had disease stabilization (cohort 2; duration, 2.7–9.1 months; 42% ORR). The recommended phase 2 dose for ficlatuzumab plus gefitinib 250 mg/day was 20 mg/kg every 2 weeks. This drug combination has shown preliminary dose‐related antitumor activity in advanced NSCLC.
Keywords: ficlatuzumab, NSCLC, gefitinib, lung adenocarcinoma, non‐small cell lung cancer
Targeted therapies are now a well‐established part of the treatment spectrum for patients with advanced non–small cell lung cancer (NSCLC).1 Currently, the most widely used agents are tyrosine kinase inhibitors (TKIs) that target the epidermal growth factor receptor (EGFR), such as erlotinib and gefitinib, and the antiangiogenic agent bevacizumab. EGFR TKIs achieve a response rate of about 10% in a population of unselected NSCLC patients, but have significantly greater activity in NSCLC patients with activating somatic mutations of the EGFR gene (primarily L858R mutations and exon 19 deletions), with a response rate of about 70%.2, 3, 4, 5, 6, 7
Although patients with advanced NSCLC and EGFR mutations show a high response rate and prolonged progression‐free survival (PFS) following treatment with EGFR TKIs, almost all patients will eventually develop resistance to these agents.8 Secondary mutations in EGFR and dysregulation of the hepatocyte growth factor (HGF)/c‐Met pathway have been identified as some of the key mechanisms of acquired resistance to gefitinib and erlotinib.9, 10, 11, 12, 13
HGF is the only known soluble ligand for the c‐Met receptor tyrosine kinase and plays a key role in regulating cell proliferation, motility, and differentiation, particularly during embryogenesis and injury repair.14, 15, 16 In patients with NSCLC, high serum and plasma levels of HGF appear to be associated with poor prognosis17 and intrinsic resistance to gefitinib.18, 19 High tumor HGF expression has also been associated with both intrinsic and acquired resistance to EGFR TKIs.13 Moreover, the presence of HGF can accelerate NSCLC‐cell resistance to EGFR TKIs by promoting clonal selection of a subpopulation of cells with c‐Met amplification.20 Preclinical studies in human lung cancer cell lines and lung tumor xenografts in transgenic mice have shown promising results with dual HGF/c‐Met and EGFR inhibition, including additive antitumor activity and restoration of EGFR‐TKI sensitivity.21, 22, 23, 24, 25, 26, 27, 28 Taken together, these results indicate that combined EGFR and HGF/c‐Met inhibition is a promising strategy to overcome intrinsic and acquired resistance and thereby to improve the outcomes of NSCLC patients.
Ficlatuzumab (AV‐299; SCH 900105) is a humanized IgG1κ inhibitory monoclonal antibody that binds to HGF with high affinity and prevents the ligand from activating the c‐Met receptor.29 Consequently, ficlatuzumab inhibits tumor growth of NSCLC xenografts, reducing angiogenesis and cell proliferation while increasing cell death.22 Ficlatuzumab in combination with erlotinib or cetuximab demonstrated increased antitumor activity compared with either agent alone, and the combination resulted in complete tumor regression in mice bearing established NSCLC xenografts.22 Ficlatuzumab decreased phospho‐c‐Met and phospho‐Akt levels in NSCLC tumor lysates when administered alone or in combination.30
Ficlatuzumab was found to have an acceptable safety/tolerability profile and preliminary clinical activity when administered either as monotherapy or in combination with erlotinib in a phase 1 study of 41 patients with advanced tumors.31 Common adverse events (AEs) in the 13 patients who received combination therapy (ficlatuzumab 20 mg/kg plus erlotinib 150 mg/day) were rash (62%) and diarrhea (46%). Pharmacokinetic (PK) profiles of ficlatuzumab and erlotinib were similar to those observed in single‐agent trials, indicating no drug–drug interaction. The most frequently reported treatment‐emergent adverse events (TEAEs) for the 15 patients receiving monotherapy 20 mg/kg in this phase 1 study were peripheral edema (8 patients), fatigue and vomiting (reported by 5 patients each), and hypokalemia and nausea (reported by 4 patients each). The most frequently reported grade 3/4 TEAE with ficlatuzumab monotherapy was hypokalemia (4 patients). Stable disease was evident in 12 of 21 efficacy‐evaluable patients who received ficlatuzumab monotherapy, including 1 patient with ongoing stable disease lasting longer than 4 years. The recommended phase 2 dose (RP2D) for the combination was 20 mg/kg intravenous ficlatuzumab every 2 weeks and oral erlotinib 150 mg/day.
In the current phase 1b study, we evaluated the safety, tolerability, PK, pharmacodynamics (PD), and antitumor activity of ficlatuzumab in combination with gefitinib in Asian patients with advanced NSCLC. The study is registered at http://www.clinicaltrials.gov as NCT01039948. A randomized phase 2 study in first‐line NSCLC patients treated with gefitinib with or without ficlatuzumab was recently completed and has recently been published.32
Material and Methods
Study Design
This was a phase 1b, open‐label, multicenter, dose‐escalation study of ficlatuzumab plus gefitinib in Asian patients with previously treated advanced NSCLC. A previous phase 1 study demonstrated that the highest administered dose of ficlatuzumab, 20 mg/kg, was well tolerated without any dose‐limiting toxicities (DLTs).31
Determination of the RP2D of Ficlatuzumab Plus Gefitinib
This phase 1b study followed a standard 3+3 dose‐escalation design, with 3 to 6 patients enrolled per dose level of ficlatuzumab to determine the RP2D. Ficlatuzumab was tested at doses of 10 and 20 mg/kg intravenously every 2 weeks, with all patients receiving oral gefitinib 250 mg/day in continuous 28‐day cycles. After the initial 6 patients completed cycle 1 at the dose selected for phase 2, an additional 6 patients were enrolled for an expanded assessment of safety and PK.
