Abstract
Objective
We estimated the incidence and prevalence of depression, anxiety disorder, bipolar disorder, and schizophrenia in a population‐based cohort with rheumatoid arthritis (RA) as compared to an age‐, sex‐, and geographically matched cohort without RA.
Methods
Using population‐based administrative health data from Manitoba, Canada, we identified persons with incident RA between 1989 and 2012, and a cohort from the general population matched 5:1 on year of birth, sex, and region of residence. We applied validated algorithms for depression, anxiety disorder, bipolar disorder, and schizophrenia to determine the annual incidence of these conditions after the diagnosis of RA, and their lifetime and annual period prevalence. We compared findings between cohorts using negative binomial regression models.
Results
We identified 10,206 incident cases of RA and 50,960 matched individuals. After adjustment for age, sex, socioeconomic status, region of residence, number of physician visits, and year, the incidence of depression was higher in the RA cohort over the study period (incidence rate ratio [IRR] 1.46 [95% confidence interval (95% CI) 1.35–1.58]), as was the incidence of anxiety disorder (IRR 1.24 [95% CI 1.15–1.34]) and bipolar disorder (IRR 1.21 [95% CI 1.00–1.47]). The incidence of schizophrenia did not differ between groups (IRR 0.96 [95% CI 0.61–1.50]). Incidence rates of psychiatric disorders declined minimally over time. The lifetime and annual period prevalence of depression and anxiety disorder were also higher in the RA than in the matched cohort over the study period.
Conclusion
The incidence and prevalence of depression, anxiety disorder, and bipolar disorder are elevated in the RA population as compared to a matched population.
Introduction
Psychiatric comorbidity adversely affects multiple outcomes in rheumatoid arthritis (RA). Depression, when comorbid with RA, for example, is associated with an increased risk of incident myocardial infarction 1, poor quality of life 2, and increased mortality 3. Among persons with recently diagnosed RA, psychiatric comorbidity at onset, particularly depression, is associated with greater pain and poorer functional status at onset, and a 40% reduced likelihood of clinical remission at 1 year 4. In a secondary analysis of a clinical trial, symptoms of depression and anxiety were associated with a reduced likelihood of RA remission 5. However, the incidence and prevalence of psychiatric disorders in RA are incompletely understood.
Significance & Innovations.
Rheumatoid arthritis (RA) is associated with an increased risk of multiple psychiatric disorders, including depression, anxiety disorder, and bipolar disorder.
The risks of depression, anxiety disorder, and bipolar disorder have not changed over time, despite substantial changes in the clinical management of RA over the 20‐year study period.
Clinicians should be aware that women and those of lower socioeconomic status are at particularly increased risk of these disorders.
In a recent systematic review, estimates of the prevalence of depression ranged broadly, from 0.04–66.3% 6. Estimates of the prevalence of anxiety range from 13–70% 7, 8, 9. However, in many prior studies, estimates of the prevalence of depression or anxiety have been of low quality because these studies were not population‐based, had low participation rates, and had small samples 6. Also, previous studies comparing the prevalence of depression in the RA population versus healthy controls have produced heterogeneous effect sizes, which varied depending on the methods used 10. In the RA population, age‐ and sex‐specific estimates have rarely been reported for depression 11 or for anxiety and other psychiatric comorbidities. As a measure of risk, incidence is more useful for the investigation of disease etiology than prevalence. Nonetheless, the incidence of psychiatric comorbidity in RA, including depression, anxiety, bipolar disorder, and schizophrenia, has been reported even less often than prevalence. Further, studies have been conducted mostly outside North America, and their findings may not apply to North American populations 11, 12, 13.
An elevated risk of psychiatric comorbidity would point toward a need for additional monitoring and clinical resources. As mental health can be modified, addressing it may be an avenue for improving outcomes in RA. Moreover, if the RA population had an increased risk for multiple psychiatric disorders, this could suggest that investigating shared risk factors or shared final common pathways would be fruitful for understanding the etiology of this elevated risk. Recent genome‐wide association studies suggest that some genetic loci associated with psychiatric disorders have pleiotropic effects and may be associated with depression, bipolar disorder, and schizophrenia. Some genetic loci are jointly associated with the risk of RA and other immune‐mediated disorders, as well as bipolar disorder and schizophrenia 14. Shared environmental factors, such as chronic stress secondary to chronic illness, could also play a role 15, 16, 17, 18, 19.
We aimed to estimate the incidence and prevalence of depression, anxiety disorder, bipolar disorder, and schizophrenia in a population‐based cohort with RA, and to provide age‐ and sex‐specific estimates. Given that the prevalence of mood and anxiety disorders has not changed over time in the general population 20, 21, we also examined temporal trends in the incidence and prevalence estimates. We compared these findings in the RA population to those in a matched cohort without RA from the general population.
Patients and methods
Setting and data sources
This retrospective matched cohort study took place in Manitoba, a Canadian province with a population of 1.3 million. As of the 2006 census, the last full census falling in the study period, more than 80% of the Manitoba population has a high school education or better, 55.9% live in a major urban center, and 11.6% are considered low income 22, 23. Nearly 10% are visible minorities, while 15.5% identify as First Nations (indigenous peoples) 24. After obtaining approval by the University of Manitoba Health Research Ethics Board and Manitoba Health Information Privacy Committee, we accessed population‐based databases in the Manitoba Population Research Data Repository housed at the Manitoba Centre for Health Policy. Since health care is publicly funded in Manitoba, these administrative (health claims) databases cover more than 98% of the population. Records are linkable at the individual level using an encrypted unique personal health identification number.
