Summary
Hepatic veno‐occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) is a potentially life‐threatening complication of haematopoietic stem cell transplant (HSCT) conditioning and chemotherapy. Defibrotide is approved for treatment of hepatic VOD/SOS with pulmonary or renal dysfunction [i.e., multi‐organ dysfunction (MOD)] after HSCT in the United States and severe VOD/SOS after HSCT in patients aged older than 1 month in the European Union. Defibrotide was available as an investigational drug by an expanded‐access treatment programme (T‐IND; NCT00628498). In the completed T‐IND, the Kaplan–Meier estimated Day +100 survival for 1000 patients with documented defibrotide treatment after HSCT was 58·9% [95% confidence interval (CI), 55·7–61·9%]. Day +100 survival was also analysed by age and MOD status, and post hoc analyses were performed to determine Day +100 survival by transplant type, timing of VOD/SOS onset (≤21 or >21 days) and timing of defibrotide treatment initiation after VOD/SOS diagnosis. Day +100 survival in paediatric patients was 67·9% (95% CI, 63·8–71·6%) and 47·1% (95% CI, 42·3–51·8%) in adults. All patient subgroups without MOD had higher Day +100 survival than those with MOD; earlier defibrotide initiation was also associated with higher Day +100 survival. The safety profile of defibrotide in the completed T‐IND study was similar to previous reports.
Keywords: defibrotide, haematopoietic stem cell transplant, multi‐organ dysfunction, veno‐occlusive disease, sinusoidal obstruction syndrome
Hepatic veno‐occlusive disease (VOD), also known as sinusoidal obstruction syndrome (SOS), is a potentially life‐threatening complication of conditioning regimens for haematopoietic stem cell transplantation (HSCT) and chemotherapy without HSCT (Bearman et al, 1992; Coppell et al, 2010; Dignan et al, 2013; Kantarjian et al, 2017). Diagnosis of VOD/SOS traditionally used the Baltimore (Jones et al, 1987) or modified Seattle criteria (McDonald et al, 1993) for both adults and paediatric patients. Both sets of criteria assess onset within the initial 20 or 21 days of HSCT (Jones et al, 1987; McDonald et al, 1993; Carreras et al, 2007; Mohty et al, 2015). However, onset after 21 days may occur in up to 20% of VOD/SOS patients (Lee et al, 1999; Carreras et al, 2007; Mohty et al, 2015; Richardson et al, 2017a; Corbacioglu et al, 2018), and the new European Society for Blood and Marrow Transplantation (EBMT) criteria for adults and children now also encompass these later‐onset cases (Mohty et al, 2016; Corbacioglu et al, 2018). Typical characteristics of VOD/SOS in adults include weight gain, painful hepatomegaly, ascites and hyperbilirubinaemia (≥34·2 μmol/l) (Mohty et al, 2016). Characteristics in paediatric patients may also include refractory thrombocytopenia and the recognition that hyperbilirubinaemia may not be present (Corbacioglu et al, 2018). The overall mean incidence of VOD/SOS was reported to be 14% in a pooled analysis of 135 studies (Coppell et al, 2010). In the setting of reduced‐intensity conditioning, one study reported an 8·8% incidence of VOD/SOS (Tsirigotis et al, 2014).
VOD/SOS may progress along a pathophysiological cascade that involves injury to hepatocytes, stellate cells and the sinusoidal endothelium, often in zone 3 of the hepatic acinus, leading to extravasation of blood into the space of Disse and extra‐luminal compression of the sinusoids. The prothrombotic state resulting from endothelial injury further promotes platelet activation, leading to fibrin deposition, augmented fibrogenesis and hepatic ischaemia. Portal hypertension is a key lesion in VOD/SOS, leading to ascites, volume overload with increased sodium avidity, and injury to pulmonary or renal tissues. Presence of multi‐organ dysfunction (MOD), also referred to as multi‐organ failure (Carreras, 2012 2012), in patients with VOD/SOS after HSCT is associated with greater than 80% mortality at Day +100 in untreated patients receiving only supportive care (Coppell et al, 2010).
Defibrotide is a complex mixture of oligodeoxyribonucleotides derived from porcine mucosal intestinal DNA, and its mechanism of action has been investigated in preclinical studies. Preclinical treatment with defibrotide has been shown to reduce endothelial cell activation; increase expression of thrombomodulin and tissue plasmin activator; decrease expression of von Willebrand factor and plasminogen activator inhibitor‐1; reduce activation of nuclear factor‐kappaB and platelets; protect endothelial cells from further damage (Bianchi et al, 1993; Palmer & Goa, 1993; Pescador et al, 2013; Richardson et al, 2013; Palomo et al, 2016); support endothelial‐cell function and angiogenesis (Benimetskaya et al, 2008); and enhance fibrinolytic activity via plasmin activity (Echart et al, 2009). Defibrotide is approved in the European Union (EU) for the treatment of patients >1 month of age who received HSCT and developed severe hepatic VOD/SOS (http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002393/WC500153150.pdf). In its recent position statement on VOD/SOS, which includes discussion of treatment options, the EBMT notes that defibrotide is the “only proven” treatment of VOD/SOS (Mohty et al, 2015). It is approved in the United States (US) for the treatment of paediatric and adult patients who received HSCT and developed hepatic VOD/SOS with concomitant renal or pulmonary dysfunction (https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208114Orig1s000Lbl.pdf). Before approval by the US Food and Drug Administration in 2016, defibrotide was available in the US through an expanded‐access treatment protocol of an investigational new drug (T‐IND; NCT00628498) (Richardson et al, 2017a). Presented here are the efficacy and safety results of the completed T‐IND study, which is the largest prospective study of defibrotide in patients with VOD/SOS.
Methods
This open‐label, single‐arm T‐IND study was designed to provide expanded access to defibrotide and assess safety and survival in patients with hepatic VOD/SOS, with or without MOD, post‐HSCT or post‐non‐transplant associated chemotherapy alone, treated with defibrotide. The primary efficacy measure was survival at Day +100 after HSCT or initiation of primary chemotherapy. Safety endpoints included treatment‐emergent adverse events (TEAEs) and treatment‐related adverse events (TRAEs).
Patient eligibility criteria
Inclusion criteria for the T‐IND were originally modelled on the defibrotide phase 3 trial (Richardson et al, 2016), which was recruiting at the time, to provide access to defibrotide for those patients not at centres participating in the phase 3 study or who had later onset VOD/SOS with MOD, and therefore were not eligible for the phase 3 study. Inclusion in the T‐IND required VOD/SOS, diagnosed by the Baltimore criteria (Jones et al, 1987) by Day +35 post‐HSCT or by a biopsy, as well as MOD (renal or pulmonary dysfunction) by Day +45 post‐HSCT (Richardson et al, 2017a). Renal dysfunction was defined as serum creatinine ≥3× baseline value, creatinine clearance or glomerular filtration rate declined to ≤40% of baseline, or dialysis dependence due to VOD/SOS. Pulmonary dysfunction was defined as oxygen saturation ≤90% on room air, requirement for supplemental oxygen to maintain oxygen saturation >90%, or ventilator dependence not due to infection. When accrual for the phase 3 study ended, the entry criteria for the T‐IND were expanded to encompass a broader population of patients, including those with VOD/SOS diagnosed by modified Seattle criteria (McDonald et al, 1993), onset of VOD/SOS after Day +35, VOD/SOS after non‐transplant associated chemotherapy, and VOD/SOS patients without MOD.
Exclusion criteria included receipt of medications with high risk of haemorrhagic complications, patients with uncontrolled acute bleeding or haemodynamic instability (need for ≥2 vasopressors or inability to maintain mean arterial pressure with a single vasopressor), or pregnancy.
Treatment
Defibrotide treatment (25 mg/kg/day: 4 divided doses of 6·25 mg/kg, 2‐h infusion) was recommended to be given for ≥21 days. Dosing was based on patient's baseline body weight prior to HSCT conditioning: doses were rounded to the nearest 10 mg for adults and paediatric patients >35 kg and to the nearest 5 mg for paediatric patients <35 kg (https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208114Orig1s000Lbl.pdf). The following blood parameters were monitored and maintained during treatment: haemoglobin >80 g/l, platelet count >30 × 109/l, international normalised ratio (INR) <1·5 with fresh frozen plasma, and fibrinogen >1·5 g/l, with factor replacement as necessary. Per protocol, treatment was recommended to be discontinued or withheld in the following situations: clinically significant bleeding defined as >15 ml/kg packed red blood cells/24 h or bleeding from a potentially life‐threatening site (pulmonary haemorrhage or central nervous system bleeding) irrespective of amount of blood loss. The cause of bleeding was to be treated, and consideration given to resuming treatment at the same dose and infusion volume after bleeding had stopped and the patient was haemodynamically stable. If severe significant bleeding recurred, defibrotide was to be permanently discontinued.
