Abstract
Background
The aim of this study was to investigate the prevalence of epidemiologic and physician‐diagnosed pollen‐induced AR (PiAR) in the grasslands of northern China and to study the impact of the intensity and time of pollen exposure on PiAR prevalence.
Methods
A multistage, clustered and proportionately stratified random sampling with a field interviewer‐administered survey study was performed together with skin prick tests (SPT) and measurements of the daily pollen count.
Results
A total of 6043 subjects completed the study, with a proportion of 32.4% epidemiologic AR and 18.5% PiAR. The prevalence was higher in males than females (19.6% vs 17.4%, P = .024), but no difference between the two major residential and ethnic groups (Han and Mongolian) was observed. Subjects from urban areas showed higher prevalence of PiAR than rural areas (23.1% vs 14.0%, P < .001). Most PiAR patients were sensitized to two or more pollens (79.4%) with artemisia, chenopodium, and humulus scandens being the most common pollen types, which were similarly found as the top three sensitizing pollen allergens by SPT. There were significant regional differences in the prevalence of epidemiologic AR (from 18.6% to 52.9%) and PiAR (from 10.5% to 31.4%) among the six areas investigated. PiAR symptoms were positively associated with pollen counts, temperature, and precipitation (P < .05), but negatively with wind speed and pressure P < .05).
Conclusion
Pollen‐induced AR (PiAR) prevalence in the investigated region is extremely high due to high seasonal pollen exposure, which was influenced by local environmental and climate conditions.
Keywords: allergy test, climate, pollen count and exposure, pollen‐induced allergic rhinitis, prevalence
Abbreviations
- 95% CI
95% confidence interval
- AI
aridity index
- AR
allergic RHINITIS
- Ar
artemisia
- Be
betula
- Ch
chenopodium
- GLM
generalized linear model
- Gr
gramineae
- Hu
humulus scandens
- Ju
juniperus chinensis var. chinensis
- OR
odds ratio
- PiAR
pollen‐induced allergic rhinitis
- Po
populus
- RR
relative risk
- Sa
salix
- SPT
skin prick test
- Ul
ulmus pumila
- Ze
zea mays
1. INTRODUCTION
Allergic rhinitis (AR) is a global health problem that causes major illness and disability. The incidence and prevalence of AR are high affecting over 10%‐40% of the population worldwide, which continue to rise rapidly in recent years.1, 2 Classically, outdoor allergens appear to constitute a greater risk for AR than indoor allergens, as outdoor pollen allergens are a major cause of seasonal AR.3, 4, 5 Mahillon et al6 showed that there was a significant association between the number of outpatient visits for AR with elevated pollen levels in Belgium. In China, Zhang et al7 found a strong association between the concentration of pollens in ambient air and the number of consultations for AR. Studies conducted in Britain, France, and Canada were able to demonstrate a strong association between the severity of AR and daily poaceae pollen counts.8, 9, 10
Sensitization to pollen species is diverse in different regions due to the nature and amount of pollens varying with the vegetation, geography, temperature, and climate.6, 11 The main sensitizing pollens are ragweed in North America, gramineae in Europe, and tree plants in Austria, New Zealand, and Japan.11, 12, 13, 14 Luo et al15 found that the main sensitizing pollens in Guangdong (Southern China) which induced AR were bermuda, timothy, and humulus. Climate change may also influence pollen production which can induce allergic manifestations and thereby the development of AR.16, 17 Weather conditions, including rainfall, atmospheric temperature, humidity, wind speed, and wind direction, may also alter the seasonality of pollen as well as the concentration of plant pollens which can subsequently influence the occurrence of AR.18, 19, 20 The Inner Mongolia grasslands, a representative of the Eurasian Steppe (one‐third of the world's grasslands), are characterized by both the high abundance of pollens and the high diversity of grass pollen species (eg, artemisia, chenopodiaceae, and gramineae). The region covers a variety of geographic characteristics and different vegetation types (meadow steppe, typical steppe, and desert steppe) from east to west.21, 22, 23 So far, there are no epidemiological studies looking at the prevalence of pollen‐induced allergic rhinitis (PiAR) and its relationship to environmental factors such as climatic variables, airborne pollen allergen intensity, and period of exposure for local residents living in this region.
The aim of this study was to investigate the prevalence of epidemiologic and clinically confirmed PiAR in this region. In addition, we also attempted to study if environmental and climate risk factors have a specific impact on pollen allergen exposure and nature of the disease. This is the first multidimensional epidemiologic study of PiAR in this region. The results of this study will provide new insights into the etiology and epidemiology of PiAR and environmental factors which influence disease manifestation that can have important implications for effective management and prevention of PiAR in this region and beyond.
2. METHODS
2.1. Study region
This study was conducted in the region of Xilingol and Horqin grasslands, located in the eastern and middle part of the Inner Mongolia Autonomous Region, China. The region is representative of the Eurasian steppe in terms of climate and vegetation composition. The steppe vegetation of the region includes meadow steppe in the eastern part, typical steppe in the middle part, and desert steppe in the northern part of the region. There were a total of six areas (municipalities): Erenhot (desert steppe), Xilinhot (typical steppe), Duolun (typical steppe), Jarud (meadow steppe), Kailu (meadow steppe), and Tongliao (meadow steppe) (Figure 1). The study area runs from 111°17 to 122°57′E in longitude and 41°45 to 45°50′N in latitude, covering an area of 262 130 km2. The elevation in the study area varies from 190 to 1280 m. The mean annual temperature ranges from 2.8 to 7.1°C, with mean monthly temperature ranging from −18.8°C in January to 24.2°C in July. As the region is a wide area far away from the ocean and resided at a high altitude above sea level, Inner Mongolia has a temperate monsoon climate which features irregular rainfall and drastic variations in temperature. The mean annual precipitation range is between 135 and 378 mm, with 60%‐90% falling during the growing season from April to August. These data were downloaded from the China Meteorological Data Sharing Service Network. (http://data.cma.cn/).
Figure 1.