The databases (and data) used included the Population Registry (dates of birth and death, dates of health care coverage, sex, and region of residence based on 6‐digit postal codes); the Discharge Abstract Database (inpatient hospitalizations, including admission and discharge dates, and up to 25 diagnoses recorded using International Classification of Diseases [ICD] codes, including codes for the Clinical Modification of the Ninth Revision [ICD‐9‐CM] until 2004 and the Canadian version of the Tenth Revision [ICD‐10‐CA] thereafter); Medical Services (physician claims, including date of service and 1 ICD‐9‐CM physician‐coded diagnosis); and the Drug Program Information Network (DPIN; all community‐dispensed prescriptions, including drug name, date of dispensation, and drug identification number [DIN]). The DIN is connected to the World Health Organization's Anatomical Therapeutic Chemical Classification System. All databases covered the period from April 1, 1985, through March 31, 2012, except DPIN, which became available in 1995.
Study populations
First, we applied a validated case definition to identify all Manitoba residents with RA during the study period (see Supplementary Table 1, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.23539/abstract) 25. This case definition has a sensitivity of 77.12% and a specificity of 90.3% as compared to physician‐recorded diagnoses in a database 26, 27. We restricted the analysis to incident cases of RA by excluding cases with any health claims for 5 years before the date of the first health claim for RA (index [diagnosis] date); therefore, the earliest incident cases had an index year of 1989. Second, we created a cohort without health claims for RA or related disorders, matched 5:1 on sex, year of birth ± 5 years, and forward sortation area (i.e., first 3 digits of postal code). As this control cohort was established as part of a larger study involving other immune‐mediated diseases, individuals with health claims for inflammatory bowel disease and demyelinating disease were also excluded. Each control was assigned the index date of its matched case.
Psychiatric disorders
As described elsewhere 28, we applied validated case definitions for identifying depression, anxiety disorder, bipolar disorder, and schizophrenia (see Supplementary Table 1, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.23539/abstract) to identify affected individuals 29, 30. To estimate the incidence of these psychiatric disorders after RA diagnosis (or the index date in matched controls), the first claim for the psychiatric disorder had to occur after the index date, and had to be preceded by a 5‐year period with no claims for that psychiatric disorder. This 5‐year period was selected on the basis of the observations that within our cohorts, among those who met the case definition for depression, the median time between depression claims was 24.0 days (interquartile range [IQR] 36.0–121.0); the 99th percentile of the distribution for time between claims was 497 days (1.36 years). Among those who met the case definition for anxiety, the median time between anxiety claims was 31.0 days (IQR 80–534); the 99th percentile of the distribution for time between claims was 1,810 days (4.96 years). The maximum period without claims was expected to be shorter for bipolar disorder and schizophrenia. We report the incidence for the period of April 1, 1989, through March 31, 2012.
To estimate lifetime (period) prevalence, once a person met the case definition for the selected comorbidity, he or she was considered affected in all subsequent years if alive and a resident in Manitoba. However, this approach would produce estimates influenced by the duration of followup, and some individuals identified could experience remission for varying periods. Therefore, we sought to estimate the annual period prevalence of these conditions requiring ongoing care each year. Once a person met the case definition, he or she was counted as an annual prevalent case if there was ≥1 hospital or ≥2 physician claims for the disorder in that year. We have shown that when this case definition is applied in the national general population in 2012, it produces comparable prevalence estimates for depression and anxiety disorder to those obtained in the Canadian Community Health Survey–Mental Health that same year 31. Since the case definitions for depression and anxiety disorder included prescription claims, which were only available as of 1995, in models (below) we included a binary covariate indicating whether the disorder occurred before or after these data were available. We age‐ and sex‐standardized the incidence and prevalence estimates to the 2010 Canadian population. We report average annual sex‐ and age‐specific incidence and prevalence estimates using the age groups 18–24, 25–44, 45–64, and ≥65 years, consistent with those used in the Canadian Community Health Survey 32 and large enough to ensure adequate cell sizes to protect participant confidentiality.
Covariates
Covariates included sex (with male as the reference group), age (18–24 [reference group], 25–44, 45–64, and ≥65 years), socioeconomic status (SES) in quintiles (best quintile as reference group), region (urban or rural [reference group]), and fiscal year. We linked participants' dissemination area–level census data by postal code to determine SES as defined by the Socioeconomic Factor Index, version 2 (SEFI‐2). The SEFI‐2 is a factor score that incorporates information regarding average household income, percentage of single‐parent households, and unemployment and high school education rates; scores <0 indicate better SES 33. Urban regions included Winnipeg (population >700,000) and Brandon (population >47,000). We included fiscal year in the regression models to evaluate temporal trends. We also included the annual number of physician visits to account for possible surveillance bias due to increased health system contacts.
Analysis
We compared incidence rates and prevalence between the 2 cohorts, adjusting for potential confounders using negative binomial regression models to account for overdispersion, for which we report rate ratios (prevalence ratio [PR] and incidence rate ratio [IRR]) and 95% confidence intervals (95% CIs). These models included the log of person‐years as an offset to account for variable followup, and the covariates defined above. Models of prevalence used generalized estimating equations with an exchangeable correlation structure to account for the dependence of repeated prevalence estimates within individuals. Additional adjusted models contained the 2‐way interaction of cohort*year to test if temporal trends differed between the 2 cohorts. To account for potentially long periods of remission in depression and anxiety disorders, we conducted a sensitivity analysis in which we required a 10‐year period before the first claim for depression or anxiety disorder without any psychiatric disorder claims. Statistical analyses were performed using SAS, version 9.4.
Results
We identified 10,206 individuals with incident RA, and 50,960 matched controls. Nearly three‐quarters were women, with a mean ± SD age at the index date of 53.7 ± 16.0 years (Table 1) 28.
