Abstract
T cell-dependent B cell responses typically develop in germinal centers. Antibodies generated during such responses are isotype-switched and high-affinity to the antigen due to somatic hypermutation of antibody genes. B cell responses to purified polysaccharides are T cell-independent, do not result in the formation of bona fide germinal centers, and the dominant antibody isotype produced during such responses is IgM with very few or no somatic mutations. Activation-induced cytidine deaminase (AID) is required for both somatic hypermutation and Ig isotype-switching in humans and mice. To test the extent to which unmutated polysaccharide-specific IgM confers protective immunity, we immunized wildtype and AID−/− mice with either heat-killed Salmonella enterica serovar Typhi (S. Typhi) or purified Vi polysaccharide (ViPS). We found that wildtype and AID−/− mice immunized with heat-killed S. Typhi generated similar anti-ViPS IgM responses. As expected, wildtype but not AID−/− mice generated ViPS-specific IgG. However, the differences in the antibody-dependent killing of S. Typhi mediated by the classical pathway of complement activation were not statistically significant. In ViPS-immunized wildtype and AID−/− mice the ViPS-specific IgM levels and S. Typhi bactericidal antibody titers at 7, but not at 28 days post-immunization were also comparable. To test the protective immunity conferred by these immunizations, mice were challenged with a chimeric S. Typhimurium strain expressing ViPS. Compared to their naïve counterparts, immunized wildtype and AID−/− mice exhibited significantly reduced bacterial burden regardless of the route of infection. These data indicate that an unmutated IgM response to ViPS contributes to protective immunity to S. Typhi.
Keywords: AID, IgM, Salmonella, B cells, Vi polysaccharide, Typhoid
Introduction
Antibodies are essential for protection against a wide range of infectious agents and toxins. In fact, protective immunity conferred by most vaccines is antibody-mediated. The capability of antibodies to recognize a broad range of antigens is attributed to diversity of the pre- and post-immune B cell antigen receptor (BCR) repertoire. Preimmune BCR diversity is generated by somatic recombination of variable (V), diversity (D), and joining (J) gene segments and by the addition and deletion of non-template nucleotides at the junctions of the V-D, D-J and V-J gene segments by terminal deoxynucleotidyl transferase (TdT) during B cell development in the bone marrow (1). Diversification of the post-immune BCR repertoire is mainly generated in the germinal centers of secondary lymphoid organs (2). In the germinal centers, somatic hypermutation (SHM) of the variable regions of the Ig genes combined with selection processes give rise to the affinity maturation of antibodies to a given antigen. In addition, DNA rearrangement can also occur in the heavy chain constant region of the Ig genes by class-switch recombination (CSR) resulting in the generation of other isotypes such as IgG. In mice as well as humans the processes of SHM and CSR require activation-induced cytidine deaminase (AID) (3, 4). The antibody diversity generated by AID following antigen encounter is considered an important component for defense against a wide range of pathogens. Protein antigens such as tetanus, diphtheria and pertussis toxins are processed and presented to T cells by antigen-presenting cells. Vaccines comprised of protein antigens typically generate affinity-matured, and isotype-switched antibody responses. Therefore, protein antigens are commonly referred to as T cell-dependent (TD) antigens.
However, there are classes of bacterial antigens that can induce antibody responses without T cell-help. These are referred to as T cell-independent (TI) antigens (5–7). TI type 1 (TI-1) antigens, the prototype of which is bacterial lipopolysaccharide (LPS), at high concentration, activate B cells primarily by stimulating mitogenic receptors e.g. Toll-like receptors (TLRs) rather than BCR. Therefore, the antibodies generated by such stimuli are not necessarily antigen-specific. On the other hand, TI type 2 (TI-2) antigens are polymeric antigens, such as capsular polysaccharides, present on clinically important human pathogens such as Streptococcus pneumoniae and Salmonella enterica serovar Typhi (S. Typhi). Unlike protein antigens, polysaccharide antigens are typically resistant to degradation and do not bind MHC II, therefore T cell help for polysaccharide-specific B cells is limited (5, 6), and germinal center reactions are not established (8, 9). Nevertheless, polysaccharide antigens can induce efficient antibody responses primarily by cross-linking the BCR of antigen-specific B cells (5, 6).
The Vi Polysaccharide (ViPS) is a TI-2 antigen expressed on S. Typhi, the causative agent of typhoid in humans (10–12). ViPS is a virulence factor (13–16) and also a protective antigen (17). Global estimates reported by the Centers for Disease Control indicate that 21.6 million cases of typhoid fever occur each year resulting in 226,000 deaths (18, 19). The rapid emergence of multiple drug-resistant strains of S. Typhi is now complicating the treatment of this disease using antibiotics (20, 21). Typhoid is a vaccine-preventable disease and vaccination of high-risk populations is considered the most promising strategy for control of S. Typhi (10–12). Two types of licensed vaccines are currently available: a subunit vaccine composed of plain ViPS, (Typhim Vi® or Typherix®) and a live attenuated S. Typhi vaccine containing a mixture of non-viable (5–50×109) and viable (2–10×109) bacteria (Vivotif®) (22).
Since antibodies produced during pure polysaccharide immunization are not affinity matured and the isotype is mainly IgM, due to the lack of a bona fide germinal center formation, we hypothesize that unmutated IgM response to ViPS should control the infection in AID−/− mice as efficiently as in wildtype mice. To test the extent to which unmutated ViPS-specific IgM confer protective immunity we immunized wildtype and AID−/− mice with either heat-killed S. Typhi or purified ViPS to determine the impact of unmutated IgM in control of Salmonella in a murine model of Typhoid.
Materials and Methods
Mice
The Institutional Animal Care and Use Committee has approved these studies. Mice were housed in micro-isolator cages with free access to food and water and were maintained in a specific pathogen-free facility. AIDCre/Cre (stock no. 007770) mice on a C57BL/6J background were purchased from The Jackson Laboratories (Bar Harbor, ME). In these mice, Cre recombinase cDNA was inserted into exon 1 of AID (23). These mice exhibit the same phenotype as those of AID−/− mice generated by neomycin insertion in exon 1 of AID (3) and we have referred the AIDCre/Cre as AID−/− mice. Disruption of the gene encoding for AID is confirmed by PCR as described (23). Age-matched (8–12 wk old) wildtype C57BL6/J mice (stock; 000664) were purchased from The Jackson Laboratories and were also bred in house.
Immunization
Two and half micrograms of Vi Polysaccharide (ViPS; Lot 5 PDMI 158299 obtained from the U.S. Food and Drug Administration, Silver Spring, MD 20993) dissolved in 100µl Dulbecco’s phosphate-buffered saline [(DPBS); Mediatech, Herndon, VA] was used to immunize mice intraperitoneally (i.p.). This ViPS was isolated from Citrobacter freundii strain WR7011 (O29 serotype)(24), and is structurally identical to the ViPS from S. Typhi (25, 26). Previously, immunization of this ViPS in the range of 0.25 – 50 µg in 100 µl PBS was shown to induce comparable anti-ViPS antibody response (26, 27). For whole bacterial immunization, mice were injected i.p. with 3×108 heat-killed S. Typhi strain Ty2. Blood samples were obtained 0, 7, 14, 21 or 28 days following immunization and stored at −20°C.
