Abstract
Background & Aims
Cholestatic liver injury is mediated by bile acid induced inflammatory responses. We hypothesized that superior therapeutic effects might be achieved by combining treatments that reduce the bile acid pool size with one that blocks inflammation.
Methods
Bile duct ligated (BDL) rats and Mdr2(Abcb4)−/− mice were treated with all-trans retinoic acid (atRA), a potent inhibitor of bile acid synthesis, 5mg/kg/day by gavage, or Cenicriviroc (CVC), a known antagonist of CCR2 and CCR5, 50mg/kg/day alone or in combination for 14-day and one month, respectively.
Results
atRA alone reduced bile acid pool size and liver necrosis in BDL rats. However, the combination with CVC further reduced liver to body weight ratio, bile acid pool size, plasma liver enzyme, bilirubin, liver necrosis and fibrosis, when compared to the atRA treatment. Assessment of hepatic hydroxyproline content further confirmed reduced liver injury while analysis of gene expression of proinflammatory cytokines was also reduced, emphasizing the synergistic effects of these two agents. Profiling of hepatic inflammatory cells revealed that the combination therapy reduced neutrophils and T cells but not macrophages. The superior therapeutic effects of the combination treatment were also confirmed in Mdr2−/− mice where significant reduction of plasma liver enzymes, bilirubin, liver fibrosis, bile duct proliferation and hepatic infiltration of neutrophils and T cells and expression of cytokines were found.
Conclusions
Multi-targeted therapy is an important paradigm for treating cholestatic liver injury. The combination of CVC with atRA or other FXR activators may warrant a clinical trial in patients with cholestatic liver disease.
Keywords: bile acid, cholestasis, inflammation, combination therapy, fibrosis
Introduction
Cholestasis is a disorder of impaired bile formation where bile acids accumulate in the liver and systemic circulation. It can be caused by genetic and developmental defects, as well as acquired diseases including the side effects of drugs, viral hepatitis, alcoholic liver disease, primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC), pregnancy, metabolic syndromes, and obstruction of bile ducts due to gallstones or tumors.1–3 Chronic cholestasis leads to hepatocyte death, liver fibrosis and cirrhosis, and eventually liver failure. Currently there is little effective therapy for cholestatic liver disorders, although ursodeoxycholic acid (UDCA) is the standard of care for patients with PBC.4 However not all patients respond to UDCA so that alternative therapies represent a major unmet medical need.5,6 Recently, Obeticholic acid has been approved by the FDA for treating PBC patients with inadequate responses or intolerance to UDCA, but pruritus and hyperlipidemia are significant side effects.7 Therefore, there is a need for developing additional therapeutic approaches for PBC and other cholestatic disorders.5,6,8
Recent studies into the pathophysiologic mechanisms of cholestasis have indicated that bile acids cause liver injury by stimulating a cytokine mediated inflammatory response.9–11 In this process, hepatocytes with elevated levels of bile acids initiate the event by releasing proinflammatory cytokines, that attract neutrophils and T cells which then lead to the tissue injury.10,12,13 Prior studies demonstrate that when either the bile acid pool size or the inflammatory response is reduced, cholestatic liver injury is diminished.14–17 Based on these observations, we hypothesized that therapeutic strategies that simultaneously reduce the bile acid pool size as well as mitigate the inflammatory response, might have superior beneficial effects.
Previously, we reported that all-trans retinoic acid (atRA) is a potent activator of the bile acid nuclear receptor FXR/NR1H4 which in turn represses the expression of cholesterol 7-alpha-hydroxylase (CYP7A1), the rate-limiting enzyme in bile acid biosynthesis.18 When used alone or in combination with UDCA, atRA significantly reduced the bile acid pool size and improved liver conditions in cholestatic rodent models.19,20 A limited clinical trial of atRA in patients with PSC supports this mechanism in humans.21
Cenicriviroc (CVC) is a novel antagonist for both C-C chemokine receptor 2 and 5 (CCR2 & CCR5) that are expressed in many inflammatory cells, including neutrophils, T cells and monocytes.22,23 It has demonstrated potent anti-inflammatory effects by blocking chemokine activated immune responses and has been shown to reduce liver fibrosis in animal models of nonalcoholic steatohepatitis (NASH), and alcoholic liver injury.24 Most importantly, it has recently demonstrated favorable therapeutic effects in patients with NASH in a clinical trial.25 However, the effect of CVC in cholestatic liver injury remains unknown.
Based on these previous observations, we speculated that CVC alone or in combination with atRA might have superior beneficial effects for cholestatic liver diseases. Here we test this hypothesis in two cholestatic rodent models, the bile duct ligated (BDL) rat and the Mdr2(Abcb4)−/− mouse, a model of Sclerosing Cholangitis.26 We found that combination therapy with atRA and CVC achieved superior protective effects than seen with either compound alone. These findings indicate that combining a drug that blocks the inflammatory response together with an agent that reduces the bile acid pool size is a novel pharmacological strategy for treating cholestatic liver diseases. Our results also suggest that the combination of CVC and atRA or other bile acid pool size lowering drugs may warrant clinical trials in patients with cholestatic disorders.
Materials and Methods
Materials
CVC was provided by Allergan plc (South San Francisco, CA). Other chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise specified.
Animal experiments
All animal experiment protocols were approved by the board of the Institutional Animal Care and Use Committee of Yale University (New Haven, CT). Male Sprague-Dawley rats (180-220g) were obtained from Charles River (Wilmington, MA). For experiments with BDL, rats were divided into five groups (n=6-9). Twenty-four hours after the surgery, BDL animals were randomly assigned to receive water (the treatment control and solvent), 50 mg/kg CVC, 5 mg/kg atRA, or 50mg/kg CVC supplemented with 5mg/kg atRA by daily gavage for 14 days. BDL rats also received 0.5 mg of vitamin K every other day through subcutaneous injection. The body weight was measured daily. For experiments with Mdr2−/− mice, 6-week-old males were randomly divided into four groups (n=10-11) and received water, 50 mg/kg CVC, 5 mg/kg atRA, or 50mg/kg CVC plus 5mg/kg atRA by gavage for 4 weeks. Body weight was measured every three days. The animals were sacrificed in random order after an overnight fast. Samples of plasma, bile, urine (from the bladder), liver and kidney were collected for further analyses.
