Abstract
Aims
To evaluate costs, effects and cost‐effectiveness of increased reach of specific smoking cessation interventions in Germany.
Design
A Markov‐based state transition return on investment model (EQUIPTMOD) was used to evaluate current smoking cessation interventions as well as two prospective investment scenarios. A health‐care perspective (extended to include out‐of‐pocket payments) with life‐time horizon was considered. A probabilistic analysis was used to assess uncertainty concerning predicted estimates.
Setting
Germany.
Participants
Cohort of current smoking population (18+ years) in Germany.
Interventions
Interventions included group‐based behavioural support, financial incentive programmes and varenicline. For prospective scenario 1 the reach of group‐based behavioral support, financial incentive programme and varenicline was increased by 1% of yearly quit attempts (= 57 915 quit attempts), while prospective scenario 2 represented a higher reach, mirroring the levels observed in England.
Measurements
EQUIPTMOD considered reach, intervention cost, number of quitters, quality‐of‐life years (QALYs) gained, cost‐effectiveness and return on investment.
Findings
The highest returns through reduction in smoking‐related health‐care costs were seen for the financial incentive programme (€2.71 per €1 invested), followed by that of group‐based behavioural support (€1.63 per €1 invested), compared with no interventions. Varenicline had lower returns (€1.02 per €1 invested) than the other two interventions. At the population level, prospective scenario 1 led to 15 034 QALYs gained and €27 million cost‐savings, compared with current investment. Intervention effects and reach contributed most to the uncertainty around the return‐on‐investment estimates. At a hypothetical willingness‐to‐pay threshold of only €5000, the probability of being cost‐effective is approximately 75% for prospective scenario 1.
Conclusions
Increasing the reach of group‐based behavioural support, financial incentives and varenicline for smoking cessation by just 1% of current annual quit attempts provides a strategy to German policymakers that improves the population's health outcomes and that may be considered cost‐effective.
Keywords: Behavioural support, cost‐effectiveness, EQUIPTMOD, Germany, pharmacotherapy, policy, smoking cessation
Introduction
Smoking constitutes a significant risk factor for various diseases, including cardiovascular disease, stroke, lung disease, autoimmune disease and cancer 1, 2, 3. Between 2000 and 2050, it is estimated that smoking will have caused approximately 450 million deaths world‐wide 4. Based on recent findings this number may even be higher, as 17% of excess mortality among smokers may, falsely, not have been attributed to smoking 5. In contrast to males, the mortality rate of lung cancer in females has been rising in Germany for more than three decades, with smoking as the biggest risk factor 6, 7. Using survey data, health‐related costs of smoking in Germany have been estimated to amount to more than €30 billion per year 8. Thus, tobacco control remains a fundamental leverage point to improve population health outcomes 9.
Smoking prevention and cessation are currently the most effective methods to avoid the detrimental consequences of smoking. Stopping smoking before the ages of 30 or 40 years has been found to reduce the associated mortality risk by approximately 97 and 90%, respectively 10. In countries with a social health‐care system such as Germany, reducing smoking rates and thus the health consequences also leads to lower health‐care costs, which reduces the burden on society. Smoking prevention includes discouraging adolescents from starting smoking, promoting smoke‐free environments and restricting use of and access to tobacco products. Smoking cessation methods include pharmacotherapy and behavioural support. However, these interventions differ in their cost‐effectiveness and may also be associated with unintended consequences. For example, tax increases may create illicit markets 11, 12, while e‐cigarettes may prevent certain smokers from quitting 13. Compared with the United Kingdom 14, smoking cessation aids are used less frequently in Germany 15, 16, while the smoking prevalence (18.0 versus 24.5%) is higher 17, 18. This underlines the need for more smoking cessation strategies in Germany. In order to reduce the number of smokers efficiently, decision‐makers must consider the cost‐effectiveness of available programmes.
Thus, the aims of this study are to evaluate incremental costs, incremental quality‐adjusted life years (QALYs), cost–effectiveness and smoking‐related return on investment (ROI) of increased reach of three smoking cessation interventions in Germany using the ROI model from the European study on Quantifying Utility of Investment in Protection from Tobacco (EQUIPT) 19. In particular, this study evaluates two prospective scenarios where the reach (= number of quit attempts made annually) through group‐based behavioural support, financial incentives and varenicline is increased by a small (prospective scenario 1) and large (prospective scenario 2) amount, while all other interventions remain unchanged. This will provide decision‐makers with important information for future resource allocation decisions. As a secondary goal, this study also aims to assess the transferability of a national tobacco control model to other countries; in this case, Germany.
Methods
Model
EQUIPTMOD is described in more detail elsewhere in this issue 20. It was developed specifically as a decision‐support tool and has a user‐friendly interface (front‐end) underpinned by a Markov state transition model (back‐end) 21. The Markov model has three health states—current smoker (smokes daily or occasionally), former smoker and death—and includes four smoking‐attributable diseases [lung cancer, chronic obstructive pulmonary disease (COPD), stroke and coronary heart disease]. The model follows a cohort of current smokers until death and assigns the costs and disutilities through annual cycles. This allows costs and effects of different tobacco control interventions to be evaluated from short‐, medium and life‐time perspectives. The age of the German cohort in EQUIPTMOD ranges from 18 to 85 years, and the cohort is followed until the age of 100. As most individuals have died by this point, the time‐frame of the model is a life‐time. New patients are not added to the cohort (i.e. it does not consider births). The analysis considers primarily a health‐care perspective, but it has been extended to include out‐of‐pocket payments that current smokers use to access cessation services. German legislation treats smoking cessation interventions as life‐style products, and therefore does not reimburse the associated costs for pharmacotherapy and only partly reimburses costs for behavioural support. A key feature of the EQUIPTMOD is that model inputs can be modified (including local demographics and intervention reach/costs) and the user is also able to add custom interventions. The model contains a base case that resembles the current investment in tobacco control activities for the selected country. The user is also able to analyse the impact of prospective changes to the current level of investment by modifying key model inputs. The predicted results allow comparisons between the current and prospective scenarios, as well as with a zero investment scenario, which includes only indoor smoking bans, brief physician advice and current levels of tobacco taxation.
Current interventions
Smoking cessation interventions in Germany can be divided into two main groups, behavioural support and pharmacotherapy. Behavioural support involves primarily specialist support, which is offered to patients either individually or in groups. Pharmacotherapy for smoking cessation in Germany includes medications such as bupropion, an antidepressant, and varenicline, a partial nicotine receptor agonist. Varenicline has been shown to be more cost‐effective than bupropion 22 for smoking cessation. However, in Germany, over‐the‐counter nicotine replacement therapies (NRT) such as nicotine patches and gums are used significantly more frequently for smoking cessation than prescription drugs (approximately 20 versus 1%) 15, although they are less effective 23, 24.
Other interventions for smoking cessation
Incentive programmes, a subgroup of behavioural support, have been shown to be effective for smoking cessation 25, 26, 27. Financial incentive programmes promote smoking cessation by providing financial incentives to quit. Certain financial incentive programmes have demonstrated their effectiveness over a 1 year horizon 25, 28. The advantage of financial incentive programmes include their ease of implementation by companies and insurance companies. Currently, most statutory insurance companies in Germany implement reward‐based bonus programmes for smoking cessation 29, 30, 31. However, these bonus programmes only offer small rewards for participating in smoking cessation programmes and do not confirm actual smoking status. E‐cigarettes are a relatively new ‘cessation’ intervention. However, long‐term data for their effects are not available.
