Abstract
Aims
To investigate whether the proven benefits of insulin degludec (IDeg) combined with insulin aspart (IAsp), known as IDegAsp, given twice daily, extend across a wide spectrum of patients with diabetes.
Materials and methods
This was a post hoc pooled analysis of 5 phase III randomized, 26‐week, open‐label, treat‐to‐target trials comparing IDegAsp twice daily (n = 1111) with one of two comparators: premixed insulin (biphasic insulin aspart 30 [BIAsp 30]) twice daily (n = 561) or IDeg once daily + IAsp (n = 136). Patient data were stratified according to baseline glycated haemoglobin (HbA1c) or fasting plasma glucose (FPG) categories, as well as by baseline duration of diabetes or body mass index (BMI) categories.
Results
We conducted a meta‐analysis of 5 clinical trials: NCT01513590, NCT01009580, NCT01059812, NCT01680341 and NCT01713530. End‐of‐trial results were broadly consistent, with differences between IDegAsp and comparators observed in phase III trials. HbA1c results were similar for IDegAsp and the comparators in all baseline characteristic (HbA1c, duration of diabetes or BMI) and category groups (number ranges). Significantly lower FPG level was observed with IDegAsp vs comparators in all baseline characteristic and most category groups (excluding FPG <5.5 mmol/L). Significantly lower insulin doses were observed with IDegAsp vs comparators in all baseline characteristic and half of the category groups, and significantly lower rates of confirmed and nocturnal confirmed hypoglycaemia were observed with IDegAsp vs comparators in all baseline variable and category groups.
Conclusions
IDegAsp retains a consistent safety and efficacy profile in patients with different baseline characteristics.
Keywords: glycaemic control, hypoglycaemia, insulin analogues, meta‐analysis, randomized trial, type 2 diabetes
1. INTRODUCTION
Type 2 diabetes (T2D) is characterized by a progressive worsening of glycaemic control, stemming from insulin resistance and gradual β‐cell failure.1, 2 After diagnosis, the management of diabetes typically involves recommendations to implement various lifestyle measures, together with the administration of oral antidiabetic drugs. Despite these recommendations, compliance with lifestyle recommendations3 and appropriate pharmacological treatment is low among patients with T2D.4 Consequently, glycaemic control often worsens, and insulin is frequently required to supplement the diminished endogenous secretory capacity.1, 2 The most common management approach is to initiate insulin therapy with once‐daily basal insulin,5 which has the following benefits: simplicity; low frequency of injections and blood glucose monitoring; low risk of hypoglycaemia; and good patient acceptability.6, 7 Although basal insulin helps regain fasting glycaemic control, prandial glucose excursions often remain unacceptably high, and the addition of 1 or more prandial insulin doses is usually required.6, 8 The need for insulin, whether initiation of basal insulin or intensification with bolus insulin, can be met with reluctance from both patients and their physicians.9, 10, 11 Intensification of insulin from basal alone to multiple daily injections is often resisted because of a perception that insulin regimens requiring multiple daily injections (particularly those using 2 types of insulin) and/or regular self‐measurement of blood glucose levels for dose adjustments are inconvenient or too complex to manage.9, 10, 12, 13 Patients may also be concerned about treatment‐emergent effects of insulin use, such as weight gain14, 15 or hypoglycaemia, the latter potentially posing a barrier to optimal insulin therapy because of patient fear generated by episodes of hypoglycaemia.16, 17 Physicians are also apprehensive of hypoglycaemia and, as a consequence, may refrain from or delay prescription of more intensive insulin regimens.16 Although most patients with T2D have a trajectory towards an intensification of their treatment, patients requiring initiation of basal insulin vary regarding their level of glycaemic control, duration of diabetes, body weight and acceptance of more complex treatment regimens. Considering this diversity, and the potential differences in insulin dose requirement and risk of hypoglycaemia, current treatment guidelines for T2D recommend that treatment is individualized according to a particular patient's needs.5 It is recommended that glycaemic targets and medications be selected after consideration of a patient's body weight/body mass index (BMI), age, duration of diabetes, risk of hypoglycaemia, attitude and expectations, and the presence of comorbidities.5
One option for patients with T2D requiring intensified insulin management is a switch from basal insulin to premixed insulin containing both rapid‐ and longer‐acting components in a single injection. Although premixed insulins can improve patient adherence as fewer injections are required,18, 19 when administered once daily, 24‐hour basal coverage is not possible20, 21, 22; as a consequence, glycaemic control is suboptimal for the majority of patients. In addition, some studies show that premixed insulins result in greater weight gain13 and higher rates of post‐meal and nocturnal hypoglycaemia compared with basal–bolus regimens,23 potentially limiting their use in some patients. Basal–bolus regimens can be considered for patients requiring a more physiological treatment, but patients must also be willing to accept the increased complexity of this regimen5 and be able to adjust their doses according to actual glucose values, anticipated food intake and physical activity.