Plasma biochemistry, liver histology and immunohistochemistry (IHC)
Plasma levels of liver enzymes, bile acids, cholesterol, triglycerides, and bilirubin were assayed as described.19 The bile salt pool size was calculated as described.19 Formalin-fixed liver tissue was paraffin embedded and sections were stained with hematoxylin and eosin (H&E) or Sirius Red. Liver histology was blindly assessed for inflammation, necrosis, bile duct proliferation, and fibrosis on a 1 to 4+ scale. For liver IHC, sections were labeled with antibodies against myeloperoxidase, F4/80 or CD3. The details are described in the supplementary materials and Table S1.
mRNA and protein expression
Tissue RNA and protein were extracted and assayed as described.10 The sequences of primer and probe for each gene are listed in Supplementary Table S2. Antibody against α-Sma for Western blotting was from Sigma-Aldrich.
Statistical Analysis
One-way ANOVA followed by student t test were used to perform the statistical analysis. Data are presented as the means ± S.D. P<0.05 was considered statistically significant.
Results
CVC supplemented with atRA improved systemic signs of cholestatic liver injury in BDL rats
The rat liver to body weight ratios were significantly increased 14-day after BDL when compared to the sham group. However, this ratio was significantly lower in the group receiving both CVC and atRA when compared to the groups receiving CVC or atRA alone or the untreated control group (Figure.1A). In contrast, the growth rates and kidney to body weight ratios were not significantly different between any of the BDL groups (Supplementary Table S3 and Figure S1A). The lower liver to body weight ratio seen with combination therapy suggested that the liver injury induced by BDL might be less. Indeed, the surface of these livers was smooth and shiny at sacrifice compared to the other BDL animals whose liver surface had an irregular nodular appearance (Supplementary Figure S2A). The volume of bile collected from the bile cyst was also less in the group with the combination treatment (Supplementary Table S3). In addition, the plasma levels of liver enzyme GGT and bilirubin were significantly lower with combination therapy although plasma ALT and ALP levels were not significantly different than the other BDL groups (Figure 1 and Supplementary Table S3). Interestingly, bile acid concentrations in the plasma as well as in the urine and bile were also significantly lower with the combination therapy when compared to the other BDL groups, as were plasma cholesterol and triglycerides levels (Figure 1 and Supplementary Table S3). These findings further suggest that cholestatic liver injury was significantly improved in these animals compared to those treated with CVC or atRA alone or the untreated BDL control animals.
Figure 1.
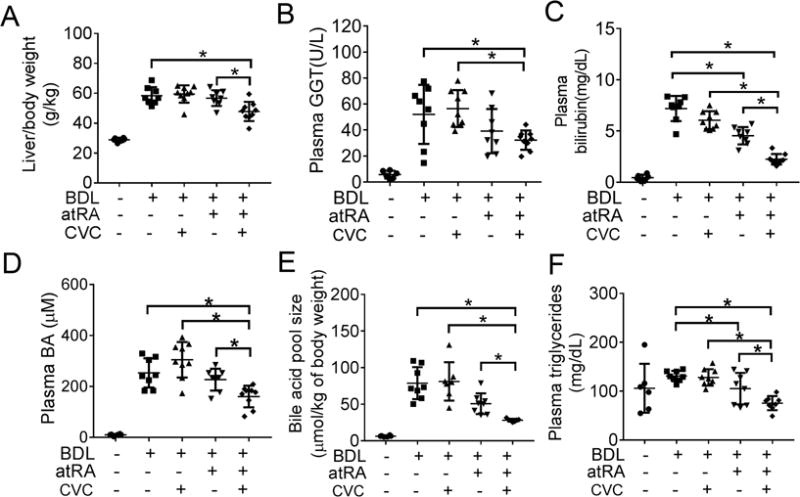
CVC in combination with atRA improved systemic signs of cholestasis in BDL rats. (A) liver/body weight ratio; (B) plasma GGT level; (C) plasma bilirubin level; (D) plasma bile acid level; (E) bile acid pool size; (F) plasma triglycerides level. * p<0.05, n=6-9.
CVC supplemented with atRA markedly improved liver histology and biochemistry in BDL rats
Blinded assessment of liver histology revealed that necrosis, fibrosis and or bile duct proliferation were significantly less in the group treated with the combination of CVC and atRA as compared to other BDL groups (Figure 2 and supplementary Figure S2B). CVC treatment alone did not significantly improve liver histology, however atRA treatment reduced necrosis when compared to the BDL controls as we previously described.19 Analysis of liver hydroxyproline content also confirmed that fibrosis was significantly reduced with atRA in combination with CVC (Figure 2B). Gene mRNA expression analysis detected lower levels of collagen 1a1(Col1a1), Tgf-β1 and cytokeratin19 (Ck19) in the livers of the group treated with atRA and CVC (Figure 3A). Western blot analysis also revealed significantly less α-Sma protein expression in the livers of the combination treatment group compared to the other BDL groups (Figure 3B). Elevated levels of bile acid were found in the livers of the BDL control group as expected (Figure 1 and Supplementary Table S3). However, combination therapy reduced liver bile acid concentrations to levels similar to values seen in the healthy sham group. Significantly lower hepatic levels of bile acids were also detected in the group treated with CVC. Further analysis indicates that both the bile acid pool size and total hepatic bile acids were lower in the combination treatment group than in the other three BDL groups. AtRA treatment also had similar although lesser effects (Figure 1E). Together, these findings confirmed that treatment of BDL rats with the combination of CVC and atRA markedly alleviated manifestations of cholestasis and reduced liver injury, effects that were superior to treatment with either atRA or CVC alone.
Figure 2.