Enzyme-linked immunosorbent assay (ELISA)
ViPS-specific IgM and IgG were measured by incubating 96-well microtiter plates (Nunc MultiSorp 467340; Nunc A/S, Roskilde, Denmark) with 2 µg/ml of ViPS in DPBS overnight at room temperature. All plates were washed and blocked with 2% Bovine serum albumin (BSA) in PBS pH 7.2 (blocking buffer) for 2 hours at room temperature. Blood from immunized mice was diluted to 1:25 for IgG detection and 1:50 for IgM detection in blocking buffer, samples were centrifuged (800 × g for 10 min.) and cell-free supernatant was used. The dilutions, 1:25 for IgG and 1:50 for IgM were chosen after evaluating various serum dilutions within the linear range by ELISA (data not shown). Bound IgM or IgG was measured using HRP-conjugated goat anti-mouse IgM or IgG (Bethyl Laboratories, Montgomery, TX). Since ViPS-specific mouse IgM and IgG reference standards are not available, and the ViPS-specific antibodies in mice are likely to be of oligoclonal nature with varying affinities, the antigen-specific antibody levels in the present study were interpreted as ng/µl “equivalents” using normal mouse serum IgM or IgG standards (Bethyl Laboratories, Montgomery, TX), as described previously (28–30).
Serum bactericidal assay (SBA)
SBA was performed as previously described (31). In brief, log-phase cultures (OD600 of 0.5 at 37°C) of S. Typhi strain Ty2 were prepared in Luria-Bertani (LB) broth with 10 mM NaCl. Bacterial cells were washed in DPBS and the bacterial cell density was adjusted to 2.5 – 5.0 × 104 colony forming units (CFU) per ml in DPBS. The expression of ViPS was assessed by slide agglutination test using a commercial Vi monoclonal antibody reagent (Statens Serum Institute diagnostica A/S, Denmark; Lot 188L-8). Serum samples were heat-inactivated by incubating at 56°C for 30 minutes prior to use in the assay. Ten microliters of S. Typhi strain Ty2 in DPBS (250–500 CFUs) were added to each well of a round-bottom polypropylene 96-well plate containing 50 µl of heat-inactivated serum in serial dilutions, 12.5 µl baby rabbit complement (Pel-Freeze, Rogers, AR), and 27.5 µl DPBS. Triplicate samples of each dilution were incubated for 120 minutes at 37°C with gentle rocking and 10 µl of this mixture were plated on LB agar plates for counting CFUs. Serum bactericidal antibody titers are defined as the reciprocal of the highest dilution that produced 50% killing in relation to control wells containing complement, but no mouse serum. Naïve mouse serum served as a negative control and serum from either mice immunized with heat-killed E. coli strain W3110 expressing pDC5 plasmid, which contains the genes necessary for the synthesis and export of ViPS (32), or S. Typhi Anti-Vi human IgG standard (Lot R1, 2011; U.S. Food and Drug Administration, Silver Spring, MD 20993) as two independent positive controls.
Infections
To test the relative protection conferred by ViPS immunization, mice were infected with a chimeric strain of S. Typhimurium (strain RC60) that expresses the S. Typhi genes necessary for ViPS synthesis, export and regulation as in S. Typhi (33). Strain RC60 was grown to an OD600 of ~1.0 in LB broth containing 10 mM NaCl. The expression of ViPS was assessed by slide agglutination test using a commercial Vi monoclonal antibody reagent (Statens Serum Institute diagnostica A/S, Denmark; Lot 188L-8). Bacteria were washed twice in DPBS and 100 µl of DPBS containing ~3×104 CFUs was injected i.p. or i.v. Three days post-infection liver and spleen were collected and the tissues were processed using a Minilys tissue homogenizer (Bertin Technologies, Montigny-le-Bretonneux, France). Blood was collected into anti-coagulant and bacterial burden in the blood and tissue homogenates was measured by counting CFUs on LB agar plates.
Histopathology analysis
Liver tissues obtained on day 3 post-infection were fixed in 10% buffered formalin and 4 µM paraffin-embedded sections were stained with hematoxylin and eosin. The specimen slides were scanned at 20× magnification on Aperio CS2 Scanscope® (Leica Biosystems Inc.), and total, necrotic and infiltration areas composed of lymphocytes and other mononuclear cells in the entire specimen was quantified using Aperio ImageScope® software (Leica Biosystems Inc.).
Statistical analysis
Data presented throughout depict pooled data from at least two independent experiments. Statistics were performed using the Prism 5 software program (GraphPad Software, Inc., La Jolla, CA).
Results
Efficient ViPS-specific IgM responses occur in mice deficient in AID
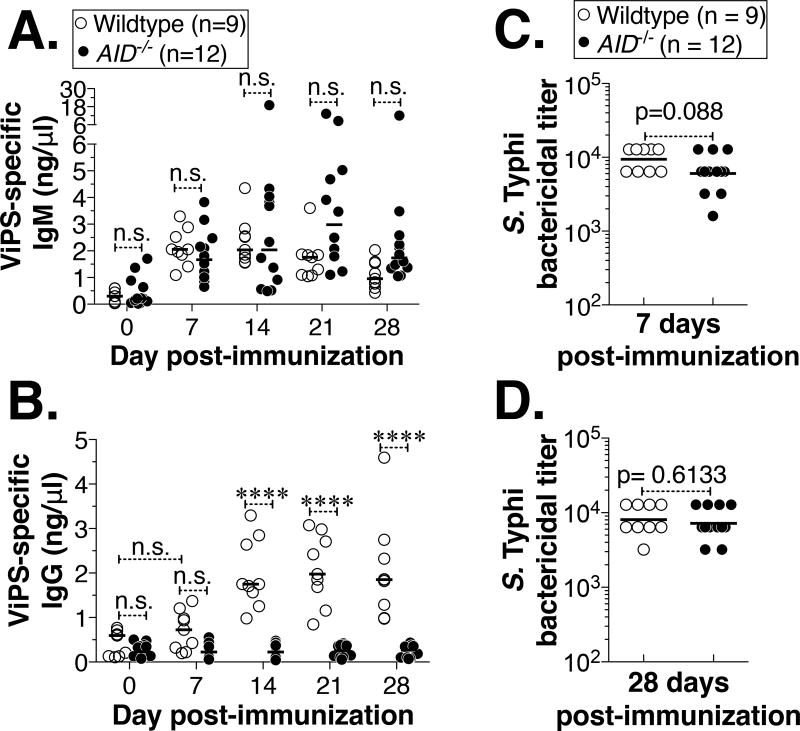
To test whether an efficient antibody response can be generated against ViPS in the complete absence of SHM and CSR, we immunized mice sufficient or deficient in AID with heat-killed S. Typhi. We found that wildtype and AID−/− mice produced comparable levels of anti-ViPS IgM (Fig. 1A). The gradual decrease of ViPS-specific IgM in wildtype mice was concurrent with a reciprocal increase of ViPS-specific IgG, indicating the occurrence of an efficient isotype switching of the antigen-specific IgM to IgG (Fig. 1B). As expected, ViPS-specific IgG was not generated in AID−/− mice (Fig. 1B).
