Abstract
Purpose of Review
Recently, there is a significant amount of progress in the research related to regenerative medicine. At the same time, the biomedical implants in orthopedics and dentistry are facing many challenges and posing clinical concerns. The purpose of this chapter is to provide an overview of the clinical applications of current regenerative strategies to the fields of dentistry and orthopedic surgery. Major research question in this review is what are the major advancement strategies in regenerative medicine that can be used for implant research?
Recent Findings
The implant surfaces can be modified through patient-specific stem cells and plasma coatings, which may provide methods to improve osseointegration and sustainability of the implant.
Summary
Overall understanding from the review suggesting that the outcome from the studies could lead to identify optimum solutions for many concerns in biomedical implants and even in drug developments as a long-term solution to orthopedic and dental patients.
Keywords: Regenerative medicine, biomedical implants, stem cells, surface modifications
1. Introduction
Diseases such as osteoarthritis, spondylosis, and tooth decay result in irreversible structural damage to the affected tissues. When non-invasive therapies fail to reduce symptoms to a manageable level, surgical intervention is often the next logical treatment option. Removal of damaged tissue and replacement with a synthetic implant is widely used to restore function and reduce pain, which includes orthopedic implants (total hip replacements, total knee replacements, intervertebral disc replacements, etc.), and dental implants. Further, in recent years, common procedures (such as total joint arthroplasty and artificial tooth replacement) have seen a gradual increase in frequency and these trends are expected to continue into the foreseeable future [1–3].
The 2017 annual report from The American Joint Replacement Registry, representing more than 4700 orthopedic surgeons, revealed that over 860,000 total hip (THA) and knee (TKA) arthroplasties were performed in 2016 alone, a significant increase from years prior [1]. THA and TKA procedures often provide symptomatic relief for the majority of recipients but also carry a moderate risk of complications. The rates of one or more complications that occur either in a hospital or after discharge are estimated to be 7% and 8%, respectively.
Complications include infection, fracture, bleeding, deep vein thrombosis, pulmonary embolism, etc. [4]. Complications may undermine implant stability and necessitate a revision procedure. Fortunately, the revision burden for both total hip and total knee replacement appears to be decreasing in recent years. Data suggests that the revision burdens for total hip and knee replacements in 2016 (8.1% and 5.6%, respectively) decreased significantly from data collected during the years 2012–2015 (mean = 13.9% and 6.5%, respectively) [1].
Similarly, a significant demand for dental implants exists. A National Health and Nutrition Examination Survey published in 2015 found that 52% of adults aged 20–64 had lost one or more permanent teeth [5]. Dental implants, while largely successful, share similar limitations that are faced by THA and TKA. Most notably, postoperative infection of the supporting tissue, known as peri-implantitis, can result in progressively worsening bone loss and is a leading cause of implant failure with an estimated moderate to severe complications of 14.5% [6,7]. A meta-analysis of the available research regarding peri-implantitis estimates the overall prevalence to be approximately 18.5% [8].
Due to increasing demand for these implants and the impact of the associated complications, resources have been directed into finding solutions that minimize their adverse effects. Promising solutions involve the integration of stem cells into the design of synthetic implants. These devices, appropriately named biomedical implants, use the principles of regenerative medicine to increase osseointegration, resistance to post-operative infection, and long-term viability of modern implants. It is the purpose of this chapter to provide an overview of the clinical applications of current biomedical implants within the fields of dentistry and orthopedic surgery.
2. Biomedical Implants
2.1. Total Joint Arthroplasty
Total joint replacement is one among the most significant developments in the history of orthopedics to relieve the pain and restore the mobility and physical activity of the patient. Between the year of 2012 and 2015, 426 K procedures have been performed, with an annual revision burden of approximately 10% [2]. The prosthetic design and biological factors equally contribute to the performance of total joint replacements. Biocompatibility and a well-functioning material-tissue interface is the pre-requisite for implant design. A total knee replacement consists of a femoral component, the tibial component and a polymer spacer. Similarly, the total hip replacement consists of a stem, neck and an acetabular component [9–11]. The materials commonly used for joint replacement includes, metals, ceramic and polymers. Metals include surgical grade stainless steel, cobalt chromium molybdenum alloys, titanium and titanium alloys. The use of stainless steel implants has been dramatically decreased due to adverse tissue reactions. Currently, cobalt chromium molybdenum alloys and titanium are generally selected by the implant manufacturers due to their better corrosion resistance and biocompatibility. Common ceramic materials used in orthopedic implants are aluminum oxide and calcium phosphate [12]. The major drawback associated with ceramic materials are their high elastic modulus compared to bone which leads to fracture of bone and loosening of ceramic acetabular sockets [13]. However, calcium phosphates are a better selection due the high biocompatibility and bio reactivity [12]. The most popular polymeric materials used in orthopedics is ultra-high molecular weight polyethylene of high density polyethylene, though wear of the polymeric material is a major concern which leads to osteolysis and adverse tissue reactions. There have been several advancements in the field of orthopedic implants field to resolve the complications facing industries today. Modifications including nickel-free stainless steel, surface modifications, introduction of metallic implants with lower modulus, etc. are some of them [14]. Aseptic loosening is the main cause of implant failure and statistics shows that approximately 70% of hip revisions and 44% of knee revisions are due to aseptic loosening. Whereas 1–2% failure occurs due to infection, which is considered as an uncommon complication. Biomechanical factors such as micromotion plays a major role in aseptic loosening. Hence, in addition to the implant material characteristics, biological factors need to be considered while accounting for means to improve biomedical implants.
2.2. Dental Implants
More than 5 million dental implants are performed every year and it is expected to increase 12–15 % in the coming years [15]. Approximately 5–11% of dental implants fail within 10–15 years and must be removed [16–21]. Major factors associated with dental implant failure are categorized as biological or mechanical factors, such as peri-implantitis (peri-implant inflammation leading to bone loss); degradation of structural materials and connections; implant design; bone density; surgical and prosthetic complications; as well as patient-specific conditions [21,22]. However, the etiology of peri-implantitis remains unclear, as well as how the risk factors interact, leading to implant failures [16,23]. Dental implant systems consist of three parts: the implant fixture, which is a metallic threaded cylinder that will be surgically placed into the jawbone; an abutment, a temporary structure that covers the top of the implant and stays until healing occurs and a crown, permanent tooth, on the top of the implant, which performs the function of the normal tooth. Titanium and its alloys are considered as the gold standard for the fabrication of endosseous dental implants, although there are various other materials such as gold, stainless steel, cobalt chromium, etc. that have been used.
Irrespective of the implant-abutment connection type [24], there will be a micro gap formed between the components in which oral fluids, glycoproteins, and microorganisms will penetrate [25–27] and form a biofilm. This biofilm will eventually act like a lubricant and reduce the mechanical integrity of the joint [27–29]. Further, micromotion associated with chewing can lead to friction and wear of the implant-abutment surfaces [30]. Osteointegration is an important requirement for the functioning of dental implants. There are several factors that dictates bone formation around the implant including the material, implant design, biomechanical factors, surface charge, surface chemistry and surface topography. Even though, implant design factors play an important role in their overall success, clinical reports also demonstrated a patient health condition and the quality of the bone make a significant impact on the healing process. The quality of the periodontal bone is the major limiting factor for the success of the dental implant.
