Abstract
Objective
Research shows that high anxiety sensitivity (AS) and dysphoria are related to poor smoking cessation outcomes. Engaging in exercise may contribute to improvement in smoking cessation outcomes through reductions in AS and dysphoria. In the current study, we examined whether exercise can aid smoking cessation through reductions in AS and dysphoria.
Method
Participants were sedentary and low activity adult daily smokers (N = 136) with elevated AS who participated in a randomized controlled trial comparing smoking cessation treatment (ST) plus an exercise intervention (ST+EX) to ST plus wellness education (ST+CTRL). Self-reported smoking status was assessed in-person weekly from baseline through week 16 (end-of-treatment; EOT), at week 22 (4-months post-quit day), and week 30 (6-months post-quit day), and verified biochemically.
Results
Results indicated that both AS and dysphoria at 6-month follow-up were significantly lower in the ST+EX group compared to the ST+CTRL group (controlling for baseline levels). Moreover, reductions in AS and dysphoria emerged as independent mechanisms of action explaining success in quitting.
Conclusions
These novel findings offer clinically significant evidence suggesting that vigorous-intensity exercise can effectively engage affective constructs in the context of smoking cessation.
Keywords: smoking cessation, Exercise, anxiety sensitivity, dysphoria
Exercise has emerged as a promising aid for smoking cessation, although benefits appear to be highly variable (Ussher, Taylor, & Faulkner, 2014). Some work indicates exercise interventions reduce cigarette craving and withdrawal symptoms (Haasova et al., 2013; Prochaska et al., 2008; Roberts, Maddison, Simpson, Bullen, & Prapavessis, 2012; Ussher, Taylor, & Faulkner, 2008). Other research indicates exercise-based smoking cessation interventions can increase rates of smoking cessation success (Prochaska et al., 2008; Ussher, Taylor, & Faulkner, 2008). Yet, other studies have found equivocal results, including small effect sizes and inconsistent results (Ussher, Taylor, & Faulkner, 2014). Limitations of such work have included potentially improper dosing, adherence challenges, individual differences, and small sample sizes (Ussher et al., 2014). Notably, recent evidence suggests that exercise interventions may be particularly effective in terms of promoting smoking abstinence among traditionally hard-to-treat smokers; namely, smokers with emotional vulnerabilities (Smits et al., 2016). Interestingly, there is little research examining mediators of treatment effects among high-risk, emotionally vulnerable smokers. This limitation is clinically important from a public health perspective, as empirical information on mechanisms of change in exercise-smoking work is needed to intervene more effectively for this high-risk and difficult-to-treat segment of the smoking population. Indeed, smokers with affective vulnerabilities represent one of the most treatment-resistant segments of the smoking population who benefit less from existing interventions (Leventhal & Zvolensky, 2015).
One means of elucidating the mechanisms of exercise interventions is to investigate the role of transdiagnostic emotional vulnerability factors that underpin affective psychopathology and smoking (Leventhal & Zvolensky, 2015). Anxiety sensitivity (AS), or the tendency to fear anxiety-related sensations (Reiss & McNally, 1985), is one such transdiagnostic construct involved in the etiology and maintenance of anxiety disorders (e.g., panic and social anxiety) and a wide variety of health behaviors (e.g., Anestis, Holm-Denoma, Gordon, Schmidt, & Joiner, 2008; Babson, Trainor, Bunaciu, & Feldner, 2008; Otto et al., 2016). AS is relatively stable, yet malleable in response to interventions (Otto & Reilly-Harrington, 1999; Smits, Berry, Tart, & Powers, 2008). Specific to exercise interventions, a number of investigations have demonstrated positive effects for exercise to effectively reduce AS (Broman-Fulks, Berman, Rabian, & Webster, 2004; Broman-Fulks & Storey, 2008; Smits et al., 2008). Exercise may impact anxiety sensitivity by way of physical sensations experienced during vigorous physical activity serving as interoceptive exposure to fearful bodily sensations in the absence of negative consequences – i.e., fear extinction (Smits, Powers, Berry, & Otto, 2007; Smits & Zvolensky, 2006).
Elevated AS is markedly common among smokers and contributes to several maladaptive smoking beliefs and behaviors. Specifically, high AS is observed in 20–33% of smokers, and therefore, is highly significant from a public health perspective (Allan et al., 2014). These emotionally vulnerable smokers perceive quitting as more difficult (Johnson, Farris, Schmidt, Smits, & Zvolensky, 2013), experience more severe withdrawal during the early phases of quitting (Johnson, Stewart, Rosenfield, Steeves, & Zvolensky, 2012; Perkins, Karelitz, Giedgowd, Conklin, & Sayette, 2010), and experience greater psychological inflexibility when confronted with stressful situations (Zvolensky, Farris, Schmidt, & Smits, 2014). AS is related to coping-oriented smoking (Battista et al., 2008; Guillot, Pang, & Leventhal, 2014), beliefs that smoking will reduce negative affect (Johnson et al., 2013), and increased positive affect after smoking (Wong et al., 2013). Furthermore, AS statistically accounts for the relation of emotional disorders with tobacco dependence, perceived barriers to cessation, and severity of problematic symptoms while quitting (Zvolensky et al., 2014). Elevated AS predicts greater odds of early lapse (Brown, Kahler, Zvolensky, Lejuez, & Ramsey, 2001) and relapse (Assayag, Bernstein, Zvolensky, Steeves, & Stewart, 2012). Furthermore, reductions in AS are related to less severe tobacco withdrawal (Bakhshaie et al., 2014) and increased rates of cessation success (Zvolensky, Bogiaizian, Salazar, Farris, & Bakhshaie, 2014; Zvolensky, Yartz, Gregor, Gonzalez, & Bernstein, 2008). However, to date, it is unknown how AS reduction in response to an exercise treatment might impact smoking cessation.
It also is important to consider other emotional vulnerability factors that may mediate exercise intervention effects on smoking cessation (Zvolensky & Leventhal, 2016). One such consideration is dysphoria—a symptom cluster of depression that is characterized by anhedonia, sadness, psychomotor disturbance, worthlessness, worry, and cognitive difficulty (Watson et al., 2007). Importantly, AS and dysphoria are related, yet distinct emotional vulnerability factors (Garey et al., In press). Extant work suggests that dysphoria may be more relevant to smoking maintenance and relapse than other depressive dimensions (Leventhal, Zvolensky, & Schmidt, 2011). For example, dysphoria is associated with several aspects of smoking behavior, including greater dependence, higher smoking rates, and more negative affect reduction smoking motives (Leventhal et al., 2011), as well as more perceived barriers to cessation (Buckner et al., 2015), greater smoking-specific experiential avoidance (Buckner et al., 2015), and more severe tobacco withdrawal (Bakhshaie, in press). Greater dysphoria is also associated with higher rates of smoking over time (Helstrom, Bell, & Pineles, 2009), and smokers who endorse greater dysphoria are more vulnerable to dependent smoking and relapse as a means to attenuate dysphoria-related symptoms (Leventhal et al., 2011). As with AS, exercise has also demonstrated positive effects on the reduction of dysphoria (Bernard et al., 2013), and may serve to enhance mood consistent with behavioral activation treatments, which have been shown to be effective in improving mood (e.g., Cuijpers, Van Straten, & Warmerdam, 2007).