Current investment and prospective scenarios
The EQUIPTMOD compares current investment with zero investment by default. Zero investment includes only the top‐level policies (i.e. indoor smoking ban in public places and tobacco taxes; both at their current levels). In other words, zero investment represents the theoretical gross costs of tobacco and is thus the baseline against which the effects of current and prospective smoking cessation interventions can be evaluated. The current investment includes all currently implemented smoking cessation interventions (see Table 1). Moreover, we evaluated how two additional prospective investment scenarios compare against the current investment. For prospective scenario 1, the rates of using group‐based behavioural support, financial incentive programmes and varenicline were increased by 1% of yearly quit attempts, while all other interventions remained unchanged. This 1 percentage point increase in reach equals 57 915 new quit attempts. Prospective scenario 2 represents a higher utilization case, where the reach of the three interventions was increased to the levels observed in England. The use of the financial incentive programme, as it is not available in England, was set to 2.9% to be comparable to the reach of group‐based behavioural support. Results for prospective scenarios 1 and 2 were calculated for each individual intervention and as aggregated results for all three interventions together.
Table 1.
Demographics, smoking status and intervention reach used for the German cohort.
| Absolute population | Percentage of total population | |
|---|---|---|
| Adult population | 67 538 844 | 100 |
| Ex‐smokers | 13 017 502 | 19.3 |
| Active smokers | 16 547 017 | 24.5 |
| Absolute attempts and reach per year | Percentage of smokers attempting to quit | |
| Yearly quit attempts among active smokers | 5 791 456 | 34.9 |
| Pharmacotherapy reach | 688 112 | 11.9 |
| Behavioural support reach | 992 636 | 17.1 |
| Combination reach | 118 146 | 2.0 |
| No support reach | 4 228 921 | 73.0 |
A detailed list of input sources is available from the authors. ‘Combination reach’ refers to quit attempts where pharmacotherapy as well as behavioural support were used; ‘No support reach’ refers to the quit attempts where no aid was used.
The three interventions (group‐based behavioural support, financial incentive programmes and varenicline) were chosen because they represent a diverse range of options available for stakeholders to improve smoking cessation in Germany. Varenicline represents an effective pharmacotherapy; group support is a well‐accepted and effective behavioural support and financial incentives have the potential to improve smoking cessation and can be implemented in Germany. Varenicline and financial incentives are currently not reimbursed in Germany, while group‐based behavioural support is partly reimbursed.
Inputs for intervention costs and effectiveness
Model inputs were taken mainly from recent relevant literature and from the Federal Statistical Office. Detailed technical documentation is available upon request from the authors. Inputs used for population, costs and effects of current interventions are shown in Tables 1 and 2. Specifically, costs in 2015 for group‐based behavioural support were based on one widely used programme in Germany. Costs for seven sessions of 90 minutes or three sessions of 180 minutes and two sessions of telephone support of 10 minutes 32 are approximately €250. The costs for a 12‐week standard treatment package (starter + maintenance) of varenicline were calculated to be €293 in Germany in 2015 (source: pharmacy pricing). Only smokers who smoke 10 cigarettes or more were assumed to be eligible for varenicline, as studies of varenicline effectiveness included only those who smoked 10 or more cigarettes per day.
Table 2.
Intervention reach and treatment cost for current investment, prospective scenario 1 and prospective scenario 2.
| Intervention | Current investment | Prospective scenario 1 | Prospective scenario 2 | |||
|---|---|---|---|---|---|---|
| Reach | Cost (€) | Reach | Cost (€) | Reach | Cost (€) | |
| Pharmacotherapy | ||||||
| Bupropion | 7393 | 1 583 063 | 7393 | 1 583 063 | 7393 | 1 583 063 |
| Varenicline | 38 102 | 11 145 978 | 96 017 (+152%) | 28 087 853 (+152%) | 839 188 (+2102%) | 245 478 666 (+2102%) |
| Mono‐NRT | 458 931 | 145 706 003 | 458 931 | 145 706 003 | 458 931 | 145 706 003 |
| Combo‐NRT | 183 686 | 54 389 425 | 183 686 | 54 389 425 | 183 686 | 54 389 425 |
| Behavioral support | ||||||
| Brief physician advice | 261 596 | 2 417 147 | 261 596 | 2 417 147 | 261 596 | 2 417 147 |
| Individual support | 102 364 | 30 197 380 | 102 364 | 30 197 380 | 102 364 | 30 197 380 |
| Group support | 51 182 | 12 795 500 | 109 097 (+113%) | 27 274 250 (+113%) | 167 954 (+228%) | 41 988 500 (+228%) |
| SMS service | 82 735 | 1 396 567 | 82 735 | 1 396 567 | 82 735 | 1 396 567 |
| Self‐help material | 756 355 | 9 900 687 | 756 355 | 9 900 687 | 756 355 | 9 900 687 |
| Financial incentives | n.a. | n.a. | 57 915 | 10 193 040 | 167 954 | 29 559 904 |
| Total | 1 942 344 | 259 500 750 | 2 116 089 | 311 145 415 | 3 028 156 | 562 617 342 |
() = numbers in brackets show the percentage increase compared with current investment.
Current investment includes all currently implemented smoking cessation interventions and their respective usage rates and costs in Germany. Prospective scenario 1 increases the usage rates of only group support, financial incentives and varenicline by 1% (= 57 915) of yearly quit attempts. Prospective scenario 2 increases the reach of group support as well as financial incentives to 2.90% and to 14.49% for varenicline. NRT = nicotine replacement therapy; n.a. = not available; SMS = short message service.
Although the financial incentive programme and respective costs are not available in Germany, our prospective scenarios included its use. Costs of the financial incentive programme, which required biochemical confirmation of smoking status, were calculated based on supplementary data from Halpern et al. 2015 25. Average costs were calculated to be $235 or €176 [gross domestic product (GDP)‐based purchasing power parity (PPP)‐adjusted, currency year 2015] per participant of the individual reward group, including a lump sum cost of €10 per participant for biochemical testing and laboratory costs. Urinary cotinine immunoassay test strips are cheap but reliable alternatives to more expensive chromatographic methods 33.
The model‐based effectiveness of interventions (likelihood to quit) compared to usual care is shown by risk ratios (RR) and originate from the Cochrane Database of Systematic Reviews for group‐based behavioural support and varenicline. The RR for group‐based behavioural support is 1.98 [95% confidence interval (CI) = 1.60, 2.46] 34, the RR for varenicline is 2.27 (95% CI = 2.02, 2.55) 35 and the RR for the individual reward‐based financial incentive programme (using biochemical testing) after 12 months is 2.17 (95% CI = 1.226, 3.851) 25. We excluded effectiveness data of cessation studies that were based solely on self‐report and lacked biochemical testing to reduce possible bias 36.
In Germany the Institute of Quality and Efficiency in Health Care (IQWiG) conducts health technology assessments on behalf of the German Statutory Health Insurance and recommends using a discount rate for costs of 3% in its methodological guidelines 37. To conduct a more conservative evaluation, all costs but also effects were discounted by 3% in this study.
Outcomes
Quitters per 1000 smokers, QALYs gained (population level), the incremental cost effectiveness ratio (ICER) and the return on investment (ROI) for smoking‐related costs are presented within the Results section. To derive the ICER, the incremental costs per smoker are divided by the incremental QALYs per smoker. The resulting value describes the cost‐effectiveness of an intervention strategy compared to another. The ROI for smoking‐related costs was calculated by dividing the incremental health‐care costs by the incremental investment costs. Therefore, ROIs greater than 1 indicate that the return is higher than the investment made. We did not include the values of health gains in the ROI, as there is no QALY affordability threshold in Germany which would allow conversion of health effects into monetary values. Despite the lack of QALY thresholds, QALYs are easy to understand and are well accepted in the scientific community. IQWiG does not exclude the use of QALYs in their assessments, but has clear standards for their use 37.