Figure 1. Immunization with heat-killed S. Typhi results in comparable bactericidal antibody responses in wildtype and AID−/− mice.
Adult (8–14 wk old) wildtype or AID−/− mice were immunized i.p. with heat-killed S. Typhi strain Ty2 (3×108 bacterial cells) and levels of ViPS-specific (A) IgM and (B) IgG were measured by ELISA. Each dot represents an individual mouse and the bar represents median. The data represents pool of two independent experiments. Statistical differences were determined using Two-way ANOVA with Bonferroni post-test. **** denotes p<0.001 and n.s. denotes not significant. Serum bactericidal antibody titers against S. Typhi strain Ty2 were determined at (C) 7 and (D) 28 days post-immunization. Each dot represents an individual mouse and the bar represents geometric mean. Statistical differences were determined by Mann-Whitney test.
For the initiation of the classical pathway of complement activation and the eventual lysis of the bacterial cells by the formation of the membrane attack complex, the binding of antibodies to target antigens on the bacterial surface is essential. Since AID−/− mice generated a comparable anti-ViPS specific IgM response by 7 days post-immunization (Fig. 1A), and ViPS-specific IgG levels at this time point in the wildtype mice were not significantly different from baseline, we tested whether the unmutated IgM antibodies present in the immune serum of AID−/− mice exert bactericidal activity against S. Typhi via the classical pathway of complement activation. Using a previously established serum bactericidal assay (31), we found that immune serum obtained on day 7 post-immunization from wildtype and AID−/− mice exerted comparable S. Typhi bactericidal activity (Fig. 1C). Although there is a difference in the levels of ViPS-specific antibody isotypes at 28 days post-immunization (Fig. 1A & B), the difference in the serum bactericidal titers between wildtype and AID−/− mice at this time point were not statistically significant (Fig. 1D).
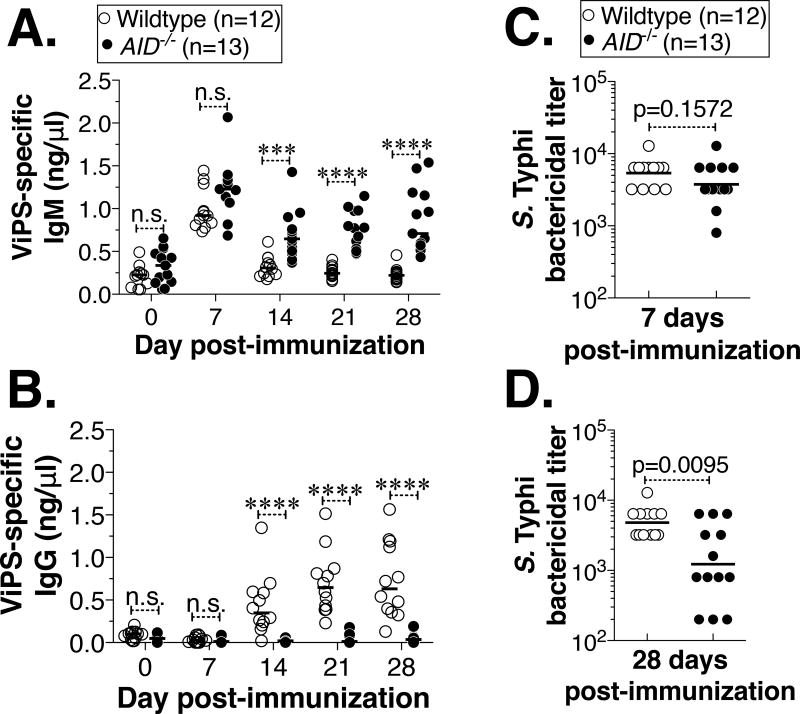
Whole bacterial immunization results in much more complex immune responses compared to the antibody response to isolated ViPS. For example, certain bacterial products can act as adjuvants and immune responses to other antigens can obscure the evaluation of protection mediated by anti-ViPS responses. Polysaccharide subunit vaccines are often contaminated with TLR ligands (34). In fact, individuals immunized with one of the current ViPS subunit vaccines, Typherix® also develop antibodies to LPS serotypes O-9, indicating the presence of S. Typhi LPS in the vaccine (35). It is important to note that TLR agonists also can induce AID expression and that BCR signaling can synergize in this process (36, 37). Thus, AID could also play a role in TI responses by promoting not only Ig isotype switching to IgG3 but also somatic mutations in V regions of polysaccharide-specific antibody genes. Since O-9 antigen-specific antibodies could interfere with our interpretations on the ViPS-specific IgM response, we have immunized mice with ViPS isolated from Citrobacter freundii (whose O serotype is 29), to test the impact of CSR and SHM under bona fide TI conditions. As observed in the whole bacterial immunization (Fig. 1A), we found that the ViPS-specific IgM responses in wildtype and AID−/− mice are comparable at 7 days post-immunization (Fig. 2A). At this peak IgM response time point, the differences in serum bactericidal titers were not statistically significant (Fig. 2C). ViPS-specific IgM in wildtype mice decreased more precipitously than in AID−/− mice (Fig. 2A) and this change was concurrent with a gradual increase of ViPS-specific IgG (Fig. 2B), indicating the occurrence of an efficient isotype switching in AID-sufficient mice. Interestingly, the serum bactericidal titers in AID−/− mice were significantly lower at 28 days post-immunization (Fig. 2D) suggesting that IgG could play an additive role at later time points under TI immunization conditions as shown previously in the pneumococcal polysaccharide response (38).
Figure 2. Immunization with Vi polysaccharide (ViPS) induces ViPS-specific IgM and bactericidal antibody responses in AID−/− mice.
Adult (8–14 wk old) wildtype or AID−/− mice were immunized i.p. with ViPS (2.5 µg) and levels of ViPS-specific (A) IgM and (B) IgG were measured by ELISA. Each dot represents an individual mouse and the bar represents median. The data represents pool of two independent experiments. Statistical differences were determined using Two-way ANOVA with Bonferroni post-test. **** denotes p<0.001; *** p<0.005, and n.s. denotes not significant. Serum bactericidal antibody titers against S. Typhi strain Ty2 were determined at (C) 7 and (D) 28 days post-immunization. Each dot represents an individual mouse and the bar represents geometric mean. Statistical differences were determined by Mann-Whitney test.
