Abstract
Aims
To evaluate the feasibility of a one‐stop microvascular screening service for the early diagnosis of diabetic distal symmetrical polyneuropathy, painful distal symmetrical polyneuropathy and the at‐risk diabetic foot.
Methods
People with diabetes attending retinal screening in hospital and community settings had their feet examined by a podiatrist. Assessment included: Toronto Clinical Neuropathy Score evaluation; a 10‐g monofilament test; and two validated, objective and quick measures of neuropathy obtained using the point‐of‐care devices ‘DPN‐Check’, a hand‐held device that measures sural nerve conduction velocity and amplitude, and ‘Sudoscan’, a device that measures sudomotor function. The diagnostic utility of these devices was assessed against the Toronto Clinical Neuropathy Score as the ‘gold standard’.
Results
A total of 236 consecutive people attending the retinal screening service, 18.9% of whom had never previously had their feet examined, were evaluated. The prevalence of distal symmetrical polyneuropathy, assessed using the Toronto Clinical Neuropathy Score, was 30.9%, and was underestimated by 10‐g monofilament test (14.4%). The prevalence of distal symmetrical polyneuropathy using DPN‐check was 51.5% (84.3% sensitivity, 68.3% specificity), 38.2% using Sudoscan foot electrochemical skin conductance (77.4% sensitivity, 68.3% specificity), and 61.9% using abnormality in either of the results (93.2% sensitivity, 52.8% specificity). The results of both devices correlated with Toronto Clinical Neuropathy Score (P<0.001). A new diagnosis of painful distal symmetrical polyneuropathy was made in 59 participants (25%), and 56.6% had moderate‐ or high‐risk foot. Participants rated the service very highly.
Conclusions
Combined, eye, foot and renal screening is feasible, has a high uptake, reduces clinic visits, and identifies painful distal symmetrical polyneuropathy and the at‐risk foot. Combined large‐ and small‐nerve‐fibre assessment using non‐invasive, quantitative and quick point‐of‐care devices may be an effective model for the early diagnosis of distal symmetrical polyneuropathy.
What's new?
A novel, one‐stop microvascular screening service in a hospital and community setting was initiated, whereby people with diabetes attending the annual eye screening, which has a high uptake, also underwent foot assessment aimed at detecting early peripheral neuropathy and the at‐risk foot requiring referral to the Foot Protection Team. Foot examination was carried out by a podiatrist. The service also identified previously undiagnosed painful neuropathy and had a high patient acceptability level.
The service used novel, validated point‐of‐care devices for combined large‐ and small‐nerve‐fibre assessment, with the aim of diagnosing peripheral neuropathy early.
What's new?
A novel, one‐stop microvascular screening service in a hospital and community setting was initiated, whereby people with diabetes attending the annual eye screening, which has a high uptake, also underwent foot assessment aimed at detecting early peripheral neuropathy and the at‐risk foot requiring referral to the Foot Protection Team. Foot examination was carried out by a podiatrist. The service also identified previously undiagnosed painful neuropathy and had a high patient acceptability level.
The service used novel, validated point‐of‐care devices for combined large‐ and small‐nerve‐fibre assessment, with the aim of diagnosing peripheral neuropathy early.
Introduction
The number of diabetes‐related amputations in England has now reached an all‐time high of 20 per day 1, with the annual number of diabetes‐related amputations at 7370 1. Although there has been a very small but significant reduction in major amputations per 10 000 people with diabetes, this has been offset by the increase in both Type 2 diabetes and minor amputations 2. Amputation is not only devastating in its impact on the person with diabetes and their family, leading to loss of independence and livelihood, but also results in a remarkable consumption of scarce medical resources and a very high mortality rate 3. Yet, it is estimated that 80% of amputations in England could be prevented through improved healthcare and management of diabetes 1.
Most amputations are preceded by foot ulceration, and diabetic distal symmetrical polyneuropathy (DPN) is the strongest initiating factor. DPN is very common, with a lifetime prevalence of 50% of all people with diabetes 4. Currently, the clinical assessments we use to screen for DPN, such as the 10‐g monofilament test, identify those at risk of foot ulceration but are not good for screening for early neuropathy 5. Unfortunately, these assessments detect the disease very late, at a time when treatment is unlikely to work. Furthermore, peripheral neurological examination using bedside instruments, such as the tuning fork and patella hammer, is not reproducible even when performed by experts 6. The situation is clearly different for the detection of early retinopathy using digital retinal photography and early nephropathy by measuring microalbuminuria. The development of early disease markers and the institution of robust screening programmes have had a tremendous impact on disease outcomes. For example, the institution of annual digital camera‐based retinal screening for all people with diabetes in the UK over the past decade has contributed to significant reduction in blindness, such that retinopathy is no longer the commonest cause of blindness in working‐age adults 7. Currently, a robust system of annual diabetes foot screening, as advocated by Diabetes UK, is not fully in place in the UK 8. Unfortunately, by the time DPN is detected it is often very well established and, consequently, it is impossible to reverse/halt the neuropathic process. Many of these patients end up in foot clinics and have very poor outcomes, with 5‐year mortality close to 50% 3.
To improve foot outcomes, there is an urgent need to develop a high‐uptake and effective diabetes foot screening programme 8. There has been a recent advance in the development of non‐invasive, objective, accurate point‐of‐care devices (POCDs) that may be able to diagnose DPN early, before overt clinical signs are apparent 9, 10. These devices do not require specialist training to use in routine clinical care and provide results within a few minutes. The aims of the present study were to examine the feasibity and patient acceptability of a combined eye, foot and renal screening clinic and to evaluate the feasibility of use and diagnostic utility of two POCDs in detecting DPN early.
