Abstract
Objective
To evaluate the safety and potential efficacy of AMG 557, a fully human antibody directed against the inducible T cell costimulator ligand (ICOSL) in patients with systemic lupus erythematosus (SLE) with arthritis.
Methods
In this phase Ib, randomized, double‐blind, placebo‐controlled study, patients received AMG 557 210 mg (n = 10) or placebo (n = 10) weekly for 3 weeks, then every other week for 10 additional doses. The corticosteroid dosage was tapered to ≤7.5 mg/day by day 85, and immunosuppressants were discontinued by day 29. Primary end points on day 169 were safety, immunogenicity, the Lupus Arthritis Response Index (LARI; defined by a reduction in the tender and swollen joint counts), ≥1‐letter improvement in the musculoskeletal domain of the British Isles Lupus Assessment Group (BILAG) index, and medication discontinuation. The secondary/exploratory end points were changes in the tender and swollen joint counts, BILAG index scores (musculoskeletal, global), and the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI).
Results
The incidence of adverse events, most of which were mild, was similar between groups. LARI responses occurred in 3 of 10 patients receiving AMG 557 and 1 of 10 patients receiving placebo (P = 0.58). More patients in the AMG 557 group achieved a ≥4‐point improvement in the SLEDAI score on day 169 (7 of 10 patients) compared with the placebo group (2 of 10 patients) (P = 0.07). Patients treated with AMG 557 (versus placebo) had greater improvements from baseline in the global BILAG index scores (–36.3% versus –24.7%) and the SLEDAI score (–47.8% versus –10.7%) and in tender (–22.8% versus –13.5%) and swollen (–62.1% versus –7.8%) joint counts on day 169.
Conclusion
AMG 557 showed safety and potential efficacy, supporting further evaluation of the clinical efficacy of ICOSL blockade in patients with SLE.
Systemic lupus erythematosus (SLE) is a complex autoimmune disease characterized by unpredictable flares of potentially destructive inflammation that can affect the skin, musculoskeletal, nervous, pulmonary, and renal systems 1. Joint involvement is a common manifestation of SLE; it has been estimated that 69–95% of patients have lupus arthritis 2. Currently available treatments are inadequate; hence, more effective therapies with fewer short‐term and long‐term toxicities are needed 1.
Deposits of autoantibody–antigen immune complexes are a hallmark of SLE and can be found in various organs, triggering complement and other inflammatory pathways 3. Autoantibodies are a key pathogenic marker of the disease and implicate immune dysregulation as a driving force for disease pathogenesis 1, 3. Production of antibodies from autoreactive B cells typically requires direct interaction with CD4+ T helper cells 4. Normal interactions between T cells and B cells are concentrated in specific anatomic areas of secondary lymphoid organs. A specialized CD4+ T helper cell subset, the follicular helper T (Tfh) cell, localizes there and is primarily responsible for promoting the B cell response leading to antibody production 5. Tfh cells express a specific pattern of cell surface receptors, including CXCR5, programmed cell death 1 (PD‐1), and inducible T cell costimulator (ICOS) 5. ICOS is a member of the CD28 superfamily that is induced on T cells upon activation 6. ICOS has a sole ligand (ICOSL; also known as B7RP‐1), which is a member of the immune system B7 family of costimulatory molecule ligands and is present primarily on the surface of antigen‐presenting cells 6. Primary immunodeficiencies in patients lacking ICOS activity further support the requirements for these molecules in humoral immune responses in humans 7, 8. Thus, inhibition of ICOS/ICOSL activity could interfere with Tfh cell–B cell interactions and modulate autoantibody production in autoimmune diseases such as SLE.
AMG 557 is a fully human IgG2 monoclonal antibody that binds with high specificity and affinity to human ICOSL to inhibit its function 9. Two phase I studies characterized the safety, tolerability, pharmacokinetics, immunogenicity, and pharmacodynamics of AMG 557 in patients with SLE after either single‐ or multiple‐dose administration 9. Multiple‐dose administration of AMG 557 was shown to selectively inhibit neoantigen‐specific IgG production following keyhole limpet hemocyanin immunization 9.