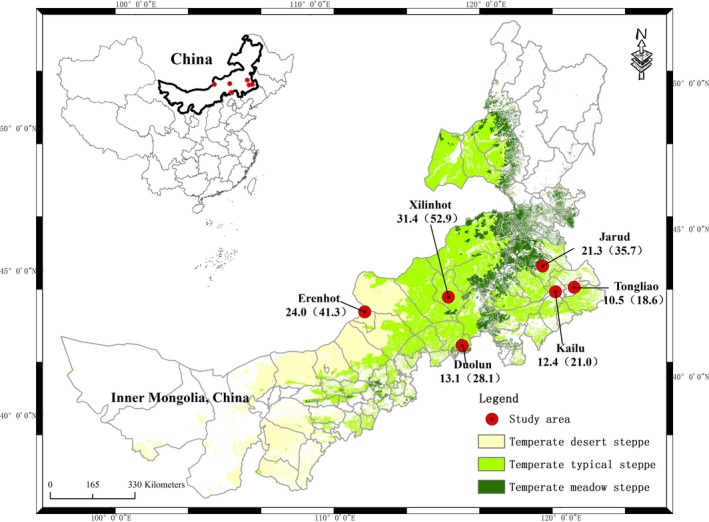
The locations of the six study areas in Inner Mongolia. The number given for each area represents the prevalence of clinical pollen‐induced allergic rhinitis (PiAR) and epidemiologic allergic rhinitis (AR) (in parenthesis)
2.2. Cross‐sectional Study
This is a multistage, clustered and proportionately stratified random sampling study. The total population in the study region is approximately 1.74 million (urban: 730928; rural: 1011696) with two predominant ethnic groups, Mongolian and Han (based on data obtained from the 2010 nationwide population census; http://www.stats.gov.cn/ztjc/zdtjgz/zgrkpc/dlcrkpc/dlcrkpczl/). The sample calculation was based on an estimated prevalence of AR of 15%; to reach a significance level (alpha) of 0.05 and error tolerance 0.10p, the estimated minimum sample size was 5000 with equal distribution in urban and rural areas. We added an additional 20% to the minimum sample size factoring in possible noncompliance rate and targeted 6000 subjects, at 1000 per survey area (municipality), with the same gender and age stratification. In order to examine ethnic variations, the study sample was stratified to include equal proportions of Mongolian and Han ethnic groups.
All subjects were investigated using an interviewer‐administered questionnaire together with SPT at a medical center or hospital near their home. Approval to conduct this study was granted by the institutional review boards of Beijing Shijitan Hospital, the affiliated hospital of the Beijing Capital Medical University, and all of six participating municipalities.
2.3. Definitions used in this study
The diagnosis of AR was made using the criteria published in the ARIA (Allergic Rhinitis and Its Impact on Asthma 2008 Update) document.1 The subjects with at least two of the four symptoms suggestive of AR (itchy nose, sneezing, runny and blocked nose) for at least 1 hour on most days during the past year were diagnosed as “epidemiological AR.” If allergic sensitization was confirmed by medical history (particularly in the pollen season), clinical examination and SPT, a diagnosis of PiAR was made.
2.4. Questionnaire and field interview
A survey questionnaire was developed based on the reports of the ISAAC24 with modifications following the ARIA's recommendations.1 The following information was collected, including demographic data (age, gender, ethnic group, place of residence, family income); nasal symptoms on a month‐to‐month basis in the past 12 months (itchy nose, sneezing, runny, and blocked nose), allergic disorders, and co‐morbidities/complications, family history of allergy, risk factors (type of heating, duration of physical activity, smoking, type of child delivery, breastfeeding, and pet ownership), allergic reactions to drugs and/or foods, medical treatments (eg, antihistamines, nasal steroids, herbal medicine) received during the past year and any symptom improvement after treatment.
The interviewers consisted of physicians and nursing staff. The survey manager prepared a standard procedure flowchart for the fieldwork at each investigational site. Two weeks before the study, a letter of introduction was sent to the study subjects to explain the purpose of the study, together with the proposed survey date. It was then followed by telephone call or a verbal reminder that was done by the study coordinator or local healthcare officers. In the selected clusters (eg, street districts from rural areas and villages from urban areas), all members from a household were invited to participate in this study. On the study day, they reported to the survey manager with their national registration identity card and signed the informed consent form before participating in this study. For children or teenagers aged less than 18 years, the informed consent was signed by their parents or guardians who accompanied them to the survey site. All study subjects returned to survey manager after they had completed both interview and skin prick test (SPT). If there were any missing information in the survey questionnaire, it will be completed immediately by the survey manager with the subject.
2.5. Skin prick test (SPT)
Skin prick test (SPTs) were conducted by trained allergy nurses or physicians. Oral antihistamines were discontinued for at least 3 days (>7 days for long‐acting antihistamines) prior to the SPT (this information was emphasized in the introduction letter). Based on our preliminary data of pollen count analyses throughout 2014, nine common pollen allergens, all present in at least one of the study areas, were chosen for SPT testing. This included artemisia (Ar), betula (Be), chenopodium (Ch), humulus scandens (Hu), salix (Sa), zea mays (Ze), juniperus chinensis var. chinensis (Ju), ulmus pumila (Ul), and populus (Po). The common house dust mite allergen of dermatophagoides pteronyssinus (DP) was also included in the test. As the SPT reagent of Gramineae (Gr) was not available, we chose zea mays as it is one of the common gramineae in this region. The SPTs were administered using standardized allergen extracts (Macro‐Union Pharmaceutical Lim, Beijing, China). The diluent was administered as a negative control and histamine hydrochloride (1 mg/mL) as a positive control. A wheal diameter ≥ 3 mm after subtracting the negative control for each of the allergens tested was considered as a positive response.1 In addition, a positive score based on the wheal size was recorded as Class 1: a wheal diameter between 3 and 5 mm; Class 2: a wheal diameter between 5 and 10 mm; Class 3: a wheal diameter between 1 and 2 cm; and Class 4: a wheal diameter ≥2 cm and present with pseudopods.
2.6. Pollen collection and pollen count
Starting January 2014, there were a total of 13 pollen‐monitoring stations established that included all six of the study areas, in collaboration with the Inner Mongolia Grassland Ecosystem Research Station, Chinese Academy, following which daily pollen counts had been analyzed in these regions. The technique used in the pollen count was a Durham pollen sampler that was valid for a perimeter of 18‐33 meters above the ground, with good ventilation and a maximum effort made to avoid roofs with surrounding obstacles. Pollen count in each study area was measured from January 1 to December 31, 2015, in order to have the daily (reported as a mean of each month) pollen count throughout the same year. Each slide was placed daily at 8 am and collected 24 hours later and mounted immediately after collection. Each slide was examined and counted by two trained examiners in a blinded manner. The result was reported as the daily total pollen grain per 1000 mm2. In this study, a mean monthly pollen count was presented.
2.7. Meteorological data
The mean monthly temperature, precipitation, wind speed, humidity, and atmospheric pressure were collected from the available monitoring networks in each area. The Aridity Index (AI) is given by AI = Pi/PET, where Pi is the monthly precipitation and PET the monthly evapotranspiration. The PET was obtained from the Global‐PET dataset (http://csi.cgiar.org/Aridity). The AI is a climatic index, which is useful for recording the evolution of the drought phenomenon.