Biostatistics
The planned analyses included Day +100 survival by Kaplan–Meier estimate for all post‐HSCT patients receiving ≥1 documented dose of defibrotide as well as stratified by age (≤16/>16 years) and MOD status. Post hoc analyses of post‐HSCT subgroups for Day +100 survival included transplant type (allogeneic transplant versus autologous transplant) and time to VOD/SOS onset (≤21 days vs. >21 days [late onset]). TEAEs were summarised.
An exploratory post hoc analysis was conducted to assess the effect of timing of defibrotide initiation post‐diagnosis of VOD/SOS on Kaplan–Meier estimate of Day +100 survival using two methods. The first method compared patients starting defibrotide before versus after each of the following days: 1, 2, 3, 4, 7 and 14 post‐diagnosis by Fisher's exact test for each cut‐off day. For the second method, subsets of patients starting defibrotide on a particular day or period only (0, 1, 2, 3, 4, 5, 6, 7, 8–14 and ≥15 post‐diagnosis) were analysed by Cochran–Armitage trend test across days. Causes of delay were not assessed.
Results
The T‐IND included a total of 1154 patients enrolled at 101 centres across the US from 2007 to 2016 who received at least 1 dose of defibrotide post‐HSCT or post‐non‐transplant associated chemotherapy (safety population). Among the 1149 patients from the safety population with available duration of treatment data, the mean (standard deviation) and median length of defibrotide treatment was 21·5 (13·51) and 21·0 days, respectively. Of those enrolled, 1137 met criteria for VOD/SOS (VOD/SOS efficacy population). The Kaplan–Meier estimated Day +100 survival for the efficacy population was 61·1% [95% confidence interval (CI), 58·2–63·9%] and for the 571 patients with MOD was 51·9% (95% CI, 47·6–55·9%). TRAEs were reported in 21·5% of the total population (N = 1154). The most common TRAEs were pulmonary haemorrhage (4·3%), gastrointestinal haemorrhage (3·0%), epistaxis (2·3%) and hypotension (2·1%). Serious TRAEs were reported in 11·5% of patients. TRAEs led to discontinuation or death in 12·0% and 2·7% of patients, respectively. The detailed analysis of the non‐transplant associated VOD/SOS subgroup is to be reported separately. All subsequent results reported here are from the analysis of the patient population diagnosed with VOD following HSCT (post‐HSCT population) (n = 1000).
Demographics of HSCT patients
Baseline characteristics for the treated HSCT patients (n = 1000) are shown in Table 1 and included 570 paediatric patients (≤16 years), of whom 28·2% were infants (aged 0–23 months, 161/570), 52·5% were children (aged 2–11 years, 299/570), and 19·3% were adolescents (aged 12–16 years, 110/570). Most of the HSCT patients who developed VOD/SOS received allogeneic transplants (84·3%; median age, 16 years), and the most common primary diseases in this group were acute myeloid leukaemia (260/843; 30·8%) and acute lymphocytic leukaemia (201/843; 23·8%). About half of patients with allografts (441/843; 52·3%) were paediatric. Most patients receiving autologous transplants were paediatric patients (127/155; 81·9%; median age, 4 years), 81·1% (103/127) of whom had a primary diagnosis of neuroblastoma. Neuroblastoma was the primary disease of 66·5% (103/155) of all autograft recipients regardless of age. Other underlying diseases are listed in Table SI. Late‐onset VOD/SOS occurred in both adults (169/430; 39·3% of all adult patients) and children (95/570; 16·7%), and 54·5% of all late‐onset VOD patients (144/264) had acute leukaemia (Table SII). Transplant types were similar for the groups with late onset and ≤21‐day onset (allogeneic: 86·0% and 83·7%; autologous: 14·0% and 16·0%, respectively). The most common graft‐versus‐host disease (GVHD) prophylaxis in the late‐onset group were regimens containing tacrolimus (61·7%), methotrexate (31·4%), ciclosporin (16·3%), and sirolimus (14·8%). Diagnosis of VOD/SOS after Day +100 occurred in 1·1% of patients, and the median day of late‐onset VOD/SOS was 29 (range, 22–335).
Table 1.
Baseline demographics and disease characteristics of the HSCT population
| Characteristic | MOD | No MOD | All HSCT | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall (n = 512) | >16 years (n = 231) | ≤16 years (n = 281) | Overall (n = 488) | >16 years (n = 199) | ≤16 years (n = 289) | Overall (n = 1000) | >16 years (n = 430) | ≤16 years (n = 570) | |
| Sex, n (%) | |||||||||
| Male | 278 (54·3) | 119 (51·5) | 159 (56·6) | 290 (59·4) | 118 (59·3) | 172 (59·5) | 568 (56·8) | 237 (55·1) | 331 (58·1) |
| Female | 234 (45·7) | 112 (48·5) | 122 (43·4) | 198 (40·6) | 81 (40·7) | 117 (40·5) | 432 (43·2) | 193 (44·9) | 239 (41·9) |
| Race, n (%) | |||||||||
| White | 343 (67·0) | 175 (75·8) | 168 (59·8) | 284 (58·2) | 135 (67·8) | 149 (51·6) | 627 (62·7) | 310 (72·1) | 317 (55·6) |
| African American | 36 (7·0) | 13 (5·6) | 23 (8·2) | 44 (9·0) | 16 (8·0) | 28 (9·7) | 80 (8·0) | 29 (6·7) | 51 (8·9) |
| Asian | 20 (3·9) | 9 (3·9) | 11 (3·9) | 34 (7·0) | 13 (6·5) | 21 (7·3) | 54 (5·4) | 22 (5·1) | 32 (5·6) |
| Latino | 71 (13·9) | 23 (10·0) | 48 (17·1) | 92 (18·9) | 27 (13·6) | 65 (22·5) | 163 (16·3) | 50 (11·6) | 113 (19·8) |
| Age at time of HSCT, years, median (range) | 14·00 (0·10, 69·00) | 41·00 (17·00, 69·00) | 4·00 (0·10, 16·00) | 12·00 (0·10, 77·00) | 43·00 (17·00, 77·00) | 3·00 (0·10, 16·00) | 14·00 (0·10, 77·00) | 42·00 (17·00, 77·00) | 4·00 (0·10, 16·00) |