Table 1.
| Characteristic | Matched cohort (n = 50,960) | RA (n = 10,206) |
|---|---|---|
| Female | 36,793 (72.2) | 7,369 (72.2) |
| Age at diagnosis, mean ± SD years | 53.7 ± 16.0 | 53.7 ± 16.0 |
| Followup duration from index date (years), median (IQR) | 9.05 (4.33–14.9) | 9.19 (4.58–14.8) |
| Region of residence | ||
| Urban | 29,870 (58.6) | 5,981 (58.6) |
| Rural | 21,090 (41.1) | 4,225 (41.4) |
| SES (SEFI‐2), mean ± SD | 0.080 ± 1.0 | 0.054 ± 1.03 |
Values are the number (%) unless otherwise indicated. RA = rheumatoid arthritis; IQR = interquartile range; SES = socioeconomic status; SEFI‐2 = Socioeconomic Factor Index, version 2.
Incidence
Among the psychiatric disorders, anxiety disorder had the highest annual incidence over the study period in both cohorts, while schizophrenia had the lowest incidence. In 2011, the crude annual incidence of depression per 1,000 persons in the RA cohort was 15.0 (95% CI 11.9–18.9) versus 9.09 (95% CI 8.03–10.3) in the matched cohort. In the same year, the crude annual incidence of anxiety per 1,000 persons was 16.7 (95% CI 13.2–21.2) in the RA cohort, versus 15.6 (95% CI 14.1–17.3) in the matched cohort. The average annual incidence of bipolar disorder (reported due to small cell sizes) was 2.6 per 1,000 persons (95% CI 2.3–3.0) in the RA cohort, versus 1.8 (95% CI 1.7–2.0) in the matched cohort. The average annual incidence of schizophrenia was 0.48 per 1,000 persons (95% CI 0.35–0.68) in the RA cohort, versus 0.41 (95% CI 0.34–0.50) in the matched cohort.
The average annual age‐specific incidence rates of the psychiatric disorders varied by age in both cohorts (Figure 1). The incidence of depression was similar in the 18–24 and 25–44 years age groups, but declined in those ages 45–64 years before rising again (Figure 1A). The incidence of anxiety disorder declined with increasing age (Figure 1B). The incidence rate of bipolar disorder was highest among those ages 25–44 years and lowest among those age ≥65 years (Figure 1C). In the RA cohort, the average annual age‐specific incidence rate of schizophrenia was highest in those ages 25–44 years (Figure 1C). The incidence of schizophrenia was higher in the RA cohort than in the matched cohort among those ages 25–44 years, but incidence rates did not differ between cohorts for the other age groups.
Figure 1.
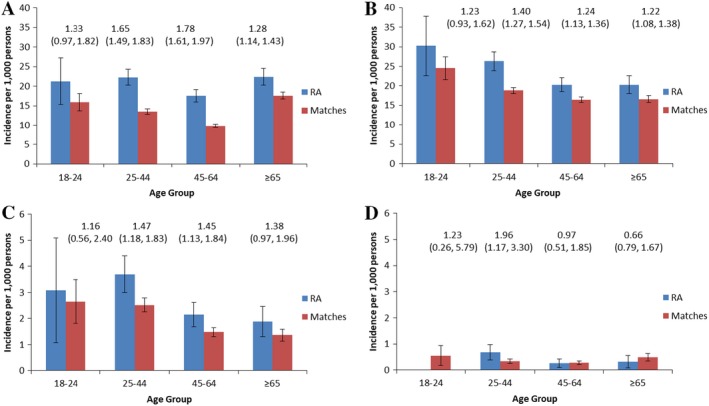
Age‐specific average annual incidence of psychiatric disorders in the rheumatoid arthritis (RA) and matched cohorts, in A, depression, B, anxiety disorder, C, bipolar disorder, and D, schizophrenia. Incidence rate ratios (95% confidence intervals) comparing the 2 cohorts are shown at the top of each panel. Results are suppressed for schizophrenia in the 18–24 years age group in the RA cohort to preserve privacy. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/doi/10.1002/acr.23539/abstract.
The average annual incidence rates of depression, anxiety disorder, and bipolar disorder were higher in women than in men (see Supplementary Table 2, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.23539/abstract). Within sex strata, the incidence of depression, anxiety disorder, and bipolar disorder was higher in the RA cohort than in the matched cohort; the incidence of schizophrenia did not differ between cohorts. As compared to the matched cohort, the age‐ and sex‐standardized incidence in 2011 of depression was higher in the RA cohort (Table 2). The standardized incidence of anxiety disorder and bipolar disorder were not statistically significantly higher in the RA cohort. The average annual incidence of schizophrenia did not differ between groups.
Table 2.
Comparison of age‐ and sex‐standardized incidence and prevalence of psychiatric disorders between rheumatoid arthritis and matched cohorts in 2011a
| Incidence | Lifetime prevalence | Annual period prevalence | |
|---|---|---|---|
| Depression | 1.71 (1.15–2.55) | 1.48 (1.35–1.64) | 1.66 (1.36–2.02) |
| Anxiety disorder | 1.21 (0.82–1.79) | 1.25 (1.13–1.37) | 1.63 (1.22–2.19) |
| Bipolar disorder | 1.30 (063–2.68) | 1.30 (1.10–1.53) | 1.19 (0.80–1.78) |
| Schizophrenia | 0.97 (0.62–1.52)b | 1.10 (0.71–1.70) | 0.71 (0.41–1.26) |
Values are the rate ratios (95% confidence intervals).
Average annual incidence over study period due to small cell sizes in a single year.
