Abstract
Aims
To assess the cost‐effectiveness of alternative smoking cessation scenarios from the perspective of the Spanish National Health Service (NHS).
Design
We used the European study on Quantifying Utility of Investment in Protection from Tobacco model (EQUIPTMOD), a Markov‐based state transition economic model, to estimate the return on investment (ROI) of: (a) the current provision of smoking cessation services (brief physician advice and printed self‐helped material + smoking ban and tobacco duty at current levels); and (b) four alternative scenarios to complement the current provision: coverage of proactive telephone calls; nicotine replacement therapy (mono and combo) [prescription nicotine replacement therapy (Rx NRT)]; varenicline (standard duration); or bupropion. A rate of 3% was used to discount life‐time costs and benefits.
Setting
Spain.
Participants
Adult smoking population (16+ years).
Measurements
Health‐care costs associated with treatment of smoking attributable diseases (lung cancer, coronary heart disease, chronic obstructive pulmonary infection and stroke); intervention costs; quality‐adjusted life years (QALYs). Costs and outcomes were summarized using various ROI estimates.
Findings
The cost of implementing the current provision of smoking cessation services is approximately €61 million in the current year. This translates to 18 quitters per 1000 smokers and a life‐time benefit–cost ratio of 5, compared with no such provision. All alternative scenarios were dominant (cost‐saving: less expensive to run and generated more QALYs) from the life‐time perspective, compared with the current provision. The life‐time benefit–cost ratios were: 1.87 (proactive telephone calls); 1.17 (Rx NRT); 2.40 (varenicline‐standard duration); and bupropion (2.18). The results remained robust in the sensitivity analysis.
Conclusions
According to the EQUIPTMOD modelling tool it would be cost‐effective for the Spanish authorities to expand the reach of existing GP brief interventions for smoking cessation, provide pro‐active telephone support, and reimburse smoking cessation medication to smokers trying to stop. Such policies would more than pay for themselves in the long run.
Keywords: Cost–effectiveness, economic evaluation, EQUIPT, smoking cessation interventions, tobacco control, Spain
Introduction
Smoking is the leading cause of preventable death 1, causing nearly 6 million deaths per year globally. Of those, more than 5 million are the result of direct tobacco use while more than 600 000 are the result of non‐smokers being exposed to second‐hand smoke world‐wide 2.
Although the European Union (EU) has made many efforts in reducing the tobacco problem, the number of smokers during the last decade is still high: 28% of the EU population smokes 3. The smoking prevalence in Spain is 27%, despite a significant drop (by five percentage points) observed since 2006 4. Therefore, smoking is still a major cause of health and social care costs faced by Spanish society 5, 6, 7, 8, 9, 10. In the Law 28/2005, the Spanish Council of State highlight that state expenditure to cover the health and social care costs caused by smoking exceed the excise duty levied on tobacco products 11.
Top‐level actions for reducing the prevalence of smoking in Spain have been a priority, e.g. the Law 42/2010. Indoor smoking bans, tobacco duties (taxation) and regulation of tobacco advertising have been found to be effective in increasing quit attempts 12. In the Spanish Health Survey 2011–12, the percentage of smokers who tried to quit during 1 year decreased from 24 to 19%. A large proportion (80%) of smokers reported not using any medication or advice/support from a doctor or trained professionals 13. In Spain, there are four main behavioural interventions that could be implemented at a national level: specialist support (one‐to‐one or group‐based); telephone support; or short message service (SMS) text messaging. Moreover, three main pharmacological options could be implemented: nicotine replacement therapy (NRT) in various forms (gum or patch), bupropion and varenicline. These interventions are cost‐effective strategies 14, 15, 16 but are not covered currently by the Spanish National Health Service (NHS) or the coverage is limited to certain groups (health‐care workers or people with limited resources). According to the World Health Organization 17, Spain has national smoke‐free legislation; a tobacco taxation policy; complete bans on tobacco advertising, promotion and sponsorship; services to treat tobacco dependence; and requirements to put health warnings on tobacco packages. However, Spanish decision‐makers currently lack information on the costs and potential benefits of investing in the new smoking cessation interventions or improving the reach of existing services. A rigorous analysis of alternative investment strategies is therefore needed.
When making funding decisions, health‐care administrators need evidence on efficacy and costs of deploying individual tobacco control measures in order to justify their investment. The ‘expert consensus document on tobacco addiction treatment in Spain’, supported by the Ministry of Health and Consumer Affairs of Spain, published in 2009, pointed out that the final definition of a common minimum care strategy in the country should be based on criteria of cost‐effectiveness. Currently, smoke‐free policies and tobacco duty are in place encouraging smokers to make a quit attempt 12, 17. Other interventions target smokers who are motivated to quit, and include printed self‐help materials and brief physician advice, although this is funded only in some regions of Spain. At the regional level, offering general practitioner (GP) brief physician advice in Spain is cost‐effective [€7260 per quality‐adjusted life years (QALYs) gained] 18. However, pharmacotherapy [such as nicotine replacement therapy (NRT), varenicline or bupropion] is not currently covered by the Spanish NHS 19, 20.
The aim of this study is therefore to estimate the cost‐effectiveness of alternative smoking cessation scenarios. We assessed the following scenarios: (a) the current provision of smoking cessation services (brief physician advice and printed self‐help material as well as an indoor smoking ban and a smoking duty at current levels); and four alternative scenarios: coverage of proactive telephone calls; nicotine replacement therapy (mono and combo) [prescription (Rx) NRT]; varenicline (standard duration); or bupropion, to complement the current provision.
Methods
Model description
We used the European study on Quantifying Utility of Investment in Protection from Tobacco model (EQUIPTMOD), a Markov‐based state transition model, to estimate the return on investment (ROI) of alternative smoking cessation scenarios. The EQUIPTMOD is described in detail elsewhere 21, but a top‐level summary of the model workings is provided here. This is a cohort model with three states: current smoker, former smokers and death. Individuals (16+ years) in the cohort can either stay within their current state or move to one of the other two states except for death, which is an absorbing state. A yearly cycle was chosen because it allowed the model to consider the natural progression and resolution of the disease. In each cycle, smokers and former smokers from the cohort model have the chance of having four conditions: lung cancer (LC); coronary heart disease (CHD); chronic obstructive pulmonary disease (COPD); and stroke (ST). Population‐weighted costs and QALYs are calculated for each age‐ and sex‐specific group during the life‐time (until 100 years of age) and then converted to various ROI estimates. Both health‐care cost‐savings and the value of willingness‐to‐pay for a QALY gain (otherwise, known as the cost‐effectiveness threshold) are used to estimate benefit–cost ratios.
