To the editor:
Allogeneic hematopoietic stem cell transplantation (ASCT) is an effective consolidative strategy to decrease relapse for patients with acute myeloid leukemia (AML) and can cure ∼30% to 50% of patients with AML.1,2 It has been recognized that survival can vary widely after transplant based on differences in population studied.3 Pivotal historical studies have shown that remission status at the time of transplant, cytogenetics, age, HLA matching, performance status, and comorbidity indices have prognostic utility.4-7 However, potential interactions among these factors have not been explored.
Herein, we hypothesized that the combination of disease and patient characteristics will better predict prognosis in patients receiving ASCT, and tested this hypothesis in a homogenous group of haploidentical transplant (HaploSCT) patients.
Patients with a diagnosis of AML/myelodysplastic syndrome (MDS) who received first HaploSCT between September 2009 and March 2015 were retrospectively evaluated. After a retrospective data review protocol and a waiver of informed consent was obtained from The MD Anderson Cancer Center (MDACC) Institutional Review Board, clinical and demographic information was obtained. Data collection included patient/donor demographics, cytomegalovirus (CMV) serostatus, cytogenetics according to SWOG/Eastern Cooperative Oncology Group (ECOG) cytogenetic risk,8 Hematopoietic Cell Transplant–Comorbidity Index (HCT-CI) score,6 source of stem cells (peripheral blood or bone marrow [BM]), and natural killer (NK) cell alloreactivity (killer immunoglobulin-like receptor [KIR]-ligand mismatch). Disease status was categorized as first or second complete remission (CR1, CR2) defined as ≤5% BM blasts (with or without count recovery, with or without minimal residual disease [MRD]), or beyond CR1/CR2.
All patients received a conditioning regimen composed of 160 mg/m2 fludarabine, 100 to 140 mg/m2 melphalan, and 5 mg/kg thiotepa or 2 Gy total body irradiation as previously described.9 Graft-versus-host disease (GVHD) prophylaxis consisted of 50 mg/kg cyclophosphamide on day +3 and +4, mycophenolate mofetil until day +100, and tacrolimus until 6 months posttransplant, unless otherwise indicated. All patients received departmentally standardized antimicrobial prophylaxis.9
Patient demographics are reported with descriptive statistics. Primary outcome of interest was progression-free survival (PFS), and secondary outcomes included overall survival (OS) and disease progression. Actuarial PFS and OS were estimated using the Kaplan-Meier method.10 The cumulative incidence of disease progression was estimated accounting for competing risks. Predictors of PFS were evaluated on univariate analysis using Cox proportional hazards regression analysis.11 To account for potential interaction and assess the independent effects of variables found to be significant on univariate analysis, multivariate analysis was performed using classification and regression tree (CART) analysis.12 Statistical significance was defined at the .05 level. Stata 9.0 (College Station, TX) was used for analysis.
One hundred five patients with a diagnosis of either AML (n = 77) or MDS/AML (n = 28) received a HaploSCT during this time. Median age of patients was 52 years (range, 18-72 years) and 51% were male. Forty-nine patients (47%) received ASCT in CR1/2. Favorable, intermediate, and unfavorable cytogenetics were seen in 9 patients (8.5%), 55 patients (52%), and 36 patients (34%), respectively. HCT-CI was <4 in 77 of 101 patients with available data (76%). MRD status before transplant by flow cytometry, cytogenetic study, or polymerase chain reaction was tested in 40 patients in CR1/2 since September 2010. MRD positivity was seen in 10 patients (25% of tested patients). The majority of the stem cell donors were male (66%); a female donor for a male recipient was used in 18 patients (17%). Ninety percent of patients and 51% of donors were CMV seropositive. Donors were siblings (n = 43; 41%), children (n = 52; 49%), parents (n = 8; 8%), or other (n = 2; 2%). A NK-alloreactive donor was found in 32 patients (30%). Myeloablative (MA) conditioning was used in 53% of the patients and BM was the predominant source of stem cells (94%). The median duration of study follow-up was 18 months (range, 12-69 months).