2.8. Statistical analyses
Categorical data were described as numbers and percentages. Continuous data were shown as median and interquartile range. Between‐group differences in subject characteristics were tested using a Wilcoxon rank‐sum test for continuous variables and chi‐square/Fisher exact test for categorical variables. Multivariate logistic regression analysis was performed to explore the risk factors related to pollen‐induced AR and estimate their odds ratio. Pollen counts and climate factors were set as categorical scales by quartiles or medians. Relative risk (RR) and 95% confidence intervals (95% CI) of pollen and climate factors to PiAR were estimated by a generalized linear model under Poisson distribution. All tests were two‐sided with a significant level of <0.05. All of the analyses were performed using SAS software version 9.4 (SAS Institute Inc. Car, NC, and USA).
3. RESULTS
3.1. Demographic characteristics
From May to August in 2015, a total of 6043 subjects completed the study (a response rate of 97.5%), 2900 males and 3143 females, age of 27.6 (19.1) years (median, interquartile range) (Figure S1 and Table 1). A total of 1958 (32.4%) had epidemiologic AR, with the highest prevalence of 52.9% in Xilinhot (Table S1). In this group, 1115 had PiAR with the highest prevalence of 31.4% also in Xilinhot (Figure 1). There were significant differences in the prevalence of both epidemiologic AR and PiAR between the six areas (P < .001). Table 1 summarizes the characteristics of the study subjects and compares this information between PiAR and non‐PiAR subjects. Other risk factors for PiAR are shown in Table S2, where the family history of people in both first‐degree and second‐degree family members had a risk factor (OR = 3.51, 95% CI: 3.06‐4.02, P < .001 and OR = 1.73, 95% CI: 1.36‐2.20, P < .001, respectively) for development of PiAR. The prevalence of PiAR was significantly (P < .001) lower in families with a pet (15.2% vs 19.5% with no pet), with breastfeeding vs mixed feeding in infants and young children only (13.0% vs 18.9%), and indoor heating with coal (10.2%) and wood (7.5%) compared to central heating (22.1%, P < .001). Table S3 showed the comparison of PiAR prevalence between the six study areas (two‐by‐two comparison).
Table 1.
Characteristics of the study subjects
| Variable | Total (n = 6043) | Non‐PiAR (n = 4928) | PiAR (n = 1115) | P value |
|---|---|---|---|---|
| Age (y),median (interquartile range) | 27.6 (19.1) | 26.1 (38.6) | 29.3(27.2) | .006 |
| Gender, n (%) | ||||
| Male | 2900 | 2331 (80.4) | 569 (19.6) | .024 |
| Female | 3143 | 2597 (82.6) | 546 (17.4) | |
| Age group (y), n (%) | ||||
| 0‐6 | 1028 | 960 (93.4) | 68 (6.6) | <.001 |
| 7‐12 | 945 | 794 (84.0) | 151 (16.0) | |
| 13‐17 | 448 | 324 (72.3) | 124 (27.7) | |
| 18‐39 | 1512 | 1054 (69.7) | 458 (30.3) | |
| 40‐59 | 1553 | 1287 (82.9) | 266 (17.1) | |
| ≥60 | 535 | 489 (91.4) | 46 (8.6) | |
| Race, n (%) | ||||
| Han | 3288 | 2699 (82.1) | 589 (17.9) | .151 |
| Mongolian | 2486 | 2002 (80.5) | 484 (19.5) | |
| Other | 269 | 227 (84.4) | 42 (15.6) | |
| Residence, n (%) | ||||
| Urban | 2961 | 2278 (76.9) | 683 (23.1) | <.001 |
| Rural | 3079 | 2647 (86.0) | 432 (14.0) | |
| Annual family income (CNY, median (interquartile range)b | 5 (5) | 5 (4) | 5 (7) | <.001 |
| Study areaa, n (%) | ||||
| Erenhot | 1008 | 766 (76.0) | 242 (24.0) | <.001 |
| Xilinhot | 842 | 578 (68.6) | 264 (31.4) | |
| Duolun | 1028 | 893 (86.9) | 135 (13.1) | |
| Jarud | 1143 | 900 (78.7) | 243 (21.3) | |
| Kailu | 1000 | 876 (87.6) | 124 (12.4) | |
| Tongliao | 1022 | 915 (89.5) | 107 (10.5) | |
| Total | 6043 | 4928 (81.5) | 1115 (18.5) | |
Chi‐square test and t test were performed in this table.
Comparison between six study areas.
The value is in Chinese Yuan (CNY).
3.2. Allergic sensitization from SPT
Among 6043 individuals, 2262 (37.4%) showed a positive SPT to at least one allergen and 708 (11.7%) were sensitive to DP. Among DP sensitized subjects, 629 (88.8%) were also sensitive to pollen allergens. Among 1958 patients with epidemiologic AR, 1134 (57.9%) had a positive SPT and 824 (42.1%) had a negative SPT. In patients with PiAR, the positive rate of SPT for pollens was shown in Figure 2, and patterns of allergic sensitization by SPT in six individual areas were shown in Figure S2. Most PiAR patients were sensitized to two (7.8%) or more than two types (71.6%) of pollens and to both grass and tree pollens (65.1%) compared to the general study population (4.8%, 20.6%, and 19.3%, respectively) (Figure S3A,B).
Figure 2.
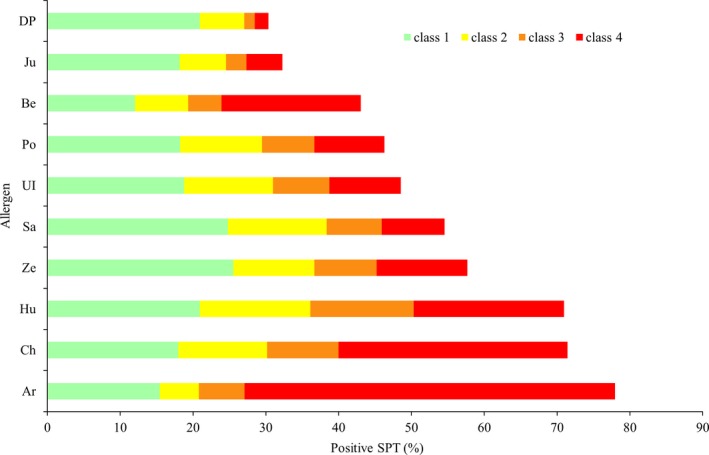
Allergic sensitization from skin prick test (SPT) in patients with pollen‐induced AR (PiAR). Results of SPT were classified as four degrees. Class 1: a wheal diameter between 3 and 5 mm; Class 2: a wheal diameter between 5 and 10 mm; Class 3: a wheal diameter between 1 and 2 cm; Class 4: a wheal diameter ≥2 cm and present with pseudopods
3.3. Relationship between allergic sensitization and PiAR symptoms
There is a significant correlation between allergy sensitization (positive SPT) to all nine pollens, with higher OR value (>20) for artemisia (OR = 27.18, 95% CI: 22.77‐32.45), chenopodium (OR = 27.35, 95% CI: 22.90‐32.66), humulus scandens (OR = 23.95, 95% CI: 20.15‐28.47), and salix (OR = 25.86, 95% CI: 20.98‐31.87) (Table S4). We have performed additional analysis by treating the number of pollen sensitization from 0 to 9 as continuous variables in the logistic regression model with age and gender adjustments. The OR value was 1.88, 95% CI: 1.82‐1.95, indicating that an increase in one pollen sensitization will add 88% of more risk for PiAR.