DLT was defined as any drug‐related grade 3 or 4 toxicity (hematologic or nonhematologic), drug‐related toxicity of any grade that resulted in a dose reduction during the first cycle of treatment, or drug‐related toxicity of any grade that resulted in an interruption of treatment for >2 weeks beyond the next scheduled dose. The following grade 3 toxicities lasting ≤48 hours were not considered DLTs: nausea and/or vomiting that could be managed with antiemetics, diarrhea that could be managed with antidiarrheals, fever without neutropenia that could be managed with antipyretics, and aspartate aminotransferase/alanine aminotransferase elevation.
The study was conducted in accordance with the Declaration of Helsinki in a manner consistent with International Conference on Harmonization and Good Clinical Practice guidelines, and all study‐related material was approved by the Institutional Review Board or Ethics Committee at the National Cancer Centre Department of Medical Oncology, Singapore, or Samsung Medical Center, Seoul, Korea. Written informed consent for participation was obtained from all patients before enrollment.
Patients
Patients were enrolled at 1 site in Hong Kong, 1 in Singapore, 3 in Malaysia, 3 in the Philippines, 3 in Thailand, 6 in Taiwan, and 7 in South Korea, for a total of 24 study sites. Men and women 18 years and older of Asian ethnicity were eligible if they met the following key criteria: diagnosis of unresectable NSCLC with or without prior therapy or other advanced solid tumor that progressed after standard therapy (however, only patients with NSCLC were enrolled in this study); Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2; adequate hematologic, hepatic, and renal function and coagulation parameters; and no active central nervous system metastases. Prior radiotherapy was allowed if completed > 14 days prior to study entry and any related toxicity was resolved. Patients were also required to have archived tumor tissue for determination of EGFR mutational status and immunohistochemistry analysis; however, this determination was not used as an eligibility criterion.
Patients were excluded from the study if they had any of the following: myocardial infarction within 6 months prior to initiation of study treatment; thrombotic or embolic events within the past 6 months; diarrhea grade ≥ 2 or active inflammatory bowel disease; severe acute or chronic medical, psychiatric, or behavioral condition or laboratory abnormality; diagnosis of interstitial lung disease; or serious active infection (grade > 2) within 14 days of starting treatment and/or uncontrolled infection requiring antibiotics, antivirals, or antifungals (including known HIV infection). Women of childbearing potential were required to have had a negative serum or urine pregnancy test within 7 days prior to study treatment.
End Points
The primary objectives of the study were to determine the safety, tolerability, and RP2D of ficlatuzumab when administered in combination with gefitinib. Secondary objectives included characterization of the PK profiles of ficlatuzumab and gefitinib when administered in combination, investigation of the effect of this combination on exploratory biomarkers, and assessment of the preliminary antitumor activity.
Safety
Patients were observed for 90 minutes following the first dose of study drug and then monitored throughout treatment and for a follow‐up period of 1 month after treatment discontinuation. Safety assessments included study drug exposure, concomitant medications, AEs, laboratory data, physical examinations, vital signs, electrocardiogram (ECG), and ECOG performance status. AEs were graded according to the National Cancer Institute Common Terminology Criteria for AEs, version 3.0, and were coded using MedDRA dictionary version 12 or later. The number and percentage of patients experiencing AEs were tabulated by system organ class and preferred term.
Efficacy
Tumor assessments via local reads were performed at baseline every 4 weeks for the first 4 cycles and then every 8 weeks. Measurements of lesions included number and location of target lesions, number and location of nontarget lesions, target lesion diameter, and sum of longest diameters. The standard method of assessment was x‐ray, computerized tomography, and magnetic resonance imaging, as appropriate. Response was determined using the Response Evaluation Criteria In Solid Tumors, version 1.1.33 The duration of response was measured from the date that the initial response was observed to the date that disease progression was observed. PFS was calculated from the time of first dose until progression or death from any cause, whichever came first, and was censored at the time of last tumor assessment if there was no documentation of progression.
Pharmacokinetics/Pharmacodynamics
PK parameters were calculated from serum or plasma levels of ficlatuzumab and gefitinib using a noncompartmental model. Levels of serum ficlatuzumab were quantified by a validated enzyme‐linked immunosorbent assay (PPD, Richmond, Virginia) as previously described.31 Gefitinib levels were analyzed by the LCMS 522 method via high‐performance liquid chromatography–tandem mass spectrometry detection (PPD, Richmond, Virginia). Whole‐blood samples were collected at each of the following times for PK evaluation of ficlatuzumab and gefitinib — cycle 1: at first dose of cycle (predose, immediately postinfusion, and 3, 6, 24, 48, and 96 hours and 1 week postinfusion), at 2 weeks (predose, immediately and 2 hours postinfusion), and prior to gefitinib dosing on days 22–28; cycles 2 and 3 and subsequent odd‐numbered cycles: predose and immediately postinfusion; cycle 4: at first dose of cycle (predose, immediately, and 3, 6, 24, 48, and 96 hours and 1 week postinfusion) and at 2 weeks (predose, immediately and 2 hours postinfusion); and at the 1‐month follow‐up visit (30 ± 3 days after last dose of study drug administration).
Serum for measuring ficlatuzumab antidrug antibody (ADA) was collected every odd‐numbered cycle on day 1 (predose), at the end of study, and at the 1‐month follow‐up visit. Peripheral blood samples to be assayed for serum HGF, a potential PD marker, were collected prior to study treatment and 3 hours postinfusion on day 1 of cycle 1, predose on days 2, 3, 8, and 22–28, predose and 2 hours postinfusion on day 15 of cycle 1, and predose on day 1 of cycle 3. Ficlatuzumab serum concentrations were measured in serum using a validated enzyme‐linked immunosorbent assay method. Briefly, ficlatuzumab was captured from serum samples by recombinant HGF (R&D Systems, Minneapolis, Minnesota) bound on a microtiter plate, and captured ficlatuzumab was detected with peroxidase‐labeled rabbit anti–human antibody (Dako, Carpinteria, California) and tetramethylbenzidine as substrate.
