Summary
Background
Routinely collected electronic health data obtained for administrative and clinical purposes are increasingly used to study atopic dermatitis (AD). Methods for identifying AD patients in routinely collected electronic health data differ, and it is unknown how this might affect study results.
Objectives
To evaluate how patients with AD have been identified in studies using routinely collected electronic health data, to determine whether these methods were validated and to estimate how the method for identifying patients with AD affected variability in prevalence estimates.
Methods
We systematically searched PubMed, Embase and Web of Science for studies using routinely collected electronic health data that reported on AD as a primary outcome. Studies of localized AD and other types of dermatitis were excluded. The protocol for this review was registered in PROSPERO (CRD42016037968).
Results
In total, 59 studies met eligibility criteria. Medical diagnosis codes for inclusion and exclusion, number of occasions of a code, type of provider associated with a code and prescription data were used to identify patients with AD. Only two studies described validation of their methods and no study reported on disease severity. Prevalence estimates ranged from 0·18% to 38·33% (median 4·91%) and up to threefold variation in prevalence was introduced by differences in the method for identifying patients with AD.
Conclusions
This systematic review highlights the need for clear reporting of methods for identifying patients with AD in routinely collected electronic health data to allow for meaningful interpretation and comparison of results.
Short abstract
What's already known about this topic?
Increasingly, studies are using routinely collected data to study atopic dermatitis (AD).
It is unclear how patients with AD are identified and whether methodological differences could have an impact on study findings.
What does this study add?
We performed a systematic review of methods for identifying patients with AD in studies using routinely collected data and found differences in methods were associated with up to a threefold variation in prevalence estimates.
We found variability in methods associated with up to a threefold variation in prevalence estimates.
We encourage validation of methods and offer suggestions for reporting to allow for meaningful interpretation and comparison of results.
Plain language summary available online
Atopic dermatitis (AD, also known as eczema or atopic eczema) affects both children and adults, and increasing data suggest it is a systemic inflammatory disease.1 There is an unmet need for additional research in large, representative populations with longitudinal follow‐up and data on comorbid conditions. ‘Routinely collected’ electronic health data obtained for administrative and clinical purposes often meet these criteria and are increasingly being used to study the epidemiology, natural history and association of AD with other diseases.2 They could include data for clinical management (e.g. primary care databases), health system planning (e.g. health administrative data), documentation of clinical care (e.g. electronic health record data repositories) or epidemiological surveillance (e.g. cancer registries and public health reporting data). Because these data are not generated specifically for research purposes, they require careful validation to ensure accuracy and reproducibility.2
Unlike some conditions for which diagnosis may be based on diagnostic tests or laboratory values easily retrievable from medical records, AD diagnosis is typically based solely on clinical signs and symptoms, and physician assessment is considered the ‘gold standard’.3, 4, 5 Moreover, AD is clinically heterogeneous, with variable morphology, severity and clinical course, all of which can present challenges to identifying patients with AD accurately in routinely collected health data. It is possible that AD prevalence and severity estimates are influenced by the method used to identify patients. Therefore, we aimed to provide an overview of AD disease definition in studies using routinely collected data. The primary objectives of this systematic review were to evaluate how patients with AD have been identified and how disease severity was defined. We also aimed to determine whether these methods were validated (i.e. whether any information was included about the accuracy of methods for identifying AD) and, when applicable, to estimate how AD disease definitions affected the variability in AD prevalence.
Materials and methods
The review protocol was registered in PROSPERO, (CRD42016037968, http://www.crd.york.ac.uk/PROSPERO). We followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) and Reporting of studies Conducted Using Observational Routinely collected Data (RECORD) guidelines, which are an extension of the STROBE guidelines.2, 6, 7
Types of studies
We included cohort, case–control and cross‐sectional studies using routinely collected health data reporting on AD as a primary outcome. Studies that examined AD as a predictor of a separate outcome (e.g. cancer diagnosis) were not included. Routinely collected health data were defined as data collected without specific a priori research questions and developed prior to utilization for research.2 Data sources designed to investigate specific questions about AD or atopic diseases, such as the International Study of Asthma and Allergies in Childhood (ISAAC), birth cohorts of patients with AD, and registries of patients with AD, were excluded. Studies of localized AD, such as hand eczema or other types of dermatitis such as contact dermatitis and seborrhoeic dermatitis, were also excluded.
Outcome
Our primary end point was the criteria used to identify patients with AD in each study. We also examined whether these criteria were validated (i.e. whether any information was included about the accuracy of methods for identifying AD), how disease severity was defined and the prevalence of AD.