2.3. Spinal Fusion
Arthrodesis, the surgical fixation of a joint to promote bone fusion, is widely considered as the gold standard treatment option for intervertebral disc degeneration in the cervical, thoracic and lumbar spine [31–33]. The surgery can be performed in a variety of ways including both anterior and posterior approaches. In either case, current techniques involve removal of the intervertebral disc, replacement with an interbody cage and the placement of supporting plates and screws. The success of the procedure depends largely upon osteoconduction (the ability of bone to grow through the implant) osteoinduction (the stimulation of pluripotent cells to develop into osteoblasts) and osteointegration (the ability of an implant to interface with living bone) [34,35]. In addition, the implant must be able to withstand biomechanical forces. Therefore, it is imperative that the implant is fitted and place properly in order to avoid unnecessary mechanical stress [36]. Traditionally, an autologous bone graft taken from the iliac crest is used due to its remarkable capacity in all three areas. Furthermore, the use of the host’s own tissue greatly reduces the risk of infection and immunoreactions. However, these benefits come at the cost of a longer procedure length and the risk of complications associated with harvesting the graft. Documented complications include infection, fracture, donor site pain, poor cosmetic outcomes, etc. [37]. These risks increase if the patient requires a multiple level fusion procedure that necessitates a larger area of donor bone to be harvested. Due to these limitations, alternative biomaterials to replace bone grafting have become the focus moving forward. Biomaterials are chosen based on biocompatibility, surface features and rigidity [34]. Optimization of these characteristics has led to combination approaches that incorporate osteoinductive capabilities of stem cells and growth factors with bio-inert scaffolds.
3. Regenerative Medicine
Regenerative medicine is an emerging field of medical science that deals with the functional restoration of specific tissues and/or organs of the patients suffering from trauma or other chronic diseases. The frontline of the regenerative medicine strategy is stem cells, which pave the foundation for all the tissues, organs and organ systems in our body. There are four different types of stem cells, i.e., unipotent, multipotent, pluripotent and toptipotent. The only totipotent stem cells in the human body are the Zygote, which gives rise to a complete organism. Based on the regenerative application, the stem cells can be categorized into embryonic stem cells (ESCs), tissue-specific progenitor stem cells (TSPSCs), mesenchymal stem cells (MSCs), umbilical cord stem cells (UCSCs), bone marrow stem cells (BMSCs), and induced pluripotent stem cells (iPSCs). Human ESCs (pluripotent) were isolated by Thomson in 1998 (38). Their pluripotency is governed by functional dynamics of the Oct4, SOX2, NANOG and other transcription factors (39). These ESCs can be differentiated into any type of cells representing three germ layers of the body and represents a promising source of regenerative medicine for tissue regeneration and therapy. However, ethical concerns limit the application of this field. TSPSCs were well studied and developed for organoid culture, which further continued by the use of MSCs as the next-generation organoid culture system along with UCSCs, BMSCs. Figure 1 describes a schematic representation of the stem cell lineage of bone marrow mesenchymal stem cells into different mesodermal cell types.
Figure 1.
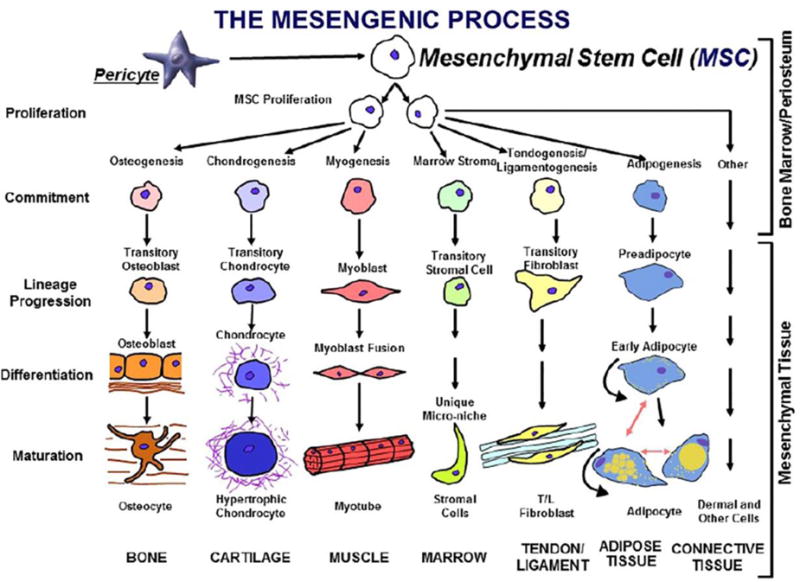
The mesengenic process was first envisioned in the late 1980s as a pathway for marrow mesenchymal stem cells (MSCs) to differentiate into a number of mesodermal cell types that could contribute to the fabrication of bone, cartilage, and muscle. It is now clear that MSCs can be isolated from many tissues because they are derived from perivascular cells and pericytes [45]. Used with permission from Elsevier.
The field of iPSCs was a novel strategy that emerged in 2006, by Takahashi and Yamanaka who generated ESCs like cells through the genetic incorporation of factors such as Sox2, Oct3/4, K1f4 and c-Myc into skin fibroblasts [40]. The generation of iPSCs opened up a new window for a potential therapeutic strategy towards age-related macular degeneration, Alzheimer’s disease and other neurodegenerative disorders [41–43]. It was speculated that by 2020, there would be a wide array of tissues, organoids, and organs from adult stem cells, with the potential of transplantation for various diseases [44]. In addition, stem cell therapies will provide considerable benefits to patients suffering from injuries. The advancement in the field of regenerative medicine also involved in the benefit of the biomedical implantation field for the betterment of implant performance. Some of the strategies already developed in this context will be described in the next section.
4. Regenerative Medicine Strategies in Biomedical Implants
4.1. Orthopedic and Dental Implants
4.1.1 Platelet Rich Plasma and Platelet-rich Fibrin
Platelet-rich plasma is defined as a volume of plasma that has a platelet count above the baseline [46]. Even though the term PRP means the combination of platelets and plasma, they are actually seen as various forms such as pure PRP, leukocyte-rich PRP, pure platelet-rich fibrin and leukocyte and platelet-rich fibrin [47–50]. Although the most visible function of platelets is coagulation, they are a rich source of growth factors, immune system messengers, enzymes and other bioactive components important for tissue healing and repair. The growth factors present in PRP includes: platelet-derived growth factor, transforming growth factor-beta, insulin-like growth factor, epidermal growth factor, fibroblast growth factor-2, and connective tissue growth factor. In addition to that cytokine, histamines, fibrinogen, fibronectin, serotonin, complement C5a, von willebrand factor, etc. are also present in a rich concentration. Hence, they have been increasingly used for a various applications such as augmentation of wound hemostasis, wound sealing and wound healing. Recently, the use of PRP was observed in arthroplasty settings. In joint arthroplasty, PRP is used in a more superficial way, ie, either injecting into the surgical area or spraying around the wound before closure of the wound [46]. However, the research information of the use of PRP in THA, TKA, total shoulder arthroplasty (TSA) and total ankle arthroplasty (TAA) is limited because of the lack of proper control. Other important information, such as the composition of the PRP and the processing condition are also unavailable in the literature, which leads to inconclusive information. However, there are reports about the decreasing post-operative pain after PRP use [46].