Pharmacokinetic parameters calculated for ficlatuzumab included minimum and maximum plasma concentration (Cmin, Cmax), time to peak plasma concentration (tmax), area under the plasma concentration–time curve from the time of dosing to the last measurable concentration and from baseline to infinity (AUC0→τ, AUC0→∞), clearance, half‐life (t1/2), apparent volume of distribution (Vd), and percent coefficient of variation (% CV), using noncompartmental analysis (Phoenix WinNonLin version 6.2; Pharsight Corporation, Mountain View, California).
EGFR Mutation Analysis
Tumor tissue samples obtained prior to treatment were assessed for EGFR mutation status using an EGFR Mutation kit EG‐04 (DxS Diagnostics, performed by Clarient, Aliso Viejo, California) or by Sanger sequencing of exons 18–21 (performed locally at the study site).
Statistical Analysis
The safety population included all patients who received at least 1 dose of either study drug. The efficacy population was defined as all patients who completed the first efficacy evaluation (cycle 1, days 25–28) or those with early progressive disease (before first scheduled efficacy evaluation) confirmed by imaging studies.
Continuous data were summarized using descriptive statistics (number of patients, mean, median, standard deviation, minimum, and maximum). Categorical data were summarized using frequencies and percentages. Safety observations and measurements, including study drug exposure, concomitant medications, AEs, DLTs, laboratory data, physical examinations, vital signs, ECGs, and ECOG performance status were summarized and presented in tables and listings. PK parameters were calculated using noncompartmental and/or compartmental models.
Best overall response was summarized by cohort and overall. Summaries included proportions of patients with best overall response of complete response (CR), CR unconfirmed, partial response (PR), PR unconfirmed, stable disease, progressive disease (or not evaluable), objective response rate (ORR), and disease control rate. Duration of response, time to progression, and PFS were calculated and presented in a listing. No formal statistical analysis was performed on efficacy data.
Results
Patient Characteristics and Disposition
A total of 15 NSCLC patients were enrolled and assessed for safety and efficacy. Their demographics and disease characteristics are summarized in Table 1.
Table 1.
Patient Demographic and Baseline Characteristics
| Characteristic | Ficlatuzumab 10 mg/kg Plus Gefitinib 250 mg (n = 3) | Ficlatuzumab 20 mg/kg Plus Gefitinib 250 mg (n = 12) | Total (n = 15) |
|---|---|---|---|
| Age (y), median (range) | 59 (54–60) | 61 (46–76) | 60 (46–76) |
| Sex, n (%) | |||
| Female | 2 (67) | 8 (67) | 10 (67) |
| Male | 1 (33) | 4 (33) | 5 (33) |
| Race, n (%) | |||
| Asian | 3 (100) | 12 (100) | 15 (100) |
| ECOG performance status, n (%) | |||
| 0 | 1 (33) | 3 (25) | 4 (27) |
| 1 | 2 (67) | 9 (75) | 11 (73) |
| Disease stage, n (%) | |||
| III | 0 | 3 (25) | 3 (20) |
| IV | 3 (100) | 9 (75) | 12 (80) |
| Smoking status, n (%) | |||
| Active | 0 | 1 (8) | 1 (7) |
| Former | 0 | 1 (8) | 1 (7) |
| Never | 3 (100) | 10 (83) | 13 (87) |
| Median number of prior chemotherapies, n (range) | 3 (1–4) | 2 (1–4) | 2 (1–4) |
| Prior EGFR TKI therapy, n (%) | |||
| Yes | 3 (100) | 7 (58) | 10 (67) |
| No | 0 | 5 (42) | 5 (33) |
| Tumor histopathology, n (%)a | |||
| NSCLC adenocarcinoma | 1 (33) | 10 (83) | 11 (73) |
| NSCLC nonadenocarcinoma | 1 (33) | 2 (17) | 3 (20) |
| Lymphoepithelial carcinoma | 1 (33) | 0 | 1 (7) |
| EGFR mutation status, n (%) | |||
| Mutation detected | 2 (67) | 1 (8) | 3 (20) |
| No mutation detected | 1 (33) | 6 (50) | 7 (47) |
| Not known | 0 | 5 (42) | 5 (33) |
ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; NSCLC, non–small cell lung cancer; TKI, tyrosine kinase inhibitor.
Percentages may not add up to 100% because of rounding.
All patients received at least 1 dose of ficlatuzumab plus gefitinib; 3 patients in cohort 1 (ficlatuzumab 10 mg/kg plus gefitinib 250 mg) and 12 patients in cohort 2 (ficlatuzumab 20 mg/kg plus gefitinib 250 mg). All patients eventually discontinued study treatment. All 3 patients in cohort 1 discontinued the study drug after cycle 1 because of disease progression. Eleven patients in cohort 2 discontinued because of disease progression (4 in cycle 1, 4 in cycles 3 and 4, 3 in cycles 6–8, and 1 in cycle 10). One patient in cohort 2 withdrew because of an AE (dyspnea) not related to the study drug.
Drug Administration
The median duration of study drug exposure was 4.0 weeks for patients in cohort 1 (4.0 weeks for all 3 patients) and 14.0 weeks for patients in cohort 2 (range, 4.0–40.0 weeks). All patients in cohort 1 received 2 infusions of ficlatuzumab, and patients in cohort 2 received a mean of 8.1 infusions. There were no dose modifications of ficlatuzumab, and all patients received approximately the intended dose. The duration of exposure to gefitinib was consistent with the duration of exposure to ficlatuzumab. Seven of the 15 patients (1 in cohort 1 and 6 in cohort 2) had at least 1 gefitinib dose modification.