After adjustment for age, sex, area‐level SES, region of residence, number of physician visits, and year, we found that the incidence of depression was higher in the RA cohort over the entire study period (IRR 1.46 [95% CI 1.35–1.58]), as were the incidences of anxiety disorder (IRR 1.24 [95% CI 1.15–1.34]) and bipolar disorder (IRR 1.21 [95% CI 1.00–1.47]) (Table 3). The incidence of schizophrenia did not differ between groups (IRR 0.96 [95% CI 0.61–1.50]).
Table 3.
Association between RA and incidence of psychiatric disordersa
| Variable | Depression | Anxiety disorder | Bipolar disorder | Schizophrenia |
|---|---|---|---|---|
| Cohort | ||||
| Matches | 1.0 | 1.0 | 1.0 | 1.0 |
| RA | 1.46 (1.35–1.58)b | 1.24 (1.15–1.34)b | 1.21 (1.00–1.47)c | 0.96 (0.61–1.50) |
| Sex | ||||
| Male | 1.0 | 1.0 | 1.0 | 1.0 |
| Female | 1.60 (1.48–1.73)b | 1.54 (1.43–1.66)b | 1.81 (1.47–2.22)b | 1.77 (1.12–2.81)b |
| Age, years | ||||
| 18–24 | 1.0 | 1.0 | 1.0 | 1.0 |
| 25–44 | 0.93 (0.80–1.08) | 0.80 (0.70–0.91)b | 1.15 (0.81–1.62) | 0.90 (0.42–1.92) |
| 45–64 | 0.73 (0.62–0.85)b | 0.68 (0.59–0.79)b | 0.76 (0.52–1.12) | 0.66 (0.29–1.54) |
| ≥65 | 1.16 (0.99–1.36) | 0.69 (0.60–0.80)b | 0.64 (0.43–0.93)b | 1.12 (0.50–2.47) |
| Socioeconomic status | ||||
| Quintile 1 (lowest) | 1.10 (1.00–1.20)b | 1.16 (1.06–1.25)b | 1.20 (0.97–1.49) | 2.79 (1.67–4.66)b |
| Quintile 2 | 1.25 (1.15–1.37)b | 1.17 (1.07–1.27)b | 1.38 (1.12–1.72)b | 1.49 (0.85–2.64) |
| Quintile 3 | 1.13 (1.03–1.24)b | 1.12 (1.03–1.22)b | 1.12 (0.90–1.41) | 1.54 (0.87–2.72) |
| Quintile 4 | 1.13 (1.04–1.24)b | 1.14 (1.05–1.24)b | 1.17 (0.94–1.46) | 1.42 (0.80–2.53) |
| Quintile 5 (highest) | 1.0 | 1.0 | 1.0 | 1.0 |
| Region | ||||
| Rural | 1.0 | 1.0 | 1.0 | 1.0 |
| Urban | 1.13 (1.06–1.19)b | 1.27 (1.20–1.34)b | 1.86 (1.60–2.15)b | 1.66 (1.21–2.29)b |
| No. physician visits | 1.00 (1.00–1.00)b | 1.00 (1.00–1.00)b | 1.00 (1.00–1.00)b | 1.00 (1.00–1.00)b |
| Yeard | 0.991 (0.984–0.997)b | 1.000 (0.993–1.006) | 0.997 (0.980–1.014) | 0.989 (0.950–1.028) |
Values are the adjusted rate ratios (95% confidence intervals) unless otherwise indicated. Models for depression and anxiety disorders also include term to adjust for whether prescription claims were used in the case definition. RA = rheumatoid arthritis.
Statistically significant.
Statistically significant at P = 0.05.
Refers to annual change.
In adjusted models, women, those of lower area‐level SES, and those living in urban settings had an increased incidence of depression, anxiety disorder, bipolar disorder, and schizophrenia (Table 3). As compared to participants ages 18–24 years, those ages 25–64 years had a reduced incidence of depression, anxiety disorder, and schizophrenia. Participants ages 65 years and older also had a reduced incidence of anxiety disorder and bipolar disorder. The incidence of depression declined minimally but statistically significantly over time in both populations, while the incidence of the other disorders did not change.
Findings for depression and anxiety disorder were similar when we required a 10‐year period without a diagnosis code for a psychiatric disorder before the first diagnosis code for depression or anxiety disorder, although confidence intervals for point estimates were broader (for cohort characteristics, see Supplementary Table 3, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.23539/abstract). Specifically, in 2011 the crude annual incidence of depression per 1,000 persons in the RA cohort was 15.1 (95% CI 11.6–19.6) versus 9.42 (95% CI 8.17–10.9) in the matched cohort. In the same year, the crude annual incidence of anxiety per 1,000 persons was 16.6 (95% CI 12.6–21.8) in the RA cohort, versus 16.3 (95% CI 14.5–18.3) in the matched cohort. After adjustment for age, sex, area‐level SES, region of residence, number of physician visits, and year, the incidence of depression was found to be higher in the RA cohort over the entire study period (IRR 1.43 [95% CI 1.29–1.59]), as was the incidence of anxiety disorder (IRR 1.26 [95% CI 1.14–1.39]).
Prevalence
Among the psychiatric disorders, anxiety disorder had the highest observed lifetime and annual period prevalence, while schizophrenia had the lowest in both cohorts. Crude prevalence estimates for 2011 are shown in Figure 2. In both cohorts, participants ages 25–44 years had the highest prevalence (current and lifetime) of depression and anxiety disorder, while participants ages 25–64 years shared the highest prevalence of bipolar disorder and schizophrenia (Figure 3). The annual period and lifetime prevalence of depression, anxiety disorder, and bipolar disorder were higher for women than men in both cohorts (see Supplementary Table 4, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.23539/abstract).