The main model outputs used in this analysis include: economic impact (loss productivity and passive smoking costs), health‐care costs savings, QALYs, the incremental cost‐effectiveness ratio (ICER); and benefit–cost ratio. ICERs measure extra costs needed to implement a scenario per QALY gain in relation to the comparator. Benefit–cost ratios measure the return (in €) on every €1 invested in the scenario, and may include only health‐care savings or also the value of the QALY gains to calculate the benefits. All ROI estimates were calculated from the perspective of the Spanish NHS. Where possible, a quasi‐societal perspective (to include productivity gains) was also considered.
Model inputs
Several sources of evidence were used to inform the model with regard to the Spanish context. Respective literature reviews were carried out between 2014 and 2016 and, where necessary, international databases were used to acquire data for the model inputs. The input data were: general population data (such as population distribution, age‐ and sex‐specific mortality rates, smoking prevalence and relative risks); disease prevalence; motivation to quit; intervention reach (varenicline; bupropion; telephone support; printed self‐help materials; brief physician advice; taxation increase; and indoor smoking ban); intervention effectiveness (varenicline; bupropion; telephone support; and printed self‐help material); disease costs; intervention costs (varenicline; bupropion telephone support; and printed self‐help material); costs attributable to passive smoking in children and adults; utilities (never smokers; current and former smoker; LC, CHD; COPD; ST); and productivity losses (work‐days lost per smoker; average hourly wage; and employment among smokers). After examination of literature reviews, diseases attributable to passive smoking in children included acute otitis media (AOM), lower respiratory tract (LRT) infections; and asthma. However, in adults, passive smoking‐related conditions included LC, CHD and asthma 21. The rate used to discount future costs and QALYs was 3%, following Spanish guidelines on health technologies assessment and economic evaluations 22. We used the cost‐effectiveness interval threshold values per QALY gain in Spain (€21 000–24 000) as derived from a recent estimation 23.The threshold value is the societal willingness‐to‐pay for a QALY gain and is used widely in cost‐effectiveness analysis. This value was also used to translate QALY gains into monetary benefits to derive the benefit–cost ratios. The mean unit and annual costs were converted to € (2015) using country‐specific interannual inflation rate from the price year to 2015 24.
Table 1 shows details of the data required to populate the model.
Table 1.
Inputs to populate the model for the Spanish context.
| Inputs | Input value (mean) | Standard error or 95% CI | Source |
|---|---|---|---|
| General data | |||
| Population | Age– and sex–dependent | – | 26 |
| Mortality rates | Age‐ and sex‐dependent | – | 27 |
| Smoking prevalence | Age‐ and sex‐dependent | – | 4 |
| Relative risks | Age‐dependent | – | 28, 29 |
| Disease prevalence | |||
| Lung cancer | Age‐ and sex‐dependent | – | 30, 31 |
| CHD | Age‐ and sex‐dependent | – | 32 |
| COPD | Age‐ and sex‐dependent | – | 33 |
| ST | Age‐ and sex‐dependent | – | 9, 34 |
| Motivation to quit | 36.49% | SE (0.0080961); CI 95% (0.3490–0.3807) | 4 |
| Costs (all expressed in €2015) | |||
| Disease costs | – | ||
| Lung cancer costs | 15 289.86 | – | 35 |
| CHD costs | 1 454.37 | – | 36, 37 |
| COPD costs | 4123.75 | – | 38 |
| ST costs | 8424.39 | – | 39, 40 |
| Passive smoking | |||
| Cost attributable to passive smoking in children (annual cost per case) | |||
| AOM | 2538 | – | 41 |
| LRT infections | 3324 | – | 41 |
| Asthma | 775 | – | 42 |
| Cost attributable to passive smoking in adults (annual cost per case) | |||
| Lung cancer | 15 290 | – | 35 |
| CHD | 1454 | – | 36, 37 |
| Asthma | 1533 | – | 43 |
| Utilities | |||
| Never smokers (include all the smoking‐attributable diseases) | 0.884 | – | 44 |
| Current smokers (include all the smoking attributable diseases) | 0.85 | – | 44 |
| Former smokers (include all the smoking attributable diseases) | 0.869 | – | 44 |
| LC | 0.56 | – | 45 |
| CHD | 0.621 | – | 45 |
| COPD | 0.732 | – | 45 |
| ST | 0.550 | – | 45 |
| Productivity loses | |||
| Work‐days lost per smoker | 6 days | – | 46 |
| Average hourly wage | 15.93 (men); 13.18 (women) | – | 47 |
| Employment among smokers | 53.52% (employed); 22.53% (unemployed); 10.86% (retired); 13.08% (others) | – | 4 |
CHD = coronary heart disease; COPD = chronic obstructive pulmonary disease; ST = stroke; CI = confidence interval; SE = standard error; AOM = acute otitis media; LRT = lower respiratory tract.
Interventions and investment scenarios
We considered the following investment scenarios.
Current provision of services
Brief physician advice and printed self‐help material were available at the current levels of reach. Although there has been no clear stated public strategy in the country for brief physician advice as part of public funding, the National Committee for Smoking Prevention (CNPT) jointly with the Ministry of Health and Consumer Affairs identified and recommended brief physician advice as an essential intervention to promote smoking cessation 12, 19, 20. In addition, provision of health‐care services is decentralized in several autonomous communities. Some of these regions are currently funding the brief physician advice interventions and a few others are not. Therefore, in order to establish the definition of the current provision of cessation services it was decided to include brief physician advice as if this was funded by all regions. As Spain already has a smoking ban and tobacco duty, the current provision was also assumed to have these two top‐level interventions as they exist currently. The current provision (brief physician advice + printed self‐help materials + smoking ban + tobacco duty at the current levels) was thus compared with the ‘baseline’ (existing smoking ban + tobacco duty at the current levels).