Safety and Tolerability
No DLTs occurred in the first 9 patients; therefore, an additional 6 patients were enrolled in the cohort 2 dose for an expanded assessment of safety and PK profile. Two DLTs were subsequently reported in 2 patients in the expanded cohort 2 population. These DLTs were a grade 3 nonserious AE of dermatitis acneiform, which the investigator considered to be probably related to study drug (either ficlatuzumab or gefitinib) and a fatal AE of diffuse alveolar damage (interstitial lung disease), which the investigator considered to be probably related to gefitinib and unlikely to be related to ficlatuzumab. Combination therapy with ficlatuzumab and gefitinib was well tolerated by the majority of patients, and most AEs were grade 1 or 2 in severity. The most common AEs included dermatitis acneiform (10 patients, 67%), decreased appetite (7 patients, 47%), diarrhea (6 patients, 40%), paronychia, fatigue, and cough (5 patients, 33%). These and other events that occurred in 3 or more patients are summarized in Table 2.
Table 2.
Summary of Adverse Events Occurring in 3 or More Patients
| Adverse Event, n (%) | Ficlatuzumab 10 mg/kg Plus Gefitinib 250 mg (n = 3) | Ficlatuzumab 20 mg/kg Plus Gefitinib 250 mg (n = 12) | Total (n = 15) |
|---|---|---|---|
| Dermatitis acneiform | 1 (33) | 9 (75) | 10 (67) |
| Decreased appetite | 1 (33) | 6 (50) | 7 (47) |
| Diarrhea | 1 (33) | 5 (42) | 6 (40) |
| Cough | 2 (67) | 3 (25) | 5 (33) |
| Fatigue | 1 (33) | 4 (33) | 5 (33) |
| Paronychia | 0 | 5 (42) | 5 (33) |
| Back pain | 1 (33) | 2 (17) | 4 (27) |
| Hemoptysis | 1 (33) | 3 (25) | 4 (27) |
| Peripheral edema | 1 (33) | 3 (25) | 4 (27) |
| Abdominal distension | 1 (33) | 2 (17) | 3 (20) |
| Dizziness | 1 (33) | 2 (17) | 3 (20) |
| Dry skin | 0 | 3 (25) | 3 (20) |
| Dyspnea | 0 | 3 (25) | 3 (20) |
| Gingival bleeding | 0 | 3 (25) | 3 (20) |
| Nausea | 0 | 3 (25) | 3 (20) |
| Chest pain (noncardiac) | 0 | 3 (25) | 3 (20) |
| Pyrexia | 0 | 3 (25) | 3 (20) |
| Vomiting | 0 | 3 (25) | 3 (20) |
Grade ≥3 drug‐related AEs occurred in 3 patients, all in cohort 2, and included peripheral edema, paronychia, diffuse alveolar damage, and dermatitis acneiform. One of these patients discontinued after cycle 1 despite clinical response of unconfirmed PR following grade 4 dyspnea and grade 5 diffuse alveolar damage, and another patient in cohort 2 discontinued after cycle 10 because of grade 2 dyspnea. No other patients discontinued treatment because of AEs. Thirteen patients died, including all 3 patients in cohort 1 and 10 patients in cohort 2. Only 1 of these deaths was attributed to an AE (diffuse alveolar damage); the remaining deaths were attributed to disease progression.
Efficacy
PRs were achieved in 5 of 15 patients (4 confirmed and 1 unconfirmed), resulting in an ORR of 33% (95%CI, 12%–62%); see Table 3. All PRs occurred in patients treated in cohort 2, and an additional 4 patients in this cohort had disease stabilization with an ORR of 42% (95%CI, 15%–72%) and a disease control rate of 75% (95%CI, 43%–95%). The remaining 3 patients in cohort 2 and all 3 patients in cohort 1 had a best overall response of progressive disease. The duration of response in the 4 patients with confirmed PR ranged from 1.9 to 6.4 months, and all had progressed at the time of data cutoff. The duration of response in the 4 patients with stable disease ranged from 2.7 to 9.1 months.
Table 3.
Best Overall Response
| Response, n (%) | Ficlatuzumab 10 mg/kg Plus Gefitinib 250 mg (n = 3) | Ficlatuzumab 20 mg/kg Plus Gefitinib 250 mg (n = 12) | Total (n = 15) |
|---|---|---|---|
| Objective response | 0 | 5 (42) | 5 (33) |
| Complete response | 0 | 0 | 0 |
| Partial response | 0 | 5 (42) | 5 (33) |
| Confirmed | 0 | 4 (33) | 4 (27) |
| Unconfirmed | 0 | 1 (8) | 1 (7) |
| Stable disease | 0 | 4 (33) | 4 (27) |
| Progressive disease | 3 (100) | 3 (25) | 6 (40) |
| Not determined/not applicable/not evaluable | 0 | 0 | 0 |
NSCLC patients with an EGFR mutation, predominantly found in never smokers or light smokers, have a high response rate and long PFS with EGFR TKI treatment.7 Detailed descriptions of EGFR mutational status, smoking status, prior EGFR TKI use, and response to ficlatuzumab with gefitinib are listed in Table 4. Of the 11 patients who had prior anti‐EGFR therapy, 10 were treated with a TKI inhibitor. Interestingly, all 5 patients without prior EGFR TKI use achieved PR to the combination therapy, including 2 patients without an EGFR mutation (6–7 cycles on study). In addition, both patients who had an L858R mutation achieved PR to the combination therapy.
Table 4.
