Abstract
Introduction
In the work‐up of patients with suspected pelvic congestion syndrome, venography is currently the gold standard. Yet if non‐invasive diagnostic tools are found to be accurate, invasive venography might no longer be indicated as necessary.
Material and methods
A literature search in Pubmed and EMBASE was performed from inception until 6 May 2017. Studies comparing non‐invasive diagnostic tools to a reference standard in the work‐up of patients with (suspected) pelvic congestion syndrome were included. Relevant data were extracted and methodological quality of individual included studies was assessed by the Quality Assessment of Diagnostic Accuracy Studies (QUADAS‐2) tool.
Results
Nine studies matched our inclusion criteria. Six studies compared ultrasonography to venography and three studies described a magnetic resonance imaging technique. In using transvaginal ultrasonography, the occurrence of a vein greater than five mm crossing the uterine body had a specificity of 91% (95% CI; 77–98%) and occurrence of pelvic varicoceles a sensitivity and specificity of 100% (95% CI; 89–100%) and 83–100% (95% CI; 66–93%), respectively. In transabdominal ultrasonography, reversed caudal flow in the ovarian vein accounted for a sensitivity of 100% (95% CI; 84–100%). Detection of pelvic congestion syndrome with magnetic resonance imaging techniques resulted in a sensitivity varying from 88 to 100%.
Conclusions
The sensitivity of ultrasonography and magnetic resonance imaging seem to be adequate, which indicates a role for both tests in an early stage of the diagnostic workup. However, due to methodological flaws and diversity in outcome parameters, more high standard research is necessary to establish a clear advice for clinical practice.
Keywords: Diagnostic imaging, magnetic resonance imaging, pelvic congestion syndrome, pelvic pain, sensitivity and specificity, ultrasonography, varicose veins
Abbreviations
- CPP
chronic pelvic pain
- CT
computed tomography
- MRI
magnetic resonance imaging
- MR PCVM
magnetic resonance phase‐contrast velocity mapping
- PCS
pelvic congestion syndrome
- QUADAS‐2
Quality Assessment of Diagnostic Accuracy Studies
Key Message.
In the work‐up of patients with suspected pelvic congestion syndrome there is a need for an accurate noninvasive diagnostic tool. The sensitivity of ultrasonography and magnetic resonance imaging seem to be adequate but more high standard research is necessary.
Introduction
Chronic pelvic pain (CPP) is a common yet underestimated condition in women of reproductive age, with a prevalence of 5.7–26.6% 1, 2, 3, 4, 5, 6, 7. By the International Association for the Study of Pain CPP is defined as pain, perceived in structures related to the pelvis, which has been continuous or recurrent for at least 6 months. In gynecology, CPP has several differential diagnoses, such as endometriosis, pelvic inflammatory disease, pelvic adhesions, ovarian pathology and the often‐neglected pelvic congestion syndrome (PCS) 1, 2, 3, 4, 5, 8.
Pelvic congestion syndrome is a clinical entity, first described in 1857, in which varicose veins are related to CPP 9. The prevalence of PCS among patients with CPP is found to be 12–33% 1, 2, 3, 4, 5, 10. Patients with PCS are mostly premenopausal, multiparous women who have complaints of CPP accompanied with dysmenorrhea, and exacerbating symptoms during and after intercourse or prolonged standing 11, 12, 13. Pelvic varices are caused by incompetence of the ovarian veins. These veins arise from the ovarian venous plexus and communicate with the uterine plexus in the broad ligament. Incompetence leads to retrograde venous flow, progressive development of pelvic varicosities and dilatation 14, 15. The origin of PCS is most likely to be multifactorial 15. Two important sources of pelvic vein insufficiency are described in the literature. First, valvular insufficiency, due to congenital absent or incompetent valves, plays a possible role in the etiology 15. Second, during pregnancy the vascular capacity of ovarian veins enlarges by up to 60 times their normal value 16. This increased capacity causes mechanical pressure and can eventually contribute to persistent venous reflux; explaining why PCS is mainly seen in multiparous women 17.
Venography is considered the reference standard test for the diagnosis of pelvic venous disorders 18, 19. The congestion is defined as extensive when wide veins are tortuous, with great variation in caliber, and when individual veins are obscured by a pool of contrast medium 18, 19. Venography is a valid method for diagnosing PCS, but is invasive, time‐consuming and exposes the pelvis of women of childbearing age to radiation 18, 19. If noninvasive diagnostic tools were found to be accurate, venography could possibly be avoided in a large number of women. Several noninvasive diagnostic tools are used in the work‐up of patients with CPP and suspected pelvic venous insufficiency, for example ultrasonography (Figure 1), computed tomography (CT) and magnetic resonance imaging (MRI) 14, 20, 21. However, the diagnostic value of these tests remains unclear. In this review, we aim to evaluate studies on noninvasive diagnostic tests in the work‐up of patients with suspected PCS.
Figure 1.