Sensitivity analysis
Both deterministic and probabilistic sensitivity analyses were performed. The cost‐effectiveness planes and the cost‐effectiveness acceptability curves of current investment, prospective scenarios 1 and prospective scenario 2 versus zero investment were each determined. For each probabilistic sensitivity analysis, utilities and reach of the interventions were calculated with a beta distribution; RR inputs were calculated with a log‐normal distribution and costs with gamma distributions. Based on probabilistic tornado diagrams for incremental QALYs and incremental costs, inputs contributing most to the variation of results were determined. Groups of inputs with similar characteristics were identified and the effects of these groups on model uncertainty were evaluated by joint probabilistic variation while leaving other inputs fixed. The examined input groups included utilities, intervention reach, intervention effects, intervention costs, other non‐intervention costs and relative risks of smoking‐attributable diseases. The results for these inputs were sorted based on the size of the 95% CI and illustrated in tornado diagrams. The deterministic sensitivity analyses examined the effect of various discount rates for both costs and effects on the outcomes calculated. EQUIPT model version 2.80 was used. Figures were designed with the software R 38 version 3.3.0 and the package ggplot2 39.
Results
Demographics, smoking status and cost
In 2011, the adult population of Germany (18+) consisted of approximately 67.5 million people 40. Of these, approximately 13.0 million were ex‐smokers, while 16.5 million were active smokers 40. Approximately 5.8 million active smokers attempt to quit per year 41. The vast majority (73.0%) of these try to quit without support (Table 1).
Current investment and prospective scenarios
Table 2 shows the intervention reach and costs of the current investment, prospective scenarios 1 and 2. In current investment, varenicline and bupropion were used in fewer than 1% of quit attempts. Individual behavioural therapy was used twice as frequently as group‐based behavioural support. Self‐help materials and mono‐NRT were the most frequently used of the evaluated interventions. Total costs calculated for the current investment were approximately €260 million. In prospective scenario 1, the reach of group‐based behavioural support, the financial incentive programme and varenicline were increased by 1% (n = 57 915) of overall quit attempts and total costs were calculated to be €311 million. In prospective scenario 2, the reach of group‐based behavioural support and varenicline was increased to the published levels for England. This resulted in an additional 800 000 quit attempts for varenicline and costs, which were approximately €234 million higher than costs calculated for varenicline in current investment. In addition, the use of the financial incentive programme was set to 2.90% to resemble the use of group‐based behavioural support.
Prospective scenario outcomes
Table 3 illustrates the outcomes for both prospective scenarios. The zero investment, which considers only effects of a smoking ban and tobacco taxes at the current level, led to 17.12 quitters per 1000 smokers and produced a total of 235 434 350 QALYs. Compared to zero investment, current investment led to an additional 55 022 QALYs and resulted in an ICER of €323 per QALY. For prospective scenario 1, group‐based behavioural support alone led to 5142 additional QALYs compared with current investment. Dividing these QALYs by the number of smokers in Germany (16 547 017) resulted in 0.0003 incremental QALYs gained per smoker. This, combined with incremental cost savings of –€0.55 per smoker, resulted in dominance–—more QALYs and lower costs—of group‐based behavioural support in prospective scenario 1 over current investment. The net ROI was calculated to be €0.63 (€1.63 – €1). Overall, prospective scenario 1 delivered 15 034 additional QALYs and saved €27 133 798 (−€1.6398 × 16 547 017). In both prospective scenarios, all reach increases were dominant. The cost savings and the ROI were highest for the financial incentive programme. In prospective scenario 2, the increased reach of varenicline by 14.5% of quit attempts added 52 104 QALYs. Together, all three interventions in prospective scenario 2 led to a gain of 82 709 QALYs and 22 quit attempts per 1000 smokers (an increase of approximately three in 1000 compared with current investment).
Table 3.
Outcomes for current investment, prospective scenarios 1 and 2.
| Investment (percentage reach) | Quitters per 1000 smokers | QALYs gained (population)a | Incremental QALYs per smokera | Incremental costs per smoker (€)a | ICER (€)a | ROI (€)a |
|---|---|---|---|---|---|---|
| Zero investment | 17.12 | 235434350b | ||||
| Current investment | 19.05 | 55 022 | 0.0033 | 1.08 | 323.33 | 0.93 |
| Prospective scenario 1 | ||||||
| GBS (1.88%) | 19.23 | 5142 | 0.0003 | – 0.55 | – 1770.35c | 1.63 |
| FIP (1.0%) | 19.26 | 6039 | 0.0004 | – 1.05 | – 2895.33c | 2.71 |
| Varenicline (1.66%) | 19.18 | 3775 | 0.0002 | – 0.02 | – 77.81c | 1.02 |
| All three | 19.58 | 15 034 | 0.0009 | – 1.64 | – 1809.70c | 1.65 |
| Prospective scenario 2 | ||||||
| GBS (2.9%) | 19.41 | 10 408 | 0.0006 | – 1.11 | – 1770.57c | 1.63 |
| FIP (2.9%) | 19.69 | 18 118 | 0.0011 | – 3.06 | – 2887.68c | 2.71 |
| Varenicline (14.49%) | 20.88 | 52 104 | 0.0031 | – 0.25 | – 77,80c | 1.02 |
| All three | 21.95 | 82 709 | 0.0050 | – 5.16 | – 1031.69c | 1.29 |
Outcomes based on lifetime horizon; Euros as absolute values. ‘All three’ depicts cases where reach was increased for all three interventions together. The sum of incremental quitters per 1000 smokers of single interventions compared with current investment add up to incremental quitters per 1000 smokers for ‘All three’.
Compared with zero investment for current investment, compared with current investment for prospective scenarios 1 and 2;
compared with no taxation, no smoking ban, no brief physician advice. GBS = group behavioural therapy; FIP = financial incentive programme; ROI = return on investment; ICER = on incremental cost‐effectiveness;
dominant [cheaper to run but generates more quality‐of‐life years (QALYs)]. The actual figure (as negative, rather than just stating ‘Dominant’) is reported here to allow readers to map this figure onto the reported ROI figure.
Sensitivity analysis
Probabilistic analyses were used to determine the uncertainty of model input groups for current investment, prospective scenario 1 and 2 compared to zero investment. Comparing current investment to zero investment, the tornado diagrams (Figs 1 and 2) showed that the intervention effects and the reach of interventions contributed most to the uncertainty of aggregated costs and QALYs. Other input groups, such as intervention costs, relative risks and utilities, had little impact on the results.
Figure 1.
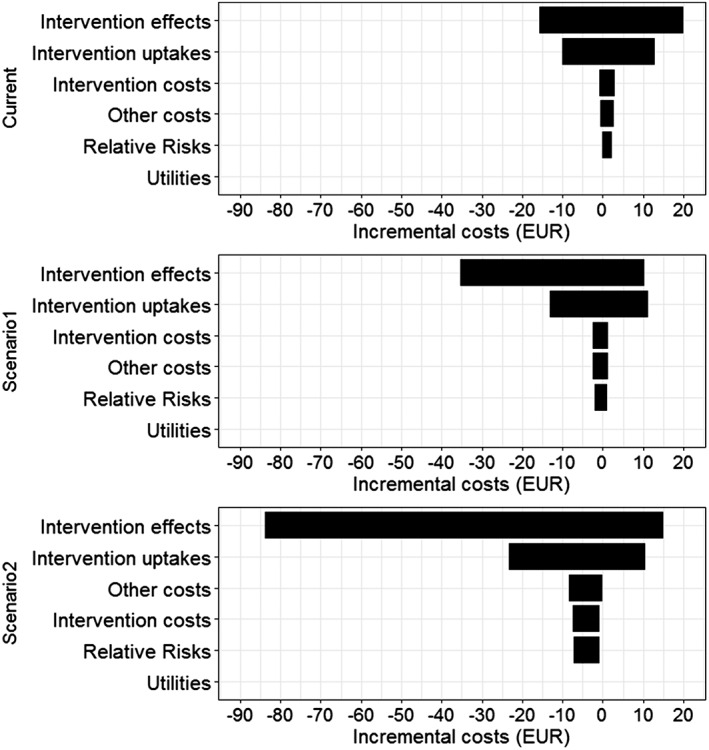
Incremental costs for current investment and prospective scenarios versus zero investment, univariate probabilistic sensitivity analyses for different selected groups. Note: In the tornado diagram, 95% confidence intervals are represented by the width of the horizontal bars
Figure 2.