3.4. The pollen count and its relation with PiAR symptoms
Although pollen counts were made on a daily basis, in this manuscript we reported a total monthly mean pollen count in all analysis. In general, nine types of pollen were counted in 94.4% of the total pollen grains. Among them, artemisia was 35.3%, populus 17.4%, chenopodium 16.7%, and humulus scandens 5.6% (Figure 3), but their patterns could be differed among the six areas (Figure S4). Artemisia had the highest pollen count in autumn (July, August, and September) and populus was the most common one in spring (March, April, and May). Taken together, the trend showed a typical two‐peak phenomenon of the total pollen count with a spring peak (March‐May) and summer/autumn peak (July‐September). The highest mean monthly pollen count was 13861.3 grains (36.1%) in August and 7841.7 grains (20.4%) in April (Figure S5).
Figure 3.
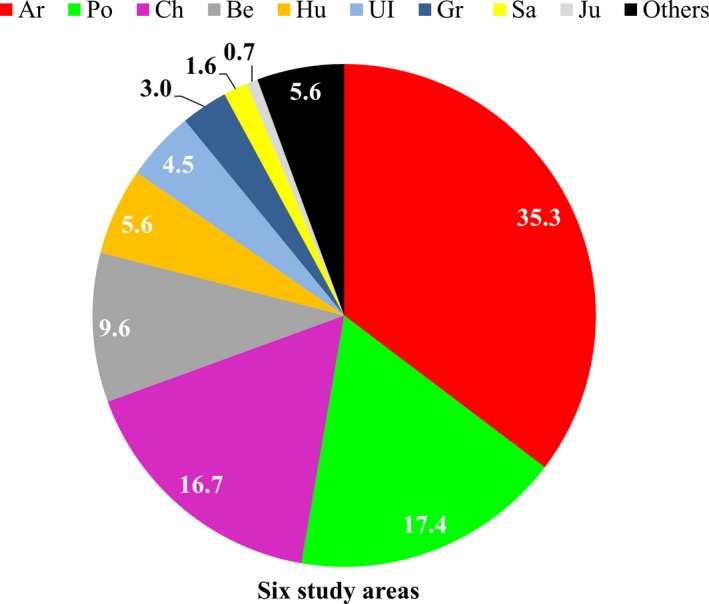
Pollen dispersal for 12 mo in the six study areas in 2015
Figure 4 showed a sum of the mean total monthly pollen counts and the presence of AR symptoms in 12 months (in 2015) in the study region (Figure 4A) and in six individual areas (Figure 4B‐G). In addition, Table 2 showed a significant and positive correlation between the total pollen count (in quartile or median number) and PiAR symptoms, and in particularly the following six pollens (eg, artemisia, chenopodium, humulus scandens, gramineae, betula, and populous) which were confirmed to be the relative risk (RRadj) for PiAR symptoms. However, the impact of these pollens on PiAR symptoms in six individual areas showed clear variations (Table S5). It is interesting to note that the top PiAR symptoms (73.0%) were presented early in July when the pollen count was far lower than those found in August (Figure 4A).
Figure 4.
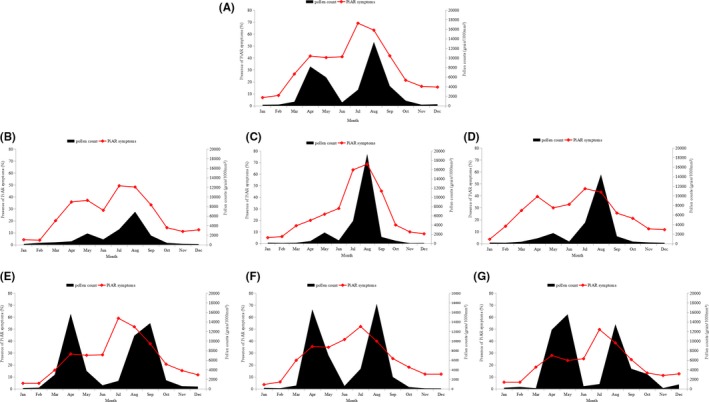
The mean total monthly pollen count and presence of rhinitis symptoms for 12 mo (2015) in the six study areas. A, represents the overall data of the six study areas. B‐G, represents the data of the six individual areas. B Erenhot, C Xilinhot, D Duolun, E Jarud, F Kailu, G Tongliao
Table 2.
Association between pollen counts and frequency of pollen‐induced allergic rhinitis (PiAR) symptoms
| Variablea (quartile/median) | PiAR symptomsb | RRc (95% CI) | P value | RRadj. d (95% CI) | P value |
|---|---|---|---|---|---|
| Total pollen counts | |||||
| ≤214.5 | 755 | 1.00 | 1.00 | ||
| ≤708.0 | 940 | 1.25 (0.97‐1.59) | .08 | 1.30 (1.04‐1.63) | .02 |
| ≤3301.5 | 1546 | 2.05 (1.54‐2.73) | <.001 | 2.02 (1.54‐2.65) | <.001 |
| >3301.5 | 1624 | 2.15 (1.57‐2.94) | .00 | 2.33 (1.71‐3.17) | <.001 |
| Artemisia | |||||
| ≤64.5 | 1163 | 1.00 | 1.00 | ||
| ≤131.0 | 864 | 0.74 (0.58‐0.96) | .02 | 0.78 (0.61‐0.99) | .04 |
| ≤526.0 | 1101 | 0.95 (0.64‐1.39) | .78 | 1.06 (0.72‐1.57) | .76 |
| >526.0 | 1737 | 1.49 (1.08‐2.07) | .01 | 1.62 (1.18‐2.22) | <.001 |
| Chenopodium | |||||
| ≤26.0 | 992 | 1.00 | 1.00 | ||
| ≤79.0 | 829 | 0.93 (0.70‐1.25) | .65 | 0.85 (0.62‐1.17) | .30 |
| ≤356.0 | 1147 | 1.22 (0.89‐1.68) | .22 | 1.13 (0.78‐1.64) | .53 |
| >356.0 | 1897 | 2.02 (1.49‐2.73) | <.001 | 1.94 (1.46‐2.59) | <.001 |
| Humulus | |||||
| ≤2.0 | 2520 | 1.00 | 1.00 | ||
| >2.0 | 2345 | 1.38 (1.04‐1.84) | .03 | 1.52 (1.14‐2.01) | .01 |
| Gramineae | |||||
| ≤4.0 | 1979 | 1.00 | 1.00 | ||
| >4.0 | 2886 | 1.63 (1.26‐2.10) | <.001 | 1.62 (1.26‐2.08) | <.001 |
| Betula | |||||
| ≤2.0 | 2358 | 1.00 | 1.00 | ||
| >2.0 | 2507 | 1.41 (1.06‐1.87) | .02 | 1.44 (1.10‐1.90) | <.001 |
| Populus | |||||
| ≤2.0 | 2146 | 1.00 | 1.00 | ||
| >2.0 | 2719 | 1.42 (1.06‐1.89) | .02 | 1.69 (1.28‐2.24) | <.001 |
| Juniperus | |||||
| ≤1.0 | 2690 | 1.00 | |||
| >1.0 | 2175 | 1.27 (0.96‐1.69) | .10 | ||
| Salix | |||||
| ≤1.0 | 2488 | 1.00 | |||
| >1.0 | 2377 | 1.19 (0.89‐1.60) | .23 | ||
| Ulmus | |||||
| <1.0 | 2537 | 1.00 | |||
| ≥1.0 | 2328 | 1.21 (0.91‐1.62) | .19 | ||
Pollen counts were set as categorical scales by quartiles or medians.
