Abstract
Octacalcium phosphate and collagen composite (OCP/Col) achieves stable bone regeneration without cell transplantation in preclinical studies. Recently, a sponsor‐initiated clinical trial was conducted to commercialize the material. The present study investigated bone regeneration by OCP/Col with the single local administration of teriparatide (parathyroid hormone 1‐34; TPTD). OCP/Col was prepared by mixing sieved granules of OCP and atelocollagen for medical use and a disk was molded. After the creation of a rodent critical‐sized calvarial defect, OCP/Col or OCP/Col with dripped TPTD solution (1.0 or 0.1 µg; OCP/Col/TPTDd1.0 or OCP/Col/TPTDd0.1) was implanted into the defect. Six defects in each group were fixed 12 weeks after implantation. Radiographic examinations indicated that radiopaque figures in defects treated with OCP/Col with TPTD (OCP/Col/TPTDd) occupied a wider range than those treated with OCP/Col. Histological results demonstrated that most of the defect in OCP/Col/TPTDd was filled with newly formed bone. A histomorphometrical examination indicated that the percentage of newly formed bone was significantly higher in the defects of OCP/Col/TPTDd 1.0 (53.6 ± 4.3%) and OCP/Col/TPTDd 0.1 (52.2 ± 7.4%) than in those of OCP/Col (40.1 ± 8.4%), whereas no significant differences were observed between OCP/Col/TPTDd1.0 and OCP/Col/TPTDd0.1. These results suggest that OCP/Col with the single local administration of TPTD enhances bone regeneration in a rodent calvarial critical‐sized bone defect. © 2017 The Authors Journal of Biomedical Materials Research Part B: Applied Biomaterials Published by Wiley Periodicals, Inc. J Biomed Mater Res Part B: Appl Biomater, 106B: 1851–1857, 2018.
Keywords: bone regeneration, bone tissue engineering, calcium phosphate, collagen, parathyroid hormone
INTRODUCTION
The restoration of bone defects represents an important issue in reconstructive surgery1 because it improves quality of life by reducing functional disturbances. Although a large number of artificial bone substitutes had been developed,2, 3 autologous bone grafting remains the gold standard, and the development of new bone regenerative material with similar bone regenerative properties to autologous bone is expected.4
Octacalcium phosphate (OCP: Ca8H2(PO4)6·5H2O) has been advocated as a precursor of biological apatite in bones,5, 6 and demonstrated superior bone formation to other bone substitutes.7 An OCP and collagen composite (OCP/Col) has been developed to overcome the limitations of OCP in moldability and handling performance.8 OCP/Col has been shown to achieve superior bone regeneration to OCP granules and other commercialized bone substitutes, and accomplished stable bone regeneration without cell transplantation.8, 9 Moreover, OCP/Col is expected to enhance physiological bone remodeling, is easy to handle, and has excellent cost performance. After the confirmation of bone regeneration by OCP/Col in various canine bone defect models,10, 11, 12, 13 a doctor‐initiated clinical study was performed on tooth extraction sockets and cyst holes.14, 15 A sponsor‐initiated clinical trial was recently conducted and the commercialization of OCP/Col appears to be forthcoming. However, bone regeneration by OCP/Col alone has limited availability, and, thus, the development of more reliable bone regeneration is anticipated.12
Parathyroid hormone (PTH) is a crucial regulator of calcium and phosphate metabolism and functions, and teriparatide (TPTD) is a recombinant form of PTH that consists of the first 34 amino acids, which contain the bioactive portion.16 TPTD has a unique mechanism of action in bone; the continuous administration of TPTD decreases bone volume (a catabolic effect), while its intermittent administration results in the formation of increased amounts of trabecular bone (an anabolic effect).17, 18 Furthermore, it is the only anabolic agent that has been approved by the U.S. Food and Drug Administration for the treatment of osteoporosis.19
Many researchers have attempted to repair experimental bone defects using the anabolic effect of the intermittent subcutaneous administration of TPTD.20, 21, 22, 23, 24 And several studies were combined with materials, such as absorbable collagen sponge (ACS),23 demineralized bone matrix (DBM),21, 23 β‐tricalcium phosphate (β‐TCP),24 or poly‐lactic acid (PLA).20 However, the optimal duration and dosage of TPTD for bone regeneration have not yet been established.25 Although it has not elucidated the effect of single local administration of TPTD for a created bone defect, it would be meaningful the enhancement of bone regeneration by single local administration of TPTD solution with OCP/Col was confirmed, because the implantation of OCP/Col is inevitably accompanied with an interventional manipulation. Since TPTD is administered by percutaneous route at approximately 1 μg/kg/week (Teribone™) to patients with osteoporosis, it might be expected that the dose of single local administration of TPTD 1.0 µg (4.0 µg/kg for 250 g rat) or TPTD 0.1 µg (0.4 µg/kg for 250 g rat) would be secured the safety. Therefore, it was investigated whether bone regeneration was achieved by OCP/Col combined with single local administration of TPTD (1.0 or 0.1 µg), if implanted into a rodent critical‐sized calvarial bone defect.