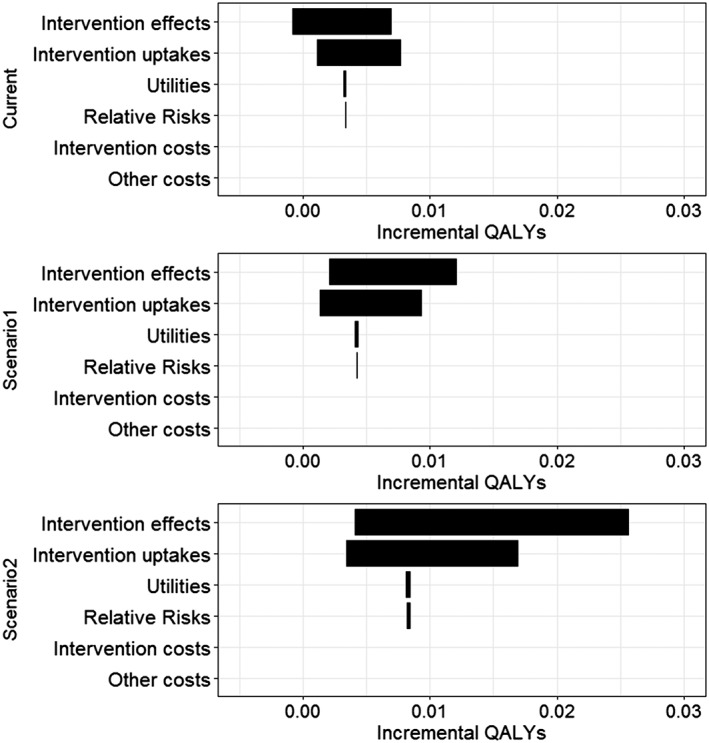
Incremental quality‐of‐life years (QALYs) for current investment and prospective scenarios versus zero investment, univariate probabilistic sensitivity analyses for different selected groups. Note: In the tornado diagram, 95% confidence intervals are represented by the width of the horizontal bars
In the multivariate probabilistic sensitivity analysis (Fig. 3), a clear trend towards fewer costs and better incremental effectiveness can be observed. While 60.8% of replications in the current investment analysis (left plot) showed positive incremental costs, only 57.6% and 53.9% did so for prospective scenarios 1 (middle plot) and 2 (right plot), respectively. Overall, 26 replications of the current investment, 12 replications of prospective scenario 1 and only three replications of prospective scenario 2 were dominated. Due to the small incremental effects and costs, which were calculated per smoker, the upper confidence interval of prospective scenario 1 appears very steep, but has an actual slope or ICER of 75 000 (north‐east quadrant) and prospective scenario 2 has 38 571 (north‐east quadrant), respectively. The latter is in the range of commonly observed willingness‐to‐pay (WTP) thresholds. Comparing the predicted point estimator (mean of the model iterations) across current investment and prospective scenarios 1 (−€1222) and 2 (−€1061) showed that it moved further away from the origin into the south‐east quadrant, resembling dominance. The corresponding cost‐effectiveness acceptability curve is shown in Fig. 4. At a WTP threshold of €15 000, the probability of being cost‐effective is around 85% for current investment, 90% for prospective scenario 1 and 92% for prospective scenario 2.
Figure 3.
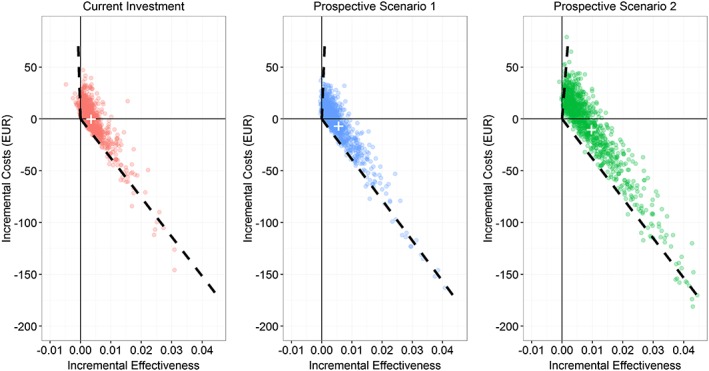
Multivariate probabilistic sensitivity analyses of incremental cost‐effectiveness ratios for current investment and prospective scenarios versus zero investment. Note: 1000 replications run in each probabilistic analysis. Predicted point estimator depicted as white cross; 95% confidence interval depicted as dashed lines. Zero investment as reference
Figure 4.
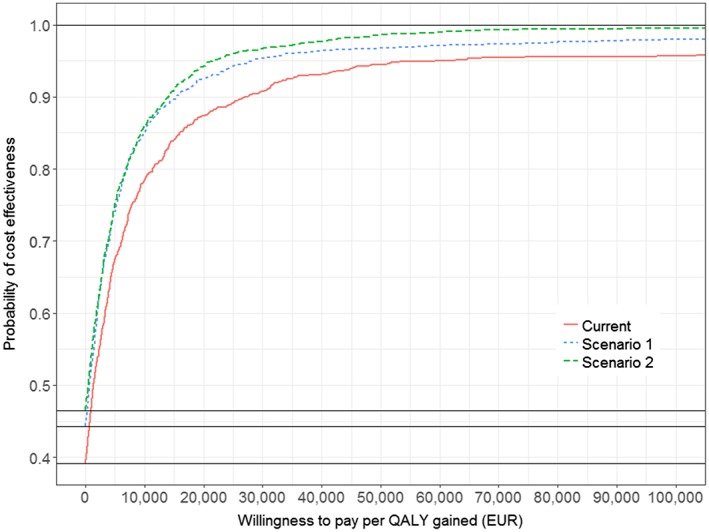
Cost‐effectiveness acceptability curve for current investment and prospective scenarios versus zero investment. Note: 1000 replications run in the probabilistic analysis
The impact of different discount rates on effects and cost‐effectiveness on current investment and prospective scenario 1 were examined using deterministic sensitivity analyses (see Table 4). Current investment was dominant only at discount rates between 0 and 2.46%, but was not dominant at higher rates. Prospective scenario 1 was found to be cost‐saving for all evaluated discount rates (0–5%). Additional analysis (Supporting information, Appendix S1) revealed that the discount rate of costs had a stronger qualitative effect on the ICER per QALY than the discount rate of effects. Furthermore, at a 5% discount rate, prospective scenario 2 delivered five times more QALYs compared with prospective scenario 1, but the ROI for smoking‐related costs was approximately one‐third lower (see Supporting information, Appendix S2 and Table 4).
Table 4.
Impact of discount rate on QALYs gained, ICER and smoking‐related ROI of current investment vs. zero investment and prospective scenario 1 versus current investment, deterministic sensitivity analysis, life‐time perspective.
| Discount rate | Outcome measures | ||
|---|---|---|---|
| QALYs gained in population | ICER per QALY gained (€) | ROI (€) | |
| Current investment versus zero investment | |||
| 0% | 106 756 | −1121.18a | 1.44 |
| 1% | 83 491 | −738.46a | 1.23 |
| 3% | 55 022 | 323.33 | 0.93 |
| 5% | 39 304 | 1725.86 | 0.75 |
| Prospective scenario 1 versus current investment | |||
| 0% | 29 193 | −2220.45a | 2.56 |
| 1% | 22 831 | −2144.04a | 2.18 |
| 3% | 15 046 | −1809.54a | 1.65 |
| 5% | 10 748 | −1259.95a | 1.33 |
ROI = return on investment; ICER = on incremental cost‐effectiveness.
