Abstract
Small-molecule inhibitors are widely used to treat a variety of inflammatory diseases. In this study, we found a novel anti-inflammatory compound, 1-[(2R,4S)-2-methyl-4-(phenylamino)-1,2,3,4-tetrahydroquinolin-1-yl]prop-2-en-1-one (MPQP). It showed strong anti-inflammatory effects in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages. These effects were exerted through the inhibition of the production of NO and pro-inflammatory cytokines, such as interleukin (IL)-6, IL-1β, and tumor necrosis factor-α (TNF-α). Furthermore, MPQP decreased the expression levels of inducible NO synthase (iNOS) and cyclooxygenase 2 (COX-2). Additionally, it mediated the inhibition of the phosphorylation of p38, c-Jun N-terminal kinase (JNK), the inhibitor of κBα (IκBα), and their upstream kinases, IκB kinase (IKK) α/β, mitogen-activated protein kinase kinase (MKK) 3/6, and MKK4. Furthermore, the expression of IL-1 receptor-associated kinase 1 (IRAK1) that regulates NF-κB, p38, and the JNK signaling pathways, was also increased by MPQP. These results indicate that MPQP regulates the IRAK1-mediated inflammatory signaling pathways by targeting IRAK1 or its upstream factors.
Keywords: Cyclooxygenase 2; IL-1 receptor-associated kinase 1; Inflammation; Inflammatory cytokine; 1-[(2R,4S)-2-methyl-4-(phenylamino)-1,2,3,4-tetrahydroquinolin-1-yl]prop-2-en-1-one
INTRODUCTION
Inflammation plays a key role in eliminating the initial causes of tissue injury and harmful stimuli such as pathogens and damaged cells (1). Inflammation is initiated by several immune cells including macrophages, dendritic cells, and monocytes upon stimulation (2). These immune cells produce various inflammatory mediators including nitric oxide (NO), prostaglandin E2 (PGE2), and pro-inflammatory cytokines such as interleukin (IL)-6, IL-1β, and tumor necrosis factor-α (TNF-α). These mediators induce the classical symptoms of inflammation including pain, heat, and swelling (3). However, the excessive production of pro-inflammatory mediators results in cellular damage and causes various inflammatory diseases (4). Therefore, the suppression of pro-inflammatory mediators is an attractive therapeutic strategy for the treatment of various inflammatory diseases.
The pro-inflammatory mediators are regulated by several signaling pathways including the nuclear factor-κB (NF-κB) and the mitogen-activated protein kinases (MAPKs). Upon stimulation of the macrophages with lipopolysaccharide (LPS), NF-κB and MAPKs are activated by cascades including IL-1 receptor-associated protein kinases (IRAKs) and the transforming growth factor-β-activated kinase 1 (TAK1). In the LPS-induced inflammation, the toll-like receptor 4 (TLR4) recognizes LPS and causes the activation of downstream signaling factors including myeloid differentiation factor 88 (MyD88), IRAKs, and TAK1 (5). When LPS binds to TLR4, the latter recruits MyD88, IRAKs, and other adaptor molecules. Association with MyD88 leads to IRAK4 activation and the phosphorylation of IRAK1 by IRAK4 (6). Phosphorylated IRAK1 activates the TRAF6/TAK1 complex to activate the downstream protein kinases, which are inhibitors of κB kinase (IKK) and MAPKs (6, 7). IRAK1 is then polyubiquitinated by the IRAK1-activated E3 ligase and degraded by proteasomes (8). Activated IKK and MAPKs upregulate various transcription factors including NF-κB and activator protein-1 (AP-1) that induce the expression of pro-inflammatory mediators.