| Conditioning agents (>10%), n (%) | |||||||||
| Cyclophosphamide | 333 (65·0) | 154 (66·7) | 179 (63·7) | 241 (49·4) | 93 (46·7) | 148 (51·2) | 574 (57·4) | 247 (57·4) | 327 (57·4) |
| Busulfan | 262 (51·2) | 100 (43·3) | 162 (57·7) | 301 (61·7) | 101 (50·8) | 200 (69·2) | 563 (56·3) | 201 (46·7) | 362 (63·5) |
| Fludarabine | 165 (32·2) | 88 (38·1) | 77 (27·4) | 177 (36·3) | 97 (48·7) | 80 (27·7) | 342 (34·2) | 185 (43·0) | 157 (27·5) |
| Anti‐thymocyte globulin | 154 (30·1) | 35 (15·2) | 119 (42·3) | 152 (31·1) | 41 (20·6) | 111 (38·4) | 306 (30·6) | 76 (17·7) | 230 (40·4) |
| Total body irradiation | 172 (33·6) | 104 (45·0) | 68 (24·2) | 115 (23·6) | 66 (33·2) | 49 (17·0) | 287 (28·7) | 170 (39·5) | 117 (20·5) |
| Melphalan | 117 (22·9) | 30 (13·0) | 87 (31·0) | 142 (29·1) | 38 (19·1) | 104 (36·0) | 259 (25·9) | 68 (15·8) | 191 (33·5) |
| Thiotepa | 64 (12·5) | 26 (11·3) | 38 (13·5) | 70 (14·3) | 20 (10·1) | 50 (17·3) | 134 (13·4) | 46 (10·7) | 88 (15·4) |
| GVHD prophylaxis, n (%) | |||||||||
| Tacrolimus | 250 (48·8) | 162 (70·1) | 88 (31·3) | 233 (47·7) | 137 (68·8) | 96 (33·2) | 483 (48·3) | 299 (69·5) | 184 (32·3) |
| Methotrexate | 168 (32·8) | 101 (43·7) | 67 (23·8) | 164 (33·6) | 82 (41·2) | 82 (28·4) | 332 (33·2) | 183 (42·6) | 149 (26·1) |
| Ciclosporin | 159 (31·1) | 38 (16·5) | 121 (43·1) | 123 (25·2) | 26 (13·1) | 97 (33·6) | 282 (28·2) | 64 (14·9) | 218 (38·2) |
| Sirolimus | 59 (11·5) | 47 (20·3) | 12 (4·3) | 30 (6·1) | 26 (13·1) | 4 (1·4) | 89 (8·9) | 73 (17·0) | 16 (2·8) |
| None | 73 (14·3) | 14 (6·1) | 59 (21·0) | 108 (22·1) | 24 (12·1) | 84 (29·1) | 181 (18·1) | 38 (8·8) | 143 (25·1) |
| Othera | 182 (35·5) | 74 (32·0) | 108 (38·4) | 160 (32·8) | 66 (33·2) | 94 (32·5) | 342 (34·2) | 140 (32·6) | 202 (35·4) |
| Transplant type,b n (%) | |||||||||
| Allogeneic | 450 (87·9) | 220 (95·2) | 230 (81·9) | 393 (80·5) | 182 (91·5) | 211 (73·0) | 843 (84·3) | 402 (93·5) | 441 (77·4) |
| Autologous | 61 (11·9) | 11 (4·8) | 50 (17·8) | 94 (19·3) | 17 (8·5) | 77 (26·6) | 155 (15·5) | 28 (6·5) | 127 (22·3) |
| Primary disease (>10%), n (%) | |||||||||
| AML | 142 (27·7) | 88 (38·1) | 54 (19·2) | 119 (24·4) | 61 (30·7) | 58 (20·1) | 261 (26·1) | 149 (34·7) | 112 (19·6) |
| ALL | 111 (21·7) | 48 (20·8) | 63 (22·4) | 90 (18·4) | 49 (24·6) | 41 (14·2) | 201 (20·1) | 97 (22·6) | 104 (18·2) |
| Neuroblastoma | 43 (8·4) | 1 (0·4) | 42 (14·9) | 62 (12·7) | 0 (0) | 62 (21·5) | 105 (10·5) | 1 (0·2) | 104 (18·2) |
| Prior HSCTs, n (%) | |||||||||
| 0 | 428 (83·6) | 189 (81·8) | 239 (85·1) | 401 (82·2) | 162 (81·4) | 239 (82·7) | 829 (82·9) | 351 (81·6) | 478 (83·9) |
| ≥1 | 82 (16·0) | 41 (17·7) | 41 (14·6) | 83 (17·0) | 35 (17·6) | 48 (16·6) | 165 (16·5) | 76 (17·7) | 89 (15·6) |
| Unknown/missing | 2 (0·4) | 1 (0·4) | 1 (0·4) | 4 (0·8) | 2 (1·0) | 2 (0·7) | 6 (0·6) | 3 (0·7) | 3 (0·5) |
| VOD/SOS onset after Day 21, n (%) | 139 (27·1) | 88 (38·1) | 51 (18·1) | 125 (25·6) | 81 (40·7) | 44 (15·2) | 264 (26·4) | 169 (39·3) | 95 (16·7) |
| Time from diagnosis to start of defibrotide, n (%) | |||||||||
| Day of diagnosis | 156 (30·4) | 68 (29·4) | 88 (31·3) | 154 (31·6) | 52 (26·1) | 102 (35·3) | 310 (31·0) | 120 (27·9) | 190 (33·3) |
| Day 1 | 135 (26·4) | 63 (27·3) | 72 (25·6) | 151 (30·9) | 49 (24·6) | 102 (35·3) | 286 (28·6) | 112 (26·0) | 174 (30·5) |
| Day 2 | 68 (13·3) | 27 (11·7) | 41 (14·6) | 57 (11·7) | 26 (13·1) | 31 (10·7) | 125 (12·5) | 53 (12·3) | 72 (12·6) |
| Day 3 | 39 (7·6) | 16 (6·9) | 23 (8·2) | 38 (7·8) | 20 (10·1) | 18 (6·2) | 77 (7·7) | 36 (8·4) | 41 (7·2) |
| Day 4 | 33 (6·4) | 15 (6·5) | 18 (6·4) | 28 (5·7) | 12 (6·0) | 16 (5·5) | 61 (6·1) | 27 (6·3) | 34 (6·0) |
| Day 5 | 17 (3·3) | 10 (4·3) | 7 (2·5) | 19 (3·9) | 12 (6·0) | 7 (2·4) | 36 (3·6) | 22 (5·1) | 14 (2·5) |
| Day 6 | 9 (1·8) | 6 (2·6) | 3 (1·1) | 6 (1·2) | 3 (1·5) | 3 (1·0) | 15 (1·5) | 9 (2·1) | 6 (1·1) |
| Day 7 | 12 (2·3) | 5 (2·2) | 7 (2·5) | 7 (1·4) | 4 (2·0) | 3 (1·0) | 19 (1·9) | 9 (2·1) | 10 (1·8) |
| Days 8–14 | 29 (5·7) | 15 (6·5) | 14 (5·0) | 16 (3·3) | 11 (5·5) | 5 (1·7) | 45 (4·5) | 29 (6·7) | 19 (3·3) |
| Day ≥15 | 14 (2·7) | 6 (2·6) | 8 (2·8) | 12 (2·5) | 10 (5·0) | 2 (0·7) | 26 (2·6) | 16 (3·7) | 10 (1·8) |
ALL, acute lymphocytic leukaemia; AML, acute myelogenous leukaemia; GVHD, graft‐versus‐host disease; HSCT, haematopoietic stem cell transplant; MOD, multi‐organ dysfunction; VOD/SOS, veno‐occlusive disease/sinusoidal obstruction syndrome.
Includes medications that were not prespecified on the case report form.
Transplant type unknown for two patients.
Efficacy
Kaplan–Meier estimated Day +100 survival of the 1000 patients who had VOD/SOS after HSCT was 58·9% (95% CI, 55·7–61·9%; Fig 1A). In patients with MOD (n = 512), Kaplan–Meier estimated Day +100 survival was lower, at 49·5% (95% CI, 45·0–53·8%), compared with 68·9% (95% CI, 64·5–72·9%) in patients without MOD (n = 488). The Kaplan–Meier estimated Day +100 survival in paediatric patients was 67·9% (95% CI, 63·8–71·6%) and 47·1% (95% CI, 42·3–51·8%) in adults (Fig 1B).
Figure 1.
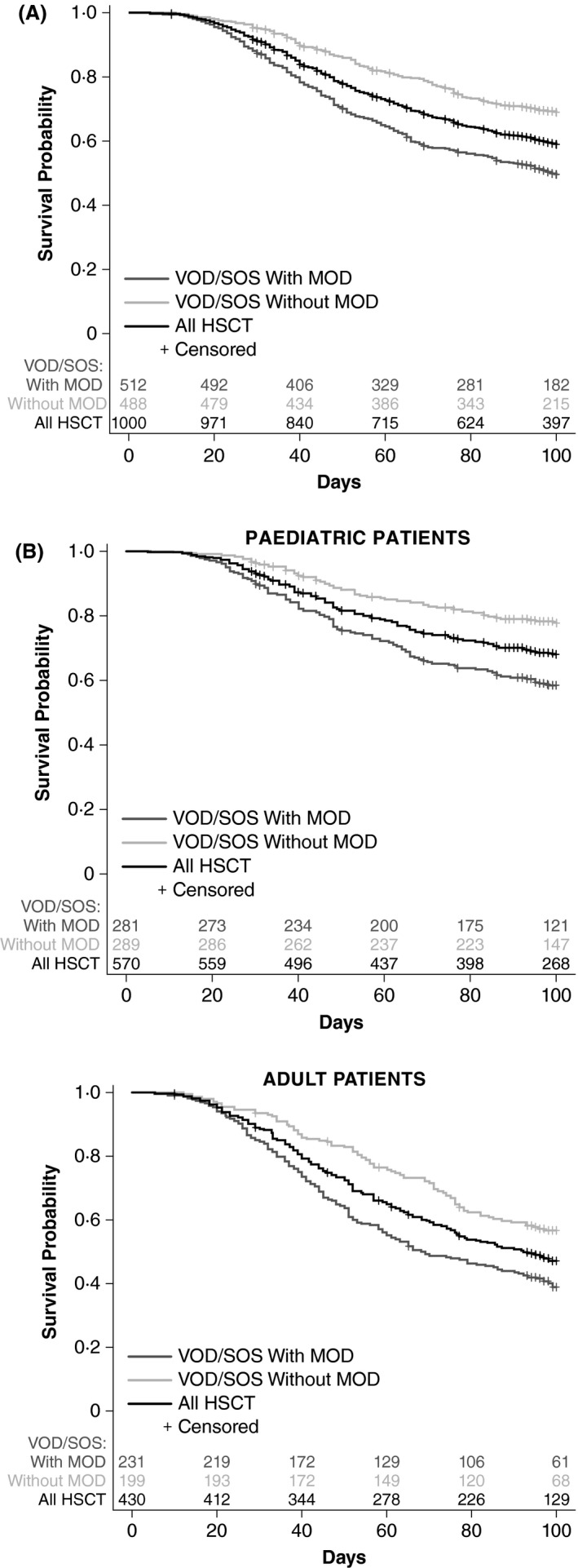
Kaplan–Meier estimated survival to Day +100 by MOD status (panel A) and age and MOD‐status subgroup (panel B), HSCT population. HSCT, haematopoietic stem cell transplant; MOD, multi‐organ dysfunction; VOD/SOS, veno‐occlusive disease/sinusoidal obstruction syndrome.