Search strategy
With the help of a professional librarian, we searched MEDLINE via PubMed, Embase and Web of Science for studies indexed until 10 April 2016. Table S1 (see Supporting Information) shows the detailed search strategy. Studies in any language were included. Because the focus of this review was to describe how AD has been defined in the mainstream published literature, we excluded literature reviews, abstracts, conference proceedings, unpublished studies, ongoing studies and the grey literature (i.e. reports and research disseminated outside of commercial publishing). We cross‐referenced review articles and reference lists to ensure completeness.
Selection and data extraction
Three authors (M.P.D. and one of A.M.Y., R.K.S.) performed the study selection independently and in duplicate. Titles and abstracts were screened for inclusion, followed by a full‐text review if abstracts were insufficient to determine whether studies met inclusion or exclusion criteria. Discrepancies were resolved through discussion and consensus with additional authors (K.A. and S.M.L.). For each eligible study, we extracted information on the database used, country of study, study objective, patient demographics, features of algorithms used to identify individuals with AD and prevalence estimates. When possible, missing data, including specific diagnosis codes, were obtained by contacting the study author(s).
Data synthesis and analysis
We described the characteristics of relevant studies. Features of algorithms used to identify individuals with AD were tabulated for the included studies, and the median prevalence and variability (interquartile range and ratio of 75th to 25th percentile) were calculated by subgroup. We also reported the proportion of studies in which these algorithms have been validated and described the methods of validation.
Risk of bias
Systematic reviews often include an assessment of the risk of bias. This involves rating each included study on the methods used for selection of the study groups, comparability of groups and ascertainment of exposure and outcome.6, 8 These categorizations were not applicable to the objective of our study (i.e. we focused exclusively on how an outcome, AD, was defined), so no risk of bias assessment was performed.
Results
Selection and characteristics of studies
Our search identified 1354 studies. Title and abstract review identified 127 articles for the full‐text review. Of these, 68 were excluded and 59 met the inclusion criteria (Fig. 1). Table 1 shows the characteristics of the included studies. The vast majority of studies (90%) included children; only six studies (10%) included only adults. Most studies (81%) included both male and female patients, 61% were from North America or Europe, and the remainder came from East Asia. The included studies were published between 1994 and 2016, and data came from the years 1967–2014. Most studies (58%) were conducted using administrative databases (e.g. insurance databases, birth/death registries or employment registries). Primary care databases, national patient registers, institutional electronic medical records and hybrid databases compiling information from multiple sources were also used.
Figure 1.
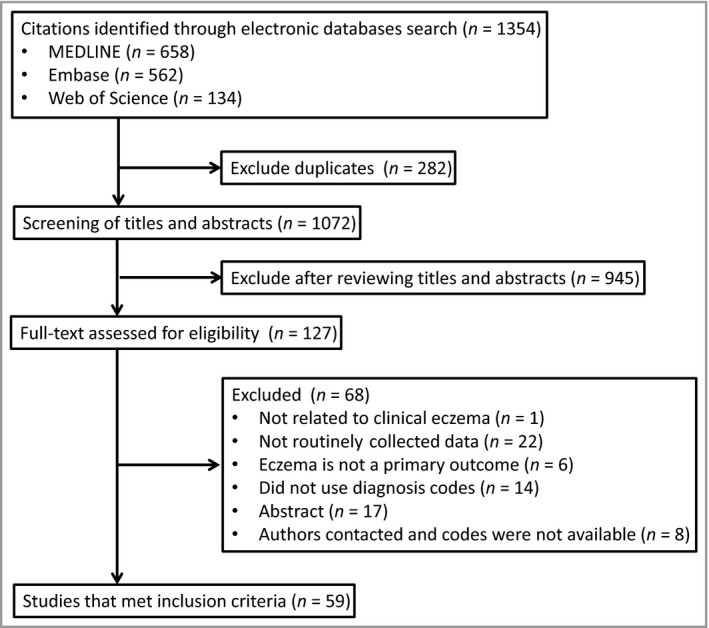
Flow diagram.
Table 1.