Immunization of AID−/− mice with heat-killed S. Typhi Ty2 or ViPS can confer protection in vivo
S. Typhi is a human-restricted pathogen and experimental models to test the efficacy of ViPS or other Typhoid vaccines are currently limited (39). Salmonella enterica serovar Typhimurium (S. Typhimurium) causes a typhoid-like systemic disease in mice. Therefore, S. Typhimurium infection in mice is widely used as an experimental model to understand certain aspects of human typhoid (40). Investigating the role of ViPS-specific antibody responses using S. Typhimurium is limited by the fact that unlike S. Typhi, S. Typhimurium does not express ViPS and possesses a longer LPS than S. Typhi. To overcome these limitations, a chimeric strain of S. Typhimurium (RC60) was previously engineered (33). RC60 expresses all genes that are necessary for ViPS synthesis, export and regulation as in S. Typhi and additionally has a deletion of FepE, which controls the length of the LPS. Strain RC60 was shown to exhibit cell surface and other characteristics of S. Typhi (33).
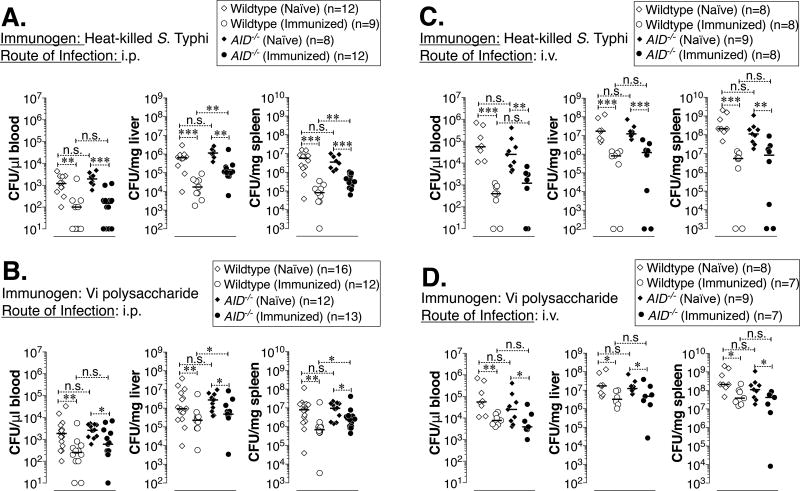
A previous study by Saxen demonstrated a role for S. Typhimurium-specific IgM in protection against Salmonella following i.p. challenge but not i.v. challenge (41). However, those IgM antibodies are generated by immunizing whole S. Typhimurium, that does not express ViPS. To test the impact of ViPS-specific IgM in protective immunity to murine typhoid, naïve or immunized wildtype and AID−/− mice were challenged with a relatively high dose (2×104 CFUs) of strain RC60 either i.p. or i.v. on day 28 post-immunization. The bacterial burden in the blood, liver, and spleen was measured 3 days later as described previously (42), since mice on C57BL6 background succumb to Salmonella infection by 5 days post-infection at this infectious dose due to the presence of a susceptible Nramp1 allele (42–44). Compared to naïve mice, we found a significant decrease in bacterial burden after immunization with either heat-killed S. Typhi Ty2 (Fig. 3A and C) or with ViPS (Fig. 3B and D) in both wildtype and AID−/− mice. Although i.v. infection resulted in an order of magnitude more bacterial burden compared to i.p. infection, the route of infection did not impact the relative control of bacterial burden (Fig. 3AB vs CD). However, compared to immunized wildtype mice, immunized AID−/− mice had significantly more bacterial load in the liver and spleen of i.p. infected (Fig 3A and B) but not i.v. infected mice (3C and D).
Figure 3. Reduced bacterial burden in wildtype and AID−/− mice immunized either with heat-killed S. Typhi or with Vi polysaccharide.
C57BL/6J mice sufficient or deficient in AID were immunized with (A&C) heat-killed S. Typhi or (B&D) ViPS as in Fig 1 and 2. Four weeks after immunization mice were infected with 3×104 CFUs of ViPS expressing S. Typhimurium strain RC60 either (A&B) i.p. or (C&D) i.v., and three days post-infection bacterial burden was determined. Each dot represents an individual mouse and the bar represents median. The data represents pool of two independent experiments. Statistical differences were determined by Mann-Whitney test. *** p<0.005; **p<0.01; *p<0.05 and n.s. denotes not significant. Note: In panels C & D, the control unimmunized wildtype and AID−/− mice were identical.
Comparable reduction of liver damage in wildtype and AID−/− mice immunized either with heat-killed Ty2 or ViPS
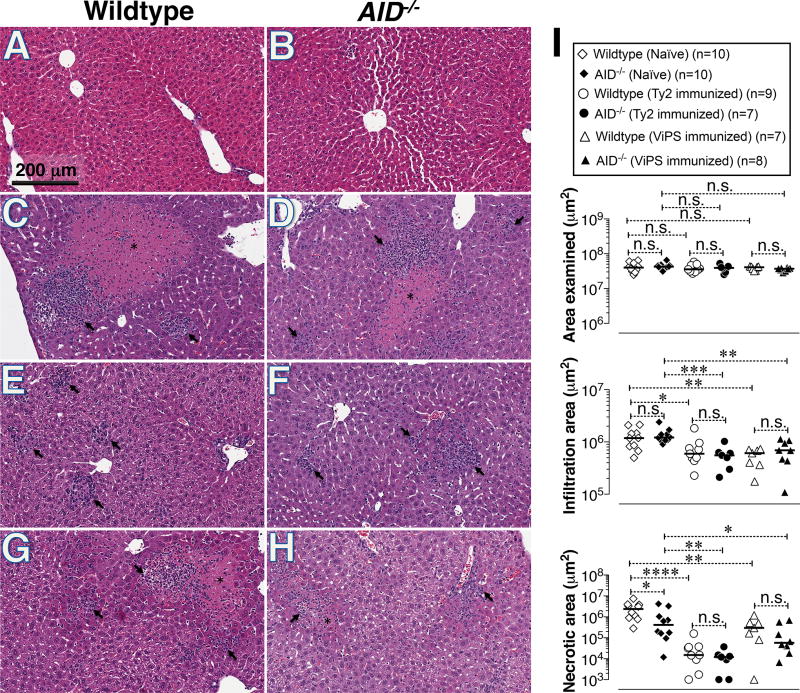
While liver biopsies are rare in typhoid-infected patients, biopsies have been collected in cases where typhoid was not initially considered (45). These rare cases provided insight into the histopathology of typhoid in humans. Typhoid-induced liver lesions consist of mononuclear cell infiltrates, congestion of sinusoids and altered staining with little/no steatosis in hepatocytes proximal to the lesion (46–48). Interestingly, the liver lesions observed in S. Typhimurium infection in mice (49–53) appear very similar to those described for human typhoid patients (46–48). We confirmed that systemic infection of naïve mice with live S. Typhimurium strain RC60 resulted in extensive liver damage as shown by large areas of hepatocyte necrosis and infiltration of mononuclear cells (Fig. 4C&D) compared to the livers of naïve uninfected mice (Fig. 4A& B). Remarkably, the reduction in mononuclear infiltration and necrotic regions in the livers of AID−/− mice immunized either with heat-killed Ty2 or ViPS were comparable to that in wildtype mice (Fig. 4E through H). This reduction in liver pathology was evaluated in several mice and was statistically significant compared to unimmunized mice (Fig. 4I). Remarkably, compared to immunization with ViPS, immunization with whole bacteria resulted in more striking decrease in liver necrosis in both wildtype and AID−/− mice, suggesting that other protective responses beside anti-ViPS antibodies, induced by whole bacterial immunization also play a role in vivo (Fig. 4I).
