Abstract
Objective
Accurate prediction of abdominal aortic aneurysm (AAA) growth in an individual can allow personalised stratification of surveillance intervals and better inform the timing for surgery. The authors recently described the novel significant association between flow mediated dilatation (FMD) and future AAA growth. The feasibility of predicting future AAA growth was explored in individual patients using a set of benchmark machine learning techniques.
Methods
The Oxford Abdominal Aortic Aneurysm Study (OxAAA) prospectively recruited AAA patients undergoing the routine NHS management pathway. In addition to the AAA diameter, FMD was systemically measured in these patients. A benchmark machine learning technique (non-linear Kernel support vector regression) was applied to predict future AAA growth in individual patients, using their baseline FMD and AAA diameter as input variables.
Results
Prospective growth data were recorded at 12 months (360 ± 49 days) in 94 patients. Of these, growth data were further recorded at 24 months (718 ± 81 days) in 79 patients. The average growth in AAA diameter was 3.4% at 12 months, and 2.8% per year at 24 months. The algorithm predicted the individual's AAA diameter to within 2 mm error in 85% and 71% of patients at 12 and 24 months.
Conclusions
The data highlight the utility of FMD as a biomarker for AAA and the value of machine learning techniques for AAA research in the new era of precision medicine.
Keywords: Abdominal aortic aneurysm, Aneurysm progression, Machine learning, Biomarker, Flow mediated dilatation
Highlights
-
•
Flow mediated dilatation of brachial artery is a biomarker of AAA progression.
-
•
It is feasible to predict future AAA growth in individuals using machine learning techniques.
-
•
Endothelial dysfunction is a key feature in human AAA disease.
Introduction
The clinical management of abdominal aortic aneurysms (AAAs) is defined by three key domains: screening/diagnosis, surveillance, and surgical intervention. With regard to the surveillance of AAAs, it is important to develop tools for the assessment of the likelihood of AAA rupture or for the prediction of future AAA growth. In the setting of clinical research, the true risk of rupture can only be established by allowing AAA rupture without intervention. In comparison, the growth rate of individual AAAs can be ascertained by repeat measurements of the AAA size during surveillance. The average growth rates of AAAs can be observed from cohort studies. However, there is currently no means of predicting the growth of an AAA in individual patients. In a recent survey of international vascular surgery colleagues, “discovering new tests for the prediction of AAA growth” was identified as the top priority for research in AAAs.1
In this regard, the novel observation that flow mediated dilatation (FMD, a marker of endothelial function) of the brachial artery is inversely correlated with the rate of future AAA growth, has recently been described. In the study, FMD of the participants was measured at baseline, and they were followed over a 12 month period. There was a significant inverse correlation between baseline FMD and the growth rate recorded over the subsequent 12 months.2 This highlights the potential utility of FMD as a novel biomarker of AAA progression.
Machine learning techniques are gaining mainstream interest in biomedical research. They are non-linear extensions of standard linear tools from medical statistics. For example, logistic regression maps a vector of values (as input) onto a class membership probability (at the output). Logistic regression finds the relationship between the multi-dimensional input and the output (in the range of [0 1] values) using a linear weighted sum of the inputs. Machine learning methods generalise this, by allowing the relationship between the same inputs and outputs to be a non-linear relationship. This allows interactions between input variables to be modelled such that the output value is closer to that which is required.
We have previously demonstrated the utility of machine learning in developing prognostic tools for the prediction of cardiovascular disease using ECG data in the China Kadoorie Biobank3 and other clinical settings.4 Here the application of a set of benchmark machine learning techniques (non-linear Kernel support vector regression) to predict future AAA growth in individual patients is described.
Methods
This study is based on a prospectively recruited cohort of patients with AAAs (Oxford Abdominal Aortic Aneurysm Study, OxAAA). Every participant gave written consent to take part in the study. The ethics approval reference for this study is SC/0250/13. AAA size data obtained by the National Health Service (NHS) AAA surveillance programme was used. AAA size was measured by the anteroposterior diameter (APD) (outer to outer) on ultrasound. FMD of the brachial artery was measured as an additional research assessment in the study participants. Annual AAA % growth was calculated by (ΔAPD/APD at baseline)/(number of days lapsed/365 days).
Full details of patient recruitment and data acquisition of the OxAAA study cohort are as reported recently.2 A significant correlation between the baseline AAA diameter and AAA growth rate recorded over the subsequent 12 months was observed, but none of the other clinical demographic parameters correlated with future AAA growth. Therefore baseline FMD and AAA diameter were included as the two variables to construct the prediction algorithm. In addition, longer term (24 months) growth data were included in the latest analysis.