Dominant [cheaper to run but generates more quality‐of‐life years (QALYs)]. The actual figure (in negative, rather than just stating ‘Dominant’) is reported here to allow readers to map this figure onto the reported ROI figure.
Discussion
The EQUIPT tool was developed to help stakeholders to evaluate new and ongoing smoking cessation strategies. We have evaluated several strategies for Germany using this model. Our evaluation showed that a scenario with increased reach of group‐based behavioural support, the financial incentive programme and varenicline resulted in long‐term cost savings and was a dominant (lower costs and higher QALYs) strategy. When uncertainty was accounted for, current investment and prospective scenarios were not dominant, although the incremental cost per QALY was low. The average incremental QALY gain per user was relatively small, but was offset by relatively low intervention costs and avoided disease costs. Sample iterations for prospective scenarios showed better cost‐effectiveness than for current investment. Overall, we found that it was possible to adjust and refine a national tobacco control model developed for England to fit the German context. This also offered a feasible alternative to creating a completely new model.
Group‐based behavioural support
Group‐based behavioural support is currently reimbursed partly by most statutory health insurances in Germany. The cost savings per invested Euro are between those of varenicline and the financial incentive programme. Some studies 32, 42 reported that specific group‐based behavioural support programmes are more effective than the effectiveness value used in our model. However, these studies did not include control groups and did not confirm smoking status with biochemical methods, only used but self‐reported status. Furthermore, these effectiveness estimates may be affected by selection bias and possible reporting bias due to social desirability, and were thus not incorporated into this evaluation. The use of group‐based behavioural support in Germany is low compared to the United Kingdom and seems to have decreased further recently. The use of support involving physicians, other health specialists and clinics fell from 7% in 2012 to 5% in 2014 43. This is unfortunate, as we find that group‐based behavioural support provides good value for money. The reach of this intervention may be improved by offering these programmes at the work‐place or by increasing reimbursement.
Financial incentive programme
The financial incentive programme is a dominant strategy, and according to our evaluation is the most cost‐saving intervention. Deposit‐based financial incentive programmes are even more cost‐effective than reward‐based programmes 25, but patients generally refrain from putting their own money at risk. Hence, proposed reach can often not be achieved for deposit‐based financial incentive programmes. One big advantage of the evaluated financial incentive programme is that participants are paid only when they actually stop smoking. This is not true for other interventions, where money is invested irrespective of smoking status and success. This also explains the relatively low average cost of financial incentive programmes. While the full costs for a successful quitter are US$960 (€681, GDP‐based PPP‐adjusted, currency year 2015), the average costs for each participant only amount to approximately US$235 (€167, GDP‐based PPP‐adjusted, currency year 2015) 25. Moreover, financial incentive programmes can be implemented easily by health insurances and private companies, as no specialist support is needed. One drawback of financial incentive programmes is their decreasing effectiveness 3 months after incentives end 26. This effect was also observed for financial incentives for weight control in obesity 44. Halpern et al. 2015 25 showed that although effectiveness was halved 6 months after stopping incentives, it was still good (RR= 2.17). As the estimated annual excess costs of employing a smoker can range around US$5816 (€4482, GDP‐based PPP‐ and inflation‐adjusted, currency year 2015) 45, smoking‐related costs are likely to be higher than financial incentive programme costs, even on an annual basis 25. Another risk of financial incentive programmes is the enrolment of non‐smokers. Providers should be aware of a patient's or employee's smoking status before offering respective programmes, and smoking status should be confirmed through biochemical testing before programme initiation.
Varenicline
Varenicline also resulted in cost savings in prospective scenarios 1 and 2, but the ROI of approximately €1 was lower than for group‐based behavioural support and financial incentive programmes. The increase in reach led to approximately 0.07 QALYs gained per additional varenicline user. These 3–4 quality‐adjusted weeks seem to be a relatively weak outcome. This is due mainly to the prerequisite that only smokers of 10 or more cigarettes per day were assumed to be eligible for varenicline, and thus the model reduced the effectiveness accordingly. Nevertheless, the investment was dominant because the avoided costs were relatively high. While varenicline offered the best effectiveness among the three interventions, it was also the most expensive. Increasing the reach of varenicline to that observed in the United Kungdom (prospective scenario 2) may not be feasible, as this would lead to an increase in usage of 2200% compared with current levels. An increase of 1 percentage point in the current reach seems more realistic. One possibility to increase reach for varenicline would be to lower costs and/or access restrictions 46. In contrast to standard NRT, where effectiveness increases with age, varenicline seems equally effective in younger and older people 47. Moreover, in a real‐world setting, varenicline together with specialist behavioural support was very effective 24, 48 and thus offers potential for transferring this evidence outside study environments.
Sensitivity analysis
To identify the most important drivers of model results, we performed a univariate probabilistic analysis using tornado diagrams. The inputs for intervention effects and reach had the highest impact on both costs and QALYs. Therefore, it is essential to find high‐quality sources for these inputs. The RRs used in our analyses were derived from international randomized controlled trials. At least for the reach, we were able to identify inputs which reflected specifically the current situation in Germany. The cost‐effectiveness plane of the current investment versus zero investment showed that approximately 40% of probability sensitivity analysis (PSA) iterations were located within the south‐east quadrant, the quadrant which indicates dominance. This amount increased to 45.7% for prospective scenario 1 and to 47.5% for prospective scenario 2. The majority of iterations were therefore within the north‐east quadrant, mainly close to the zero point. The upper boundary of the 95% CI for the ICERs excluded dominated replications for both scenarios, and was located close to the y‐axis for prospective scenarios 1 and 2 (Fig. 3). Using exemplary WTP thresholds of €5000, €10 000 and €15 000 (as Germany does not state any threshold) the conservative prospective scenario 1 had a probability of being cost‐effective of approximately 75, 85 and 90% respectively (Fig. 4).
Assisted versus unassisted quitting
One general criticism of assisted smoking cessation is that the vast majority of ex‐smokers quit without assistance 49. According to the Eurobarometer 2012, 76% of ex‐smokers in the EU27 and 75% of ex‐smokers in Germany quit without assistance 50. Only approximately 0.8% seemed to use prescription medication for quit attempts in Germany 15. Possibly, many more people would have quit smoking if they had used smoking cessation interventions. This question requires more research.
Implications for German stakeholders
This analysis provides information to policy/decision‐makers and wider stakeholders to justify the extent of health and economic returns that a policy change in current tobacco cessation in Germany could generate. The intention to use an economic tool such as the EQUIPTMOD in policymaking has been examined elsewhere 51. Overall, German stakeholders were less inclined to use a tobacco ROI tool compared with stakeholders from other countries under consideration. Thus, it is important to plan dissemination strategies that account for factors that facilitate or hinder stakeholder acceptance 52. Close collaboration between expert users and wider stakeholders will therefore be needed to implement the findings presented here.