Recipient age (>50 vs ≤50: hazard ratio [HR], 1.3; confidence interval, 0.7-2.1; P = .4), recipient sex (male vs female: HR, 1.4; confidence interval, 0.8-2.4; P = .2), donor sex (male vs female: HR, 0.9; confidence interval, 0.5-1.6; P = .8) donor CMV serostatus (negative/positive: HR, 0.97; confidence interval, 0.6-1.6; P = .9), and recipient CMV serostatus (negative/positive: HR, 0.8; confidence interval, 0.3-2.1; P = .7) were not significant predictors of PFS. However, HCT-CI (score, >4 vs ≤4: HR, 2.05; confidence interval, 1.1-3.9; P = .03) was a significant negative prognostic factor (supplemental Table 1, see supplemental Data available on the Blood Web site).
Individuals with unfavorable cytogenetics had a worse PFS (HR, 2.1; confidence interval, 1.2-3.7; P = .006) in comparison with those with favorable/intermediate-risk cytogenetics. Also, individuals transplanted in CR1/2 had a more favorable outcome compared with those transplanted beyond CR1/2 (HR, 0.2; confidence interval, 0.1-0.5; P < .001). MRD status (positive vs negative) for patients in CR1/2 and the intensity of conditioning regimen (non-MA vs MA: HR, 1.2; confidence interval, 0.7-1.9; P = .6) did not significantly influence PFS.
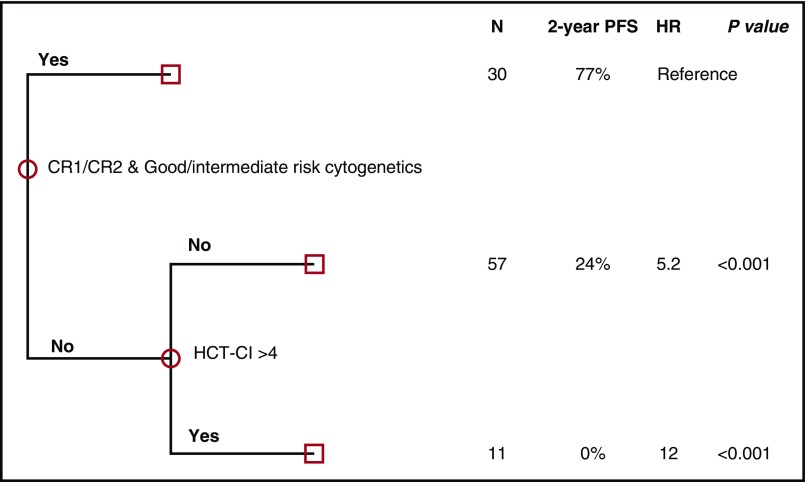
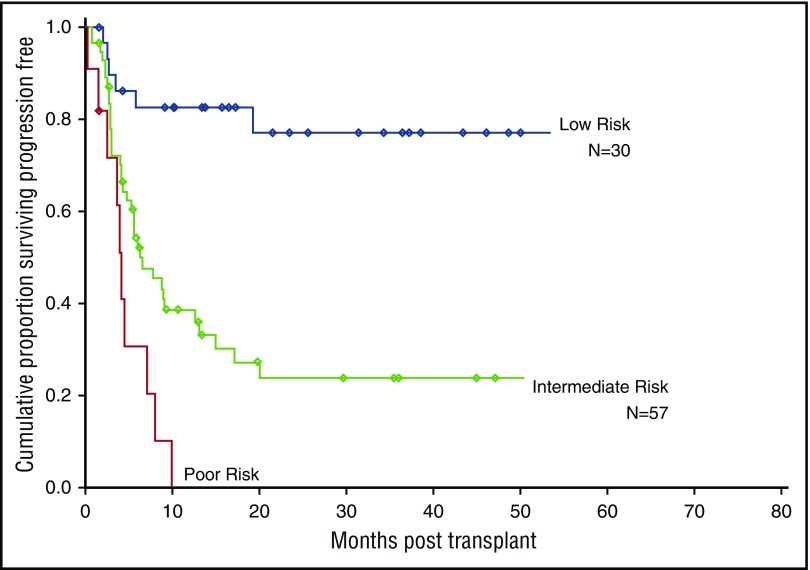
To account for potential interaction effects among the 3 factors (disease status, cytogenetics, and HCT-CI) found on univariate analysis to significantly impact PFS, multivariate analysis was performed using CART (Figure 1; supplemental Table 2). Based on this analysis, 3 mutually exclusive risk groups were identified (Figure 2; supplemental Table 3): a low-risk group (patients in CR1/2 with intermediate/favorable-risk cytogenetics, irrespective of HCT-CI [N = 30]), an intermediate-risk group (patients beyond CR1/2 and/or with adverse-risk cytogenetics and HCT-CI score ≤4 [N = 57]), and a high-risk group (patients with at least 1 negative prognostic factor: beyond CR1/2 and/or with adverse-risk cytogenetics and a HCT-CI score >4 [N = 11]) (Figure 1). The 2-year OS for the 3 groups was 83% (HR, reference), 30% (HR, 4.6; P = .002), and 12% (HR, 9.3; P < .001), respectively, and the actuarial 2-year PFS for these 3 groups was 77% (HR, reference), 24% (HR, 5.2; P < .001), and 0% (HR, 12.2; P < .001), respectively (Figure 2). The cumulative incidence of disease progression at 2 years was 5% (reference), 48% (P = .004), and 51% (P = .005) in low-, intermediate- and high-risk groups respectively.
Figure 1.
CART analysis (multivariate) for PFS using disease risk category and HCT-CI.
Figure 2.
PFS based on the 3 risk groups.