Monthly pollen counts recorded in 6 study areas from January 1 to December 31, 2015. The pollen count is shown as grain per 1000 mm2.
Frequency of symptoms in the past 12 mo in PiAR patients.
Univariate generalized linear model was conducted, and RR and 95% CI of pollens to Pi AR were estimated under Poisson distribution.
Multivariate generalized linear model was conducted and adjusted region and month under Poisson distribution.
In six individual areas, tree pollens were predominant in the eastern areas (eg, Jarud, Kailu, and Tongliao) (Figure S5E,F,G), while grass/weed pollens were higher in the western areas (eg, Xilinhot, Erenhot, and Duolun) (Figure S5B,C,D). Although the absolute pollen counts were less in the western than eastern areas, the prevalence of PiAR symptoms was similar or even higher in the Western areas (Figure 4). For example, in Erenhot (Figure 4B), a desert area with the lowest pollen count, the PiAR symptoms were the second highest in the study region (Table 1).
3.5. Relationship between climate and PiAR symptoms
Pollen‐induced allergic rhinitis (PiAR) symptoms were positively correlated with temperature and precipitation (P < .05) but negatively correlated with wind speed and pressure (P < .05) across the six study areas (Table 3). The relationships between PiAR symptoms and humidity and AI were not statistically significant (Table 3). However, the impact of climate factors calculated as a relative risk (RR) factor on PiAR symptoms in the six individual areas differed from each other (Table S6). When the data were analyzed within each of the six areas, PiAR symptoms were consistently and significantly correlated with air pressure, wind speed, temperature, and precipitation, while AI was significantly correlated with AR symptoms in the eastern region of Jarud and Duolun (P < .01, Table S6).
Table 3.
Association between frequency of pollen‐induced allergic rhinitis (PiAR) symptoms and climate factors (temperature, wind speed, humidity, precipitation, pressure, AI)
| Variablea (quartile) | PiAR symptomsb | RRc (95% CI) | P value | RRadj. d (95% CI) | P value |
|---|---|---|---|---|---|
| Temperature (°C) | |||||
| ≤−7.9 | 688 | 1.00 | — | 1.00 | |
| ≤7.4 | 979 | 1.42 (1.11‐1.83) | .01 | 1.32 (1.08‐1.60) | .01 |
| ≤17.0 | 1311 | 1.81 (1.35‐2.41) | <.001 | 1.46 (1.10‐1.96) | .01 |
| >17.0 | 1887 | 2.00 (2.16‐3.90) | <.001 | 1.84 (1.29‐2.63) | <.001 |
| Humidity (%) | |||||
| ≤44.0 | 1350 | 1.00 | — | — | |
| ≤52.0 | 975 | 1.06 (0.69‐1.64) | .80 | ||
| ≤61.8 | 1323 | 1.27 (0.86‐1.88) | .24 | ||
| >61.8 | 1217 | 1.10 (0.79‐1.53) | .57 | ||
| Wind speed (m/s) | |||||
| ≤3.0 | 1869 | 1.00 | — | 1.00 | — |
| ≤3.5 | 1009 | 0.85 (0.57‐1.27) | .42 | 0.77 (0.63‐0.95) | .01 |
| ≤3.9 | 1133 | 0.67 (0.46‐0.98) | .04 | 0.67 (0.55‐0.81) | <.001 |
| >3.9 | 854 | 0.63 (0.44‐0.89) | .01 | 0.58 (0.47‐0.73) | <.001 |
| Pressure (h Pa) | |||||
| ≤897.7 | 1487 | 1.00 | — | 1.00 | — |
| ≤942.1 | 1459 | 0.98 (0.69‐1.40) | .92 | 0.90 (0.66‐1.21) | .47 |
| ≤988.8 | 1364 | 0.92 (0.63‐1.33) | .65 | 0.67 (0.51‐0.88) | .01 |
| >988.8 | 555 | 0.37 (0.27‐0.53) | <.001 | 0.50 (0.51‐0.88) | <.001 |
| Precipitation (mm) | |||||
| ≤2.6 | 704 | 1.00 | — | 1.00 | — |
| ≤12.6 | 1030 | 1.46 (1.12‐1.92) | <.001 | 1.24 (1.02‐1.51) | .03 |
| ≤31.8 | 1230 | 1.47 (1.29‐2.37) | <.001 | 1.36 (1.05‐1.77) | .02 |
| >31.8 | 1901 | 2.70 (1.99‐3.66) | <.001 | 1.56 (1.11‐2.19) | .01 |
| AIe | |||||
| ≤0.2 | 1123 | 1.00 | |||
| ≤0.2 | 1165 | 1.04 (0.74‐1.46) | .83 | ||
| ≤0.4 | 1036 | 0.92 (0.61‐1.39) | .70 | ||
| >0.4 | 1541 | 1.37 (0.96‐1.96) | .08 | ||
Climate factors were from monthly mean data.
Frequency of symptoms in the past 12 mo in PiAR patients.
Univariate generalized linear model was conducted, and RR and 95% CI of climate factors to PiAR were estimated under Poisson distribution.
Multivariate generalized linear model was conducted and adjusted region, month, and total pollen count under Poisson distribution.