Characteristics of the included studies
| n (%) | |
|---|---|
| Age groups included in study sample | |
| Children | 32 (54) |
| Adults | 6 (10) |
| Both | 21 (36) |
| Sex of patients included in study sample | |
| Male only | 4 (7) |
| Female only | 0 |
| Both male and female | 48 (81) |
| Not reported | 7 (12) |
| Countries represented in studiesa | |
| Taiwan | 19 |
| South Korea | 2 |
| U.S.A. | 7 |
| Canada | 1 |
| Sweden | 5 |
| Denmark | 3 |
| Norway | 1 |
| Finland | 1 |
| U.K. | 11 |
| The Netherlands | 4 |
| Germany | 4 |
| Australia | 2 |
| Type of routinely collected datab | |
| National patient register | 3 (5) |
| Administrative databases (insurance, birth/death, employment) | 34 (58) |
| Primary care databases | 15 (25) |
| Institution‐specific electronic medical record | 3 (5) |
| Hybridb | 4 (7) |
| Provided an estimate of atopic dermatitis prevalence | |
| Yes | 40 (68) |
| No | 19 (32) |
aFor some studies, data came from more than one country; bhybrid datasets included patient data from sources spanning multiple categories (i.e. both primary care databases and administrative databases). National patient registers include hospital records in countries with government‐funded universal healthcare. They are not specific to insurance claims or prescriptions nor are they limited to primary care.
Algorithm features
We categorized algorithms that were used to identify patients with AD into: (i) diagnosis codes, (ii) number of occasions of a code, (iii) type of provider associated with a code, and (iv) prescription data.
Diagnosis codes
Multiple codes from multiple coding systems, including International Statistical Classification of Diseases and Related Health Problems (ICD)‐9/10 and Read/OXMIS, were used. We grouped studies into those that used codes specifically for AD, and those that used codes referring to a broader group of dermatitis‐related disorders (including, for example, contact dermatitis and eczema not otherwise specified) (Table S2; see Supporting Information). When we compared the terminology used in each published study (i.e. ‘atopic dermatitis’ vs. ‘eczema’) to the types of codes, we found imperfect overlap (Table 2). Only one study directly incorporated codes for exclusionary diagnoses (‘diaper or napkin rash’ and ‘contact dermatitis and other eczema’) as part of their algorithm for AD.9
Table 2.
Comparison of manuscript terminology and medical codes
| Terms used in manuscript | Total | Atopic dermatitis codes onlya | Atopic dermatitis plus other dermatitis codesa |
|---|---|---|---|
| Atopic dermatitis | 31 | 26 (84) | 5 (16) |
| Atopic dermatitis/eczema | 3 | 0 | 3 (100) |
| Atopic eczema | 5 | 5 (100) | 0 |
| Dermatitis/eczema | 3 | 0 | 3 (100) |
| Eczema | 17 | 4 (24) | 13 (76) |
Variables are n (%). aA full listing of codes is detailed in Table S2 (see Supporting Information).
Number of occasions of a code
A total of 10 studies used algorithms that required multiple instances of codes to identify patients as having AD. Two studies10, 11 used unique algorithms that identified individuals if they had at least one inpatient claim or two outpatient claims associated with diagnosis codes for AD. Nine studies specified codes associated with AD must occur on a minimum of two occasions,12, 13, 14, 15, 16, 17, 18, 19, 20 and one study required three medical visits coded for AD to identify patients.21
Type of provider associated with a code
A total of seven studies, all of which were conducted in Taiwan using the National Health Insurance Research Database, specified the type of provider required to enter a diagnosis to define an individual with AD. Three studies required a diagnosis by a dermatologist,14, 17, 22 three studies required a diagnosis by a dermatologist or a paediatrician15, 23, 24 and one study required a diagnosis by a ‘specialist.’18
Prescription data
Five studies included medication prescriptions in the algorithms used to identify patients with AD. One study required a patient to have a diagnosis code and a recorded prescription of a treatment for AD (e.g. emollients, topical corticosteroids and topical calcineurin inhibitors).25 Two studies specified diagnosis either by diagnosis code or a prescription for calcineurin inhibitors or topical corticosteroids26, 27 and one study used only prescription codes for either topical corticosteroids or topical calcineurin inhibitors to identify patients with eczema.28
Validation information
Only two studies described methods to validate the algorithms used to identify individuals with AD. The first study assessed the validity of ICD‐9‐CM codes by conducting a detailed chart review of randomly selected visits of 1000 patients.29 The ICD‐9‐CM diagnosis was confirmed in 93% of cases; however, the study included a range of skin diseases and was not limited to AD. The criteria used in the chart review to confirm diagnoses were not described. The second study calculated the positive predictive value of filled prescriptions of topical corticosteroids or immunosuppressants for identifying patients with an ‘umbrella diagnosis’ of dermatitis or eczema based on diagnosis codes, and found positive predictive values of 82% and 45%, respectively.30 The study described specific clinical criteria used to validate asthma diagnoses in a subset of paediatric patients through review of medical records; however, coded diagnoses for dermatitis and eczema were not evaluated against clinical criteria.