Alternative scenarios
Alternative scenarios included proactive telephone calls; prescription nicotine replacement therapy (Rx NRT: mono and combo); varenicline; or bupropion; used separately to complement the current provision. Spanish experts have recommended proactive telephone calls and cessation medications for national implementation from efficacy/effectiveness perspectives, but not considering cost‐effectiveness 12. This analysis will address this lack of information on the cost‐effectiveness of those interventions if they were to be implemented to complement the current provision of services.
Details on intervention reach, relative effectiveness and cost of intervention are provided in Table 2.
Table 2.
Data on reach, relative effectiveness and costs of interventions.
| Interventions | Reach (source) | Relative effectiveness (SD)[Link] (source) | Cost per smoker (€ 2015) (source) |
|---|---|---|---|
| Current provision | |||
| Top‐level interventions | |||
| Brief physician advice | 21% of smokers not previously prepared to make a quit attempt receive brief physician advice 48 | 1.40 (0.3910) 49 | 26.67 50 |
| Tobacco duty | All (100%) smokers are exposed to tobacco duty 48 | 1.20 (0.1637) 51 | 0 |
| Indoor smoking ban | All (100%) smokers are exposed to indoor smoking ban 48 | 1.10 (0.1562) 52 | 0 |
| Pharmacological interventions | – | – | – |
| Behavioural interventions | |||
| Printed self‐help materials | 1% of smokers who make quit attempts receive self‐help materials 48 | 1.19 (0.2701) 53 | 17.62 54 |
| Alternative scenario with proactive telephone support (current provision + telephone support) | |||
| Behavioural interventions | |||
| Telephone support (proactive) | 0.5% of smokers who make quit attempts make use of the telephone support 48 | 1.40 (0.1709) 55 | 205.04 56 |
| Alternative scenario with medications (current provision + varenicline/bupropion/Rx NRT) | |||
| Varenicline (standard duration) | 5% of smokers motivated to quit use varenicline (standard duration) 48 | 2.30 (0.2283) 57 | 298.33 19, 58, 59 |
| Bupropion | 1% of smokers motivated to quit use bupropion 48 | 1.60 (0.2267) 60 | 151.28 19, 58, 59 |
| Rx mono NRT | 5% of smokers motivated to quit use OTC mono NRT 48 | 1.60 (0.0917) 61 | 276.38 19, 58, 59 |
| Rx combo NRT | 2% of smokers motivated to quit use OTC combo NRT 48 | 2.14 (0.2417) 61 | 554.19 19, 58, 59 |
SD = standard deviation; NRT = nicotine replacement therapy; OTC = over‐the‐counter.
Sensitivity analysis
We analysed the uncertainty around model inputs. Deterministic univariate sensitivity analysis was conducted by changing the discount rate from 0 to 5%. Probabilistic sensitivity analysis (PSA) was undertaken by sampling randomly from the distribution of each of the input value and calculating the expected costs and expected QALYs for that combination of input values. Details on distributions and parameters uncertainty are provided elsewhere 21. One thousand Monte Carlo simulations were conducted from these distributions 25. Transition probabilities were characterized by beta distributions; relative risks and odds ratios by log‐normal distributions; utility values specific to smoking status by beta distributions; utility decrements associated with smoking‐related disease by normal distributions; and costs by gamma distributions, although intervention costs were assumed to be fixed, as were population‐level data. When no uncertainty boundaries were obtained for the included input estimates in the model, analysis then adopts standard methods for defining uncertainty concerning input values 25. The PSA was conducted to compare the alternative scenarios with the baseline, as allowed by the EQUIPTMOD.
Results
The results (model outputs) described in this section and in Table 3 refer to the Spanish adult (16+ years) population of approximately 39.2 million, of whom approximately 10.5 million (26.9%) are smokers and 7.7 million (19.6%) are former smokers. Of all current smokers throughout Spain, approximately 3.1 million (30%) would make a quit attempt in the next 12 months. Approximately €61 million would be required to spend on services to deliver the current provision (brief physician advice and self‐help material on top of smoking ban and tobacco duty at current levels) nationally in Spain (currently, this happens in only some regions). This investment would lead to 193 128 successful quitters, i.e. 18 per 1000 smokers. Every £1 invested in the current provision nationally has the potential to generate £5 during the life‐time compared to the baseline scenario, where no brief advice and printed materials were available (Table 3).
Table 3.
Model outputs for current provision and alternative scenarios (€ 2015).
| Estimates | Current provisiona | Alternative scenario involving proactive telephone callsb | Alternative scenariosc involving | ||
|---|---|---|---|---|---|
| Rx NRT (mono and combo) | Varenicline (standard duration) | Bupropion | |||
| Investment (current year) | |||||
| Top‐level interventions (€ 2015) | 58 981 985 | 58 981 985 | 58 981 985 | 58 981 985 | 58 981 985 |
| Cessation interventions (€ 2015) | 1 855 597 | 12 652 183 | 262 256 091 | 158 943 943 | 17 787 196 |
| Total (€ 2015) | 60 837 582 | 71 634 168 | 323 093 674 | 217 925 929 | 77 769 181 |
| Outputs (over the life‐time unless stated otherwise ) | |||||
| Successful quitters (current year) | 193 128 | 194 181 (+1053) | 209 087 (+6905) | 212 744 (+5312) | 194 939 (+1811) |
| Quitters per 1000 smokers (current year) | 18.18 | 18.28 (+1.6) | 19.68 (+2.15) | 20.03 (+2.00) | 18.35 (+1.67) |
| Average QALYs gained (per smoker) | 16.1136 | 16.1138 | 16.1156 | 16.1161 | 16.1138 |
| Average cost (€ 2015) (per smoker) | 41196.91 | 41196.02 | 41192.72 | 41176.06 | 41195.12 |
| Productivity losses (€ 2015) | 3980.54 | 3980.14 (−0.4) | 3974.40 (−2.65) | 3972.99 (−2.04) | 3979.85 (−0.69) |
| Passive smoking costs in children (€ 2015) | 88.15 | 88.15 (−0.14) | 88.02 (−0.19) | 87.99 (−0.18) | 88.14 (−0.15) |
| Passive smoking costs in adults (€ 2015) | 607.68 | 607.62 (−0.93) | 606.74 (−1.34) | 606.52 (−1.24) | 607.57 (−1.04) |
| Incremental QALYs per smoker | − | +0.0002 | +0.002 | +0.0025 | +0.0002 |
| Incremental costs (€ 2015) per smoker | − | ‐‐0.89 | −4.19 | −20.85 | −1.79 |
| ICER (€/QALY) (€ 2015) | |||||
| Time horizon: 10 years | Dominantc | 7968 €/QALY | 23 816€/QALY | 2215€/QALY | 4241€/QALY |
| Time horizon: life‐time | Dominantd | Dominantd | Dominantd | Dominantd | Dominantd |
| ROI (€ 2015) (per 1€ invested) | |||||
| Time horizon: 10 years | 1.87 | 0.70 | 0.43 | 0.89 | 0.81 |
| Time horizon: life‐time | 5.01 | 1.87 | 1.17 | 2.40 | 2.18 |
Current provision (brief physician advice + self‐help material + tobacco duty + smoking ban) versus the baseline (tobacco duty + smoking ban).