Small-molecule inhibitors are used for the treatment of various diseases and as tools to study the mechanisms of various cellular processes through the regulation of specific targets (9). In addition, small-molecule inhibitors are more stable than larger molecules (such as monoclonal antibodies), are generally cell-permeable, and can be easily used to compare, in vivo, the activity of analogs with diverse in vitro activities (9, 10). Previous studies have revealed a variety of small-molecule inhibitors with anti-inflammatory properties (11, 12). In this study, we screened 4,160 chemicals using NO production assays and found a novel small-molecule inhibitor, 1-[(2R,4S)-2-methyl-4-(phenylamino)-1,2,3,4-tetrahydroquinoli n-1-yl] prop-2-en-1-one, named MPQP (Fig. 1A). Its inhibitory effects and the possible mechanism of action were studied by assessing the inflammatory signal transduction in LPS-stimulated RAW 264.7 macrophages.
Fig. 1.
Chemical structure and inhibitory effects of MPQP on the NO production without cytotoxicity. (A) Chemical structure of MPQP. (B, C, D, E, and F) RAW 264.7 macrophages were incubated with MPQP (1, 2, 5, and 10 μM) for 2 h and then stimulated with LPS for the indicated time periods. (B) After LPS (1 μg/ml) stimulation for 24 h, cell viability was measured using EZ-Cytox solution. Cell viability values are shown as bar graphs compared to the LPS-treated group (100%). (C) After 24 h LPS (1 μg/ml) stimulation, the NO production levels were measured using Griess reagents and are shown as bar graphs according to the standard curve calculated on the basis of the nitrite standard solution. (D and E) After 3 h LPS (100 ng/ml) stimulation, total RNA was extracted and reverse transcribed to cDNA. (D) iNOS was amplified by qPCR and the expression levels of iNOS are shown as bar graphs compared to the LPS-treated group (100%). (E) iNOS was amplified by PCR and visualized using EtBr staining. The relative expression level of iNOS was normalized to GAPDH levels. Quantitative analyses of mRNA expression levels are shown as fold changes following normalization. (F) Total cell lysates were prepared after 24 h LPS (1 μg/ml) stimulation and analyzed by immunoblot analysis. The expression levels of iNOS were detected using specific antibodies. The relative expression levels of iNOS were normalized to α-tubulin levels. Quantitative analyses of the protein levels are shown as fold changes following normalization. The relative expression levels of iNOS are presented as a bar graph. All bar graphs are represented as the mean ± SEM and analyzed using one-way ANOVA for the significance between the three independent experiments. #P < 0.0001 vs. LPS-untreated control groups. aP < 0.01 and bP < 0.001 vs. LPS-treated groups.
RESULTS AND DISCUSSION
MPQP inhibits NO production in RAW 264.7 macrophages
Changes in the LPS-induced expression of pro-inflammatory mediators at the mRNA and protein levels in RAW 264.7 macrophages were measured by dose-dependent analysis to establish appropriate experimental conditions for evaluation of the anti-inflammatory effect of the chemicals. The mRNA expression levels of pro-inflammatory mediators (iNOS, COX-2, IL-6, IL-1β, and TNF-α) were increased until LPS concentration reached 100 ng/ml, but they were not increased any more at higher concentrations (Supplementary Fig. 1A). However, protein expression levels of iNOS and COX-2 were increased until LPS concentration reached 1 μg/ml of LPS (Supplementary Fig. 1B). We, therefore, used 100 ng/ml LPS for detection of mRNA and 1 μg/ml LPS for detection of protein in subsequent experiments, respectively.
NO assay was used as the primary screening tool to discover novel inflammatory inhibitors since NO analysis is less expensive, easier, and faster than other methods. We screened 4,160 chemicals for inhibitory effects on LPS-induced NO production and cell viability using RAW 264.7 macrophages at a constant concentration of 10 μM. Among candidates that inhibited NO production and showed no effect on cell viability, MPQP exhibited strong inhibitory effect on NO production compared to the LPS-control group.