The Aridity Index (AI) is given by AI = Pi/PET, where Pi is the monthly precipitation and PET is the monthly evapotranspiration. PET obtained from Global‐PET dataset (http://csi.cgiar.org/Aridity).
3.6. Relationship between climate and pollen count
Similar to the data shown in Table 3, pollen counts were positively correlated with temperature (r = .729, P < .001) and precipitation (r = .656, P < .001), but negatively correlated with wind speed (r = −.205, P < .001) across the six study areas (Table S7). Due to a significant difference in pollen count and climate characteristics among the six individual areas, the relationship between pollen counts and climate factors was again not consistent among these six areas (Table S8).
4. DISCUSSION
The present study showed an extremely high prevalence of epidemiologic AR (from 18.6% to 52.9%) and physician‐diagnosed PiAR (from 10.5% to 31.4%) in the grasslands of northern China. This was possibly one of the very few epidemiological studies which combined a field interviewer‐administered questionnaire survey with SPT in a randomly selected urban and rural population, and daily pollen analysis to assess the intensity and time of pollen allergen exposure in the same area. In addition, meteorological and climatological data had been collected and analyzed which will be helpful in understanding the potential climate factors that may influence pollen distribution and pollen allergen exposure in patients with PiAR. Although the seasonal onset of PiAR symptoms due to pollen allergy is a typical disease pattern in this region, most patients suffer from a persistent type of AR as most of them (65.1%) were allergic to both tree and grass pollens. Multisensitization and exposure to a very high concentration of pollens in the high pollen seasons may impact the severity of disease and create challenges for currently available treatments thus encouraging the development of new therapeutic strategies for patients. In addition, a significant (P < .001) regional difference in PiAR prevalence was found in this large regional area of approximately 262 130 km2.
The gold standard in diagnosis of AR is based on the combination of a typical history of allergic symptoms and diagnostic tests.1, 25 These criteria are often neglected in clinical practice especially when allergy testing is not available. A report of the local pollen count, an important indicator of pollen exposure, is even less available in most part of the world. There is insufficient epidemiologic data using allergy tests, so that more data are needed to establish etiologic risk factors and the natural history of AR. Data from this study confirmed that there was a significant correlation between allergic sensitization and the level of sensitization (by SPT scores) with pollen counts and PiAR symptoms. For example, artemisia, chenopodium, and humulus scandens were the top three pollens found in August/September (Figure S5), where at the same time, they were also the top three sensitized pollen allergens by SPT (Figure 2). In both the EAACI (European Academy of Allergy and Clinical Immunology) position paper and WAO (World Allergy Organization) taskforce report, it was recently emphasized that for standardization of clinical trials with allergen‐specific immunotherapy for respiratory allergy, there should be a report of the relevant exposure for these allergens as a baseline, during and at the end of a trial.25, 26 In addition, the MACVIA‐ARIA Sentinel Network for allergic rhinitis (MASK‐rhinitis) has introduced an electronic reporting of daily symptoms during pollen season via mobile devices in 13 European countries.27 Our data contributed significantly to the establishment of a similar network in China by providing valuable information with regard to pollen exposure in the 13 pollen‐monitoring stations which were established in the grasslands of northern China.
The role of airborne allergens on AR symptoms is well known, but the effect of pollens differs based on biogeographic conditions.20 Globally, due to the difference of geographic position and vegetation, the types and concentrations of airborne pollens will differ.8, 11, 28 Prior to this study (2014), pollen‐monitoring stations were established in the six study areas. The results from 2 years of pollen analysis showed a similar pattern and seasonal variation of pollen counts in the same areas. For example (from west to east), Erenhot, Xilinhot, and Duolun had a typical one‐peak pollen season in summer/autumn (dominant with weed and grass pollens); while in the East, Jarud, Kailu, and Tongliao had two‐peak pollen seasons in spring (dominant with tree pollens) and summer/autumn (dominant with weed and grass pollens). In our study, it showed that PiAR symptoms started early in July when the pollen counts were far lower than those assessed in August and September. The main reason could be due to the priming effect caused by allergy and mucosal inflammation to spring pollens. There were other possible explanations such as representativeness of the pollen trap, exposure to other pollens carrying the same allergen, psychological reasons linked to pollen forecasts that announce the start of the season, and others.25 More importantly, a determination of the threshold of pollen exposure (eg, type of pollen, single, or in combination) for symptoms in allergic individuals (eg, sensitization pattern and nature of diseases) needs to be carried out in order to improve the level of disease control, enhance patient satisfaction, and increase effectiveness of preventive interventions.29
Climate elements could also play an important role in local pollen allergen exposure that will directly affect the onset and severity of PiAR.30, 31 Several recent studies proposed that both direct effects of climate factors and their indirect effects (through their effect on physical, chemical, and biological aerosols) on intensity or period of pollen exposure should be explored in detail.17, 32, 33, 34, 35, 36, 37 In our study, temperature and precipitation were risk factors (RR > 1), while wind speed and air pressure were protective factors (RR < 1, Table 3). AI was not statistically associated with PiAR symptoms, but AI in humid areas showed a risk trend for PiAR. We presumed that AI had a threshold which will affect PiAR so that when AI is above a certain value, there would be no linear correlation. Additionally, climate factors varied among different regions and therefore should be considered separately. A study by Silverberg17 found a negative effect from humidity and AI, but a positive effect from temperature and precipitation. Weiland36 also analyzed the effects of climate and the prevalence of allergic symptoms and found a negative effect from temperature and humidity. In our study, the northern grassland of China has a high latitude and narrow range of humidity, and thus, humidity appeared to have no effect on AR. Studies along the Pacific rim demonstrated that high nonsummer temperatures were positively related to the prevalence of physician‐diagnosed AR in both sexes.38 Our study demonstrated a positive association with temperature and an inverse association in all seasons with PiAR prevalence. This was in contrast to eczema, where higher temperatures were associated with lower disease prevalence.39 Therefore, while high temperatures may aggravate AR, the humidity along with higher temperatures may contribute to the remission of eczema which could explain the inverted result.
There are some limitations in this study. Firstly, the severity and quality‐of‐life impairment from PiAR symptoms were not evaluated, and therefore, their association with indices of disease severity could not be determined. Secondly, there was no epidemiological data on AR prevalence in this region and, therefore, it was not possible to assess whether there was a trend toward a higher incidence of allergic disease in this region. Thirdly, we were aware of the limitations of the Durham Sampler technique such as (i) the volume of the air sampled is unknown, so the catch of pollen cannot be converted to a volumetric measure of concentration; (ii) the efficiency cannot be determined; (iii) the catch is relatively low; and (iv) the catch is a function of wind speed, turbulence, and orientation of the sampler with respect to wind direction as well as concentration of pollen in the air. However, as it was inexpensive and easy to manage, coupled with the fact that this sampling technique has been used in almost all studies in China, it provides the possibility to compare the data from previous studies. The comparisons were important in contributing to the understanding of the evolution and possible changes in the background pollen concentration and exposure in China. On the other hand, we had also started using a Hirst‐type volumetric pollen trap in some stations and will be able to report local pollen concentration in future studies.