MATERIALS AND METHODS
Preparation of OCP/Col
We previously described the preparation of OCP/Col.9 Briefly, OCP was prepared by direct precipitation,26 and sieved granules (particle sizes of 300–500 µm) of OCP were prepared. Collagen was prepared from NMP collagen PS (Nippon Meat Packers, Tsukuba, Ibaraki, Japan), a lyophilized powder of pepsin‐digested atelocollagen isolated from the porcine dermis. The sieved granules of OCP were added to concentrated collagen and mixed, and the weight percentage of OCP in OCP/Col became 77%. The OCP/Col mixture was then lyophilized, and a disk was molded (diameter of 9 mm, thickness of 1.5 mm). OCP/Col disks were prepared by a dehydrothermal treatment (150°C, 24 h) in a Vacuum Drying Oven. OCP/Col disks were sterilized using electron beam irradiation (15 kGy). And the elastic modulus of OCP/Col is 0.40 MPa.
Preparation of TPTD solution
Chemically synthesized TPTD acetate, an active ingredient of Teribone Inj. 56.5 µg (Asahi Kasei Pharma Corp., Tokyo, Japan), was used. Lyophilized TPTD acetate was reconstituted with saline at a concentration of 50 µg/mL. In order to prepare TPTD solution (1.0 µg/0.1 mL), 20 µL of TPTD solution (50 µg/mL) was dispensed into separate 0.5‐mL siliconized polymerase chain reaction (PCR) tubes and diluted with saline to give 100 µL of TPTD solution (1.0 µg/0.1 mL). TPTD solution (0.1 µg/0.1 mL) was prepared by diluting TPTD solution (1.0 µg/0.1 mL) with saline. The TPTD solution (1.0 µg/0.1 mL or 0.1 µg/0.1 mL) prepared was stored in a freezer (−20°C) until immediately before use.
Implantation procedure
Twelve‐week‐old male Wistar rats (SLC Corp., Hamamatsu, Shizuoka, Japan) were used. The principles of laboratory animal care as well as national laws were followed. All procedures were approved by the Animal Research Committee of Tohoku University (2013‐Biomedical Engineering Animal‐003).
After body weight measurements, experimental rats were anesthetized with intraperitoneal dexmedetomidine hydrochloride (0.15–0.4 mg/kg), midazolam (2 mg/kg), and butorphanol tartrate (2.5–5 mg/kg). A skin incision was made aseptically along the bilateral temporal line and the middle of the forehead, and the dissection was carried down to the calvarium. The periosteum of the calvarium was ablated, and a full‐thickness standardized trephine defect, 9 mm in diameter, was made in the calvarium under continuous saline buffer irrigation. Extreme care was exercised to avoid injury to the midsagittal blood sinus and dura mater. After saline irrigation of the trephine defect, an OCP/Col disk was implanted into the trephine defect. An OCP/Col disk was implanted in every experimental animal. In each animal of OCP/Col/TPTDd1.0 group, TPTD solution (1.0 µg/0.1 mL) was dripped onto a disk of OCP/Col immediately after implantation. In each animal of OCP/Col/TPTDd0.1 group, TPTD solution (0.1 µg/0.1 mL) was dripped onto a disk of OCP/Col immediately after implantation. As a control, an OCP/Col disk was implanted onto the trephine defect without the dripping of TPTD solution. After the defects had been treated, the ablated periosteum and skin were repositioned and sutured, respectively. In order to prevent infection, cephalexin (15 mg/kg) was subcutaneously administered postoperatively. Eighteen experimental rats were randomly divided into three groups (OCP/Col/TPTDd1.0, OCP/Col/TPTDd0.1, and OCP/Col), and six defects in each group were fixed 12 weeks after implantation.