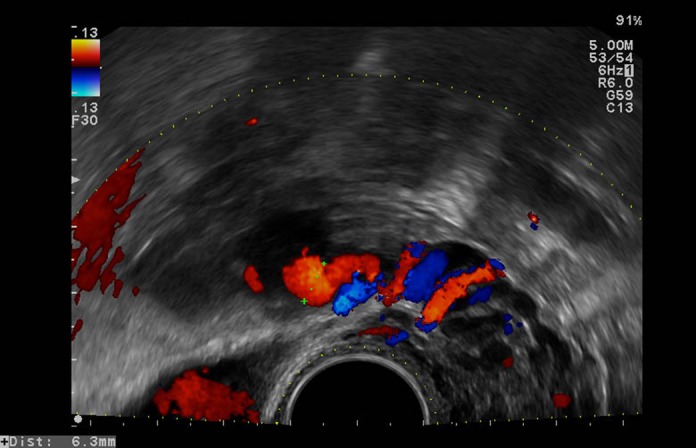
An example of tortuous veins visualized with pulsed Doppler during transvaginal ultrasonography, a possible sign of pelvic congestion syndrome. [Color figure can be viewed at http://wileyonlinelibrary.com].
Material and methods
A computer‐aided search in PubMed and EMBASE was performed, in collaboration with a medical librarian. The databases were searched from inception until 6 May 2017, the search strategy can be found in the Supplementary material (Appendix S1). To identify additional suitable studies, the reference lists of reviews and included studies were cross‐searched manually.
Titles and abstracts were independently screened by two reviewers (TN and MS) to identify suitable articles for the first selection. Full manuscripts of the remaining citations were obtained and reviewed by TN and MS. We excluded articles from the search if they were not relevant to the goals of the review, not published in the English language, included fewer than four patients, did not include a reference test, did not involve human participants or were reviews, letters or conference abstracts. Selection disagreements were resolved by consensus in cooperation with one of the co‐authors.
Data were extracted from manuscripts using a data extraction form. On these data extraction forms information on study characteristics were noted. These included study design, sample size, inclusion and exclusion criteria, and baseline characteristics of included patients, such as age, ethnicity, parity and menopausal status. Information about the setting, parameters, thresholds and outcomes of the index and reference test was also extracted. Sensitivity and specificity of pelvic venous dilatation associated with CPP were set as main outcomes and, when necessary, these were calculated based on available data. A p‐value < 0.05 was considered statistically significant. The methodological quality of included studies was assessed systematically using the Second Quality Assessment of Diagnostic Accuracy Studies (QUADAS‐2) tool. This 12‐item scale, applicable to diagnostic accuracy studies, is designed to assess the risk of bias and possible concerns regarding applicability 22.
Results
The initial search yielded 588 articles; cross‐searching reference lists yielded another 21 records. After screening on title and abstract we selected 58 articles. These articles were assessed for eligibility based on the full text manuscript. Eventually, nine articles fully matched our eligibility criteria; six studies described ultrasonography 11, 20, 23, 24, 25, 26 and three studies described MRI 12, 14, 27. The process of study identification is displayed in Figure 2, using a prisma flow diagram 28. Two studies, describing 131Xenon clearance and applied potential tomography, were excluded because the diagnostic tools are now considered obsolete (no published data since 1981 and 1991, respectively) 29, 30.
Figure 2.
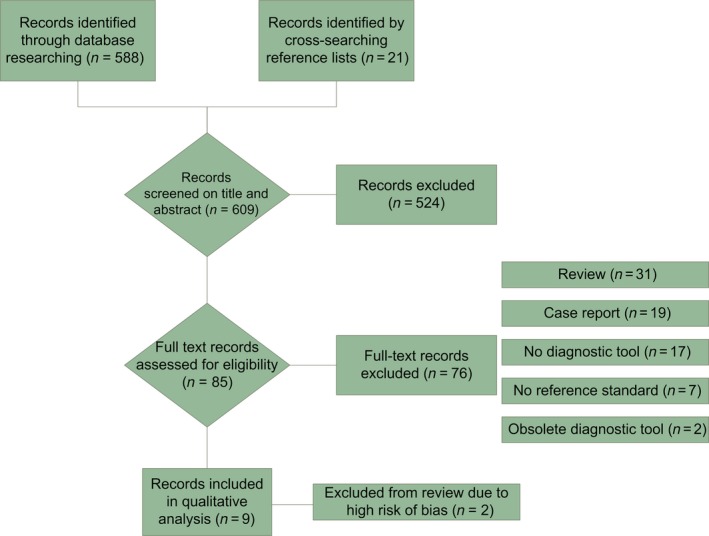
PRISMA flow chart. [Color figure can be viewed at http://wileyonlinelibrary.com].
The nine included studies were each assessed on 12 items. In total, 60 items were scored with an “L” (low risk of bias), eight items with an “H” (high risk of bias) and four items with a “U” (unclear risk of bias). Overall, seven of the included studies met most of the quality indicators of the QUADAS‐2 tool (Table 1). Regarding two studies the quality assessment could not be completed because of a lack of information, for this reason we concluded that the results could not be substantially reviewed 25, 26.
Table 1.