Methods
Study design and participants
Peripheral neurological examination
A total of 244 consecutive patients with either Type 1 or Type 2 diabetes attending for annual eye screening in a hospital (Northern General Hospital, Sheffield) and primary care (Jordanthorpe Medical Centre, Sheffield) setting were recruited between January 2015 and December 2016 into this service development project, funded by Sheffield Teaching Hospitals NHS Foundation Trust. Before undergoing retinal photography and whilst the mydriatic was taking effect, the feet were examined by a podiatrist (O.B.H.) in an adjacent room for any abnormality, including deformity, callus and ulceration. The presence of dorsalis pedis and posterior tibial pulses were also assessed. Participants then underwent Toronto Clincal Neuropathy Score (TCNS) assessment 11 and the 10‐g monofilament test (at five sites in each foot, with an inability to feel ≥2 sites taken to indicate DPN). The TCNS has been found to be a valid instrument to reflect the presence and severity of DPN as measured by sural nerve morphology and electrophysiology 11, and was used in the present study as the ‘gold standard’ for the diagnosis of DPN against which all other measures of DPN were compared. It takes ~15 min to perform the TCNS assessment, which includes evaluation of symptoms (score 0–6) and reflexes (0–8), and a sensory examination (0–10), with a maximum score of 24, and a minimum score of 5 to diagnose DPN. A score of 5–8 was regarded as mild DPN, 9–11 as moderate DPN and ≥12 as severe DPN. Finally, participants underwent assessment of large‐fibre function using ‘DPN‐Check’ (Neurometrix Inc., Waltham, MA, USA) 12 and small‐fibre function using ‘Sudoscan’ (Impeto Medical, Paris, France) 13 as described below. These POCDs were chosen as they are both easily portable, provide quick, objective quatitative results and have been validated to detect DPN. As DPN involves both large and small fibres, an objective assessment of both seemed reasonable.
Assessment of peripheral neuropathy using DPN‐Check
Participants underwent sural sensory nerve conduction velocity (SNCV; m/s) and amplitude [sural nerve action potential (SNAP); μV] measurement in both the left and right leg (to insure symmetry) using DPN‐Check, and the average was calculated 9. These tests were conducted by the same podiatrist, without any technical expertise in standard nerve conduction study protocol, and with only 1‐h training in the use of this device. DPN‐Check is a handheld POCD with stimulating probes at one end and a disposable biosensor 9.22 cm at the other end. The biosensor covered a wide area of the lateral aspect of the lower limb just above the ankle to reliably record SNAP responses without the need for careful positioning over the sural nerve by an expert electrophysiologist. The device had a built‐in infrared thermometer just below the stimulating probes to measure skin temperature near the ankle. Measured skin temperature was used to normalize SNCV to an equivalent measured at 28°C. The device had a screen that displayed SNCV and SNAP. Unlike conventional electrophysiology equipment, DPN‐Check stimulated the sural nerve orthodromically. A single recording took ~2 min. We used proprietary thresholds for the diagnosis of DPN if either SNAP was >4 μV and/or SNCV was < 40 m/s 12. An abnormality in both legs was required for a diagnosis of DPN 9, 12.
Assessment of peripheral neuropathy using Sudoscan
Participants then underwent assessment of small‐fibre neuropathy using Sudoscan, a device developed to provide a quick, non‐invasive and reproducible 13, quantitative assessment of sudomotor function 10. This was performed with participants placing their hands and bare feet on electrode plates for 3 min 14. Measurement was based on an electrochemical reaction between electrodes and chloride ions, after stimulation of small fibres innervating sweat glands by a low‐voltage current (<4 V) 10, 13. A measurement of electrochemical skin conductance (ESC; μS) for the hands and feet was generated from the derivative current associated with the applied voltage 9. Sudomotor dysfunction, and hence DPN, was diagnosed according to the ESC measured on the feet with Sudoscan: foot ESC >60μS = no DPN and <60μS = DPN. These threshold values were defined on the basis of previous studies 14. No special preparation of participants was required.
Assessment of foot risk
In addition to neuropathy evaluation, the feet were also carefully examined for deformity, callus, ulceration, amputations, gangrene, infection/inflammation, the presence of foot pulses and Charcot arthropathy, and foot risk was determined according to National Institute for Health and Care Excellence (NICE) guidelines (NG19) 16.
Renal screening and questionnaires
Blood (urea and electrolytes, creatinine, estimated GFR) and urine (albumin:creatinine ratio) tests for renal screening, HbA1c, vitamin B12, liver function tests and lipid profile were carried out if these had not been measured in the previous 6 months. These results were readily available in shared primary and secondary care records. The presence of painful DPN was determined on the basis of the presence of bilateral lower limb painful neuropathic symptoms (e.g. paraethesia, burning, sharp shooting, deep aching, numbness and contact hypersensitivity) for at least 3 months and a TCNS > 5. Where this was the case, the average intensity of neuropathic pain over the previous 24 h, with 0 indicating no pain and 10 indicating worst pain imaginable (numeric rating scale), was obtained. Finally a patient satisfaction survey for this one‐stop service (strongly against, against, neutral, in favour, strongly in favour) was completed.