4.1.2. Smart Biomaterial-tissue Interface
The development of a smart biomaterial-tissue interface to modulate cellular response is another less explored area. The drug-eluting implant is already established for the controlled release of the drug, in order to reduce the pain and infection. However, this strategy has a very good application for generating a smart material-tissue interface, where a controlled in situ cell delivery and regeneration, cell transplantation therapy or release of biological cues for the activation of stem cells to early differentiation and secretion of a bone matrix can be achievable. Unlike tissue-engineered scaffolds, where the cell-seeded matrix can regenerate a bone matrix, biomedical metal implants cannot carry cells for a prolonged duration. However, the application of dynamic cell-based devices or interfaces can be adopted to develop a reservoir of cells or biomolecules whenever needed [51,52]. Another important problem associated with metal implants are the corrosion of the implant and subsequent release of metal ions and particles to the surrounding leading to inflammation and osteolysis. Stimuli-sensitive hydrogels, which respond to the inflammation to release proteins or molecule to block the pro-inflammatory proteins and thereby reduce the signaling of inflammation can eventually prevent the progression of inflammation. It was established that trafficking mesenchymal stem cells to the peri-implant sites would enhance the regenerative process. Hence, several researchers have been involved in injecting MSC pool to the injured site.
4.1.3. Stem Cell Therapy
Recent advancements in regenerative medicine has opened up a new therapeutic strategy for osteoarthritis. The inability of articular cartilage to self-repair requires an additional option to improve chondrogenesis. The delivery of mesenchymal stem cells will be a good attempt to overcome the challenge [53–55].
Adult stem cells, such as bone marrow-derived mesenchymal stem cells, adipose-derived stem cells and synovium-derived stem cells have the potential to generate cartilage in the presence of specific growth factors such as TGF-b and BMPs. The stem cell therapy for arthritis treatment is a new concept pioneered by advanced regenerative medicine. The strategy utilizes the body’s natural ability to heal the degenerated joint by transplanting new stem cells and other factors. According to this strategy, invasive surgery can be avoided. However, the method may not be efficient to those who already at the end stage of osteoarthritis and hip replacement will be the only option to maintain the physical activity. However, a combined effort of stem cell therapy with that of joint replacement will open a new solution to reduce the time of bone integration effectively. One of the major challenges associated with stem cell therapy is the presence of a high level of pro-inflammatory cytokines, which may interfere with the stem cell functionality towards chondrogenic differentiation [55],[56]. Reports suggest that the presence of pro-inflammatory cytokines affects the mature tissue engineered constructs as well and leads to degradation [55,57]. Recently, Brunger et al. developed engineered stem cells for autonomously regulated closed looped delivery of anti-inflammatory cytokines in response to pro-inflammatory cytokines [58]. The study used genome editing with CRISPR/Cas9 system to create stem cells that generates anti-inflammatory factors in an autoregulated manner. This development is a breakthrough in regenerative medicine, which has a high potential for the efficient recovery of orthopedic patients with total joint replacements [58].
Another approach is to use bone marrow mesenchymal stem cell associated metal implants. Zhao et al., 2014 reported a successful method for the treatment of young patients with osteonecrosis of the femoral head. In this study, a vascularized iliac bone graft was implanted along with a tantalum rod and assessed on an average of 65–70 months compared to a control. There was a promising outcome in end-stage ONFH patients with a vascularized iliac graft. The study suggested that the involvement of a vascularized fibular graft on the articular surface helped the primary callus formation, which has osteoinductive and osteoconductive factors [59].
A stem cell strategy has been widely used in dentistry [60]. As discussed previously, one of the limitations associated with the initial placement of the dental implant is the availability of adequate bone volume to maintain biomechanical loading. In several cases, the loss of alveolar bone will lead to poor bonding of dental implants on site. Even though autogenous bone grafting was considered as a gold standard, the availability of graft and donor site morbidity may limit their success. However, stem cells have the potential to regenerate bone using a tissue-engineered scaffold with specific growth factors. Several studies have reported the successful application of this strategy in dental implantation as it significantly improved bone formation and presented adequate weight and height of the bone for implant placement [61–66]. Figure 2 depicts the overall regenerative medicine strategies applicable for the biomedical field.
Figure 2.
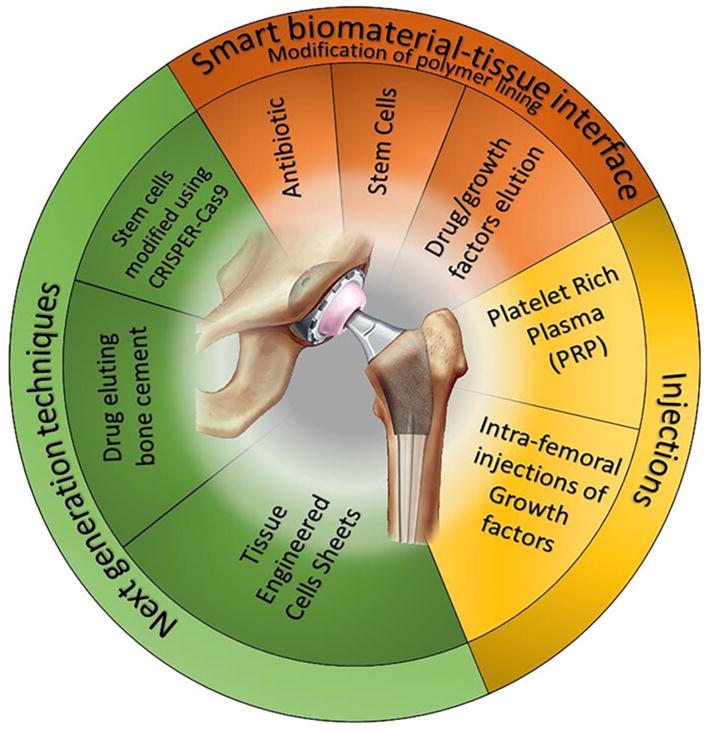
Schematic diagram represents different regenerative medicine strategies with potential applications in the biomedical implant field.