Severity information
None of the studies included in the review reported on the severity of AD within the study population.
Variation in prevalence estimates
Of the 59 included studies, 40 (68%) provided a prevalence estimate of AD, which ranged from 0·18% to 38·33%. Estimates varied by features used to identify patients with AD, study characteristics and the duration of time used to calculate the period prevalence. Of note, the variation in prevalence introduced by differences in the methods used to identify patients was similar in magnitude to the variation in prevalence introduced by study characteristics (e.g. prevalence in studies where the algorithm included prescription data was 16·9% vs. no prescription data at 4·5%; prevalence among studies including children only was 11% vs. adults and/or children at 4%, Table 3).
Table 3.
Prevalence estimates by subgroup
| Studies, n | Studies with prevalence estimates, n | Median prevalence, % | IQR, % | Ratio of 75th to 25th percentile | |
|---|---|---|---|---|---|
| Overall | 59 | 40 | 4·91 | 2·64–11·51 | 4·36 |
| Features of algorithms used to identify AD patients | |||||
| Diagnosis code category | |||||
| Limited to AD | 35 | 26 | 4·30 | 2·14–6·70 | 3·13 |
| AD plus others | 23 | 13 | 11·53 | 9·20–30·23 | 3·29 |
| Algorithm included number of visits/codes | |||||
| Required multiple occasions | 8 | 8 | 4·27 | 3·85–5·61 | 1·46 |
| No | 50 | 31 | 5·60 | 2·24–12·37 | 5·52 |
| Algorithm specified the type of provider associated with a code | |||||
| Yes | 5 | 5 | 4·53 | 4·39–6·70 | 1·53 |
| No | 53 | 34 | 4·91 | 2·50–11·50 | 4·60 |
| Algorithm included prescription data | |||||
| Yes | 5 | 3 | 16·93 | 12·37–32·49 | 2·63 |
| No | 54 | 37 | 4·53 | 2·50–10·94 | 4·38 |
| Study characteristics | |||||
| Age of patient population | |||||
| Children only | 32 | 19 | 10·94 | 4·69–19·00 | 4·05 |
| Adults +/− children | 27 | 21 | 3·80 | 2·14–5·60 | 2·62 |
| Continent | |||||
| Europe | 28 | 21 | 10·35 | 3·80–16·93 | 4·46 |
| North America | 8 | 4 | 6·05 | 2·85–9·05 | 3·18 |
| Asia | 21 | 15 | 4·39 | 2·21–6·70 | 3·03 |
| Type of routinely collected data | |||||
| National patient register | 3 | 1 | 12·37 | n/a | n/a |
| Administrative database | 34 | 26 | 4·27 | 2·24–6·70 | 2·99 |
| Primary care database | 15 | 11 | 13·24 | 2·79–31·40 | 11·25 |
| Hybrid | 4 | 2 | 6·81 | 4·42–9·20 | 2·08 |
| Terminology | |||||
| Atopic dermatitis | 31 | 21 | 4·69 | 2·24–10·94 | 4·88 |
| Atopic dermatitis/eczema | 3 | 2 | 2·85 | 2·50–3·20 | 1·28 |
| Atopic eczema | 5 | 4 | 3·85 | 2·25–7·13 | 3·17 |
| Eczema | 17 | 13 | 11·50 | 4·42–30·23 | 6·84 |
| Prevalence calculation | |||||
| Time period | |||||
| 1‐year period prevalence | 9 | 9 | 2·79 | 2·24–3·43 | 1·53 |
| Multiyear period prevalence | 29 | 29 | 8·90 | 4·15–13·21 | 3·18 |
IQR, interquartile range; AD, atopic dermatitis; n/a; not applicable.
Studies that used only diagnosis codes specific to AD to identify patients found a lower median prevalence than studies that used more general dermatitis codes (4·3% vs. 11·5%), and the amount of variability was similar (ratio of 75th to 25th percentile 3·1 vs. 3·3). Studies that required patients to have specified diagnosis codes on multiple occasions found a lower median prevalence (4·3% vs. 5·6%) and less variability (ratio of 75th to 25th percentile 1·5 vs. 5·5) than studies that only required one instance of a code. The same was true of studies that required patients to have specified diagnosis codes on multiple occasions vs. only one instance of a code found a lower median prevalence (43% vs. 56%) and less variability (ratio of 75th to 25th percentile 15 vs. 55). Finally, studies that used prescription data to identify patients found a higher median prevalence (16·9%, vs. 4·5%), versus studies that did not specify any restrictions on provider type possibly because of misclassification of patients receiving medications for other conditions.