Statistical analysis
Statistical analysis was carried out using spss (version 21, IBM). Group differences with regard to demographic characteristics, and clinical and neurophysiological measures were compared using Student's t‐tests/anova for normally distributed variables and the Mann–Whitney U‐test for non‐normally distributed variables. For categorical variables the chi‐squared test for frequencies was used. Associations of POCDs with DPN severity (TCNS score) were assessed using Pearson's coefficient correlation and linear regression. The diagnostic validity of POCDs was analysed using receiver‐operating characteristic (ROC) curves to determine sensitivity, specificity and area under the curve (AUC). The optimal thesholds for POCDs to distinguish between the presence or absence of DPN was determined by calculating the Youden's index. Using the optimized POCDs thresholds, ROC curves were generated to compare the POCD results [results from each POCD and combined POCD results (either Sudoscan or DPN‐Check results below threshold)] with DPN status, as defined by TCNS. The TCNS threshold used for this analysis was the presence of DPN regardless of severity, i.e. a TCNS score >5. We used a second approach to develop an algorithm for the use of POCDs in a clinical context. Two threshold values were determined, one that maximized sensitivity and the other that maximized specificity, such that the negative likelihood ratio would approach 0.1, while the positive likelihood ratio would approach 10. A sample size of at least 106 (53 DPN cases) had > 97.5% probability to discriminate a conservatively modelled AUC 0.80 from the null hypothesis in which the diagnostic accuracy was no different from chance alone (AUC 0.5) 17. An α‐level of 0.05 was used for tests of statistical significance. Normality distribution of study measures was assessed graphically and using the Kolmogorov–Smirnov test.
Results
Altogether, data from 236 participants were analysed [mean age (sd) 63.5(14.1) years; 61.4% men; 97.8% with Type 2 diabetes (Table 1)]. Eight participants [mean (sd) age 72 (11.2) years] were excluded because of missing data for one or both POCDs: seven were missing DPN‐Check results (three were non‐compliant, two had severe lower‐limb oedema and two underwent amputations) and five were missing Sudoscan results (technical issues and amputations). A total of 33 participants (14.1%) had previous foot ulcers and four (2.1%) had undergone previous amputations. Just under half of the participants (43%) recalled being provided with basic foot care education 18. Only 46 participants (18.9%) had documented evidence of foot screening completed in the previous 12 months, five of whom (10.9%) had a history of foot ulceration. Foot risk assessment showed that 43.4% had a low, 38.5% a moderate and 18.1% a high risk of foot ulceration 16.
Table 1.
Clinical characteristics and demographics of the study population
| Total study population | |
|---|---|
| Number of participants | 236 |
| Mean (sd) age, years | 63.5(14.1) |
| Gender: male, n (%) | 145(61.4) |
| Type 2 diabetes, n (%) | 231(97.8) |
| No DPN | Mild DPN | Moderate DPN | Severe DPN | |
|---|---|---|---|---|
| Number of participants, n (%) | 163 (69.1) | 34 (14.4) | 19 (8.1) | 20 (8.5) |
| Mean (sd) age, years* | 61.6 (14.3) | 66.8 (12.9) | 66.1 (13.9) | 67.9 (12.7) |
| Type 2 diabetes, n (%) | 154 (95.1) | 33 (100) | 18 (94.7) | 19 (95.0) |
| Previous ulcer†, n (%) | 11 (6.7) | 7 (20.0) | 4 (21.1) | 11 (55.0) |
| Amputation†, n (%) | 0 | 1 (2.9) | 0 | 3 (15.0) |
| Mean (sd) HbA1c | ||||
| mmol/mol | 60.7 (18.8) | 61.3 (19.8) | 58.4 (22.1) | 68.1 (23.2) |
| % | 7.7 (3.9) | 7.8 (4) | 7.5 (4.2) | 8.4 (4.3) |
| Mean (sd) albumin:creatinine ratio | 6.5 (21.4) | 22.7 (54.4) | 2.4 (2.1) | 8.5 (14.9) |
| Systolic blood pressure, mmHg | 139.3 | 139.4 | 137.3 | 135.1 |
| Diastolic blood pressure, mmHg | 80.1 | 82.0 | 77.7 | 82.3 |
| Positive 10‐g monofilament test, n (%) | 12 (7.4) | 4 (11.8) | 11 (57.9) | 7 (35.0) |
| DPN‐Check | ||||
| Mean (sd) right sural SNAP‡, μV | 10.2 (6.3) | 5.4 (3.2) | 4.8 (5.5) | 2.2 (1.1) |
| Mean (sd) right sural SNCV‡, m/s | 51.3 (11.9) | 39.2 (12.4) | 33.6 (14.8) | 27.6 (15.5) |
| Mean (sd) left sural SNAP‡, μV) | 10.2 (6.0) | 5.3 (3.4) | 4.3 (5.3) | 1.8 (0.98) |
| Mean (sd) left sural SNCV‡, m/s | 49.7 (11.3) | 43.0 (10.7) | 34.3 (17.3) | 23.9 (10.3) |
| Sudoscan | ||||
| Mean (sd) hand ESC‡, μS | 66.7 (13.3) | 63.0 (12.5) | 51.7 (17.9) | 52.3 (18.8) |
| Mean (sd) foot ESC‡, μS | 65.2 (14.5) | 53.5 (16.6) | 49.5 (14.4) | 48.4 (21.7) |
DPN, diabetic distal symmetrical polyneuropathy; ESC, electrochemical skin conductance; SNAP, sural nerve action potential; SNCV, sural nerve conduction velocity.
*t‐test comparison between no DPN and any DPN (mild, moderate and severe as one group) P<0.01; †chi‐squared test P<0.001; ‡Mann–Whitney U‐test P<0.001.