Figure 4. Reduced liver damage in wildtype and AID−/− mice immunized either with heat-killed S. Typhi or with Vi polysaccharide (ViPS).
C57BL/6J mice sufficient or deficient in AID were immunized with heat-killed S. Typhi or ViPS as in Fig 1 and 2. Four weeks after immunization mice were infected i.p. with 3 × 104 CFUs of ViPS expressing S. Typhimurium strain RC60 and three days post-challenge livers were analyzed for histopathology. (A & B) naïve uninfected; (C&D) naïve infected; (E&F) heat-killed S. Typhi (Ty2) immunized and infected; (G&H) ViPS immunized and infected. Necrotic lesions are indicated with asterisk and cellular infiltration areas are indicated with arrows with in the picture. (I) Quantification of liver damage in multiple mice. Each dot represents an individual mouse and the bar represents median. The data represents pool of two independent experiments. Statistical differences were determined by Mann-Whitney test. **** p<0.0001; *** p<0.005; **p<0.01; *p<0.05 and n.s. denotes not significant.
Discussion
B cell responses to purified polysaccharide antigens are T cell-independent and do not induce sustained GC formation (8, 9). Although the antibody isotypes produced during such responses are predominantly of the IgM isotype, IgG3 is also considered as a signature for TI responses (54). A low frequency of somatic mutations in V regions can occur with BCR signaling even in the absence of T cell-help (55–57). Nevertheless, we found that anti-ViPS IgM can be generated even in the absence of AID and these antibodies were efficient in killing S. Typhi via the classical complement pathway. S. Typhi expresses O9 LPS, and induction of anti-O9 antibodies occur in humans immunized with ViPS subunit vaccine, due to contaminated LPS in ViPS purified directly from S. Typhi (35). Since we immunized mice with purified ViPS from C. freundii (25, 26) not S. Typhi, a role for anti-O9 antibodies in the observed serum bactericidal activity (Fig. 2C or D) is unlikely. Moreover, under high levels of ViPS expressing conditions such as our S. Typhi culture conditions, O9 antibodies are not expected to exert anti-bacterial activity as demonstrated previously (58). AID−/− immunized with ViPS also showed a significantly reduced bacterial burden (Fig. 3B&D) and liver damage (Fig.4). Together, our results suggest that CSR and SHM of antibody genes are not absolutely essential for antibody-mediated protection, and that unmutated antibodies to ViPS contribute significantly towards controlling murine typhoid.
The absence of functional AID in patients is characterized by the absence of immunoglobulin CSR and SHM, and significantly increased serum IgM levels (4). Similarly, AID−/− mice also have a 2–3-fold increase in basal levels of IgM (3). A striking observation was that the necrotic regions of unimmunized AID−/− mice were significantly smaller than that in unimmunized wildtype mice (Fig. 4). This can be attributed to naïve AID−/− mice having 2–3 fold higher levels of total IgM than naïve wildtype mice (3). This finding is consistent with the fact that “natural” IgM produced in mice without any apparent antigen stimulation has a significant role in protective immunity (59, 60). In fact, passive immunization of mice with high doses of polyclonal human IgM can reduce S. Typhimurium burden in various tissues (61). Since Salmonella antigens recognized by “natural” IgM are not known, it remains to be determined how high levels of natural IgM contribute to protective immunity to typhoid.
Although AID is well characterized for its selective expression in activated mature B cells such as those in germinal centers, AID expression in developing B cells in bone marrow has been discovered. This expression appears to regulate central tolerance of B cells in both mice and humans and alter the BCR repertoire (62–65). For example, AID−/− mice are unable to purge certain auto-reactive B cells (62) and have significantly increased anti-DNA antibodies compared to wildtype mice. Unlike DNA, ViPS is not a self-antigen and the IgM of naïve wildtype or AID−/− mice show similar background reactivity to ViPS (Fig. 1). However, upon immunization with either ViPS or heat-killed S. Typhi both wildtype and AID−/− mice generate comparable levels of anti-ViPS IgM by 7 days post-immunization, suggesting that the altered preimmune BCR repertoire of AID−/− mice is unlikely to influence the conclusions of the present study.
It has been shown that Salmonella induces a significant B cell response at extra-follicular sites, without notable GCs (66, 67). At these sites SHM occurs efficiently leading to the generation of high affinity antibodies to Salmonella antigens (66). Although we found that antibody responses generated by ViPS or whole bacterial immunization in AID−/− mice can exert anti-Salmonella activity ex vivo (Figs. 1 C&D, 2 C&D), in the in vivo challenge model, we found that wildtype mice had significantly lower bacterial burden in liver and spleen compared to that in AID−/− mice (Fig. 3A&B). This indicates that isotype switched antibodies could also contribute to protective immunity in murine typhoid. In fact, it has recently been shown that IgG1 is required for optimal protection in the murine model of S. Typhimurium infection (68).
We have previously shown that B1b cells, a subset of mature B cells can generate a long-lasting T cell independent IgM memory to Borrelia hermsii and that unmutated IgM specific to B. hersmii is sufficient to control bacteremia in vivo (28). Recently, it has been reported that IgM+ memory B cells that have a lesser amount of SHM were found to be longer-lived than hypermutated IgG+ memory B cells, indicating that antigen-specific unmutated IgM B cells can persist as memory B cells and confer long-lasting immunity (69). Although ViPS and NP-Ficoll, a widely used synthetic model polysaccharide antigen are also recognized by B1b cells in mice (29, 70, 71), the antibody response induced by these antigens is short-lived, suggesting that hypermutated and isotype-switched antibodies influence IgM memory to TI-2 antigens. In fact, it has previously been shown that AID−/− mice can generate a heightened antigen-specific IgM response to NP-Ficoll upon reimmunization, which is indicative of the persistence of unmutated IgM memory B cells to TI-2 antigens (72).
While polysaccharide conjugate vaccines induce prolonged immunity, they require administration of multiple doses to boost the primary response, and are prohibitively expensive for countries where Typhoid and pneumococcal diseases are endemic. Identification of the mechanisms persistence of long-lasting unmutated antibody responses could help in the development of subunit vaccines that are safe, cost-effective, require fewer doses, and will likely result in greater global accessibility and higher compliance in the broader population.
Acknowledgments
This work was supported by NIH AI105724 and AI121270 grant to K.R.A. and by Cancer Center Support Grant 5P30CA056036-17 to Translational Research/Pathology Shared Resources.