Prevalence estimates also varied by region and age group, with the median prevalence higher in studies that included only children. The duration of period prevalence ranged from 1 to 39 years; among the nine estimates that calculated a 1‐year prevalence, the median prevalence was lower (2·8%) than in studies that calculated a multiyear period prevalence (8·9%).
Discussion
This review demonstrates variability in the way patients with AD are identified in studies using routinely collected data. It also highlights a lack of standardization in terminology, validation studies and information on disease severity, which are all crucial to allow for comparison of study results. These issues are not specific to AD; however, they are of particular importance in AD because it is a common condition and misclassification of even a relatively small percentage of patients could result in large absolute errors.
Much has been written about the inconsistent use of terminology in allergic disorders, and efforts are under way to improve the classification system and standardize terminology used in coding.31, 32, 33, 34 To ensure we captured all relevant studies, we used multiple terms in our search, including ‘atopic dermatitis’, ‘dermatitis’, ‘eczema’ and other variants thereof (Table S1; see Supporting Information). Inconsistent use of terminology can be seen in Table 2, with some studies of ‘eczema’ including only patients with AD‐specific diagnosis codes and some studies of ‘atopic dermatitis’ including patients with diagnosis codes for other types of inflammatory skin conditions such as contact dermatitis. We found that use of broader diagnosis codes increased the median prevalence from 4·3% to 11·5% (Table 3). Such ‘lumping together’ of different disease entities could inflate AD prevalence estimates, although using more limited AD code sets might underestimate the true prevalence. For example, a recent validation study using electronic medical record data found that 42% of patients with the nonspecific diagnosis code of 692·9 and no AD‐specific code of 691·8 had a final diagnosis of AD after chart review.35 If a study focuses on more than one type of dermatitis, authors should clearly delineate how each condition was defined, including which codes were used. Ideally, studies examining multiple types of dermatitis would report estimates separately by subgroups to facilitate comparison with the existing literature.
This systematic review highlights the frequent use of nonvalidated algorithms to identify patients with AD in routinely collected data; only two of 59 studies described any attempt to validate the algorithms used. Validation research is a high priority to ensure patients are accurately identified and avoid misclassification bias,36 and since completion of our search two new validation studies of AD using routinely collected data have been published.35, 37 Both highlighted the potential magnitude of misclassification bias, even when using physician‐defined codes. For example, using a single code for ‘atopic dermatitis/eczema’, rather than one of five AD‐related codes in a primary care database from the U.K., could result in a 50% reduction in prevalence estimates.38
The performance of coding algorithms for identifying patients with AD is inherently context‐specific. For example, the performance of an algorithm may depend on the baseline prevalence and the way in which diagnosis codes and pharmacy codes are entered in a given setting. Moreover, in any given context, the choice of coding algorithms may be related to the goals of the study, as there is often a trade‐off between maximizing the number of true positives and reducing the number of false positives, and the value of optimizing sensitivity or specificity may depend on whether the study is aiming to identify all possible cases or to identify only those with definite disease. Therefore, each study should discuss evidence for the validity of the methods used to identify patients with AD.2 When possible, researchers also may consider showing how changes in their definition of AD could affect their estimates. Additional research is necessary to understand the generalizability of coding algorithms and the extent to which these might be standardized across settings.
A secondary objective of this systematic review was to examine methods used to describe AD severity in studies using routinely collected electronic health data, however, none of the studies meeting our inclusion criteria evaluated disease severity. Current approaches to measuring AD severity are often complex and not routinely documented in the medical record39 or are not standardized,40 and therefore are difficult to use in routinely collected electronic health data studies. Treatment data and/or frequency of healthcare visits has been used to define severity in studies of psoriasis and asthma using routinely collected data and may be applicable to AD.41, 42, 43, 44 Such an approach, if applied to AD, should be carefully validated.
Strengths of this systematic review include a predefined and registered protocol and adherence to reporting standards.6 We included 59 studies of varying designs from a variety of settings to show all of the ways patients with AD have been defined in the literature to date. We included only studies with AD as a primary outcome because we were most interested in whether differences in AD definitions would affect prevalence estimates, but were unable to synthesize these estimates using meta‐analysis because of a lack of standardized reporting of prevalence. Nonetheless, we include unadjusted median and interquartile prevalence ranges, which demonstrate variation in these estimates.