For the entire post‐HSCT population, a post hoc trend analysis examining the relationship between Day +100 survival and time to start of defibrotide treatment after VOD/SOS diagnosis found that earlier initiation of defibrotide was associated with higher Day +100 survival (P < 0·001, Cochran–Armitage test for trend; Fig 2). The analysis also shows that earlier treatment resulted in improved survival in paediatric patients (P < 0·001) and may suggest a similar relationship in adults (P = 0·055). Another analysis by cut‐off day (Fisher's exact test) showed that earlier treatment initiation compared with later initiation after VOD/SOS diagnosis was associated with significantly higher Day +100 survival (P ≤ 0·001) at all time points except Day 14 post‐diagnosis (2·6% of patients started after Day 14 post‐diagnosis). Individual cut‐off days were significant (P ≤ 0·003) for all days except Day 14 post‐diagnosis in the MOD subgroup, and in the subgroup without MOD (P < 0·041) except for Day 2 post‐diagnosis. Reasons for treatment delay were not assessed.
Figure 2.
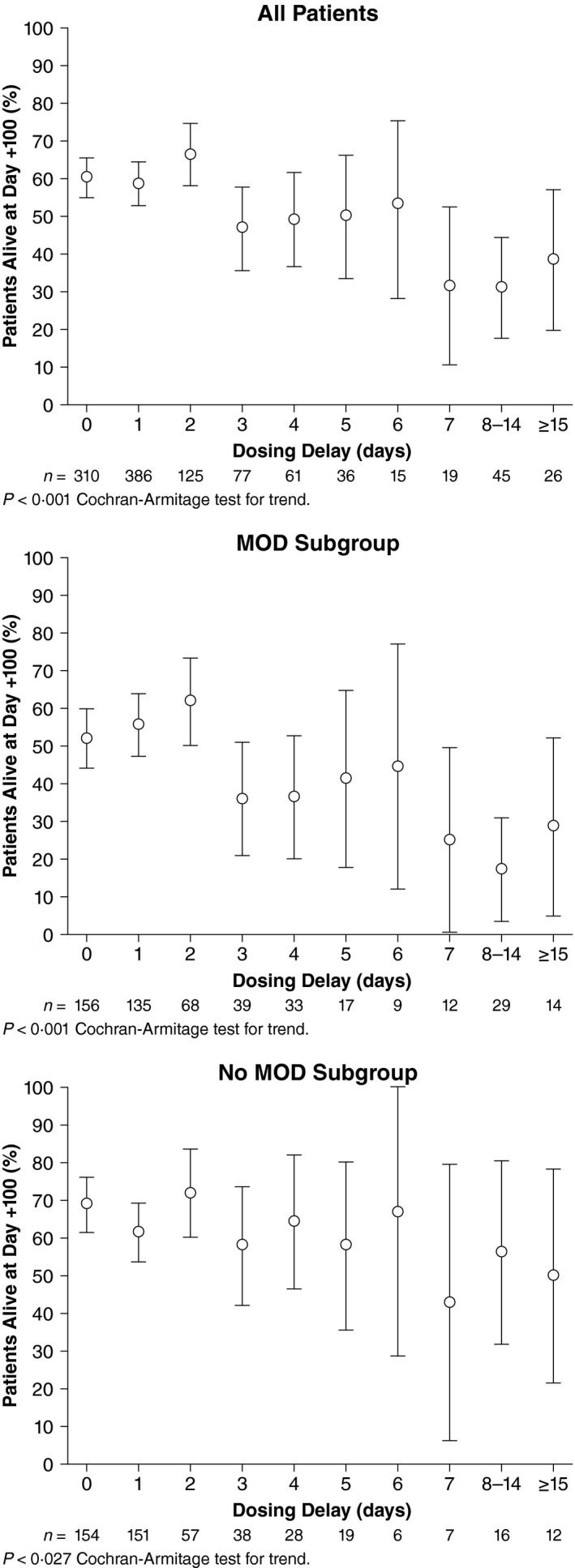
Timing of initiation of defibrotide after VOD/SOS diagnosis. MOD, multi‐organ dysfunction; VOD/SOS, veno‐occlusive disease/sinusoidal obstruction syndrome.
Regardless of graft source, patients without MOD had higher survival than patients with MOD. Paediatric patients with autologous grafts and VOD/SOS, regardless of MOD status, had higher survival than those with allogeneic grafts. The pattern was generally similar for adult patients; however, only 7·0% received autologous grafts (Fig 3).
Figure 3.
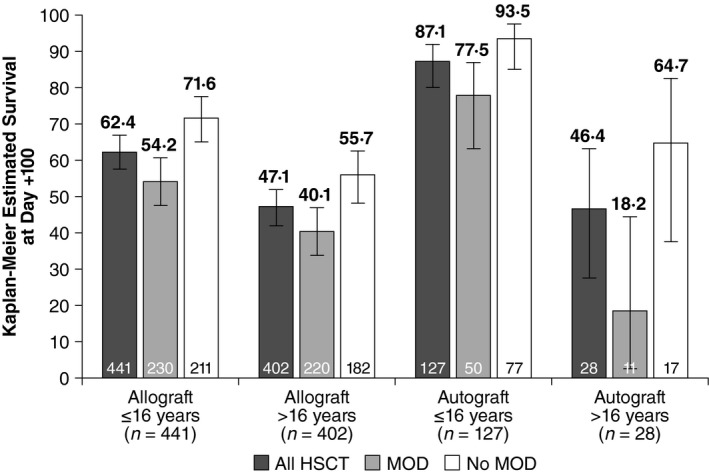
Kaplan–Meier estimated Day +100 survival by transplant type. HSCT, haematopoietic stem cell transplant; MOD, multi‐organ dysfunction.
Paediatric patients with late‐onset VOD/SOS had a Kaplan–Meier estimated Day +100 survival of 60·4% (95% CI, 49·5–69·7%), and adults, 48·7% (95% CI, 40·9–56·0%) (Fig 4). In patients with late‐onset VOD/SOS and MOD, Kaplan–Meier estimated survival in the paediatric subgroup was 45·4% (95% CI, 31·0–58·6%) and 43·0% (95% CI, 32·5–53·0%) in adults, while estimated survival in patients with MOD was 78·4% (95% CI, 62·5–88·1%) in paediatric patients and 55·1% (95% CI, 43·5–65·2%) in adults. Paediatric patients who were diagnosed with late‐onset VOD/SOS with MOD had numerically lower Day +100 survival (45·4%; 95% CI, 31·0–58·6%) than paediatric patients diagnosed in ≤21 days (60·9%; 95% CI, 54·2–66·9%); however, similar Kaplan–Meier estimated Day +100 survival was observed in both onset subgroups of adults (Fig 4).
Figure 4.
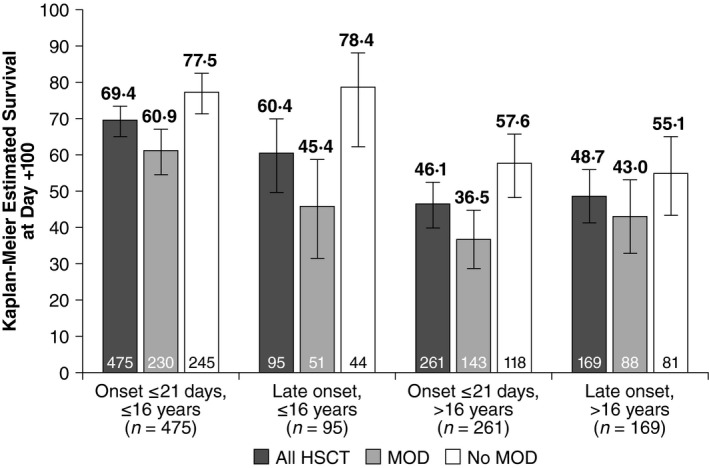
Comparison of Kaplan–Meier estimated Day +100 survival by time of VOD/SOS onset post‐HSCT. HSCT, haematopoietic stem cell transplant; MOD, multi‐organ dysfunction; VOD/SOS, veno‐occlusive disease/sinusoidal obstruction syndrome.