4.1.4 Tissue-engineered Cell Sheet
Stem cell therapy usually requires isolation of mesenchymal stem cells from various sources in vitro, which is a tedious procedure. Recently, new tissue engineering approaches have developed to use cell sheets for bone regeneration. In this technique, enzymatic digestion and isolation of the cells is not required. In addition, cell-cell contact will be intact in the cell sheet which provides a better environment for the regeneration of bone. Several studies have been performed to observe the applicability of this methodology for bone integration [67–70]. In the case of orthopedic implants, several approaches have been adopted to modify the surface of implants, such as physical, chemical and biochemical approaches. Most of these methods show significant improvements in osteointegration. However, at times, when pre-existing bone has weak osteogenic capacity and vasculature, obtaining osteointegration with surface modification alone is difficult. Though tissue-engineering approaches to develop bone grafts can be adopted to heal small bone defects, the strategy can be attempted to improve bone integration around orthopedic implants as well. Zhou et al developed a MSC-implant complex in vitro using partially mineralized cell sheets. After 4 weeks of in vitro culture under osteogenic medium, ECM deposition was observed under SEM. Further, in vivo evaluation confirmed an increase in bone formation as well as enhanced expression of VEGF to improve vascularization [68]. The advantage of this method is to use patient-specific cells to prepare an MSC-implant with their own MSC sheets to improve osteointegration. A lot more research has to be done in this area to utilize this method for actual clinical applications. Le et al. developed a bio-implant ensheathed in multi-layered cell sheets [71]. They co-cultured immortalized human cementoblasts and human cementifying fibroma cells. In addition, GFP (Green Fluorescent Protein) tagged human umbilical vein endothelial cells and epithelial cells were also employed to generate multi-layered cell sheets. The results of the study clearly demonstrated the involvement of each cell type in a fixture of the implant with newly formed calcified tissue on the surface of the fixture. In addition, the presence of oxytalan fibers was evident on the bi-layered and tri-layered cell sheets similar to periodontal tissue. This study provides us clear evidence for the involvement of regenerative medicine strategies in biomedical implantation (Figure 3).
Figure 3.
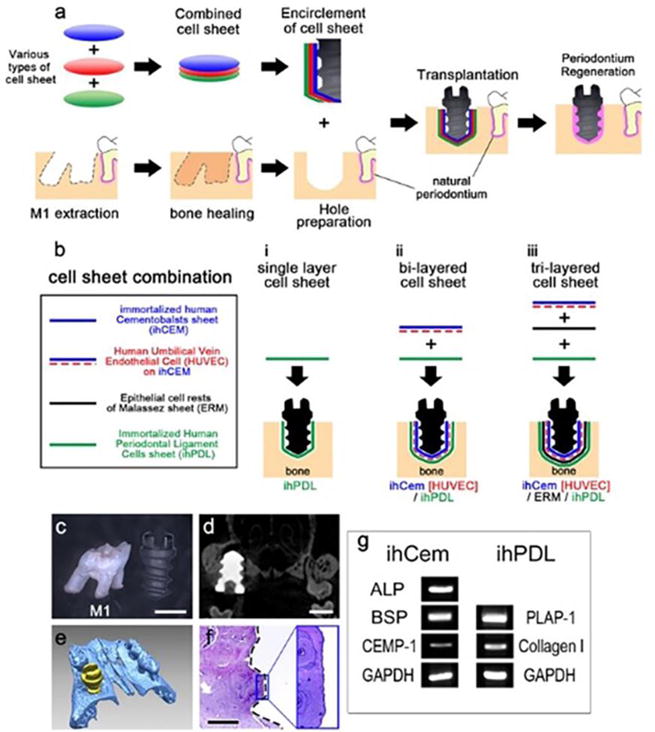
a) Schematic representation of the bio-implant procedure. The main purpose of this study is periodontium regeneration around the implant fixture. (b) A combination of cell sheets used in this study. Various types of cell sheets (panel a) are described. (c) Maxillary first molar and HA-coated dental implant fixture. (d) Micro CT image of 8 weeks after transplantation of the HA-coated fixture shows the alveolar bone compactly healed around the fixture. (e) 3D reconstruction of the micro CT results at 8 weeks after transplantation of the HA-coated fixture. (f) HE-stained image at 8 weeks after transplantation of the HA-coated fixture showing osseointegration between the alveolar bone and the fixture. Black dashed line shows the margin of the removed implant fixture. (g) RT-PCR results of immortalized human cells (cropped gel image). The ihCEMs expressed ALP, BSP and CEMP-1 [71].
4.2. Spinal Fusion
Throughout the history of spinal fusion procedures, it was widely accepted that autologous bone grafts harvested from the iliac crest was the “gold standard” disc replacement due to its osteoconductive, osteointegrative and osteogenic properties [34,72,73]. However, research has shown that bone graft harvesting is associated with an increased morbidity rates, numerous complications and increased surgical time. Moreover, a systematic review of outcomes associated with procedures that commonly use bone harvesting suggests the overall morbidity rate to be 19.37% (37). Due to these limitations, biocompatible materials such as titanium have become more popular. However, titanium, while having similar efficacy as autologous bone grafts, has limitations due to its mechanical properties as well as its radiopacity [74]. Therefore, research has been directed toward alternative biomaterials and bioactive substances such as polyester scaffolds, growth factors and stem cells in order to decrease the rate of nonunions and other complications.
Bone morphogenetic proteins (BMPs), belonging to the transforming growth factor β (TGF-β) superfamily, are growth factors implicated in postnatal osteogenesis as well as normal growth in utero [75,76]. BMPs act through a multitude of pathways that involve the regulation of hormones and the stimulation of mesenchymal stem cells to differentiate into osteoprogenitor cells [75–77]. Since their first discovery in 1965 by Marshall Urist, BMPs have been modified to maximize their clinical applications in orthopedic surgery [78]. Approved by the FDA in 2002 for anterior lumbar interbody fusion, the INFUSE bone graft delivers rhBMP-2 through an absorbable collagen sponge that is placed in the intervertebral space [79,80]. This eliminates the need for an additional surgery for bone harvesting and its associated complications. Many studies have suggested that rhBMP-2 discs outcompeted autologous bone grafts and reported fusion rates to be as high as 100% [81]. Eager to eliminate the risk and morbidity associated with autologous bone grafting, the widespread usage of rhBMP-2 began as did off label usage [77,79,80]. Years later, studies began to report severe complications in both the cervical and lumbar spine associated with rhBMP-2, most likely related to the very high concentrations used. This prompted the FDA to place a warning on the use of rhBMP-2 for cervical fusions. Complications in the cervical neck included increased risk of death, severe dysphagia, and compromising of the airway [79]. As a result, research focused on changing the delivery mechanism of rhBMP-2 so that the dosage and tissue exposure can be finely tuned. Extensive research has provided a plethora of delivery strategies including metallic implants, injectable hydrogels, polymer scaffolds, ceramic cages as well as combination products [82–86]. Due to the vast amount of available materials, these studies are ongoing and each year many more products come to light. The overall goal of these studies is to create an efficient, reproducible, and effective substitute for current gold standard disc replacements so that their complications can be avoided.
Similar to the work that is previously described, mesenchymal stromal cells, have been extensively studied for potential applications in vertebral fusion. MSCs are capable of releasing osteogenic compounds that recruit neighboring cells and induce the formation of bone. Results even suggest that these BMPs released from implanted MSCs may be more effective than direct delivery of rhBMP-2 via scaffolding. Therefore, the bulk of research regarding their application in spinal fusion revolves around finding an acceptable carrier that allows adequate exposure and differentiation that is required for fusion. A vast diversity of materials have been studied including metal cages, ceramics, synthetic polymers, hydrogels, nano scaffolds, etc. [73,87–91]; all with varying results. The results of these studies are highly variable depending on the scaffold and the controls they are measured against. Currently, there does not seem to be one single-handed winner for either the delivery mechanism or tissue from which MSCs are derived.