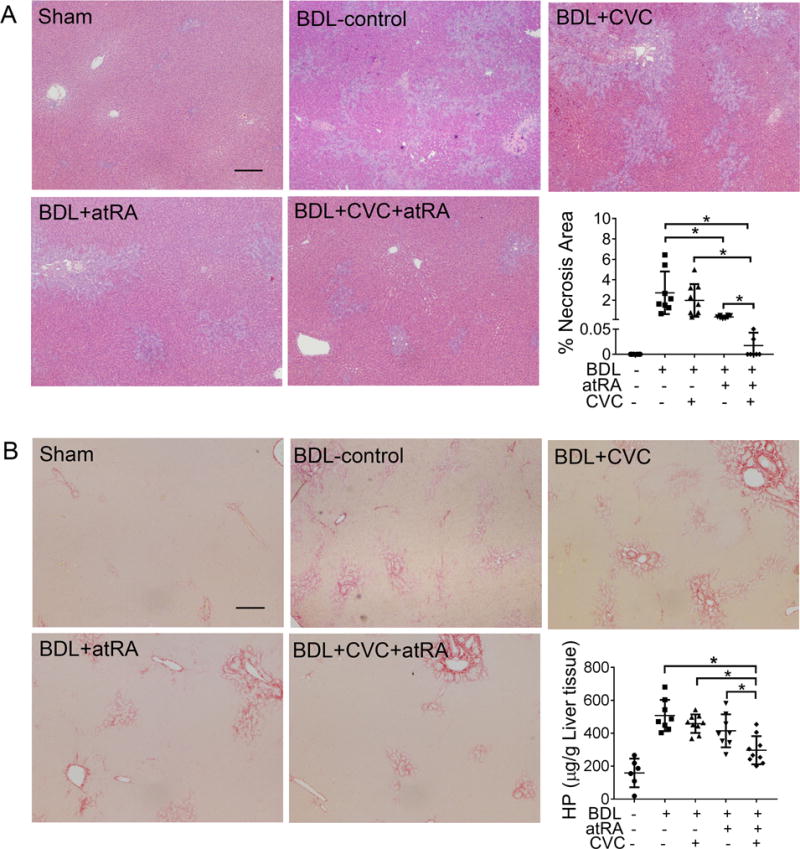
CVC in combination with atRA significantly reduced liver injury in BDL rats. (A) representative photomicrographs of hematoxylin and eosin-stained liver histology and quantitative measurement of necrotic area in all livers; (B) representative images of liver section stained with Sirius Red and detection of liver hydroxyproline content. Scale bar = 100 μm. * p<0.05, n=6-9.
Figure 3.
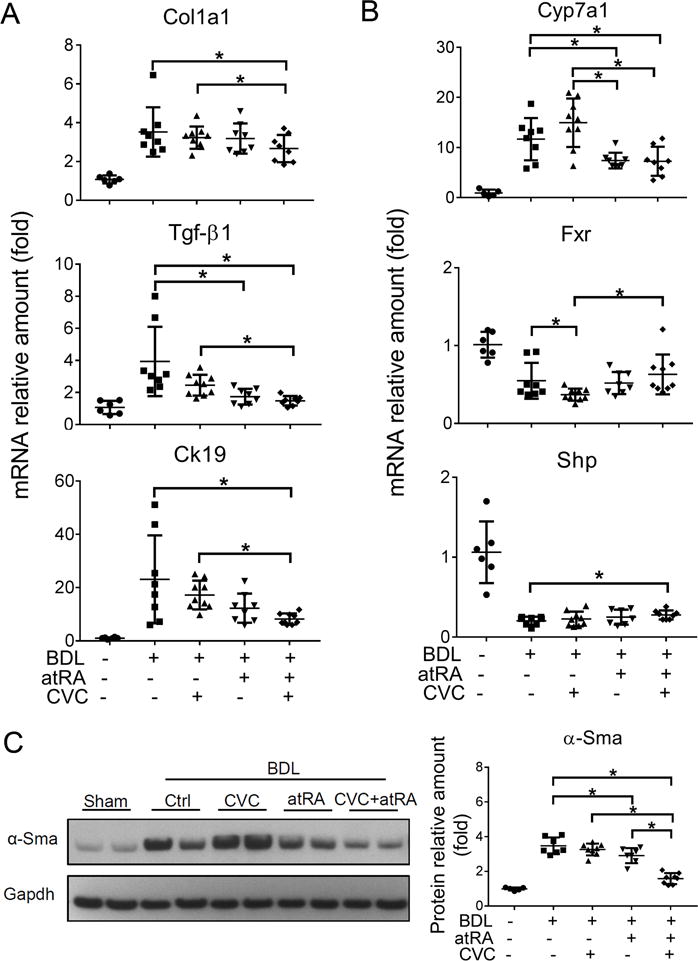
The combination of CVC and atRA significantly reduced the expression of marker genes for liver fibrosis, bile duct proliferation and bile acid synthesis in BDL rats. (A) liver mRNA expression of Col1a1, Tgf-β1 and Ck19; (B) liver mRNA expression of Cyp7a1, Fxr and Shp; and (C) liver α-Sma protein expression detected by Western blot. Data were normalized to the house keeper gene Gapdh and quantified using Image J software. * p<0.05, n=6-9.
atRA with or without CVC altered gene expression in the liver of BDL rats
To gain mechanistic insight into how these agents improved liver structure and function in 14-day BDL rats, we analyzed hepatic expression of genes involved in bile acid metabolism and cytokine mediated inflammation. Interestingly there was no effect of CVC, atRA or their combination on the expression (mRNA) of various bile acid transporters among the different BDL groups, including Ntcp/Slc10a1, Bsep/Abcb11, Ostα/Slc51a and Mrp2/Abcc2 (Supplementary Figure S3). However, atRA alone or when supplemented with CVC significantly repressed hepatic Cyp7a1 mRNA expression when compared to BDL control and CVC treatment groups (Figure 3C). In contrast, CVC alone lowered the expression of Fxr in the treated BDL livers while higher levels of hepatic small heterodimer partner (Shp/Nr0b2) was found in the combination treatment group. Importantly, significant reductions were seen in the inflammatory cytokine IL-1β mRNA expression in atRA treatment alone or in combination with CVC (Figure 4A). However, Tnf-α expression did not differ among the BDL groups (Figure 4A). Interestingly, significant reductions in mRNA expression of the pro-inflammatory genes IL-6 and Icam-1 were detected with either CVC and atRA alone or in combination, whereas the anti-inflammatory gene IL-10 expression did not differ among the treated BDL groups (Figure 4A and Supplementary Figure S3).
Figure 4.