To further evaluate the inhibitory effects of MPQP in LPS-induced inflammation, RAW 264.7 macrophages were pre-treated with MPQP at the indicated concentrations. MPQP was observed to significantly reduce the LPS-induced NO production in a dose-dependent manner without causing cytotoxicity (Fig. 1B and C). Since NO is synthesized by inducible NO synthase (iNOS) on LPS stimulation, we tested the effects of MPQP on iNOS mRNA expression. The mRNA expression levels of iNOS were measured using RT-qPCR and semiquantitative PCR, as previously described (13). The decrease in NO levels was due to the reduced mRNA expression of the iNOS gene as shown by RT-qPCR (Fig. 1D). Semiquantitative PCR data verified the inhibitory effects of MPQP on the LPS-induced iNOS mRNA expression (Fig. 1E). Reduced iNOS mRNA expression resulted in the decreased expression of iNOS at the protein level (Fig. 1F). These results suggest that MPQP suppresses the LPS-induced NO production in RAW 264.7 macrophages through the regulation of the iNOS gene expression.
MPQP reduces cyclooxygenase 2 (COX-2) expression in RAW 264.7 macrophages
Next, we measured the COX-2 expression levels in the MPQP-treated macrophages since COX-2 is a general inflammatory mediator. As an enzyme that synthesizes PGE2, it leads to inflammatory responses such as redness, swelling, and pain (14). MPQP significantly decreased the COX-2 gene expression as determined by RT-qPCR (Fig. 2A). Additionally, semiquantitative RT-PCR showed that MPQP reduced the LPS-induced COX-2 mRNA expression levels (Fig. 2B). Due to the decreased mRNA expression of COX-2, MPQP treatment led to a decreased expression of COX-2 at the protein level (Fig. 2C). These results indicate that MPQP may play a role in the regulation of PGE2 production by inhibiting the COX-2 gene expression at the transcription level.
Fig. 2.

Inhibitory effects of MPQP on the expression levels of COX-2 and the production of pro-inflammatory cytokines. RAW 264.7 macrophages were incubated with MPQP (1, 2, 5, and 10 μM) for 2 h and stimulated with LPS for the indicated time periods. (A, B, E, and F) After 3 h LPS (100 ng/ml) stimulation, total RNA was extracted and reverse transcribed to cDNA. (A) COX-2 was amplified by qPCR and the expression levels of COX-2 are shown as bar graphs compared to the LPS-treated group (100%). (B) COX-2 was amplified by PCR and visualized by EtBr staining. The relative expression level of COX-2 was normalized to the GAPDH levels. Quantitative analyses of mRNA expression levels are shown as fold changes following normalization. (C) Total cell lysates were prepared after 24 h LPS (1 μg/ml) stimulation and analyzed by immunoblot analysis. The expression levels of COX-2 were detected using specific antibodies. The relative expression levels of COX-2 were normalized to α-tubulin levels. Quantitative analyses of protein levels are shown as fold changes after normalization. (D) After 24 h LPS (1 μg/ml) stimulation, ELISA was carried out to measure the levels of TNF-α and IL-6. The production of each cytokine was measured using a standard curve. (E) IL-1β, IL-6, and TNF-α were amplified by qPCR and their expression levels are shown as bar graphs compared to the LPS-treated group (100%). (F) IL-1β, IL-6, and TNF-α were amplified by PCR and visualized by EtBr staining. The relative expression levels of IL-1β, IL-6, and TNF-α were normalized to GAPDH levels. Quantitative analyses of the mRNA expression levels are shown as fold changes following normalization. All bar graphs are represented as the mean ± SEM and analyzed using one-way ANOVA from three independent experiments. #P < 0.0001 vs. LPS-untreated control groups. aP < 0.01 and bP < 0.001 vs LPS-treated groups.