Figure 2.
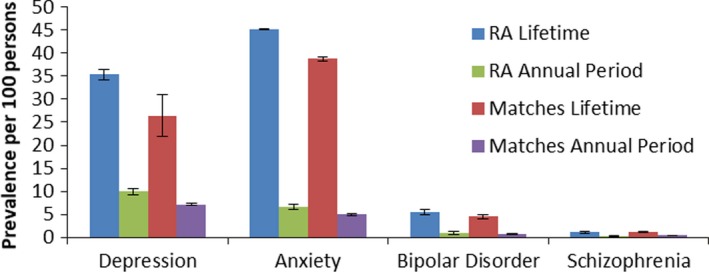
Crude lifetime and annual period prevalence (95% confidence intervals) of psychiatric disorders in the rheumatoid arthritis (RA) and matched cohorts in 2011.
Figure 3.
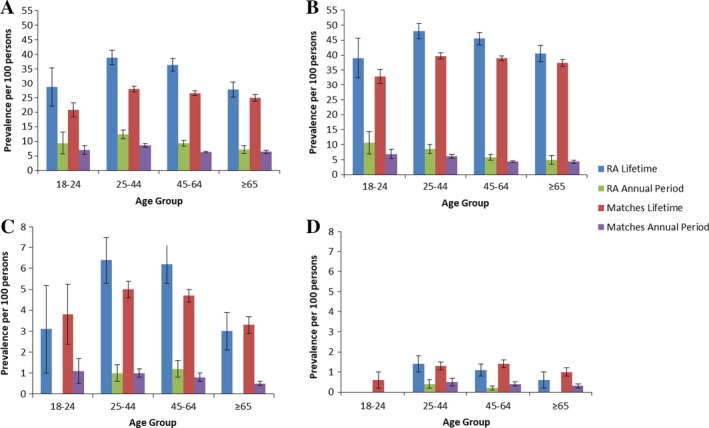
Age‐specific lifetime and annual period prevalence (95% confidence intervals) of psychiatric disorders in the rheumatoid arthritis (RA) and matched cohorts in 2011, in A, depression, B, anxiety disorder, C, bipolar disorder, and D, schizophrenia. Results are suppressed for schizophrenia and bipolar disorder in the 18–24 years age group in the RA cohort to preserve privacy.
As compared to the matched cohort, the age‐ and sex‐standardized lifetime prevalence (in 2011) of depression, anxiety disorder, and bipolar disorder were higher in the RA cohort (Table 2). The lifetime prevalence of schizophrenia did not differ between groups (PR 1.10 [95% CI 0.71–1.70]). Similarly, the annual period prevalence of depression and anxiety disorder were higher in the RA cohort (Table 2). The annual period prevalence of bipolar disorder and schizophrenia did not differ between the 2 cohorts.
After adjustment for age, sex, area‐level SES, region of residence, and year, the lifetime prevalence of depression (PR 1.35 [95% CI 1.26–1.45]) and anxiety disorder (PR 1.20 [95% CI 1.13–1.27]) were higher in the RA cohort than in the matched cohort over the entire study period (see Supplementary Table 5, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.23539/abstract). The lifetime prevalence of bipolar disorder (PR 1.13 [95% CI 0.95–1.36]) and schizophrenia did not differ between the RA and matched cohorts (PR 1.02 [95% CI 0.72–1.43]). Similarly, after adjustment, the annual period prevalence of depression (PR 1.36 [95% CI 1.26–1.47]), anxiety disorder (PR 1.30 [95% CI 1.19–1.41]), and bipolar disorder were higher in the RA cohort than the matched cohort over the study period (see Supplementary Table 6, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.23539/abstract). The annual period prevalence of bipolar disorder (PR 1.06 [95% CI 0.86–1.31]) and schizophrenia did not differ between the RA and matched cohorts (PR 0.68 [95% CI 0.40–1.15]).
Discussion
We conducted a large, population‐based study and found that incidence and prevalence of multiple psychiatric disorders, including depression, anxiety, and bipolar disorder, were higher in the RA population than in the matched cohort from the general population. For depression and anxiety disorders, the relative increase in annual period prevalence in the RA population was higher than the relative increase in lifetime prevalence. This suggests that differences between the RA and matched populations may diminish over a lifetime as these conditions accumulate in the general population. We reported age‐ and sex‐specific estimates, which illustrate the variation in the incidence and prevalence of these conditions across demographic groups; prior research regarding this variation is limited 11.
Similar to our study, 2 previous studies in Taiwan reported an increased incidence of depression in the RA population as compared to controls 13, 34. Although the prevalence of anxiety disorders is reportedly increased in RA 7, we could not identify prior studies reporting the incidence of anxiety disorders. Our findings regarding the increased incidence of bipolar disorder in RA are consistent with 1 previous study which evaluated the association of RA and bipolar disorder 12, 35, 36. In that study of 2,570 individuals with RA from Taiwan and 2,570 individuals without RA, matched on age, sex, comorbidities, and date of enrollment in the national health insurance plan, RA was associated with a 2‐fold increased incidence of bipolar disorder 12. Two other studies failed to showed associations between RA and bipolar disorder 35, 36. These differences may reflect differences in study design or study populations. We did not identify an association between RA and schizophrenia in our population, possibly due to the relatively small number of affected individuals, but the preponderance of prior studies suggests that schizophrenia occurs less often than expected in RA 37.
For both cohorts, the incidence of depression, anxiety disorder, bipolar disorder, and schizophrenia did not change meaningfully over the more than 20‐year study period, while the prevalence rose minimally. We were unable to identify any comparable studies examining temporal trends in the incidence of these disorders in RA. Evaluation of changes in the prevalence of mood and anxiety disorders over time in the general population has suggested that, although access to treatment has improved, the prevalence of these disorders has not decreased 38.