“IDegAsp,” insulin degludec (IDeg) and insulin aspart (IAsp), is the first soluble co‐formulation of basal (70% IDeg) and bolus (30% IAsp) insulin analogues, available in a single injection pen.24 IDegAsp provides flat and stable 24‐hour basal coverage that allows flexibility and once‐daily or twice‐daily use.21, 25, 26 The IDegAsp co‐formulation has been demonstrated to result in less interference in patients’ day‐to‐day lives compared with intensified insulin therapy in a basal–bolus regimen, potentially improving treatment adherence and glycaemic control.27 Across the phase III clinical development programme, IDegAsp has been shown to provide effective glycaemic control and reduced rates of hypoglycaemia vs the premixed insulin biphasic insulin aspart 30 (BIAsp 30) in patients with T2D.28, 29, 30 With the advantages of IDegAsp having been proven vs some of the phase III trial comparators, it is of interest to investigate whether these benefits apply to patients with different baseline characteristics. As the phase III studies were of similar design (Table 1 27, 28, 29, 30, 31), it is possible to pool the data into a single post hoc analysis cohort to investigate the efficacy and safety of IDegAsp across a large population of patients.
Table 1.
Trial designs
| Study name | START TWICE DAILY28 | INTENSIFY PREMIX30 | INTENSIFY ALL29 | SIMPLE vs STEP‐WISE TWICE DAILY31 | TWICE DAILY vs BASAL—BOLUS27 |
|---|---|---|---|---|---|
| Clinical trial number | NCT01513590 | NCT01009580 | NCT01059812 | NCT01680341 | NCT01713530 |
| Trial duration, weeks | 26 | 26 | 26 | 26 | 26 |
| Age, years | ≥18 | ≥18 | ≥18 | ≥18 | ≥18 |
| BMI, kg/m2 | ≤40 | ≤40 | ≤35 | ≤40 | ≤40 |
| HbA1c, mmol/mol | 53.0–85.8 | 53.0–85.8 | 53.0–85.8 | 53.0–85.8 | 53.0–85.8 |
| HbA1c, % | 7.0–10.0 | 7.0–10.0 | 7.0–10.0 | 7.0–10.0 | 7.0–10.0 |
| Diabetes duration, months | T2D ≥ 6 | T2D ≥ 6 | T2D ≥ 6 | T2D ≥ 6 | T2D ≥ 6 |
| Previous treatment | Insulin‐naïve treated with metformin ±1 OAD for ≥3 months | Previously treated with basal–bolus, premixed or self‐mixed insulin ± metformin ≥3 months | Asian patients previously treated with basal, premixed or self‐mixed insulin ± metformin ≥3 months | Currently treated with stable doses of IGlar U100 + ≤3 OADs for ≥3 months | Currently treated with basal insulin ± OADs for ≥3 months |
| Trial treatments | IDegAsp twice daily + metformin vs BIAsp 30 twice daily + metformin | IDegAsp twice daily ± OADs vs BIAsp 30 twice daily ± OADsa | IDegAsp twice daily ± OADs vs BIAsp 30 twice daily ± OADsa | Simple vs step‐wise titration of IDegAsp twice daily ± OADsb c | IDegAsp twice daily ± OADs vs IDeg once daily + IAsp (2‐4 injections/day) ± OADsb |
Abbreviations: BIAsp 30, biphasic insulin aspart 30; DPP‐4, dipeptidyl peptidase‐4; IAsp, insulin aspart; IDeg, insulin degludec; IDegAsp, insulin degludec/insulin aspart; IGlar U100, insulin glargine U100; OAD, oral antidiabetic drug; SMPG, self‐monitored plasma glucose; T2D, type 2 diabetes.
OADs used included ± metformin (NCT01059812) or metformin, DPP‐4 inhibitors and pioglitazone (NCT01009580).
OADs used during this trial included metformin, DPP‐4 inhibitors and alpha‐glucosidase inhibitors, while sulphonylureas or glinides were discontinued at randomization.
In this trial, 2 different titration regimens were compared: simple titration (following a single pre‐breakfast and pre‐dinner SMPG measurement) involving twice‐weekly up‐ or down‐titration by 2 U; and step‐wise titration (based on the lowest of 3 pre‐breakfast and 3 pre‐evening meal SMPG values) involving once‐weekly up‐ or downtitration of 2 to 8 U and 2 to 4 U, respectively.
2. MATERIALS AND METHODS
This post hoc pooled analysis of 5 phase III randomized, 26‐week, open‐label, treat‐to‐target trials aimed to assess whether IDegAsp twice daily had consistent safety and efficacy benefits in patients with different baseline characteristics (BMI, duration of diabetes and glycaemic control) when compared with BIAsp 30 twice daily or IDeg once daily + IAsp (2‐4 injections). Data were pooled from 5 clinical trials in the IDegAsp clinical development programme that included use of IDegAsp twice daily. All trials were registered with http://www.clinicaltrials.gov and conducted according to the Declaration of Helsinki32 and Good Clinical Practice Guidelines (as defined by the International Conference on Harmonisation).33 All participants provided prior written consent before the onset of trial‐related activities.
Trial designs have been reported previously and are shown in Table 1,27, 28, 29, 30, 31 along with descriptions of individual study designs.