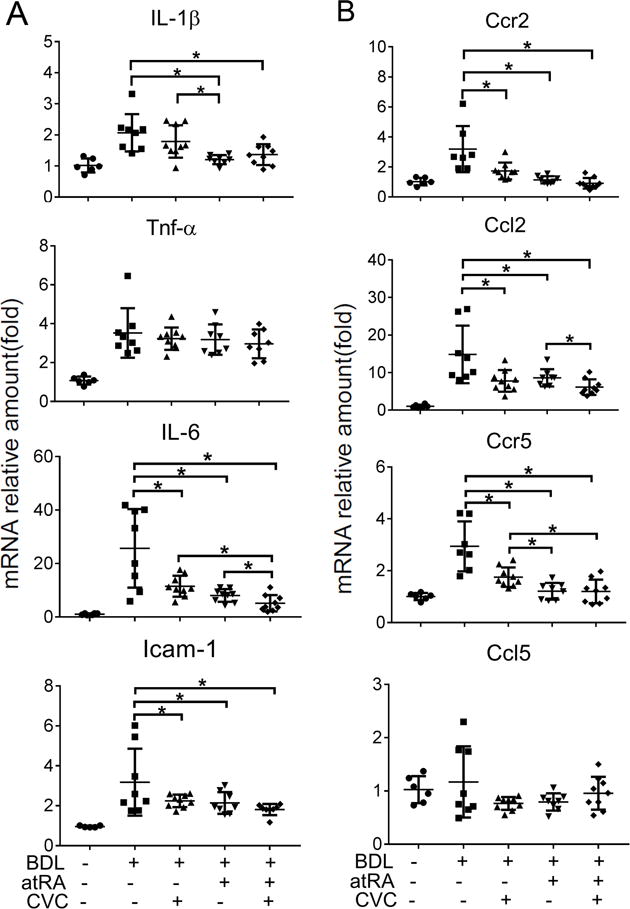
The treatment of CVC or atRA alone or in combination reduced hepatic mRNA expression of genes involved in the inflammatory response in BDL rats, including (A) Tnf-α, IL-1β, IL-6, and Intercellular Adhesion Molecule 1(Icam-1); (B) Ccr2 Ccl2, Ccr5 and Ccl5. * p<0.05, n=6-9.
Because CVC is a dual CCR2 and CCR5 antagonist, we also determined the effects of these treatments on mRNA expression of Ccr2 and Ccr5 and their ligands Ccl2 and Ccl5. As shown in Figure 4B, the expression of Ccr2, Ccl2 and Ccr5 were significantly reduced by CVC or atRA alone or their combination when compared to the untreated BDL controls, whereas there were no significant changes in Ccl5 mRNA expression among all five groups (Figure 4B). Collectively, these data demonstrated that treatments with CVC or atRA alone or in combination reduced the expression of many genes involved in bile acid synthesis and the cytokine mediated inflammatory response, consistent with the reduced liver injury phenotype seen in these animals.
Inflammatory cells (neutrophils and T cells) were reduced in the liver of BDL rats treated with CVC and or atRA
To investigate changes in the cellular immune response induced by CVC and or atRA treatment in the livers of these BDL rats, we first detected the expression of the leukocyte marker, CD11b.27 As shown in Figure 5A, CD11b mRNA expression was significantly reduced by treatment with CVC and atRA alone or in combination, suggesting that fewer neutrophils and or macrophages were present in these livers. Subsequent IHC labeling with a neutrophil specific marker, MPO,28,29 revealed that there were markedly more neutrophils infiltrating the necrotic areas in the BDL control livers. Quantitative assessment indicated that there were significantly fewer neutrophils in the livers treated with atRA alone or in combination with CVC (Figure 5D). This observation was further substantiated by reductions in another neutrophil marker, Elane (an elastase) (Figure 5C).28 In contrast, IHC labeling of F4/80 showed no significant difference among the BDL groups, indicating that there were no changes in the amount of macrophages (Figure 5B). In addition, IHC labeling of CD3 further indicated that T cells were also mostly increased in the necrotic areas in the liver of BDL rats when compared to sham livers. Treatment with CVC or atRA alone or in combination again significantly reduced the population of T cells in these BDL groups (Figure 5E). Together, these findings indicated that treatments with CVC or atRA alone or in combination significantly reduced the recruitment of neutrophils and T cells in the liver of these BDL rats. These findings suggest that each of these drugs were mitigating the infiltration of the very same inflammatory cells thought to be producing bile acid induced liver injury.10,12,13
Figure 5.
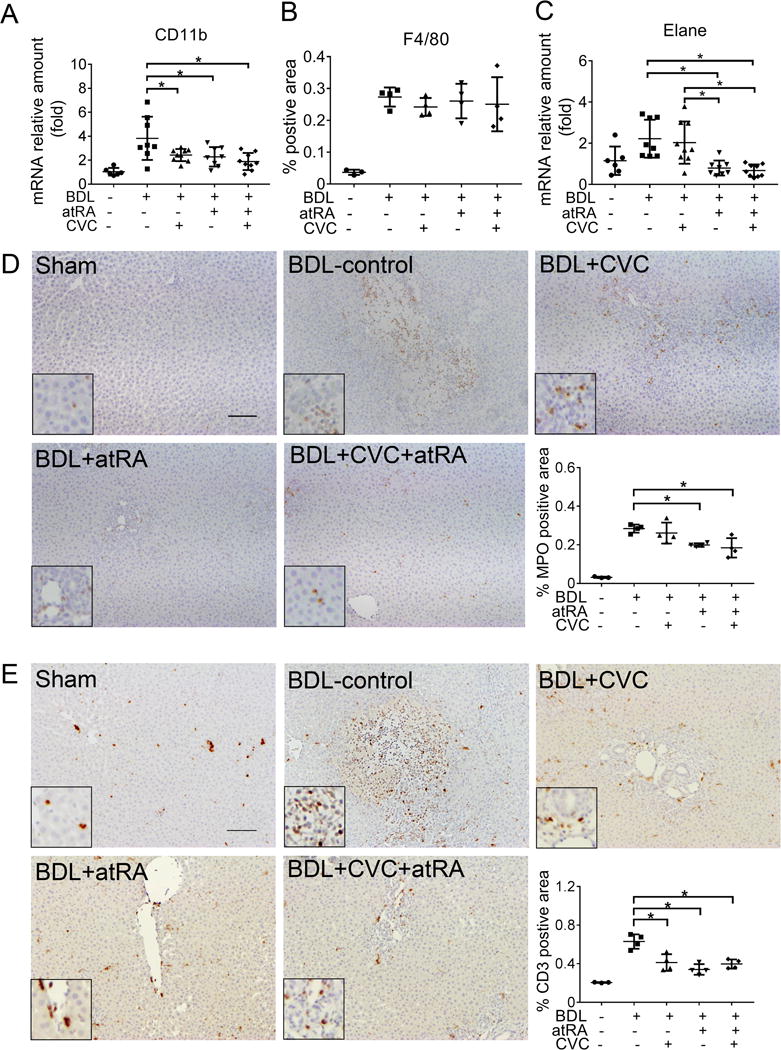
The treatment of CVC or atRA alone or in combination significantly reduced neutrophil and T cell infiltration in the liver of BDL rats. (A) Liver mRNA expression of CD11b; (B) Quantitative analysis of immunohistochemical detection of liver macrophages using an antibody against F4/80; (C) Liver mRNA expression of Elane, a neutrophil specific elastase; (D) Representative image of immunohistochemical labeling of liver neutrophils using an antibody against myeloperoxidase (MPO) (brown, with a zoom-in area in the insert) and quantitative analysis of neutrophil positive areas in the liver section. (E) Representative image of immunohistochemical labeling of T cells using an anti-CD3 antibody (brown, with a zoom-in area in the insert). Of note, 3-4 representative animals from each group were assayed. For quantification, 10 pictures were randomly taken from each liver section for obtaining the raw data and the average of these 10 data points was presented here. The area of positive cells was measured using Image J software (version 1.46r, NIH). Scale bar = 100 μm. *p<0.05, n=3-4.