Methodological quality assessment (QUADAS‐2 criteria)
| Author, year | Risk of bias | Applicability concerns | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | ||||||
| Consecutive or random sample of patients enrolled | Case–control design avoided | Inappropriate exclusion avoided | Blind interpretation | Prespecified threshold | Correctly classify PCS | Appropriate interval (index and reference standard) | The same reference standard in all patients | All patients included in analysis | ||||
| Ultrasonography | ||||||||||||
| Adams, 198726 | Ua | H | U | L | H | U | U | L | H | U | U | U |
| Campbell, 200320 | L | L | L | L | H | L | L | L | L | L | L | L |
| Giacchetto, 199024 | U | L | U | U | L | L | L | L | L | L | L | L |
| Halligan, 200023 | L | H | L | H | H | L | L | H | L | U | L | L |
| Park, 200411 | U | H | L | L | U | L | L | H | L | L | L | L |
| Rae, 199025 | U | L | U | U | H | U | U | L | U | U | U | U |
| Magnetic resonance imaging | ||||||||||||
| Asciutto, 200812 | L | L | L | L | L | L | L | L | L | L | L | L |
| Meneses, 201027 | U | L | L | L | L | L | L | L | L | L | L | L |
| Yang, 201214 | L | L | L | L | L | L | L | L | L | L | L | L |
PCS, pelvic congestion syndrome; QUADAS‐2, Quality Assessment of Diagnostic Accuracy Studies.
L, low risk; H, high risk; U, unclear risk.
The characteristics of the study populations are presented in Table 2 and study characteristics are shown in Table 3. Some form of venography was used in all studies as reference standard. A variety of diagnostic criteria and thresholds were used, as shown in Table 3.
Table 2.
Characteristics of the study population
| Author, year | Patients with (suspected) PCS | Healthy controls | Inclusion criteria for PCS | Median age (range) PCS/controls | Parity PCS/controls | Premenopausal/postmenopausal | Previous treatment |
|---|---|---|---|---|---|---|---|
| Ultrasonography | |||||||
| Campbell, 200320 | 42 | – | CPPa | 29 (22–52)/‐ | NM | NM | NM |
| Giacchetto, 199024 | 35 | – |
CPP not cyclic no dysmenorrhea |
(16–53) | 0 (n = 19), ≥ 1 (n = 16) | 34/1 | NM |
| Halligan, 200023 | 36 | 19 | Transuterine venographic congestion score ≥ 6 pointsb |
29 (22–44)/ 39 (24–51) |
NM | NM | NM |
| Park, 200411 | 32 | 35 |
CPPa
increased with prolonged standing ovarian point tenderness positive venographyc |
Mean 39 (26–64)/mean 39 (27–57) | ≥ 1(n = 32)/NM | NM | NM |
| Magnetic resonance imaging | |||||||
| Asciutto, 200812 | 23 | – |
CPPa
vulval varices increase of symptoms during intercourse dyspareunia surgery for recurrent varices |
51 (29–71)/– | 0 (n = 3), 1 (n = 9), >1 (n = 11)/– | NM | Oophorectomy (n = 2), hysterectomy (n = 7) |
| Meneses, 201027 | 9 | – |
CPP > 3 months functional disability due to pain exacerbation with standing associated with bladder irritability, dyspareunia or varicosities in vulva, buttocks or thighs |
Mean 44 (26–48)/– |
NM | NM | NM |
| Yang, 201214 | 19 | – | CPPa | Mean (42)/– | 0 (n = 2), ≥ 1 (n = 17)/NM | 19/0 | NM |
CPP, chronic pelvic pain; NM, not mentioned; PCS, pelvic congestion syndrome.
Chronic pelvic pain: dull pelvic pain of variable intensity, persisting for at least 6 months.
Venographic congestion score = Ovarian vein diameter 1–4 mm (1 point), 5–8 mm (2), > 9 mm 3; Contrast clearance < 20 s (1), 21–40 s (2), > 40 s 3; Congestion absent (1), moderate (2), severe 3.
Ovarian vein diameter > 5–10 mm, uterine vein engorgement, congestion of ovarian plexuses, filling of pelvic veins across the midline, filling of vulvovaginal and thigh varicosities.
Table 3.