Using the TCNS as the gold standard for the diagnosis of DPN, we divided the participants into those with no DPN (n=163, 69.1%) and those with DPN [mild DPN, n=34 (14.4%); moderate DPN, n=19 (8.1%) and severe DPN n=20 (8.5%)]. Participants with DPN were older (P=0.01; Table 1) and were more likely to have had a previous foot ulcer (chi‐squared test 36.4, P<0.001) and/or amputation (chi‐squared test 24.2, P<0.001). There were no differences in HbA1c (P=0.75) or urine albumin:creatinine ratio (anova, P=0.07). Participants with DPN had significantly lower SNCV (P<0.001) and SNAP (P<0.001) and Sudoscan hand (P<0.001) and foot (P<0.001) ESC (Table 1). The prevalence of DPN based on TCNS (>5) was 30.9%. A positive 10‐g monofilament test was present in only 34 participants (14.4%). The mean (sd) TCNS was significantly higher in participants who had a positive 10‐g monofilament test vs those with a negative test [8.35 (4.6) vs 4.18 (3.7); P<0.001]. The prevalence rates of DPN based on DPN‐Check (abnormal SNCV and/or SNAP) and Sudoscan foot ESC were 51.5% and 38.2%, respectively.
When choosing a Sudoscan foot ESC threshold of ≤58.5 μS (optimal Youden index), sensitivity was 77.4%, specificity was 68.3% and the Youden index was 0.45 (Fig. 1). The area under the ROC curve was 0.75. The diagnostic performance of hand ESC (Youden index 0.30, sensitivity 73.6%) was poorer in comparison with foot ESC. DPN‐Check SNAP (threshold ≤ 4.3 μV, Youden index 0.53) and SNCV (≤ 46.3 m/s, 0.52) had sensitivity (84.3%, 72.3%) and specificity (68.3%, 80.0%), respectively. The area under the ROC curve was 0.84 and 0.81 for SNAP and SNCV, respectively (Fig. 1). The 10‐g monofilament test sensitivity, specificity and AUC were 30.0%, 92.7% and 0.61, respectively.
Figure 1.
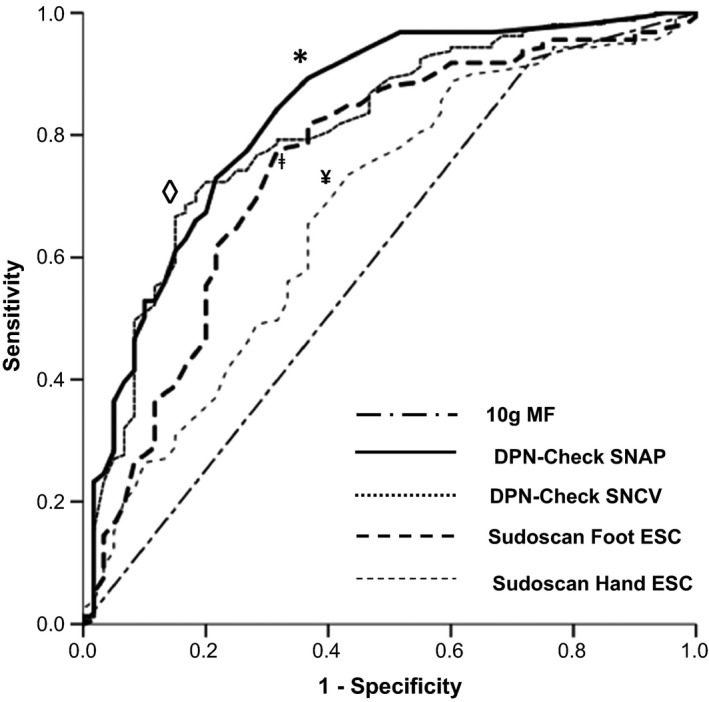
Receiver‐operating characteristic curve for the identification of diabetic distal symmetrical polyneuropathy (DPN) using point‐of‐care devices DPN‐Check and Sudoscan. ESC, electrochemical skin conductance; MF, monofilament; SNAP, sural nerve action potential amplitude; SNCV, sural nerve conduction velocity. Symbols ◊, *, ǂ and ¥ represent thresholds for DPN‐Check SNCV, SNAP, Sudoscan foot and hand ESC, respectively.
Next, we examined the efficiency of combined Sudoscan and DPN‐Check assessments using the proprietary thresholds for normality. The sensitivity of combined assessments improved to 93.2%, but specificity fell to 52.8% (Youden index 0.46, AUC 0.73). Both Sudoscan and DPN‐Check results were significantly correlated with TCNS score (P<0.001; Table 2). To further explore the relationship of POCDs, age and DPN, we examined POCDs values (e.g. DPN‐Check SNCV or SNAP) across a range of DPN severities [mild, moderate and severe; Fig. 2 (DPN‐Check SNCV) and Fig. 3 (DPN‐Check SNAP), respectively], subcategorized into different age ranges (20–49, 50–59, 60–69 and 70–99 years). All POCD results had a significant, stepwise, inverse linear relationship with ordinal categories of increasing DPN severity (linear regression: DPN‐Check SNAP β=–0.43, P<0.001; DPN‐Check SNCV β=–0.56, P<0.001; Sudoscan foot β=–0.36; P<0.001). There was also stepwise reduction in each POCD result with each DPN category.
Table 2.
Spearman correlations (r) between Toronto Clinical Neuropathy Score and point of care device results
ESC, electrochemical skin conductance; SNAP, sural nerve action potential amplitude; SNCV, sural nerve conduction velocity; TCNS, Toronto Clinical Neuropathy Score
P<0.001.