Combination treatment of CVC with atRA also reduced cholestatic liver injury in Mdr2−/− mice
To further confirm the therapeutic potential of CVC and or atRA in cholestatic liver injury, we conducted similar studies in Mdr2−/− mice, a rodent model for Progressive Familial Intrahepatic Cholestasis 3 (PFIC3) and PSC.26,30 After four weeks treatment of 6-week-old males, blinded assessment of liver histology (at 10 weeks) revealed that there was significantly less bile duct proliferation and inflammation in the mice treated with the combination of CVC and atRA than seen in the untreated control group (Supplementary Figure S4). Histologic evidence of liver fibrosis also trended to be lower in the combination treatment group, although these findings were not statistically significant. No hepatic necrosis was seen in the liver of these animals. Plasma biochemistry analyses demonstrated that the levels of liver enzymes ALT, ALP and bilirubin were all significantly lower in Mdr2−/− mice when treated with the combination of CVC and atRA (Figure 6A-C). Lower levels of bile acid and hydroxyproline were also found in the liver tissue of the combination treatment group, where the protein expression of α-Sma and mRNA expression of Col1a1 and Ck19 were found to be less as well (Figure 6 and Figure 7A&B). These results confirmed that the treatment of CVC in combination with atRA also reduced cholestatic liver injury in Mdr2−/− mice that was superior to either drug alone.
Figure 6.
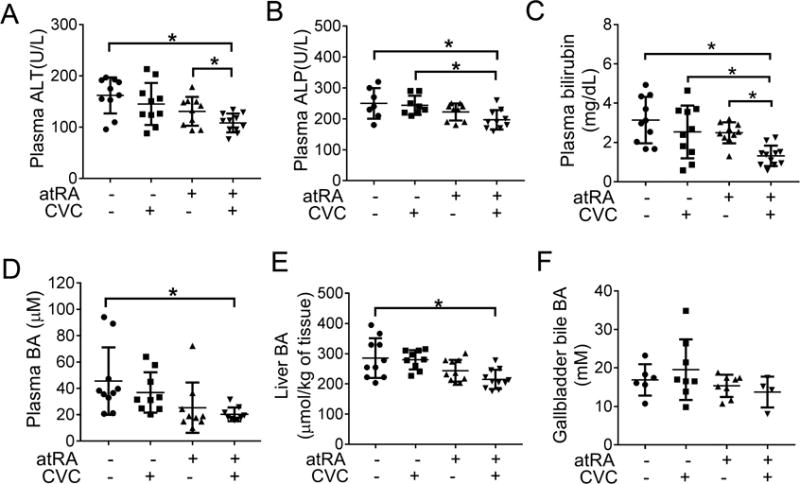
Combination treatment of atRA and CVC improved liver test levels in Mdr2−/− mice. (A) plasma ALT; (B) plasma ALP; (C) plasma bilirubin; (D) plasma bile acids; (E) liver bile acid; and (F) gallbladder bile acid. * p<0.05, n=9-11.
Figure 7.
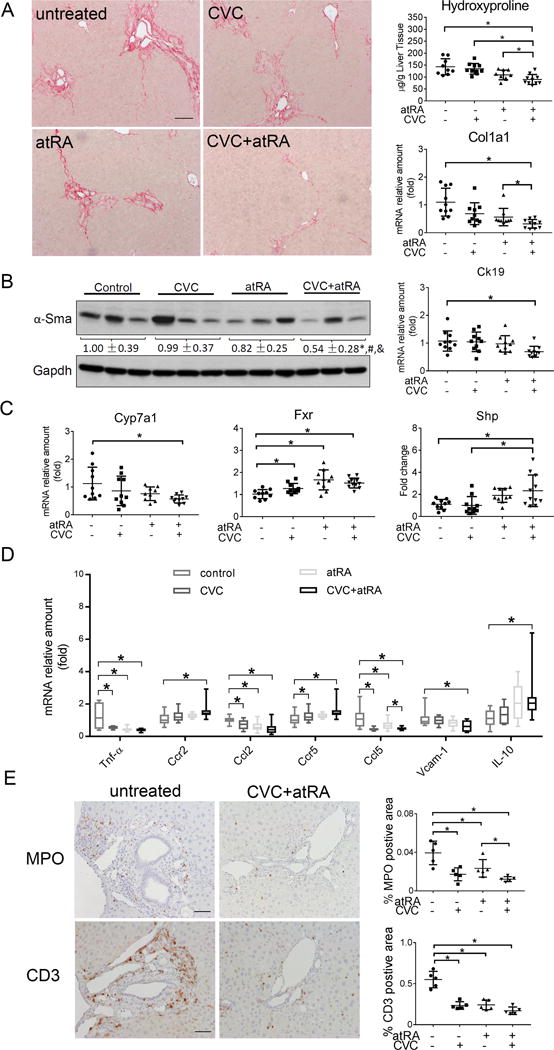
The combination treatment of atRA and CVC reduced liver injury in cholestatic Mdr2−/− mice. (A) liver Sirius Red staining, hydroxyproline content and mRNA expression of Col1a1, Scale bar = 100 μm; (B) liver protein expression of α-Sma and mRNA expression of Ck19; (C) liver mRNA expression of Cyp7a1, Fxr and Shp; (D) liver mRNA expression of inflammation related genes, i.e. Tnf-α, Ccr2, Ccl2, Ccr5, Ccl5, Vcam-1 and IL-10; and (E) immunohistochemistry for the neutrophil marker myeloperoxidase (MPO) and T cells marker CD3. Representative photomicrographs taken with a 20X objective are shown on the left, and quantitative analysis of the positive areas in the liver sections are shown on the right. Scale bar = 50 μm. * p<0.05, n=9-11.