Several demographic factors were found to be associated with the risk of psychiatric disorders. The peak incidence of the psychiatric disorders studied was generally among those ages 18–44 years, and declined at older ages, which is in keeping with the typical age of onset of these disorders. The incidence and prevalence of depression, anxiety, and bipolar disorder was increased in women. In the general population, female sex is associated with an increased prevalence of depression and anxiety globally 39. Prior studies in RA have also found that female sex is associated with an increased incidence of depression 11, 13, although this has not always been found 8. Bipolar I disorder does not demonstrate a sex predilection, but bipolar II disorder may affect women more often than men 40. Therefore, our findings raise the possibility that bipolar II disorder may be more common in RA than bipolar I disorder. However, we could not distinguish bipolar I from bipolar II disorder using administrative data. A previous meta‐analysis reported an increased prevalence of several psychiatric disorders among members of the general population living in urban areas 41. Consistent with that observation, urban residence was associated with an increased incidence of all the psychiatric disorders studied. Previously it has been proposed that, due to the presence of greater psychosocial and environmental stressors, the urban environment may contribute to the risk of disorders such as schizophrenia in genetically susceptible individuals 42. Similarly, lower SES was associated with an increased incidence of all the psychiatric disorders, consistent with prior findings in RA 8.
Strengths of this study include the large study population and the use of population‐based data sources. However, limitations should be considered. Although we evaluated multiple psychiatric disorders, we did not evaluate psychiatric multimorbidity, a common and clinically relevant issue which may affect outcomes 43, 44, nor did we evaluate medical comorbidity. Because physician claims in Manitoba include only a single diagnosis, administrative data are not ideal to evaluate this issue, which deserves future study. We used administrative data, which may limit the accuracy of the diagnostic codes reported. However, we employed a validated case definition for RA that was highly specific. The sensitivity of this definition is 77%, and therefore some cases were likely missed, but this would bias findings toward the null. The case definitions used to identify psychiatric comorbidity have not been validated in RA, but they have been validated in 2 other immune‐mediated inflammatory diseases and demonstrated stable performance characteristics across diseases 29, 30.
Moreover, we identified the expected demographic relationships with the psychiatric disorders studied. Administrative data only identify health conditions for which individuals seek medical treatment 45 and will not identify care provided by nonphysician providers such as psychologists. Conditions that cause symptoms but do not meet diagnostic criteria may not be captured. Also, underdiagnosis of psychiatric disorders in the general and RA populations is recognized 46. All of these factors may have led to underestimation of the incidence and prevalence of psychiatric disorders, but this is unlikely to affect the relative risk of psychiatric comorbidity between cohorts. Because psychiatric disorders may be lifelong, recurrent conditions, the use of only a 5‐year period of no claims may have misclassified some prevalent cases as incident, rather than recurrent, particularly for depression and anxiety disorders. This would tend to overestimate incidence rates, but would be unlikely to affect comparisons between cohorts. However, the median time between claims, of 24–31 days, and the consistency of our findings with the longer 10‐year run‐in period in the complementary analysis suggest that the effects of any such misclassification are likely to be small. As administrative data lack clinical details, we could not evaluate the associations between characteristics of RA or its treatments and the risk of psychiatric comorbidity. Future studies should explore these issues in population‐based clinical cohorts that comprehensively evaluate multiple psychiatric disorders. Finally, the generalizability of the findings should be considered. We conducted this study in Manitoba, the Canadian province with the highest proportion of First Nations Canadians, a group at increased risk of RA. However, access to health care is similar to that in other Canadian provinces, as is educational attainment. Educational attainment is slightly higher in Canada than the Organization for Economic Cooperation and Development average 22.
We observed that RA is associated with an increased risk of multiple psychiatric disorders, including depression, anxiety disorder, and bipolar disorder, and that these risks have not changed over time, despite changes in the clinical management of RA over the 20‐year study period. Clinicians should be aware that women and those of lower SES are at particularly increased risk of these disorders.
Author contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Marrie had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Marrie, Hitchon, Patten, Sareen, Walker, Lix, El‐Gabalawy, Katz, Fisk, Bernstein.
Acquisition of data
Marrie, Singer, Katz, Bernstein.
Analysis and interpretation of data
Marrie, Hitchon, Walld, Patten, Bolton, Walker, Singer, Lix, El‐Gabalawy, Katz, Bernstein.
Supporting information
Appendix A. Members of the Canadian Institutes of Health Research Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Inflammatory Disease
In addition to the authors, the members of the Canadian Institutes of Health Research Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease are Lesley Graff, Lindsay Berrigan, Ryan Zarychanski, Christine Peschken, and James Marriott.
Supported by the Canadian Institutes of Health Research (THC‐135234). Dr. Marrie is supported by the Waugh Family Chair in Multiple Sclerosis. Dr. Sareen is supported by the Canadian Institutes of Health Research (333252). Dr. Lix is supported by a Research Manitoba Chair. Dr. Bernstein is supported in part by the Bingham Chair in Gastroenterology.
Members of the Canadian Institutes of Health Research Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease are shown in Appendix A.
Dr. Hitchon has received research support from UCB Canada. Dr. Sareen holds stock in Johnson and Johnson. Dr. Bernstein has received consulting fees from AbbVie Canada, Ferring Canada, Janssen Canada, Napo Pharmaceuticals, Pfizer Canada, Shire Canada, Takeda Canada, and Mylan Pharmaceuticals (less than $10,000 each) and has received speaker's bureau fees from AbbVie Canada, Ferring Canada, and Shire Canada (less than $10,000 each).