MPQP suppresses the production of pro-inflammatory cytokines in RAW 264.7 macrophages
The production of pro-inflammatory cytokines is an important process that accompanies LPS-stimulation in macrophages (15). Hence, we measured the effects of MPQP on the production of these pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α, in LPS-stimulated RAW 264.7 macrophages. As shown in Fig. 2D, MPQP treatment showed a dose-dependent decrease in TNF-α production. In addition, MPQP strongly inhibited the production of IL-6 (Fig. 2D). To verify whether MPQP regulates the LPS-induced production of the cytokines at the mRNA expression level, we tested its effects on the gene expression by RT-qPCR and semiquantitative RT-PCR. The mRNA expression of IL-1β, IL-6, and TNF-α in the RAW 264.7 macrophages was significantly reduced by MPQP treatment (Fig. 2E and F). These results suggest that MPQP modulates the transcription factors that are involved in the production of the pro-inflammatory cytokines in the LPS-stimulated RAW 264.7 macrophages.
Interestingly, MPQP showed differential inhibitory effects on the LPS-induced production of pro-inflammatory mediators (Fig. 1 and 2). In particular, the inhibitory effects on NO, COX-2, and IL-6 production was observed to be higher than those of the other mediators including IL-1β and TNF-α (Fig. 1 and 2). Since these effects were regulated at the mRNA expression level by several signaling pathways, we estimated that MPQP might differentially regulate each LPS-stimulated signaling pathway. There are several signaling pathways such as NF-κB and MAPKs, including extracellular signal-regulated kinase (ERK), p38, and c-Jun N-terminal kinases (JNK), that are activated by LPS stimulation. When these signaling pathways are suppressed by each specific inhibitor, different inhibitory effects on the production of the pro-inflammatory mediators were observed. For example, SP600125, a specific JNK inhibitor, significantly inhibits the LPS-induced production of NO, IL-6, IL-1β, and TNF-α, but not COX-2. On the other hand, specific ERK inhibitors (U0126 and PD98059) strongly decrease the production of COX-2 but have little effect on the production of IL-6 and TNF-α (16–18). Similar to these studies, our results suggest that MPQP probably has anti-inflammatory effects through the selective inhibition of specific signaling pathways.
MPQP inhibits IκBα, p38, and JNK phosphorylation in LPS-stimulated RAW 264.7 macrophages
In LPS-stimulated inflammation, the two major transcription factors, NF-κB and AP-1, are activated by LPS-induced upstream kinases such as IκBα and MAPKs. LPS-induced NF-κB activation occurs via the phosphorylation of IκBα at Ser-32/36 followed by degradation, which results in the release and nuclear translocation of NF-κB (19). AP-1 is a dimeric transcription factor composed of Jun, Fos, or ATF (activating transcription factor), and is upregulated through phosphorylation by MAPKs (20). MAPKs are activated through phosphorylation in the activation loops by MAPK kinases (21). To test the effects of MPQP on the LPS-induced activation of the NF-κB and MAPK signaling pathways, we analyzed the phosphorylation levels of IκBα and MAPKs in the LPS-stimulated RAW 264.7 macrophages upon treatment with MPQP. The phosphorylation levels of IκBα were attenuated and the IκBα expression was increased upon MPQP treatment in a dose-dependent manner (Fig. 3A). Furthermore, the phospho (p)-p38 and p-JNK levels were decreased after MPQP treatment without changing the total p38 and JNK levels, whereas p-ERK levels were observed to be unchanged (Fig. 3B). These results suggest that MPQP selectively inhibits pro-inflammatory responses through the inhibition of NF-κB, p38, and JNK signaling pathways.
Fig. 3.