2.1. Pooled analysis
Comparators for the individual trials were pooled (“comparators” group) and included BIAsp 30 twice daily (n = 561) or IDeg once daily + IAsp (n = 136). Patient data for end‐of‐trial (EOT) HbA1c, confirmed hypoglycaemia, nocturnal confirmed hypoglycaemia and insulin dose were stratified according to: baseline HbA1c category: 53.0 to <58.5 mmol/mol (7.0‐7.5% [n = 263]), ≥58.5 to <69.4 mmol/mol (≥7.5 to <8.5% [n = 760]), ≥69.4 to <74.9 mmol/mol (≥8.5 to <9.0% [n = 309]) or ≥74.9 mmol/mol (≥9.0% [n = 476]); duration of diabetes: ≤10 years (n = 728) or >10 years (n = 1080); and BMI: ≤30 kg/m2 (n = 1000) or >30 kg/m2 (n = 808). Patient data for EOT fasting plasma glucose (FPG) were stratified according to duration of diabetes and BMI as described above, and also by baseline FPG: <5.5 mmol/L (<99 mg/dL [n = 170]), ≥5.5 to <7.0 mmol/L (≥99 to <126 mg/dL [n = 318]), ≥7.0 to <10.0 mmol/L (≥126 to <180 mg/dL [n = 787]) or ≥10.0 mmol/L (≥180 mg/dL [n = 523]). Hypoglycaemic episodes were analysed using a negative binomial regression model with treatment, trial, sex, region, antidiabetic medicine at screening, and age as explanatory variables. Overall confirmed hypoglycaemia was classified as: “confirmed” (a plasma glucose measurement of <3.1 mmol/L [<56.0 mg/dL], or severe events requiring assistance from another person), plus “nocturnal confirmed” (any confirmed event between 12:01 am and 5:59 am inclusive). The EOT insulin doses (after 26 weeks) were analysed using an ANOVA method with treatment, trial, antidiabetic therapy at screening, sex and region as fixed factors, and age and baseline value as covariates. Pooled data and statistical analyses were for the full analysis set. Data reported are mean (SEM). Rates of hypoglycaemia were compared using rate ratios of IDegAsp vs comparator (per 100 patient‐years of exposure).
3. RESULTS
3.1. Baseline characteristics
A total of 1808 patients were included in the pooled analysis of the 5 randomized, 26‐week, treat‐to‐target, phase III clinical trials in patients with T2D. Baseline characteristics of patients enrolled in the trials are shown in Table S1 in File S1.27, 28, 29, 30, 31 There was no difference between the IDegAsp twice daily and comparator arms in the mean age of patients stratified by duration of diabetes (Figure S1 in File S1), nor was there any difference in mean EOT body weight of patients stratified by baseline BMI (Figure S2 in File S1).
3.2. Glycated haemoglobin
In terms of EOT HbA1c, no significant differences were observed between IDegAsp twice daily and the comparator group when comparing across HbA1c categories (53.0‐<58.5 mmol/mol, ≥58.5 to <69.4 mmol/mol, ≥69.4 to <74.9 mmol/mol and ≥74.9 mmol/mol; Figure 1A), duration of diabetes (≤10 and >10 years; Figure 1B) and BMI groups (≤30 and >30 kg/m2; Figure 1C) in these treat‐to‐target trials (Table S2 in File S1).
Figure 1.
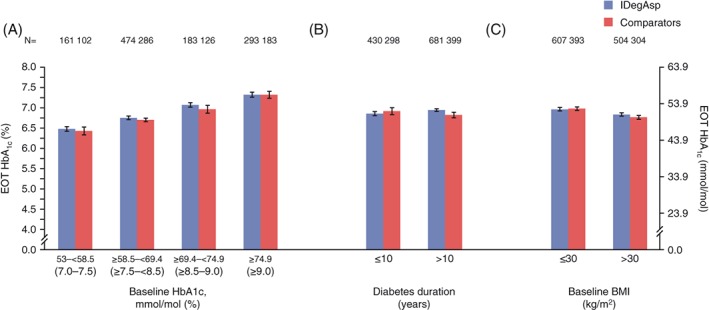
Glycated haemoglobin (HbA1c) at end of trial stratified by: A, HbA1c category (mmol/mol); B, diabetes duration (years); and C, BMI (kg/m2). Data are mean (SEM) from full analysis set, LOCF. Comparators: biphasic insulin aspart 30 (BIAsp 30; NCT01009580, NCT01059812, NCT01513590), insulin degludec (IDeg) once daily + insulin aspart (IAsp; NCT01713530); Trial NCT01680341: insulin degludec/insulin aspart (IDegAsp) Simple and IDegAsp Step‐wise arms are considered within the IDegAsp group. EOT, end of trial
3.3. Fasting plasma glucose
Fasting plasma glucose at EOT (Figure 2 and Table S2 in File S1) was numerically lower with IDegAsp twice daily than with comparators in patients in all baseline FPG categories, reaching statistical significance (P < .001) in those with a baseline FPG level of ≥5.5 to <7.0, ≥7.0 to <10.0 or ≥10.0 mmol/L (Figure 2A). Significantly lower (P < .0001) EOT FPG values were observed with IDegAsp twice daily vs comparators in patients with a duration of diabetes of ≤10 or >10 years (Figure 2B) and in those with a BMI ≤30 or >30 kg/m2 (Figure 2C).
Figure 2.