Micro‐CT examination
Four, eight, and twelve weeks after implantation, an in vivo micro‐computed tomography (CT) analysis of the rat calvarium was performed using an X‐ray CT system (Latheta LCT‐200; Hitachi Aloka Medical, Tokyo, Japan) under an intraperitoneal injection of sodium pentobarbital (50 mg/kg). After body weight measurements, the calvarium were scanned continuously with increments in thickness of 120 µm for slices and the pixel size was 60 µm. CT images were acquired using the following parameters: 50 kVp tube voltage, 500 μA tube current, 3.6 ms integration time, and 120 mm axial field of view. After the completion of the CT analysis at 12 weeks, rats were euthanized by an intraperitoneal injection of an overdose of sodium pentobarbital. After sacrifice, the implants were resected together with the surrounding bones and tissues and fixed with 4% paraformaldehyde in 0.1M phosphate‐buffered saline, pH 7.4.
Radiographic analysis
Skulls were radiographed using a microradiography unit (Softex CMR Unit, Softex, Tokyo, Japan) with an X‐ray film (FR, Fuji photo film, Tokyo, Japan) under standardized conditions (20 kV, 5 mA), because OCP/Col disks before implantation showed little radiopacity in this condition.9
Tissue preparations and a quantitative micrograph analysis
Although OCP/Col itself hardly showed radiopacity, it exhibited radiopacity by initiation of bone regeneration and the irreversible conversion from OCP to apatitic phase.27 Because it is quite difficult to distinguish newly formed bone and the converted apatite in radiographic examination,8, 9 it would be more suitable to apply histomorphometrical analysis of newly formed bone than radiomorphometric analysis by micro‐CT images. Therefore, the samples were decalcified in 10% ethylenediamimetetraacetic acid (EDTA) in 0.01M phosphate buffer, pH 7.4 at 4°C for 2–4 weeks after radiographs had been taken. Specimens were embedded in paraffin and the center of the defect was extracted and sectioned coronally. Sections were stained with hematoxylin and eosin and photographs were taken with a photomicroscope (Leica DM2500, Leica Microsystems Japan, Tokyo, Japan).
The procedures used in the histomorphometrical analysis have been described previously.7, 9 The percentage of newly formed bone in the defect (n‐Bone%) was calculated as the area of newly formed bone/area of the defect originally created by trephination × 100. n‐Bone% was quantified two‐dimensionally using version 1.43 of ImageJ public‐domain software.
Statistical analysis
The body weights of experimental animals before implantation and 4, 8, and 12 weeks after implantation as well as n‐Bone% were statistically analyzed using Excel v. X. (Microsoft Co., Redmond, WA). All values are reported as mean ± standard deviation (SD). The chi‐squared test was used to investigate whether each group had a normal distribution, and Bartlett's test was employed to examine the homogeneity of variance across samples. A one‐way analysis of variance (ANOVA) or the Kruskal–Wallis test was used to compare means among groups. Significance was accepted at p < 0.05. If significant differences were detected in the mean values, the Tukey–Kramer or Scheffé's multiple comparison analysis was used as a post hoc test.
RESULTS
Body weight changes during the experimental period (Figure 1)
Figure 1.
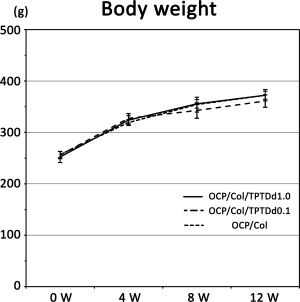
Body weight changes in experimental animals. The body weights of experimental animals are measured before implantation and 4, 8, and 12 weeks after implantation in OCP/Col/TPTDd1.0, OCP/Col/TPTDd0.1, and OCP/Col. No significant difference is observed among the groups at each period.
The body weights of experimental animals before implantation in OCP/Col/TPTDd1.0, OCP/Col/TPTDd0.1, and OCP/Col were 252.2 ± 10.7, 256.0 ± 7.1, and 257.0 ± 5.6 g, respectively. Body weights in OCP/Col/TPTDd1.0, OCP/Col/TPTDd0.1, and OCP/Col 4 weeks after implantation were 323.8 ± 8.7, 327.2 ± 9.3, and 319.2 ± 5.8 g, respectively. Body weights in OCP/Col/TPTDd1.0, OCP/Col/TPTDd0.1, and OCP/Col 8 weeks after implantation were 355.5 ± 8.6, 342.5 ± 15.0, and 354.0 ± 14.4, respectively. Body weights in OCP/Col/TPTDd1.0, OCP/Col/TPTDd0.1, and OCP/Col 12 weeks after implantation were 372.3 ± 11.1, 361.0 ± 12.2, and 372.2 ± 7.9 g, respectively (Figure 1). No significant differences were observed among the groups at any period. Furthermore, no marked differences were found in local conditions, and no noticeable inflammation or skin symptoms were observed.