Treatment with sirolimus has been associated with development of VOD/SOS (Cutler et al, 2008), and among patients receiving sirolimus at baseline (n = 90; primarily adults), 66·7% (60/90) had MOD, compared with 49·7% (452/910) in patients who did not receive sirolimus (majority paediatric). A post hoc analysis showed that Day +100 survival was 53·8% (95% CI, 48·2–63·6%), compared with 59·4% (95% CI, 56·0–62·5%) for patients not receiving sirolimus. Per protocol, concomitant use of sirolimus was not recommended.
Safety profile
Age, MOD status, and transplant type were associated with some apparent variations in safety profiles. Adult patients had higher rates of TEAEs than paediatric patients, while patients with MOD had higher rates than patients without MOD (Table 2). Two hundred and ten patients (21%) had ≥1 TEAE as assessed by investigators as being at least possibly related to treatment (TRAE). Similar rates of TRAEs were observed in the late‐onset (20·8%) and ≤21‐days onset (21·1%) subgroups. TRAEs led to discontinuation in 124 patients (12·4%) and death in 28 patients (2·8%). TRAEs that occurred in ≥2% of patients were pulmonary haemorrhage (4·6%), gastrointestinal haemorrhage (3·0%), epistaxis (2·3%) and hypotension (2·0%). The incidence of TRAEs appeared to be generally similar in paediatric and adult patients, although the incidence of specific TRAEs varied by age. TRAEs that occurred in ≥2% of adults were epistaxis (3·5%), gastrointestinal haemorrhage (3·5%), pulmonary haemorrhage (2·3%), haematuria (2.1%) and hypotension (2·1%). TRAEs that occurred in ≥2% of paediatric patients were pulmonary haemorrhage (6·3%) and gastrointestinal haemorrhage (2·6%). Epistaxis and gastrointestinal haemorrhage appeared to be more frequent in adult patients than in paediatric patients (epistaxis: 1·4% in paediatric patients). Pulmonary haemorrhage and lower gastrointestinal haemorrhage appeared to be more frequent in paediatric patients than adult patients (lower gastrointestinal haemorrhage: 1·1% in paediatric patients and 0.5% in adults). By transplant type, TRAEs occurred in 21·2% of patients who received an allogeneic HSCT and 20·0% of those who received an autologous HSCT. The most common TRAEs (>2%) in either graft subgroup were pulmonary haemorrhage (allogeneic 4·9%; autologous 3·2%), gastrointestinal haemorrhage (allogeneic 3·3%; autologous 1·3%), epistaxis (allogeneic 2·4%; autologous 1·9%) and hypotension (allogeneic 1·9%; autologous 2·6%). The safety profile of defibrotide in the completed T‐IND study was similar to previous reports.
Table 2.
Safety profile of total HSCT population
| TEAE, n (%) | MOD | No MOD | All HSCT | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall (n = 512) | >16 years (n = 231) | ≤16 years (n = 281) | Overall (n = 488) | >16 years (n = 199) | ≤16 years (n = 289) | Overall (n = 1000) | >16 years (n = 430) | ≤16 years (n = 570) | |
| ≥1 TEAE | 385 (75·2) | 187 (81·0) | 198 (70·5) | 324 (66·4) | 148 (74·4) | 176 (60·9) | 709 (70·9) | 335 (77·9) | 374 (65·6) |
| ≥1 Serious TEAE | 310 (60·5) | 151 (65·4) | 159 (56·6) | 227 (46·5) | 105 (52·8) | 122 (42·2) | 537 (53·7) | 256 (59·5) | 281 (49·3) |
| ≥1 Treatment‐related TEAE | 118 (23·0) | 50 (21·6) | 68 (24·2) | 92 (18·9) | 38 (19·1) | 54 (18·7) | 210 (21·0) | 88 (20·5) | 122 (21·4) |
| ≥1 Haemorrhage | 166 (32·4) | 77 (33·3) | 89 (31·7) | 124 (25·4) | 55 (27·6) | 69 (23·9) | 290 (29·0) | 132 (30·7) | 158 (27·7) |
| ≥1 Hypotension | 78 (15·2) | 47 (20·3) | 31 (11·0) | 42 (8·6) | 23 (11·6) | 19 (6·6) | 120 (12·0) | 70 (16·3) | 50 (8·8) |
HSCT, haematopoietic stem cell transplant; MOD, multi‐organ dysfunction; TEAE, treatment‐emergent adverse event.
Discussion
The findings of the completed T‐IND in the 1000 patients who received HSCT, all of whom received defibrotide, showed an overall Kaplan–Meier estimated Day +100 survival of 58·9%. Day +100 survival was 67·9% in paediatric patients and 47·1% in adults and, consistent with prior reports (Richardson et al, 2010, 2017a; Corbacioglu et al, 2016; Strouse et al, 2016), was higher in patients without MOD (68·9%) than in patients with MOD (49·5%). Post hoc analyses suggest that time of VOD/SOS onset and timing of initiation of defibrotide treatment after diagnosis affected patient outcomes.
The HSCT population of the final report (n = 1000) is substantially larger than for the interim analysis (n = 573, data up to 5 December 2014) (Richardson et al, 2017a), and the findings were consistent. Importantly, the 427 additional patients were enrolled between 31 December 2013, and study closure on 4 April 2016, and thus represent a near‐contemporary clinical population. The Kaplan–Meier estimates of Day +100 survival for post‐HSCT patients of the interim and completed T‐IND analyses were 53·8% (Richardson et al, 2017a) and 58·9%, respectively. The higher proportion of patients without MOD in the completed T‐IND (49%) than in the interim report (39%) (Richardson et al, 2017a), presumably due to change in enrolment criteria, may have contributed to the somewhat higher survival in the completed study. The patient population at the interim and completed time points were similar in age (age ≤16: 56% vs. 57%) and experienced similar rates and types of TRAEs (Richardson et al, 2017a).
The T‐IND results in patients with and without MOD who were treated with defibrotide are consistent with other reports, including the Compassionate Use Program (CUP) results (Corbacioglu et al, 2016). The CUP programme collected data on patients with VOD/SOS with or without MOD who were treated with defibrotide (median dose, 25 mg/kg/day; range, 10–80 mg/kg/day); 89% of patients had received HSCT. The overall Day +100 survival was 54% and was 58% in patients who received defibrotide 25 mg/kg/day, which is a result similar to that in the T‐IND HSCT patients. The patients without MOD from the CUP study had a survival at Day +100 of 65% (Corbacioglu et al, 2016), comparable to the 68·9% survival of the T‐IND subgroup without MOD. Further, the T‐IND also included detailed results by age group as well as post hoc subgroup analyses by transplant type and time to onset of VOS/SOS, in addition to analysing timing of initiation of defibrotide after VOD/SOS diagnosis. The similar overall findings in the present report build on those of the CUP, because the T‐IND included a formal protocol and one dose level of defibrotide with mandatory reporting of safety and survival, while reporting in the CUP was voluntary and data were provided for 710 of the 1129 patients provided with defibrotide at a range of suggested doses (Corbacioglu et al, 2016).
Among patients with MOD, the CUP study reported a Day +100 survival of 39.7% (Corbacioglu et al, 2016), which is similar to that reported for the treatment group in the phase 3 historically controlled trial (38·2%) (Richardson et al, 2016). In the phase 2 dose‐finding study of defibrotide, Day +100 survival was 46% in the 25 mg/kg/day arm and 42% in the 40 mg/kg/day arm (Richardson et al, 2010). In addition, a retrospective analysis of the Center for International Blood & Marrow Transplant Research (CIBMTR) database of patients with VOD/SOS and MOD who were treated with defibrotide reported a Day +100 survival of 39% (Strouse et al, 2016). Overall, the results from these other studies are generally supportive of the 49·5% Day +100 survival in the MOD subgroup of this T‐IND study.