Clinicians should be aware that estimates from studies using routinely collected data may vary depending on the algorithms used to identify patients, and should be wary of studies that do not provide data on the validity of these measures. The international Harmonising Outcome Measures for Eczema (HOME) initiative was founded in response to the lack of standardization and validation of methods used to measure outcomes in randomized clinical trials, and the initiative has resulted in multiple publications suggesting standardized methods of measurement and reporting.45, 46, 47, 48, 49 Similar international efforts are needed for questionnaire‐based studies and studies of AD using routinely collected electronic health data. In the meantime, we encourage authors to report on their methods clearly, including: specific codes used to identify patients or exclude patients, whether there was a minimum number of codes or visits required, whether there were any restrictions on type of provider associated with the code or visit, and whether prescription data were used to identify patients. In addition, whenever possible, we encourage authors to report on the annual period prevalence of visits and/or prescriptions for AD by age to enable comparison across studies. All studies should include information on the validity of the algorithm used in their particular locale and practice setting.
Supporting information
Table S1 Search strategy.
Table S2 Classification system.
Table S3 List of included studies.
Powerpoint S1 Journal Club Slide Set.
Acknowledgments
The authors would like to thank Elaine Allen for her statistical advice regarding study design and analysis, Evans Whitaker for helping us developing the search strategy, and Jochen Schmitt for his assistance in reviewing and extracting data from German‐language articles.
Funding sources This study was supported in part by the University of California, San Francisco Resource Allocation Program for Trainees (RAPtr). K.A. is supported by grants from the Dermatology Foundation and National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under Award Number KL2TR001870. Research reported in this publication is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. C.F. is funded through a UK National Institute for Health Research (NIHR) Career Development Fellowship (CDF‐2014‐07‐037). The views expressed are those of the author(s) and not necessarily those of the UK National Health Service, the UK NIHR, or the UK Department of Health. S.M.L. is supported by a Wellcome senior clinical fellowship in science (205039/Z/16/Z). J.I.S. is supported by grants from the AHRQ K12 HS023011 and Dermatology Foundation. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflicts of interest J.W. receives research funding from the National Institutes of Health (NIH), the Dermatology Foundation and the American Skin Association. D.J. M. receives research funding from the NIH, Leo Pharma and Valeant, and he is a consultant for Sanofi and GlaxoSmithKline. K.A. receives research funding from the NIH, the Dermatology Foundation and the Robert Wood Johnson Foundation.
S.M.L. and K.A. contributed equally to the work and should be considered joint senior authors.
Plain language summary available online
References
- 1. Brunner PM, Silverberg JI, Guttman‐Yassky E et al Increasing comorbidities suggest that atopic dermatitis is a systemic disorder. J Invest Dermatol 2017; 137:18–25. [DOI] [PubMed] [Google Scholar]
- 2. Benchimol EI, Smeeth L, Guttmann A et al [The REporting of studies Conducted using Observational Routinely‐collected health Data (RECORD) statement]. Z Evid Fortbild Qual Gesundhwes 2016; 116:33–48 (in German). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hanifin JM. Diagnostic features of atopic dermatitis. Acta Derm Venereol 1980; 92 (Suppl.):44–7. [Google Scholar]
- 4. Asher MI, Keil U, Anderson HR et al International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J 1995; 8:483–91. [DOI] [PubMed] [Google Scholar]
- 5. Eichenfield LF, Tom WL, Chamlin SL et al Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol 2014; 70:338–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liberati A, Altman DG, Tetzlaff J et al The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vandenbroucke JP, von Elm E, Altman DG et al Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surgery 2014; 12:1500–24. [DOI] [PubMed] [Google Scholar]
- 8. Wells GA, Shea B, O'Connell D, Peterson J et al The Newcastle‐Ottawa Scale (NOS) for assessing the quality of non‐randomized studies in meta‐analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (last accessed 12 April 2018).