5. Conclusion
The chapter summarizes some recent advancements in the biomedical implant area by incorporating regenerative medicine strategies. Both metallic implants and the tissue engineering research field face challenges when reaching orthopedic applications in terms of bio-integration and mechanical properties respectively. Combining the advantages of both fields will assist in overcoming the challenges and clinical limitations, which has already been shown significant positive results by recent studies.
Footnotes
Compliance with Ethical Guidelines
Conflict of Interest
Divya Bijukumar, Clay McGeehan and Mathew Mathew declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
••Of major importance
- 1.Rankin EA. AJRR: becoming a national US joint registry. Orthopedics. 2013;36(3):175–176. doi: 10.3928/01477447-20130222-02. [DOI] [PubMed] [Google Scholar]
- 2.Maradit Kremers H, Larson DR, Crowson CS, Kremers WK, Washington RE, Steiner CA, et al. Prevalence of Total Hip and Knee Replacement in the United States. J Bone Joint Surg Am. 2015 Sep 2;97(17):1386–97. doi: 10.2106/JBJS.N.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson NA, Schneller ES, Montgomery K, Bozic KJ. Hip And Knee Implants: Current Trends And Policy Considerations. Health Aff (Millwood) 2008 Nov 1;27(6):1587–98. doi: 10.1377/hlthaff.27.6.1587. [DOI] [PubMed] [Google Scholar]
- 4.Cushner F, Agnelli G, Fitzgerald G, Warwick D. Complications and functional outcomes after total hip arthroplasty and total knee arthroplasty: results from the Global Orthopaedic Registry (GLORY) 2010 [PubMed] [Google Scholar]
- 5.Products - Data Briefs - Number 197-May 2015 [Internet] [cited 2017 Dec 24]. Available from: https://www.cdc.gov/nchs/products/databriefs/db197.htm.
- 6.Zitzmann NU, Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodontol. 2008 Sep 1;35:286–91. doi: 10.1111/j.1600-051X.2008.01274.x. [DOI] [PubMed] [Google Scholar]
- 7.Ramseier CA, Eick S, Brönnimann C, Buser D, Brägger U, Salvi GE. Host-derived biomarkers at teeth and implants in partially edentulous patients. A 10-year retrospective study. Clin Oral Implants Res. 2016 Feb;27(2):211–7. doi: 10.1111/clr.12566. [DOI] [PubMed] [Google Scholar]
- 8.Rakic M, Galindo-Moreno P, Monje A, Radovanovic S, Wang H-L, Cochran D, et al. How frequent does peri-implantitis occur? A systematic review and meta-analysis. Clin Oral Investig [Internet] 2017 Dec 7; doi: 10.1007/s00784-017-2276-y. [cited 2017 Dec 24]; Available from: http://link.springer.com/10.1007/s00784-017-2276-y. [DOI] [PubMed]
- 9.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007 Apr;89(4):780–5. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 10.Harding P, Holland AE, Delany C, Hinman RS. Do activity levels increase after total hip and knee arthroplasty? Clin Orthop. 2014 May;472(5):1502–11. doi: 10.1007/s11999-013-3427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones CA, Beaupre LA, Johnston DWC, Suarez-Almazor ME. Total Joint Arthroplasties: Current Concepts of Patient Outcomes after Surgery. Rheum Dis Clin N Am. 2007 Feb;33(1):71–86. doi: 10.1016/j.rdc.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Simon JP, Fabry G. An overview of implant materials. Acta Orthop Belg. 1991;57(1):1–5. [PubMed] [Google Scholar]
- 13.Osman K, Panagiotidou AP, Khan M, Blunn G, Haddad FS. Corrosion at the head-neck interface of current designs of modular femoral components: essential questions and answers relating to corrosion in modular head-neck junctions. Bone Jt J. 2016 May;98–B(5):579–84. doi: 10.1302/0301-620X.98B5.35592. [DOI] [PubMed] [Google Scholar]
- 14.Mantripragada VP, Lecka-Czernik B, Ebraheim NA, Jayasuriya AC. An overview of recent advances in designing orthopedic and craniofacial implants. J Biomed Mater Res A. 2013 Nov 1;101(11):3349–64. doi: 10.1002/jbm.a.34605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dental Implant Prosthetics - 2nd Edition [Internet] [cited 2017 Dec 29]. Available from: https://www.elsevier.com/books/dental-implant-prosthetics/misch/978-0-323-07845-0.
- 16.Albrektsson T, Buser D, Chen ST, Cochran D, DeBruyn H, Jemt T, et al. Statements from the Estepona consensus meeting on peri-implantitis, February 2–4, 2012. Clin Implant Dent Relat Res. 2012 Dec;14(6):781–2. doi: 10.1111/cid.12017. [DOI] [PubMed] [Google Scholar]
- 17.Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol. 2015 Apr;42(Suppl 16):S158–171. doi: 10.1111/jcpe.12334. [DOI] [PubMed] [Google Scholar]
- 18.Levin L. Editorial: peri-implantitis: the disease of the future. Quintessence Int Berl Ger 1985. 2013 Oct;44(9):643. doi: 10.3290/j.qi.a30508. [DOI] [PubMed] [Google Scholar]
- 19.Norowski PA, Bumgardner JD. Biomaterial and antibiotic strategies for peri-implantitis: a review. J Biomed Mater Res B Appl Biomater. 2009 Feb;88(2):530–43. doi: 10.1002/jbm.b.31152. [DOI] [PubMed] [Google Scholar]
- 20.Bumgardner JD, Adatrow P, Haggard WO, Norowski PA. Emerging antibacterial biomaterial strategies for the prevention of peri-implant inflammatory diseases. Int J Oral Maxillofac Implants. 2011 Jun;26(3):553–60. [PubMed] [Google Scholar]
- 21.Adler L, Liedholm E, Silvegren M, Modin C, Buhlin K, Jansson L. Patient satisfaction 8–14 years after dental implant therapy - a questionnaire study. Acta Odontol Scand. 2016 Jul;74(5):423–9. doi: 10.1080/00016357.2016.1177661. [DOI] [PubMed] [Google Scholar]
- 22.Testori T, Clauser C, Deflorian M, Capelli M, Zuffetti F, Fabbro MD. A Retrospective Analysis of the Effectiveness of the Longevity Protocol for Assessing the Risk of Implant Failure. Clin Implant Dent Relat Res. 2016 Dec;18(6):1113–8. doi: 10.1111/cid.12428. [DOI] [PubMed] [Google Scholar]
- 23.Jemt T, Karouni M, Abitbol J, Zouiten O, Antoun H. A retrospective study on 1592 consecutively performed operations in one private referral clinic. Part II: Peri-implantitis and implant failures. Clin Implant Dent Relat Res. 2017 Jun;19(3):413–22. doi: 10.1111/cid.12481. [DOI] [PubMed] [Google Scholar]