Contributor Information
Ruth Ann Marrie, Email: rmarrie@hsc.mb.ca.
for the Canadian Institutes of Health Research Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease:
Lesley Graff, Lindsay Berrigan, Ryan Zarychanski, Christine Peschken, and James Marriott
References
- 1. Scherrer JF, Virgo KS, Zeringue A, Bucholz KK, Jacob T, Johnson RG, et al. Depression increases risk of incident myocardial infarction among Veterans Administration patients with rheumatoid arthritis. Gen Hosp Psychiatry 2009;31:353–9. [DOI] [PubMed] [Google Scholar]
- 2. Rupp I, Boshuizen HC, Roorda LD, Dinant HJ, Jacobi CE, van den Bos G. Poor and good health outcomes in rheumatoid arthritis: the role of comorbidity. J Rheumatol 2006;33:1488–95. [PubMed] [Google Scholar]
- 3. Ang DC, Choi H, Kroenke K, Wolfe F. Comorbid depression is an independent risk factor for mortality in patients with rheumatoid arthritis. J Rheumatol 2005;32:1013–9. [PubMed] [Google Scholar]
- 4. Hitchon CA, Boire G, Haraoui B, Keystone E, Pope J, Jamal S, et al. Self‐reported comorbidity is common in early inflammatory arthritis and associated with poorer function and worse arthritis disease outcomes: results from the Canadian Early Arthritis Cohort. Rheumatology (Oxford) 2016;55:1751–62. [DOI] [PubMed] [Google Scholar]
- 5. Matcham F, Norton S, Scott DL, Steer S, Hotopf M. Symptoms of depression and anxiety predict treatment response and long‐term physical health outcomes in rheumatoid arthritis: secondary analysis of a randomized controlled trial. Rheumatology (Oxford) 2016;55:268–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matcham F, Rayner L, Steer S, Hotopf M. The prevalence of depression in rheumatoid arthritis: a systematic review and meta‐analysis. Rheumatology (Oxford) 2013;52:2136–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Isik A, Koca SS, Ozturk A, Mermi O. Anxiety and depression in patients with rheumatoid arthritis. Clin Rheumatol 2007;26:872–8. [DOI] [PubMed] [Google Scholar]
- 8. Lok EY, Mok CC, Cheng CW, Cheung EF. Prevalence and determinants of psychiatric disorders in patients with rheumatoid arthritis. Psychosomatics 2010;51:338–e8. [DOI] [PubMed] [Google Scholar]
- 9. VanDyke MM, Parker JC, Smarr KL, Hewett JE, Johnson GE, Slaughter JR, et al. Anxiety in rheumatoid arthritis. Arthritis Rheum 2004;51:408–12. [DOI] [PubMed] [Google Scholar]
- 10. Dickens C, McGowan L, Clark‐Carter D, Creed F. Depression in rheumatoid arthritis: a systematic review of the literature with meta‐analysis. Psychosom Med 2002;64:52–60. [DOI] [PubMed] [Google Scholar]
- 11. Wang S‐L, Chang C‐H, Hu L‐Y, Tsai S‐J, Yang AC, You Z‐H. Risk of developing depressive disorders following rheumatoid arthritis: a nationwide population‐based study. PLoS One 2014;9:e107791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hsu CC, Chen SC, Liu CJ, Lu T, Shen CC, Hu YW, et al. Rheumatoid arthritis and the risk of bipolar disorder: a nationwide population‐based study. PLoS One 2014;9:e107512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin MC, Guo HR, Lu MC, Livneh H, Lai NS, Tsai TY. Increased risk of depression in patients with rheumatoid arthritis: a seven‐year population‐based cohort study. Clinics (Sao Paulo) 2015;70:91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Q, Yang C, Gelernter J, Zhao H. Pervasive pleiotropy between psychiatric disorders and immune disorders revealed by integrative analysis of multiple GWAS. Hum Genet 2015;134:1195–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Winkel Rv, Nierop Mv, Myin‐Germeys I, van Os J. Childhood trauma as a cause of psychosis: linking genes, psychology, and biology. Can J Psychiatry 2013;58:44–51. [DOI] [PubMed] [Google Scholar]
- 16. Cohen S, Janicki‐Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci 2012;109:5995–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schmeer KK, Yoon A. Socioeconomic status inequalities in low‐grade inflammation during childhood. Arch Dis Child 2016;101:1043–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Calcia MA, Bonsall DR, Bloomfield PS, Selvaraj S, Barichello T, Howes OD. Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology (Berl) 2016;233:1637–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pervanidou P, Chrousos GP. Metabolic consequences of stress during childhood and adolescence. Metabolism 2012;61:611–9. [DOI] [PubMed] [Google Scholar]
- 20. Patten SB, Williams JV, Lavorato DH, Fiest KM, Bulloch AG, Wang J. The prevalence of major depression is not changing. Can J Psychiatry 2015;60:31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kessler RC, Demler O, Frank RG, Olfson M, Pincus HA, Walters EE, et al. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med 2005;352:2515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Statistics Canada . Table 477‐0135: educational attainment of the population aged 25 to 64, by age group and sex. Organisation for Economic Co‐operation and Development Canada, provinces and territories, occasional (percent), CANSIM (database). Ottawa 2006. URL: https://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/demo52b-eng.htm. [Google Scholar]
- 23. Statistics Canada . Profile for Canada, provinces, territories, census divisions, and census subdivisions, 2006 Census Ottawa 2006. URL: http://www12.statcan.gc.ca/census-recensement/2006/dp-pd/prof/rel/Rp-eng.cfm?LANG=E&APATH=3&DETAIL=1&DIM=0&FL=A&FREE=1&GC=0&GID=0&GK=0&GRP=1&PID=94533&PRID=0&PTYPE=89103&S=0&SHOWALL=No&SUB=0&Temporal=2006&THEME=81&VID=0&VNAMEE=&VNAMEF=.