We thank Dr. Andreas Bäumler for providing S. Typhi strain Ty2, S. Typhimurium strain RC60, E. coli W3110 (pDC5), and suggestions on the Salmonella infection model. We thank Dr. Tim Manser for discussion and comments on the manuscript, Dr. David Abraham for sharing the AID−/− mice, and Drs. Shousun Szu and John Cipollo for providing Vi Polysaccharide and S. Typhi Anti-Vi human IgG standard.
References
- 1.Benedict CL, Gilfillan S, Thai TH, Kearney JF. Terminal deoxynucleotidyl transferase and repertoire development. Immunol Rev. 2000;175:150–157. [PubMed] [Google Scholar]
- 2.Chan TD, Brink R. Affinity-based selection and the germinal center response. Immunol Rev. 2012;247:11–23. doi: 10.1111/j.1600-065X.2012.01118.x. [DOI] [PubMed] [Google Scholar]
- 3.Muramatsu M, Kinoshita S, Fagarasan S, Yamada Y, Shinkai Y, Honjo T. Class switch recombination and hypermutation require Activation-induced cytidine demainase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 4.Revy P, Muto T, Levy Y, Geissmann F, Plebani A, Sanal O, Catalan N, Forveille M, Dufourcq-Lagelouse R, Gennery A, Tezcan I, Ersoy F, Kayserili H, Ugazio AG, Brousse N, Muramatsu M, Notarangelo LD, Kinoshita K, Honjo T, Fischer A, Durandy A. Activation-induced cytidine Deaminase (AID) deficiency causes the autosomal recessive form of the hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 5.Vos Q, Lees A, Wu Z, Snapper CM, Mond JJ. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol. Rev. 2000;176:154–170. doi: 10.1034/j.1600-065x.2000.00607.x. [DOI] [PubMed] [Google Scholar]
- 6.Lesinski GB, Westerink MA. Novel vaccine strategies to T-independent antigens. J. Microbiol. Methods. 2001;47:135–149. doi: 10.1016/s0167-7012(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 7.Alugupalli KR. A distinct role for B1b lymphocytes in T cell-independent immunity. Curr Top Microbiol Immunol. 2008;319:105–130. doi: 10.1007/978-3-540-73900-5_5. [DOI] [PubMed] [Google Scholar]
- 8.Manser T. Textbook germinal centers? J Immunol. 2004;172:3369–3375. doi: 10.4049/jimmunol.172.6.3369. [DOI] [PubMed] [Google Scholar]
- 9.de Vinuesa CG, Cook MC, Ball J, Drew M, Sunners Y, Cascalho M, Wabl M, Klaus GG, MacLennan IC. Germinal centers without T cells. J Exp Med. 2000;191:485–494. doi: 10.1084/jem.191.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhutta ZA. Current concepts in the diagnosis and treatment of typhoid fever. BMJ. 2006;333:78–82. doi: 10.1136/bmj.333.7558.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Jong HK, Parry CM, van der Poll T, Wiersinga WJ. Host-pathogen interaction in invasive Salmonellosis. PLoS Pathog. 2012;8:e1002933. doi: 10.1371/journal.ppat.1002933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaur J, Jain SK. Role of antigens and virulence factors of Salmonella enterica serovar Typhi in its pathogenesis. Microbiol Res. 2012;167:199–210. doi: 10.1016/j.micres.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Raffatellu M, Chessa D, Wilson RP, Dusold R, Rubino S, Baumler AJ. The Vi capsular antigen of Salmonella enterica serotype Typhi reduces Toll-like receptor-dependent interleukin-8 expression in the intestinal mucosa. Infect Immun. 2005;73:3367–3374. doi: 10.1128/IAI.73.6.3367-3374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson RP, Raffatellu M, Chessa D, Winter SE, Tukel C, Baumler AJ. The Vi-capsule prevents Toll-like receptor 4 recognition of Salmonella. Cell Microbiol. 2008;10:876–890. doi: 10.1111/j.1462-5822.2007.01090.x. [DOI] [PubMed] [Google Scholar]
- 15.Wilson RP, Winter SE, Spees AM, Winter MG, Nishimori JH, Sanchez JF, Nuccio SP, Crawford RW, Tukel C, Baumler AJ. The Vi capsular polysaccharide prevents complement receptor 3-mediated clearance of Salmonella enterica serotype Typhi. Infect Immun. 2011;79:830–837. doi: 10.1128/IAI.00961-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wangdi T, Lee CY, Spees AM, Yu C, Kingsbury DD, Winter SE, Hastey CJ, Wilson RP, Heinrich V, Baumler AJ. The Vi capsular polysaccharide enables Salmonella enterica serovar typhi to evade microbe-guided neutrophil chemotaxis. PLoS Pathog. 2014;10:e1004306. doi: 10.1371/journal.ppat.1004306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Felix A, Pitt RM. Virulence and Immunogenic Activities of B. typhosus in Relation to its Antigenic Constituents. J Hyg (Lond) 1935;35:428–436. doi: 10.1017/s0022172400032459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crump JA, Mintz ED. Global trends in typhoid and paratyphoid Fever. Clin Infect Dis. 2010;50:241–246. doi: 10.1086/649541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346–353. [PMC free article] [PubMed] [Google Scholar]
- 20.Mirza SH, Beeching NJ, Hart CA. Multi-drug resistant typhoid: a global problem. J Med Microbiol. 1996;44:317–319. doi: 10.1099/00222615-44-5-317. [DOI] [PubMed] [Google Scholar]
- 21.Karki S, Shakya P, Cheng AC, Dumre SP, Leder K. Trends of etiology and drug resistance in enteric fever in the last two decades in Nepal: a systematic review and meta-analysis. Clin Infect Dis. 2013;57:e167–176. doi: 10.1093/cid/cit563. [DOI] [PubMed] [Google Scholar]
- 22.Levine MM. Typhoid Fever Vaccine. Saunders Elsevier; 2008. [Google Scholar]
- 23.Robbiani DF, Bothmer A, Callen E, Reina-San-Martin B, Dorsett Y, Difilippantonio S, Bolland DJ, Chen HT, Corcoran AE, Nussenzweig A, Nussenzweig MC. AID is required for the chromosomal breaks in c-myc that lead to c-myc/IgH translocations. Cell. 2008;135:1028–1038. doi: 10.1016/j.cell.2008.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snellings NJ, Johnson EM, Kopecko DJ, Collins HH, Baron LS. Genetic regulation of variable Vi antigen expression in a strain of Citrobacter freundii. J Bacteriol. 1981;145:1010–1017. doi: 10.1128/jb.145.2.1010-1017.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szu SC, Li XR, Schneerson R, Vickers JH, Bryla D, Robbins JB. Comparative immunogenicities of Vi polysaccharide-protein conjugates composed of cholera toxin or its B subunit as a carrier bound to high- or lower-molecular-weight Vi. Infect Immun. 1989;57:3823–3827. doi: 10.1128/iai.57.12.3823-3827.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szu SC, Li XR, Stone AL, Robbins JB. Relation between structure and immunologic properties of the Vi capsular polysaccharide. Infect Immun. 1991;59:4555–4561. doi: 10.1128/iai.59.12.4555-4561.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szu SC, Stone AL, Robbins JD, Schneerson R, Robbins JB. Vi capsular polysaccharide-protein conjugates for prevention of typhoid fever. Preparation, characterization, and immunogenicity in laboratory animals. J Exp Med. 1987;166:1510–1524. doi: 10.1084/jem.166.5.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b Lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 29.Shriner AK, Liu H, Sun G, Guimond M, Alugupalli KR. IL-7-dependent B lymphocytes are essential for the anti-polysaccharide response and protective immunity to Streptococcus pneumoniae. J Immunol. 2010;185:525–531. doi: 10.4049/jimmunol.0902841. [DOI] [PubMed] [Google Scholar]