Micro‐CT analysis (Figure 2)
Figure 2.
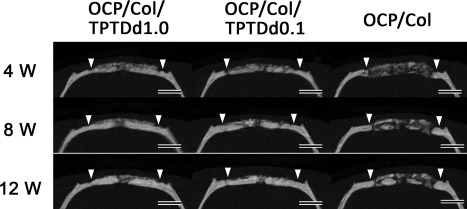
Micro‐CT of OCP/Col/TPTDd1.0, OCP/Col/TPTDd0.1, and OCP/Col. In OCP/Col/TPTDd1.0 and OCP/Col/TPTDd0.1, most of the defect is occupied by a radiopaque figure after 4 weeks or more. It increases with time, and repairs the defect. Furthermore, the width of radiopacity is similar to that of original bone. In the OCP/Col group, small radiopaque masses are scattered throughout the defect 4 weeks after implantation. They increase in size and fuse with each other over time. Closed triangle: Defect margin, Bars: 3 mm
Figure 2 shows coronal sections of the central part of the defect. Although a previous study reported that OCP/Col disks have negligible radiopacity,9 implanted OCP/Col demonstrated a radiopaque figure by converting the apatitic phase or regenerating bone.10 In the OCP/Col/TPTDd1.0 and OCP/Col/TPTDd0.1 groups, most of the defect including the central part was occupied by radiopaque figures after 4 weeks or more. Radiopaque figures increased with time, and repaired the defects. The border between original bone and the margin of the defect was indistinguishable after 4 weeks or more. The width of radiopacity was similar to that of original bone. In the OCP/Col group, small radiopaque masses were scattered throughout the defect 4 weeks after implantation. Radiopaque masses enlarged and fused with each other over time. The width of radiopacity was similar to that of original bone.
Radiographic examinations (Figure 3)
Figure 3.
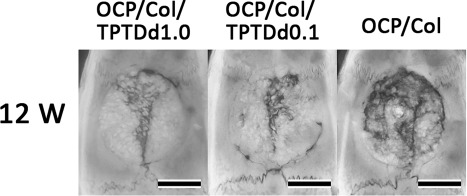
Radiographic examination. In the OCP/Col/TPTDd1.0 and OCP/Col/TPTDd0.1 groups, most of the defect is covered by a radiopaque figure, which is denser, more granulous, and has relatively uniform radiopacity. In OCP/Col, most of the defect is occupied by radiopacity, which consists of the fusion of small radiopaque masses. Bars: 4 mm.
In the OCP/Col/TPTDd1.0 and OCP/Col/TPTDd0.1 groups, most of the defect was covered by a radiopaque figure, which was denser and more granulous and had relatively uniform radiopacity, similar to that of original bone. In the OCP/Col group, most of the defect was occupied by radiopacity, which consisted of the fusion of small radiopaque masses.
Histological results of implants (Figure 4)
Figure 4.
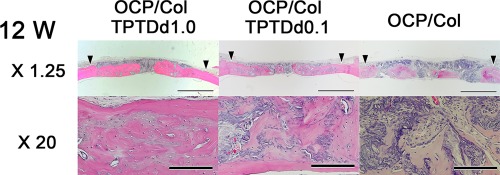
Histological findings of implants. In the OCP/Col/TPTDd1.0 and OCP/Col/TPTDd0.1 groups, most of the defect is filled with newly formed bone, and a part of regenerated bone has a cortical bone‐like structure. The width of new bone is similar to that of original bone. Most of the implanted OCP/Col is resorbed. In the OCP/Col group, a wide range of the defect is filled with regenerated bone, which has a mosaic pattern. Implanted OCP granules became smaller. Hematoxylin eosin stain, Closed triangle: Defect margin, Bars: 3 mm (upper row), 200 µm (lower row)
The upper side of Figure 4 indicates the skin side, while the lower side is the dura mater side. In the OCP/Col/TPTDd1.0 and OCP/Col/TPTDd0.1 groups, most of the defect was filled with newly formed bone, except for the area situated at the sagittal suture. The width of new bone was similar to that of original bone. Furthermore, the border between original bone and the defect margin was indistinguishable. In the OCP/Col/TPTDd1.0 group, a part of regenerated bone had a cortical bone‐like structure, and the implanted OCP/Col was almost resorbed. In the OCP/Col/TPTDd0.1 group, a small part of OCP/Col still remained, whereas a part of regenerated bone had a cortical bone‐like structure. In the OCP/Col group, a wide range of the defect was filled with regenerated bone. Newly formed bone, which had integrated with implanted OCP/Col, had a mosaic pattern and the implanted OCP granules had also become smaller.