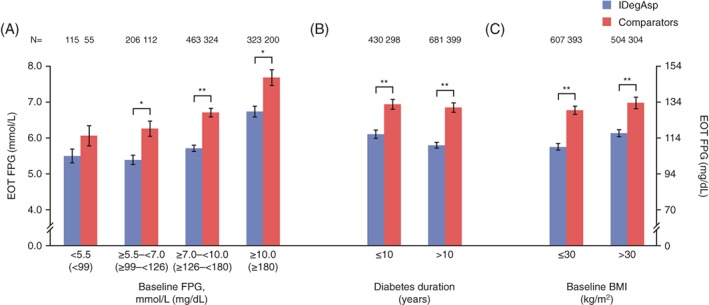
Fasting plasma glucose (FPG, mmol/L) at end of trial (EOT) by: A, baseline FPG (mmol/L), B, diabetes duration (years) and C, body mass index (BMI, kg/m2). Data are mean (SEM) from the full analysis set, LOCF. **P < .001; ***P < .0001. Comparator: biphasic insulin aspart 30 (BIAsp 30; NCT01009580, NCT01059812, NCT01513590), insulin degludec (IDeg) once daily + insulin aspart (IAsp; NCT01713530). Trial NCT01680341: IDegAsp Simple and IDegAsp Step‐wise arms are considered within the IDegAsp group
3.4. Hypoglycaemia
Among all baseline characteristic and category groups, significantly lower rates of confirmed and nocturnal confirmed hypoglycaemia were observed with IDegAsp twice daily vs comparators. Rates of confirmed and nocturnal confirmed hypoglycaemia were significantly lower (P < .05) with IDegAsp twice daily vs comparators in all 4 baseline HbA1c categories (53.0‐<58.5 mmol/mol, ≥58.5 to <69.4 mmol/mol, ≥69.4 to <74.9 mmol/mol and ≥74.9 mmol/mol) with rate ratios of 0.66, 0.72, 0.68 and 0.74, respectively, for confirmed hypoglycaemia and 0.54, 0.43, 0.41 and 0.36 for nocturnal confirmed hypoglycaemia (Figures 3A and 4A and Table S3 in File S1). In patients with differing duration of diabetes, IDegAsp twice daily was also associated with significantly lower rates of confirmed hypoglycaemia (P < .01; Figure 3B) and nocturnal confirmed hypoglycaemia (P < .0001; Figure 4B) vs comparators, with rate ratios for those with diabetes for ≤10 and >10 years of 0.61 and 0.76 for confirmed hypoglycaemia, and 0.31 and 0.48 for nocturnal confirmed hypoglycaemia, respectively. Likewise, regardless of baseline BMI, IDegAsp twice daily was associated with lower rates of confirmed hypoglycaemia (Figure 3C) and nocturnal confirmed hypoglycaemia (Figure 4C) than comparators; rate ratios for ≤30 and >30 kg/m2 were 0.81 (P = .0288) and 0.55 (P < .0001) for confirmed hypoglycaemia and 0.43 (P < .0001) and 0.38 (P < .0001) for nocturnal confirmed hypoglycaemia, respectively. As the rate of severe hypoglycaemia was low in both IDegAsp (0‐0.87 events per 100 patient‐years of exposure) and comparator groups (0‐1.15 events per 100 patient‐years of exposure), meaningful comparisons were not possible and therefore these data are not presented.
Figure 3.
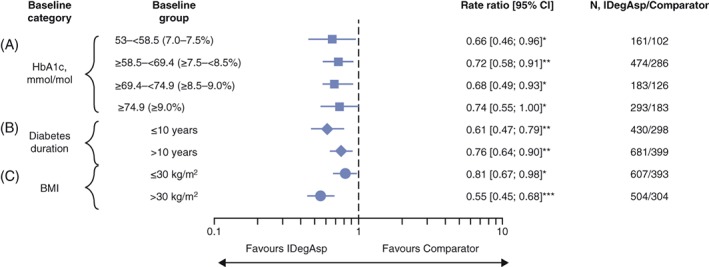
Rate ratios and 95% confidence intervals (CIs) for confirmed hypoglycaemia (per 100 patient‐years of exposure) stratified by: A, glycated haemoglobin (HbA1c) category (mmol/mol); B, diabetes duration (years); and C, body mass index (BMI, kg/m2). Data are rate ratios, insulin degludec/insulin aspart (IDegAsp) vs comparator (95% CI), and are from the full analysis set, LOCF. *P < .05; **P < .01; ***P < .0001. Comparator: biphasic insulin aspart 30 (BIAsp 30; NCT01009580, NCT01059812, NCT01513590), insulin degludec (IDeg) once daily + insulin aspart (IAsp; NCT01713530). Trial NCT01680341: IDegAsp Simple and IDegAsp Step‐wise arms are considered within the IDegAsp group. Confirmed hypoglycaemia: subject unable to treat himself/herself and/or has a recorded plasma glucose <3.1 mmol/L (56 mg/dL)
Figure 4.