The goal of the current study was to assess the safety and potential efficacy of ICOSL blockade in patients with active lupus arthritis. Historically, clinical studies in patients with SLE have been difficult to interpret because of the heterogeneity of immune pathology, clinical symptoms, and background medications 10. To overcome such difficulties, recent trials have used more discriminatory end points 11 and have enrolled large patient populations 12, 13. For smaller studies, alternative approaches may be necessary, such as the use of lupus organ–specific end points and withdrawal/tapering of background medications 14. In the current study, using a small number of patients, a treatment withdrawal design and a focus on single‐organ assessment (arthritis) were used to improve the detection of the clinical effects of AMG 557 relative to placebo.
Patients and Methods
This was a phase Ib, randomized, double‐blind, parallel‐group, placebo‐controlled, multiple‐dose study in patients with active lupus arthritis. The study was performed at 8 centers in the US, Europe, and Malaysia. All patients provided written informed consent.
Key eligibility criteria. Patients were adults ages 18–65 years with a diagnosis of SLE for ≥6 months as defined by the American College of Rheumatology criteria 15, 16, including positivity for antinuclear antibodies (ANAs) at screening or as previously documented. A positive ANA test was defined as a titer ≥1:80. Patients had inflammatory lupus arthritis with ≥4 tender joints and ≥4 swollen joints (28 joints assessed) and an SLE Disease Activity Index (SLEDAI) 17 score of ≥6 at screening. A requirement to be receiving immunosuppressants (methotrexate, azathioprine, or mycophenolate mofetil) for ≥12 weeks before study entry was subsequently removed to improve enrollment. Prednisone treatment was permitted if patients received a stable dosage of ≤20 mg/day (or comparable dose of another corticosteroid) for ≥4 weeks before screening. Antimalarial therapy was permitted, provided the dose had been stable for ≥4 weeks before the start of the trial.
Study design. Patients were randomized to receive 210 mg AMG 557 or equivalent placebo subcutaneously once weekly for 3 weeks, followed by 10 additional doses of AMG 557 or placebo every other week. The study initially required patients to be receiving an immunosuppressant agent at baseline, to be ≤55 years of age, and to have been immunized with H1N1 vaccine; the corticosteroid dosage permitted was ≤10 mg/day. Because of low enrollment, these criteria were later changed to remove the requirement for prestudy immunosuppressant treatment, increase the maximum age at enrollment to 65 years, require only relevant influenza vaccines during flu season, and increase the maximum corticosteroid dosage at entry to 20 mg/day. Withdrawal of immunosuppressants and corticosteroids was carried out according to a prespecified schedule (see Supplementary Figure 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40479/abstract).
Immunosuppressant therapy was discontinued by day 29 in patients treated with an immunosuppressant agent at baseline. Prednisone at a dosage of ≤20 mg/day was permitted at the start of the trial, and the dosage was not to exceed 20 mg/day through day 29, not to exceed 15 mg/day through day 57, and not to exceed 7.5 mg/day by day 85. Patients received AMG 557 or placebo until the end of the study unless it was discontinued by the investigator because of adverse events (AEs), clinical laboratory abnormalities, physical examination findings, the inability to withdraw immunosuppressants by day 29, or the need to resume immunosuppressant treatment after day 29.
Assessments. The SLEDAI and British Isles Lupus Assessment Group (BILAG) index 18 were assessed at screening and on a monthly basis thereafter. Tender and swollen joint counts (68 joints and 66 joints, respectively) were assessed at screening and on days 1, 8, 15, and 29 and then monthly thereafter. Treatment response based on the Lupus Arthritis Responder Index (LARI) was used as a primary end point and was defined as follows: ≥50% decrease in the combined tender and swollen joint counts on day 169 compared with baseline, ≥1‐letter improvement in the BILAG musculoskeletal system score on day 169 compared with baseline, and a reduction in and maintenance of the dosage of prednisone (or its equivalent) to ≤50% of baseline or ≤7.5 mg/day, whichever is lower, from day 85 to day 169 in patients not treated with immunosuppressants at baseline and ≤7.5 mg/day from day 85 to day 169 and discontinuation of immunosuppressants by day 29 in patients treated with immunosuppressants at baseline. AEs were monitored at each visit, graded according to the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0, and coded using Medical Dictionary for Regulatory Activities version 19.0 (or a later version).