Patient Characteristics and Treatment Response
| Patient | Age | Sex | PS | Number of Prior Treatments | Prior EGFR Treatments | Prior Best Response on EGFR Treatment | Ficlatuzumab Dose (mg/kg) | Best Response on Study | Time on Study (Cycles) | EGFR Mutation Status | Smoking Status |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 59 | M | 1 | 1 | Gefitinib | PD | 10 | PD | 1 | Exon 19 del | Never |
| 2 | 60 | F | 0 | 4 | Gefitinib | PD | 10 | PD | 1 | No mutation detected | Never |
| 3 | 54 | F | 1 | 3 | Gefitinib | SD | 10 | PD | 1 | Exon 19 del | Never |
| 4 | 54 | F | 0 | 4 | Gefitinib | PR | 20 | SD | 4 | N/A | Never |
| 5 | 46 | F | 1 | 3 | Erlotinib | SD | 20 | PD | 1 | No mutation detected | Never |
| 6 | 70 | F | 1 | 4 | Gefitinib | SD | 20 | SD | 10 | No mutation detected | Never |
| 7 | 70 | M | 1 | 2 | Erlotinib | SD | 20 | SD | 4 | N/A | Never |
| 8 | 68 | F | 1 | 1 | Gefitinib | PR | 20 | PD | 1 | No mutation detected | Never |
| 9 | 59 | F | 1 | 4 | Gefitinib | SD | 20 | PD | 1 | N/A | Never |
| 10 | 59 | F | 0 | 4 | Gefitinib | PR | 20 | SD | 3 | N/A | Never |
| 11 | 62 | M | 1 | 2 | No | N/A | 20 | PR | 8 | L858R | Never |
| 12 | 48 | M | 1 | 2 | Cetuximab | SD | 20 | PR | 3 | N/A | Former |
| 13 | 60 | M | 0 | 1 | No | N/A | 20 | PR | 8 | No mutation detected | Smoker |
| 14 | 63 | F | 1 | 1 | No | N/A | 20 | PR | 6 | No mutation detecteda | Never |
| 15 | 76 | F | 1 | 1 | No | N/A | 20 | PR | 1 | L858Ra | Never |
N/A, not applicable; PD, progressive disease; PR, partial response; PS, Eastern Cooperative Oncology Group (ECOG) performance status; SD, stable disease.
Mutation results by Sanger sequencing; all others by DxS Diagnostics, performed by Clarient.
Pharmacokinetics
The PK properties of ficlatuzumab and gefitinib are summarized in Table 5. Ficlatuzumab drug exposure (maximal plasma concentration [Cmax] and area under the plasma concentration–time curve [AUC0–last]) was approximately proportional to dose during cycle 1. The mean Cmax after administration of 20 mg/kg ficlatuzumab was 544 μg/mL in cycle 1 and 1070 μg/mL in cycle 4. Mean AUC0–last increased from 81.3 mg·h/mL in cycle 1 to 212 mg·h/mL in cycle 4. Cmax was reached at or after the end of ficlatuzumab intravenous infusion. The mean half‐life was approximately 15 days for the first dose in cycle 1 and 18 days for the first dose in cycle 4. Plots of average ficlatuzumab concentrations of all measurements taken in cycles 1–4 are shown in Figure 1.
Table 5.
Mean (SD) Pharmacokinetic Parameters of Ficlatuzumab (10 or 20 mg/kg) and Gefitinib (250 mg Daily)
| Ficlatuzumab dose | Cycle, Day | n | t1/2 (Days) | Cmax (μg/mL) | Tmax (h) | AUC0–last (mg·h/mL) |
|---|---|---|---|---|---|---|
| Ficlatuzumab parameters | ||||||
| 10 mg/kg | Cycle 1, day 1 | 3 | 15.2 (4.7) | 229 (8.9) | — | 39.2 (4.5) |
| 20 mg/kg | Cycle 1, day 1 | 12 | 11.3 (3.2) | 544 (141) | — | 81.3 (16.5) |
| 20 mg/kg | Cycle 4, day 1 | 6a | 17.8 (6.3)b | 1070 (220) | — | 212 (47.3) |
| Gefitinib parameters | ||||||
| 10 mg/kg | Cycle 1, day 1 | 3 | — | 250 (44.1) | 4.0 (0) | 3706 (1109) |
| 20 mg/kg | Cycle 1, day 1 | 12 | — | 245 (89.5) | 5.4 (1.9) | 3960 (1588) |
| 20 mg/kg | Cycle 4, day 1 | 6c | — | 377 (224) | 9.9 (6.7) | 6591 (4824) |
AUC0–last, area under the serum concentration–time curve from the time of dosing to the last measurable concentration; Cmax, maximal plasma concentration; SD, standard deviation; t1/2, half‐life; Tmax, time to Cmax.
Only 6 patients enrolled in the 20 mg/kg dosage group were treated in cycle 4.
Patient 6501‐000107 had a t1/2 of 74.0 days and was not included in the calculation of mean (SD) t1/2.
Only 6 patients enrolled in the 20 mg/kg dosage group were dosed in cycle 4.
Figure 1.
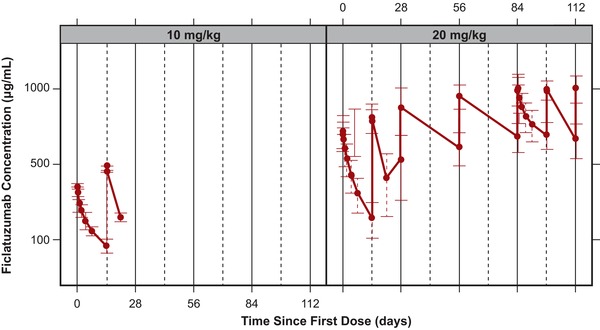
Mean ficlatuzumab concentrations (μg/mL) versus time by 10 and 20 mg/kg doses. Error bars reflect standard deviation. Solid vertical lines reflect separate treatment cycles, and dashed vertical lines reflect nominal ficlatuzumab dosing times.