Alternative scenario (proactive telephone calls + current provision) versus the current provision alone (brief physician advice + self‐help material + tobacco duty + smoking ban).
Alternative scenario (medication + current provision) versus the current provision alone (brief physician advice + self‐help material + tobacco duty + smoking ban).
Dominant = cost saving (scenario is less expensive and produces more QALYs). ICER = incremental cost effectiveness ratio; ROI = return on investment; QALY = quality‐adjusted life years; NRT = nicotine replacement therapy.
Implementing proactive telephone calls to complement the current provision would require an additional investment of approximately €12.6 million. However, this would generate 1053 additional successful quitters. For every €1 spent on providing this intervention to smokers who are trying to stop smoking, the NHS could recoup €1.87 in health‐care cost savings during the long term (Table 3).
Providing cessation medications to complement the current provision would generate additional quitters. Including Tx NRT (mono and combo), for example, would generate 6905 additional quitters at an additional investment of approximately €260 million. For every €1 spent on providing this intervention, the NHS could recoup €1.17 in health‐care cost savings during the long term (Table 3). The corresponding figure for other alternative scenarios was, respectively, €2.40 (varenicline) and €2.18 (bupropion).
Table 3 also shows that all scenarios were ‘dominant’ (i.e. cost‐saving: less expensive to run but generates more QALYs than the comparator) on the life‐time horizon. ICERs calculated for the 10‐year horizon shows that all scenarios are cost‐effective, as the incremental costs for a QALY gain (e.g. €7968 for proactive calls) are well below or within the Spanish cost‐effectiveness interval threshold of €21 000–24 000 for all scenarios.
The effect of scenarios on productivity gains and reduction of passive smoking costs was small at per‐smoker level (Table 3). However, when translated to the population level, these effects can deliver notable cost‐savings. For example, if only 2% of the 10.5 million smokers were reached via proactive telephone calls, we would gain €84 000 in productivity and €29 400 in the treatment of passive smoking‐related diseases in children.
On deterministic sensitivity analysis, the scenario involving proactive telephone calls was cost‐effective compared to the current provision for a 3% discount rate after 10 years, but for a 0% discount rate it was cost‐effective after 5 years. For alternative scenarios involving varenicline and bupropion, decisions based on life‐time cost‐effectiveness remained the same regardless of the discount rate used. The results from the PSA are shown in Figure 1, where incremental costs and incremental QALYs are plotted. As seen in the figure, most of the dots fell into the cost‐saving quadrant. The cost‐effectiveness acceptability curve (CEAC) is presented in Figure 2. At the Spanish interval threshold value from €21 000 to 24 000 per QALY, the probability that the current provision is cost‐effective compared to the baseline is approximately 96%.
Figure 1.
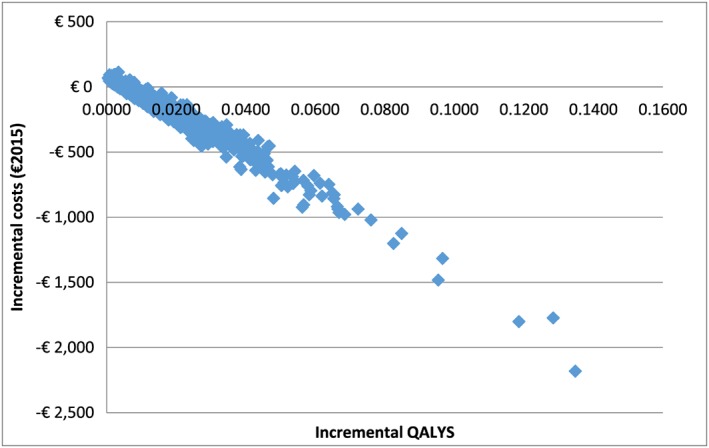
Cost‐effectiveness plane plotting incremental costs and incremental quality‐adjusted life years (QALYs) (current provision versus the baseline)
Figure 2.