Biomarker analysis. Serum and whole blood samples were collected and sent to central (ICON plc) locations in the US, Ireland, and Singapore, for autoantibody, complement, and lymphocyte cell population measurements. Anti–AMG 557 antibodies were measured using a bridging assay that used AMG 557 to capture serum anti‐drug antibodies. Any binding antibody–positive samples were subsequently tested for the presence of neutralizing antibodies 9. Lymphocyte populations were measured in whole blood, using a validated flow cytometry panel (see Supplementary Table 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40479/abstract). Flow Cytometry Standard file data were analyzed centrally using a prespecified analysis template. All analytes were measured as a percentage of parent and as cells/μl (if TruCount) or as relative absolute counts (using the TBNK TruCount values as a denominator).
Statistical analysis. The primary end points were as follows: 1) safety, tolerability, and immunogenicity to day 309, and 2) response according to the LARI on day 169. Key secondary end points included the proportion of patients achieving a response according to the BILAG index musculoskeletal system score (≥1‐letter improvement and a score of C or better) on day 169 and changes in tender and swollen joint counts on day 169. Exploratory end points included but were not limited to changes in the BILAG index score and the SLEDAI score by day 169 compared with placebo, proportion of patients achieving a ≥4‐point decrease in the SLEDAI relative to baseline, and changes in biomarker end points including complement, autoantibodies, and lymphocyte subsets.
Descriptive statistics were reported for each end point, with no formal hypothesis testing. The LARI and other categorical end points were summarized using proportions and the associated 90% confidence intervals by treatment group. An analysis of covariance model was used to compare AMG 557 with placebo for joint counts, BILAG index, and SLEDAI on day 169 after adjusting for the baseline difference. A baseline‐adjusted mixed‐effects repeated measures regression model, with time and treatment as fixed factors, baseline as a covariate, patient as a random factor, and a time‐by‐treatment interaction term was used to compare AMG 557 with placebo for blood analyte levels. Values below the assay lower limit of quantitation (LoQ) were set to one‐half the LoQ. P values were reported for descriptive purposes.
Results
Patients. Twenty SLE patients with active lupus arthritis enrolled and received AMG 557 or placebo. The baseline demographics and disease characteristics of the enrolled patients are shown in Table 1. Most patients (95%) were women, the median age was 43 years in the AMG 557 group and 46 years in the placebo group, and the median duration of SLE was 7.8 years and 3.0 years, respectively. The mean baseline SLEDAI score was 10.4 in the AMG 557 group and 7.5 in the placebo group. Baseline use of immunosuppressants and antimalarials was similar between the 2 groups. Four patients discontinued the investigational product: 2 because of AEs (both in the placebo group) and 2 because of increasing disease activity (1 each in the AMG 557 and placebo groups). Nineteen of 20 patients completed the study; 1 patient was lost to follow‐up.
Table 1.