Regulatory and practical implications
The German Pharmaceutical Directive 53 states that pharmacotherapy for smoking cessation is a life‐style product, as smoking is a free life‐style choice. Thus, these medications are not reimbursed and consumers have to pay out of their own pocket. Therefore, increasing the use of pharmacotherapy by increasing reimbursement is not possible and probably will not be in the near future. A recent court decision even excluded the reimbursement of pharmacotherapy for smoking cessation for patients registered in disease‐management programmes for COPD 54, despite their seemingly good cost‐effectiveness 55. Unlike pharmacotherapy, statutory and private health insurances often reimburse a certain proportion of group‐based behavioural support costs for smoking cessation (source: clinic correspondence). Financial incentive programmes would have to be financed by employers or insurance companies. Although deposit‐based financial incentive programmes also exist, as the patient's own money is partially at stake, participation rates in these programmes are relatively low 25. Other ways to increase the reach of these interventions include improving German medical students’ knowledge of how to treat smoking 56, but also to encourage companies to offer respective seminars or financial incentives. Intervention usage can only be increased if decision‐makers are willing to act and if they recognize the need for smoking cessation. The smoking cessation interventions included in our analysis are reasonable options that policymakers might want to consider.
Study strengths and limitations
A strength of this study is the evaluation of several investment scenarios involving different smoking cessation interventions for Germany. Like any other model, EQUIPTMOD also has limitations (Coyle et al. 2016), and therefore any weaknesses of the current analysis should be considered in that context. Although prospective scenarios included additional within‐model costs (e.g. increased cost of varenicline due to increased reach), we recognize that extra costs over and above those within‐model costs (e.g. costs of policy change to improve reach of varenicline) may be necessary to implement the scenarios. Furthermore, the model does not include possible costs or effects of adverse events of varenicline. However, recent studies have not shown an adverse safety profile for varenicline 57, 58, 59, 60, 61, 62. Close collaboration with stakeholders and experts in the field of smoking cessation both nationally and internationally allowed us to evaluate interventions for which no effectiveness data could be identified for Germany. Thus, our model is the first, to our knowledge, to evaluate the cost‐effectiveness of financial incentive programmes in Germany. For missing inputs, Cochrane effectiveness inputs from a wide range of studies world‐wide were used, and represented rigorous and well‐accepted evidence. Some reported but controversial protective effects of smoking, such as those regarding Parkinson disease 63, 64, 65, 66, 67, 68, 69, 70, 71, were not accounted for in this model. Some diseases attributable to smoking 5 were also not included in the model, as the true impact of smoking could not be estimated, and some interventions available only in the United Kingdom but not in Germany were also not considered. Another important limitation concerns PSA. The economic model was developed primarily to underpin a ROI tool for decision‐making purposes. This objective inevitably required not only the provision of a simple generalized user interface (GUI) and granularity of outputs (a number of ROI metrics), but also subjected us significantly to consider Microsoft Excel's own limitations to handle such a large model. The PSA functionality was therefore restricted to provide uncertainty estimates for current investment versus zero investment scenarios. Thus, the current PSA functionality does not lend itself directly to the evaluation of uncertainty concerning current policy changes (i.e. prospective investment versus current investment scenarios). In such cases, indirect comparison may still be performed by subjecting both current and projective investments to the baseline (i.e. zero investment scenario). Future updates of the EQUIPTMOD should consider improving upon this PSA functionality.
It should be noted that increasing reach for varenicline or group‐based behavioural support does not increase the reach of those interventions in isolation. Increasing varenicline reach will also have an impact upon the subpopulation of potential quitters who receive a combination of varenicline and a behavioural support (and vice versa for changes to group‐based behavioural support). While the number of smokers affected will be small, it cannot be asserted that the improvements in outcomes are associated only with the increase in reach of the individual therapies. Moreover, the ROI is based on smoking‐related costs and does not include other costs associated with quitters living longer. While the health‐care costs of increased life‐span are not accounted for, they are generally far into the future and, through discounting, would have only a minor impact on results.
Conclusion
Increasing the reach of group‐based behavioural support, financial incentives and varenicline for smoking cessation by just 1% of current annual quit attempts has a 75% probability of being cost‐effective, at a WTP threshold of only €5000 per QALY. To German policymakers, this may provide a strategy that is both economically acceptable and beneficial to the population's health outcomes.
Ethics approval
None required for this study. However, the EQUIPT project was approved by Brunel University Research Ethics Committee.
Data sharing
The model and accompanying documents are freely available to download from http://equipt.eu.
Declaration of interests
None.
Supporting information
Appendix S1 Impact of differential discounting on incremental cost‐effectiveness (ICER) for current investment versus zero investment, life‐time perspective
Appendix S2 Impact of discount rate on quality‐of‐life years (QALY) gained, incremental cost‐effectiveness (ICER) and return on investment (ROI) for prospective scenario 2 versus current investment over a life‐time perspective.
Acknowledgements
The funding was received from the European Community's Seventh Framework Programme under grant agreement no. 602270 (EQUIPT).
Huber, M. B. , Präger, M. , Coyle, K. , Coyle, D. , Lester‐George, A. , Trapero‐Bertran, M. , Nemeth, B. , Cheung, K. L. , Stark, R. , Vogl, M. , Pokhrel, S. , and Leidl, R. (2018) Cost‐effectiveness of increasing the reach of smoking cessation interventions in Germany: results from the EQUIPTMOD. Addiction, 113: 52–64. https://doi.org/10.1111/add.14062.
References
- 1. National Center for Chronic Disease , Prevention and Health Promotion (US) Office on Smoking and Health . Reports of the Surgeon General. The Health Consequences of Smoking–50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2014. [PubMed] [Google Scholar]
- 2. Thun M. J., Carter B. D., Feskanich D., Freedman N. D., Prentice R., Lopez A. D. et al 50‐year trends in smoking‐related mortality in the United States. N Engl J Med 2013; 368: 351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Perricone C., Versini M., Ben‐Ami D., Gertel S., Watad A., Segel M. J. et al Smoke and autoimmunity: the fire behind the disease. Autoimmun Rev 2016; 15: 354–374. [DOI] [PubMed] [Google Scholar]
- 4. Jha P. Avoidable global cancer deaths and total deaths from smoking. Nat Rev Cancer 2009; 9: 655–664. [DOI] [PubMed] [Google Scholar]
- 5. Carter B. D., Abnet C. C., Feskanich D., Freedman N. D., Hartge P., Lewis C. E. et al Smoking and mortality—beyond established causes. N Engl J Med 2015; 372: 631–640. [DOI] [PubMed] [Google Scholar]
- 6. Islami F., Torre L. A., Jemal A. Global trends of lung cancer mortality and smoking prevalence. Transl Lung Cancer Res 2015; 4: 327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leidl R., Wacker M., Schwarzkopf L. Better understanding of the health care costs of lung cancer and the implications. Expert Rev Respir Med 2016; 10: 373–375. [DOI] [PubMed] [Google Scholar]
- 8. Wacker M., Holle R., Heinrich J., Ladwig K. H., Peters A., Leidl R. et al The association of smoking status with healthcare utilisation, productivity loss and resulting costs: results from the population‐based KORA F4 study. BMC Health Serv Res 2013; 13: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Colditz G. A. Smoke alarm—tobacco control remains paramount. N Engl J Med 2015; 372: 665–666. [DOI] [PubMed] [Google Scholar]
- 10. Pirie K., Peto R., Reeves G. K., Green J., Beral V. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet 2013; 381: 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shelley D., Cantrell M. J., Moon‐Howard J., Ramjohn D. Q., VanDevanter N. The $5 man: the underground economic response to a large cigarette tax increase in New York City. Am J Public Health 2007; 97: 1483–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Callaghan R. C., Tavares J., Taylor L. Another example of an illicit cigarette market: a study of psychiatric patients in Toronto, Ontario. Am J Public Health 2008; 98: 4–5; author reply. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kalkhoran S., Glantz S. A. E‐cigarettes and smoking cessation in real‐world and clinical settings: a systematic review and meta‐analysis. Lancet Respir Med 2016; 4: 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kotz D., Fidler J. A., West R. Did the introduction of varenicline in England substitute for or add to the use of other smoking cessation medications? Nicotine Tob Res 2011; 13: 793–799. [DOI] [PubMed] [Google Scholar]
- 15. Kroger C. B., Gomes de Matos E., Piontek D., Wenig J. R. Quitting attempts and utilisation of smoking cessation aids among smokers in Germany: results from the 2012 epidemiological survey of substance abuse. Gesundheitswesen 2015; 78: 752–758. [DOI] [PubMed] [Google Scholar]
- 16. Borland R., Li L., Driezen P., Wilson N., Hammond D., Thompson M. E. et al Cessation assistance reported by smokers in 15 countries participating in the international tobacco control (ITC) policy evaluation surveys. Addiction 2012; 107: 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Public Health England . Local tobacco control profiles for England 2014. Available at: https://www.gov.uk/government/collections/local-tobacco-control-profiles-for-england (accessed on 22 August 2017) (archived at https://www.webcitation.org/6su6WAKdq on 22 August 2017).