Characteristics of included studies
| Author, year | Design | Index test | Parameters and thresholds | Reference test | Parameters and thresholds | Position of patient index test/reference test |
|---|---|---|---|---|---|---|
| Ultrasonography | ||||||
| Campbell, 200320 | Prospective cohort study | TVUS + power Doppler |
Ultrasound congestion score: ‐ diameter of the largest vein measured < 2 mm (1), 2–5 mm (2), > 5 mm 3 ‐ number of veins in the sector 0–2 (1), 3–6 (2), ≥ 7 (3) ‐ subjective assessment of congestion normal (1), moderate (2), severe (3) Total: 3 = normal, 9 = severe congestion |
Transuterine venography |
Venographic congestion score: ‐ ovarian vein diameter 1–4 mm (1), 5–8 mm (2), > 9 mm (3) ‐ contrast clearance < 20 s (1), 21–40 s (2), > 40 s (3) ‐ congestion absent (1), moderate (2), severe (3) Total: 3 = normal, 9 = severe congestion |
Supine/supine |
| Giacchetto, 199024 | Prospective cohort study | TVUS |
Pelvic varcies: Circular or linear anechogenic structures with a diameter > 5 mm found in transverse and oblique scan sections of the lateral fornices Vascular nature of these structures confirmed with Valsalva's maneuver and upright position |
Retrograde phlebography | Passive reflux in homolateral ovarian vein | Supine |
| Halligan, 200023 | Prospective case‐control study | TVUS + power Doppler |
Ultrasound congestion score: ‐ diameter of the largest vein measured < 2 mm (1), 2–5 mm (2), > 5 mm (3) ‐ number of veins in the sector 0–2 (1), 3–6 (2), ≥ 7 (3) ‐ subjective assessment of congestion normal (1), moderate (2), severe (3) |
Transuterine venography |
Venographic congestion score: ‐ ovarian vein diameter 1–4 mm (1), 5–8 mm (2), > 9 mm (3) ‐ Contrast clearance < 20 s (1), 21–40 s (2), > 40 s (3) ‐ Congestion absent (1), moderate (2), severe (3) PCS: ≥ 6 points |
Supine/supine |
| Park,200411 | Prospective case‐control study |
TVUS + TAUS + color Duplex |
Pelvic varicoceles: tortuous vein. Dilated ovarian vein: > 5 mm. Flow direction of ovarian vein |
Selective ovarian venography |
PCS: ‐ ovarian vein diameter > 5–10 mm ‐ or uterine vein engorgement ‐ or congestion of ovarian plexuses ‐ or filling of pelvic veins across the midline ‐ or filling of vulvovaginal and thigh varicosities |
Supine/unknown |
| Magnetic resonance imaging | ||||||
| Asciutto, 200812 | Prospective cohort study | MRV |
Pelvic venous insufficiency: ‐ dilatation ≥ 1.5 times the contralateral vessel ‐ or contrast depicting the pelvic plexus ‐ or varicose dilatation of hypogastric veins (tortuosity) |
Phlebography |
‐ Mild congestion; small, straight, similar in caliber and easily visualized ‐ Moderate congestion; vein variable in caliber, tortuous, difficult to see separately, diameter 0.5 –1.0 cm. ‐ Severe congestion; wide veins, great variation in caliber, markedly tortuous, diameter > 1.0 cm |
Supine/35° reverse Trendelenburg |
| Meneses, 201027 | Prospective cohort study | MR PCVM |
PCS: ‐ slow anterograde flow velocity (peak velocity < 5 cm/s ‐ or retrograde flow |
Direct venography |
PCS: ‐ ovarian vein diameter > 6 mm ‐ congestion of the ovarian plexus ‐ filling of the pelvic veins across the midline ‐ or filling of vulvovaginal and thigh varicosities |
Unknown/unknown |
| Yang, 201214 | Retrospective cohort study | TR‐MRA |
‐ Grade I: Reflux in the left ovarian vein or left parauterine veins ‐ Grade II: Grade I with reflux in the right ovarian vein, reflux in left or right internal iliac vein, or varicosities of vulva or thighs |
Selective ovarian venography |
‐ Grade I: Reflux in the left ovarian vein or left parauterine veins. ‐ Grade II: Grade I with reflux in the right ovarian vein, reflux in left or right internal iliac vein, or varicosities of vulva or thighs |
Supine/supine |
MR PCVM, magnetic resonance phase‐contrast velocity mapping; MRV, magnetic resonance venography; PCS, pelvic congestion syndrome; TAUS, transabdominal ultrasonography; TR‐MRA, time‐resolved magnetic resonance angiography; TVUS, transvaginal ultrasonography.
The main results are summarized in Table 4. Campbell et al. 20 and Halligan et al. 23 studied the same ultrasound congestion score and power Doppler assessment of vascularity, but were not able to discriminate between women with PCS and control women. The occurrence of pelvic varicoceles on transvaginal ultrasonography had sensitivity and specificity of 100% and 83–100%, respectively, according to Giacchetto et al. 24 and Park et al. 11. Aside from varicoceles, Park et al. 11 found a specificity of 91% when communication between bilateral pelvic varicosities via transuterine crossing veins, > 5 mm, was observed. Reversed caudal flow, seen with transabdominal ultrasonography, was found in all patients with PCS proven by venography, resulting in a sensitivity of 100%. An ovarian vein diameter > 5 or > 6 mm seen on transabdominal ultrasonography had a positive predictive value of 71.2% or 83.3%, respectively 11. As secondary outcome, women with PCS had statistically significantly more and smaller ovarian follicles, smaller uterine volume and thinner endometrium in comparison with healthy control women 20, 23.
Table 4.