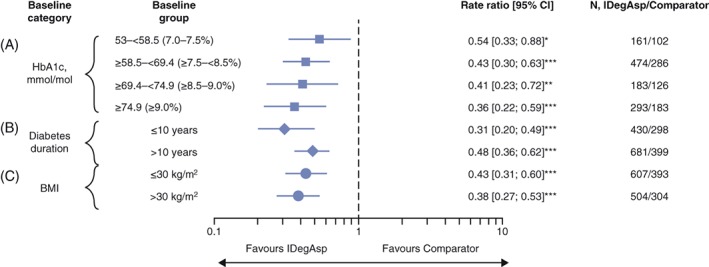
Rate ratios and 95% confidence intervals (CIs) for nocturnal confirmed hypoglycaemia (per 100 patient‐years of exposure) stratified by: A, glycated haemoglobin (HbA1c) category (mmol/mol); B, diabetes duration (years); and C, body mass index (BMI, kg/m2). Data are rate ratios, insulin degludec/insulin aspart (IDegAsp) vs comparator (95% CI), and are from the full analysis set, LOCF. *P < .05; **P < .01; ***P < .0001. Comparators: biphasic insulin aspart 30 (BIAsp 30; NCT01009580, NCT01059812, NCT01513590), insulin degludec (IDeg) once daily + insulin aspart (IAsp; NCT01713530). Trial NCT01680341: IDegAsp Simple and IDegAsp Step‐wise arms are considered within the IDegAsp group. Confirmed hypoglycaemia: subject unable to treat himself/herself and/or has a recorded plasma glucose <3.1 mmol/L (56 mg/dL). Nocturnal period: the period between 12:01 am and 5:59 am (both inclusive)
3.5. Insulin dose
The EOT daily insulin dose (Figure S3 and Table S4 in File S1) was numerically lower with IDegAsp twice daily vs comparators in all baseline HbA1c, diabetes duration and BMI categories, reaching statistical significance in patients with baseline HbA1c of 53.0 to <58.5 mmol/mol (P < .01) and ≥58.5‐<69.4 mmol/mol (P < .05; Figure S3A and Table S4 in File S1), diabetes duration of >10 years (P < .0001) and baseline BMI of ≤30 kg/m2 (P < .0001). Similar differences were found when comparing EOT daily insulin doses for IDegAsp vs BIAsp 30, while EOT daily insulin doses were significantly lower in those with baseline HbA1c ≥58.5 to <69.4 mmol/mol vs IDeg once daily + IAsp (P < .05; data not shown).
4. DISCUSSION
This post hoc pooled analysis of 5 phase III randomized trials found that IDegAsp compares favourably across all baseline criteria (BMI, duration of diabetes and glycaemic control) when compared with the comparators in the pooled population (BIAsp 30 twice daily or IDeg once daily + IAsp [2‐4 injections]) in terms of safety and efficacy. In terms of glycaemic efficacy, IDegAsp compared favourably with these comparators; no differences were observed in EOT HbA1c (across all baseline groups), and EOT FPG levels were significantly lower in patients using IDegAsp vs comparators in all but 1 category (baseline FPG <5.5 mmol/L) when stratified by baseline FPG, diabetes duration and BMI. These findings are in broad agreement with those of the total population from the individual treat‐to‐target phase III trials comparing IDegAsp with BIAsp 30 twice daily or IDeg once daily + IAsp. In addition, the safety results in the present study echo those of the individual trials, showing significantly lower rates of hypoglycaemia with IDegAsp than with comparators.27, 28, 29, 30, 31 IDegAsp twice daily was associated with lower rates of confirmed and nocturnal confirmed hypoglycaemia vs comparators, independently of baseline HbA1c, duration of diabetes and baseline BMI. As demonstrated previously, rates of hypoglycaemia were higher in those with a lower BMI (<30 kg/m2) and longer duration of diabetes, with no apparent association between baseline HbA1c and rates of hypoglycaemia.34 Notably, EOT insulin dose was significantly lower in patients on IDegAsp with baseline HbA1c 53.0 to <58.5 mmol/mol and ≥58.5‐<69.4 mmol/mol, diabetes duration >10 years or BMI ≤30 kg/m2. The latter 2 findings (diabetes duration and BMI) may be influenced by the results from INTENSIFY ALL, which compared IDegAsp with BIAsp 30 in Asian patients with a longer duration of diabetes and lower BMI compared with the other phase III trials.
These positive results (similar HbA1c levels, lower EOT FPG levels and lower rates of confirmed and nocturnal hypoglycaemia with IDegAsp vs comparators) are consistent with the results from the BEGIN trials35, 36, 37, 38, 39 of IDeg (both comparing IDeg and IGlar) and may be largely attributed to the actions of IDeg in the IDegAsp formulation (ie, lower variability and a longer duration of action compared with the 70% protamine crystallized IAsp in BIAsp 30).21, 25 This shows that the actions of IDeg are clearly preserved in the IDegAsp co‐formulation, and thus the lower rates of hypoglycaemia (vs comparators such as BIAsp 30) may give clinicians greater confidence to prescribe doses at which lower HbA1c targets are more likely to be achieved. Furthermore, the dual benefits of having simplified diabetes management (basal and prandial glycaemic control in a single injection) with less weight gain, may also lead to improved patient adherence and outcomes.40
In summary, the findings of this pooled analysis show that IDegAsp is an effective treatment across a spectrum of patient baseline characteristics (HbA1c, duration of diabetes and BMI). Further, these findings support the use of IDegAsp to address the clinical need to improve patient adherence and outcomes by providing a simpler treatment, with lower rates of hypoglycaemia, at a similar dose with no excess weight gain when compared with a mixed population of patients treated with BIAsp 30 and IDeg + IAsp.