Inhibitory effects of MPQP on LPS-induced NF-κB and MAPK pathways. RAW 264.7 macrophages were pre-incubated with MPQP (1, 2, 5, and 10 μM) for 2 h and stimulated with LPS (1 μg/ml) for 5 min (for detection of IκBα, IKKα/β, and IRAK1) or 15 min (for detection MAPKs, MKK3/6, and MKK4). Total cell lysates were prepared and analyzed by immunoblot analysis. The expression levels of (A) p-IκBα, IκBα, (B) p-JNK, JNK, p-ERK, ERK, p-p38, p38, (C) p-MKK3/6, MKK3/6, p-MKK4, MKK4, (D) IRAK1, p-IKKα/β, and IKKα/β were detected using specific antibodies. The relative expression levels of p-IκBα, IκBα, and IRAK1 were normalized to the α-tubulin levels. The phosphorylation levels of MAPKs, MKK3/6, MKK4, and IKKα/β were normalized to the corresponding MAPKs, MKK3/6, MKK4, and IKKα/β levels. Quantitative analyses of phosphorylation and protein levels are shown as bar graphs after normalization. All bar graphs are represented as the mean ± SEM and analyzed using one-way ANOVA from three independent experiments. #P < 0.0001 vs. LPS-untreated control groups. aP < 0.01 and bP < 0.001 vs LPS-treated groups.
MPQP suppresses the activation of upstream kinases including IKKα/β, MAPK kinase (MKK) 3/6, and IRAK1 in LPS-stimulated RAW 264.7 macrophages
Since MPQP inhibited the phosphorylation of IκBα, p38, and JNK, these molecular targets of MPQP were further investigated by examining their upstream kinases. Upon LPS stimulation, the phosphorylation of IκBα, p38, and JNK requires the activation of upstream kinases including IKKα/β, MKK3/6, and MKK4, respectively. Therefore, we examined whether MPQP was able to inhibit the phosphorylation of these upstream kinases. Treatment of RAW 264.7 macrophages with MPQP decreased p-IKKα/β, p-MKK3/6, and p-MKK4 in a dose-dependent manner. These results suggest that MPQP regulates a further upstream regulator that commonly modulates these signals (Fig. 3C and D). Since the activation of IKKα/β, MKK3/6, and MKK4 occurs after LPS-induced activation and degradation of IRAK1 (6, 7), we examined whether MPQP regulates the expression level of IRAK1. In RAW 264.7 macrophages, the expression level of IRAK1 was measured after LPS treatment. As a result, the expression level of IRAK1 decreased after 5 min of LPS treatment (Supplementary Fig. 1). When RAW 264.7 macrophages were pre-treated with MPQP prior to the LPS stimulation, MPQP suppressed the degradation of IRAK1 (Fig. 3D). These data suggest that MPQP might regulate the expression of pro-inflammatory mediators by acting on far upstream signaling proteins such as IRAK1 or its upstream factors (Fig. 4).
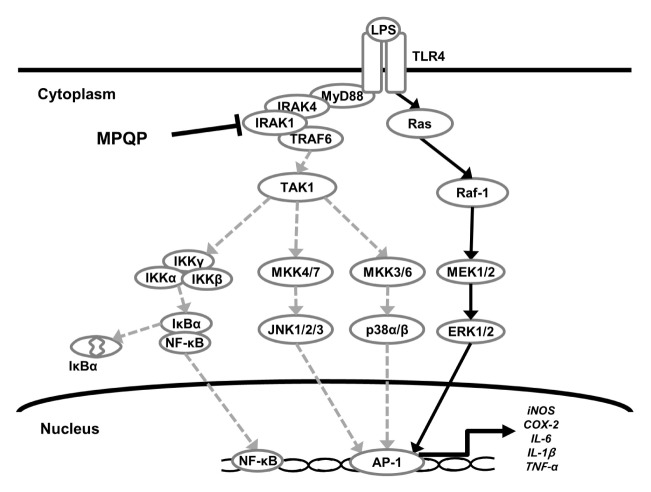
Fig. 4.
Schematic representation of the anti-inflammatory effects of MPQP on the LPS-induced inflammatory pathway. MPQP inhibited LPS-induced activation of IRAK1 or its upstream factors and sequentially suppressed the activation of downstream kinases. As a result of IRAK1 inhibition, the expression of pro-inflammatory mediators was decreased.