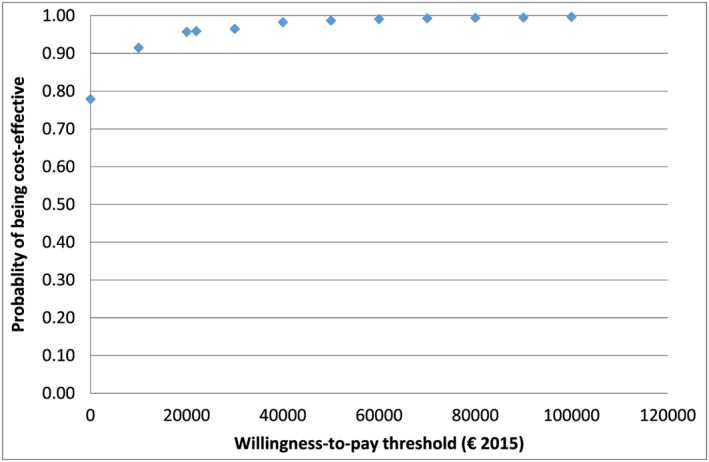
Cost‐effectiveness acceptability curve (current provision versus the baseline)
Discussion
Decision analytical models are necessary to inform decision‐making by bringing together existing evidence to assess the probable cost‐effectiveness of competing forms of smoking cessation programmes. This study is the first to evaluate the cost‐effectiveness of the current and potential investment scenarios on smoking cessation in Spain. The analysis based on the EQUIPTMOD, presented in this paper, incorporated the full range of assumptions used to assess cost‐effectiveness during the life‐time in the Spanish population. This study has demonstrated a practical application of the EQUIPT ROI tool in Spain to evaluate both the existing and several potential investment scenarios to improve smoking cessation 62. Our findings show that the current provision is cost‐saving, showing that tobacco control in Spain appears to move in the right direction. However, we evaluated the current provision on the assumption that brief physician advice would be extended and implemented in all Spanish regions, which is not the case currently 19, 20. This implies that a definitive action needs to be taken by the Government in order to increase the reach of this intervention to cover the whole country.
Our findings also point to a new direction of travel for Spanish decision‐makers. The alternative scenario with proactive telephone calls implied an extra investment of €10.8 million to generate 1053 successful quitters. For each one extra successful quitter, the Government will therefore have to pay €10 253. After 10 years, this intervention would be cost‐saving. If the Government is willing to pay an extra €15.9 million, instead of €10.8 million, to implement a national strategy with bupropion, this would generate an extra 1811 successful quitters. In this case, gaining one extra successful quitter will cost the Government €8797, allowing the Government to save €1455 per successful quitter. However, from the viewpoint of cost‐effectiveness alone, bupropion is not the most efficient intervention; varenicline (standard duration) is the most efficient. Implementing the varenicline scenario would require an extra investment of almost €157 million, which is a much larger investment than the extra €15.9 million needed to fund bupropion. Our analysis thus provides decision‐makers with alternative policy options with a potential budgetary impact.
The model presented some limitations that need to be considered in order to interpret the results. First, the model did not include possible adverse events of any of the medications considered. Conversely, for some particular diseases the model did not include any potential protective effects of smoking. It is fair to assume a trivial impact of this on our conclusions, given the scale of estimated health‐care savings and QALY gains in our analysis. Secondly, due to the lack of data on smoking status‐specific relative risks, it was not possible to ‘split’ former smokers into ‘recent’ and ‘long‐term’ categories. It is reasonable to assume that the use of a large sample size (7.7 million former smokers) included in the analysis might have mitigated the impact of this, although to what extent remains unclear. Thirdly, within the model it is assumed that smokers only try to stop smoking once within the first year. In subsequent years, the background quit rate was assumed to reflect the balance of quitting and relapsing. In real life, smokers who fail to quit smoking with one intervention may be more likely to repeat the same intervention or try a number of different interventions 63. Furthermore, smokers who do not stop may do so in later years, thus partly lowering the incremental effect of cessation. Fourthly, no country‐specific effectiveness data could be retrieved for the evaluated interventions in Spain. However, Cochrane effectiveness inputs used in this model represent rigorous evidence from a wide range of studies world‐wide and are well accepted in the research community. Fifthly, interactions between pharmaceuticals and behavioural interventions were included within the model using a multiplicative assumption, as specific data regarding the combinations were not available in the literature. Finally, an important limitation of EQUIPTMOD was the restriction posed by its PSA functionality. The economic model was developed primarily to underpin a ROI tool for decision‐making purposes. This objective inevitably required the tool developers to not only provide a simple generalized user interface (GUI) and granularity of outputs (a number of ROI metrics) but, significantly, also subjected them to consider Microsoft Excel's own limitations to handle such a large model. The PSA functionality available to the users was therefore restricted to providing sensitivity estimates for the current provision compared to the baseline. Future research will benefit from further development on the PSA functionality of the EQUIPTMOD.
Treatment costs used in the model only took into account the hospitalization costs, because such costs in Spain are available only at hospital level. Therefore, there is a need to calculate health‐care costs attributable to smoking‐related diseases by including not only hospital‐related costs, but also the downstream costs relevant to the entire disease episode.
Conclusion
Analysis based on the EQUIPTMOD has provided Spanish decision‐makers with policy options for tobacco control. It would be cost‐effective to expand the reach of GP brief interventions to all areas of Spain, provide proactive telephone support and reimburse smoking cessation medication to smokers trying to stop. During a life‐time, these policies would be cost‐saving.
Ethical approval
None required for this analysis. However, the EQUIPT study, on which the current analysis is based, received full ethical clearance from Brunel University Research Ethics Committee.
Declaration of interests
None.
Acknowledgements
We have received funding from the European Community's Seventh Framework Programme (the EQUIPT Project; grant agreement 602 270). The funders had no influence in the conducting of this study or the drafting of this manuscript. The authors are grateful to all members of the EQUIPT consortium and the Steering Committee for their invaluable help and support.
Trapero‐Bertran, M. , Muñoz, C. , Coyle, K. , Coyle, D. , Lester‐George, A. , Leidl, R. , Németh, B. , Cheung, K.‐L. , Pokhrel, S. , and Lopez‐Nicolás, Á. (2018) Cost‐effectiveness of alternative smoking cessation scenarios in Spain: results from the EQUIPTMOD. Addiction, 113: 65–75. https://doi.org/10.1111/add.14090.