Main outcomes
| Author, year | Diagnostic tool | Characteristic | Main outcome | Characteristic | Secondary outcome |
|---|---|---|---|---|---|
| Ultrasonography | |||||
| Campbell, 200320 | TVUS | Ultrasound congestion score | Weak positive correlation between ultrasound and venographic congestion scores (r = 0.29; p = 0.06)a . Unable to discriminate between women with PCS and control women | Number of ovarian follicles | Weak positive correlation with venographic congestion score (r = 0.31; p = 0.04) |
| Power Doppler | Unable to discriminate between women with PCS and control women | Diameter of ovarian follicles | Moderate negative correlation with venographic congestion score (r = –0.48; p = 0.001) | ||
| Giacchetto, 199024 | TVUS | Pelvic varicoceles | Sens 100% (95% CI 95–100%), Spec 100% (95% CI 95–100%) | ||
| Halligan, 200023 | TVUS | Ultrasound congestion score | Unable to discriminate between women with PCS and control women | Uterine volume | Uterine volume significantly less in women with PCS compared with control women (61.4 cm3 vs. 88,3 cm3) |
| Power Doppler | Unable to discriminate between women with PCS and control women | Endometrium thickness | Endometrium significantly thinner in women with PCS compared with control women (3.9 mm vs. 8.4 mm) | ||
| Number of ovarian follicles | Number of ovarian follicles significantly higher in women with PCS compared with control women (4.6 vs. 2.8) | ||||
| Park, 200411 | TVUS | Pelvic varicoceles | Sens 100% (95% CI 89–100%), Spec 83% (95% CI 66–93%) | Uterine volume | Uterine volume less in women with PCS compared with control women (61.4 cm3 vs. 88.3 cm3; p > 0.05) |
| Vein > 5 mm crossing uterine body | Sens 25% (95% CI 17–59%), Spec 91% (95% CI 77–98%) | Polycystic changes | Polycystic changes more frequent in patients with PCS compared with control women (40.6% vs. 11.4%; p‐value not provided) | ||
| TAUS | Ovarian vein diameter > 5 mm | Positive predictive value 71.2% | |||
| Ovarian vein diameter > 6 mm | Positive predictive value 83.3% | ||||
| Reversed caudal flow in ovarian vein | Sens 100% (95% CI 84–100%), Spec 75% (95% CI 48–93%) | ||||
| Magnetic resonance imaging | |||||
| Asciutto, 2008 12 | MRV | Ovarian vein insufficiency | Sens 88%, Spec 67% | ||
| Hypogastric vein insufficiency | Sens 100%, Spec 38% | ||||
| Pelvic plexus insufficiency | Sens 91%, Spec 42% | ||||
| Meneses, 2010 27 | MR PCVM | Pelvic congestion | Sens 100% (95% CI 77–100%), Spec 50% (95% CI 7–93%) | ||
| Yang, 2012 14 | TR‐MRA | Left ovarian venous reflux | Sens 100% (95% CI 82–100%) | grade I vs. grade II, observer 1 | Sens 67% (95% CI 35–90%), Spec 100% (95% CI 59–100%) |
| Grade I vs. grade II, observer 2 | Sens 70% (95% CI 43–94%), Spec 100% (95% CI 59–100%) | ||||
MR PCVM, magnetic resonance phase‐contrast velocity mapping; MRV, magnetic resonance venography; PCS, pelvic congestion syndrome; TAUS, transabdominal ultrasound; TR‐MRA, time‐resolved magnetic resonance angiography; TVUS, transvaginal ultrasound; Sens: sensitivity; Spec: specificity, both followed by 95% confidence intervals between brackets.
95% CI, when not reported in literature, was calculated based upon reported results.
Classification of correlation by Dancey and Reidy (2004) [31].
Three studies regarding MRI were included in this review; in each study a slightly different technique was applied. Asciutto et al. studied the occurrence of venous insufficiency in the pelvic plexus, ovarian vein and hypogastric vein, using magnetic resonance venography 12. This resulted in sensitivities of 91%, 88% and 100%, respectively. The specificities were found to be 42%, 67% and 38%, respectively. Meneses et al. used a different kind of MRI technique, based upon phase‐contrast velocity mapping 27. They found a sensitivity of 100% and specificity of 50%, based upon nine patients with suspected PCS, in whom both left and right side were evaluated 27. Time‐resolved magnetic resonance angiography, as studied by Yang et al., resulted in a sensitivity of 100% among the 19 patients included 14. As secondary outcome, they made a distinction between grade I and II, as demonstrated in Table 3, which resulted in a specificity of 100%.
Discussion
In this systematic review, we aimed to identify the value of noninvasive diagnostic tools in the work‐up of patients suspected of PCS. In ultrasonography, a vein > 5 mm crossing the uterine body, pelvic varicoceles and reversed caudal flow appeared to be the most indicative of PCS. Due to the limited number of patients in MRI studies, no firm conclusions could be drawn from these studies.