When we examined the effects of the IRAK specific inhibitor (N-[1-[2-(4-Morpholinyl)ethyl]-1H-benzimidazol-2-yl]-3-nitrobenzamide) on LPS-stimulated inflammation in RAW 264.7 macrophages, the IRAK inhibitor showed anti-inflammatory effects similar to MPQP in LPS-stimulated RAW 264.7 macrophages (Supplementary Fig. 3A and B). It reduced the phosphorylation of IκBα, p38, JNK, IKKα/β, MKK4, and MKK3/6 as well as degradation of IRAK1. In addition, the IRAK inhibitor significantly inhibited NO production without cytotoxicity (Supplementary Fig. 4A and B). Furthermore, the IRAK inhibitor showed stronger inhibitory effects on the LPS-induced expression of IL-6, IL-1β, and iNOS than COX-2 and TNF-α (Supplementary Fig. 4C and D), suggesting similar effects as MPQP.
In this study, we investigated the anti-inflammatory effects of MPQP, which is one of the many quinoline derivatives that possess diverse pharmacological properties including anti-microbial, analgesic, and anti-carcinogenic effects (22–24). There are several studies reporting the anti-inflammatory effects of quinoline derivatives (25). In general, quinoline derivatives modulate the inflammatory responses associated with TNF-α, thereby suppressing chronic inflammatory diseases such as psoriasis, Crohn’s disease, and rheumatoid arthritis (26, 27). However, the anti-inflammatory effects of MPQP are exhibited through the inhibition of LPS-induced IRAK1 activation. Reduction of inflammation by the inhibition of IRAK1 signaling may contribute to the regulation of chronic as well as acute inflammatory responses (28, 29).
MPQP inhibited IL-6 production more strongly than other inflammatory cytokines. IL-6 is involved in the development of various inflammatory diseases, including rheumatoid arthritis, cancer, and osteoporosis (30–32). So far, various peptide drugs including tocilizumab, which blocks the effect of IL-6 by inhibiting its binding to IL-6 receptor for the treatment of RA and systemic juvenile idiopathic arthritis, have been available (33, 34). However, the limitation of administration site based on the dosage form of peptide drugs led to the trial for the discovery of IL-6-inhibiting chemicals. Since MPQP significantly inhibited LPS-induced IL-6 production at low dose (1 μM) with little regulatory effects on the other inflammatory mediators in RAW 264.7 cells, MPQP has the potential as a therapeutic candidate for the treatment of IL-6-dominant inflammatory diseases.
Based on previous studies and our results, it is expected that MPQP could provide a new perspective on the regulation of LPS-stimulated inflammation by quinoline derivatives through the inhibition of LPS-induced IRAK1 activation. In conclusion, the results of this study suggest that MPQP might be useful in the treatment of various inflammatory diseases through the inhibition of IRAK1-mediated inflammatory signals.