Figure 2.
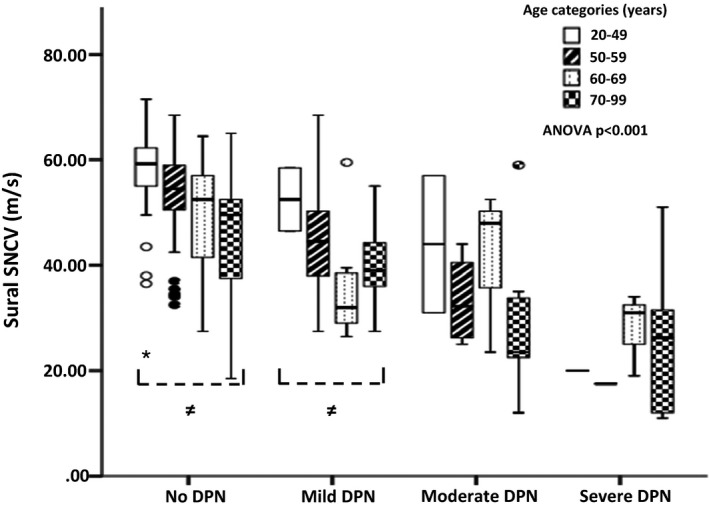
Box and whisker plots of point‐of‐care device DPN‐Check mean of left and right sural sensory nerve conduction velocity (SNCV) for each neuropathy group, subdivided into to age ranges. ≠ anova P<0.05.
Figure 3.
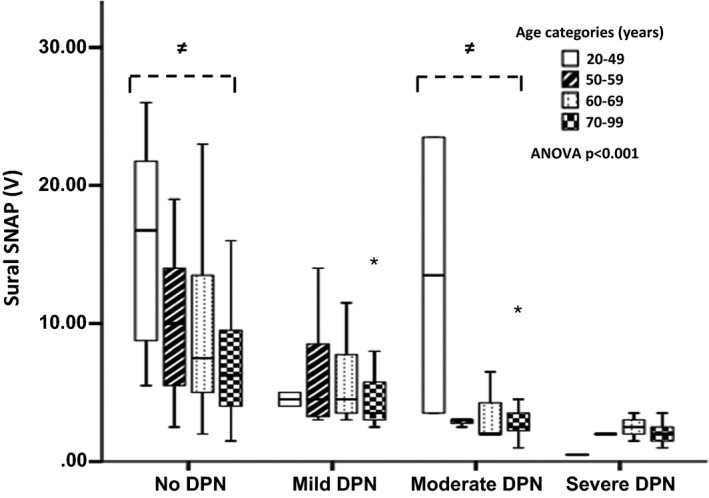
Box and whisker plots of point‐of‐care device DPN‐Check mean of left and right sural sensory nerve amplitude (SNAP) for each neuropathy group, subdivided into to age ranges. ≠ anova P<0.05.
Finally, to develop an algorithm for using POCDs within a clinical context, we sought two additional thresholds for each POCD, one that maximized sensitivity and one that maximized specificity (Fig. 4). For DPN‐Check the SNAP and SNCV values used to rule in DPN were <3.75 μV (negative likelihood ratio 0.11) and <24.3 m/s (negative likelihood ratio 0.1), respectively. For Sudoscan, the foot ESC used to rule in DPN was 46.5 μS (negative likelihood ratio 0.20). To exclude DPN we used a SNAP value of >16.75 μV (positive likelihood ratio 10.32), an SNCV value of >59.7 m/s (positive likelihood ratio 10.32) and a Sudoscan foot ESC value of >79.5 μS (positive likelihood ratio 4.33). The following clinical algorithm for using POCDs was proposed: (1) no DPN – SNAP >16.75 μV or SNCV > 59.7 m/s or Sudoscan foot > 79.5 μS (sensitivity 94.6% and negative predictive value 97.1%) and (2) DPN – SNAP <3.75 μV or SNCV <24.3 m/s or Sudoscan foot < 46.5 μS (specificity 82.2% and positive predictive value 70.1%). Using this diagnostic algorithm, 153 of 236 participants (64.8%) were classified as having DPN (n=84) or no DPN (n=69) by the POCDs, while 83 participants (35.2%) were left unclassified.
Figure 4.
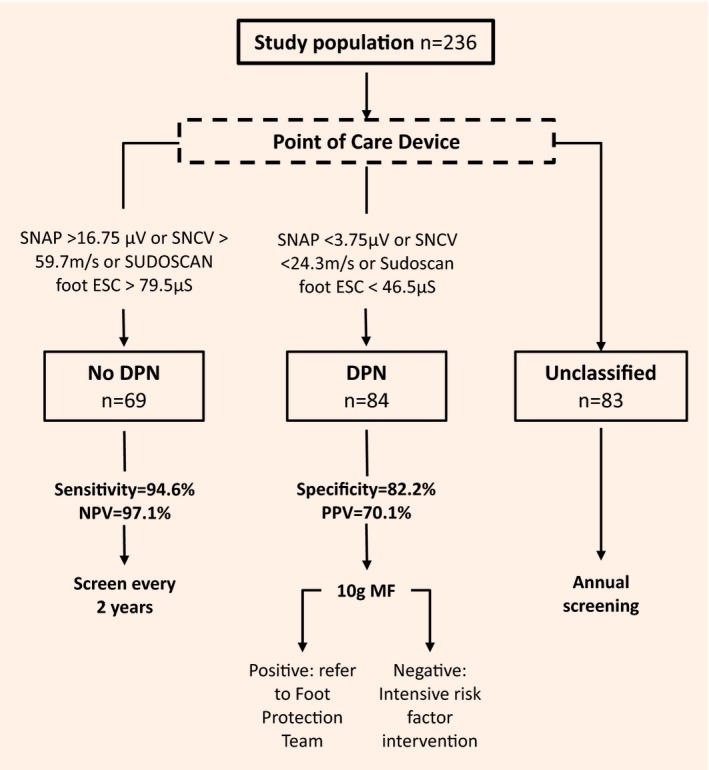
Proposed diagnostic algorithm for the clinical application of the point‐of‐care device (DPN‐Check or Sudoscan). DPN, diabetic peripheral neuropathy; ESC, electrochemical skin conductance; MF, monofilament; NPV, negative predictive value; PPV, positive predictive value; SNAP, sural nerve action potential; SNCV, sural nerve conduction velocity.