Baseline patient demographics and disease characteristicsa
| Placebo (n = 10) | AMG 557 (n = 10) | |
|---|---|---|
| Women | 10 (100) | 9 (90) |
| Race | ||
| White | 6 (60) | 7 (70) |
| Asian | 3 (30) | 3 (30) |
| Black | 1 (10) | 0 |
| Age, median years | 46.0 | 43.0 |
| SLE duration, mean years | 3.0 | 7.8 |
| ANA positive | 10 (100) | 8 (80) |
| Anti‐dsDNA ≥30 IU/ml | 4 (40) | 6 (60) |
| BILAG global score, mean ± SD | 5.3 ± 2.4 | 6.6 ± 3.9 |
| SLEDAI score, mean ± SD | 7.5 ± 2.0 | 10.4 ± 3.9 |
| No. of tender joints (68 assessed), mean ± SD | 22.9 ± 17.3 | 19.2 ± 12.5 |
| No. of swollen joints (66 assessed), mean ± SD | 15.5 ± 18.7 | 12.6 ± 13.1 |
| Complement | ||
| C3 <0.9 gm/liter | 3 (30) | 6 (60) |
| C4 <100 mg/liter | 0 | 4 (40) |
| Treatment with prednisone >7.5 mg/day or equivalent | 2 (20) | 4 (40) |
| Immunosuppressant treatment | 8 (80) | 8 (80) |
| Methotrexate | 6 (60) | 7 (70) |
| Azathioprine | 2 (20) | 1 (10) |
| Etanerceptb | 0 | 1 (10) |
| Treatment with antimalarial agents | 6 (60) | 7 (70) |
Except where indicated otherwise, values are the number (%). ANA = antinuclear antibody; anti‐dsDNA = anti–double‐stranded DNA; BILAG = British Isles Lupus Assessment Group; SLEDAI = Systemic Lupus Erythematosus Disease Activity Index.
One patient received azathioprine and etanercept; etanercept was started after AMG 557 therapy was completed, because of a disease flare.
Safety. The most common events were headache and upper respiratory tract infection (see Supplementary Table 2, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40479/abstract). The majority of AEs were CTCAE grade 1 or grade 2; 7 grade 3 events occurred in 4 patients. In the AMG 557 group, 3 grade 3 events occurred in 3 patients: depression, upper respiratory tract infection (neither considered treatment related), and myalgia (considered treatment related). In the placebo group, 1 patient experienced grade 3 headache, pyrexia, inflammation, and rash (not considered treatment related). No grade 4 events or deaths occurred during the study. Serious AEs were reported in 1 patient in the AMG 557 group (depression) and in 3 patients in the placebo group (lymphadenopathy, deafness, and pulmonary hypertension); none of these AEs was considered treatment related. No anti–AMG 557 antibodies were detected at screening or at any time during the study.
Efficacy. The primary efficacy end point was the LARI, a composite measure incorporating improvements in the joint count and the musculoskeletal system component of the BILAG, discontinuation of immunosuppressants, when applicable, and the successful reduction of prednisone according to protocol. The numbers of LARI responders in both groups were relatively small. Three of 10 patients in the AMG 557 group met the criteria for a LARI response compared with 1 of 10 patients in the placebo group (P = 0.58) (Table 2).
Table 2.
Efficacy and change from baseline in the SLEDAI, BILAG, and tender/swollen joint scores on day 169a
| Variable | Treatment | |
|---|---|---|
| Placebo | AMG 557 | |
| Efficacy end points | ||
| LARI responders | 1 (10) [2.3–34.8] | 3 (30) [12.7–55.8] |
| Patients achieving a ≥4‐point decrease in the SLEDAI | 2 (20) [7.6–49.6] | 7 (70) [44.2–87.3] |
| Index scores | ||
| SLEDAI | −1.0 (−10.7) | −5.0 (−47.8) |
| BILAG global | −1.8 (−24.7) | −2.7 (−36.3) |
| No. of tender joints (28 assessed) | 1.2 (−13.5) | −9.4 (−22.8) |
| No. of swollen joints (28 assessed) | 2.0 (−7.8) | −8.9 (−62.1) |
Values for efficacy end points are the number (%) [90% confidence interval]. Values for index scores are the mean (% change from baseline). The mean change from baseline was calculated as the mean of individual changes from baseline. SLEDAI = Systemic Lupus Erythematosus Disease Activity Index; BILAG = British Isles Lupus Assessment Group; LARI = Lupus Arthritis Response Index.