In conclusion, this is the first study that combined epidemiological study of AR with allergy testing and pollen counts in the northern grassland regions of China, where the prevalence of PiAR in this region is extremely high with a persistent type of AR being common. Patients with PiAR suffer from symptoms as they are exposed to a very high amount of pollen allergens in season. Additionally, with the acceleration in global warming, the drying trend in northern China could deteriorate the local climate and vegetation distribution, and thus, it may further accelerate the prevalence of allergic diseases. Therefore, while it remains unknown, it is important to know how the pollen distribution in season affects allergic disease and the levels where the effects can be observed. The intensity of disease‐related sensitization of each pollen and climate factors contributing to it needs to be studied in the future.
CONFLICTS OF INTERESTS
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
W‐XY, YY, ZL, W‐DY, B‐YF, ZY, S‐HY, B‐YF, and S‐GL conceived the study. W‐XY, ZY, M‐TT, Z‐YF, Z‐TJ, J‐DZ, L‐XL, K‐ZX, Y‐WJ, Y‐BT, B‐XZ, S‐SH, Z‐FF, Y‐WH, B‐CL, WT, YT, M‐TQ, W‐XB, L‐JG, and DH collected the data. ZB, S‐QK, M‐TT, and W‐XY analyzed and interpreted the data. W‐XY, M‐TT, W‐DY, W‐XD YD, and H‐HD drafted the manuscript. W‐XY, ZL, W‐DY, and W‐XD edited the final approval of the manuscript. All authors had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. W‐XY is guarantor for the study.
ETHICAL APPROVAL
Approval to conduct this study was granted by the institutional review boards of Beijing Shijitan Hospital, the affiliated hospital of the Beijing Capital Medical University, and all of six participating municipalities.
Supporting information
ACKNOWLEDGMENTS
We wish to thank colleagues and research team members in (i) Department of Epidemiology and Statistics, Institute of Basic Medical Sciences Chinese Academy of Medical Sciences, School of Basic Medicine Peking Union Medical College, Beijing, China; (ii) State Key Laboratory of Vegetation and Environmental Change, Institute of Botany, Chinese Academy of Sciences, Beijing, China; (iii) Tongliao Hospital, Tongliao, Inner Mongolia, China; (iv) Jarud People's Hospital, Jarud Banner, Tongliao, Inner Mongolia, China; (v) Kailu People's Hospital, Kailu County, Tongliao, Inner Mongolia, China; (vi) Erenhot Community Health Service Center, Erenhot, Inner Mongolia, China; (vii) Duolun People's Hospital, Duolun, Inner Mongolia, China; (viii) Xiwu People's Hospital, Xiwu Banner, Inner Mongolia, China; (ix) Xilingol Mongolian hospital, Xilinhot, Inner Mongolia, China.; (x) The government of Tongliao, Jarud, Kailu, Erenhot, Duolun, Xiwu, Xilingol, Inner Mongolia, China; (xi)Inner Mongolia Grassland Ecosystem Research Station, Chinese Academy of Sciences; (xii) Departments of Otolaryngology and Allergy, Head and Neck Surgery, Beijing Tongren Hospital; and (xiii) We also wish to thank Prof James Smith for kindly reviewing this article.
Wang X‐Y, Ma T‐T, Wang X‐Y, et al. Prevalence of pollen‐induced allergic rhinitis with high pollen exposure in grasslands of northern China. Allergy. 2018;73:1232–1243. https://doi.org/10.1111/all.13388
Funding information
This study was supported by the National Key Specialty Funding of China.
Contributor Information
X.‐Y. Wang, Email: allergy_wxy@126.com
L. Zhang, Email: dr.luozhang@139.com
Y. Yan, Email: yyshijitan@126.com
D.‐Y. Wang, Email: entwdy@nus.edu.sg.
REFERENCES
- 1. Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63(suppl 86):8‐160. [DOI] [PubMed] [Google Scholar]
- 2. Wang XD, Zheng M, Lou HF, et al. An increased prevalence of self‐reported allergic rhinitis in major Chinese cities from 2005 to 2011. Allergy. 2016;71:1170‐1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singh N, Singh U, Singh D, Daya M, Singh V. Correlation of pollen counts and number of hospital visits of asthmatic and allergic rhinitis patients. Lung India. 2017;34:127‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alexandropoulos T, Haidich AB, Pilalas D, Dardavessis T, Daniilidis M, Arvanitidou M. Characteristics of patients with allergic rhinitis in an outpatient clinic: a retrospective study. Allergol Immunopathol. 2013;41:194‐200. [DOI] [PubMed] [Google Scholar]
- 5. Erkara IP, Cingi C, Ayranci U, Gurbuz KM, Pehlivan S, Tokur S. Skin prick test reactivity in allergic rhinitis patients to airborne pollens. Environ Monit Assess. 2009;151:401‐412. [DOI] [PubMed] [Google Scholar]
- 6. Mahillon V, Saussez S, Michel O. High incidence of sensitization to ornamental plants in allergic rhinitis. Allergy. 2006;61:1138‐1140. [DOI] [PubMed] [Google Scholar]
- 7. Zhang F, Krafft T, Zhang D, Xu J, Wang W. The association between daily outpatient visits for allergic rhinitis and pollen levels in Beijing. Sci Total Environ. 2012;417–418:39‐44. [DOI] [PubMed] [Google Scholar]
- 8. Ross AM, Corden JM, Fleming DM. The role of oak pollen in hay fever consultations in general practice and the factors influencing patients’ decisions to consult. Br J Gen Pract. 1996;46:451‐455. [PMC free article] [PubMed] [Google Scholar]
- 9. Cakmak S, Dales RE, Burnett RT, Judek S, Coates F, Brook JR. Effect of airborne allergens on emergency visits by children for conjunctivitis and rhinitis. Lancet. 2002;359:947‐948. [DOI] [PubMed] [Google Scholar]
- 10. Fuhrman C, Sarter H, Thibaudon M, et al. Short‐term effect of pollen exposure on antiallergic drug consumption. Ann Allergy Asthma Immunol. 2007;99:225‐231. [DOI] [PubMed] [Google Scholar]
- 11. Mesa JAS, Smith M, Emberlin J, Allitt U, Caulton E, Galan C. Characteristics of grass pollen seasons in areas of southern Spain and the United Kingdom. Aerobiologia. 2003;19:243‐250. [Google Scholar]
- 12. D'Amato G, Cecchi L, Bonini S, et al. Allergenic pollen and pollen allergy in Europe. Allergy. 2007;62:976‐990. [DOI] [PubMed] [Google Scholar]