The phase 3 trial and the CIBMTR registry analysis also included Day +100 survival results for patients with MOD who did not receive defibrotide and reported survival of 25% and 30·9%, respectively (Richardson et al, 2016; Strouse et al, 2016). In addition, a Japanese registry reported survival in a cohort (84% aged ≥16 years) treated with plasma, ursodiol, antithrombin III and/or heparin (>30% each), but only 5% received defibrotide (Yakushijin et al, 2016). In that analysis, the Day +100 survival was 15% in patients with VOD/SOS and MOD and 44% in patients without MOD (Yakushijin et al, 2016). Similarly, the CIBMTR analysis of VOD/SOS with MOD included patients not receiving defibrotide and found survival of 27·3% in adults and 45·5% in paediatric patients (Strouse et al, 2016). Of note, untreated patients in the CIBMTR analysis had somewhat lower rates of organ dysfunction than treated patients (pulmonary: 43·6% vs. 53·7%; renal: 67·3% vs. 70·7%) (Strouse et al, 2016).
More than 30% of patients were treated on the day of VOD/SOS diagnosis (more than 70% within the first 2 days), and earlier initiation of defibrotide treatment after diagnosis yielded higher Day +100 survival by cut‐off days and for the trend test, which is similar to the T‐IND interim results (Richardson et al, 2017b). Per protocol, treatment was to begin as soon as the patient met eligibly requirements; the causes of delay in treatment initiation were not assessed. These findings suggest that defibrotide initiation should not be delayed once VOD/SOS has been diagnosed, which is consistent with guidelines from the British Committee for Standards in Haematology/British Society for Blood and Marrow Transplantation and the EBMT, which emphasise prompt initiation of treatment “at a stage when the disease is in a more favourable response state” (Dignan et al, 2013; Mohty et al, 2016). Although these results suggest that it may be beneficial to initiate treatment as early as possible in patients meeting EBMT diagnostic criteria, irrespective of MOD status, rather than waiting for further development of the disease to MOD, the absence of a control limits interpretation of these data.
By transplant type, the survival in recipients of autologous transplants probably reflects the younger age of patients in this group (82% age ≤16 years) and diagnosis of a solid tumour (80% neuroblastoma, a disease of very young children) compared with allogeneic transplants (52% age ≤16 years), with primary diagnoses of acute leukaemia (54%). A single institution retrospective report showed very high responses to high‐dose methylprednisolone in 9 paediatric patients with VOD/SOS (5 allogeneic and 4 autologous transplants), 4 of whom also received defibrotide (Myers et al, 2013). Overall survival was 78%, with a Day +100 survival of 89%. In a subsequent retrospective study at the same institution (Gloude et al, 2018), 15 additional patients with VOD/SOS (8 allogeneic and 7 autologous transplants) were treated with both high‐dose methylprednisolone and defibrotide and had a Day +100 survival of 73%. Of note, 6 of the patients had a primary diagnosis of neuroblastoma. The authors suggest further study of defibrotide with high‐dose steroids (Gloude et al, 2018).
Late‐onset (>21 days after HSCT) VOD/SOS Day +100 estimated survival was 52·8%, which was similar to the overall HSCT population (58·9%). Late‐onset and ≤Day‐21 onset VOD/SOS Day +100 estimated survival was similar in the paediatric subgroups and in the adult subgroups. In recognition of the occurrence of VOD/SOS >21 days (Jones et al, 1987; Mohty et al, 2015), EBMT criteria for adults now include a late‐onset category (Mohty et al, 2016). In addition, onset >21 days occurs in approximately 20% of paediatric cases (Corbacioglu et al, 2016, 2018) and thus, the EBMT paediatric criteria do not include time parameters for diagnosis, to acknowledge and include the presence of VOD/SOS onset before and after Day 21 (Corbacioglu et al, 2018). The frequency of VOD/SOS after Day 21 found in this study (26·4%) as well as others (Lee et al, 1999; Carreras et al, 2007; Mohty et al, 2015; Corbacioglu et al, 2018), reinforces the importance of continued vigilance for occurrence of late‐onset VOD/SOS. Importantly, in one study, diagnosis of late‐onset VOD/SOS in adult patients ranged from 8 to 43 days after hospital discharge and was more prevalent in patients conditioned with busulfan and melphalan (Bu/MEL; 52%) than other conditioning regimens (Carreras et al, 2007). Ten of 11 patients in the Bu/MEL subgroup who developed VOD/SOS after hospital discharge required readmission (Carreras et al, 2007). The finding that late‐onset VOD/SOS was more frequent in adults than paediatric patients was unexpected because late‐onset VOD/SOS is generally thought to be more common in paediatric patients (Corbacioglu et al, 2018). However, this study may underestimate the true incidence of late‐onset VOD/SOS due to restrictions in the initial protocol requiring diagnosis by Day +35 or could reflect differences in the disease or treatment response by age group.
The safety profile in this completed T‐IND is consistent with prior studies (Richardson et al, 2010, 2016, 2017a; Corbacioglu et al, 2012a, 2016), including the general overlap of safety profile and types of TRAEs seen between adult patients and paediatric patients (Corbacioglu et al, 2016; Richardson et al, 2017a). An important consideration when assessing any possible causal association between TEAEs and defibrotide therapy is the high rate of reported TEAEs in this very ill patient population, which is illustrated by control populations in prior defibrotide clinical trials (Corbacioglu et al, 2012b; Richardson et al, 2016). In the historically controlled treatment study, overall TEAEs occurred in virtually all patients (101/102), with hypotension and haemorrhagic events occurring in 39% and 64% in the defibrotide arm and in 50% and 75% of historical controls (Richardson et al, 2016). In the randomised prophylaxis study, haemorrhagic TEAEs occurred in similar proportions of the defibrotide (22%) and control (21%) groups, and overall adverse event rates were 87% and 88% in the prophylaxis and control groups, respectively (Corbacioglu et al, 2012b).
Among the key strengths of this T‐IND study are its size, as it is the largest prospective analysis of defibrotide treatment of patients with VOD/SOS after HSCT (n = 1000); real‐world use (reflected by the diversity of its patients by age, conditioning regimen, type of transplant, presence or absence of MOD and treated at 101 centres); and its essentially contemporary population, with >40% enrolled between December 2013 and April 2016. Acknowledged limitations include that this T‐IND was designed to provide access to defibrotide and was therefore a single‐arm study with limited data monitoring; however, it followed a larger population of defibrotide‐treated patients with MOD (n = 512) than the treated population in the combined phase 2 and phase 3 studies (n = 251) (Richardson et al, 2010, 2016). The results of this larger defibrotide‐treated population were consistent with previous studies, and the large population allowed a wide range of subgroup analyses for uncommon presentations.
Conclusions
This large prospective expanded‐access study found efficacy of defibrotide treatment of VOD/SOS in multiple patient subgroups: paediatric (≤16 years) and adult (>16 years) patients, patients with VOD/SOS with or without MOD, patients receiving allogeneic and autologous transplants, and patients with VOD/SOS onset ≤21 days and >21 days post‐HSCT. Moreover, earlier treatment after diagnosis of VOD/SOS was associated with higher Day +100 survival. The safety profile of defibrotide treatment was similar to previous studies, with no new types of TRAEs.
Author contributions
P.G.R., R.J.S. and R.H. were responsible for the study conception and design. N.A.K., S.A.G., A.R.S., S.A., B.M.T., J.H.A., L.L., T.S., V.T.H., N.B., M.I., W.L., R.H., W.T., R.J.S. and P.G.R. were involved in the collection and assembly of data. All authors participated in the study data analysis and interpretation, contributed to the manuscript writing, and provided their final approval of this manuscript.
Disclosures
Nancy A. Kernan received grants from Gentium during the conduct of the study, and her research was supported by National Cancer Institute of the National Institutes of Health under award number P30 CA008748; the content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health. Stephan A. Grupp has served as a consultant to Jazz Pharmaceuticals. Angela R. Smith, Sally Arai, Brandon M. Triplett, Leslie Lehmann, Tsiporah Shore, and Nancy Bunin have no relevant conflicts of interest to disclose. Joseph H. Antin has served on advisory committees with Jazz Pharmaceuticals. Vincent T. Ho has served as a consultant to Jazz Pharmaceuticals. Massimo Iacobelli was an employee of Gentium SpA during the conduct of the study. Wei Liang, Robin Hume, and William Tappe are employees of Jazz Pharmaceuticals and hold stock and/or stock options in Jazz Pharmaceuticals plc. Robert J. Soiffer has served on advisory committees with Jazz Pharmaceuticals. Paul G. Richardson has served on advisory committees and as a consultant, and received research funding from Jazz Pharmaceuticals.
Supporting information
Table SI. Underlying primary diseases for HSCT.
Table SII. Demographics of the patient subgroups by late‐onset or non‐late‐onset VOD/SOS.