Histomorphometrical examination (Figure 5)
Figure 5.
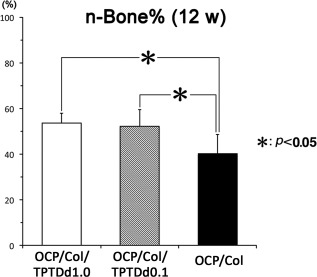
Quantitative analysis of newly formed bone in defects. The percentage of newly formed bone in defects (n‐Bone%) ± SD is shown in OCP/Col/TPTDd1.0 (white square), OCP/Col/TPTDd0.1 (hatched square), and OCP/Col (black square). A significant difference is observed in mean values between OCP/Col/TPTDd1.0 and OCP/Col, and between OCP/Col/TPTDd0.1 and OCP/Col. Data are shown as the mean ± SD of six specimens.
The n‐Bone% of OCP/Col/TPTDd1.0, OCP/Col/TPTDd0.1, and OCP/Col were 53.6 ± 4.3, 52.2 ± 7.4, and 40.1 ± 8.4%, respectively. Significant differences were observed in the mean values of n‐Bone% (ANOVA, F = 6.86; df = 2; p = 0.008). The Tukey–Kramer multiple comparison analysis as a post hoc test revealed significant differences between OCP/Col/TPTDd1.0 and OCP/Col and between OCP/Col/TPTDd0.1 and OCP/Col.
DISCUSSION
There are several studies which have applied TPTD to bone defects.20, 21, 23, 24 These studies employed the intermittent subcutaneous administration of TPTD because it has been established that TPTD has a unique mechanism of action in bone and its intermittent subcutaneous administration leads to bone formation.17, 18 Thus, few studies have used TPTD combined with bone substitutes to investigate bone regeneration in bone defects.
The results of the present study revealed OCP/Col disks with dripped TPTD solution (1.0 µg/0.1 mL or 0.1 µg/0.1 mL) enhanced bone regeneration significantly more than those without TPTD. Radiographic examinations including a micro‐CT analysis indicated that radiopaque figures in defects treated with OCP/Col/TPTDd1.0 or OCP/Col/TPTDd0.1 occupied a wider range than in those treated with OCP/Col at every experimental period. Radiopaque figures increased with time and repaired the defects. Bone regeneration by implanted OCP/Col has been shown to originate from the defect margin and around implanted OCP/Col if it has been implanted into a critical‐sized calvarial defect.8 Thus, the addition of TPTD with OCP/Col appears to synergistically augment the repair of bone defects. Furthermore, histological results demonstrated that most of the defect in the OCP/Col/TPTDd groups was filled with newly formed bone, and the width of regenerated bone was similar to that of original bone. And a part of regenerated bone had a cortical bone‐like structure. In addition, the border between original bone and the defect margin was indistinguishable, and implanted OCP/Col was almost resorbed. In the OCP/Col group, a wide range of the defect was filled with regenerated bone, and implanted OCP granules became smaller. The results of the histomorphometrical examination indicated that the n‐Bone% of OCP/Col/TPTDd1.0 and OCP/Col/TPTDd0.1 was significantly greater than that of OCP/Col, whereas no significant differences were observed between OCP/Col/TPTDd1.0 and OCP/Col/TPTDd0.1. We previously reported that OCP combined with bone morphogenetic protein‐2 (BMP‐2) synergistically enhanced bone regeneration more than OCP alone, while the dose of BMP‐2 used did not affect bone regeneration by OCP.28 Furthermore, a proteome analysis by liquid chromatography tandem mass spectrometry indicated that OCP granules incubated in rat serum proteins selectively adsorbed bone formation‐related proteins, such as apolipoprotein E.29 These findings suggest that some bone formation‐related proteins adsorb OCP/Col, and adsorbed proteins may then promote bone formation.