Receiver operating characteristic curves (ROC) were plotted first, using two variables (baseline FMD and AAA diameter) to analyse the performance of the generalised linear logistic regression model for discerning growth against a predefined growth rate threshold.
A set of benchmark machine learning techniques was then applied for the prediction of AAA diameter in individual patients at 12 and 24 months from baseline. These included non-linear kernel support vector regression (SVR) using two features (FMD, AAA diameter) and hyperparameter optimisation using nested fivefold cross validations5, 6 (Matlab, V2016b, Natick, MA, USA).
SVR is a regression technique which can be used in the context of linear or non-linear regression, where the linear relation assumption would not be optimal or sufficient to characterise the dynamics of input feature patterns versus model outcome. A Kernel trick can be used in SVR to learn a non-linear function and map feature input into desired model output. Here, non-linear kernel SVR including a Gaussian kernel has been used to non-linearly map the feature input (FMD, AAA size) into the future AAA growth rate.
Cross validation is a well established approach where the data are partitioned into a training set used to train the predictive model and a test set to evaluate the model. In “k-fold”, the data is partitioned into “k” equal sized subset (folds). Then, k-1 folds of the data are used for training the model and a single fold is used for testing the trained model. The process is then repeated k times (the folds) and the average error has been calculated to assess the model. Based on this model, all the observations are used for both training and testing the model.
A 2 mm error margin was allowed in the algorithm because this is the accepted technical variability between ultrasound antero-posterior diameter measurements in AAAs.7, 8
Results
Baseline demographic data of the study participants are presented in Table 1. Prospective growth data were recorded at 12 months (360 ± 49 days) for 94 patients. Of these, growth data were further recorded at 24 months (718 ± 81 days) in 79 patients. The average growth in AAA diameter was 3.4% at 12 months, and 2.8% per year at 24 months.
Table 1.
Summary of participant characteristics at the baseline assessment.
| Number (male) | 94 (82) |
| Age at consent, years (SD) | 74 (8) |
| AAA size, mm (IQR) | 43 (36–48) |
| Height, m (SD) | 1.72 (0.08) |
| Weight, kg (SD) | 83.5 (14) |
| BMI median (IQR) | 27 (24–31) |
| Blood pressure SBP/DBP, mmHg (SD) | 137/77 (15/11) |
| Smoking status, n (%) | |
| Current smoker | 14 (15) |
| Past history of smoking (>1 month) | 66 (70) |
| Never smoked | 14 (15) |
| History of ischaemic heart disease, n (%) | 38 (40) |
| MI/ACS | 33 (35) |
| Stable angina | 18 (19) |
| Coronary intervention/bypass | 34 (36) |
| History of peripheral arterial disease, n (%) | 24 (26) |
| History of cerebral arterial disease, n (%) | 12 (13) |
| History of hypertension, n (%) | 62 (66) |
| History of hypercholesterolemia, n (%) | 57 (61) |
| Total cholesterol, mmol/L (IQR) | 4 (3.4–5) |
| High density lipoprotein, mmol/L (IQR) | 1.1 (1–1.4) |
| Low density lipoprotein, mmol/L (IQR) | 2.2 (1.7–3.1) |
| Triglycerides, mmol/L (IQR) | 1.3 (0.9–1.9) |
| History of diabetes mellitus, n (%) | 14 (15) |
| HbA1C%, mean (SD) | 41 (8) |
| Oral anti-hyperglycaemics, n (%) | 11 (12) |
| Insulin, n | 0 |
| Chronic kidney disease (eGFR< 60), n (%) | 21 (22) |
| Creatinine μmol/L (IQR) | 80 (68–96) |
| Chronic respiratory disease, n (%) | 15 (16) |
| Family history of AAA, n (%) | 20 (21) |
| History of treated neoplasms, n (%) | 14 (15) |
| Regular medication, n (%) | |
| Aspirin | 56 (60) |
| Thienopyridine/cyclopentyltriazolopyrimidine | 14 (15) |
| Anticoagulants | 11 (12) |
| Statin | 71 (76) |
| β blocker | 35 (37) |
| ACE inhibitor/ARB | 60 (64) |
| C-reactive protein (mg/L, IQR) | 2.9 (1.1–7.3) |
| Median FMD (%, IQR) | 2.0 (0.75–4.02) |
Note. For variables which demonstrate Gaussian distribution, mean and standard deviation (SD) are presented. For variables which demonstrate non-Gaussian distribution, median and interquartile range (IQR) are presented. AAA = abdominal aortic aneurysm; IQR = interquartile range; BMI = body mass index; SBP = systolic blood pressure; DBP = diastolic blood pressure; MI = myocardial infarction; ACS = acute coronary syndrome; PAD = peripheral arterial disease; TC = total cholesterol; TG = triglycerides; DM = diabetes mellitus; HbA1C = glycated haemoglobin; CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate; ARB = angiotensin II receptor blocker; CRP = C-reactive protein; FMD = flow mediated dilatation of brachial artery.