Analysis of the impact of AMG 557 administration on the SLEDAI and BILAG index scores showed evidence of improvement. A greater number of patients in the AMG 557 group achieved a ≥4‐point improvement in SLEDAI on day 169 (7 of 10 patients) compared with the placebo group (2 of 10 patients) (P = 0.07) (Table 2). The mean percent changes (improvement) in SLEDAI scores from baseline were –47.8% in patients in the AMG 557 group and –10.7% in the placebo group (P = 0.14). Global BILAG index scores improved by –36.3% and –24.7%, respectively, from baseline (P = 0.89) (Table 2). There was no discernible difference associated with AMG 557 treatment compared with placebo in the BILAG index musculoskeletal system score.
Evidence of improvement was observed with AMG 557 administration as reflected by changes in the tender and swollen joint counts from baseline (Table 2). The mean percent change in the tender joint count on day 169 was –22.8% in the AMG 557 group and –13.5% in the placebo group (P = 0.11), and the mean percent changes in the swollen joint count were –62.1% and –7.8%, respectively (P = 0.03).
Biomarkers. Although the current study was limited by a small sample size and high variability, there appeared to be differences between the treatment and placebo groups in the lupus‐related biomarker end points C3, C4, total hemolytic complement, and anti‐dsDNA antibodies (Figure 1), which is consistent with a beneficial treatment effect. Other autoantibodies were also measured at baseline and assessed over the course of treatment with AMG 557 or placebo (see Supplementary Figure 2, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40479/abstract). No differences in the relative percentages of CD4+ T cells, Tfh cells, B cells, and plasma cells/plasmablasts were detected between day 29 and the end of the study.
Figure 1.
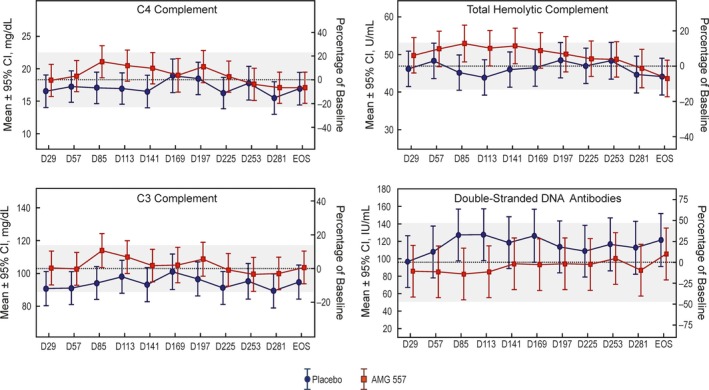
Biomarker analyses showing postdose changes in complement and double‐stranded DNA. The dotted lines show the estimated pretreatment (baseline) mean analyte level in the study population (n = 20). The shaded bands represent the 95% confidence intervals (95% CIs) for the baseline means. The x‐axis indicates the nominal time points in days. The y1‐axis (left) is the analyte level, and the y2‐axis (right) is the estimated study population mean analyte level as a percentage of baseline. All available clinical study samples were analyzed, typically resulting in 10 observations per time point per dose. For some time points, there are as few as 8 observations.
Discussion
The interaction between ICOS and ICOSL is important in T cell function, including cytokine production and differentiation to Tfh cells 6. Tfh cells play an integral role in selecting B cells for clonal expansion; hyperactivity of the ICOS pathway may lead to an imbalance in antibody‐producing B cells 5. Dysregulation of T cells and B cells contributes to autoimmunity in SLE patients 3. The observed trends toward improvement in the levels of complement and anti‐dsDNA in our study, and prior results demonstrating inhibition of antibody responses 9, support the hypothesis that AMG 557 blocks ICOSL and its biologic effects in an autoimmune setting.
Many biologics that target pathways supported by strong scientific rationale have failed to prove efficacy in SLE 10; trial design is suspected to play a role in these disappointing results. Because of the heterogeneous disease manifestations of lupus, multiple aggressive background medications have been allowed in trials that are suitable for patients with the most serious disease, which may yield high placebo response rates. This in turn results in the requirement for a large trial population to identify a significant treatment effect.