A total of 59 participants (25%) were diagnosed with painful DPN with a mean 24‐h pain score of 5.3 (2.8). Overall, 215 participants (91.1%) reported they were either ‘in favour’ (18.6%) or ‘strongly in favour’ (72.6%) of a ‘one‐stop’ microvascular screening service, with 8.9% being ‘neutral’ and no one ‘against’ this service. No adverse events or discomfort during and after measurements were reported.
Discussion
The Toronto Consensus meeting 19 defined DPN as a symmetrical and length‐dependent sensorimotor polyneuropathy that may involve motor, sensory and autonomic nerves as a result of chronic hyperglycaemia and vascular risk factors 20. It is now well recognized that DPN and its sequelae have major impacts on quality of life, morbidity and mortality and confer considerable healthcare costs 3. Unfortunately, DPN has an insidious onset and the majority of people with DPN will have no symptoms. For this reason, the recent American Diabetes Association (ADA) position statement on diabetic neuropathy recommends annual assessment for DPN using simple bedside instruments, starting at diagnosis of Type 2 diabetes and 5 years after diagnosis of Type 1 diabetes 21. The NICE guidance on prevention and management of diabetic foot problems makes a similar recommendation (NG19) 16. The present study and a recent report 8, however, have shown that this recommendation is not currently being adhered to. There may be many reasons for this. First, the diabetes consultation is currently centred on glucose control, the management of hypertension and elevated cholesterol level, and the careful examination of the feet with shoes and socks taken off may not be considered a priority. Even when foot risk has been assessed, the management of diabetic foot disease, as advised by NICE, may not be undertaken appropriately 8. Second, in the UK primary care setting, foot screening may not always be undertaken by healthcare professionals who have the necessary clinical skills. Third, the ADA recommendation of assessing either temperature or pinprick sensation (small‐fibre function) and vibration sensation using a 128‐Hz tuning fork (large‐fibre function) in addition to the use of the 10‐g monofilament, which is a good way of diagnosing foot ulcer risk, is not routinely undertaken 21. This contrasts with screening for retinopathy and renal disease, for which there are clearly established screening methods aimed at detecting the complications early and integrated management pathways, and, in the case of retinopathy, the screening has a very high uptake.
Given the rising tide of amputations in the UK 2, 3, there may be a good rationale for a robust, high‐uptake DPN screening service, aimed at diagnosing the disease early at a point when it can be halted, in both Type 1 (level A evidence) and Type 2 diabetes (level B evidence) 21, and insuring the implementation of the NICE guideline NG19 for the ‘at‐risk diabetic foot’ 21. The results of the present study show that combined eye, foot and renal screening in a one‐stop microvascular screening clinic 22 was feasible and had high patient acceptability and uptake (91.1% of patients in favour of this service). Moreover, this service was conducted by a podiatrist with the skills to assess foot risk and footwear and to refer at‐risk patients appropriately.
Current bedside assessments for DPN, such as the 10‐g monofilament test, are primarily aimed at screening for those at risk of foot ulceration and can be used to diagnose DPN when it is well established 21, but late diagnosis hampers the benefits of early identification, the focus on early, intensified diabetes control, and the prevention of neuropathy‐related sequelae 21. The Toronto Consensus Panel recommended that to confirm a diagnosis of DPN, abnormal nerve conduction studies and/or a validated measure of small‐fibre neuropathy (with level A evidence) are required 19; however, measurement of a complete set of electrophysiological variables requires an expert neurophysiologist and is time‐consuming and expensive, and access to care is hindered by the limited number of clinics available to perform standard nerve conduction studies in the face of the increasing prevalence of diabetes. A novel point‐of‐care nerve conduction device, DPN‐Check, has been developed that has the potential to serve as an acceptable proxy to standard nerve conduction studies 9, 23 for screening and identification of DPN in clinical practice and can be performed within 5 min 24. Recent studies have also shown early small‐fibre involvement in DPN 25. Sudomotor dysfunction, a measure of small‐fibre neuropathy, is one of the earliest detectable abnormalities in DPN 26. Sweat glands are innervated by sudomotor, postganglionic, thin unmyelinated cholinergic sympathetic C‐fibres, and a number of skin biopsy studies have shown a reduction in the epidermal C‐nerve fibres in people with diabetes 27; therefore, a rapid assessment of sudomotor function may provide an attractive tool to evaluate peripheral small‐fibre neuropathy in diabetes 25. Indeed as DPN involves both small and large fibres early in its course 28, there is a good rationale for combining DPN‐Check and Sudoscan to screen for DPN.