Gefitinib exposure (Cmax and AUC0–last) was similar in patients administered ficlatuzumab 10 or 20 mg/kg, indicating that the gefitinib PK parameters are unlikely to be altered by ficlatuzumab. Gefitinib was slowly absorbed, with time to Cmax observed 4–10 hours after dosing. Daily oral treatment with gefitinib resulted in approximately 2‐fold accumulation at steady state (cycle 4).
Pharmacodynamics
All patients showed increasing levels of total HGF, starting on day 2 after ficlatuzumab administration and continuing through the observation period to days 22–28 in a time‐dependent manner (Figure 2). All patients experienced increases in total HGF levels on ficlatuzumab administration. The highest HGF level observed was ≈14 ng/mL, which was a fraction of the mean trough level of ficlatuzumab at the beginning of cycle 4 of 413.1 μg/mL (n = 8) in the 20 mg/kg cohort.
Figure 2.
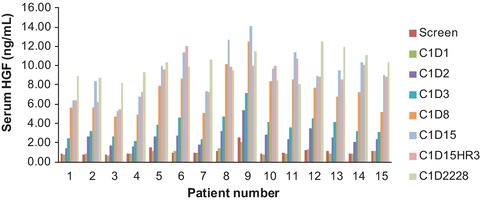
Serum HGF levels (ng/mL) after ficlatuzumab and gefitinib treatment by patient number.
Antidrug Antibodies
All the 41 postficlatuzumab samples from 14 patients measured for ADA were negative. The median time from first dose was 112 days (range, 28–252 days). Ficlatuzumab was not immunogenic in any patients during the observation period.
Discussion
Anti‐EGFR‐targeted therapies, such as the EGFR TKI gefitinib, have demonstrated superior efficacy (PFS and ORR) compared with chemotherapy in the first‐line setting for the NSCLC patient with an EGFR mutation.7 However, all patients who initially respond will eventually progress on therapy.8 Dysregulation of the HGF/c‐Met pathway, via c‐Met amplification10, 11 and/or HGF upregulation,13 has been identified as one of the major mechanisms of resistance to EGFR TKIs. Recently, the phase 3 METLung study of onartuzumab, a c‐Met‐targeted monovalent monoclonal antibody, in combination with erlotinib in c‐Met‐positive advanced NSCLC was halted because of lack of efficacy,34 and in 2012 the phase 3 MARQUEE trial investigating the selective MET inhibitor tivantinib in NSCLC was discontinued. Agents targeting the HGF/c‐Met pathway (such as rilotumumab and ficlatuzumab) are actively being investigated in the clinic, and may benefit patients with NSCLC who would likely have had poor outcomes with EGFR TKI therapy alone.35, 36
Ficlatuzumab, the focus of the current investigation, binds specifically to the HGF ligand and blocks HGF‐mediated activation. In this phase 1b study, ficlatuzumab was investigated in combination with the EGFR TKI gefitinib. Based on the dose‐escalation phase of this phase 1b trial, the higher dose of ficlatuzumab, 20 mg/kg, was determined to be the RP2D for further evaluation, and 12 patients in total were treated in this cohort. Five of the 12 patients in cohort 2 achieved an objective response (42%), and an additional 4 patients had disease stabilization, resulting in a disease control rate of 75%. Moreover, all 5 patients without prior EGFR TKI treatment had PR, including 2 patients without an EGFR mutation. Although the response rate to EGFR TKIs when used alone in NSCLC patients without an EGFR mutation has been found to be as low as 1%,7 the clinical activity observed in these patients may suggest that ficlatuzumab in combination with gefitinib may be active in NSCLC without an EGFR mutation. Of note, mutations were analyzed by Sanger sequencing in 1 of these 2 patients, which introduces the possibility of false‐negative results.
A phase 2, open‐label, randomized trial to compare ficlatuzumab plus gefitinib versus gefitinib alone as first‐line treatment in Asian patients with lung adenocarcinoma who were never smokers or former light smokers was recently published. In the phase 2 study, the addition of ficlatuzumab did not provide significant improvement over gefitinib therapy. However, the biomarker data from that study did suggest that patients classified as VeriStrat poor may benefit from ficlatuzumab combination therapy.
The ficlatuzumab/gefitinib combination was well tolerated by most patients. Treatment‐related grade 3/4 AEs occurred in 2 patients and included peripheral edema, paronychia, and dermatitis acneiform. One patient died because of respiratory failure despite achieving PR after 4 weeks with gefitinib. Two patients discontinued treatment following AEs (dyspnea); in one of these patients the primary cause of withdrawal was attributed to progressive disease. The most common AEs (dermatitis acneiform, decreased appetite, diarrhea, paronychia, fatigue, and cough) were as expected in a study including gefitinib.
The PK profiles of ficlatuzumab and gefitinib showed no indication of drug–drug interactions. The observation of a time‐dependent increase in circulating HGF after drug treatment was consistent with that reported in previous studies with ficlatuzumab as a single agent or in combination with erlotinib.37, 38 All patients experienced the expected increase in total HGF levels on ficlatuzumab administration. This was likely because of the stabilization and/or induction of HGF expression in the presence of ficlatuzumab, suggesting the predicted engagement of HGF by study drug. The highest HGF level observed was ≈14 ng/mL, which was a fraction of the mean trough level of ficlatuzumab at the beginning of cycle 4 of 413.1 μg/mL (n = 8) in the 20 mg/kg cohort. Given the high affinity of ficlatuzumab to HGF with a KD of 3 pM,32 the majority of serum HGF was in an HGF–ficlatuzumab complex. Similar increases in HGF were observed in clinical studies of rilotumumab, another monoclonal antibody targeting the HGF ligand.38, 39 Ficlatuzumab was not immunogenic in any of the patients treated.
In summary, the findings from this study suggest that the combination of ficlatuzumab and gefitinib is well tolerated and has promising antitumor activity in Asian patients with advanced NSCLC. In combination with the recently published phase 2 results of the study, these results suggest that ficlatuzumab may potentially improve outcomes for patients who have responded poorly to EGFR TKIs.