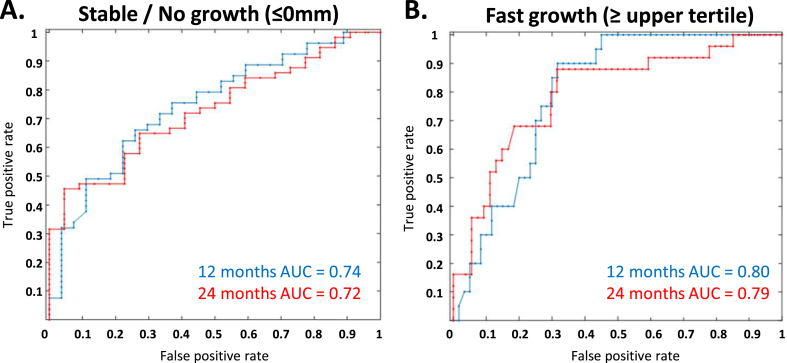
The ROC curves are plotted with the threshold of “stable/no growth” (defined as growth ≤0%/year) or “fast growth” (defined as ≥ the upper tertile of growth within the group, during the respective period) for both 12 and 24 months (blue and red line respectively; Fig. 1A and B). The area under ROC (AUROC) metrics show good discrimination based on FMD, AAA diameter, and the prediction of future growth rates at 12 and 24 months against the predefined thresholds.
Figure 1.
Receiver operating curve (ROC) demonstrating the ability of the logistic regression model to discern future growth at predefined growth rate thresholds. ROC curves were first plotted using two variables (baseline FMD and AAA diameter) to analyse the performance of the generalised linear logistic regression model. The ROC curves are plotted with the threshold of “stable/no growth“ (A) (defined as growth ≤ 0mm/year) or “fast growth” (B) (defined as upper tertile of growth within the group, during the respective period) at both 12 and 24 months (blue and red line respectively).
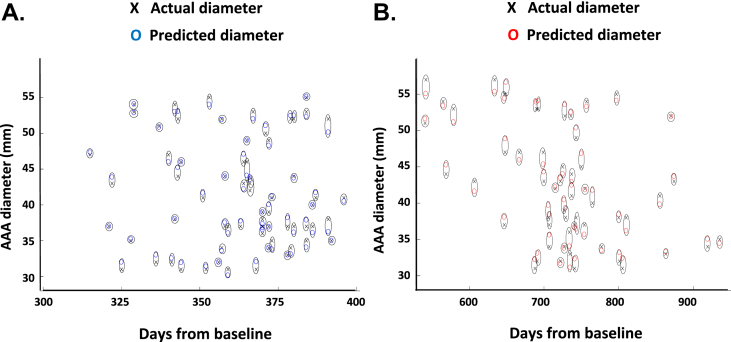
Using the machine learning techniques as described, the individual's AAA diameter was predicted to within 2 mm error in 85% and 71% of patients at 12 and 24 months, respectively (with root mean square error of 1.7 and 2.4, respectively) (Fig. 2C and D; black cross, actual AAA diameter measured at 12 and 24 months; blue and red circles, machine predicted diameter at 12 (blue) and 24 (red) months).
Figure 2.
Applying machine learning techniques for the prediction of AAA growth in individual patients. For the prediction of AAA diameter in individual patients at 12 (A) and 24 (B) months from baseline, non-linear kernel support vector regression (SVM) was applied using two features (FMD, AAA diameter), and hyperparameter optimisation using nested fivefold cross validations. The SVM method is a non-linear regression which can potentially improve the accuracy of predicting AAA diameter by considering non-linear functions of the input features. A 2 mm error margin was allowed because this is accepted technical variability between ultrasound diameter measurements in AAAs. The algorithm predicted the individual's AAA diameter to within a 2 mm error in 85% and 71% of patients at 12 and 24 months, respectively (with root mean square error of 1.7 and 2.4, respectively). Note. The figure includes only data points that are within the 2 mm error tolerance. Black cross, actual AAA diameter measured at 12 and 24 months; blue and red circles, machine predicted diameter at 12 (blue) and 24 (red) months.