The present study showed that both POCDs were patient‐friendly, and easy and quick to use in a hospital‐ and community‐based, one‐stop microvascular screening service. With combined assessment using DPN‐Check and Sudoscan, an abnormality in the results of one or both indicating DPN correctly classified 73% of patients with 93.2% sensitivity. Moreover there was good correlation between POCD results and TCNS, indicating diagnostic utility across the range of DPN severity. We propose the following clinical algorithm to enable easy adoption with the potential to personalize treatment pathways. All patients with abnormal POCDs confirming DPN could be offered intensive, target‐driven risk factor reduction including glucose control, optimization of vascular risk factors and lifestyle modification 21. In addition, those with loss of protective foot sensation (positive 10‐g monofilament) could also be referred to the Foot Protection Team (podiatry and orthotics) for further assessment and treatment. We would also suggest that, whilst people who are unclassified by this algorithm will require annual screening, those who have no evidence of DPN may require screening every 2 years. Finally, compared with standardized clinical examination, which is less reproducible whilst being more time‐consuming, requires additional training and patient cooperation, there may be a good rationale for using POCDs.
Early identification of participants with insipient neuropathy using these validated, yet novel non‐invasive methods will allow targeted intensified metabolic control and other potential new treatment interventions in order to prevent clinical DPN or halt disease progression 21. Ultimately, the prevention of DPN may have the greatest impact on reducing amputations dramatically because >80% of patients attending the diabetic foot clinic with foot ulcers 29 and virtually all diabetes amputees have DPN. Clearly, in those with established DPN, careful foot ulcer risk assessment (including peripheral vascular status, deformity, callus etc.) and appropriate management (provision of foot information and contacts, footwear, podiatry etc.) are warranted 16. A one‐stop microvascular screening service will employ a specialist podiatrist to perform this task, assess level of risk and manage patients appropriately in order to prevent foot ulceration and amputation. The widespread implementation of retinal screening in the UK has had a dramatic impact on reducing working‐age blindness, and a similar robust approach to initiating high‐uptake foot screening may prove to be a game‐changer.
Several critical lines of future research emerge from the present study including: further large, longitudinal studies looking at hard outcomes such as the development of clinical DPN; hospital admissions with foot‐related problems; incident foot ulceration and amputations; quality of life; and the cost‐effectiveness of such an approach.
The study also led to significant detection of undiagnosed painful DPN (25%). Many of these patients reported moderate to severe painful symptoms and were referred to the painful neuropathy clinic for treatment. People with painful DPN often do not make the connection between their lower limb neuropathic symptoms and diabetes. This results in underdiagnosis, and hence undertreatment and considerable suffering 30. The one‐stop service described in the present study provides a valuable oppportunity to identify such patients and intervene appropriately.
In conclusion, a one‐stop microvascular assessment, recently highlighted by Vas and Edmonds 22, is feasible, has a high uptake and reduces clinic visits and identifies painful DPN. The study has also shown that the use of the POCDs DPN‐Check and Sudoscan, which provide objective and quantitative measures of DPN, was feasible within this one‐stop service. An abnormality in the results of either (abnormal SNCV and/ or SNAP and/or Sudoscan foot ESC) had a sensitivity of 93.2% for detecting DPN defined by TCNS, and there was a strong correlation of PCOD results with the TCNS. Widespread implementation of a one‐stop microvascular screening service may therefore be an effective model for the early diagnosis of DPN and foot complications.
Funding sources
The service development project was funded by Sheffield Teaching Hospitals NHS Foundation Trust.
Competing interests
The POCDs tested (DPN‐Check manufactured by Neurometrix; and Sudoscan manufactured by ImpetoMedical) were provided free of charge; however, the companies had no input into the design of the study or analysis of the data. S.T. and D.S. served on the Scientific Advisory Board of ImpetoMedical until 2015. S.T. also served on the Scientific Advisory Board of Neurometrix until 2015.
Diabet. Med. 35, 887–894 (2018)
References
- 1. Diabetes UK . Twenty devastating amputations ever day. Available at https://www.diabetes.org.uk/About_us/News/Twenty-devastating-amputations-every-day/. Last accessed 1 January 2018.
- 2. Public Health England . Diabetes foot care profile. 2017. Available at https://app.box.com/s/pmdl91gf2d6pscttb9avqwan6mcbs296/file/220021723534. Last accessed 1 January 2018.
- 3. Kerr M. Foot care in diabetes: the human and financial cost. 2017. Available at http://www.londonscn.nhs.uk/wp-content/uploads/2017/04/dia-foot-care-mtg-kerr-27042017.pdf. Last accessed 1 January 2018.