MATERIALS AND METHODS
Supplementary Information
ACKNOWLEDGEMENTS
We thank the cooperation of Korea Chemical Bank to use chemical library for screening (http://www.chembank.or.kr). This work was supported by the National Research Foundation of Korea (NRF) grants funded by the Korean government (Ministry of Science and ICT) (NRF-2018R1A2B6005084 and 2015R1A5A1008958).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Kim E, Yang WS, Kim JH, et al. Lancemaside A from Codonopsis lanceolata modulates the inflammatory responses mediated by monocytes and macrophages. Mediators Inflamm. 20142014:405158–405158. doi: 10.1155/2014/405158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nowarski R, Gagliani N, Huber S, Flavell RA. Innate immune cells in inflammation and cancer. Cancer Immunol Res. 2013;1:77–84. doi: 10.1158/2326-6066.CIR-13-0081. [DOI] [PubMed] [Google Scholar]
- 3.Wu LC, Fan NC, Lin MH, et al. Anti- inflammatory effect of spilanthol from Spilanthes acmella on murine macrophage by down-regulating LPS-induced inflammatory mediators. J Agric Food Chem. 2008;56:2341–2349. doi: 10.1021/jf073057e. [DOI] [PubMed] [Google Scholar]
- 4.Libby P. Inflammatory mechanisms: the molecular basis of inflammation and disease. Nutr Rev. 2007;65:S140–S146. doi: 10.1301/nr.2007.dec.S140-S146. [DOI] [PubMed] [Google Scholar]
- 5.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–1525. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 6.Ordureau A, Smith H, Windheim M, et al. The IRAK-catalysed activation of the E3 ligase function of Pellino isoforms induces the Lys63-linked polyubiquitination of IRAK1. Biochem J. 2008;409:43–52. doi: 10.1042/BJ20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avila M, Gonzalez-Espinosa C. Signaling through Toll-like receptor 4 and mast cell-dependent innate immunity responses. IUBMB Life. 2011;63:873–880. doi: 10.1002/iub.555. [DOI] [PubMed] [Google Scholar]
- 8.Jiang Z, Ninomiya-Tsuji J, Qian Y, Matsumoto K, Li X. Interleukin-1 (IL-1) receptor-associated kinase-dependent IL-1-induced signaling complexes phosphorylate TAK1 and TAB2 at the plasma membrane and activate TAK1 in the cytosol. Mol Cell Biol. 2002;22:7158–7167. doi: 10.1128/MCB.22.20.7158-7167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schreiber SL. Chemical genetics resulting from a passion for synthetic organic chemistry. Biorg Med Chem. 1998;6:1127–1152. doi: 10.1016/S0968-0896(98)00126-6. [DOI] [PubMed] [Google Scholar]
- 10.Thompson LA, Ellman JA. Synthesis and applications of small molecule libraries. Chem Rev. 1996;96:555–600. doi: 10.1021/cr9402081. [DOI] [PubMed] [Google Scholar]
- 11.Chen W, Wang J, Luo Y, et al. Ginsenoside Rb1 and compound K improve insulin signaling and inhibit ER stress-associated NLRP3 inflammasome activation in adipose tissue. J Ginseng Res. 2016;40:351–358. doi: 10.1016/j.jgr.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cha BJ, Park JH, Shrestha S, et al. Glycosyl glycerides from hydroponic Panax ginseng inhibited NO production in lipopolysaccharide-stimulated RAW264. 7 cells. J Ginseng Res. 2015;39:162–168. doi: 10.1016/j.jgr.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho YC, Kim YR, Kim BR, Cho S. Thunbergia alata inhibits inflammatory responses through the inactivation of ERK and STAT3 in macrophages. Int J Mol Med. 2016;38:1596–1604. doi: 10.3892/ijmm.2016.2746. [DOI] [PubMed] [Google Scholar]
- 14.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Sci. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 15.Feghali CA, Wright TM. Cytokines in acute and chronic inflammation. Front Biosci. 1997;2:d12–d26. doi: 10.2741/A171. [DOI] [PubMed] [Google Scholar]
- 16.Ma P, Liu HT, Wei P, et al. Chitosan oligosaccharides inhibit LPS-induced over-expression of IL-6 and TNF-α in RAW264. 7 macrophage cells through blockade of mitogen-activated protein kinase (MAPK) and I3K/Akt signaling pathways. Carbohydr Polym. 2011;84:1391–1398. doi: 10.1016/j.carbpol.2011.01.045. [DOI] [Google Scholar]