- 9. Sun H‐L, Yeh C‐J, Ku M‐S et al Coexistence of allergic diseases: patterns and frequencies. Allergy Asthma Proc 2012; 33:e1–4. [DOI] [PubMed] [Google Scholar]
- 10. Wang J‐Y, Liu L‐F, Chen C‐Y et al Acetaminophen and/or antibiotic use in early life and the development of childhood allergic diseases. Int J Epidemiol 2013; 42:1087–99. [DOI] [PubMed] [Google Scholar]
- 11. Hua T‐C, Hwang C‐Y, Chen Y‐J et al The natural course of early‐onset atopic dermatitis in Taiwan: a population‐based cohort study. Br J Dermatol 2014; 170:130–5. [DOI] [PubMed] [Google Scholar]
- 12. Schmitt J, Schmitt NM, Kirch W et al Significance of atopic dermatitis in outpatient medical care. Analysis of health care data from Saxony. Hautarzt 2009; 60:320–7. [DOI] [PubMed] [Google Scholar]
- 13. Schmitt J. Medical care and health‐economic relevance of atopic eczema. Allergologie 2010; 33:279–88. [Google Scholar]
- 14. Chung S‐D, Keller JJ, Lin H‐C. Association of erectile dysfunction with atopic dermatitis: a population‐based case‐control study. J Sex Med 2012; 9:679–85. [DOI] [PubMed] [Google Scholar]
- 15. Chen M‐H, Su T‐P, Chen Y‐S et al Attention deficit hyperactivity disorder, tic disorder, and allergy: is there a link? A nationwide population‐based study. J Child Psychol Psychiatry 2013; 54:545–51. [DOI] [PubMed] [Google Scholar]
- 16. Hwang C‐Y, Hwang Y‐Y, Chen Y‐J et al Atopic diathesis in patients with Kawasaki disease. J Pediatrics 2013; 163:811–15. [DOI] [PubMed] [Google Scholar]
- 17. Tien K‐J, Chou C‐W, Lee S‐Y et al Obstructive sleep apnea and the risk of atopic dermatitis: a population‐based case control study. PLOS ONE 2014; 9:e89656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen YT, Chen YJ, Hwang CY et al Comorbidity profiles in association with vitiligo: a nationwide population‐based study in Taiwan. J Eur Acad Dermatol Venereol 2015; 29:1362–69. [DOI] [PubMed] [Google Scholar]
- 19. Hirsch AG, Yan XS, Sundaresan AS et al Five‐year risk of incident disease following a diagnosis of chronic rhinosinusitis. Allergy 2015; 70:1613–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wei C‐C, Lin C‐L, Shen T‐C et al Occurrence of common allergic diseases in children with idiopathic nephrotic syndrome. J Epidemiol 2015; 25:370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsai J‐D, Chang S‐N, Mou C‐H et al Association between atopic diseases and attention‐deficit/hyperactivity disorder in childhood: a population‐based case‐control study. Ann Epidemiol 2013; 23:185–8. [DOI] [PubMed] [Google Scholar]
- 22. Chu S‐Y, Chen Y‐J, Tseng W‐C et al Comorbidity profiles among patients with alopecia areata: the importance of onset age, a nationwide population‐based study. J Am Acad Dermatol 2011; 65:949–56. [DOI] [PubMed] [Google Scholar]
- 23. Chen M‐H, Su T‐P, Chen Y‐S et al Comorbidity of allergic and autoimmune diseases among patients with ADHD: a nationwide population‐based study. J Attent Disord 2013; 21:219–27. [DOI] [PubMed] [Google Scholar]
- 24. Woon PY, Chang WC, Liang C‐C et al Increased risk of atopic dermatitis in preschool children with Kawasaki disease: a population‐based study in Taiwan. Evid Based Complement Alternat Med 2013; 2013:605123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schmitt J, Schmitt NM, Kirch W et al Early exposure to antibiotics and infections and the incidence of atopic eczema: a population‐based cohort study. Ped Allergy Immunol 2010; 21:292–300. [DOI] [PubMed] [Google Scholar]
- 26. Henriksen L, Simonsen J, Haerskjold A et al Incidence rates of atopic dermatitis, asthma, and allergic rhinoconjunctivitis in Danish and Swedish children. J Allergy Clin Immunol 2015; 136:360–66.e2. [DOI] [PubMed] [Google Scholar]
- 27. Egeberg A, Andersen YMF, Gislason G et al Neonatal risk factors of atopic dermatitis in Denmark – results from a nationwide register based study. Pediatr Allergy Immunol 2016; 27:368–74. [DOI] [PubMed] [Google Scholar]
- 28. Mulder B, Schuiling‐Veninga CCM, Bos HJ et al Prenatal exposure to acid‐suppressive drugs and the risk of allergic diseases in the offspring: a cohort study. Clin Experim Allergy 2014; 44:261–9. [DOI] [PubMed] [Google Scholar]
- 29. Henderson MD, Abboud J, Cogan CM et al Skin‐of‐color epidemiology: a report of the most common skin conditions by race. Pediatr Dermatol 2012; 29:584–89. [DOI] [PubMed] [Google Scholar]
- 30. Ortqvist AK, Lundholm C, Wettermark B et al Validation of asthma and eczema in population‐based Swedish drug and patient registers. Pharmacoepidemiol Drug Saf 2013; 22:850–60. [DOI] [PubMed] [Google Scholar]