- 24.Apaza-Bedoya K, Tarce M, Benfatti CAM, Henriques B, Mathew MT, Teughels W, et al. Synergistic interactions between corrosion and wear at titanium-based dental implant connections: A scoping review. J Periodontal Res. 2017 Dec;52(6):946–54. doi: 10.1111/jre.12469. [DOI] [PubMed] [Google Scholar]
- 25.Quirynen M, van Steenberghe D. Bacterial colonization of the internal part of two-stage implants. An in vivo study. Clin Oral Implants Res. 1993 Sep;4(3):158–61. doi: 10.1034/j.1600-0501.1993.040307.x. [DOI] [PubMed] [Google Scholar]
- 26.Dibart S, Warbington M, Su MF, Skobe Z. In vitro evaluation of the implant-abutment bacterial seal: the locking taper system. Int J Oral Maxillofac Implants. 2005 Oct;20(5):732–7. [PubMed] [Google Scholar]
- 27.Prado AM, Pereira J, Henriques B, Benfatti CA, Magini RS, López-López J, et al. Biofilm Affecting the Mechanical Integrity of Implant-Abutment Joints. Int J Prosthodont. 2016 Aug;29(4):381–3. doi: 10.11607/ijp.4759. [DOI] [PubMed] [Google Scholar]
- 28.Souza JCM, Henriques M, Oliveira R, Teughels W, Celis J-P, Rocha LA. Biofilms inducing ultra-low friction on titanium. J Dent Res. 2010 Dec;89(12):1470–5. doi: 10.1177/0022034510378428. [DOI] [PubMed] [Google Scholar]
- 29.Pereira J, Morsch CS, Henriques B, Nascimento RM, Benfatti CA, Silva FS, et al. Removal Torque and Biofilm Accumulation at Two Dental Implant-Abutment Joints After Fatigue. Int J Oral Maxillofac Implants. 2016 Aug;31(4):813–9. doi: 10.11607/jomi.4173. [DOI] [PubMed] [Google Scholar]
- 30.Schwarz MS. Mechanical complications of dental implants. Clin Oral Implants Res. 2000;11(Suppl 1):156–8. doi: 10.1034/j.1600-0501.2000.011s1156.x. [DOI] [PubMed] [Google Scholar]
- 31.Phan K, Mobbs RJ. Evolution of Design of Interbody Cages for Anterior Lumbar Interbody Fusion. Orthop Surg. 2016 Aug;8(3):270–7. doi: 10.1111/os.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Kunder S, Rijkers K, Caelers IJMH, de Bie RA, Koehler PJ, van Santbrink H. Lumbar Interbody Fusion, A Historical Overview and A Future Perspective. Spine [Internet] 2017 Dec 27; doi: 10.1097/BRS.0000000000002534. [cited 2017 Dec 29];Publish Ahead of Print. Available from: http://journals.lww.com/spinejournal/Abstract/publishahead/Lumbar_Interbody_Fusion,_A_Historical_Overview_and.95245.aspx. [DOI] [PubMed]
- 33.Weinstein JN, Lurie JD, Olson PR, Bronner KK, Fisher ES. United States’ trends and regional variations in lumbar spine surgery: 1992–2003. Spine. 2006 Nov 1;31(23):2707–14. doi: 10.1097/01.brs.0000248132.15231.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duarte RM, Varanda P, Reis RL, Duarte ARC, Correia-Pinto J. Biomaterials and Bioactive Agents in Spinal Fusion. Tissue Eng Part B Rev. 2017 Dec;23(6):540–51. doi: 10.1089/ten.TEB.2017.0072. [DOI] [PubMed] [Google Scholar]
- 35.Albrektsson T, Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2001 Oct;10(Suppl 2):S96–101. doi: 10.1007/s005860100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du L, Sun X, Zhou T, Li Y, Chen C, Zhao C, et al. The role of cage height on the flexibility and load sharing of lumbar spine after lumbar interbody fusion with unilateral and bilateral instrumentation: a biomechanical study. BMC Musculoskelet Disord [Internet] 2017 Nov 21;18 doi: 10.1186/s12891-017-1845-1. [cited 2017 Dec 29]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5696757/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury. 2011 Sep;42(Suppl 2):S3–15. doi: 10.1016/j.injury.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 38.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998 Nov 6;282(5391):1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 39.Hogan MS, Parfitt D-E, Zepeda-Mendoza CJ, Shen MM, Spector DL. Transient pairing of homologous Oct4 alleles accompanies the onset of embryonic stem cell differentiation. Cell Stem Cell. 2015 Mar 5;16(3):275–88. doi: 10.1016/j.stem.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006 Aug 25;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 41.Tsai Y, Lu B, Bakondi B, Girman S, Sahabian A, Sareen D, et al. Human iPSC-Derived Neural Progenitors Preserve Vision in an AMD-Like Model. Stem Cells Dayt Ohio. 2015 Aug;33(8):2537–49. doi: 10.1002/stem.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colasante G, Lignani G, Rubio A, Medrihan L, Yekhlef L, Sessa A, et al. Rapid Conversion of Fibroblasts into Functional Forebrain GABAergic Interneurons by Direct Genetic Reprogramming. Cell Stem Cell. 2015 Dec 3;17(6):719–34. doi: 10.1016/j.stem.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Denton KR, Lei L, Grenier J, Rodionov V, Blackstone C, Li X-J. Loss of spastin function results in disease-specific axonal defects in human pluripotent stem cell-based models of hereditary spastic paraplegia. Stem Cells Dayt Ohio. 2014 Feb;32(2):414–23. doi: 10.1002/stem.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahla RS. Stem Cells Applications in Regenerative Medicine and Disease Therapeutics. Int J Cell Biol [Internet] 2016;2016 doi: 10.1155/2016/6940283. [cited 2017 Dec 26]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4969512/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DiMarino AM, Caplan AI, Bonfield TL. Mesenchymal Stem Cells in Tissue Repair. Front Immunol [Internet] 2013;4 doi: 10.3389/fimmu.2013.00201. [cited 2017 Dec 26]; Available from: https://www.frontiersin.org/articles/10.3389/fimmu.2013.00201/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46••.Frank RM, Mascarenhas R, Romeo AA. The use of platelet-rich plasma in joint replacement surgery. Semin Arthroplasty. 2014 Mar 1;25(1):28–37. This review article describes about autologous platelet rich plasma (PRP) and their use in orthopedic surgery. The subject is controversial and the manuscript seeks attention towards the basic science and experimental evidence for PRP use in joint replacement surgery. [Google Scholar]