Engaging in exercise may contribute to improvement in smoking cessation outcomes through reductions in AS and dysphoria. Indeed, extensive theoretical work on the negative reinforcing properties of substance use purports that the attenuation of negative affective symptoms, including symptoms of anxiety and depression, serves as a primary motive for use and may explain substance use behavior change, including abstinence (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004). To date, however, no work has examined AS or dysphoria as mediators of the effect of an exercise intervention to promote smoking cessation. Such work has the potential to advance intervention development for smoking cessation for a particularly difficult to treat segment of the smoking population (emotionally at-risk smokers).
The primary goal of the current study was to address clinically important limitations in exercise-smoking research (e.g., lack of consideration of individual difference factors) and build upon findings by Smits et al. (2016) by testing the hypothesis that change in AS and dysphoria (independently) mediated the efficacy of exercise treatment to facilitate abstinence among high AS smokers. We modeled both mediators simultaneously to test the unique contributions of AS and dysphoria reductions, above the other, on quit success (Hayes, 2013).
Methods
Participants
Data were collected as part of a larger randomized controlled trial examining the efficacy of a smoking cessation intervention described below in the Procedure section (Smits et al., 2016). Between January 2010 and July 2014, 136 participants interested in participating in the trial were recruited from the Dallas area and attended a baseline appointment. Participants were recruited through the community and physician referrals. Recruitment techniques included newspaper and radio advertisements, fliers in community-based organizations, and internet-based advertising, including our laboratory website and Craigslist. Study eligibility was assessed through: (1) an initial pre-screen (both online and via telephone), and (2) an in-person psychiatric diagnostic screening visit. Eligibility criteria included: (1) adult daily smokers (at least 1 year of smoking, at a minimum rate of 10 cigarettes per day over the past year); (2) elevated AS (prescreen score of ≥ 20 on the 16-item Anxiety Sensitivity Index; Reiss, Peterson, Gursky, & McNally, 1986); (3) sedentary (moderate-intensity exercise less than twice a week for 30 minutes or less); and (4) an authored-generated motivated to quit screening scale used in past smoking trials (reporting a motivation of a least 5 on a 10-point scale; 0= no interest in quitting to 10= desire to quit today; Schmidt, Raines, Allan, & Zvolensky, 2016). A comprehensive list of exclusion criteria and screening procedures is provided in the study protocol (Smits et al., 2012). See Table 1 for participant characteristics.
Table 1.
Baseline Participant Characteristics
| Age | 42.25 (SD = 11.2) |
| Race/Ethnicity | |
| White/Caucasian | 73.4% |
| Black/African American | 20.3% |
| Asian | 2.3% |
| Hispanic/Latinx | 8.6% |
| “Other” | 3.1% |
| No response | 0.8% |
| Sex | 52.2% (female) |
| Education | |
| Junior high school | 1.6% |
| Some high school | 3.9% |
| High school graduate | 17.2% |
| Some college | 35.2% |
| College degree | 31.9% |
| Graduate school | 10.2% |
| Smoking Characteristics | |
| Age of first cigarette | M =16.2 (SD= 4.9) |
| Age of regular smoking onset | M =18.9 (SD= 5.1) |
| Cigarettes per day | M =19.4 (SD= 9.7) |
| FTND | M =5.3 (SD= 2.0) |
| Expired CO | M =15.4 (SD= 8.2) |
| Percentage with prior quit attempt | 88.0% |
| Number of past quit attempts | M=3.8 (SD= 2.8) |
| Psychopathology | |
| Percent meeting criteria for 1+ disorder | 60.2% |
| Average number of diagnoses | M=2.0 (SD= 1.1) |
| Major depressive disorder | 11.7% |
| Alcohol use disorder | 10.2% |
| Specific phobia | 10.2% |
| Study Variables | |
| ASI | |
| Baseline | 18.32 |
| Post-Treatment | 10.80 |
| 4-month Follow-up | 10.41 |
| 6-month Follow-up | 11.43 |
| Dysphoria | |
| Baseline | 18.30 |
| Post-Treatment | 16.88 |
| 4-month Follow-up | 17.22 |
| 6-month Follow-up | 17.84 |
| PPA | |
| Baseline | 0.0% |
| Post-Treatment | 32.2% |
| 4-month Follow-up | 26.6% |
| 6-Month Follow-up | 17.7% |
Note. N = 136 at baseline. FTND = Fagerström Test for Nicotine Dependence (Heatherton et al., 1991); Expired CO = Expired Carbon Monoxide measured with a CMD/CO Series Carbon Monoxide Monitor.
Procedure
Detailed protocol and intervention information is reported elsewhere (Smits et al., 2012). The study involved five phases: pre-screen via the phone for basic eligibility criteria, in-person prescreen for psychological and medical eligibility testing, baseline assessment and randomization, intervention delivery, and follow-up assessments. Participants eligible at the pre-screen were invited to an in-person screening appointment. Upon arrival, participants received an informed consent form explaining the details of the study, potential benefits and risks of participation, and the procedures they might undergo if they chose to participate. Participants then completed self-report questionnaires and a diagnostic interview (SCID; First, Spitzer, Gibbon, & Williams, 2002), a medical examination comprising of a physical exam, laboratory work, and maximal exercise testing. Eligible participants completed the baseline assessment within 3 weeks of completing their screening visits wherein they were randomized to one of the two arms of the study.
Both conditions provided the same smoking cessation treatment (ST; Fiore et al., 2008; Zvolensky et al., 2008), consisting of 7 weekly 60-minute group sessions of cognitive behavioral therapy for smoking cessation and optional nicotine replacement therapy, which commenced at week 6 (target quit day). Congruently, participants were randomized to receive either a vigorous-intensity exercise intervention (ST+EX) or a wellness education control intervention (ST+CTRL; e.g., lectures on wellness topics). Both interventions were equivalent in time, consisting of 3 weekly 25-minute (exercise or wellness) sessions for 15 weeks, and began at week 1. In terms of ST+EX, one week following the baseline session, participants in the exercise condition initiated a 15-week program involving three 45-minute exercise sessions each week. During the first session, clinicians provide participants with a rationale for exercise as a treatment for smoking cessation. They are given a model of the role of AS in the maintenance of smoking, and told how interoceptive exposure plays a role in the treatment of AS. Exercise is then introduced as a means of systematic interoceptive exposure. Participants work together with their therapist to develop an exercise training progression schedule which allows participants to gradually increase their intensity across weeks to reach their prescribed dose by week 4. By week 4, participant’s target exercise intensity dose is 77–85% of their maximum heart rate achieved during the pretreatment maximal exercise test. Trained staff supervised exercise sessions. During the session, participants wear a heart rate monitor to confirm that they are training at the targeted exercise intensity.
The ST+CTRL is a 15- week program involving three 45-minute sessions each week. During the first session, participants receive a thorough description of the wellness program along with a rationale for the intervention. Participants were then given the rationale that, because people who quit smoking often adopt healthy lifestyle changes after smoking, we have opted to include these changes from the onset of the quit attempt to determine whether the addition of these lifestyle changes during a quit attempt has an effect on smoking cessation outcomes. They are also advised that focusing on small wellness goals each week and gaining small successes may carry over into their smoking cessation goals. Wellness education sessions focus on discussing a variety of healthy lifestyle topics, such as healthy eating, stress and time management, recommended health screenings, and cancer and cardiovascular prevention. The content in each session is delivered using a combination of lectures, videos, handouts, and discussions with the means of allowing participants to set their own realistic wellness goals, which they can gradually incorporate into their lives.
Smoking cessation outcomes were measured up to 6-months following the quit date. Participants were compensated $25 for the baseline and each of the 6 assessment visits that occurred during the follow-up period; see Abstinence description below. Participants who attended at least 90% of the sessions were compensated with an additional $125. The Institutional Review Board of Southern Methodist University approved the study and a Data Safety and Monitoring Board provided ongoing monitoring.