The limitations of the present pooled analysis stem partly from its constituent studies, such as the inclusion of open‐label studies, variability in study design and study duration of <1 year, which limits our conclusions to the short term. To be able to draw conclusions from the subgroups of the individual trials, data were pooled from the studies comparing IDegAsp twice daily with BIAsp 30 twice daily and IDeg + IAsp once daily. In addition, the patient population in the INTENSIFY ALL trial differed from the other phase III trials, and its Asian population of patients had a lower mean BMI and longer duration of diabetes compared with patients in the other phase III trials, potentially influencing the results.
In conclusion, this pooled analysis shows that IDegAsp retains the efficacy and safety benefits shown in the phase III studies when compared with either basal–bolus or premixed insulin among a wide range of patients with T2D with different baseline characteristics. This finding gives confidence to prescribers that IDegAsp is a reliable option for patients with T2D in need of insulin intensification.
Supporting information
Figure S1 Mean age by disease duration (years).
Figure S2 Body weight (kg) EOT by baseline BMI (kg/m2).
Figure S3 Total daily insulin dose (U) at EOT, stratified by: A, HbA1c categories (%); B, diabetes duration (years); and c) BMI (kg/m2).
Table S1 Baseline characteristics.
Table S2 Summary of efficacy data.
Table S3 Summary of hypoglycaemia data.
Table S4 Summary of insulin dose data.
ACKNOWLEDGMENTS
Prof. Jens Sandahl Christiansen, who was an investigator in the present study, died whilst the manuscript was in preparation. His contribution to the work is noted here, as is the very significant contribution he made to the field of endocrinology and the diabetes community. His positive attitude, enthusiasm and kindness will be remembered by all those who had the opportunity to work with him.
The authors acknowledge medical writing and submission support provided by Dr Sam Mason and Richard McDonald of Watermeadow Medical, an Ashfield company, part of UDG Healthcare plc. This support was funded by Novo Nordisk A/S.
Conflict of interest
M.H. has received research support from Eli Lilly, Bristol‐Myers Squibb and AstraZeneca, and personal fees as consultant or lecturer from AstraZeneca, Eli Lilly, Sanofi Aventis, Novartis and Novo Nordisk. G.F. has received honoraria, teaching and research sponsorship/grants from AstraZeneca, Boehringer Ingelheim, Janssen, MSD, Novartis, Novo Nordisk, Sanofi Aventis and Servier SA. T.R.P. has received research support from Novo Nordisk (paid directly to the Medical University of Graz), and personal fees as a consultant from AstraZeneca, Bristol‐Myers Squibb, Eli Lilly, Novo Nordisk and Roche Diabetes Care. He is also the CSO of CBmed (Center for Biomarker Research in Medicine), a public‐funded research company. L.B. is an employee of, and holds stock in, Novo Nordisk A/S. D.T. is an employee of, and holds stock in, Novo Nordisk A/S. H.W.R. has served on advisory panels for Amylin Pharmaceuticals Inc., AstraZeneca Pharmaceuticals LP, Biodel Inc., Bayer Health Care LLC, Merck, Novo Nordisk A/S, Roche Pharmaceuticals and Sanofi, as a consultant for Biodel Inc., Merck, Roche Pharmaceuticals and Takeda Pharmaceuticals USA Inc, Merck and Sanofi, has received research support from AstraZeneca Pharmaceuticals LP, Biodel Inc., Boehringer Ingelheim Pharmaceuticals Inc., Hamni, Janssen Pharmaceuticals, Eli Lilly and Company, Merck, Novartis Pharmaceuticals Corporation, Novo Nordisk A/S, Roche Pharmaceuticals and Sanofi, and has served as a speaker for AstraZeneca Pharmaceuticals LP, BMS, Boehringer Ingelheim Pharmaceuticals Inc., Janssen, Eli Lilly and Company, Merck, Novo Nordisk A/S, Sanofi and Takeda Pharmaceuticals USA Inc.
Author contributions
M.H., G.F., T.R.P. and H.W.R. contributed to the interpretation of data, critical revision of the article and final approval for submission. D.T. contributed to the design, interpretation of data, critical revision of the article and final approval for submission. L.B. contributed to the analysis and interpretation of data, critical revision of the article and final approval for submission.
Haluzík M, Fulcher G, Pieber TR, Bardtrum L, Tutkunkardas D, Rodbard HW. The co‐formulation of insulin degludec and insulin aspart lowers fasting plasma glucose and rates of confirmed and nocturnal hypoglycaemia, independent of baseline glycated haemoglobin levels, disease duration or body mass index: A pooled meta‐analysis of phase III studies in patients with type 2 diabetes. Diabetes Obes Metab. 2018;20:1585–1592. https://doi.org/10.1111/dom.13261
Funding information The phase III programme for insulin degludec/insulin aspart was funded by Novo Nordisk A/S.