CVC and atRA alone or in combination reduced neutrophils and T cells and hepatic expression of proinflammatory cytokines in Mdr2−/− mice
To verify whether the beneficial effects seen in Mdr2−/− livers from these treatments were mechanistically similar to the findings in BDL rats, we again analyzed the hepatic expression of genes involved in bile acid metabolism and the inflammatory response. While there were no substantial changes in bile acid transporters (e.g. Ntcp, Ostα, Bsep and Mrp2, supplementary Figure S5), we found significant reductions in Cyp7a1 mRNA expression along with increased Fxr and Shp expression in the combination treatment group (Figure 7C). The expression (mRNA) of proinflammatory cytokine Tnf-α, Ccl2, and Ccl5 were significantly reduced with either CVC or atRA treatment alone or in combination when compared with the untreated control animals (Figure 7D), whereas IL-1β, IL-6, and Icam-1 were not altered among the different treatment groups (data not shown). In contrast, both Ccr2 and Ccr5 mRNA expression levels were higher in CVC treatment alone or in combination with atRA. Interestingly, the anti-inflammatory gene IL-10 was significantly increased in the combination group when compared to the untreated control group (Figure 7D). Characterization of the inflammatory cell types in Mdr2−/− livers using immunohistochemical staining revealed that both MPO positive neutrophil and CD3+ T cells were enriched around portal region in untreated control livers (Figure 7E). Treatments with CVC or atRA alone or in combination significantly reduced the number of these cells when compared to the untreated control livers. Furthermore, mRNA expression analysis revealed significantly less Elane (another neutrophil specific marker) and CD3, consistent with the immunohistochemistry results (supplementary Figure S6). In contrast, there was no difference in the infiltration of macrophages among the different Mdr2−/− groups when assayed for either expression of protein (using immunohistochemistry staining) or mRNA (using Q-PCR) of F4/80 (supplementary Figure S6). Together, these findings indicate that CVC and atRA act by similar mechanisms in both Mdr2−/− mouse and BDL rat cholestatic models.
Discussion
Recent studies into the pathogenic mechanism of cholestatic liver injury suggested to us that strategies for treating cholestasis might be advanced by targeting both the bile acid pool size and the inflammatory response.9,10,31 In this report, we evaluated this hypothesis in two cholestatic rodent models by reducing the bile acid pool size with an inhibitor of Cyp7a1 and mitigating the inflammatory response with a cytokine receptor antagonist (Figure 8). Specifically, to inhibit Cyp7a1, we used atRA, which we previously have shown is a potent inhibitor for both rodent and human CYP7A1.18,19 To target cytokine receptors, we used CVC, a known CCR2 and CCR5 antagonist, now in phase 3 trials for NASH.24,25 We indeed found that the best therapeutic effects were achieved in the BDL rat and Mdr2−/− mouse, only when both the bile acid pool size and the inflammatory response were reduced by combining atRA together with CVC. Although each agent alone also showed some beneficial effects, the combination treatment far exceeded the response of monotherapy with these two agents (see summary of these comparative effects in supplementary Table S4&S5). These observations emphasize the principal that multi-target directed therapy should be a clinical therapeutic goal for cholestatic diseases.
Figure 8.
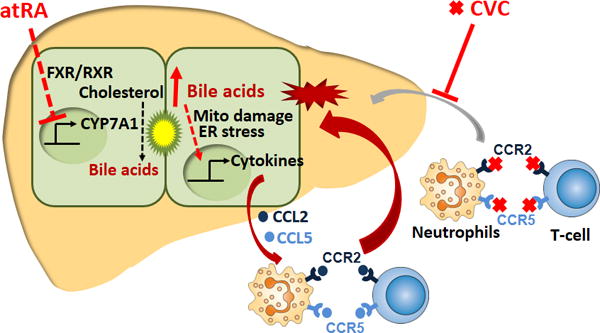
An illustration of the proposed mechanisms of action of atRA and CVC in the treatment of cholestatic liver injury. atRA activates the nuclear receptor FXR/RXR which in turn represses the expression of CYP7A1 (the rate limiting enzyme in bile acid synthesis) resulting in a lower bile acid pool size. At the same time, CVC antagonizes the chemokine receptors CCR2 and CCR5 that are present on neutrophils and T cells thereby reducing their attraction to the stressed hepatocytes that are being stimulated by bile acids to release chemokines CCL2 and CCL5.
This conclusion is supported by the following findings: 1) levels of bile acid in both plasma and liver were significantly lower in the combination treatment groups when compared to their untreated controls, indicating that these animals are less cholestatic (Figure 1 and 6); 2) plasma liver enzyme assays and or liver histology assessment indicated that there was less liver injury in the combination treatment groups (Figure 1, 2, 6 and supplementary Figure S1 and S2); 3) Alteration of the expression of genes involved in hepatic injury, bile acid metabolism and inflammatory response not only further confirmed reduced liver injury in animals received the combination treatment but also provided a molecular explanation for the actions of these two agents (Figure 3–7). Finally, these two agents effectively reduced the hepatic recruitment of neutrophils and T cells in both BDL rats and Mdr2−/− mice, the same inflammatory cell types that are seen as the primary cell infiltrates that causes cholestatic liver injury.10,12,13 These findings strongly support our hypothesis that atRA or a similar Fxr activator in combination with CVC might be an effective novel therapy for treating patients with chronic cholestatic liver diseases. Since our studies do not demonstrate a specific mechanistic difference to explain the better effects of combination therapy than seen with each drug alone, we propose that the superior benefits come from quantitative synergistic effects rather than specific qualitative differences.