The administration of TPTD is expected to accelerate the remodeling of newly formed bone because it enhances osteogenic and osteoclastic activities.19 Therefore, the addition of TPTD with OCP/Col may promote bone regeneration significantly more than OCP/Col. The results of the present study indicate that bone regeneration by OCP/Col/TPTDd did not occur in a dose‐dependent manner because no significant differences were observed in bone regenerative properties between OCP/Col/TPTDd1.0 and OCP/Col/TPTDd0.1. These results suggest that a lower dose of TPTD combined with OCP/Col stimulates bone regeneration, and, thus, warrants further study.
When the intermittent subcutaneous administration of TPTD (15–40 µg/kg/day) has been applied to the bone repair of a critical‐sized defect, various kinds of materials, such as an ACS,23 DBM,21, 23 β‐TCP,24 or PLA20 have been implanted into the defect. The effects of the intermittent subcutaneous administration of TPTD on the repair of a critical‐sized defect after an 8‐ to 9‐week observation period remain controversial; some researchers advocated a significant effect,20, 24 whereas others denied any effect.21, 23 When a biomaterial was implanted into a rat calvarial critical‐sized defect, which was the most similar to that used in the present study, the effects of the intermittent subcutaneous TPTD administration for bone repair were not strongly emphasized.23, 24 The findings of our previous studies indicated that the bone regenerative properties of OCP/Col significantly better than on that of β‐TCP granules or collagen sponge.8, 30 TPTD solution dripped on OCP/Col may elicit the bone‐promoting ability of PTH and achieve excellent bone regeneration in addition to the superior bone regenerative properties of OCP/Col; however, it is not possible to unconditionally compare these studies due to the different administration routes of PTH and types of scaffolds used.
No significant differences were observed in the body weights of the experimental animals in each group (OCP/Col/TPTDd1.0, OCP/Col/TPTDd0.1, and OCP/Col) at any measured period. These results suggest that the systemic effects of dripped TPTD solution (1.0 or 0.1 µg) on OCP/Col are negligible. In the present study, the total doses of TPTD were 3.97 ± 0.17 µg/kg in the OCP/Col/TPTDd1.0 group and 0.391 ± 0.01 µg/kg in the OCP/Col/TPTDd0.1 group when converted to the average weight of each group at the implantation of OCP/Col. The doses of PTH used in previous studies vary from a total amount of 70 µg/kg (10 µg/kg/day × 1 week)22 to 2520 µg/kg (40 µg/kg/day × 9 weeks). Therefore, the total dose of PTH in the present study (0.391–3.97 µg/kg) was within a few tenths to a few thousandths of those used in previous studies, indicating a low dosage.
When TPTD was administered subcutaneously to rats daily for 2 years, the incidence of osteosarcoma was increased at 13.6 µg/kg/day (total amount of approximately 10,000 µg/kg), whereas the noncarcinogenic dose was 4.5 µg/kg/day (total amount of approximately 3300 µg/kg).31 Since TPTD is typically administered at approximately 1 μg/kg/week (Teribone) or 0.2–0.5 μg/kg/day (Forteo®) to patients with osteoporosis, the dose of TPTD used in the present study is safe for clinical use. The dose of TPTD used in this study was markedly lower as a single dose or total amount than previously reported; therefore, OCP/Col combined with TPTD, which have synergistic effects, may be safe for practical use.
This study indicated that the single local administration of TPTD with OCP/Col might be enhanced bone regeneration and would be easy to apply for the clinical situation more than the intermittent subcutaneous administration of TPTD with biomaterials.20, 23, 24 Because the commercialization of OCP/Col appears to be forthcoming for bone defects in oral and maxillofacial surgery, such as sinus floor elevation and alveolar clefts, this newly developed OCP/Col/TPTD, which has superior bone regenerative properties to OCP/Col, has potential as a therapeutic option for refractory bone defects, such as segmental bone defects.
Conflict of interest
One of the authors (S.K.) obtained a patent on OCP/Col in Japan (#5046511). And some of the authors (F.K., A.I., H.T., and S.K.) have applied for a patent on OCP/Col/TPTDd.
How to cite this article: Kajii, F , Iwai, A , Tanaka, H , Matsui, K , Kawai, T , Kamakura, S 2018. Single‐dose local administration of teriparatide with a octacalcium phosphate collagen composite enhances bone regeneration in a rodent critical‐sized calvarial defect. J Biomed Mater Res Part B 2018:106B:1851–1857.