Declaration of Conflicting Interests
The following authors were employed at AVEO during the time of the study: F. Payumo (currently employed at Unum Therapeutics Inc.), S. Agarwal (currently employed at Tesaro Bio), M. Credi (currently employed at Novartis Institutes for Biomedical Research), J. Jac (currently employed at Chiltern International), K. McKee (currently employed at Takeda Pharmaceuticals International Co.), and W. Yin (currently employed at Biogen). D. Tan discloses personal fees from Pfizer, Bayer, Boehringer Ingelheim, Merck, and Novartis, as well as grant funding from Bayer, GSK, and Novartis. Q. Ng has been an advisory board participant for AstraZeneca, Celgene, and Boehringer Ingelheim, and K. Park has been an advisory board participant for AVEO. J. Ahn, W. Lim, J. Sun, E. Tan, and M. Ahn report nothing to disclose.
Funding
This study was supported by AVEO Oncology. Editorial assistance was provided by Melissa Kirk, PhD, of Scientific Connexions, Lyndhurst, New Jersey, an Ashfield Company, part of UDG Healthcare, and was funded by AVEO Oncology.
Author Contributions
Conception and design: E. Tan, M. Han, F. Payumo, J. Jac, K. Park. Development of methodology: E. Tan, K. Park. Acquisition of data: E. Tan, W. Lim, M. Ahn, Q. Ng, D. Tan, J. Sun, K. McKee, K. Park. Analysis and interpretation of data: E. Tan, W. Lim, M. Ahn, Q. Ng, J. Ahn, M. Han, W. Yin, J. Jac, K. Park. Writing, review, and/or revision of the manuscript: E. Tan, W. Lim, M. Ahn, Q. Ng, J. Ahn, D. Tan, J. Sun, M. Han, F. Payumo, K. McKee, W. Yin, M. Credi, S. Agarwal, J. Jac, K. Park. Administrative, technical, or material support: F. Payumo, M. Credi, J. Jac. Study supervision: F. Payumo, K. McKee, S. Agarwal, J. Jac, K. Park.
Trial registration ID: NCT01039948
Contributor Information
Eng‐Huat Tan, Email: tan.eng.huat@singhealth.com.sg.
Keunchil Park, Email: kpark@skku.edu.
References
- 1. NCCN Clinical Practice Guidelines in Oncology . Non‐small cell lung cancer. V7.2017. 2017. http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed July 13, 2017.
- 2. Amann J, Kalyankrishna S, Massion PP, et al. Aberrant epidermal growth factor receptor signaling and enhanced sensitivity to EGFR inhibitors in lung cancer. Cancer Res. 2005;65:226–235. [PubMed] [Google Scholar]
- 3. Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non‐small‐cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. [DOI] [PubMed] [Google Scholar]
- 4. Sequist LV, Martins RG, Spigel D, et al. First‐line gefitinib in patients with advanced non‐small‐cell lung cancer harboring somatic EGFR mutations. J Clin Oncol. 2008;26:2442–2449. [DOI] [PubMed] [Google Scholar]
- 5. Miller VA, Riely GJ, Zakowski MF, et al. Molecular characteristics of bronchioloalveolar carcinoma and adenocarcinoma, bronchioloalveolar carcinoma subtype, predict response to erlotinib. J Clin Oncol. 2008;26:1472–1478. [DOI] [PubMed] [Google Scholar]
- 6. Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non‐small‐cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900–5909. [DOI] [PubMed] [Google Scholar]
- 7. Mok TS, Wu Y‐L, Thongprasert S, et al. Gefitinib or carboplatin‐paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. [DOI] [PubMed] [Google Scholar]
- 8. Riely GJ, Pao W, Pham D, et al. Clinical course of patients with non‐small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:839–844. [DOI] [PubMed] [Google Scholar]
- 9. Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non‐small‐cell lung cancer to gefitinib. N Engl J Med. 2005; 352:786–792. [DOI] [PubMed] [Google Scholar]
- 10. Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Engelman JA, Jänne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non‐small cell lung cancer. Clin Cancer Res. 2008;14:2895–2899. [DOI] [PubMed] [Google Scholar]
- 12. Yano S, Wang W, Li Q, et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor‐activating mutations. Cancer Res. 2008;68:9479–9487. [DOI] [PubMed] [Google Scholar]
- 13. Yano S, Yamada T, Takeuchi S, et al. Hepatocyte growth factor expression in EGFR mutant lung cancer with intrinsic and acquired resistance to tyrosine kinase inhibitors in a Japanese cohort. J Thorac Oncol. 2011;6:2011–2017. [DOI] [PubMed] [Google Scholar]
- 14. Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. [DOI] [PubMed] [Google Scholar]
- 15. Trusolino L, Comoglio PM. Scatter‐factor and semaphorin receptors: cell signalling for invasive growth. Nat Rev Cancer. 2002;2:289–300. [DOI] [PubMed] [Google Scholar]
- 16. Weidner KM, Sachs M, Birchmeier W. The Met receptor tyrosine kinase transduces motility, proliferation, and morphogenic signals of scatter factor/hepatocyte growth factor in epithelial cells. J Cell Biol. 1993;121:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Siegfried JM, Weissfeld LA, Luketich JD, Weyant RJ, Gubish CT, Landreneau RJ. The clinical significance of hepatocyte growth factor for non‐small cell lung cancer. Ann Thorac Surg. 1998;66:1915–1918. [DOI] [PubMed] [Google Scholar]