Acknowledgements
Clinical research was funded by Jazz Pharmaceuticals. The authors thank Katherine Molnar‐Kimber, PhD, and John Norwood of The Curry Rockefeller Group, LLC, of Tarrytown, NY, USA, for providing medical writing and editorial support, which were funded by Jazz Pharmaceuticals in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). Jazz Pharmaceuticals also reviewed and edited the manuscript for scientific accuracy. The authors would like to thank all of the study investigators, study staff, nursing team, and patients for their participation in this research.
References
- Bearman, S.I. , Shuhart, M.C. , Hinds, M.S. & McDonald, G.B. (1992) Recombinant human tissue plasminogen activator for the treatment of established severe venocclusive disease of the liver after bone marrow transplantation. Blood, 80, 2458–2462. [PubMed] [Google Scholar]
- Benimetskaya, L. , Wu, S. , Voskresenskiy, A.M. , Echart, C. , Zhou, J.F. , Shin, J. , Iacobelli, M. , Richardson, P. , Ayyanar, K. & Stein, C.A. (2008) Angiogenesis alteration by defibrotide: implications for its mechanism of action in severe hepatic veno‐occlusive disease. Blood, 112, 4343–4352. [DOI] [PubMed] [Google Scholar]
- Bianchi, G. , Barone, D. , Lanzarotti, E. , Tettamanti, R. , Porta, R. , Moltrasio, D. , Cedro, A. , Salvetti, L. , Mantovani, M. & Prino, G. (1993) Defibrotide, a single‐stranded polydeoxyribonucleotide acting as an adenosine receptor agonist. European Journal of Pharmacology, 238, 327–334. [DOI] [PubMed] [Google Scholar]
- Carreras, E. (2012) Early complications after HSCT In: EBMT‐ESH Handbook, Chapter 11. 2012. https://ebmtonline.forumservice.net/media/11/tex/content_alt/EBMT_Handbook2012_CHAP11.pdf [Google Scholar]
- Carreras, E. , Rosinol, L. , Terol, M.J. , Alegre, A. , de Arriba, F. , Garcia‐Larana, J. , Bello, J.L. , Garcia, R. , Leon, A. , Martinez, R. , Penarrubia, M.J. , Poderos, C. , Ribas, P. , Ribera, J.M. , San Miguel, J. , Blade, J. , Lahuerta, J.J. & Spanish Myeloma Group/PETHEMA . (2007) Veno‐occlusive disease of the liver after high‐dose cytoreductive therapy with busulfan and melphalan for autologous blood stem cell transplantation in multiple myeloma patients. Biology of Blood and Marrow Transplantation, 13, 1448–1454. [DOI] [PubMed] [Google Scholar]
- Coppell, J.A. , Richardson, P.G. , Soiffer, R. , Martin, P.L. , Kernan, N.A. , Chen, A. , Guinan, E. , Vogelsang, G. , Krishnan, A. , Giralt, S. , Revta, C. , Carreau, N.A. , Iacobelli, M. , Carreras, E. , Ruutu, T. , Barbui, T. , Antin, J.H. & Niederwieser, D. (2010) Hepatic veno‐occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biology of Blood and Marrow Transplantation, 16, 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbacioglu, S. , Kernan, N. , Lehmann, L. , Brochstein, J. , Revta, C. , Grupp, S. , Martin, P. & Richardson, P.G. (2012a) Defibrotide for the treatment of hepatic veno‐occlusive disease in children after hematopoietic stem cell transplantation. Expert Review of Hematology, 5, 291–302. [DOI] [PubMed] [Google Scholar]
- Corbacioglu, S. , Cesaro, S. , Faraci, M. , Valteau‐Couanet, D. , Gruhn, B. , Rovelli, A. , Boelens, J.J. , Hewitt, A. , Schrum, J. , Schulz, A.S. , Muller, I. , Stein, J. , Wynn, R. , Greil, J. , Sykora, K.W. , Matthes‐Martin, S. , Fuhrer, M. , O'Meara, A. , Toporski, J. , Sedlacek, P. , Schlegel, P.G. , Ehlert, K. , Fasth, A. , Winiarski, J. , Arvidson, J. , Mauz‐Korholz, C. , Ozsahin, H. , Schrauder, A. , Bader, P. , Massaro, J. , D'Agostino, R. , Hoyle, M. , Iacobelli, M. , Debatin, K.M. , Peters, C. & Dini, G. (2012b) Defibrotide for prophylaxis of hepatic veno‐occlusive disease in paediatric haemopoietic stem‐cell transplantation: an open‐label, phase 3, randomised controlled trial. Lancet, 379, 1301–1309. [DOI] [PubMed] [Google Scholar]
- Corbacioglu, S. , Carreras, E. , Mohty, M. , Pagliuca, A. , Boelens, J.J. , Damaj, G. , Iacobelli, M. , Niederwieser, D. , Olavarria, E. , Suarez, F. , Ruutu, T. , Verdonck, L. , Hume, R. , Nejadnik, B. , Lai, C. , Finetto, G. & Richardson, P. (2016) Defibrotide for the treatment of hepatic veno‐occlusive disease: final results from the international compassionate‐use program. Biology of Blood and Marrow Transplantation, 22, 1874–1882. [DOI] [PubMed] [Google Scholar]
- Corbacioglu, S. , Carreras, E. , Ansari, M. , Balduzzi, A. , Cesaro, S. , Dalle, J.H. , Dignan, F. , Gibson, B. , Guengoer, T. , Gruhn, B. , Lankester, A. , Locatelli, F. , Pagliuca, A. , Peters, C. , Richardson, P.G. , Schulz, A.S. , Sedlacek, P. , Stein, J. , Sykora, K.W. , Toporski, J. , Trigoso, E. , Vetteranta, K. , Wachowiak, J. , Wallhult, E. , Wynn, R. , Yaniv, I. , Yesilipek, A. , Mohty, M. & Bader, P. (2018) Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno‐occlusive disease in pediatric patients: a new classification from the European society for blood and marrow transplantation. Bone Marrow Transplantation, 53, 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler, C. , Stevenson, K. , Kim, H.T. , Richardson, P. , Ho, V.T. , Linden, E. , Revta, C. , Ebert, R. , Warren, D. , Choi, S. , Koreth, J. , Armand, P. , Alyea, E. , Carter, S. , Horowitz, M. , Antin, J.H. & Soiffer, R. (2008) Sirolimus is associated with veno‐occlusive disease of the liver after myeloablative allogeneic stem cell transplantation. Blood, 112, 4425–4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignan, F.L. , Wynn, R.F. , Hadzic, N. , Karani, J. , Quaglia, A. , Pagliuca, A. , Veys, P. , Potter, M.N. , Haemato‐oncology Task Force of British Committee for Standards in Haematology & British Society for Blood and Marrow Transplantation . (2013) BCSH/BSBMT guideline: diagnosis and management of veno‐occlusive disease (sinusoidal obstruction syndrome) following haematopoietic stem cell transplantation. British Journal of Haematology, 163, 444–457. [DOI] [PubMed] [Google Scholar]
- Echart, C.L. , Graziadio, B. , Somaini, S. , Ferro, L.I. , Richardson, P.G. , Fareed, J. & Iacobelli, M. (2009) The fibrinolytic mechanism of defibrotide: effect of defibrotide on plasmin activity. Blood Coagulation & Fibrinolysis, 20, 627–634. [DOI] [PubMed] [Google Scholar]
- Gloude, N.J. , Jodele, S. , Teusink‐Cross, A. , Grimley, M. , Davies, S.M. , Lane, A. & Myers, K.C. (2018) Combination of high‐dose methylprednisolone and defibrotide for veno‐occlusive disease in pediatric hematopoietic stem cell transplant recipients. Biology of Blood and Marrow Transplantation, 24, 91–95. [DOI] [PubMed] [Google Scholar]
- Jones, R.J. , Lee, K.S. , Beschorner, W.E. , Vogel, V.G. , Grochow, L.B. , Braine, H.G. , Vogelsang, G.B. , Sensenbrenner, L.L. , Santos, G.W. & Saral, R. (1987) Venoocclusive disease of the liver following bone marrow transplantation. Transplantation, 44, 778–783. [DOI] [PubMed] [Google Scholar]
- Kantarjian, H.M. , DeAngelo, D.J. , Advani, A.S. , Stelljes, M. , Kebriaei, P. , Cassaday, R.D. , Merchant, A.A. , Fujishima, N. , Uchida, T. , Calbacho, M. , Ejduk, A.A. , O'Brien, S.M. , Jabbour, E.J. , Zhang, H. , Sleight, B.J. , Vandendries, E.R. & Marks, D.I. (2017) Hepatic adverse event profile of inotuzumab ozogamicin in adult patients with relapsed or refractory acute lymphoblastic leukaemia: results from the open‐label, randomised, phase 3 INO‐VATE study. The Lancet Haematology, 4, e387–e398. [DOI] [PubMed] [Google Scholar]