Being a noninvasive diagnostic tool used in the work‐up of numerous gynecological symptoms, transvaginal ultrasonography would theoretically be the ideal first step in the diagnostics of PCS. The ultrasound congestion score however, based on vein diameter, number of veins in the sector and a subjective assessment of congestion, appeared to be unable to discriminate patients with PCS from healthy control women 20, 23. Park et al. found a high specificity (91%) when a vein > 5 mm was crossing the uterine body, yet the low sensitivity (25%) will subsequently result in a high chance of false‐negative results 11. Pelvic varicoceles on transvaginal ultrasound appeared to have a high sensitivity (100%), assuming a good ability to rule out PCS when pelvic varicoceles are not identified 11, 24. Park et al. also studied the role of transabdominal ultrasonography next to the transvaginal approach, and concluded that an ovarian vein diameter of > 6 mm accounted for a positive predictive value of 83.3% 11. However, the positive predictive value is only a relevant outcome when the study population reflects the real prevalence of disease and due to the case–control design used in the Park et al. study, this outcome may not be relevant. Reversed caudal flow, seen in the ovarian vein, appeared to be highly sensitive (100%) in detecting PCS with a conventional ultrasound Doppler technique 11. The flow in congested adnexal veins is typically low; power Doppler assigns different color tones and brightness to the total energy of the Doppler signal, consequently making it more sensitive to motion 31. Despite this hypothesis, the two studies included in this review that investigated power Doppler assessment of adnexal veins, were unable to discriminate women with PCS from healthy control women 20, 23. Furthermore, Malgor et al. demonstrated a compensatory right ovarian vein dilatation in the case of left ovarian vein reflux 32. This is clinically relevant regarding the fact that treatment should be based not only on the veins’ size but also the amount of reflux.
Magnetic resonance imaging allows the demonstration of the ovarian and gonadal veins in a complete examination of the pelvic anatomy, due to the multiplanar imaging capability. Three studies regarding MRI‐based methods were included in this review; in each study a slightly different technique of MRI was applied 12, 14, 26. Yang et al. investigated the feasibility of time‐resolved magnetic resonance angiography 14. Time‐resolved magnetic resonance angiography is a quick and noninvasive technique to visualize physiological blood flow dynamics 14. It is widely used and proven to be highly sensitive, when compared with conventional angiography for detecting pathology in a variety of blood vessels. High sensitivity was also demonstrated in the study of Yang et al. when reflux was detected in the ovarian veins of patients diagnosed with PCS 14. The specificity could not be assessed due to the absence of controls (without pelvic pathology on venography) 14.
Asciutto et al. studied the application of magnetic resonance venography in the assessment of women with PCS 12. Magnetic resonance venography appeared to be highly sensitive for insufficiency in pelvic plexus, ovarian or hypogastric veins. Especially hypogastric vein insufficiency accounted for a high sensitivity (100%), but the specificity was low, which results in a high prevalence of false‐positive results 12.
Computed tomography might account for a less expensive alternative to MRI, but at this moment there are no studies available describing the value of CT in the work‐up of patients suspected of PCS. A case report and a retrospective study performed in asymptomatic patients assume that it may be possible to diagnose PCS when incompetence or dilatation is visualized using CT 33, 34. CT and MRI are both operator dependent, but CT is easier to read and so less operator dependent. The downside is the limited amount of information that CT provides; MRI has the possibility to provide information on the differential diagnosis of PCS, which includes endometriosis and adenomyosis.
Meneses et al. investigated the role of magnetic resonance phase‐contrast velocity mapping (MR PCVM) in patients with suspected PCS 27. MR PCVM is a modality used to assess anomalous venous flow, and not solely anatomical changes. Hypothetically, PCS is an entity caused by dynamic disturbance of venous flow, the anatomical changes are the result of this anomalous flow. Meneses et al. found a high sensitivity, but the specificity could not reliably be determined due to the wide 95% confidence interval 27. It is possible that MR PCVM can detect an earlier stage of PCS, in which the slow venous flow has not yet caused any dilatation of the ovarian veins. On the other hand, slow venous flow may not result in dilatation, varicosities, or pain in all patients.
This review has some potential limitations. First, the included studies yielded different parameters and thresholds, which made a formal meta‐analysis impossible. Due to publication bias, relevant data may be missing. The studies of Park et al. and Halligan et al. were based upon a case–control design, which is a possible source for bias in results 11, 23. For example, a bias in the selection of patient population and verification may occur because the healthy control women did not undergo the reference standard. Consequently, it is important to note that the reference standard, venography, solely used in the studies included in this review is not 100% accurate. Based on the study of Beard et al., the sensitivity and specificity of venography were found to be 91% and 89%, respectively 19. This represents a fundamental flaw in the test accuracy design of included studies. The index test is unable to perform better than the reference standard, and hence its value may be underestimated. Finally, all index tests described in this review are performed with the patients in supine position. It is likely that ovarian and pelvic varices may not be as prominent on images in this position. Especially in early stages, with mild changes in venous anatomy, ultrasonography could reach a higher diagnostic accuracy when the patient is examined in reverse Trendelenburg or when Valsalva's maneuver is applied. Furthermore, to enhance discriminative ability it could be of added value to examine patients later in the afternoon, which is also common practice in the work‐up of patients with pelvic organ prolapse. Labropoulos et al. described a more complete protocol on how patients can be examined with ultrasonography, including the position of the patient with a head elevation of 30 degrees 35. Their technique aids in the overall visibility of the relevant veins in the lower abdomen 35. However, this technique requires that patients have been fasting overnight, which is of course not a usual preparation for a visit to the outpatient gynecological department 35.