REFERENCES
- 1. Fishero BA, Kohli N, Das A, Christophel JJ, Cui Q. Current concepts of bone tissue engineering for craniofacial bone defect repair. Craniomaxillofac Trauma Reconstr 2015;8:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bohner M, Tadier S, van Garderen N, de Gasparo A, Dobelin N, Baroud G. Synthesis of spherical calcium phosphate particles for dental and orthopedic applications. Biomatter 2013;3:e25103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dorozhkin SV. Biocomposites and hybrid biomaterials based on calcium orthophosphates. Biomatter 2011;1:3–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Habraken W, Habibovic P, Epple M, Bohner M. Calcium phosphates in biomedical applications: materials for the future? Mater Today 2016;19:69–87. [Google Scholar]
- 5. Brown W, Smith J, Lehr J, Frazier A. Crystallographic and chemical relations between octacalcium phosphate and hydroxyapatite. Nature 1962;196:1050–1055. [Google Scholar]
- 6. Crane N, Popescu V, Morris M, Steenhuis P, Ignelzi MJ. Raman spectroscopic evidence for octacalcium phosphate and other transient mineral species deposited during intramembranous mineralization. Bone 2006;39:434–442. [DOI] [PubMed] [Google Scholar]
- 7. Kamakura S, Sasano Y, Shimizu T, Hatori K, Suzuki O, Kagayama M, Motegi K. Implanted octacalcium phosphate is more resorbable than beta‐tricalcium phosphate and hydroxyapatite. J Biomed Mater Res 2002;59:29–34. [DOI] [PubMed] [Google Scholar]
- 8. Kamakura S, Sasaki K, Honda Y, Anada T, Suzuki O. Octacalcium phosphate combined with collagen orthotopically enhances bone regeneration. J Biomed Mater Res Part B 2006;79:210–217. [DOI] [PubMed] [Google Scholar]
- 9. Kamakura S, Sasaki K, Homma T, Honda Y, Anada T, Echigo S, Suzuki O. The primacy of octacalcium phosphate collagen composites in bone regeneration. J Biomed Mater Res Part A 2007;83:725–733. [DOI] [PubMed] [Google Scholar]
- 10. Iibuchi S, Matsui K, Kawai T, Sasaki K, Suzuki O, Kamakura S, Echigo S. Octacalcium phosphate (OCP) collagen composites enhance bone healing in a dog tooth extraction socket model. Int J Oral Maxillofac Surg 2010;39:161–168. [DOI] [PubMed] [Google Scholar]
- 11. Matsui K, Matsui A, Handa T, Kawai T, Suzuki O, Kamakura S, Echigo S. Bone regeneration by octacalcium phosphate collagen composites in a dog alveolar cleft model. Int J Oral Maxillofac Surg 2010;39:1218–1225. [DOI] [PubMed] [Google Scholar]
- 12. Miura K, Matsui K, Kawai T, Kato Y, Matsui A, Suzuki O, Kamakura S, Echigo S. Octacalcium phosphate collagen composites with titanium mesh facilitate alveolar augmentation in canine mandibular bone defects. Int J Oral Maxillofac Surg 2012;41:1161–1169. [DOI] [PubMed] [Google Scholar]
- 13. Tanuma Y, Anada T, Honda Y, Kawai T, Kamakura S, Echigo S, Suzuki O. Granule size‐dependent bone regenerative capacity of octacalcium phosphate in collagen matrix. Tissue Eng Part A 2012;18:546–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kawai T, Suzuki O, Matsui K, Tanuma Y, Takahashi T, Kamakura S. Octacalcium phosphate collagen composite facilitates bone regeneration of large mandibular bone defect in humans. J Tissue Eng Regen Med 2017;11:1641–1647. [DOI] [PubMed] [Google Scholar]
- 15. Kawai T, Echigo S, Matsui K, Tanuma Y, Takahashi T, Suzuki O, Kamakura S. First clinical application of octacalcium phosphate collagen composite in human bone defect. Tissue Eng Part A 2014;20:1336–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Niall HD, Sauer RT, Jacobs JW, Keutmann HT, Segre GV, O'Riordan JL, Aurbach GD, Potts JT Jr. The amino‐acid sequence of the amino‐terminal 37 residues of human parathyroid hormone. Proc Natl Acad Sci USA 1974;71:384–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest 1999;104:439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tam CS, Heersche JN, Murray TM, Parsons JA. Parathyroid hormone stimulates the bone apposition rate independently of its resorptive action: differential effects of intermittent and continuous administration. Endocrinology 1982;110:506–512. [DOI] [PubMed] [Google Scholar]