Here, two approaches were used to examine proof of concept in a small trial. Tender and swollen joint counts have been used successfully as an exploratory, organ‐specific end point in smaller trials of SLE 19. In addition, withdrawal of background therapy has been performed safely in SLE populations with non–organ‐threatening disease. This approach in a stable population has a number of advantages, as follows: supporting less‐noisy immunologic profiling, exploring the pharmacodynamic effects of new agents, and lessening the high rates of placebo response to allow a small trial 14.
This study was designed as a pilot project to identify a clinical signal. The limited size of the patient population contributed to imbalances in demographics; therefore, limited conclusions can be made regarding the efficacy of AMG 557 in patients with SLE. However, changes in the LARI and SLEDAI, tender and swollen joint counts, and the proportion of responders who exhibited a 4‐point drop in the SLEDAI score suggest that AMG 557 may be efficacious. Double‐stranded DNA and complement measures also showed trends consistent with clinical benefit and the mechanism of action of AMG 557. AMG 557 was well tolerated, and no anti‐drug antibodies were observed.
Previous phase I trials showed that AMG 557 inhibited the IgG anti–keyhole limpet hemocyanin response and had an acceptable safety profile in patients with mild, stable SLE 9. Our study extends these findings by examining patients with more significant disease over a longer period of time, providing additional data to suggest a promising safety profile as well as indicators of biologic activity and potential efficacy.
In conclusion, the safety, efficacy, and pharmacodynamic biomarker results from this small study support further evaluation of ICOSL blockade with AMG 557 as a potential therapeutic option for patients with SLE.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Chung had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Sullivan, Tsuji, Merrill, Chung.
Acquisition of data
Cheng, Amoura, Cheah, Hiepe, Sullivan, Tsuji, Merrill, Chung.
Analysis and interpretation of data
Cheng, Sullivan, Zhou, Arnold, Merrill, Chung.
Role of The Study Sponsor
Amgen and AstraZeneca‐MedImmune funded the study and were involved in the design, data collection, data analysis, and writing of the manuscript. They reviewed and approved the manuscript prior to submission. The authors independently interpreted the results and had the final approval and decision to submit the manuscript for publication. Amgen provided writing assistance (performed by Dikran Toroser, Amgen Inc., and by Miranda Tradewell, and Rick Davis, Complete Healthcare Communications, a CHC Group company, Chadds Ford, PA). Publication of this article was not contingent upon approval by the sponsors.
Supporting information
Acknowledgment
We would like to thank Deepali Mitragotri, PhD, for biostatistics contributions to the study.
ClinicalTrials.gov identifier: NCT01683695.
Supported by Amgen and AstraZeneca‐MedImmune.
Dr. Amoura has received consulting fees from GlaxoSmithKline, Roche, Amgen, and AstraZeneca (less than $10,000 each) and research grants from GlaxoSmithKline, Bristol‐Myers Squibb, Amgen, and AstraZeneca. Dr. Tsuji has received consulting fees from Celimmune, Seattle Genetics, and Resolve Therapeutics (less than $10,000 each), owns stock or stock options in Amgen and Johnson & Johnson, and is an owner/partner of the Cascadia Drug Development Group. Dr. Merrill has received consulting fees from UCB Pharma, GlaxoSmithKline, Questcore, EMD Serono, Pfizer, Celgene, Exagen, Bristol‐Myers Squibb, MedImmune, AstraZeneca, Eli Lilly and Company, Takeda, Xencor, Biogen, Neovacs, Anthera, Boehringer Ingelheim, Boston Pharmaceuticals, Baxalta, Incyte, ReAlta, Amgen, Eisai, KPI Therapeutics, and ImmuPharma (less than $10,000 each) and research grants from GlaxoSmithKline and Bristol‐Myers Squibb. Drs. Cheng, Sullivan, Zhou, Arnold, and Chung are employees of and own stock or stock options in Amgen.