- 47.Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004 Jan;91(1):4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 48.Anitua E, Andí I, Sanchez M, Azofra J, del Mar Zalduendo M, de la Fuente M, et al. Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF production by human tendon cells in culture. J Orthop Res. 2005 Mar 1;23(2):281–6. doi: 10.1016/j.orthres.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 49.El-Sharkawy H, Kantarci A, Deady J, Hasturk H, Liu H, Alshahat M, et al. Platelet-Rich Plasma: Growth Factors and Pro- and Anti-Inflammatory Properties. J Periodontol. 2007 Mar 15;78(4):661–9. doi: 10.1902/jop.2007.060302. [DOI] [PubMed] [Google Scholar]
- 50.Harris NL, Huffer WE, von Stade E, Larson AI, Phinney S, Purnell ML. The effect of platelet-rich plasma on normal soft tissues in the rabbit. J Bone Joint Surg Am. 2012 May 2;94(9):786–93. doi: 10.2106/JBJS.J.00984. [DOI] [PubMed] [Google Scholar]
- 51.Fong ELS, Chan CK, Goodman SB. Stem cell homing in musculoskeletal injury. Biomaterials. 2011 Jan;32(2):395–409. doi: 10.1016/j.biomaterials.2010.08.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruder SP, Kraus KH, Goldberg VM, Kadiyala S. The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J Bone Joint Surg Am. 1998 Jul;80(7):985–96. doi: 10.2106/00004623-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 53.Mardones R, Larrain C. Cartilage restoration technique of the hip. J Hip Preserv Surg. 2015 Oct 1;3(1):30–6. doi: 10.1093/jhps/hnv061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells Dayt Ohio. 2014 May;32(5):1254–66. doi: 10.1002/stem.1634. [DOI] [PubMed] [Google Scholar]
- 55.Diekman BO, Guilak F. Stem cell-based therapies for osteoarthritis: challenges and opportunities. Curr Opin Rheumatol. 2013 Jan;25(1):119–26. doi: 10.1097/BOR.0b013e32835aa28d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ando W, Heard BJ, Chung M, Nakamura N, Frank CB, Hart DA. Ovine synovial membrane-derived mesenchymal progenitor cells retain the phenotype of the original tissue that was exposed to in-vivo inflammation: evidence for a suppressed chondrogenic differentiation potential of the cells. Inflamm Res Off J Eur Histamine Res Soc Al. 2012 Jun;61(6):599–608. doi: 10.1007/s00011-012-0450-x. [DOI] [PubMed] [Google Scholar]
- 57.Boeuf S, Graf F, Fischer J, Moradi B, Little CB, Richter W. Regulation of aggrecanases from the ADAMTS family and aggrecan neoepitope formation during in vitro chondrogenesis of human mesenchymal stem cells. Eur Cell Mater. 2012 May 4;23:320–32. doi: 10.22203/ecm.v023a25. [DOI] [PubMed] [Google Scholar]
- 58.Brunger JM, Zutshi A, Willard VP, Gersbach CA, Guilak F. Genome Engineering of Stem Cells for Autonomously Regulated, Closed-Loop Delivery of Biologic Drugs. Stem Cell Rep. 2017 May 9;8(5):1202–13. doi: 10.1016/j.stemcr.2017.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59•.Zhao D, Liu B, Wang B, Yang L, Xie H, Huang S, et al. Autologous Bone Marrow Mesenchymal Stem Cells Associated with Tantalum Rod Implantation and Vascularized Iliac Grafting for the Treatment of End-Stage Osteonecrosis of the Femoral Head. BioMed Res Int [Internet] 2015;2015 doi: 10.1155/2015/240506. [cited 2017 Dec8]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4352743/ The study provides experimental evidence for the regeneration of end stage osteonecrotic femoral head by the application of autologous bone marrow cells along with tantalum implant, which is a regenerative strategy in biomedical implantation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Filho Cerruti H, Kerkis I, Kerkis A, Tatsui NH, da Costa Neves A, Bueno DF, et al. Allogenous bone grafts improved by bone marrow stem cells and platelet growth factors: clinical case reports. Artif Organs. 2007 Apr;31(4):268–73. doi: 10.1111/j.1525-1594.2007.00374.x. [DOI] [PubMed] [Google Scholar]
- 61.Kaigler D, Avila-Ortiz G, Travan S, Taut AD, Padial-Molina M, Rudek I, et al. Bone Engineering of Maxillary Sinus Bone Deficiencies Using Enriched CD90+ Stem Cell Therapy: A Randomized Clinical Trial. J Bone Miner Res Off J Am Soc Bone Miner Res. 2015 Jul;30(7):1206–16. doi: 10.1002/jbmr.2464. [DOI] [PubMed] [Google Scholar]
- 62.McAllister BS. Stem cell-containing allograft matrix enhances periodontal regeneration: case presentations. Int J Periodontics Restorative Dent. 2011 Apr;31(2):149–55. [PubMed] [Google Scholar]
- 63.McAllister BS, Eshraghi VT. Alveolar Ridge Augmentation With Allograft Stem Cell–Based Matrix and Titanium Mesh. Clin Adv Periodontics. 2012 Apr 23;3(1):1–7. [Google Scholar]
- 64.Koo S, Alshihri A, Karimbux NY, Maksoud M. Cellular Allograft in the Treatment of a Severe Periodontal Intrabony Defect: A Case Report. Clin Adv Periodontics. 2012 Jan 30;2(1):35–9. doi: 10.1902/cap.2011.110017. [DOI] [PubMed] [Google Scholar]
- 65.Rickert D, Sauerbier S, Nagursky H, Menne D, Vissink A, Raghoebar GM. Maxillary sinus floor elevation with bovine bone mineral combined with either autogenous bone or autogenous stem cells: a prospective randomized clinical trial. Clin Oral Implants Res. 2011 Mar;22(3):251–8. doi: 10.1111/j.1600-0501.2010.01981.x. [DOI] [PubMed] [Google Scholar]
- 66.Gonshor A, McAllister BS, Wallace SS, Prasad H. Histologic and histomorphometric evaluation of an allograft stem cell-based matrix sinus augmentation procedure. Int J Oral Maxillofac Implants. 2011 Feb;26(1):123–31. [PubMed] [Google Scholar]
- 67.Zhou Y, Chen F, Ho ST, Woodruff MA, Lim TM, Hutmacher DW. Combined marrow stromal cell-sheet techniques and high-strength biodegradable composite scaffolds for engineered functional bone grafts. Biomaterials. 2007 Feb 1;28(5):814–24. doi: 10.1016/j.biomaterials.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 68.Zhou W, Han C, Song Y, Yan X, Li D, Chai Z, et al. The performance of bone marrow mesenchymal stem cell – Implant complexes prepared by cell sheet engineering techniques. Biomaterials. 2010 Apr 1;31(12):3212–21. doi: 10.1016/j.biomaterials.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 69.Nakamura A, Akahane M, Shigematsu H, Tadokoro M, Morita Y, Ohgushi H, et al. Cell sheet transplantation of cultured mesenchymal stem cells enhances bone formation in a rat nonunion model. Bone. 2010 Feb 1;46(2):418–24. doi: 10.1016/j.bone.2009.08.048. [DOI] [PubMed] [Google Scholar]