Measures
Demographics Questionnaire
Demographic information collected included gender, age, ethnicity, and highest level of education attained.
Structured Clinical Interview-Non-Patient Version for DSM-IV (SCID-I/NP; First et al., 2002)
Diagnostic assessments of past year Axis I disorders were conducted using the SCID-I/NP, which was administered by doctoral level staff or trained research assistants and supervised by licensed clinical psychologists.
Smoking History Questionnaire (SHQ; Brown, Lejuez, Kahler, & Strong, 2002)
The SHQ is a self-report questionnaire used to assess smoking history (e.g., onset of daily smoking) and pattern (e.g., smoking rate). In the present study, the SHQ was used at baseline to describe the sample on smoking history and patterns of use.
Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991)
The FTND is a 6-item scale that assesses an individual smoker’s tobacco dependence (Heatherton et al., 1991). The FTND has shown adequate internal consistency (Cronbach’s α = .61 in the current study), positive relations with key smoking variables (e.g., saliva cotinine), and high test-retest reliability (Heatherton et al., 1991). In the present study, the FTND was used at baseline to describe the sample.
Anxiety Sensitivity Index (ASI)
To determine study eligibility at the pre-screen, we used the 16-item Anxiety Sensitivity Index (ASI; Reiss, Peterson, Gursky, & McNally, 1986), which demonstrates good retest reliability, internal consistency, and convergent validity with other measures (Zinbarg, Mohlman, & Hong, 1999). At baseline, as well as throughout treatment and follow-up period, we assessed AS using the Anxiety Sensitivity Index-3 (ASI-3; Taylor et al., 2007). The ASI-3 is an 18-item self-report measure, based in part upon the original ASI (Reiss et al., 1986), that assesses the sensitivity to, and fear of, the potential negative consequences of anxiety-related symptoms and sensations. Respondents are asked to indicate, on a 5-point Likert-type scale (0 = "very little" to 4 = "very much"), the degree to which they are concerned about these possible negative consequences (Cronbach's α = .90 in the current study). The ASI-3 has been validated among smokers (Farris et al., 2015). At the inception of the study, no cut-offs for the ASI-3 were available, which is why the original ASI was used for eligibility (i.e., screening).
Inventory of Depression and Anxiety Symptoms (IDAS; Watson et al., 2007) Dysphoria subscale
The IDAS is a 64-item self-report instrument that assesses distinct affect symptom dimensions within the past two weeks. Items are answered on a 5-point Likert scale ranging from “not at all” to “extremely.” The IDAS subscales show strong internal consistency, convergent, and discriminant validity with psychiatric diagnoses and self-report measures, and short-term retest reliability(Watson et al., 2007). In the present study, we employed the 10-item dysphoria subscale (e.g., “I felt depressed;” Cronbach’s α = .92 in the current study), as this subscale assesses the core emotional and cognitive symptoms of anxiety and depression (Watson et al., 2007). The IDAS was administered at baseline as well as throughout treatment and follow-up.
Abstinence
Self-reported smoking status was assessed in-person weekly from baseline through week 16 (end-of-treatment; EOT), at week 22 (4-months post-quit day), and week 30 (6-months post-quit day). In addition, we also examined abstinence at post-quit appointments that occurred during the active treatment phase, including week 8 (2-weeks post-quit), week 10 (4-weeks post-quit), and week 14 (8-weeks post-quit). We used the timeline follow-back (TLFB) procedure at all assessments to assess cigarette consumption at each day since the previous assessment. We have previously used the TLFB to assess cigarette use among high AS smokers (McLeish, Zvolensky, & Bucossi, 2007) and the assessment has demonstrated good reliability and validity with biochemical indices of smoking (Sobell & Sobell, 1996). Self-reported abstinence at every assessment was verified by expired carbon monoxide (CO) using a CMD/CO Series Carbon Monoxide Monitor (Model 3110; Spirometrics, Inc., Auburn, ME). Abstinence at 16- and 24-weeks following the quit day was additionally verified with saliva cotinine that was analyzed by Salimetrics. Self-reported abstinence was overridden by a positive expired CO reading (> 8ppm) or saliva cotinine verification (>10 ng/mL). If neither expired CO nor cotinine levels were available to verify abstinence at an assessment, abstinence was considered missing data. As recommended and consistent with past work (Hughes et al., 2003; Smits et al., 2016), we employed point prevalence abstinence (PPA) and prolonged abstinence (PA) as the primary outcomes. PPA was defined as no smoking, not even a puff, in the 7 days prior to any assessment and biochemical verification of abstinence at the time of the assessment. Failure to maintain PA at any assessment was defined by 7 or more consecutive days of smoking (starting with quit day) or smoking at least 1 cigarette over the 2 consecutive weeks prior to the assessment and biochemical verification of abstinence at the time of the assessment.
Data Analytic Strategy
Following the recommendations of the Society for Nicotine and Tobacco Research (Hall et al., 2001) and others (Hamer & Simpson, 2009), hypotheses were tested using multilevel modeling (MLM), which is an intent-to-treat analysis that includes all subjects, regardless of missing data. In order to avoid inflation of Type I error from conducting duplicate univariate analyses for the two smoking outcomes (PPA and PA), we used multivariate MLM (MMLM; see Heck, Thomas, & Tabata, 2013; Hox, Moerbeek, & van de Schoot, 2010). MMLM has the additional advantage of including data from all assessments from which any of the outcome data is obtained. We used a logistic linking function in the analyses in which abstinence was the dependent variable to accommodate the dichotomous outcomes, thus performing a generalized MMLM. Robust standard errors (robust to violations of multivariate normality) were used.
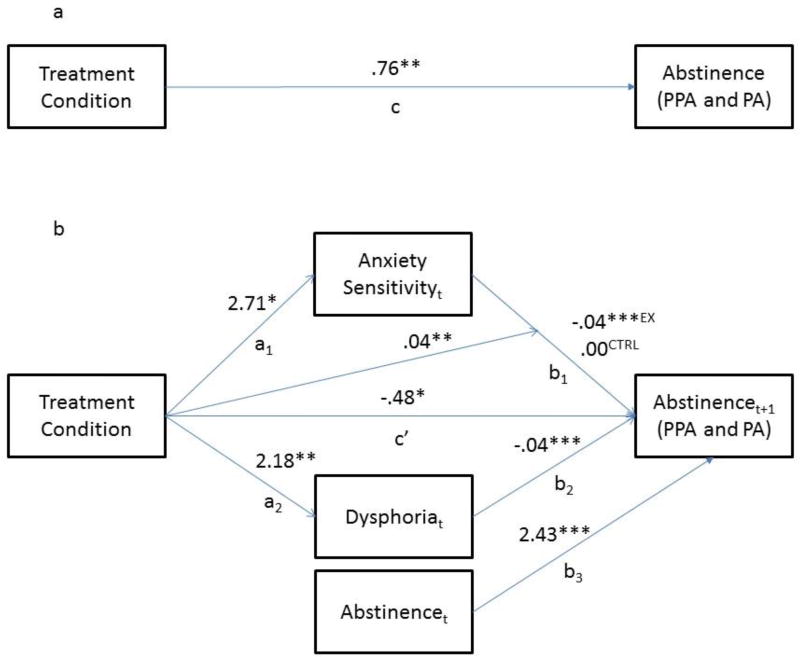
The aim of the present investigation was to examine whether AS and/or dysphoria mediated the treatment condition differences in abstinence at the final (6-month) follow-up (the “c” path; Figure 1a). Thus, the “a” paths in the mediation analysis were the effects of treatment on AS and dysphoria (at 6-month follow-up), and the “b” paths in the mediation analysis were the relations between the mediators (as time-varying predictors) and abstinence (see Figure 1b). We examined mediation of the effect of treatment on outcomes at the 6-month follow-up because the primary goal of the intervention was to increase long-term smoking. Sensitivity analyses showed similar results for analyses that examined mediation of treatment condition differences in abstinence at post-treatment.