In patients with CPP and symptoms directing towards PCS, a good diagnostic work‐up must be readily available. This systematic review emphasizes the current gap in literature and lack of standardized criteria regarding PCS. With this review, we add important data on the accuracy of noninvasive diagnostic tools in the daily practice of gynecologists in the work‐up of patients with CPP. Pelvic veins > 5 mm, a vein crossing the uterine body from left to right and communicating with both ovarian plexus and reversed caudal flow seem to be the most indicative for PCS. Therefore, gynecologists should pay extra attention to these parameters and the possibility of PCS.
Based on this systematic review, a validated noninvasive diagnostic tool is currently not available. Ultrasonography and MRI are the most investigated modalities. A vein > 5 mm crossing the uterine body, pelvic varicoceles and reversed caudal flow shown with ultrasonography seem to indicate PCS. Additionally, this review highlights important gaps in the available literature. Future studies should ideally investigate the role of transvaginal ultrasonography with the patient in different positions, such as reverse Trendelenburg and using the Valsalva's maneuver and correlate these with the typical complaints of PCS and the current reference standard for diagnosis, being venography. There is an urgent need for methodologically adequate diagnostic accuracy studies in patients with a suspicion of PCS.
Supporting information
Appendix S1. Search strategy.
Acknowledgments
The authors thank Rosemary Schadenberg for her valuable contributions on spelling and grammar issues.
Steenbeek MP, van der Vleuten CJM, Schultze Kool LJ, Nieboer TE. Noninvasive diagnostic tools for pelvic congestion syndrome: a systematic review. Acta Obstet Gynecol Scand 2018;97:776–786.
Conflict of interest
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
References
- 1. Hebbar S, Chawla C. Role of laparoscopy in evaluation of chronic pelvic pain. J Minim Access Surg. 2005;1:116–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Papathanasiou K, Papageorgiou C, Panidis D, Mantalenakis S. Our experience in laparoscopic diagnosis and management in women with chronic pelvic pain. Clin Exp Obstet Gynecol. 1999;26:190–2. [PubMed] [Google Scholar]
- 3. Santosh A, Liaquat HB, Fatima N, Liaquat S, Anwar MA. Chronic pelvic pain: a dilemma. J Pak Med Assoc. 2010;60:257–60. [PubMed] [Google Scholar]
- 4. Sharma D, Dahiya K, Duhan N, Bansal R. Diagnostic laparoscopy in chronic pelvic pain. Arch Gynecol Obstet. 2011;283:295–7. [DOI] [PubMed] [Google Scholar]
- 5. Swanton A, Iyer L, Reginald PW. Diagnosis, treatment and follow up of women undergoing conscious pain mapping for chronic pelvic pain: a prospective cohort study. BJOG. 2006;113:792–6. [DOI] [PubMed] [Google Scholar]
- 6. Durham JDML. Pelvic congestion syndrome. Semin Intervent Radiol. 2013;30:372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ahangari A. Prevalence of chronic pelvic pain among women: an updated review. Pain Physician. 2014;17:141–7. [PubMed] [Google Scholar]
- 8. Farquhar CM, Hoghton GB, Beard RW. Pelvic pain – pelvic congestion or the irritable bowel syndrome? Eur J Obstet Gynecol Reprod Biol. 1990;37:71–5. [DOI] [PubMed] [Google Scholar]
- 9. O'Kane C, Chinnadurai A, Johnston K. Case report: pelvic congestion syndrome – successful treatment of a nulliparous patient with ovarian vein embolization. J Min Invasive Gynecol. 2010;1:S177. [Google Scholar]
- 10. Milingos S, Protopapas A, Kallipolitis G, Drakakis P, Makrigiannakis A, Liapi A, et al. Laparoscopic evaluation of infertile patients with chronic pelvic pain. Reprod Biomed Online. 2006;12:347–53. [DOI] [PubMed] [Google Scholar]
- 11. Park SJ, Lim JW, Ko YT, Lee DH, Yoon Y, Oh JH, et al. Diagnosis of pelvic congestion syndrome using transabdominal and transvaginal sonography. AJR Am J Roentgenol. 2004;182:683–8. [DOI] [PubMed] [Google Scholar]
- 12. Asciutto G, Mumme A, Marpe B, Koster O, Asciutto KC, Geier B. MR venography in the detection of pelvic venous congestion. Eur J Vasc Endovasc Surg. 2008;36:491–6. [DOI] [PubMed] [Google Scholar]
- 13. Hansrani V, Morris J, Caress AL, Payne K, Seif M, McCollum CN. Is pelvic vein incompetence associated with symptoms of chronic pelvic pain in women? A pilot study. Eur J Obstet Gynecol Reprod Biol. 2016;196:21–5. [DOI] [PubMed] [Google Scholar]