- 70.Iwata T, Yamato M, Tsuchioka H, Takagi R, Mukobata S, Washio K, et al. Periodontal regeneration with multi-layered periodontal ligament-derived cell sheets in a canine model. Biomaterials. 2009 May 1;30(14):2716–23. doi: 10.1016/j.biomaterials.2009.01.032. [DOI] [PubMed] [Google Scholar]
- 71••.Lee D-J, Lee J-M, Kim E-J, Takata T, Abiko Y, Okano T, et al. Bio-implant as a novel restoration for tooth loss. Sci Rep. 2017 Aug 7;7(1):7414. doi: 10.1038/s41598-017-07819-z. In this study, the authors generate a multi layered cell sheets by utilizing three different engineered cell corresponds to periodontium and wrapped around hydroxyapatite coated titanium screw as a novel restoration bio-implant for tooth loss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pape HC, Evans A, Kobbe P. Autologous Bone Graft: Properties and Techniques. J Orthop Trauma [Internet] 2010 Mar 1;24 doi: 10.1097/BOT.0b013e3181cec4a1. [cited 2017 Dec 29]; Available from: https://insights-ovid-com.proxy.cc.uic.edu/pubmed?pmid=20182233. [DOI] [PubMed] [Google Scholar]
- 73.Hsu WK, Goldstein CL, Shamji MF, Cho SK, Arnold PM, Fehlings MG, et al. Novel Osteobiologics and Biomaterials in the Treatment of Spinal Disorders. Neurosurgery. 2017 Mar 1;80(3S):S100–7. doi: 10.1093/neuros/nyw085. [DOI] [PubMed] [Google Scholar]
- 74.Hassan N, McCarville K, Morinaga K, Mengatto CM, Langfelder P, Hokugo A, et al. Titanium biomaterials with complex surfaces induced aberrant peripheral circadian rhythms in bone marrow mesenchymal stromal cells. PloS One. 2017;12(8):e0183359. doi: 10.1371/journal.pone.0183359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen D, Zhao M, Mundy GR. Bone Morphogenetic Proteins. Growth Factors. 2004 Dec;22(4):233–41. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 76.Chen G, Deng C, Li Y-P. TGF-β and BMP Signaling in Osteoblast Differentiation and Bone Formation. Int J Biol Sci. 2012 Jan 21;8(2):272–88. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hustedt JW, Blizzard DJ. The Controversy Surrounding Bone Morphogenetic Proteins in the Spine: A Review of Current Research. Yale J Biol Med. 2014 Dec 12;87(4):549–61. [PMC free article] [PubMed] [Google Scholar]
- 78.Urist MR. Bone: Formation by Autoinduction. Science. 1965;150(3698):893–9. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 79.Mroz TE, Wang JC, Hashimoto R, Norvell DC. Complications related to osteobiologics use in spine surgery: a systematic review. Spine. 2010;35(9S):S86–S104. doi: 10.1097/BRS.0b013e3181d81ef2. [DOI] [PubMed] [Google Scholar]
- 80.Lao L, Cohen JR, Buser Z, Brodke DS, Youssef JA, Park J-B, et al. Trends Analysis of rhBMP Utilization in Single-Level Posterior Lumbar Interbody Fusion in the United States. Glob Spine J. 2017 Oct;7(7):624–8. doi: 10.1177/2192568217699387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boden SD, Zdeblick TA, Sandhu HS, Heim SE. The use of rhBMP-2 in interbody fusion cages: definitive evidence of osteoinduction in humans: a preliminary report. Spine. 2000;25(3):376–381. doi: 10.1097/00007632-200002010-00020. [DOI] [PubMed] [Google Scholar]
- 82.Agrawal V, Sinha M. A review on carrier systems for bone morphogenetic protein-2. J Biomed Mater Res B Appl Biomater. 2017 May 1;105(4):904–25. doi: 10.1002/jbm.b.33599. [DOI] [PubMed] [Google Scholar]
- 83.Bouyer M, Guillot R, Lavaud J, Plettinx C, Olivier C, Curry V, et al. Surface delivery of tunable doses of BMP-2 from an adaptable polymeric scaffold induces volumetric bone regeneration. Biomaterials. 2016 Oct;104:168–81. doi: 10.1016/j.biomaterials.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shiels SM, Talley AD, McGough MAP, Zienkiewicz KJ, Kalpakci K, Shimko D, et al. Injectable and compression-resistant low-viscosity polymer/ceramic composite carriers for rhBMP-2 in a rabbit model of posterolateral fusion: a pilot study. J Orthop Surg [Internet] 2017 Jul 11;12 doi: 10.1186/s13018-017-0613-0. [cited 2017 Dec 29]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5504717/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hu T, Abbah SA, Toh SY, Wang M, Lam RWM, Naidu M, et al. Bone marrow-derived mesenchymal stem cells assembled with low-dose BMP-2 in a three-dimensional hybrid construct enhances posterolateral spinal fusion in syngeneic rats. Spine J. 2015 Dec;15(12):2552–63. doi: 10.1016/j.spinee.2015.08.063. [DOI] [PubMed] [Google Scholar]
- 86.Seo B-B, Koh J-T, Song S-C. Tuning physical properties and BMP-2 release rates of injectable hydrogel systems for an optimal bone regeneration effect. Biomaterials. 2017 Apr;122:91–104. doi: 10.1016/j.biomaterials.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 87.Vergroesen P-PA, Kroeze R-J, Helder MN, Smit TH. The Use of Poly(L-lactide-co-caprolactone) as a Scaffold for Adipose Stem Cells in Bone Tissue Engineering: Application in a Spinal Fusion Model. Macromol Biosci. 2011 Jun 14;11(6):722–30. doi: 10.1002/mabi.201000433. [DOI] [PubMed] [Google Scholar]
- 88.Liu Z, Zhu Y, Ge R, Zhu J, He X, Yuan X, et al. Combination of bone marrow mesenchymal stem cells sheet and platelet rich plasma for posterolateral lumbar fusion. Oncotarget. 2017 Jul 31;8(37):62298–311. doi: 10.18632/oncotarget.19749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang W, Dong Y, Hong Y, Guang Q, Chen X. Evaluation of anterior vertebral interbody fusion using osteogenic mesenchymal stem cells transplanted in collagen sponge. Clin Spine Surg. 2016;29(4):E201–E207. doi: 10.1097/BSD.0b013e31825ca123. [DOI] [PubMed] [Google Scholar]
- 90.Kroeze RJ, Smit TH, Vergroesen PP, Bank RA, Stoop R, van Rietbergen B, et al. Spinal fusion using adipose stem cells seeded on a radiolucent cage filler: a feasibility study of a single surgical procedure in goats. Eur Spine J. 2015 May 1;24(5):1031–42. doi: 10.1007/s00586-014-3696-x. [DOI] [PubMed] [Google Scholar]
- 91.Duarte RM, Varanda P, Reis RL, Duarte ARC, Correia-Pinto J. Biomaterials and Bioactive Agents in Spinal Fusion. Tissue Eng Part B Rev. 2017 May 17;23(6):540–51. doi: 10.1089/ten.TEB.2017.0072. [DOI] [PubMed] [Google Scholar]