While the types of cholestatic liver disease are numerous and their initial pathogenesis often unknown, a common feature is the hepatic retention of bile acids. When bile acids accumulate in the liver, they cause mitochondrial damage and ER stress to the hepatocytes which results in activation of signaling pathways that trigger a cytokine mediated inflammatory response, which further aggravates liver injury, leading to cell death.9,10,13,32 This process involves activation to TLR9 and EGR1 as well as other signaling pathways yet to be determined.9,10,33 In the current study, atRA reduced bile acid pool size by inhibiting Cyp7a1 and repressing de novo bile acid synthesis, whereas CVC mitigated inflammation by blocking the infiltration of neutrophils and T cells (Figure 8). Both these populations of inflammatory cells express CCR2 and CCR5,23 (and supplementary Figure S7) helping to explain why the best therapeutic effects were obtained from the combination treatment of atRA with CVC in these rodent cholestatic models. Liver injury in these cholestatic animal models treated with atRA alone as observed in this study is consistent with our previously published reports,19,20 in which atRA also moderately reduced the bile acid pool size. However, because atRA did not reduce the bile acid pool size to normal levels, liver injury was attenuated but not eliminated by focusing only on this single target. To further reduce liver injury, mitigating the inflammatory response seems necessary. Indeed, addition of CVC potentiated atRA therapeutic effects in these animals, resulting in further reduction of the bile acid pool size and liver fibrosis (Supplementary Table S4 and S5). The synergistic effects of CVC and atRA also confirm that the inflammatory response (infiltration of neutrophils and T cells) plays an important role in cholestatic liver injury as reported previously.12 Interestingly, treatment with CVC alone did not reduce cholestatic liver injury in either rodent models, including an absence of an effect on liver necrosis, fibrosis and even bile duct proliferation. This observation is consistent with reports from clinical trials in PBC patients where there was a lack of efficacy when immunosuppressive drugs were tested.8 However, at the molecular level, CVC treatment alone did reduce hepatic expression of proinflammatory cytokine Ccl2, Ccl5, Tnf-α, and IL-6 in these cholestatic animals (Figure 4&7). These observations suggest that the reduction of these cytokines alone is not sufficient to diminish liver injury. Most likely, there are additional signaling pathways that are involved in this bile acid induced inflammatory response because CVC only blocked Ccr2 and Ccr5 mediated pathways. Indeed, recent studies demonstrated that a number of Cxcl chemokines are also induced by bile acid in hepatocytes in addition to Ccl2 and Ccl5.9,10 Therefore, it would be difficult to improve cholestatic liver injury by only blocking the effect of these specific cytokines as there are undoubtedly additional pathways in vivo that are stimulating inflammation. On the other hand, by repressing bile acid synthesis and reducing the bile acid pool size as seen with atRA or other FXR activators such as obeticholic acid or blocking the intestinal uptake of bile acid in the ileum using an ASBT (SLC10A2) inhibitor, liver injury should be further reduced.15,16 Hence, it is conceivable that cholestatic liver injury could be eliminated if an agent or combination of agents could normalize the bile acid pool size. When the agent cannot normalize the bile acid pool size, addition of anti-inflammation drug(s) may potentiate the therapeutic effect of the agent as seen in the present study. Future studies will be needed to test this speculation.
In summary, we assessed the therapeutic effects of CVC and atRA separately and together in vivo in BDL rats and Mdr2−/− mice. We found that the best therapeutic effects were obtained by combining CVC with atRA, where CVC inhibited the inflammatory response while atRA reduced the bile acid pool size. These findings provide a novel strategy for the treatment of cholestatic liver injury and establish a paradigm to encourage clinical trials with multi-target therapy. Investigation of the therapeutic effect of these two agents in patients with cholestasis seems warranted.
Supplementary Material
Key points.
Cholestasis results in liver injury, but therapeutic interventions are limited.
Recent studies indicate that both the bile acid pool size and the innate inflammatory response play major pathophysiologic roles.
Here we demonstrate that reducing bile acid pool size using retinoic acid (a CYP7A1 expression repressor) in combination with an anti-inflammatory drug Cenicriviroc (an antagonist of chemokine receptor CCR2 and CCR5) has a superior therapeutic effect in reducing liver injury in cholestatic rodent models.
A drug that blocks the inflammatory response together with an agent that reduces the bile acid pool size is a novel strategy for treating cholestatic liver diseases.
Acknowledgments
We are grateful to Kathy Harry for excellent technical support. We also thank Dr. David N. Assis for constructive discussions and critical review of the manuscript.
Funding information: This study was supported by National Institutes of Health Grants DK34989 (Yale Liver Center), DK25636 (to J.L.B.)
Abbreviations
- ALT
alanine aminotransferase
- ALP
alkaline phosphatase
- atRA
all-trans retinoic acid
- BDL
bile duct ligation
- CA
cholic acid
- CVC
Cenicriviroc
- Cyp7a1
cholesterol 7-alpha-hydroxylase
- FXR/Fxr
Farnesoid X receptor (NR1H4)
- IHC
immunohistochemistry
- Mdr2
Multidrug resistance protein 2 (Abcb4)
Footnotes
Conflict of interest: DKY, SYC, AM, and JLB have declared that no conflict of interest exists. PV was affiliated with Allergan plc.