Figure 1. Mediation Model.
Note. *p ≤.05, **p ≤ .01, ***p ≤ .001. Treatment condition differences in Abstinence were at the 6-month follow-up. PPA = point prevalence abstinence and PA = prolonged abstinence. Treatment condition effects are at the 6-month follow-up. The subscripts “t” (for AS and Dysphoria) and “t+1” (for Abstinence) indicate that the mediators at each assessment were used to predict abstinence at the next assessment. Estimated parameters that were not central to the test of mediation are omitted to improve interpretability. The superscripts “CTRL” and “EX” refer to the values of the path from anxiety sensitivity to abstinence (b1) in the control condition and in the exercise condition, respectively.
The mediation analysis that we performed is commonly referred to as “level-1 mediation of a level-2 effect” (Bauer, Preacher, & Gil, 2006). “Level 1” mediation refers to the fact that the mediators were assessed repeatedly throughout the study, and hence the relations between the mediators and abstinence were within-subjects, longitudinal relations over time. The “level 2 effect” here is the significant between-subjects effect of treatment condition on abstinence. Our approach, which we elaborate on below, is similar to the approach taken in other papers with repeated measurements of the mediators and outcomes (e.g., Meuret, Rosenfield, Seidel, Bhaskara, & Hofmann, 2010; Moscovitch, Hofmann, Suvak, & In-Albon, 2005; Olatunji et al., 2013; Smits, Rosenfield, McDonald, & Telch, 2006).
To determine whether mediation effects (i.e., the a*b pathways; see Figure 1b) are meaningful, it is first important to determine if the intervention does indeed improve outcomes (the “c” path; Figure 1a). In a previous paper that focused on efficacy of this treatment (Smits et al., 2016), we reported on the “c” path employing a model that best fit the study procedures and data (“best fit” models are crucial to avoiding misleading results; Liu, Rovine, & Molenaar, 2012). We will thus adopt the model reported in Smits et al. (2016) for the “c” path in the current investigation. Specifically, we modeled abstinence (i.e., the outcome) using a 3-phase piecewise growth curve, with the first phase reflecting pre-quit treatment phase (weeks 0–6), the second phase reflecting the post-quit treatment phase (weeks 7–16), and the third and last phase reflecting the follow-up phase (weeks 17–30). We modeled a discontinuity in the growth curve between the first and second phase to reflect the increase in abstinence during the scheduled quit week (week 6). To be consistent with Smits et al., we also included treatment adherence (operationalized as session attendance) as a covariate, which was related to abstinence. Also, since Smits et al. (2016) found that the effect of treatment depended on AS (treatment led to improved abstinence only for those with high AS), and since Smits et al. included AS as well as the treatment × AS interaction in their analyses, we included AS and the treatment × AS interaction in our model. Lastly, because extant work suggests that other constructs, including sex, education, and ethnicity, relate to the putative mediators and/or outcomes (Leventhal & Zvolensky, 2015), we entered these as covariates in all analyses, both to control for these potential third-variable confounds and to decrease error variance.
As reported in Smits et al. (2016) ST+EX was superior to ST+CTRL, but only for participants who had high AS (defined as those with ASI-3 ≥ 23 in accordance with previous work by (Allan et al., 2014). Based on the findings that treatment efficacy was specific to those with elevated AS, we estimated all the effects in the present investigation centered at the cut-off for high AS (ASI-3 = 23) as per the technique developed by Aiken and West (1991).
To investigate the mediators of this effect of treatment on outcome, we next calculated the “a” paths in the mediation analysis (Figure 1b). To calculate the effect of treatment on the mediators, we used univariate MLM (rather than generalized MMLM) because the mediators were normally distributed, continuous measures. As is typical in mediation analyses, the model that we used to calculate this effect of treatment on the mediators was the same growth curve model used for calculating the effect of treatment on outcome (the “c” path).
We next estimated the “b” paths in the mediation analysis (Figure 1b). In level-1 mediation of a level-2 effect, the “b” path is the within-subjects, over time relation between the mediator and the outcome, or the effect of a person’s individual change (within-subject) in the mediator on that person’s individual change (within-subject) in the outcome. These “b” paths were calculated by adding both AS and dysphoria to the model for the “c” path. And since we were estimating within-subjects, longitudinal relations between the mediators and outcome, AS and dysphoria were added as time-varying predictors (TVPs) to the model. To enhance causal inference, the time-varying effect for our putative mediators on the outcome was lagged such that AS and dysphoria at each session were modeled to predict abstinence at the following assessment. In other words, the mediators at baseline predicted outcomes at week 1; mediators at week 1 predicted outcome at week 2, etc. Abstinence at each session was also included as a predictor of abstinence at the next session (see Figure 1b). This creates a within-subjects cross-lag association between the mediators and outcome, thus controlling for potential “reverse causality” (the possibility that outcome caused the mediators rather than vice-versa). Each mediated pathway (a*b for each mediator) was tested for significance using the program RMediation (Tofighi & MacKinnon, 2011), which calculates the 95% confidence interval (CI) for the mediated effect (a*b). If the 95% CI did not include 0, the mediated pathway was significant.
Results
Descriptive Data
Initial examination of the data indicated that continuous variables in the analysis appeared to be normally distributed, with skewness less than 1, and no substantial outliers. Further, examination of scattergrams did not suggest the presence of heteroscedasticity. There were no significant treatment condition differences in baseline levels of AS (ASI-3; MST+EX=18.04; MST+CTRL=18.63; p=.776) or dysphoria (IDAS-dysphoria; MST+EX=18.78; MST+CTRL=17.77; p=.465). Most participants who did not drop out before quit week (81%) used nicotine replacement therapy (NRT; i.e., patch). Use of NRT did not vary by treatment condition (p=.496).
Dropouts and Missing Data
Forty-eight of the original 136 participants (35.2%) dropped out of the study before the end of the treatment phase (16 weeks after inception). An additional 22 participants (16.2%) dropped out between EOT and the 6-month follow-up. Dropout rates did not differ between treatments at either EOT or the 6-month follow-up (p=.37 and p=.31, respectively). Dropouts did not differ from completers on baseline levels of AS, dysphoria, nor any of the demographics (p’s>.33–.86), nor were there any significant differences between treatment dropouts, follow-up dropouts, or completers (p’s=.12–.93). Also, participants with missing data (some missed assessments but did not drop out) did not differ from those with no missing data on baseline levels of these variables (n = 41 with no missing data; p’s>.20).
This pattern of missing data shows no specific evidence that the data are “missing not at random”, and is consistent with the data being “missing at random”, an assumption of all MLM analyses. Further evidence of the nature of the missing data can be derived by examining “missing not at random” models to determine if our findings vary between participants with missing data and those without missing data. One such technique is pattern mixture modelling (see, for example, Enders, 2011), which examines whether different patterns of missing data have different growth models. Hence, we coded participants as either completers, treatment dropouts, or follow-up dropouts, and included these different dropout patterns as moderators of the slopes and intercepts in the growth curve model for abstinence. Results indicated that the growth curves of abstinence did not differ between these different dropout categories. Hence, our results indicate the dropout did not seem to affect our results.