REFERENCES
- 1. Weir GC, S‐Weir B. Five stages of evolving beta‐cell dysfunction during progression to diabetes. Diabetes. 2004;53(suppl. 3):S16‐S21. [DOI] [PubMed] [Google Scholar]
- 2. U.K. Prospective Diabetes Study Group . Overview of 6 years’ therapy of type ii diabetes: a progressive disease (UKPDS 16). Diabetes. 1995;44(11):1249‐1258. [PubMed] [Google Scholar]
- 3. Saleh F, Mumu SJ, Ara F, Hafez MA, Ali L. Non‐adherence to self‐care practices & medication and health related quality of life among patients with type 2 diabetes: a cross‐sectional study. BMC Public Health. 2014;14:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. García‐Pérez L‐E, Álvarez M, Dilla T, Gil‐Guillén V, Orozco‐Beltrán D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013;4(2):175‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140‐149. [DOI] [PubMed] [Google Scholar]
- 6. Moghissi E, King AB. Individualizing insulin therapy in the management of type 2 diabetes. Am J Med. 2014;127(suppl. 10):S3‐10. [DOI] [PubMed] [Google Scholar]
- 7. Riddle MC, Rosenstock J, Gerich J, Insulin Glargine 4002 Study Investigators . The treat‐to‐target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26(11):3080‐3086. [DOI] [PubMed] [Google Scholar]
- 8. Garber AJ. Insulin intensification strategies in type 2 diabetes: when one injection is no longer sufficient. Diabetes Obes Metab. 2009;11(suppl. 5):14‐18. [DOI] [PubMed] [Google Scholar]
- 9. Vijan S, Hayward RA, Ronis DL, Hofer TP. Brief report: the burden of diabetes therapy: implications for the design of effective patient‐centered treatment regimens. J General Intern Med. 2005;20(5):479‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peyrot M, Rubin RR, Kruger DF, Travis LB. Correlates of insulin injection omission. Diabetes Care. 2010;33(2):240‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vaag A, Lund SS. Insulin initiation in patients with type 2 diabetes mellitus: treatment guidelines, clinical evidence and patterns of use of basal vs premixed insulin analogues. Eur J Endocrinol. 2012;166(2):159‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient‐centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2010;35(6):1364‐1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mosenzon O, Raz I. Intensification of insulin therapy for type 2 diabetic patients in primary care: basal–bolus regimen versus premix insulin analogs: when and for whom? Diabetes Care. 2013;36(suppl. 2):S212‐S218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holman RR, Thorne KI, Farmer AJ, et al. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. New Engl J Med. 2007;357:1716‐1730. [DOI] [PubMed] [Google Scholar]
- 15. Robbins D, Beisswenger P, Ceriello A, et al. Mealtime 50/50 basal + prandial insulin analogue mixture with a basal insulin analogue, both plus metformin, in the achievement of target HbA1c and pre‐ and postprandial blood glucose levels in patients with type 2 diabetes: a multinational, 24‐week, randomized, open‐label, parallel‐group comparison. Clin Ther. 2007;29(11):2349‐2364. [DOI] [PubMed] [Google Scholar]
- 16. Peyrot M, Barnett AH, Meneghini LF, Schumm‐Draeger PM. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med. 2012;29(5):682‐689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leiter LA, Yale JF, Chiasson J‐L, Harris SB, Kleinstiver P, Sauriol L. Assessment of the impact of fear of hypoglycemic episodes on glycemic and hypoglycemic management. Can J Diabetes. 2005;29(3):186‐192. [Google Scholar]
- 18. Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296‐1310. [DOI] [PubMed] [Google Scholar]
- 19. Garber AJ, Ligthelm R, Christiansen JS, Liebl A. Premixed insulin treatment for type 2 diabetes: analogue or human? Diabetes Obes Metab. 2007;9(5):630‐639. [DOI] [PubMed] [Google Scholar]
- 20. Thorisdottir R, Parkner T, Chen J, Ejskjaer N, Christiansen J. A comparison of pharmacokinetics and pharmacodynamics of biphasic insulin aspart 30, 50, 70 and pure insulin aspart: a randomized, quadruple crossover study. Basic Clin Pharmacol Toxicol. 2009;104(3):216‐221. [DOI] [PubMed] [Google Scholar]
- 21. Heise T, Nosek L, Klein O, Coester H, Svendsen AL, Haahr H. Insulin degludec/insulin aspart produces a dose‐proportional glucose‐lowering effect in subjects with type 1 diabetes mellitus. Diabetes Obes Metab. 2015;17(7):659‐664. [DOI] [PubMed] [Google Scholar]
- 22. NovoMix®30 . Summary of Product Characteristics (SPC). Bagsværd D, Denmark: Novo Nordisk A/S; July 2010. [Google Scholar]
- 23. Liebl A, Prusty V, Valensi P, et al. Ten years of experience with biphasic insulin aspart 30: from drug development to the latest clinical findings. Drugs. 2012;72(11):1495‐1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ryzodeg® . Summary of Product Characteristics (SPC). Bagsværd D, Denmark: Novo Nordisk A/S; February 2014. [Google Scholar]