- 31. Johansson SG, Bieber T, Dahl R et al Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol 2004; 113:832–6. [DOI] [PubMed] [Google Scholar]
- 32. Demoly P, Tanno LK, Akdis CA et al Global classification and coding of hypersensitivity diseases – An EAACI – WAO survey, strategic paper and review. Allergy 2014; 69:559–70. [DOI] [PubMed] [Google Scholar]
- 33. Tanno LK, Calderon MA, Goldberg BJ et al Categorization of allergic disorders in the new World Health Organization International Classification of Diseases. Clin Transl Allergy 2014; 4:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kantor R, Thyssen JP, Paller AS et al Atopic dermatitis, atopic eczema, or eczema? A systematic review, meta‐analysis, and recommendation for uniform use of ‘atopic dermatitis’. Allergy 2016; 71:1480–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hsu DY, Dalal P, Sable KA et al Validation of international classification of disease ninth revision codes for atopic dermatitis. Allergy 2017; 72:1091–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. De Coster C, Quan H, Finlayson A et al Identifying priorities in methodological research using ICD‐9‐CM and ICD‐10 administrative data: report from an international consortium. BMC Health Serv Res 2006; 6:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Abuabara K, Magyari AM, Hoffstad O et al Development and validation of an algorithm to accurately identify atopic eczema patients in primary care electronic health records from the UK. J Invest Dermatol 2017; 137:1655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Abuabara K, Hoffstad O, Troxel AB et al Patterns and predictors of atopic dermatitis disease control past childhood: an observational cohort study. J Allergy Clin Immunol 2018; 141:778–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rehal B, Armstrong A. Health outcome measures in atopic dermatitis: a systematic review of trends in disease severity and quality‐of‐life instruments 1985–2010. PLOS ONE 2011; 6:e17520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Futamura M, Leshem YA, Thomas KS et al A systematic review of Investigator Global Assessment (IGA) in atopic dermatitis (AD) trials: many options, no standards. J Am Acad Dermatol 2016; 74:288–94. [DOI] [PubMed] [Google Scholar]
- 41. Fuhlbrigge AL, Carey VJ, Finkelstein JA et al Validity of the HEDIS criteria to identify children with persistent asthma and sustained high utilization. Am J Manag Care 2005; 11:325–30. [PubMed] [Google Scholar]
- 42. Bennett AV, Lozano P, Richardson LP et al Identifying high‐risk asthma with utilization data: a revised HEDIS definition. Am J Manag Care 2008; 14:450–56. [PMC free article] [PubMed] [Google Scholar]
- 43. Schatz M, Zeiger RS, Yang SJ et al Persistent asthma defined using HEDIS versus survey criteria. Am J Manag Care 2010; 16:e281–8. [PubMed] [Google Scholar]
- 44. Seminara NM, Abuabara K, Shin DB et al Validity of The Health Improvement Network (THIN) for the study of psoriasis. Br J Dermatol 2011; 164:602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schmitt J, Williams H; HOME Development Group . Harmonising Outcome Measures for Eczema (HOME). Report from the First International Consensus Meeting (HOME 1), 24 July 2010, Munich, Germany. Br J Dermatology 2010; 163:1166–8. [DOI] [PubMed] [Google Scholar]
- 46. Schmitt J, Spuls P, Boers M et al Towards global consensus on outcome measures for atopic eczema research: results of the HOME II meeting. Allergy 2012; 67:1111–17. [DOI] [PubMed] [Google Scholar]
- 47. Chalmers JR, Schmitt J, Apfelbacher C et al Report from the third international consensus meeting to harmonise core outcome measures for atopic eczema/dermatitis clinical trials (HOME). Br J Dermatol 2014; 171:1318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schmitt J, Apfelbacher C, Spuls PI et al The Harmonizing Outcome Measures for Eczema (HOME) roadmap: a methodological framework to develop core sets of outcome measurements in dermatology. J Invest Dermatol 2015; 135:24–30. [DOI] [PubMed] [Google Scholar]
- 49. Chalmers JR, Simpson E, Apfelbacher CJ et al Report from the fourth international consensus meeting to harmonize core outcome measures for atopic eczema/dermatitis clinical trials (HOME initiative). Br J Dermatol 2016; 175:69–79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Search strategy.
Table S2 Classification system.
Table S3 List of included studies.
Powerpoint S1 Journal Club Slide Set.