- Lee, J.L. , Gooley, T. , Bensinger, W. , Schiffman, K. & McDonald, G.B. (1999) Veno‐occlusive disease of the liver after busulfan, melphalan, and thiotepa conditioning therapy: incidence, risk factors, and outcome. Biology of Blood and Marrow Transplantation, 5, 306–315. [DOI] [PubMed] [Google Scholar]
- McDonald, G.B. , Hinds, M.S. , Fisher, L.D. , Schoch, H.G. , Wolford, J.L. , Banaji, M. , Hardin, B.J. , Shulman, H.M. & Clift, R.A. (1993) Veno‐occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Annals of Internal Medicine, 118, 255–267. [DOI] [PubMed] [Google Scholar]
- Mohty, M. , Malard, F. , Abecassis, M. , Aerts, E. , Alaskar, A.S. , Aljurf, M. , Arat, M. , Bader, P. , Baron, F. , Bazarbachi, A. , Blaise, D. , Ciceri, F. , Corbacioglu, S. , Dalle, J.H. , Duarte, R.F. , Fukuda, T. , Huynh, A. , Masszi, T. , Michallet, M. , Nagler, A. , NiChonghaile, M. , Pagluica, T. , Peters, C. , Petersen, F.B. , Richardson, P.G. , Ruutu, T. , Savani, B.N. , Wallhult, E. , Yakoub‐Agha, I. & Carreras, E. (2015) Sinusoidal obstruction syndrome/veno‐occlusive disease: current situation and perspectives‐a position statement from the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplantation, 50, 781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohty, M. , Malard, F. , Abecassis, M. , Aerts, E. , Alaskar, A.S. , Aljurf, M. , Arat, M. , Bader, P. , Baron, F. , Bazarbachi, A. , Blaise, D. , Ciceri, F. , Corbacioglu, S. , Dalle, J.H. , Dignan, F. , Fukuda, T. , Huynh, A. , Masszi, T. , Michallet, M. , Nagler, A. , NiChonghaile, M. , Okamoto, S. , Pagliuca, A. , Peters, C. , Petersen, F.B. , Richardson, P.G. , Ruutu, T. , Savani, B.N. , Wallhult, E. , Yakoub‐Agha, I. , Duarte, R.F. & Carreras, E. (2016) Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno‐occlusive disease in adult patients: a new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplantation, 51, 906–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers, K.C. , Lawrence, J. , Marsh, R.A. , Davies, S.M. & Jodele, S. (2013) High‐dose methylprednisolone for veno‐occlusive disease of the liver in pediatric hematopoietic stem cell transplantation recipients. Biology of Blood and Marrow Transplantation, 19, 500–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, K.J. & Goa, K.L. (1993) Defibrotide. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in vascular disorders. Drugs, 45, 259–294. [DOI] [PubMed] [Google Scholar]
- Palomo, M. , Mir, E. , Rovira, M. , Escolar, G. , Carreras, E. & Diaz‐Ricart, M. (2016) What is going on between defibrotide and endothelial cells? Snapshots reveal the hot spots of their romance. Blood, 127, 1719–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pescador, R. , Capuzzi, L. , Mantovani, M. , Fulgenzi, A. & Ferrero, M.E. (2013) Defibrotide: properties and clinical use of an old/new drug. Vascular Pharmacology, 59, 1–10. [DOI] [PubMed] [Google Scholar]
- Richardson, P.G. , Soiffer, R.J. , Antin, J.H. , Uno, H. , Jin, Z. , Kurtzberg, J. , Martin, P.L. , Steinbach, G. , Murray, K.F. , Vogelsang, G.B. , Chen, A.R. , Krishnan, A. , Kernan, N.A. , Avigan, D.E. , Spitzer, T.R. , Shulman, H.M. , Di Salvo, D.N. , Revta, C. , Warren, D. , Momtaz, P. , Bradwin, G. , Wei, L.J. , Iacobelli, M. , McDonald, G.B. & Guinan, E.C. (2010) Defibrotide for the treatment of severe hepatic veno‐occlusive disease and multiorgan failure after stem cell transplantation: a multicenter, randomized, dose‐finding trial. Biology of Blood and Marrow Transplantation, 16, 1005–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, P.G. , Corbacioglu, S. , Ho, V.T. , Kernan, N.A. , Lehmann, L. , Maguire, C. , Maglio, M. , Hoyle, M. , Sardella, M. , Giralt, S. , Holler, E. , Carreras, E. , Niederwieser, D. & Soiffer, R. (2013) Drug safety evaluation of defibrotide. Expert Opinion on Drug Safety, 12, 123–136. [DOI] [PubMed] [Google Scholar]
- Richardson, P.G. , Riches, M.L. , Kernan, N.A. , Brochstein, J.A. , Mineishi, S. , Termuhlen, A.M. , Arai, S. , Grupp, S.A. , Guinan, E.C. , Martin, P.L. , Steinbach, G. , Krishnan, A. , Nemecek, E.R. , Giralt, S. , Rodriguez, T. , Duerst, R. , Doyle, J. , Antin, J.H. , Smith, A. , Lehmann, L. , Champlin, R. , Gillio, A. , Bajwa, R. , D'Agostino, R.B. Sr , Massaro, J. , Warren, D. , Miloslavsky, M. , Hume, R.L. , Iacobelli, M. , Nejadnik, B. , Hannah, A.L. & Soiffer, R.J. (2016) Phase 3 trial of defibrotide for the treatment of severe veno‐occlusive disease and multi‐organ failure. Blood, 127, 1656–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, P.G. , Smith, A.R. , Triplett, B.M. , Kernan, N.A. , Grupp, S.A. , Antin, J.H. , Lehmann, L. , Shore, T. , Iacobelli, M. , Miloslavsky, M. , Hume, R. , Hannah, A.L. , Nejadnik, B. , Soiffer, R.J. & Defibrotide Study Group . (2017a) Defibrotide for patients with hepatic veno‐occlusive disease/sinusoidal obstruction syndrome: interim results from a treatment IND study. Biology of Blood and Marrow Transplantation, 23, 997–1004. [DOI] [PubMed] [Google Scholar]
- Richardson, P.G. , Smith, A.R. , Triplett, B.M. , Kernan, N.A. , Grupp, S.A. , Antin, J.H. , Lehmann, L. , Miloslavsky, M. , Hume, R. , Hannah, A.L. , Nejadnik, B. & Soiffer, R.J. (2017b) Earlier defibrotide initiation post‐diagnosis of veno‐occlusive disease/sinusoidal obstruction syndrome improves Day +100 survival following haematopoietic stem cell transplantation. British Journal of Haematology, 178, 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strouse, C. , Richardson, P. , Prentice, G. , Korman, S. , Hume, R. , Nejadnik, B. , Horowitz, M.M. & Saber, W. (2016) Defibrotide for treatment of severe veno‐occlusive disease in pediatrics and adults: an exploratory analysis using data from the Center for International Blood and Marrow Transplant Research. Biology of Blood and Marrow Transplantation, 22, 1306–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsirigotis, P.D. , Resnick, I.B. , Avni, B. , Grisariu, S. , Stepensky, P. , Or, R. & Shapira, M.Y. (2014) Incidence and risk factors for moderate‐to‐severe veno‐occlusive disease of the liver after allogeneic stem cell transplantation using a reduced intensity conditioning regimen. Bone Marrow Transplantation, 49, 1389–1392. [DOI] [PubMed] [Google Scholar]
- Yakushijin, K. , Atsuta, Y. , Doki, N. , Yokota, A. , Kanamori, H. , Miyamoto, T. , Ohwada, C. , Miyamura, K. , Nawa, Y. , Kurokawa, M. , Mizuno, I. , Mori, T. , Onizuka, M. , Taguchi, J. , Ichinohe, T. , Yabe, H. , Morishima, Y. , Kato, K. , Suzuki, R. & Fukuda, T. (2016) Sinusoidal obstruction syndrome after allogeneic hematopoietic stem cell transplantation: incidence, risk factors and outcomes. Bone Marrow Transplantation, 51, 403–409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Underlying primary diseases for HSCT.
Table SII. Demographics of the patient subgroups by late‐onset or non‐late‐onset VOD/SOS.