- 18. Masago K, Togashi Y, Fujita S, et al. Clinical significance of serum hepatocyte growth factor and epidermal growth factor gene somatic mutations in patients with non‐squamous non‐small cell lung cancer receiving gefitinib or erlotinib. Med Oncol. 2012;29:1614–1621. [DOI] [PubMed] [Google Scholar]
- 19. Han JY, Kim JY, Lee SH, Yoo NJ, Choi BG. Association between plasma hepatocyte growth factor and gefitinib resistance in patients with advanced non‐small cell lung cancer. Lung Cancer. 2011;74:293–299. [DOI] [PubMed] [Google Scholar]
- 20. Turke AB, Zejnullahu K, Wu YL, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17:77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang YW, Staal B, Essenburg C, et al. MET kinase inhibitor SGX523 synergizes with epidermal growth factor receptor inhibitor erlotinib in a hepatocyte growth factor‐dependent fashion to suppress carcinoma growth. Cancer Res. 2010;70:6880–6890. [DOI] [PubMed] [Google Scholar]
- 22. Meetze KA, Boudrow A, Connolly K, Huang R, Rideout W, Gyuris J. Anti‐tumor activity of SCH 900105 (AV299), an anti‐HGF antibody, in non‐small cell lung cancer models [AACR abstract C173]. Mol Cancer Ther. 2009;8(suppl 1). [Google Scholar]
- 23. Meetze KA, Connolly K, Boudrow A, et al. Preclinical efficacy and pharmacodynamics of SCH 900105 (AV‐299) an anti‐HGF antibody in an intracranial glioblastoma model [AACR abstract C181]. Mol Cancer Ther. 2009;8(suppl 1). [Google Scholar]
- 24. Sano Y, Hashimoto E, Nakatani N, et al. Combining onartuzumab with erlotinib inhibits growth of non‐small cell lung cancer with activating EGFR mutations and HGF overexpression. Mol Cancer Ther. 2015;14(2):533–541. [DOI] [PubMed] [Google Scholar]
- 25. Stabile LP, Rothstein ME, Keohavong P, et al. Targeting of both the c‐Met and EGFR pathways results in additive inhibition of lung tumorigenesis in transgenic mice. Cancers (Basel). 2010;2:2153–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang W, Li Q, Takeuchi S, Yamada T, et al. Met kinase inhibitor E7050 reverses three different mechanisms of hepatocyte growth factor‐induced tyrosine kinase inhibitor resistance in EGFR mutant lung cancer. Clin Cancer Res. 2012;18:1663–1671. [DOI] [PubMed] [Google Scholar]
- 27. Okamoto W, Okamoto I, Tanaka K, et al. TAK‐701, a humanized monoclonal antibody to hepatocyte growth factor, reverses gefitinib resistance induced by tumor‐derived HGF in non‐small cell lung cancer with an EGFR mutation. Mol Cancer Ther. 2010;9:2785–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tan E, Park K, Lim WT, et al. Phase Ib study of ficlatuzumab (formerly AV‐299), an anti‐hepatocyte growth factor (HGF) monoclonal antibody (MAb) in combination with gefitinib (G) in Asian patients (pts) with NSCLC [ASCO abstract 7571]. J Clin Oncol. 2011;29(suppl). [Google Scholar]
- 29. Han M, Breault L, Wright K, et al. In vivo genetic screens designed to complement loss of HER2 or kRas functions identify c‐Met/HGF pathway as potent driver of growth/survival in solid tumor models [AACR abstract 4887]. Cancer Res. 2007;67(suppl 9). [Google Scholar]
- 30. Meetze KA, Connolly K, Zi T, Heyer J, Gyuris J, Han M. Antitumor activity of ficlatuzumab in combination with cetuximab on squamous cell carcinomas of the head and neck. Eur J Cancer. 2012;48(suppl 6):92. [Google Scholar]
- 31. Patnaik A, Weiss GJ, Papadopoulos K, et al. Phase I ficlatuzumab monotherapy or with erlotinib for refractory advanced solid tumors and multiple myeloma. Br J Cancer. 2014;111:272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mok TS, Geater SL, Su WC, et al. A randomized phase 2 study comparing the combination of ficlatuzumab and gefitinib with gefitinib alone in Asian patients with advanced stage pulmonary adenocarcinoma. J Thorac Oncol. 2016;11(10):1736–1744. [DOI] [PubMed] [Google Scholar]
- 33. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 34. Spigel DR, Edelman MJ, Mok T, et al. The MetLUNG study: A randomized, double‐blind, phase III study of onartuzumab (MetMAb) plus erlotinib in patients with advanced, MET‐positive non‐small cell lung cancer (NSCLC) [ASCO abstract TPS7616]. J Clin Oncol. 2012:30(suppl). [DOI] [PubMed] [Google Scholar]
- 35. Feng Y, Thiagarajan PS, Ma PC. MET signaling: novel targeted inhibition and its clinical development in lung cancer. J Thorac Oncol. 2012:7;459–467. [DOI] [PubMed] [Google Scholar]
- 36. Gozdzik‐Spychalska J, Szyszka‐Barth K, Spychalski L, et al. c‐MET inhibitors in the treatment of lung cancer. Curr Treat Options Oncol. 2014;15:670–682. [DOI] [PubMed] [Google Scholar]
- 37. Scagliotti GV, Novello S, von Pawel J. The emerging role of MET/HGF inhibitors in oncology. Cancer Treat Rev. 2013;39:793–801. [DOI] [PubMed] [Google Scholar]
- 38. Tabernero J, Elez ME, Herranz M, et al. A pharmacodynamic/pharmacokinetic study of ficlatuzumab in patients with advanced solid tumors and liver metastases. Clin Cancer Res. 2014;20:2793–2804. [DOI] [PubMed] [Google Scholar]
- 39. Gordon MS, Sweeney CS, Mendelson DS, et al. Safety, pharmacokinetics, and pharmacodynamics of AMG 102, a fully human hepatocyte growth factor–neutralizing monoclonal antibody, in a first‐in‐human study of patients with advanced solid tumors. Clin Cancer Res. 2010;16;699–710. [DOI] [PubMed] [Google Scholar]