The overall effect of treatment on abstinence (the “c” path)
As reported by Smits et al. (2016), our multivariate generalized MLM found that participants in ST+EX exhibited higher abstinence at the 6-month follow-up than participants in ST+CTRL (b = .76, t [4878] = 3.31, p < .001; see Figure 1a). This result justifies subsequent investigation into the mediators of this difference in abstinence at the 6-month follow-up. To avoid redundancy with past reporting of these outcome analyses, please see Smits et al. (2016) for details of the slopes during each phase and the impact of other variables such as adherence.
The effects of treatment on the mediators (the “a” paths)
As expected, our growth curve model showed that treatment condition was a significant predictor of both mediators in the mediation model (the “a” paths in the mediation model; Figure 1b). Specifically, both AS and dysphoria were lower in ST+EX than in ST+CTRL at the 6-month follow-up (b = 2.71, p = .032 for AS; b = 2.18, p = .003 for dysphoria).
The relation between putative mediators and outcome (the “b” paths)
The next step was to calculate the “b” paths in the mediation analyses. This was accomplished by adding the mediators (AS and dysphoria) as TVPs to the growth curve model for abstinence, along with adding prior levels of abstinence. We modeled the cross lag longitudinal relation between the mediators (and abstinence) and outcome by modeling mediators (and abstinence) at each time point (time “t”) predicting abstinence at the next time point (time “t+1”).
Results showed that the relation between AS and abstinence varied by treatment condition (AS × treatment condition: b = .04, t [124] = 2.69, p = .007), such that lower levels of AS were related to higher abstinence at the next session for participants in the ST+EX condition (b = −.04, t[124] = −4.06, p < .001), but not for those in ST+CTRL condition. With respect to dysphoria, lower levels of dysphoria were related to higher abstinence at the next session (b = −.04, t [124] = 3.42, p < .001), and this relation was consistent across treatment conditions.
The test of mediation (the a*b pathway)
Mediation is present if the joint, mediated pathway, a*b, is significant. Using the program RMediation (Tofighi, West, & MacKinnon, 2013), we found that the mediated pathway from treatment condition to dysphoria to abstinence was significant (a*b = −.087, 99.5% CI: [−.216, −.003], p < .005). Since AS was related to abstinence only for participants in the ST+EX condition, AS was not a significant overall mediator of the effect of exercise treatment condition on abstinence. However, since AS was related to abstinence for those in ST+EX, improvements in AS that occurred for participants in ST+EX would likely be transmitted to abstinence because AS is related to abstinence for those in ST+EX.
Discussion
The current study sought to address notable limitations in exercise-smoking research (e.g., lack of consideration of change in individual difference factors) and to systematically build upon past work on emotionally vulnerable smokers. Specifically, we tested the hypothesis that AS and dysphoria independently mediated the efficacy of exercise for facilitating abstinence in high AS smokers. Treatment condition differences were observed in AS and dysphoria, such that participants in the exercise intervention evidenced lower average levels of AS and dysphoria at the 6-month follow-up assessment relative to participants in the control intervention. These findings are in accord with past work suggesting that exercise is an efficacious and tolerable treatment modality with which to target and reduce depressive symptoms, including dysphoria (Bernard et al., 2013; Stathopoulou, Powers, Berry, Smits, & Otto, 2006; Stubbs et al., 2016) and AS (Broman-Fulks et al., 2004; Broman-Fulks & Storey, 2008; Smits et al., 2008). Yet, this study uniquely extends past work by demonstrating that vigorous-intensity aerobic exercise, as an adjunct to a smoking cessation intervention, is effective at addressing AS and dysphoria among a sedentary, emotionally vulnerable population of smokers.
Additionally, when participants had lower levels of dysphoria, they were more likely to be abstinent at the next session, regardless of treatment condition. This observation is consistent with past research documenting the relevance of dysphoria to smoking maintenance and relapse, and tobacco withdrawal (Leventhal et al., 2011). The present research provides further support for this effect by showing that dysphoria is related to abstinence over and above AS and current abstinence (and, as a result, over and above other variables that are highly related to AS and current abstinence). Also, by controlling for concurrent levels of abstinence in predicting future abstinence, the findings show that the relation between dysphoria and future abstinence is not due to “reverse causation” (the possibility that dysphoria and later abstinence are related because abstinence causes current dysphoria and later abstinence).
Consistent with prediction and earlier work (Broman-Fulks et al., 2004), exercise effectively reduced AS in treatment-seeking smokers, yielding clinically meaningful effects up to 6 months following the quit attempt. Although reductions in AS were related to greater abstinence in the exercise intervention, the relation between AS and abstinence was not significant in the control condition. Additionally, when participants had lower levels of AS, they were more likely to be abstinent only in the ST+EX condition. Thus, AS was a mechanism of change for only the ST+EX condition. These data suggest that engaging AS via exercise in the context of smoking cessation is clinically important for improved smoking outcome.
Together, the current findings provide insight into putative mechanisms (AS and dysphoria) underlying the effects of exercise on smoking cessation among high AS smokers. Such data have the potential to guide the development of novel cessation treatments to optimize the application of exercise interventions for high-risk emotionally vulnerable smokers. Specifically, the present study suggests that among high AS smokers: a) participating in a smoking cessation treatment plus vigorous-intensity exercise successfully lowers levels of AS and dysphoria; and b) reductions in dysphoria and AS due to exercise significantly attenuate the risk of smoking cessation failure. Collectively, these findings are consistent with past theory and research that highlight AS and dysphoria as cognitive-based transdiagnostic mechanisms involved in smoking lapse and relapse (Leventhal & Zvolensky, 2015).
We should point out that, although the “a” paths in our analyses were from a randomized independent variable and hence were causal, the “b” paths in the mediation analysis were not from a randomized mediator and hence were not causal. This problem is endemic to all mediation analyses in RCTs in which the mediators are not manipulated/randomized (Imai, Keele, & Tingley, 2010). We attempted to enhance the possibility of causal inference in the “b” path by 1) “lagging” the “b” path in our analysis (modeling the mediators at each time point as impacting abstinence at the next time point), 2) controlling for concurrent levels of abstinence when predicting abstinence at the next time point (effectively performing a within-subjects cross lag analysis), 3) controlling for other putative mediators (AS and dysphoria simultaneously), and by 4) calculating a within-subjects relation between the mediators and abstinence, which eliminates the potential confounding effects of third-variable differences between subjects(Smits, Julian, Rosenfield, & Powers, 2012). But since the “b” paths were not causal, the significant mediated pathway is not proof of causal mediation. Rather, our significant mediated pathway can only indicate that our results are consistent with the mechanism illustrated in our mediation model.