- 14. Yang DM, Kim HC, Nam DH, Jahng GH, Huh CY, Lim JW. Time‐resolved MR angiography for detecting and grading ovarian venous reflux: comparison with conventional venography. Br J Radiol. 1014;2012:e117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borghi C, Dell'Atti L. Pelvic congestion syndrome: the current state of the literature. Arch Gynecol Obstet. 2016;293:291–301. [DOI] [PubMed] [Google Scholar]
- 16. Hodgkinson CP. Physiology of the ovarian veins during pregnancy. Obstet Gynecol. 1953;1:26–37. [PubMed] [Google Scholar]
- 17. Soysal ME, Soysal S, Vicdan K, Ozer S. A randomized controlled trial of goserelin and medroxyprogesterone acetate in the treatment of pelvic congestion. Hum Reprod. 2001;16:931–9. [DOI] [PubMed] [Google Scholar]
- 18. Gloviczki P. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Form. J Vasc Surg. 2011;53:2S–48S. [DOI] [PubMed] [Google Scholar]
- 19. Beard RW, Highman JH, Pearce S, Reginald PW. Diagnosis of pelvic varicosities in women with chronic pelvic pain. Lancet. 1984;2:946–9. [DOI] [PubMed] [Google Scholar]
- 20. Campbell D, Halligan S, Bartram CI, Rogers V, Hollings N, Kingston K, et al. Transvaginal power Doppler ultrasound in pelvic congestion. Acta Radiol. 2003;44:269–74. [DOI] [PubMed] [Google Scholar]
- 21. Coakley FV, Varghese SL, Hricak H. CT and MRI of pelvic varices in women. J Comput Assist Tomogr. 1999;23:429–34. [DOI] [PubMed] [Google Scholar]
- 22. Whiting PF. Quadas‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–36. [DOI] [PubMed] [Google Scholar]
- 23. Halligan S, Campbell D, Bartram CI, Rogers V, El‐Haddad C, Patel S, et al. Transvaginal ultrasound examination of women with and without pelvic venous congestion. Clin Radiol. 2000;55:954–8. [DOI] [PubMed] [Google Scholar]
- 24. Giacchetto G, Cotroneo GB, Marincolo F, Cammisuli F, Caruso G, Catizone F. Ovarian varicocele: ultrasonic and phlebographic evaluation. J Clin Ultrasound. 1990;18:551–5. [DOI] [PubMed] [Google Scholar]
- 25. Rae T, Stones RW, Beard RW. Investigation of chronic pelvic pain in women: a comparison of venography and transabdominal pelvic ultrasound scanning. Br J Radiol. 1990;63:389. [Google Scholar]
- 26. Adams J, Beard RW, Reginald PW, Franks S. Pelvic ultrasound findings in women with chronig pelvic pain – correlation with laparoscopy and venography. Br J Radiol. 1987;60:620–1. [Google Scholar]
- 27. Meneses LQ, Uribe S, Tejos C, Andia ME, Fava M, Irarrazaval P. Using magnetic resonance phase‐contrast velocity mapping for diagnosing pelvic congestion syndrome. Phlebology. 2011;26:157–61. [DOI] [PubMed] [Google Scholar]
- 28. Mother D. Preferred reporting items for systematic reviews and meta‐analysis: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pellegri PP, Montanari GD. The preoperative diagnosis of pelvic congestion by means of 133Xenon injected into the cervical myometrium. Acta Obstet Gynecol Scand. 1981;60:447–9. [DOI] [PubMed] [Google Scholar]
- 30. Thomas DC, McArdle FJ, Rogers VE, Beard RW, Brown BH. Local blood volume changes in women with pelvic congestion measured by applied potential tomography. Clin Sci. 1991;81:401–4. [DOI] [PubMed] [Google Scholar]
- 31. Hudson‐Dixon CM, Long BW, Cox LA. Power Doppler imaging: principles and applications. Radiol Techol. 1999;70:235–43. [PubMed] [Google Scholar]
- 32. Malgor RD, Adrahtas D, Spentzouris G, Gasparis AP, Tassiopoulos AK, Labropoulos N. The role of duplex ultrasound in the workup of pelvic congestion syndrome. J Vasc Surg Venous Lymphat Disord. 2014;2:34–8. [DOI] [PubMed] [Google Scholar]
- 33. Desimpelaere JH, Seynaeve PC, Hagers YM, Appel BJ, Mortelmans LL. Pelvic congestion syndrome: demonstration and diagnosis by helical CT. Abdom Imaging. 1999;24:100–2. [DOI] [PubMed] [Google Scholar]
- 34. Rozenblit AM, Ricci ZJ, Tuvia J, Amis ES Jr. Incompetent and dilated ovarian veins: a common CT finding in asymptomatic parous women. AJR Am J Roentgenol. 2001;176:119–22. [DOI] [PubMed] [Google Scholar]
- 35. Labropoulos N, Jasinski PT, Adrahtas D, Gasparis AP, Meissner MH. A standardized ultrasound approach to pelvic congestion syndrome. Phlebology. 2016; 31: 1–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Search strategy.