- 19. Morimoto T, Kaito T, Kashii M, Matsuo Y, Sugiura T, Iwasaki M, Yoshikawa H. Effect of intermittent administration of teriparatide (parathyroid hormone 1–34) on bone morphogenetic protein‐induced bone formation in a rat model of spinal fusion. J Bone Joint Surg Am 2014;96:e107. [DOI] [PubMed] [Google Scholar]
- 20. Jacobson JA, Yanoso‐Scholl L, Reynolds DG, Dadali T, Bradica G, Bukata S, Puzas EJ, Zuscik MJ, Rosier R, O'Keefe RJ, Schwarz EM, Awad HA. Teriparatide therapy and beta‐tricalcium phosphate enhance scaffold reconstruction of mouse femoral defects. Tissue Eng Part A 2011;17:389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pensak M, Hong SH, Dukas A, Bayron J, Tinsley B, Jain A, Tang A, Rowe D, Lieberman JR. Combination therapy with PTH and DBM cannot heal a critical sized murine femoral defect. J Orthop Res 2015;33:1242–1249. [DOI] [PubMed] [Google Scholar]
- 22. Rowshan HH, Parham MA, Baur DA, McEntee RD, Cauley E, Carriere DT, Wood JC, Demsar WJ, Pizarro JM. Effect of intermittent systemic administration of recombinant parathyroid hormone (1–34) on mandibular fracture healing in rats. J Oral Maxillofac Surg 2010;68:260–267. [DOI] [PubMed] [Google Scholar]
- 23. Stancoven BW, Lee J, Dixon DR, McPherson JC 3rd, Bisch FC, Wikesjo UM, Susin C. Effect of bone morphogenetic protein‐2, demineralized bone matrix and systemic parathyroid hormone (1–34) on local bone formation in a rat calvaria critical‐size defect model. J Periodontal Res 2013;48:243–251. [DOI] [PubMed] [Google Scholar]
- 24. Yun JI, Wikesjo UM, Borke JL, Bisch FC, Lewis JE, Herold RW, Swiec GD, Wood JC, McPherson JC 3rd. Effect of systemic parathyroid hormone (1–34) and a beta‐tricalcium phosphate biomaterial on local bone formation in a critical‐size rat calvarial defect model. J Clin Periodontol 2010;37:419–426. [DOI] [PubMed] [Google Scholar]
- 25. Chan HL, McCauley LK. Parathyroid hormone applications in the craniofacial skeleton. J Dent Res 2013;92:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Suzuki O, Nakamura M, Miyasaka Y, Kagayama M, Sakurai M. Bone formation on synthetic precursors of hydroxyapatite. Tohoku J Exp Med 1991;164:37–50. [DOI] [PubMed] [Google Scholar]
- 27. Suzuki O, Kamakura S, Katagiri T, Nakamura M, Zhao BH, Honda Y, Kamijo R. Bone formation enhanced by implanted octacalcium phosphate involving conversion into Ca‐deficient hydroxyapatite. Biomaterials 2006;27:2671–2681. [DOI] [PubMed] [Google Scholar]
- 28. Kamakura S, Nakajo S, Suzuki O, Sasano Y. New scaffold for recombinant human bone morphogenetic protein‐2. J Biomed Mater Res A 2004;71:299–307. [DOI] [PubMed] [Google Scholar]
- 29. Kaneko H, Kamiie J, Kawakami H, Anada T, Honda Y, Shiraishi N, Kamakura S, Terasaki T, Shimauchi H, Suzuki O. Proteome analysis of rat serum proteins adsorbed onto synthetic octacalcium phosphate crystals. Anal Biochem 2011;418:276–285. [DOI] [PubMed] [Google Scholar]
- 30. Tanuma Y, Matsui K, Kawai T, Matsui A, Suzuki O, Kamakura S, Echigo S. Comparison of bone regeneration between octacalcium phosphate/collagen composite and beta‐tricalcium phosphate in canine calvarial defect. Oral Surg Oral Med Oral Pathol Oral Radiol 2013;115:9–17. [DOI] [PubMed] [Google Scholar]
- 31. Watanabe A, Yoneyama S, Nakajima M, Sato N, Takao‐Kawabata R, Isogai Y, Sakurai‐Tanikawa A, Higuchi K, Shimoi A, Yamatoya H, Yoshida K, Kohira T. Osteosarcoma in Sprague‐Dawley rats after long‐term treatment with teriparatide (human parathyroid hormone (1–34)). J Toxicol Sci 2012;37:617–629. [DOI] [PubMed] [Google Scholar]