References
- 1.Hirschfield GM. Genetic determinants of cholestasis. Clin Liver Dis. 2013;17:147–159. doi: 10.1016/j.cld.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Keitel V, Droge C, Stepanow S, et al. Intrahepatic cholestasis of pregnancy (ICP): case report and review of the literature. Z Gastroenterol. 2016;54:1327–1333. doi: 10.1055/s-0042-118388. [DOI] [PubMed] [Google Scholar]
- 3.Pollheimer MJ, Fickert P, Stieger B. Chronic cholestatic liver diseases: clues from histopathology for pathogenesis. Mol Aspects Med. 2014;37:35–56. doi: 10.1016/j.mam.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–1273. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
- 5.Ali AH, Tabibian JH, Carey EJ, Lindor KD. Emerging drugs for the treatment of Primary Biliary Cholangitis. Expert Opin Emerg Drugs. 2016;21:39–56. doi: 10.1517/14728214.2016.1150999. [DOI] [PubMed] [Google Scholar]
- 6.Trauner M, Fuchs CD, Halilbasic E, Paumgartner G. New therapeutic concepts in bile acid transport and signaling for management of cholestasis. Hepatology. 2017;65:1393–1404. doi: 10.1002/hep.28991. [DOI] [PubMed] [Google Scholar]
- 7.Markham A, Keam SJ. Obeticholic Acid: First Global Approval. Drugs. 2016;76:1221–1226. doi: 10.1007/s40265-016-0616-x. [DOI] [PubMed] [Google Scholar]
- 8.Floreani A, Sun Y, Zou ZS, et al. Proposed therapies in primary biliary cholangitis. Expert Rev Gastroenterol Hepatol. 2016;10:371–382. doi: 10.1586/17474124.2016.1121810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen K, Jaeschke H, Copple BL. Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am J Pathol. 2011;178:175–186. doi: 10.1016/j.ajpath.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai SY, Ouyang X, Chen Y, et al. Bile acids initiate cholestatic liver injury by triggering a hepatocyte-specific inflammatory response. JCI Insight. 2017;2:e90780. doi: 10.1172/jci.insight.90780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woolbright BL, Jaeschke H. Novel insight into mechanisms of cholestatic liver injury. World J Gastroenterol. 2012;18:4985–4993. doi: 10.3748/wjg.v18.i36.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gujral JS, Farhood A, Bajt ML, Jaeschke H. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology. 2003;38:355–363. doi: 10.1053/jhep.2003.50341. [DOI] [PubMed] [Google Scholar]
- 13.O’Brien KM, Allen KM, Rockwell CE, Towery K, Luyendyk JP, Copple BL. IL-17A synergistically enhances bile acid-induced inflammation during obstructive cholestasis. Am J Pathol. 2013;183:1498–1507. doi: 10.1016/j.ajpath.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soroka CJ, Ballatori N, Boyer JL. Organic solute transporter, OSTalpha-OSTbeta: its role in bile acid transport and cholestasis. Semin Liver Dis. 2010;30:178–185. doi: 10.1055/s-0030-1253226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miethke AG, Zhang W, Simmons J, et al. Pharmacological inhibition of apical sodium-dependent bile acid transporter changes bile composition and blocks progression of sclerosing cholangitis in multidrug resistance 2 knockout mice. Hepatology. 2016;63:512–523. doi: 10.1002/hep.27973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baghdasaryan A, Fuchs CD, Osterreicher CH, et al. Inhibition of intestinal bile acid absorption improves cholestatic liver and bile duct injury in a mouse model of sclerosing cholangitis. J Hepatol. 2016;64:674–681. doi: 10.1016/j.jhep.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 17.Seki E, de MS, Inokuchi S, et al. CCR2 promotes hepatic fibrosis in mice. Hepatology. 2009;50:185–197. doi: 10.1002/hep.22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai SY, He H, Nguyen T, Mennone A, Boyer JL. Retinoic acid represses CYP7A1 expression in human hepatocytes and HepG2 cells by FXR/RXR-dependent and independent mechanisms. J Lipid Res. 2010;51:2265–2274. doi: 10.1194/jlr.M005546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He H, Mennone A, Boyer JL, Cai SY. Combination of retinoic acid and ursodeoxycholic acid attenuates liver injury in bile duct-ligated rats and human hepatic cells. Hepatology. 2011;53:548–557. doi: 10.1002/hep.24047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai SY, Mennone A, Soroka CJ, Boyer JL. All-trans-retinoic acid improves cholestasis in alpha-naphthylisothiocyanate-treated rats and Mdr2−/− mice. J Pharmacol Exp Ther. 2014;349:94–98. doi: 10.1124/jpet.113.209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Assis DN, Abdelghany O, Cai SY, et al. Combination Therapy of All-Trans Retinoic Acid With Ursodeoxycholic Acid in Patients With Primary Sclerosing Cholangitis: A Human Pilot Study. J Clin Gastroenterol. 2017;51:e11–e16. doi: 10.1097/MCG.0000000000000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baba M, Takashima K, Miyake H, et al. TAK-652 inhibits CCR5-mediated human immunodeficiency virus type 1 infection in vitro and has favorable pharmacokinetics in humans. Antimicrob Agents Chemother. 2005;49:4584–4591. doi: 10.1128/AAC.49.11.4584-4591.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mack M, Cihak J, Simonis C, et al. Expression and characterization of the chemokine receptors CCR2 and CCR5 in mice. J Immunol. 2001;166:4697–4704. doi: 10.4049/jimmunol.166.7.4697. [DOI] [PubMed] [Google Scholar]
- 24.Lefebvre E, Moyle G, Reshef R, et al. Antifibrotic Effects of the Dual CCR2/CCR5 Antagonist Cenicriviroc in Animal Models of Liver and Kidney Fibrosis. PLoS One. 2016;11:e0158156. doi: 10.1371/journal.pone.0158156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedman SL, Ratziu V, Harrison SA, et al. A Randomized, Placebo-Controlled Trial of Cenicriviroc for Treatment of Nonalcoholic Steatohepatitis with Fibrosis. Hepatology. 2017 Aug 17; doi: 10.1002/hep.29477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fickert P, Fuchsbichler A, Wagner M, et al. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2004;127:261–274. doi: 10.1053/j.gastro.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Sherwood ER, Toliver-Kinsky T. Mechanisms of the inflammatory response. Best Pract Res Clin Anaesthesiol. 2004;18:385–405. doi: 10.1016/j.bpa.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Amanzada A, Malik IA, Nischwitz M, Sultan S, Naz N, Ramadori G. Myeloperoxidase and elastase are only expressed by neutrophils in normal and in inflamed liver. Histochem Cell Biol. 2011;135:305–315. doi: 10.1007/s00418-011-0787-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krohn N, Kapoor S, Enami Y, et al. Hepatocyte transplantation-induced liver inflammation is driven by cytokines-chemokines associated with neutrophils and Kupffer cells. Gastroenterology. 2009;136:1806–1817. doi: 10.1053/j.gastro.2009.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davit-Spraul A, Gonzales E, Baussan C, Jacquemin E. The spectrum of liver diseases related to ABCB4 gene mutations: pathophysiology and clinical aspects. Semin Liver Dis. 2010;30:134–146. doi: 10.1055/s-0030-1253223. [DOI] [PubMed] [Google Scholar]
- 31.Woolbright BL, Jaeschke H. Therapeutic targets for cholestatic liver injury. Expert Opin Ther Targets. 2016;20:463–475. doi: 10.1517/14728222.2016.1103735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65:166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 33.Kim ND, Moon JO, Slitt AL, Copple BL. Early growth response factor-1 is critical for cholestatic liver injury. Toxicol Sci. 2006;90:586–595. doi: 10.1093/toxsci/kfj111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