- 30.Dickinson GS, Sun G, Bram RJ, Alugupalli KR. Efficient B cell responses to Borrelia hermsii infection depend on BAFF and BAFFR but not TACI. Infect Immun. 2014;82:453–459. doi: 10.1128/IAI.01147-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyd MA, Tennant SM, Saague VA, Simon R, Muhsen K, Ramachandran G, Cross AS, Galen JE, Pasetti MF, Levine MM. Serum bactericidal assays to evaluate typhoidal and nontyphoidal Salmonella vaccines. Clin Vaccine Immunol. 2014;21:712–721. doi: 10.1128/CVI.00115-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raffatellu M, Santos RL, Chessa D, Wilson RP, Winter SE, Rossetti CA, Lawhon SD, Chu H, Lau T, Bevins CL, Adams LG, Baumler AJ. The capsule encoding the viaB locus reduces interleukin-17 expression and mucosal innate responses in the bovine intestinal mucosa during infection with Salmonella enterica serotype Typhi. Infect Immun. 2007;75:4342–4350. doi: 10.1128/IAI.01571-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crawford RW, Wangdi T, Spees AM, Xavier MN, Tsolis RM, Baumler AJ. Loss of very-long O-antigen chains optimizes capsule-mediated immune evasion by Salmonella enterica serovar Typhi. MBio. 2013;4:e00232–00213. doi: 10.1128/mBio.00232-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sen G, Khan AQ, Chen Q, Snapper CM. In vivo humoral immune responses to isolated pneumococcal polysaccharides are dependent on the presence of associated TLR ligands. J Immunol. 2005;175:3084–3091. doi: 10.4049/jimmunol.175.5.3084. [DOI] [PubMed] [Google Scholar]
- 35.Kantele A, Pakkanen SH, Karttunen R, Kantele JM. Head-to-head comparison of humoral immune responses to Vi capsular polysaccharide and Salmonella Typhi Ty21a typhoid vaccines--a randomized trial. PLoS One. 2013;8:e60583. doi: 10.1371/journal.pone.0060583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pone EJ, Zhang J, Mai T, White CA, Li G, Sakakura JK, Patel PJ, Al-Qahtani A, Zan H, Xu Z, Casali P. BCR-signalling synergizes with TLR-signalling for induction of AID and immunoglobulin class-switching through the non-canonical NF-kappaB pathway. Nat Commun. 2012;3:767. doi: 10.1038/ncomms1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuraoka M, Snowden PB, Nojima T, Verkoczy L, Haynes BF, Kitamura D, Kelsoe G. BCR and Endosomal TLR Signals Synergize to Increase AID Expression and Establish Central B Cell Tolerance. Cell Rep. 2017;18:1627–1635. doi: 10.1016/j.celrep.2017.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLay J, Leonard E, Petersen S, Shapiro D, Greenspan NS, Schreiber JR. Gamma 3 gene-disrupted mice selectively deficient in the dominant IgG subclass made to bacterial polysaccharides. II. Increased susceptibility to fatal pneumococcal sepsis due to absence of anti-polysaccharide IgG3 is corrected by induction of anti-polysaccharide IgG1. J Immunol. 2002;168:3437–3443. doi: 10.4049/jimmunol.168.7.3437. [DOI] [PubMed] [Google Scholar]
- 39.Darton TC, Blohmke CJ, Pollard AJ. Typhoid epidemiology, diagnostics and the human challenge model. Curr Opin Gastroenterol. 2014;30:7–17. doi: 10.1097/MOG.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 40.McSorley SJ. Immunity to intestinal pathogens: lessons learned from Salmonella. Immunol Rev. 2014;260:168–182. doi: 10.1111/imr.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saxen H. Mechanism of the protective action of anti-Salmonella IgM in experimental mouse salmonellosis. J Gen Microbiol. 1984;130:2277–2283. doi: 10.1099/00221287-130-9-2277. [DOI] [PubMed] [Google Scholar]
- 42.Loomis WP, Johnson ML, Brasfield A, Blanc MP, Yi J, Miller SI, Cookson BT, Hajjar AM. Temporal and anatomical host resistance to chronic Salmonella infection is quantitatively dictated by Nramp1 and influenced by host genetic background. PLoS One. 2014;9:e111763. doi: 10.1371/journal.pone.0111763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sancho-Shimizu V, Malo D. Sequencing, expression, and functional analyses support the candidacy of Ncf2 in susceptibility to Salmonella typhimurium infection in wild-derived mice. J Immunol. 2006;176:6954–6961. doi: 10.4049/jimmunol.176.11.6954. [DOI] [PubMed] [Google Scholar]
- 44.Torii I, Oka S, Hotomi M, Benjamin WH, Jr, Takai T, Kearney JF, Briles DE, Kubagawa H. PIR-B-deficient mice are susceptible to Salmonella infection. J Immunol. 2008;181:4229–4239. doi: 10.4049/jimmunol.181.6.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khosla SN. Typhoid hepatitis. Postgrad Med J. 1990;66:923–925. doi: 10.1136/pgmj.66.781.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mert A, Tabak F, Ozaras R, Ozturk R, Aki H, Aktuglu Y. Typhoid fever as a rare cause of hepatic, splenic, and bone marrow granulomas. Intern Med. 2004;43:436–439. doi: 10.2169/internalmedicine.43.436. [DOI] [PubMed] [Google Scholar]
- 47.Narechania S, Duran M, Karivedu V, Gopalakrishna KV. A case of typhoid Fever with hepatic granulomas and enteritis. Case Rep Pathol. 2015;2015:745461. doi: 10.1155/2015/745461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramachandran S, Godfrey JJ, Perera MV. Typhoid hepatitis. JAMA. 1974;230:236–240. [PubMed] [Google Scholar]
- 49.Nakoneczna I, Hsu HS. The comparative histopathology of primary and secondary lesions in murine salmonellosis. Br J Exp Pathol. 1980;61:76–84. [PMC free article] [PubMed] [Google Scholar]