- 18. Mikrozensus, Fragen zur Gesundheit, Rauchgewohnheiten der Bevölkerung 2013: Statistisches Bundesamt; 2014. [Microcensus—Health Issues. Smoking Habits of the Population. Federal Office of Statistics. Available at: https://www.destatis.de/DE/Publikationen/Thematisch/Gesundheit/Gesundheitszustand/Rauchgewohnheiten5239004139004.pdf?__blob=publicationFile
- 19. Pokhrel S., Evers S., Leidl R., Trapero‐Bertran M., Kalo Z., Vries H. et al EQUIPT: protocol of a comparative effectiveness research study evaluating cross‐context transferability of economic evidence on tobacco control. BMJ Open 2014; 4: e006945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coyle K., Coyle D., Lester‐George A., West R., Nemeth B., Hiligsmann M. et al Development and application of an economic model (EQUIPTMOD) to assess the impact of smoking cessation. Addiction; https://doi.org/10.1111/add.14001. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. National Institute for Health and Care Excellence (NICE) . Tobacco return on investment tool 2014. Available at: http://www.webcitation.org/6su4HhNqa.
- 22. Mahmoudi M., Coleman C. I., Sobieraj D. M. Systematic review of the cost‐effectiveness of varenicline vs. bupropion for smoking cessation. Int J Clin Pract 2012; 66: 171–182. [DOI] [PubMed] [Google Scholar]
- 23. Kotz D., Brown J., West R. Prospective cohort study of the effectiveness of varenicline versus nicotine replacement therapy for smoking cessation in the ‘real world’. BMC Public Health 2014; 14: 1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kotz D., Brown J., West R. ‘Real‐world’ effectiveness of smoking cessation treatments: a population study. Addiction 2014; 109: 491–499. [DOI] [PubMed] [Google Scholar]
- 25. Halpern S. D., French B., Small D. S., Saulsgiver K., Harhay M. O., Audrain‐McGovern J. et al Randomized trial of four financial‐incentive programs for smoking cessation. N Engl J Med 2015; 372: 2108–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mantzari E., Vogt F., Shemilt I., Wei Y., Higgins J. P., Marteau T. M. Personal financial incentives for changing habitual health‐related behaviors: a systematic review and meta‐analysis. Prev Med 2015; 75: 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cahill K., Hartmann‐Boyce J., Perera R. Incentives for smoking cessation. Cochrane Database Syst Rev 2015; 5: CD004307. [DOI] [PubMed] [Google Scholar]
- 28. Volpp K. G., Troxel A. B., Pauly M. V., Glick H. A., Puig A., Asch D. A. et al A randomized, controlled trial of financial incentives for smoking cessation. N Engl J Med 2009; 360: 699–709. [DOI] [PubMed] [Google Scholar]
- 29. German: DAK‐Gesundheit . Bonuspunktekatalog: Gesundheitsmaßnahmen und ‐erfolge 2016 English: DAK Health. Catalogue of Bonus points: Health activities and achievements 2016. Available at: https://www.dak.de/dak/leistungen/gesundheitsmassnahmen-und--erfolge-1738992.html (accessed 22 August 2017) (archived at http://www.webcitation.org/6su3W1Wkz on 22 August 2017).
- 30. German: Techniker Krankenkasse . Maßnahmen 2016. English: Techniker Health Insurance company. Activities 2016. Available at: https://www.tk.de/tk/bonusprogramm/tk-bonusprogramm/massnahmen/140976 (accessed 22 August 2017) (archived at https://www.webcitation.org/6su3kd3WN on 22 August 2017).
- 31. German: AOK-PLUS. Teilnahmebedingungen AOK PLUS Bonusprogramm 2016. English: AOK‐PLUS. Requirements for participation in the AOK PLUS Bonusprogram 2016. Available at: https://plus.aok.de/fileadmin/user_upload/AOK-PLUS/05-Content-PDF/Bonusprogramm-Infoblatt-1-UEbersicht-Massnahmen-und-Teilnahme.pdf (accessed 22 August 2017).
- 32. Wenig J. R., Erfurt L., Kroger C. B., Nowak D. Smoking cessation in groups—who benefits in the long term? Health Educ Res 2013; 28: 869–878. [DOI] [PubMed] [Google Scholar]
- 33. Yeh E., Levasseur G., Kaiserman M. J. Evaluation of urinary cotinine immunoassay test strips used to assess smoking status. Nicotine Tob Res 2011; 13: 1045–1051. [DOI] [PubMed] [Google Scholar]
- 34. Stead L. F., Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database Syst Rev 2005; 2: CD001007 http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD001007.pub2/epdf [DOI] [PubMed] [Google Scholar]
- 35. Cahill K., Stead L. F., Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev 2012; 4: CD006103. [DOI] [PubMed] [Google Scholar]
- 36. Gerritsen M., Berndt N., Lechner L., de Vries H., Mudde A., Bolman C. Self‐reporting of smoking cessation in cardiac patients: How reliable is it and is reliability associated with patient characteristics? J Addict Med 2015; 9: 308–316. [DOI] [PubMed] [Google Scholar]
- 37. German: Institute of Quality and Efficiency in Health Care (IQWiG) . Allgemeine Methoden: Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen; 2015. English: Institute of Quality and Efficiency in Health Care (IQWiG). General methods: Institute of Quality and Efficiency in Health Care; 2015. Available at: https://www.iqwig.de/download/IQWiG_Methoden_Version_4-2.pdf (accessed 22 August 2017).
- 38. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation; 2015. [Google Scholar]
- 39. Wickham H. ggplot2: Elegant Graphics For Data Analysis. New York, NY: Springer; 2009. [Google Scholar]
- 40. German: Statistisches Bundesamt . Ausgangsdaten der Bevölkerungsfortschreibung aus dem Zensus 2011. Wiesbaden: Statistisches Bundesamt; 2015. English: Federal Statistical Office of Germany. Initial data of the population update 2011. Wiesbaden: Statistisches Bundesamt; 2015.