References
- 1. Jha P. Avoidable global cancer deaths and total deaths from smoking. Nat Rev Cancer 2009; 9: 655–664. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization (WHO) . Tobacco. Fact Sheet 339 [internet]. Geneva: WHO; 2016. . Available at: http://www.who.int/mediacentre/factsheets/fs339/en (accessed 18 July 2016). [Google Scholar]
- 3. European Comission Tobacco or Health in the European Union. Past, present and future. Brussels: The ASPECT Consortium and European Commission Directorate‐General for Health and Consumer Protection; 2004. Available at: http://ec.europa.eu/health/ph_determinants/life_style/Tobacco/Documents/tobacco_fr_en.pdf (accessed February 2017). [Google Scholar]
- 4. Instituto Nacional de Estadística (INE) . [Spanish National Health Survey (SNHS) 2011–2012] [internet]. Madrid: National Statistics Institute; 2011. Available at: http://www.ine.es/dyngs/INEbase/en/operacion.htm?c=Estadistica_C&cid=1254736176783&menu=resultados&secc=1254736194721&idp=1254735573175 (accessed 14 December 2016). [Google Scholar]
- 5. Banegas Banegas J. R., Rodriguez Artalejo F., Martin‐Moreno J. M., Gonzalez Enriquez J., Villar Alvarez F., Guasch Aguilar A. Projections of the impact of the smoking habit on the health of the Spanish population and on the potential benefits from its control. Med Clin (Barc) 1993; 101: 644–649. [PubMed] [Google Scholar]
- 6. Gonzalez Enriquez J., Villar Alvarez F., Banegas Banegas J. R., Rodriguez Artalejo F., Martin Moreno J. M. Trends in the mortality attributable to tobacco use in Spain, 1978–1992: 600,000 deaths in 15 years. Med Clin (Barc) 1997; 109: 577–582. [PubMed] [Google Scholar]
- 7. Banegas Banegas J. R., Diez Ganan L., Rodriguez‐Artalejo F., Gonzalez Enriquez J., Graciani Perez‐Regadera A., Villar Alvarez F. Smoking‐attributable deaths in Spain in 1998. Med Clin (Barc) 2001; 117: 692–694. [DOI] [PubMed] [Google Scholar]
- 8. Pardell H., Saltó E., Jané M., Salleras L. Spanish: En profundidad: Coste Sociosanitario del Tabaquismo Impacto sanitario y económico del tabaquismo. English: In depth: Social health cost of smoking and healthcare and economic impact of smoking. Prevención del Tabaquismo 2001; 3: 245–250. [Google Scholar]
- 9. Gonzalez‐Enriquez J., Salvador‐Llivina T., Lopez‐Nicolas A., Anton De Las Heras E., Musin A., Fernandez E., et al The effects of implementing a smoking cessation intervention in Spain on morbidity, mortality and health care costs. Gac Sanit 2002; 16: 308–317. [DOI] [PubMed] [Google Scholar]
- 10. Banegas Banegas J. R., Diez Ganan L., Gonzalez Enriquez J., Villar Alvarez F., Rodriguez‐Artalejo F. Recent decrease in smoking‐attributable mortality in Spain. Med Clin (Barc) 2005; 124: 769–771. [DOI] [PubMed] [Google Scholar]
- 11. Consejo Económico y Social . Spanish: Dictamen sobre el anteproyecto de Ley reguladora de la venta, publicidad, promoción y consumo público de tabaco. English: Opinion on the preliminary draft of the Law regulating the sale, advertising, promotion and public consumption of tobacco. Madrid: Consejo Económico y Social; 2005. Dictamen 2/05. Available at: http://www.ces.es/documents/10180/18507/Dic022005 (accessed February 2017). [Google Scholar]
- 12. Córdoba García R. Spanish: Impacto potencial en la prevalencia y en la mortalidad de las medidas de prevención y control del tabaquismo: informe del CNPT. English: Potential impact on the prevalence and mortality of smoking prevention and control measures: report of the National Committee for Smoking Prevention (CNPT). Madrid: National Committee for Smoking Prevention; 2010. Available at: http://www.cnpt.es/doc_pdf/IMPACTO%20MEDIDAS%20CONTROL%20TABACO_DEFINITIVO_Enero_2011.pdf (accessed February 2017). [Google Scholar]
- 13. European Commission . Attitudes of Europeans towards Tobacco and Electronic Cigarettes. Brussels: European Commission: Directorate‐General for Health and Food Safety; 2015. Special Eurobarometer 429. Available at: http://ec.europa.eu/public_opinion/archives/ebs/ebs_429_en.pdf (accessed February 2017). [Google Scholar]
- 14. Cornuz J., Gilbert A., Pinget C., McDonald P., Slama K., Salto E., et al Cost‐effectiveness of pharmacotherapies for nicotine dependence in primary care settings: a multinational comparison. Tob Control 2006; 15: 152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fernandez de Bobadilla Osorio J., Sanchez‐Maestre C., Brosa Riestra M., Arroyo O., Sanz de Burgoa V., Wilson K. Cost effectiveness analysis of varenicline (Champix) for the treatment of smoking in Spain. An Med Interna 2008; 25: 342–348. [DOI] [PubMed] [Google Scholar]
- 16. Jimenez‐Ruiz C. A., Solano‐Reina S., Signes‐Costa J., de Higes‐Martinez E., Granda‐Orive J. I., Lorza‐Blasco J. J., et al Budgetary impact analysis on funding smoking‐cessation drugs in patients with COPD in Spain. Int J Chron Obstruct Pulmon Dis 2015; 10: 2027–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization (WHO) WHO Report on the Global Tobacco Epidemic. Geneva: WHO; 2015. Country Profile: Spain, p. 2015 Available at: http://www.who.int/tobacco/surveillance/policy/country_profile/esp.pdf?ua=1. [Google Scholar]
- 18. López‐Nicolás A., Trapero‐Bertran M., Muñoz C. Spanish: Coste‐utilidad del consejo médico para dejar de fumar en la Región de Murcia. English: Cost‐utility of medical advice to stop smoking in the Region of Murcia. Atención Primaria 2017; 49: 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Camarelles Guillem F., Dalmau González‐Gallarza R., Clemente Jiménez L., Cascán Herreros M. P., Gallego Valdeiglesia A., Díaz‐Maroto Muñoz J. L., et al Spanish: Documento de Consenso para la Atención Clínica del Tabaquismo en España. English: Consensus Document for the Clinical Care of Smoking in Spain. Madrid: National Committee for Smoking Prevention; 2013. Available at: http://www.cnpt.es/documentacion/publicaciones/ec34e5d56ba572d76297484cb6eb6a3f9dd91ac750db1addf646305eccae0f6a.pdf. [DOI] [PubMed] [Google Scholar]