References
- 1. Lisnevskaia L, Murphy G, Isenberg D. Systemic lupus erythematosus. Lancet 2014;384:1878–88. [DOI] [PubMed] [Google Scholar]
- 2. Grossman JM. Lupus arthritis. Best Pract Res Clin Rheumatol 2009;23:495–506. [DOI] [PubMed] [Google Scholar]
- 3. Kaul A, Gordon C, Crow MK, Touma Z, Urowitz MB, van Vollenhoven R, et al. Systemic lupus erythematosus. Nat Rev Dis Primers 2016;2:16039. [DOI] [PubMed] [Google Scholar]
- 4. Craft JE. Follicular helper T cells in immunity and systemic autoimmunity. Nat Rev Rheumatol 2012;8:337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vinuesa CG, Linterman MA, Yu D, MacLennan IC. Follicular helper T cells. Annu Rev Immunol 2016;34:335–68. [DOI] [PubMed] [Google Scholar]
- 6. Dong C, Nurieva RI. Regulation of immune and autoimmune responses by ICOS. J Autoimmun 2003;21:255–60. [DOI] [PubMed] [Google Scholar]
- 7. Robertson N, Engelhardt KR, Morgan NV, Barge D, Cant AJ, Hughes SM, et al. Astute clinician report: a novel 10 bp frameshift deletion in exon 2 of ICOS causes a combined immunodeficiency associated with an enteritis and hepatitis. J Clin Immunol 2015;35:598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grimbacher B, Hutloff A, Schlesier M, Glocker E, Warnatz K, Drager R, et al. Homozygous loss of ICOS is associated with adult‐onset common variable immunodeficiency. Nat Immunol 2003;4:261–8. [DOI] [PubMed] [Google Scholar]
- 9. Sullivan BA, Tsuji W, Kivitz A, Peng J, Arnold GE, Boedigheimer MJ, et al. Inducible T cell co‐stimulator ligand (ICOSL) blockade leads to selective inhibition of anti‐KLH IgG responses in subjects with systemic lupus erythematosus. Lupus Sci Med 2016;3:e000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gatto M, Saccon F, Zen M, Bettio S, Iaccarino L, Punzi L, et al. Success and failure of biological treatment in systemic lupus erythematosus: a critical analysis. J Autoimmun 2016;74:94–105. [DOI] [PubMed] [Google Scholar]
- 11. Furie RA, Petri MA, Wallace DJ, Ginzler EM, Merrill JT, Stohl W, et al. Novel evidence‐based systemic lupus erythematosus responder index. Arthritis Rheum 2009;61:1143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzová D, et al. A phase III, randomized, placebo‐controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum 2011;63:3918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Navarra SV, Guzman RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo‐controlled, phase 3 trial. Lancet 2011;377:721–31. [DOI] [PubMed] [Google Scholar]
- 14. Merrill JT, Immermann F, Whitley M, Zhou T, Hill A, O'Toole M, et al. The Biomarkers of Lupus Disease study: a bold approach may mitigate interference of background immunosuppressants in clinical trials. Arthritis Rheumatol 2017;69:1257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–7. [DOI] [PubMed] [Google Scholar]
- 16. Hochberg MC, for the Diagnostic and Therapeutic Criteria Committee of the American College of Rheumatology . Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus [letter]. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 17. Bombardier C, Gladman DD, Urowitz MB, Caron D. Chang DH, and the Committee on Prognosis Studies in SLE. Derivation of the SLEDAI: a disease activity index for lupus patients. Arthritis Rheum 1992;35:630–40. [DOI] [PubMed] [Google Scholar]
- 18. Yee CS, Farewell V, Isenberg DA, Prabu A, Sokoll K, Teh LS, et al. Revised British Isles Lupus Assessment Group 2004 index: a reliable tool for assessment of systemic lupus erythematosus activity. Arthritis Rheum 2006;54:3300–5. [DOI] [PubMed] [Google Scholar]
- 19. Illei GG, Shirota Y, Yarboro CH, Daruwalla J, Tackey E, Takada K, et al. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open‐label phase I dosage‐escalation study. Arthritis Rheum 2010;62:542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