- 17.Kwak HJ, Song JS, Heo JY, Yang SD, Nam JY, Cheon HG. Roflumilast inhibits lipopolysaccharide-induced inflammatory mediators via suppression of nuclear factor-κB, p38 mitogen-activated protein kinase, and c-Jun NH2-terminal kinase activation. J Pharmacol Exp Ther. 2005;315:1188–1195. doi: 10.1124/jpet.105.092056. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Tae N, Lee JJ, Kim T, Lee JH. Eupatolide inhibits lipopolysaccharide-induced COX-2 and iNOS expression in RAW264. 7 cells by inducing proteasomal degradation of TRAF6. Eur J Pharmacol. 2010;636:173–180. doi: 10.1016/j.ejphar.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 19.Finco TS, Beg AA, Baldwin AS. Inducible phosphorylation of I kappa B alpha is not sufficient for its dissociation from NF-kappa B and is inhibited by protease inhibitors. Proc Natl Acad Sci U S A. 1994;91:11884–11888. doi: 10.1073/pnas.91.25.11884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karin M, Liu Zg, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/S0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 21.Plotnikov A, Zehorai E, Procaccia S, Seger R. The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim Biophys Acta. 20111813:1619–1633. doi: 10.1016/j.bbamcr.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Neu HC. Quinolone antimicrobial agents. Annu Rev Med. 1992;43:465–486. doi: 10.1146/annurev.me.43.020192.002341. [DOI] [PubMed] [Google Scholar]
- 23.Abadi AH, Hegazy GH, El-Zaher AA. Synthesis of novel 4-substituted-7-trifluoromethylquinoline derivatives with nitric oxide releasing properties and their evaluation as analgesic and anti-inflammatory agents. Biorg Med Chem. 2005;13:5759–5765. doi: 10.1016/j.bmc.2005.05.053. [DOI] [PubMed] [Google Scholar]
- 24.Solomon VR, Lee H. Quinoline as a privileged scaffold in cancer drug discovery. Curr Med Chem. 2011;18:1488–1508. doi: 10.2174/092986711795328382. [DOI] [PubMed] [Google Scholar]
- 25.Kumar S, Bawa S, Gupta H. Biological activities of quinoline derivatives. Mini Rev Med Chem. 2009;9:1648–1654. doi: 10.2174/138955709791012247. [DOI] [PubMed] [Google Scholar]
- 26.Dinarello CA. Anti-inflammatory agents: present and future. Cell. 2010;140:935–950. doi: 10.1016/j.cell.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palladino MA, Bahjat FR, Theodorakis EA, Moldawer LL. Anti-TNF-α therapies: the next generation. Nature Reviews Drug Discovery. 2003;2:736–742. doi: 10.1038/nrd1175. [DOI] [PubMed] [Google Scholar]
- 28.Kim SJ, Cha JY, Kang HS, et al. Corosolic acid ameliorates acute inflammation through inhibition of IRAK-1 phosphorylation in macrophages. BMB Rep. 2016;49:276–281. doi: 10.5483/BMBRep.2016.49.5.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li M, Yu D, Ni B, Hao F. Interleukin-1 receptor associated kinase 1 is a potential therapeutic target of anti-inflammatory therapy for systemic lupus erythematosus. Mol Immunol. 2017;87:94–101. doi: 10.1016/j.molimm.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 30.Nishimoto N. Interleukin-6 in rheumatoid arthritis. Curr Opin Rheumatol. 2006;18:277–281. doi: 10.1097/01.bor.0000218949.19860.d1. [DOI] [PubMed] [Google Scholar]
- 31.Anestakis D, Petanidis S, Kalyvas S, et al. Mechanisms and applications of interleukins in cancer immunotherapy. Int J Mol Sci. 2015;16:1691–1710. doi: 10.3390/ijms16011691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheidt-Nave C, Bismar H, Leidig-Bruckner G, et al. Serum interleukin 6 is a major predictor of bone loss in women specific to the first decade past menopause. J Clin Endocrinol Metab. 2001;86:2032–2042. doi: 10.1210/jcem.86.5.7445. [DOI] [PubMed] [Google Scholar]
- 33.Smolen JS, Maini RN. Interleukin-6: a new therapeutic target. Arthrit Res Ther. 2006;8:S5. doi: 10.1186/ar1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emery P, Keystone E, Tony H, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67:1516–1523. doi: 10.1136/ard.2008.092932. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.