- 20. Camarelles Guillem F., Dalmau González‐Gallarza R., Clemente Jiménez L., Díaz‐Maroto Muñoz J. L., Lozano Polo A., Pinet Ogué M. C. Spanish: Documento de consenso para la atención clínica al tabaquismo en España. English: Consensus document for clinical attention to smoking in Spain. Med Clin (Barc) 2013; 140: 272.e1–272e12. [DOI] [PubMed] [Google Scholar]
- 21. Coyle K., Coyle D., Lester‐George A., West R., Trapero‐Bertran M., Leidl R., et al On behalf of the EQUIPT study group. EQUIPTMOD—development and application of an economic model to assess the impact of smoking cessation. Addiction 2017; https://doi.org/10.1111/add.14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lopez Bastida J., Oliva J., Antonanzas F., Garcia‐Altes A., Gisbert R., Mar J., et al A proposed guideline for economic evaluation of health technologies. Gac Sanit 2010; 24: 154–170. [DOI] [PubMed] [Google Scholar]
- 23. Vallejo‐Torres L., Garcia‐Lorenzo B., Castilla I., Valcarcel‐Nazco C., Garcia‐Perez L., Linertova R., et al On the estimation of the cost‐effectiveness threshold: why, what, how? Value Health 2016; 19: 558–566. [DOI] [PubMed] [Google Scholar]
- 24. National Statistics Institute . National Price Index (IPC) [internet]. Madrid: National Statistics Institute; 2015. Available at: http://www.ine.es/jaxiT3/Tabla.htm?t=10013&L=0 (accessed 14 December 2015). [Google Scholar]
- 25. Briggs A. H. Handling uncertainty in cost‐effectiveness models. Pharmacoeconomics 2000; 17: 479–500. [DOI] [PubMed] [Google Scholar]
- 26. Instituto Nacional de Estadística (INE) . [Population figures] [internet]. Madrid: National Statistics Institute; 2013]. Available at: http://www.ine.es/jaxi/tabla.do?path=/t20/p321/serie/def/l0/&file=02001.px&type=pcaxis&L=0 (accessed 4 December 2014). [Google Scholar]
- 27. Instituto Nacional de Estadística (INE) . [Main Demographic Indicators: Mortality] [internet]. Madrid: National Statistics Institute; 2013. Available at: http://www.ine.es/jaxiT3/Tabla.htm?t=1412&L=0 (accessed 9 December 2014). [Google Scholar]
- 28. Thun M. J., Carter B. D., Feskanich D., Freedman N. D., Prentice R., Lopez A. D., et al 50‐year trends in smoking‐related mortality in the United States. N Engl J Med 2013; 368: 351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. US Department of Health and Human Services . The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centres for Disease Control and Prevention, National Centre for Chronic disease Prevention and Health Promotion, Office on Smoking and Health; 2014. Available at: https://www.surgeongeneral.gov/library/reports/50-years-of-progress. [Google Scholar]
- 30. International Agency for Research on Cancer (IARC) . GLOBOCAN 2012 [internet]. Paris: IARC; 2012. Available at: http://globocan.iarc.fr/Pages/online.aspx (accessed 14 December 2016). [Google Scholar]
- 31. Instituto Nacional de Estadística (INE) . [Hospital Morbidity Survey] [internet]. Madrid: National Statistics Institute; 2013. Available at: http://www.ine.es/jaxi/tabla.do?path=/t15/p414/a2013/l0/&file=01021.px&type=pcaxis&L=0 (accessed 14 December 2016). [Google Scholar]
- 32. Alonso J. J., Muniz J., Gomez‐Doblas J. J., Rodriguez‐Roca G., Lobos J. M., Permanyer‐Miralda G. et al Prevalence of stable angina in Spain. Results of the OFRECE study. Rev Esp Cardiol (English edn) 2015;68:691–699. [DOI] [PubMed] [Google Scholar]
- 33. Miravitlles M., Soriano J. B., Garcia‐Rio F., Munoz L., Duran‐Tauleria E., Sanchez G., et al Prevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activities. Thorax 2009; 64: 863–868. [DOI] [PubMed] [Google Scholar]
- 34. Diaz‐Guzman J., Egido J. A., Gabriel‐Sanchez R., Barbera‐Comes G., Fuentes‐Gimeno B., Fernandez‐Perez C., et al Stroke and transient ischemic attack incidence rate in Spain: the IBERICTUS study. Cerebrovasc Dis 2012; 34: 272–281. [DOI] [PubMed] [Google Scholar]
- 35. Corral J., Espinas J. A., Cots F., Pareja L., Sola J., Font R., et al Estimation of lung cancer diagnosis and treatment costs based on a patient‐level analysis in Catalonia (Spain). BMC Health Serv Res 2015; 15: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ramirez de Arellano A., Coca A., de la Figuera M., Rubio‐Terres C., Rubio‐Rodriguez D., Gracia A., et al Economic evaluation of cardio inCode(R), a clinical‐genetic function for coronary heart disease risk assessment. Appl Health Econ Health Policy 2013; 11: 531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sicras‐Mainar A., Velasco‐Velasco S., Gonzalez‐Rojas Guix N., Rodriguez‐Cid J. L. Clinical and economic evaluation in accordance with the level of cardiovascular risk in subjects appertaining to Spanish population setting. Med Clin (Barc). 2008; 131: 158–159. [DOI] [PubMed] [Google Scholar]
- 38. Izquierdo J. L. The burden of COPD in Spain: results from the confronting COPD survey. Respir Med 2003; 97: S61–S69. [DOI] [PubMed] [Google Scholar]
- 39. Álvarez‐Sabin J. Los cuidados del paciente tras el ictus: Estudio CONOCES [oral communication]. English: Patient care after stroke: CONOCES study [oral communication]. Pamplona: Health Economics Association Conference; 2014. [Google Scholar]
- 40. Mar J., Alvarez‐Sabin J., Oliva J., Becerra V., Casado M. A., Yebenes M., et al The costs of stroke in Spain by aetiology: the CONOCES study protocol. Neurologia 2013; 28: 332–339. [DOI] [PubMed] [Google Scholar]