- 50.Moncure CW, Guo YN, Xu HR, Hsu HS. Comparative histopathology in mouse typhoid among genetically diverse mice. Int J Exp Pathol. 1998;79:183–192. doi: 10.1046/j.1365-2613.1998.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alam MS, Akaike T, Okamoto S, Kubota T, Yoshitake J, Sawa T, Miyamoto Y, Tamura F, Maeda H. Role of nitric oxide in host defense in murine salmonellosis as a function of its antibacterial and antiapoptotic activities. Infect Immun. 2002;70:3130–3142. doi: 10.1128/IAI.70.6.3130-3142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sebastiani G, Blais V, Sancho V, Vogel SN, Stevenson MM, Gros P, Lapointe JM, Rivest S, Malo D. Host immune response to Salmonella enterica serovar Typhimurium infection in mice derived from wild strains. Infect Immun. 2002;70:1997–2009. doi: 10.1128/IAI.70.4.1997-2009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gonzalez-Escobedo G, La Perle KM, Gunn JS. Histopathological analysis of Salmonella chronic carriage in the mouse hepatopancreatobiliary system. PLoS One. 2013;8:e84058. doi: 10.1371/journal.pone.0084058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin F, Kearney JF. B1 cells: similarities and differences with other B cell subsets. Curr. Opin. Immunol. 2001;13:195–201. doi: 10.1016/s0952-7915(00)00204-1. [DOI] [PubMed] [Google Scholar]
- 55.Weller S, Faili A, Garcia C, Braun MC, Le Deist FF, de Saint Basile GG, Hermine O, Fischer A, Reynaud CA, Weill JC. CD40-CD40L independent Ig gene hypermutation suggests a second B cell diversification pathway in humans. Proc Natl Acad Sci U S A. 2001;98:1166–1170. doi: 10.1073/pnas.98.3.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Faili A, Aoufouchi S, Gueranger Q, Zober C, Leon A, Bertocci B, Weill JC, Reynaud CA. AID-dependent somatic hypermutation occurs as a DNA single-strand event in the BL2 cell line. Nat Immunol. 2002;3:815–821. doi: 10.1038/ni826. [DOI] [PubMed] [Google Scholar]
- 57.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hart PJ, O'Shaughnessy CM, Siggins MK, Bobat S, Kingsley RA, Goulding DA, Crump JA, Reyburn H, Micoli F, Dougan G, Cunningham AF, MacLennan CA. Differential Killing of Salmonella enterica Serovar Typhi by Antibodies Targeting Vi and Lipopolysaccharide O:9 Antigen. PLoS One. 2016;11:e0145945. doi: 10.1371/journal.pone.0145945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boes M. Role of natural and immune IgM antibodies in immune responses. Mol Immunol. 2000;37:1141–1149. doi: 10.1016/s0161-5890(01)00025-6. [DOI] [PubMed] [Google Scholar]
- 60.Panda S, Ding JL. Natural antibodies bridge innate and adaptive immunity. J Immunol. 2015;194:13–20. doi: 10.4049/jimmunol.1400844. [DOI] [PubMed] [Google Scholar]
- 61.Bioley G, Monnerat J, Lotscher M, Vonarburg C, Zuercher A, Corthesy B. Plasma-Derived Polyreactive Secretory-Like IgA and IgM Opsonizing Salmonella enterica Typhimurium Reduces Invasion and Gut Tissue Inflammation through Agglutination. Front Immunol. 2017;8:1043. doi: 10.3389/fimmu.2017.01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuraoka M, Holl TM, Liao D, Womble M, Cain DW, Reynolds AE, Kelsoe G. Activation-induced cytidine deaminase mediates central tolerance in B cells. Proc Natl Acad Sci U S A. 2011;108:11560–11565. doi: 10.1073/pnas.1102571108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuraoka M, Liao D, Yang K, Allgood SD, Levesque MC, Kelsoe G, Ueda Y. Activation-induced cytidine deaminase expression and activity in the absence of germinal centers: insights into hyper-IgM syndrome. J Immunol. 2009;183:3237–3248. doi: 10.4049/jimmunol.0901548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuraoka M, McWilliams L, Kelsoe G. AID expression during B-cell development: searching for answers. Immunol Res. 2011;49:3–13. doi: 10.1007/s12026-010-8185-7. [DOI] [PubMed] [Google Scholar]
- 65.Meyers G, Ng YS, Bannock JM, Lavoie A, Walter JE, Notarangelo LD, Kilic SS, Aksu G, Debre M, Rieux-Laucat F, Conley ME, Cunningham-Rundles C, Durandy A, Meffre E. Activation-induced cytidine deaminase (AID) is required for B-cell tolerance in humans. Proc Natl Acad Sci U S A. 2011;108:11554–11559. doi: 10.1073/pnas.1102600108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Di Niro R, Lee SJ, Vander Heiden JA, Elsner RA, Trivedi N, Bannock JM, Gupta NT, Kleinstein SH, Vigneault F, Gilbert TJ, Meffre E, McSorley SJ, Shlomchik MJ. Salmonella Infection Drives Promiscuous B Cell Activation Followed by Extrafollicular Affinity Maturation. Immunity. 2015;43:120–131. doi: 10.1016/j.immuni.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cunningham AF, Gaspal F, Serre K, Mohr E, Henderson IR, Scott-Tucker A, Kenny SM, Khan M, Toellner KM, Lane PJ, MacLennan IC. Salmonella induces a switched antibody response without germinal centers that impedes the extracellular spread of infection. J Immunol. 2007;178:6200–6207. doi: 10.4049/jimmunol.178.10.6200. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Y, Dominguez-Medina C, Cumley NJ, Heath JN, Essex SJ, Bobat S, Schager A, Goodall M, Kracker S, Buckley CD, May RC, Kingsley RA, MacLennan CA, Lopez-Macias C, Cunningham AF, Toellner KM. IgG1 Is Required for Optimal Protection after Immunization with the Purified Porin OmpD from Salmonella Typhimurium. J Immunol. 2017;199:4103–4109. doi: 10.4049/jimmunol.1700952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gitlin AD, von Boehmer L, Gazumyan A, Shulman Z, Oliveira TY, Nussenzweig MC. Independent Roles of Switching and Hypermutation in the Development and Persistence of B Lymphocyte Memory. Immunity. 2016;44:769–781. doi: 10.1016/j.immuni.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marshall JL, Flores-Langarica A, Kingsley RA, Hitchcock JR, Ross EA, Lopez-Macias C, Lakey J, Martin LB, Toellner KM, Maclennan CA, Maclennan IC, Henderson IR, Dougan G, Cunningham AF. The Capsular Polysaccharide Vi from Salmonella Typhi Is a B1b Antigen. J Immunol. 2012;189:5527–5532. doi: 10.4049/jimmunol.1103166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hsu MC, Toellner KM, Vinuesa CG, Maclennan IC. B cell clones that sustain long-term plasmablast growth in T-independent extrafollicular antibody responses. Proc Natl Acad Sci U S A. 2006;103:5905–5910. doi: 10.1073/pnas.0601502103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Obukhanych TV, Nussenzweig MC. T-independent type II immune responses generate memory B cells. J Exp Med. 2006;203:305–310. doi: 10.1084/jem.20052036. [DOI] [PMC free article] [PubMed] [Google Scholar]