Discussion
To prevent AAA rupture related mortalities, AAA screening programmes have been established in the UK, Sweden, and Germany. International guidelines state that small AAAs (<55 mm in diameter) require regular ultrasound scans to monitor growth until the 55 mm threshold is reached.9, 10, 11 For AAAs between 30 and 45 mm diameter, monitoring with an annual ultrasound scan is recommended; for AAAs between 45 and 55 mm in diameter, monitoring with 6 monthly scans is recommended. In the UK, the total number of screen detected AAAs requiring surveillance increases by ∼2,000 each year.12 The total number of surveillance scans performed each year therefore increases accordingly.
In 2016, a survey of all AAA patients at the Oxford Regional Vascular Service was conducted to ascertain their experience during AAA care. Among the comments raised by the participants (n = 194), “frequency/regularity of AAA monitoring” and “explanation regarding their management” emerged as the most important issues. This was further underpinned by the fact that 52% of the respondents felt highly preoccupied by the size of their AAAs.13
A tool for predicting the future growth of an AAA will impact clinical practice. Firstly, an explanation and reassurance to our patients can be provided regarding the “future” of their AAAs. More so, accurate prediction of AAA growth in an individual will allow personalised stratification of surveillance intervals. Those with a predicted slow growing AAA would not require as frequent monitoring, whereas the opposite is true for those with a predicted fast growing AAA. By applying benchmark machine learning techniques to an established dataset, an algorithm is derived that is able to predict the future growth of AAA in an individual patient.
Patients with small AAAs are typically monitored through dedicated AAA surveillance programmes. In real life settings, more than one operator would have performed the surveillance scans during the course of AAA surveillance. The pragmatic approach was taken of utilising the diameter measurements obtained at the clinical scan by the NHS AAA surveillance programme. In the prediction algorithm, clinically acceptable inter-observer variation was allowed in the ultrasound measurements. These measures help to improve the external validity and generalisability of the results.
The predictive accuracy of any forecasting algorithm would be expected a priori to decrease as the look ahead duration is increased. The degree of the decrease will vary according to the dynamics of the data for the individual application.14 It is noted here that a decrease from 85% at 12 months to 71% at 24 months is a relatively small decrease, considered informally, compared with equivalent doubling of the look ahead duration for many applications.
The study is designed to examine biomarkers predictive of future AAA growth as it utilises the documented size measurements during the natural history of AAA surveillance. It is also important for studies to examine biomarkers predictive of the future risk of AAA rupture. However, in order to examine the correlations between baseline biomarkers and the risk of future AAA rupture, a study should ideally establish the actual rupture rate observed in a prospectively recruited cohort, instead of the assumed risk of rupture. This can only happen if the recruited patients (with small AAAs) are allowed to progress to rupture without intervention, and therefore presents practical and ethical challenges. If FMD becomes a widely adopted biomarker in the setting of AAA surveillance, it may one day be possible to examine its value for predicting AAA rupture at a population level.
There is emerging evidence that the geometric/volumetric measurements of an AAA may provide better information regarding AAA growth. In particular, volumetric measurement by 3D ultrasound is particularly applicable for AAA surveillance.15 For future validation work, it will be useful to acquire geometric/volumetric measurements of the AAA to assess its effect on the prediction algorithm.
Conclusion
Biomarkers for the prediction of AAA rupture or growth can have important implications in the management of AAAs. The data highlight the utility of FMD as a biomarker for AAA growth, and the value of machine learning techniques in the new era of precision medicine. Given the international opinion regarding the importance of biomarkers for the prediction of AAA growth, the findings serve as a primer to stimulate interest for further validation of this biomarker by external cohorts.
Acknowledgement
We acknowledge the support from the following: Medical Sciences Division, University of Oxford Medical Research Fund; Jackie Walton Vascular Studies Unit, Oxford University Hospitals NHS Foundation Trust; Oxford Regional Vascular Services, Oxford University Hospitals NHS Foundation Trust; National Institute of Health Research (NIHR) Oxford Biomedical Research Centre. Academy of Medical Sciences (AMS_SGL013∖1015).
Contributors to this OxAAA Study: Kirthi Bellamkonda, Felicity Woodgate, Nicholas Killough, Niveshni Maistry, Anirudh Chandrashekar, and the Oxford Regional Vascular Service (Chris R. Darby, Alison Halliday, Linda J. Hands, Patrick Lintott, Tim R. Magee, Andrew Northeast, Jeremy Perkins, Ediri Sideso).