- 41. Spanish: Ministerio de Sanidad Política Social e Igualdad (MSSSI) . Conjunto Mínimo Básico de Datos—Hospitalización (CMBD‐H) [internet]. English: Ministerio de Sanidad Política Social e Igualdad (MSSSI). Basic Minimum Data Set‐Hospitalization (CMBD‐H) [internet]. Madrid: MSSSI; 2012. Available at: http://pestadistico.msc.es/PEMSC25/ArbolNodos.aspx (accessed 9 December 2014). [Google Scholar]
- 42. Blasco Bravo A. J., Perez‐Yarza E. G., Lazaro y de Mercado P., Bonillo Perales A., Diaz Vazquez C. A., Moreno Galdo A. Cost of childhood asthma in Spain: a cost evaluation model based on the prevalence. An Pediatr (Barc) 2011; 74: 145–153. [DOI] [PubMed] [Google Scholar]
- 43. Martinez‐Moragon E., Serra‐Batlles J., De Diego A., Palop M., Casan P., Rubio‐Terres C., et al Economic cost of treating the patient with asthma in Spain: the AsmaCost study. Arch Bronconeumol 2009; 45: 481–486. [DOI] [PubMed] [Google Scholar]
- 44. Vogl M., Wenig C. M., Leidl R., Pokhrel S. Smoking and health‐related quality of life in English general population: implications for economic evaluations. BMC Public Health 2012; 12: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sullivan P. W., Slejko J. F., Sculpher M. J., Ghushchyan V. Catalogue of EQ‐5D scores for the United Kingdom. Med Decis Making 2011; 31: 800–804. [DOI] [PubMed] [Google Scholar]
- 46. Comité National para la Prevención del Tabaquismo (CNPT) . Spanish: Evaluación del Control del Tabaquismo sobre los costes empresariales y sanitarios. English: Evaluation of Smoking Control on business and healthcare costs. Madrid: National Committee for Smoking Prevention; 2009. Available at: http://www.cnpt.es/doc_pdf/Informe_fiscalidad_01.pdf (accessed February 2017). [Google Scholar]
- 47. Instituto Nacional de Estadística (INE) . [Wage Structure Survey] [internet]. Madrid: National Statistics Institute; 2012. Available at: http://www.ine.es/jaxi/tabla.do?path=/t22/p133/cno11/serie/l1/&file=04001.px&type=pcaxis&L=1 (accessed 23 December 2014). [Google Scholar]
- 48. West R., Coyle K., Owen L., Coyle D., Pokhrel S., EQUIPT Study Group . Estimates of effectiveness and reach for ‘return on investment' modelling of smoking cessation interventions using data from England. Addiction 2017; https://doi.org/10.1111/add.14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Aveyard P., Begh R., Parsons A., West R. Brief opportunistic smoking cessation interventions: a systematic review and meta‐analysis to compare advice to quit and offer of assistance. Addiction 2012; 107: 1066–1073. [DOI] [PubMed] [Google Scholar]
- 50. Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: Personal Social Services Research Unit; 2014. Available at: http://www.pssru.ac.uk/project-pages/unit-costs/2014/index.php?file=full (accessed December 2015). [Google Scholar]
- 51. Jha P., Peto R. Global effects of smoking, of quitting, and of taxing tobacco. N Engl J Med 2014; 370: 60–68. [DOI] [PubMed] [Google Scholar]
- 52. Hackshaw L., McEwen A., West R., Bauld L. Quit attempts in response to smoke‐free legislation in England. Tob Control 2010; 19: 160–164. [DOI] [PubMed] [Google Scholar]
- 53. Hartmann‐Boyce J., Lancaster T., Stead L. F. Print‐based self‐help interventions for smoking cessation. Cochrane Database Syst Rev 2014; 6: CD001118. [DOI] [PubMed] [Google Scholar]
- 54. Blyth A., Maskrey V., Notley C., Barton G. R., Brown T. J., Aveyard P., et al Effectiveness and economic evaluation of self‐help educational materials for the prevention of smoking relapse: randomised controlled trial. Health Technol Assess 2015; 19: 1–70; v–vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stead L. F., Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database Syst Rev 2005; 2: CD001007. [DOI] [PubMed] [Google Scholar]
- 56. Hollis J. F., McAfee T. A., Fellows J. L., Zbikowski S. M., Stark M., Riedlinger K. The effectiveness and cost effectiveness of telephone counselling and the nicotine patch in a state tobacco quitline. Tob Control 2007; 16: i53–i59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cahill K., Stead L. F., Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev 2012; 4: CD006103. [DOI] [PubMed] [Google Scholar]
- 58. Spanish: Colegio Oficial de Farmacéuticos de Pontevedra (COFPO) . Listado de Precios de los Medicamentos (October 2015) [internet]. English: Price List of Drugs (October 2015) [internet]. Pontevedra: COFPO; 2015. Available at: http://www.cofpo.org/index.php/medic-es.html (accessed 20 December 2015). [Google Scholar]
- 59. Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) . Spanish: Centro de Información online de Medicamentos de la AEMPS: CIMA [internet]. English: Online Information Center for Medicines of the Spanish Agency of Medicines and Medical Devices (AEMPS): CIMA [internet]. Madrid: AEMPS. Ministerio de Sanidad, Servicios Sociales e Igualdad; 2016. [accessed http://08.02.2016]. Available at: http://www.aemps.gob.es/cima/fichasTecnicas.do?metodo=detalleForm. [Google Scholar]
- 60. Hughes J. R., Stead L. F., Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev 2007; 1; CD000031. [DOI] [PubMed] [Google Scholar]
- 61. Stead L. F., Perera R., Bullen C., Mant D., Hartmann‐Boyce J., Cahill K., et al Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2012; 11; CD000146. [DOI] [PubMed] [Google Scholar]
- 62. Pokhrel S., Evers S., Leidl R., Trapero‐Bertran M., Kalo Z., Vries H., et al EQUIPT: protocol of a comparative effectiveness research study evaluating cross‐context transferability of economic evidence on tobacco control. BMJ Open 2014; 4; e006945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hajek P., Stead L. F., West R., Jarvis M., Lancaster T. Relapse prevention interventions for smoking cessation. Cochrane Database Syst Rev 2009; 1: CD003999. [DOI] [PubMed] [Google Scholar]