There are several interpretive caveats to the present study. First, the sample consisted of a relatively small sample of community-recruited, treatment-seeking sedentary cigarette smokers, with generally elevated baseline AS. Thus, the generalizability is limited in overall sample size and to the sampled population and may not extend to other types of smokers (e.g., low AS smokers, older more heavily dependent smokers). Second, this study focused primarily on AS and dysphoria as mediating variables. These cognitive-affective factors are only two of many possible emotional risk candidates that may account for the observed treatment effects on cessation outcomes. Future work could usefully continue to build multi-risk factor models that help to explain how certain treatments, such as exercise, affect the ability to quit smoking, including such factors as distress tolerance and smoking cessation self-efficacy. The study compared two active treatments. Future work should examine effects with treatment as usual as a comparison. Third, the current study utilized a vigorous-intensity exercise intervention. Future work should experimentally manipulate exercise intensity to examine effects of intensity as well as other dose parameters (e.g., frequency, duration of bout, duration of intervention). It is possible that reduction of AS and dysphoria may depend on exercise intensity (e.g., minimum heart rate threshold) or experience ceiling effects (i.e., limited benefit after a certain intensity level). Additional work in this area may examine moderators of exercise intensity to develop personalized treatments (e.g., matching age, sex, AS level to an optimal exercise program for smoking cessation). Research also could examine whether exercise interventions impact behavior changes post-treatment (e.g., frequency and intensity of exercise) and whether it does so to a greater degree than control conditions.
Fourth, the sample was relatively highly educated, majority White, started smoking late in life (see Table 1), and were only required to report moderate motivation for smoking on an author-generated scale. Thus, future work should examine exercise interventions for smoking cessation among underserved populations such as low-income racial/ethnic minorities. Future research also could conduct a more comprehensive motivation to quit smoking assessment to determine eligibility using evidenced-based assessments. Finally, dysphoria, AS, and smoking behavior were measured at specified assessment intervals. Although multi-method approaches were employed, an important next step in this area of research would be to evaluate affective symptoms and smoking outcomes using time sampling approaches wherein a more comprehensive evaluation of the temporal ordering of these factors and treatment outcome could be obtained.
Overall, the present investigation sheds light on specific mechanisms of action during a vigorous-intensity exercise treatment as an aid to cessation in a high-risk, emotionally vulnerable population of smokers. This work documents the clinical importance of engaging psychological mechanisms in the context of exercise-based interventions to offset the risk of poor health behaviors such as tobacco smoking.
Supplementary Material
Public Health Statements.
An adjunct exercise treatment component offered in the context of smoking cessation actively engaged anxiety sensitivity and dysphoria symptoms and produced greater reductions in symptoms at a 6-month follow-up. Reductions in AS and dysphoria emerged as independent mechanisms of action explaining success in quitting. These novel findings offer clinically significant evidence suggesting that vigorous-intensity exercise can effectively engage affective constructs in the context of smoking cessation and the unique roles that each construct may play in the dynamic process of quitting smoking.
Acknowledgments
Funding: This study was funded by grants from the National Institute on Drug Abuse: R01DA027533 (awarded to Drs. Zvolensky and Smits; MPI) and K01DA035930 (awarded to Dr. Powers; PI).
Dr. Smits and Otto receive royalties from Oxford University Press for books on exercise as a treatment for mood and anxiety disorders. Dr. Smits has served as a paid consultant for Microtransponder for work unrelated to the research reported in this manuscript.
Footnotes
Trial Registration: ClinicalTrials.gov, NCT01065506, http://clinicaltrials.gov/ct2/show/NCT01065506
Protocol: The full trial protocol can be found at pubmed.gov; PMCID: PMC3522063
Declarations of Interest: Drs. Zvolensky, Rosenfield, Powers, Langdon, Marcus, Church, Frierson, Hopkins, Ms. Garey, Ms. Kauffman, Ms. Davis, MS. Baird, and Mr. Paulus report no financial relationships with commercial interests.
References
- Aiken LS, West SG. Mulitple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Allan NP, Raines AM, Capron DW, Norr AM, Zvolensky MJ, Schmidt NB. Identification of anxiety sensitivity classes and clinical cut-scores in a sample of adult smokers: Results from a factor mixture model. J Anxiety Disord. 2014;28(7):696–703. doi: 10.1016/j.janxdis.2014.07.006. doi: http://dx.doi.org/10.1016/j.janxdis.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anestis MD, Holm-Denoma JM, Gordon KH, Schmidt NB, Joiner TE. The role of anxiety sensitivity in eating pathology. Cognitive Therapy & Research. 2008;32(3):370–385. doi: 10.1007/s10608-006-9085-y. [DOI] [Google Scholar]
- Assayag Y, Bernstein A, Zvolensky MJ, Steeves D, Stewart SS. Nature and role of change in anxiety sensitivity during NRT-aided cognitive-behavioral smoking cessation treatment. Cogn Behav Ther. 2012;41(1):51–62. doi: 10.1080/16506073.2011.632437. [DOI] [PubMed] [Google Scholar]
- Babson KA, Trainor CD, Bunaciu L, Feldner MT. An examination of anxiety sensitivity as a moderator of the relation between sleep anticipatory anxiety and sleep onset latency. Journal of Cognitive Psychotherapy. 2008;22(3):258–270. doi: 10.1891/0889-8391.22.3.258. [DOI] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111(1):33–51. doi: 10.1037/0033-295x.111.1.33. [DOI] [PubMed] [Google Scholar]
- Bakhshaie J, Kulesz PA, Langdon KJ, Businelle MS, Leventhal AM, Gallagher MW, Schmidt NB, Manning K, Goodwin R, Zvolensky MJ. A prospective investigation of the synergistic effect of change in anxiety sensitivity and dysphoria on tobacco withdrawal. Journal of consulting and clinical psychology. doi: 10.1037/ccp0000256. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhshaie J, Zvolensky MJ, Brandt CP, Vujanovic AA, Goodwin R, Schmidt NB. The role of anxiety sensitivity in the relationship between emotional non-acceptance and panic, social anxiety, and depressive symptoms among treatment-seeking daily smokers. International Journal of Cognitive Therapy. 2014;7(2):175–191. [Google Scholar]
- Battista SR, Stewart SH, Fulton HG, Steeves D, Darredeau C, Gavric D. A further investigation of the relations of anxiety sensitivity to smoking motives. Addict Behav. 2008;33(11):1402–1408. doi: 10.1016/j.addbeh.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Bauer DJ, Preacher KJ, Gil KM. Conceptualizing and testing random indirect effects and moderated mediation in multilevel models: new procedures and recommendations. Psychological Methods. 2006;11(2):142. doi: 10.1037/1082-989X.11.2.142. [DOI] [PubMed] [Google Scholar]
- Bernard P, Ninot G, Moullec G, Guillaume S, Courtet P, Quantin X. Smoking cessation, depression, and exercise: empirical evidence, clinical needs, and mechanisms. Nicotine & Tobacco Research. 2013;15(10):1635–1650. doi: 10.1093/ntr/ntt042. [DOI] [PubMed] [Google Scholar]