Contributor Information
R. Lee, Email: regent.lee@nds.ox.ac.uk.
the Oxford Abdominal Aortic Aneurysm Study and:
K. Bellamkonda, F. Woodgate, N. Killough, N. Maistry, and A. Chandrashekar
the Oxford Regional Vascular Service:
C.R. Darby, A. Halliday, L.J. Hands, P. Lintott, T.R. Magee, A. Northeast, J. Perkins, and E. Sideso
References
- 1.Lee R., Jones A., Cassimjee I., Handa A. International opinion on priorities in research for small abdominal aortic aneurysms and the potential path for research to impact clinical management. Int J Cardiol. 2017;245:253–255. doi: 10.1016/j.ijcard.2017.06.058. [DOI] [PubMed] [Google Scholar]
- 2.Lee R., Bellamkonda K., Jones A., Killough N., Woodgate F., Williams M. Flow mediated dilatation and progression of abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2017;53:820–829. doi: 10.1016/j.ejvs.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yanting S., Yang Y., Parish S., Zhengming C., Clarke R., Clifton D.A. Risk prediction for cardiovascular disease using ECG data in the China Kadoorie Biobank. Conf Proc IEEE Eng Med Biol Soc. 2016;2016:2419–2422. doi: 10.1109/EMBC.2016.7591218. [DOI] [PubMed] [Google Scholar]
- 4.Johnson A.E., Ghassemi M.M., Nemati S., Niehaus K.E., Clifton D.A., Clifford G.D. Machine Learning and decision support in critical care. Proc IEEE Inst Electr Electron Eng. 2016;104:444–466. doi: 10.1109/JPROC.2015.2501978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito K., Nakano R. Optimizing support vector regression hyperparameters based on cross-validation. Proc Intl Jt Conf Neural Netw. 2003;3:2077–2082. [Google Scholar]
- 6.Arlot S., Celisse A. A survey of cross-validation procedures for model selection. Stat Surv. 2010;4:40–79. [Google Scholar]
- 7.Jaakkola P., Hippelainen M., Farin P., Rytkonen H., Kainulainen S., Partanen K. Interobserver variability in measuring the dimensions of the abdominal aorta: comparison of ultrasound and computed tomography. Eur J Vasc Endovasc Surg. 1996;12:230–237. doi: 10.1016/s1078-5884(96)80112-2. [DOI] [PubMed] [Google Scholar]
- 8.Singh K., Jacobsen B.K., Solberg S., Bonaa K.H., Kumar S., Bajic R. Intra- and interobserver variability in the measurements of abdominal aortic and common iliac artery diameter with computed tomography. The Tromso study. Eur J Vasc Endovasc Surg. 2003;25:399–407. doi: 10.1053/ejvs.2002.1856. [DOI] [PubMed] [Google Scholar]
- 9.Erbel R., Aboyans V., Boileau C., Bossone E., Bartolomeo R.D., Eggebrecht H. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC) Eur Heart J. 2014;35:2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 10.Anderson J.L., Halperin J.L., Albert N.M., Bozkurt B., Brindis R.G., Curtis L.H. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA guideline recommendations): a report of the American College of Cardiology Foundation/American Heart association task force on practice guidelines. Circulation. 2013;127:1425–1443. doi: 10.1161/CIR.0b013e31828b82aa. [DOI] [PubMed] [Google Scholar]
- 11.Moll F.L., Powell J.T., Fraedrich G., Verzini F., Haulon S., Waltham M. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011;41(Suppl. 1):S1–S58. doi: 10.1016/j.ejvs.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 12.NHS England . NHS; 2017. Abdominal aortic aneurysm screening – NHS Choices.https://www.nhs.uk/condictions/abdominal-aortic-aneurysm-screening [Google Scholar]
- 13.Lee R., Jones A., Woodgate F., Bellamkonda K., Killough N., Fulford-Smith L. The experience of patients during the clinical management pathway of abdominal aortic aneurysms at a NHS trust. J Patient Exp. 2017;4:202–209. doi: 10.1177/2374373517715010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bishop C. Springer; 2006. Pattern recognition and machine learning. ISBN 0387310738. [Google Scholar]
- 15.Lowe C., Ghulam Q., Bredahl K., Rogers S., Ghosh J., Sillesen H. Three-dimensional Ultrasound in the Management of abdominal aortic aneurysms: a topical review. Eur J Vasc Endovasc Surg. 2016;52:466–474. doi: 10.1016/j.ejvs.2016.06.009. [DOI] [PubMed] [Google Scholar]