- 4. Dyck PJ, Kratz KM, Karnes JL, Litchy WJ, Klein R et al The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population‐based cohort: the Rochester Diabetic Neuropathy Study. Neurology 1993; 43: 817–824. [DOI] [PubMed] [Google Scholar]
- 5. Tan LS. The clinical use of the 10g monofilament and its limitations: a review. Diabetes Res Clin Pract 2010; 90: 1–7. [DOI] [PubMed] [Google Scholar]
- 6. Dyck PJ, Overland CJ, Low PA, Litchy WJ, Davies JL, Dyck PJ et al Signs and symptoms versus nerve conduction studies to diagnose diabetic sensorimotor polyneuropathy: Cl vs. NPhys trial. Muscle Nerve 2010; 42: 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liew G, Michaelides M, Bunce C. A comparison of the causes of blindness certifications in England and Wales in working age adults (16‐64 years), 1999‐2000 with 2009‐2010. BMJ Open 2014; 4: e004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mayor S. Half of NHS services do not provide recommended care for diabetic foot ulcers, shows audit. BMJ 2017; 356: j1274. [Google Scholar]
- 9. Lee JA, Halpern EM, Lovblom LE, Yeung E, Bril V, Perkins BA. Reliability and validity of a point‐of‐care sural nerve conduction device for identification of diabetic neuropathy. PLoS One 2014; 9: e86515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Casellini CM, Parson HK, Richardson MS, Nevoret ML, Vinik AI. Sudoscan, a noninvasive tool for detecting diabetic small fiber neuropathy and autonomic dysfunction. Diabetes Technol Ther 2013; 15: 948–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bril V, Perkins BA. Validation of the Toronto Clinical Scoring System for diabetic polyneuropathy. Diabetes Care 2002; 25: 2048–2052. [DOI] [PubMed] [Google Scholar]
- 12. Kong X, Schoenfeld DA, Lesser EA, Gozani SN. Implementation and evaluation of a statistical framework for nerve conduction study reference range calculation. Comput Methods Programs Biomed 2010; 97: 1–10. [DOI] [PubMed] [Google Scholar]
- 13. Bordier L, Dolz M, Monteiro L, Névoret ML, Calvet JH, Bauduceau B. Accuracy of a Rapid and Non‐Invasive Method for the Assessment of Small Fiber Neuropathy Based on Measurement of Electrochemical Skin Conductances. Front Endocrinol (Lausanne) 2016; 7: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Selvarajah D, Cash T, Davies J, Sankar A, Rao G, Grieg M et al Sudoscan: A Simple, Rapid, and Objective Method with Potential for Screening for Diabetic Peripheral Neuropathy. PLoS One 2015; 10: e0138224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mayaudon H, Miloche PO, Bauduceau B. A new simple method for assessing sudomotor function: relevance in type 2 diabetes. Diabetes Metab 2010; 36: 450–454. [DOI] [PubMed] [Google Scholar]
- 16. NICE guideline [NG19] Diabetic foot problems: prevention and management. 2015. Available at https://www.nice.org.uk/guidance/ng19. Last accessed 1 January 2018.
- 17. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver‐operating characteristic (ROC) curve. Radiology 1982; 143: 29–36. [DOI] [PubMed] [Google Scholar]
- 18. Taking Care of your feet. Available at https://www.diabetes.org.uk/Guide-to-diabetes/Complications/Feet/Taking-care-of-your-feet/. Last accessed 1 January 2018.
- 19. Tesfaye S, Boulton AJM, Dyck PJ, Freeman R, Horowitz M, Kempler P et al Diabetic Neuropathies: Update on Definitions, Diagnostic Criteria, Estimation of Severity and Treatments. Diabetes Care 2010; 33: 2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tesfaye S, Chaturvedi N, Eaton SE, Ward JD, Manes C, Ionescu Tirgoviste C et al Vascular risk factors and diabetic neuropathy. N Engl J Med 2005; 352: 341–350. [DOI] [PubMed] [Google Scholar]
- 21. Pop‐Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA et al Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care 2017; 40: 136–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vas PR, Edmonds ME. Early recognition of diabetic peripheral neuropathy and the need for one‐stop microvascular assessment. Lancet Diabetes Endocrinol 2016; 4: 723–725. [DOI] [PubMed] [Google Scholar]
- 23. Perkins BA, Grewal J, Ng E, Ngo M, Bril V. Validation of a novel point‐of‐care nerve conduction device for the detection of diabetic sensorimotor polyneuropathy. Diabetes Care 2006; 29: 2023–2027. [DOI] [PubMed] [Google Scholar]
- 24. Chatzikosma G, Pafili K, Demetriou M, Vadikolias K, Maltezos E, Papanas N. Evaluation of sural nerve automated nerve conduction study in the diagnosis of peripheral neuropathy in patients with type 2 diabetes mellitus. Arch Med Sci 2016; 12: 390–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kempler P, Amarenco G, Freeman R, Frontoni S, Horowitz M, Stevens M et al Gastrointestinal autonomic neuropathy, erectile‐, bladder‐ and sudomotor dysfunction in patients with diabetes mellitus: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev 2011; 27: 665–677. [DOI] [PubMed] [Google Scholar]
- 26. McArthur JC, Stocks EA, Hauer P, Cornblath DR, Griffin JW. Epidermal nerve fiber density: normative reference range and diagnostic efficiency. Arch Neurol 1998; 55: 1513–1520. [DOI] [PubMed] [Google Scholar]
- 27. Fealey RD, Low PA, Thomas JE. Thermoregulatory sweating abnormalities in diabetes mellitus. Mayo Clin Proc 1989; 64: 617–628. [DOI] [PubMed] [Google Scholar]
- 28. Ziegler D, Papanas N, Zhivov A, Allgeier S, Winter K, Ziegler I et al Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes. 2014; 63:2454–2463. [DOI] [PubMed] [Google Scholar]
- 29. National Diabetes Foot Care Audit Report 2014‐2015 England and Wales. Version V1, 31 March 2016. Available at file://sth/user/Downloads/055/stesfaye/nati‐diab‐foot‐care‐audit‐14‐16‐rep%20(2).pdf. Last accessed 1 January 2018.
- 30. Daousi C, MacFarlane IA, Woodward A, Nurmikko TJ, Bundred PE, Benbow SJ. Chronic painful peripheral neuropathy in an urban community: a controlled comparison of people with and without diabetes. Diabet Med 2004; 21: 976–982. [DOI] [PubMed] [Google Scholar]