- Broman-Fulks JJ, Berman ME, Rabian BA, Webster MJ. Effects of aerobic exercise on anxiety sensitivity. Behav Res Ther. 2004;42(2):125–136. doi: 10.1016/s0005-7967(03)00103-7. [DOI] [PubMed] [Google Scholar]
- Broman-Fulks JJ, Storey KM. Evaluation of a brief aerobic exercise intervention for high anxiety sensitivity. Anxiety Stress Coping. 2008;21(2):117–128. doi: 10.1080/10615800701762675. [DOI] [PubMed] [Google Scholar]
- Brown RA, Kahler CW, Zvolensky MJ, Lejuez CW, Ramsey SE. Anxiety sensitivity: Relationship to negative affect smoking and smoking cessation in smokers with past major depressive disorder. Addict Behav. 2001;26(6):887–899. doi: 10.1016/s0306-4603(01)00241-6. doi: http://dx.doi.org/10.1016/S0306-4603(01)00241-6. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lejuez C, Kahler CW, Strong DR. Distress tolerance and duration of past smoking cessation attempts. Journal of abnormal psychology. 2002;111(1):180. [PubMed] [Google Scholar]
- Buckner JD, Farris SG, Zvolensky MJ, Shah SM, Leventhal AM, Minnix JA, Schmidt NB. Dysphoria and smoking among treatment seeking smokers: the role of smoking-related inflexibility/avoidance. American Journal Of Drug & Alcohol Abuse. 2015;41(1):45–51. doi: 10.3109/00952990.2014.927472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P, Van Straten A, Warmerdam L. Behavioral activation treatments of depression: A meta-analysis. Clinical psychology review. 2007;27(3):318–326. doi: 10.1016/j.cpr.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Enders CK. Missing not at random models for latent growth curve analyses. Psychological Methods. 2011;16(1):1. doi: 10.1037/a0022640. [DOI] [PubMed] [Google Scholar]
- Farris SG, DiBello AM, Allan NP, Hogan J, Schmidt NB, Zvolensky MJ. Evaluation of the Anxiety Sensitivity Index-3 among treatment-seeking smokers. Psychological Assessment. 2015;27(3):1123–1128. doi: 10.1037/pas0000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz N, Curry SJ. Treating tobacco use and dependence: 2008 update U.S. Public Health Service Clinical Practice Guideline executive summary. Respir Care. 2008;53(9):1217–1222. [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: New York State Psychiatric Institute; 2002. [Google Scholar]
- Garey L, Bakhshaie J, Brandt CP, Langdon KJ, Kauffman BY, Schmidt NB, Zvolensky MJ. Interplay of dysphoria and anxiety sensitivity in relation to emotion regulatory cognitions of smoking among treatment-seeking smokers. The American Journal on Addictions. doi: 10.1111/ajad.12379. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot CR, Pang RD, Leventhal AM. Anxiety sensitivity and negative urgency: A pathway to negative reinforcement-related smoking expectancies. Journal of addiction medicine. 2014;8(3):189. doi: 10.1097/ADM.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haasova M, Warren FC, Ussher M, Janse Van Rensburg K, Faulkner G, Cropley M, Taylor AH. The acute effects of physical activity on cigarette cravings: systematic review and meta-analysis with individual participant data. Addiction. 2013;108(1):26–37. doi: 10.1111/j.1360-0443.2012.04034.x. [DOI] [PubMed] [Google Scholar]
- Hall SM, Delucchi KL, Tsoh JY, Velicer WF, Kahler CW, Moore JR, Niaura R. Statistical analysis of randomized trials in tobacco treatment: longitudinal designs with dichotomous outcome. Nicotine & Tobacco Research. 2001;3(3):193–202. doi: 10.1080/14622200110050411. [DOI] [PubMed] [Google Scholar]
- Hamer RM, Simpson PM. Last observation carried forward versus mixed models in the analysis of psychiatric clinical trials. American Journal of Psychiatry. 2009;166(6):639–641. doi: 10.1176/appi.ajp.2009.09040458. [DOI] [PubMed] [Google Scholar]
- Hayes A. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. Methodology in the social sciences. New York, NY: The Guilford Press; 2013. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K-O. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British journal of addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heck RH, Thomas SL, Tabata LN. Multilevel and longitudinal modeling with IBM SPSS. Routledge: 2013. [Google Scholar]
- Helstrom AW, Bell ME, Pineles SL. Feeling Better and Smoking Less: The Relationship Between Trauma Symptoms and Smoking Over Time. Cognitive Therapy and Research. 2009;33(2):235–240. doi: 10.1007/s10608-008-9183-0. [DOI] [Google Scholar]
- Hox JJ, Moerbeek M, van de Schoot R. Multilevel analysis: Techniques and applications. Routledge: 2010. [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine & Tobacco Research. 2003;5(1):13–25. [PubMed] [Google Scholar]
- Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. Psychological Methods. 2010;15(4):309. doi: 10.1037/a0020761. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Farris SG, Schmidt NB, Smits JA, Zvolensky MJ. Panic attack history and anxiety sensitivity in relation to cognitive-based smoking processes among treatment-seeking daily smokers. Nicotine & Tobacco Research. 2013;15(1):1–10. doi: 10.1093/ntr/ntr332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Stewart S, Rosenfield D, Steeves D, Zvolensky MJ. Prospective evaluation of the effects of anxiety sensitivity and state anxiety in predicting acute nicotine withdrawal symptoms during smoking cessation. Psychol Addict Behav. 2012;26(2):289–297. doi: 10.1037/a0024133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Zvolensky MJ. Anxiety, depression, and cigarette smoking: A transdiagnostic vulnerability framework to understanding emotion-smoking comorbidity. Psychological Bulletin. 2015 doi: 10.1037/bul0000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Zvolensky MJ, Schmidt NB. Smoking-related correlates of depressive symptom dimensions in treatment-seeking smokers. Nicotine & Tobacco Research. 2011;13(8):668–676. doi: 10.1093/ntr/ntr056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Rovine MJ, Molenaar P. Selecting a linear mixed model for longitudinal data: repeated measures analysis of variance, covariance pattern model, and growth curve approaches. Psychological Methods. 2012;17(1):15. doi: 10.1037/a0026971. [DOI] [PubMed] [Google Scholar]
- McLeish AC, Zvolensky MJ, Bucossi MM. Interaction between smoking rate and anxiety sensitivity: relation to anticipatory anxiety and panic-relevant avoidance among daily smokers. J Anxiety Disord. 2007;21(6):849–859. doi: 10.1016/j.janxdis.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Meuret AE, Rosenfield D, Seidel A, Bhaskara L, Hofmann SG. Respiratory and cognitive mediators of treatment change in panic disorder: Evidence for intervention specificity. Journal of consulting and clinical psychology. 2010;78(5):691. doi: 10.1037/a0019552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch DA, Hofmann SG, Suvak MK, In-Albon T. Mediation of changes in anxiety and depression during treatment of social phobia. Journal of consulting and clinical psychology. 2005;73(5):945. doi: 10.1037/0022-006X.73.5.945. [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Rosenfield D, Tart CD, Cottraux J, Powers MB, Smits JA. Behavioral versus cognitive treatment of obsessive-compulsive disorder: An examination of outcome and mediators of change. Journal of consulting and clinical psychology. 2013;81(3):415. doi: 10.1037/a0031865. [DOI] [PubMed] [Google Scholar]
- Otto MW, Eastman A, Lo S, Hearon BA, Bickel WK, Zvolensky M, Doan SN. Anxiety sensitivity and working memory capacity: Risk factors and targets for health behavior promotion. Clinical psychology review. 2016;49:67–78. doi: 10.1016/j.cpr.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Otto MW, Reilly-Harrington NA. The impact of treatment on anxiety sensitivity. In: Taylor S, editor. Anxiety sensitivity: Theory, research, and treatment of the fear of anxiety. Hillsdale, NJ: Lawrence Erlbaum Associates; 1999. [Google Scholar]
- Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA, Sayette MA. Differences in negative mood-induced smoking reinforcement due to distress tolerance, anxiety sensitivity, and depression history. Psychopharmacology. 2010;210(1):25–34. doi: 10.1007/s00213-010-1811-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JJ, Hall SM, Humfleet G, Muňoz RF, Reus V, Gorecki J, Hu D. Physical activity as a strategy for maintaining tobacco abstinence: A randomized trial. Preventive Medicine. 2008;47(2):215–220. doi: 10.1016/j.ypmed.2008.05.006. doi: http://dx.doi.org/10.1016/j.ypmed.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss S, McNally RJ. Expectancy model of fear-Theoretical issues in behavior therapy ***(pp. 107-121) In: Reiss S, Bootsin RR, editors. Theoretical issues in behavior therapy. San Diego, CA: Academic Press; 1985. pp. 107–121. [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behav Res Ther. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Roberts V, Maddison R, Simpson C, Bullen C, Prapavessis H. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect, and smoking behaviour: systematic review update and meta-analysis. Psychopharmacology (Berl) 2012;222(1):1–15. doi: 10.1007/s00213-012-2731-z. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Raines AM, Allan NP, Zvolensky MJ. Anxiety sensitivity risk reduction in smokers: a randomized control trial examining effects on panic. Behaviour Research and Therapy. 2016;77:138–146. doi: 10.1016/j.brat.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JA, Berry AC, Tart CD, Powers MB. The efficacy of cognitive-behavioral interventions for reducing anxiety sensitivity: a meta-analytic review. Behav Res Ther. 2008;46(9):1047–1054. doi: 10.1016/j.brat.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Smits JA, Julian K, Rosenfield D, Powers MB. Threat reappraisal as a mediator of symptom change in cognitive-behavioral treatment of anxiety disorders: A systematic review. Journal of consulting and clinical psychology. 2012;80(4):624. doi: 10.1037/a0028957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JA, Powers MB, Berry AC, Otto MW. Translating empirically supported strategies into accessible interventions: the potential utility of exercise for the treatment of panic disorder. Cognitive and Behavioral Practice. 2007;14(4):364–374. [Google Scholar]
- Smits JA, Rosenfield D, McDonald R, Telch MJ. Cognitive mechanisms of social anxiety reduction: An examination of specificity and temporality. Journal of Consulting and Clinical Psychology. 2006;74(6):1203. doi: 10.1037/0022-006X.74.6.1203. [DOI] [PubMed] [Google Scholar]
- Smits JA, Zvolensky MJ. Emotional vulnerability as a function of physical activity among individuals with panic disorder. Depression and anxiety. 2006;23(2):102–106. doi: 10.1002/da.20146. [DOI] [PubMed] [Google Scholar]
- Smits JA, Zvolensky MJ, Davis ML, Rosenfield D, Marcus BH, Church TS, Baird SO. The Efficacy of Vigorous-Intensity Exercise as an Aid to Smoking Cessation in Adults With High Anxiety Sensitivity: A Randomized Controlled Trial. Psychosom Med. 2016;78(3):354–364. doi: 10.1097/psy.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JA, Zvolensky MJ, Rosenfield D, Marcus BH, Church TS, Frierson GM, Briceno NF. The efficacy of vigorous-intensity exercise as an aid to smoking cessation in adults with elevated anxiety sensitivity: Study protocol for a randomized controlled trial. Trials. 2012;13:207. doi: 10.1186/1745-6215-13-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JAJ, Berry AC, Rosenfield D, Powers MB, Behar E, Otto MW. Reducing anxiety sensitivity with exercise. Depression and Anxiety. 2008;25(8):689–699. doi: 10.1002/da.20411. [DOI] [PubMed] [Google Scholar]
- Sobell MB, Sobell LC. Problem drinkers: Guided self-change treatment. New York, NY: Guilford Press; 1996. [Google Scholar]
- Stathopoulou G, Powers MB, Berry AC, Smits JAJ, Otto MW. Exercise interventions for mental health: A quantitative and qualitative review. Clinical Psychology: Science and Practice. 2006;13(2):179–193. doi: 10.1111/j.1468-2850.2006.00021.x. [DOI] [Google Scholar]
- Stubbs B, Vancampfort D, Rosenbaum S, Ward PB, Richards J, Soundy A, Schuch FB. Dropout from exercise randomized controlled trials among people with depression: A meta-analysis and meta regression. Journal of Affective Disorders. 2016;190:457–466. doi: 10.1016/j.jad.2015.10.019. [DOI] [PubMed] [Google Scholar]
- Taylor S, Zvolensky MJ, Cox BJ, Deacon B, Heimberg RG, Ledley DR, Cardenas SJ. Robust dimensions of anxiety sensitivity: development and initial validation of the Anxiety Sensitivity Index-3. Psychol Assess. 2007;19(2):176–188. doi: 10.1037/1040-3590.19.2.176. [DOI] [PubMed] [Google Scholar]
- Tofighi D, MacKinnon DP. RMediation: An R package for mediation analysis confidence intervals. Behavior Research Methods. 2011;43(3):692–700. doi: 10.3758/s13428-011-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofighi D, West SG, MacKinnon DP. Multilevel mediation analysis: The effects of omitted variables in the 1–1–1 model. British Journal of Mathematical and Statistical Psychology. 2013;66(2):290–307. doi: 10.1111/j.2044-8317.2012.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ussher MH, Taylor A, Faulkner G. Exercise interventions for smoking cessation. Cochrane Database of Systematic Reviews. 2008;(4):Cd002295. doi: 10.1002/14651858.CD002295.pub3. [DOI] [PubMed] [Google Scholar]
- Ussher MH, Taylor AH, Faulkner GE. Exercise interventions for smoking cessation. Cochrane Database Syst Rev. 2014;8:Cd002295. doi: 10.1002/14651858.CD002295.pub5. [DOI] [PubMed] [Google Scholar]
- Ussher MH, Taylor AH, Faulkner GE. Exercise interventions for smoking cessation. The Cochrane Library. 2014 doi: 10.1002/14651858.CD002295.pub5. [DOI] [PubMed] [Google Scholar]
- Watson D, O'Hara MW, Kotov R, Simms LJ, Chmielewski M, McDade-Montez EA, Stuart S. Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS) Psychological Assessment. 2007;19(3):253–268. doi: 10.1037/1040-3590.19.3.253. [DOI] [PubMed] [Google Scholar]
- Wong M, Krajisnik A, Truong L, Lisha NE, Trujillo M, Greenberg JB, Leventhal AM. Anxiety sensitivity as a predictor of acute subjective effects of smoking. Nicotine & Tobacco Research. 2013;15(6):1084–1090. doi: 10.1093/ntr/nts208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinbarg RE, Mohlman J, Hong NN. Dimensions of anxiety sensitivity. In: Taylor S, editor. Anxiety sensitivity: Theory, research, and treatment of the fear of anxiety. New Jersey: 1999. pp. 83–114. [Google Scholar]
- Zvolensky MJ, Bogiaizian D, Salazar PL, Farris SG, Bakhshaie J. An Anxiety Sensitivity Reduction Smoking-Cessation Program for Spanish-Speaking Smokers (Argentina) Cognitive and Behavioral Practice. 2014;21(3):350–363. doi: http://dx.doi.org/10.1016/j.cbpra.2013.10.005. [Google Scholar]
- Zvolensky MJ, Farris SG, Schmidt NB, Smits JA. The role of smoking inflexibility/avoidance in the relation between anxiety sensitivity and tobacco use and beliefs among treatment-seeking smokers. Experimental and Clinical Psychopharmacology. 2014;22(3):229–237. doi: 10.1037/a0035306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Leventhal AM. Affect and health behavior co-occurrence the emerging roles of transdiagnostic factors and fociocultural factors. Behavior Modification. 2016 doi: 10.1177/0145445515627307. 0145445515627307. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Yartz AR, Gregor K, Gonzalez A, Bernstein A. Interoceptive exposure-based cessation intervention for smokers high in anxiety sensitivity: A case series. Journal of Cognitive Psychotherapy. 2008;22(4):346–365. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.