- 25. Heise T, Nosek L, Roepstorff C, Chenji S, Klein O, Haahr H. Distinct prandial and basal glucose‐lowering effects of insulin degludec/insulin aspart (IDegAsp) at steady state in subjects with type 1 diabetes mellitus. Diabetes Ther. 2014;5(1):255‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dardano A, Bianchi C, Prato DS, Miccoli R. Insulin degludec/insulin aspart combination for the treatment of type 1 and type 2 diabetes. Vasc Health Risk Man. 2014;10:465‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rodbard HW, Cariou B, Pieber TR, Endahl LA, Zacho J, Cooper JG. Treatment intensification with an insulin degludec (IDeg)/insulin aspart (IAsp) co‐formulation twice daily compared with basal IDeg and prandial IAsp in type 2 diabetes: a randomized, controlled phase III trial. Diabetes Obes Metab. 2016;18(3):274‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Franek E, Haluzik M, Varzic CS, et al. Twice‐daily insulin degludec/insulin aspart provides superior fasting plasma glucose control and a reduced rate of hypoglycaemia compared with biphasic insulin aspart 30 in insulin‐naive adults with Type 2 diabetes. Diabet Med. 2016;33(4):497‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaneko S, Chow F, Choi DS, et al. Insulin degludec/insulin aspart versus biphasic insulin aspart 30 in Asian patients with type 2 diabetes inadequately controlled on basal or pre‐/self‐mixed insulin: a 26‐week, randomised, treat‐to‐target trial. Diabetes Res Clin Pract. 2015;107(1):139‐147. [DOI] [PubMed] [Google Scholar]
- 30. Fulcher GR, Christiansen JS, Bantwal G, et al. Comparison of insulin degludec/insulin aspart and biphasic insulin aspart 30 in uncontrolled, insulin‐treated type 2 diabetes: a phase 3a, randomized, treat‐to‐target trial. Diabetes Care. 2014;37(8):2084‐2090. [DOI] [PubMed] [Google Scholar]
- 31. Gerety G, Bebakar WM, Chaykin L, et al. Treatment intensification with insulin degludec/insulin aspart twice daily: randomized study to compare simple and step‐wise titration algorithms. Endocr Pract. 2016;22(5):546‐554. [DOI] [PubMed] [Google Scholar]
- 32. World Medical Association . Declaration of Helsinki: ethical principles for medical research involving human subjects. Last amended by the 64th WMA general assembly; October 16‐19, 2013; Fortaleza, Brazil. [Google Scholar]
- 33. International Conference on Harmonisation . ICH harmonised tripartite guideline. Good clinical practice May 01, 1996.
- 34. Cariou B, Fontaine P, Eschwege E, et al. Frequency and predictors of confirmed hypoglycemia in type 1 and insulin‐treated type 2 diabetes mellitus patients in a real‐life setting: results from the DIALOG study. Diabetes Metab. 2015;41(2):116‐125. [DOI] [PubMed] [Google Scholar]
- 35. Garber AJ, King AB, Del Prato S, et al. Insulin degludec, an ultra‐long‐acting basal insulin, versus insulin glargine in basal‐bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal‐Bolus Type 2): a phase 3, randomised, open‐label, treat‐to‐target non‐inferiority trial. Lancet. 2012;379:1498‐1507. [DOI] [PubMed] [Google Scholar]
- 36. Zinman B, Philis‐Tsimikas A, Cariou B, et al. Insulin degludec versus insulin glargine in insulin‐naive patients with type 2 diabetes: a 1‐year, randomized, treat‐to‐target trial (BEGIN Once Long). Diabetes Care. 2012;35:2464‐2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gough SC, Bhargava A, Jain R, Mersebach H, Rasmussen S, Bergenstal R. Low‐volume insulin degludec 200 U/ml once‐daily improves glycemic control similar to insulin glargine with a low risk of hypoglycemia in insulin‐naïve patients with type 2 diabetes: a 26‐week, randomized, controlled, multinational, treat‐to‐target trial: the BEGIN LOW VOLUME trial. Diabetes Care. 2013;36:2536‐2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Onishi Y, Iwamoto Y, Yoo SJ, Clauson P, Tamer SC, Park S. Insulin degludec compared with insulin glargine in insulin‐naïve patients with type 2 diabetes: a 26‐week, randomised, controlled, Pan‐Asian, treat‐to‐target trial. J Diabetes Investig. 2013;4(6):605‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meneghini L, Atkin SL, Gough SC, et al. The efficacy and safety of insulin degludec given in variable once‐daily dosing intervals compared with insulin glargine and insulin degludec dosed at the same time daily: a 26‐week, randomized, open‐label, parallel‐group, treat‐to‐target trial in people with type 2 diabetes. Diabetes Care. 2013;36:858‐864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Farsaei S, Radfar M, Heydari Z, Abbasi F, Qorbani M. Insulin adherence in patients with diabetes: risk factors for injection omission. Prim Care Diabetes. 2014;8:338‐345. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Mean age by disease duration (years).
Figure S2 Body weight (kg) EOT by baseline BMI (kg/m2).
Figure S3 Total daily insulin dose (U) at EOT, stratified by: A, HbA1c categories (%); B, diabetes duration (years); and c) BMI (kg/m2).
Table S1 Baseline characteristics.
Table S2 Summary of efficacy data.
Table S3 Summary of hypoglycaemia data.
Table S4 Summary of insulin dose data.