- 41. Thyrian J. R., Panagiotakos D. B., Polychronopoulos E., West R., Zatonski W., John U. The relationship between smokers’ motivation to quit and intensity of tobacco control at the population level: a comparison of five European countries. BMC Public Health 2008; 8: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Erfurt L., Kroger C. B. Use of medication in combination with a modern group programme for smoking cessation. Gesundheitswesen 2015; 77: 74–80. [DOI] [PubMed] [Google Scholar]
- 43. TNS Opinion and Social at the request of Directorate‐General Health and consumers. Special Eurobarometer 429—Attitudes of Europeans towards Tobacco and Electronic Cigarettes 2015. Available at: http://ec.europa.eu/public_opinion/archives/ebs/ebs_429_en.pdf
- 44. Paul‐Ebhohimhen V., Avenell A. Systematic review of the use of financial incentives in treatments for obesity and overweight. Obes Rev 2008; 9: 355–367. [DOI] [PubMed] [Google Scholar]
- 45. Berman M., Crane R., Seiber E., Munur M. Estimating the cost of a smoking employee. Tob Control 2014; 23: 428–433. [DOI] [PubMed] [Google Scholar]
- 46. Galaznik A., Cappell K., Montejano L., Makinson G., Zou K. H., Lenhart G. Impact of access restrictions on varenicline utilization. Expert Rev Pharmacoecon Outcomes Res 2013; 13: 651–656. [DOI] [PubMed] [Google Scholar]
- 47. Scholz J., Lima Santos P. C., Buzo C. G., Moreira Lopes N. H., Abe T. M., Gaya P. V. et al Effects of aging on the effectiveness of smoking cessation medication. Oncotarget 2016; 7: 30032–30036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pascual F. P., Fontoba Ferrandiz J., Gil Sanchez M. C., Ponce Lorenzo F., Botella E. C. Two‐year therapeutic effectiveness of varenicline for smoking cessation in a real world setting. Subst Use Misuse 2016; 51: 131–140. [DOI] [PubMed] [Google Scholar]
- 49. Chapman S., MacKenzie R. The global research neglect of unassisted smoking cessation: causes and consequences. PLOS Med 2010; 7: e1000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. TNS Opinion and Social at the request of Directorate‐General Health and consumers. Special Eurobarometer 385—Attitudes of Europeans towards Tobacco 2012. Available at: http://ec.europa.eu/health/tobacco/docs/eurobaro_attitudes_towards_tobacco_2012_en.pdf
- 51. Voko Z., Cheung K. L., Jozwiak‐Hagymasy J., Wolfenstetter S., Jones T., Munoz C. et al Similarities and differences between stakeholders’ opinions on using health technology assessment (HTA) information across five European countries: results from the EQUIPT survey. Health Res Policy Syst 2016; 14: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cheung K. L., Evers S. M., Hiligsmann M., Voko Z., Pokhrel S., Jones T. et al Understanding the stakeholders’ intention to use economic decision‐support tools: a cross‐sectional study with the tobacco return on investment tool. Health Policy 2016; 120: 46–54. [DOI] [PubMed] [Google Scholar]
- 53. German: Gemeinsamer Bundesausschuss . Anlage II zum Abschnitt F der Arzneimittel‐Richtlinie 2016. English: Federal joint committee. Appendix II to Paragraph F of the Pharmaceutical Directive 2016. Available at: https://www.g-ba.de/downloads/83-691-397/AM-RL-II-Life%20style-2016-02-03.pdf (accessed 22 August 2017).
- 54. Landessozialgericht Berlin Brandenburg . Court decision: L 9 KR 309/12 KL. 2015.
- 55. Kirsch F. A systematic review of quality and cost‐effectiveness derived from Markov models evaluating smoking cessation interventions in patients with chronic obstructive pulmonary disease. Expert Rev Pharmacoecon Outcomes Res 2015; 15: 301–316. [DOI] [PubMed] [Google Scholar]
- 56. Strobel L., Schneider N. K., Krampe H., Beissbarth T., Pukrop T., Anders S. et al German medical students lack knowledge of how to treat smoking and problem drinking. Addiction 2012; 107: 1878–1882. [DOI] [PubMed] [Google Scholar]
- 57. Wu Q., Gilbody S., Peckham E., Brabyn S., Parrott S. Varenicline for smoking cessation and reduction in people with severe mental illnesses: systematic review and meta‐analysis. Addiction 2016; 111: 1554–1567. [DOI] [PubMed] [Google Scholar]
- 58. Cunningham F. E., Hur K., Dong D., Miller D. R., Zhang R., Wei X. et al A comparison of neuropsychiatric adverse events during early treatment with varenicline or a nicotine patch. Addiction 2016; 111: 1283–1292. [DOI] [PubMed] [Google Scholar]
- 59. Raich A., Ballbe M., Nieva G., Cano M., Fernandez T., Bruguera E. et al Safety of Varenicline for smoking cessation in psychiatric and addicts patients. Subst Use Misuse 2016; 51: 649–657. [DOI] [PubMed] [Google Scholar]
- 60. Kotz D., Viechtbauer W., Simpson C., van Schayck O. C., West R., Sheikh A. Cardiovascular and neuropsychiatric risks of varenicline: a retrospective cohort study. Lancet Respir Med 2015; 3: 761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sterling L. H., Windle S. B., Filion K. B., Touma L., Eisenberg M. J. Varenicline and adverse cardiovascular events: a systematic review and meta‐analysis of randomized controlled trials. J Am Heart Assoc 2016; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Eisenberg M. J., Windle S. B., Roy N., Old W., Grondin F. R., Bata I. et al Varenicline for smoking cessation in hospitalized patients with acute coronary syndrome. Circulation 2016; 133: 21–30. [DOI] [PubMed] [Google Scholar]
- 63. Checkoway H., Powers K., Smith‐Weller T., Franklin G. M., Longstreth W. T. Jr., Swanson P. D. Parkinson's disease risks associated with cigarette smoking, alcohol consumption, and caffeine intake. Am J Epidemiol 2002; 155: 732–738. [DOI] [PubMed] [Google Scholar]
- 64. van der Mark M., Nijssen P. C., Vlaanderen J., Huss A., Mulleners W. M., Sas A. M. et al A case–control study of the protective effect of alcohol, coffee, and cigarette consumption on Parkinson disease risk: time‐since‐cessation modifies the effect of tobacco smoking. PLOS ONE 2014; 9: e95297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Quik M., Parameswaran N., McCallum S. E., Bordia T., Bao S., McCormack A. et al Chronic oral nicotine treatment protects against striatal degeneration in MPTP‐treated primates. J Neurochem 2006; 98: 1866–1875. [DOI] [PubMed] [Google Scholar]
- 66. Hernan M. A., Takkouche B., Caamano‐Isorna F., Gestal‐Otero J. J. A meta‐analysis of coffee drinking, cigarette smoking, and the risk of Parkinson's disease. Ann Neurol 2002; 52: 276–284. [DOI] [PubMed] [Google Scholar]
- 67. Powers K. M., Kay D. M., Factor S. A., Zabetian C. P., Higgins D. S., Samii A. et al Combined effects of smoking, coffee, and NSAIDs on Parkinson's disease risk. Mov Disord 2008; 23: 88–95. [DOI] [PubMed] [Google Scholar]
- 68. Hancock D. B., Martin E. R., Stajich J. M., Jewett R., Stacy M. A., Scott B. L. et al Smoking, caffeine, and nonsteroidal anti‐inflammatory drugs in families with Parkinson disease. Arch Neurol 2007; 64: 576–580. [DOI] [PubMed] [Google Scholar]
- 69. Morens D. M., Grandinetti A., Davis J. W., Ross G. W., White L. R., Reed D. Evidence against the operation of selective mortality in explaining the association between cigarette smoking and reduced occurrence of idiopathic Parkinson disease. Am J Epidemiol 1996; 144: 400–404. [DOI] [PubMed] [Google Scholar]
- 70. Thacker E. L., O'Reilly E. J., Weisskopf M. G., Chen H., Schwarzschild M. A., McCullough M. L. et al Temporal relationship between cigarette smoking and risk of Parkinson disease. Neurology 2007; 68: 764–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Scott W. K., Zhang F., Stajich J. M., Scott B. L., Stacy M. A., Vance J. M. Family‐based case–control study of cigarette smoking and Parkinson disease. Neurology 2005; 64: 442–447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Impact of differential discounting on incremental cost‐effectiveness (ICER) for current investment versus zero investment, life‐time perspective
Appendix S2 Impact of discount rate on quality‐of‐life years (QALY) gained, incremental cost‐effectiveness (ICER) and return on investment (ROI) for prospective scenario 2 versus current investment over a life‐time perspective.