- 24. Statistics Canada . Visible minority population, by province and territory (2006 Census) (Quebec, Ontario, Manitoba, Saskatchewan). Ottawa 2006. URL: https://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/demo52b-eng.htm. [Google Scholar]
- 25. Hitchon CA, Khan S, Elias B, El‐Gabalawy HS, Katz A, Peschken CA. First Nations persons have an increased risk of developing rheumatoid arthritis with an early onset age but are seen less frequently by rheumatologists: a population‐based study. ACR/ARHP Annual Meeting; 2014 Nov 14–19; Boston, MA: American College of Rheumatology; 2014.
- 26. Peschken CA, Hitchon CA, Garland A, Bernstein CN, Chen H, Fransoo R, et al. A population‐based study of intensive care unit admissions in rheumatoid arthritis. J Rheumatol 2016;43:26–33. [DOI] [PubMed] [Google Scholar]
- 27. Hitchon C, Khan S, Elias B, Peschken C. Increased prevalence and incidence of rheumatoid arthritis in Manitoba First Nations: a population‐based study. J Rheumatol In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marrie RA, Walld R, Bolton JM, Sareen J, Walker JR, Patten SB, et al. Increased incidence of psychiatric disorders in immune‐mediated inflammatory disease. J Psychosom Res 2017;101:17–23. [DOI] [PubMed] [Google Scholar]
- 29. Marrie RA, Fisk JD, Yu BN, Leung S, Elliott L, Caetano P, et al. Mental comorbidity and multiple sclerosis: validating administrative data to support population‐based surveillance. BMC Neurol 2013;13:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marrie RA, Walker JR, Graff LA, Lix LM, Bolton JM, Nugent Z, et al. Performance of administrative case definitions for depression and anxiety in inflammatory bowel disease. J Psychosom Res 2016;89:107–13. [DOI] [PubMed] [Google Scholar]
- 31. Marrie RA, Walld R, Bolton JM, Sareen J, Walker JR, Patten SB, et al. Estimating annual prevalence of depression and anxiety disorder in multiple sclerosis using administrative data. BMC Res Notes 2017;10:619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Statistics Canada . Canadian Community Health Survey–Mental Health (CCHS). Ottawa, 2013. URL: http://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&Id=119789.
- 33. Chateau D, Metge C, Prior H, Soodeen RA. Learning from the census: the Socio‐economic Factor Index (SEFI) and health outcomes in Manitoba. Can J Public Health 2012;103 Suppl 2:S23–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lu MC, Guo HR, Lin MC, Livneh H, Lai NS, Tsai TY. Bidirectional associations between rheumatoid arthritis and depression: a nationwide longitudinal study. Sci Rep 2016;6:20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Farhi A, Cohen AD, Shovman O, Comaneshter D, Amital H, Amital D. Bipolar disorder associated with rheumatoid arthritis: a case‐control study. J Affect Disord 2016;189:287–9. [DOI] [PubMed] [Google Scholar]
- 36. Sellgren C, Frisell T, Lichtenstein P, Landèn M, Askling J. The association between schizophrenia and rheumatoid arthritis: a nationwide population‐based Swedish study on intraindividual and familial risks. Schizophr Bull 2014;40:1552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Eaton WW, Hayward C, Ram R. Schizophrenia and rheumatoid arthritis: a review. Schizophr Res 1992;6:181–92. [DOI] [PubMed] [Google Scholar]
- 38. Jorm AF, Patten SB, Brugha TS, Mojtabai R. Has increased provision of treatment reduced the prevalence of common mental disorders? Review of the evidence from four countries. World Psychiatry 2017;16:90–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Steel Z, Marnane C, Iranpour C, Chey T, Jackson JW, Patel V, et al. The global prevalence of common mental disorders: a systematic review and meta‐analysis 1980–2013. Int J Epidemiol 2014;43:476–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arnold LM. Gender differences in bipolar disorder. Psychiatr Clin N Am 2003;26:595–620. [DOI] [PubMed] [Google Scholar]
- 41. Peen J, Schoevers RA, Beekman AT, Dekker J. The current status of urban‐rural differences in psychiatric disorders. Acta Psychiatr Scand 2010;121:84–93. [DOI] [PubMed] [Google Scholar]
- 42. Krabbendam L, van Os J. Schizophrenia and urbanicity: a major environmental influence—conditional on genetic risk. Schizophr Bull 2005;31:795–9. [DOI] [PubMed] [Google Scholar]
- 43. Mittal D, Fortney JC, Pyne JM, Edlund MJ, Wetherell JL. Impact of comorbid anxiety disorders on health‐related quality of life among patients with major depressive disorder. Psychiatric Services 2006;57:1731–7. [DOI] [PubMed] [Google Scholar]
- 44. Dunner DL. Management of anxiety disorders: the added challenge of comorbidity. Depress Anxiety 2001;13:57–71. [DOI] [PubMed] [Google Scholar]
- 45. Mojtabai R, Olfson M, Sampson NA, Jin R, Druss B, Wang PS, et al. Barriers to mental health treatment: results from the National Comorbidity Survey Replication. Psychol Med 2011;41:1751–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hider SL, Tanveer W, Brownfield A, Mattey DL, Packham JC. Depression in RA patients treated with anti‐TNF is common and under‐recognized in the rheumatology clinic. Rheumatology (Oxford) 2009;48:1152–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