- 13. Rönmark E, Bjerg A, Perzanowski M, Platts‐Mills T, Lundbäck B. Major increase in allergic sensitization in schoolchildren from 1996 to 2006 in northern Sweden. J Allergy Clin Immunol. 2009;124:357‐363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sakashita M, Hirota T, Harada M, et al. Prevalence of allergic rhinitis and sensitization to common aeroallergens in a Japanese population. Int Arch Allergy Immunol. 2010;151:255‐261. [DOI] [PubMed] [Google Scholar]
- 15. Luo W, Huang H, Zheng P, et al. Major grass pollen allergens and components detected in a southern Chinese cohort of patients with allergic rhinitis and/or asthma. Mol Immunol. 2016;78:105‐112. [DOI] [PubMed] [Google Scholar]
- 16. Lake IR, Jones NR, Agnew M, et al. Climate change and future pollen allergy in Europe. Environ Health Perspect. 2017;125:385‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Silverberg JI, Braunstein M, Lee‐Wong M. Association between climate factors, pollen counts, and childhood hay fever prevalence in the United States. J Allergy Clin Immunol. 2015;135:463‐469. [DOI] [PubMed] [Google Scholar]
- 18. Beggs PJ. Adaptation to impacts of climate change on aeroallergens and allergic respiratory diseases. Int J Environ Res Public Health. 2010;7:3006‐3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parry ML. Climate change 2007 impacts, adaptation and vulnerability: contribution of Working Group II to the fourth assessment report of the Intergovernmental Panel on Climate Change. J Environ Qual. 2007;37:2407. [Google Scholar]
- 20. D'Amato G, Cecchi L. Effects of climate change on environmental factors in respiratory allergic diseases. Clin Exp Allergy. 2008;38:1264‐1274. [DOI] [PubMed] [Google Scholar]
- 21. Lan Z, Bai Y. Testing mechanisms of N‐enrichment‐induced species loss in a semiarid Inner Mongolia grassland: critical thresholds and implications for long‐term ecosystem responses. Philos Trans R Soc Lond B Biol Sci. 2012;367:3125‐3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu N, Wilske B, Ni J, John R, Chen J. Climate change in Inner Mongolia from 1955 to 2005‐trends at regional, biome and local scales. Environ Res Lett. 2009;4:45006. [Google Scholar]
- 23. Hu Q, Pan F, Pan X, et al. Spatial analysis of climate change in Inner Mongolia during 1961–2012. China. Appl Geogr. 2015;60:254‐260. [Google Scholar]
- 24. Strachan D, Sibbald B, Weiland S, et al. Worldwide variations in prevalence of symptoms of allergic rhinoconjunctivitis in children: the International Study of Asthma and Allergies in Childhood (ISAAC). Pediatr Allergy Immunol. 1997;8:161. [DOI] [PubMed] [Google Scholar]
- 25. Pfaar O, Bastl K, Berger U, et al. Defining pollen exposure times for clinical trials of allergen immunotherapy for pollen‐induced rhinoconjunctivitis ‐ an EAACI position paper. Allergy. 2017;72:713‐722. [DOI] [PubMed] [Google Scholar]
- 26. Canonica GW, Baena‐Cagnani CE, Bousquet J, et al. Recommendations for standardization of clinical trials with Allergen Specific Immunotherapy for respiratory allergy. A statement of a World Allergy Organization (WAO) taskforce. Allergy. 2007;62:317‐324. [DOI] [PubMed] [Google Scholar]
- 27. Bousquet J, Schunemann HJ, Hellings PW, et al. MACVIA clinical decision algorithm in adolescents and adults with allergic rhinitis. J Allergy Clin Immunol. 2016;138:367‐374. [DOI] [PubMed] [Google Scholar]
- 28. Blando J, Bielory L, Nguyen V, Diaz R, Jeng HA. Anthropogenic climate change and allergic diseases. Atmosphere. 2012;3:200‐212. [Google Scholar]
- 29. Hellings PW, Fokkens WJ, Bachert C, et al. Positioning the principles of precision medicine in care pathways for allergic rhinitis and chronic rhinosinusitis‐A EUFOREA‐ARIA‐EPOS‐AIRWAYS ICP statement. Allergy. 2017;72:1297‐1305. [DOI] [PubMed] [Google Scholar]
- 30. Hong S, Son DK, Kwon HJ. Climate change and allergic disease. Ann Allergy Asthma Immunol. 2010;109:166‐172. [DOI] [PubMed] [Google Scholar]
- 31. Lake IR, Jones NR, Agnew M, et al. Climate change and future pollen allergy in Europe. Environ Health Perspect. 2016;125:385‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ho WC, Hartley WR, Myers L, et al. Air pollution, weather, and associated risk factors related to asthma prevalence and attack rate. Environ Res. 2007;104:402‐409. [DOI] [PubMed] [Google Scholar]
- 33. Makra L, Tombácz S, Bálint B, Sümeghy Z, Sánta T, Hirsch T. Influences of meteorological parameters and biological and chemical air pollutants on the incidence of asthma and rhinitis. Clim Res. 2008;37:99‐119. [Google Scholar]
- 34. Matyasovszky I, Makra L, Bálint B, Guba Z, Sümeghy Z. Multivariate analysis of respiratory problems and their connection with meteorological parameters and the main biological and chemical air pollutants. Atmos Environ. 2011;45:4152‐4159. [Google Scholar]
- 35. Kim H, Park Y, Park K, Yoo B. Association between pollen risk indexes, air pollutants, and allergic diseases in Korea. Osong Public Health Res Perspect. 2016;7:172‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weiland SK, Husing A, Strachan DP, Rzehak P, Pearce N. Climate and the prevalence of symptoms of asthma, allergic rhinitis, and atopic eczema in children. Occup Environ Med. 2004;61:609‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang Y, Bielory L, Georgopoulos PG. Climate change effect on Betula (birch) and Quercus (oak) pollen seasons in the United States. Int J Biometeorol. 2014;58:909‐919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hughes AM, Lucas RM, Ponsonby AL, et al. The role of latitude, ultraviolet radiation exposure and vitamin D in childhood asthma and hayfever: an Australian multicenter study. Pediatr Allergy Immunol. 2011;22:327‐333. [DOI] [PubMed] [Google Scholar]
- 39. Silverberg JI, Hanifin J, Simpson EL. Climatic factors are associated with childhood eczema prevalence in the United States. J Invest Dermatol. 2013;133:1752‐1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