Here, we evaluated disease (cytogenetics and disease status at transplant) and patient-related (HCT-CI) variables in a uniform group of AML/MDS patients receiving HaploSCT using posttransplant cyclophosphamide for GVHD prophylaxis to determine the combined effect of these variables and develop a predictive model for PFS. Our findings revealed that HCT-CI of patients in CR1/2 with favorable/intermediate cytogenetic risk did not impact PFS. In contrast, in patients who were beyond CR1/2 and/or had adverse-risk cytogenetics, a high comorbidity index (HCT-CI >4) was associated with significantly lower PFS. Finally, by integrating these significant predictors of PFS in multivariate models (HCT-CI, remission status, and cytogenetic data), we identified 3 risk groups (low, intermediate, and high) with very distinct survival rates ranging from 0% to ∼80%.
These findings could have important clinical and therapeutic implications. First, the highest survival rate was observed in patients in morphologic remission at time of transplant with intermediate/good-risk cytogenetics. In this group, only 1 patient relapsed and HCT-CI did not appear to significantly impact survival. Therefore, utilization of posttransplant maintenance therapies in this population may not significantly impact treatment outcomes. Another group of patients for whom HCT-CI appeared to be crucially important was the group of patients at high risk for disease relapse (beyond CR1/CR2 or with high-risk cytogenetics). A high HCT-CI in these patients resulted in uniformly negative outcomes. This group of patients with very poor prognosis should probably not be transplanted. Lastly, the intermediate-risk group could be the group that would benefit the most from posttransplant interventions to decrease relapse rate.
This analysis is limited by a relatively small number of patients treated with haploidentical transplants only. Factors included in the risk model were selected from significant factors identified in the multivariable model for PFS without weighting of the prognostic impact of each component. This model needs to be validated in a larger number of patients, including HLA-matched transplants, as it could have major implications in treatment decisions.
In conclusion, risk stratification models combining disease and patient characteristics could further refine prognosis for potential ASCT patients, better identify patients who could benefit from maintenance therapies posttransplant, as well as serve as a tool to further compare results among different studies.
Supplementary Material
The online version of this article contains a data supplement.
Authorship
Contribution: L.S.B. and R.R. collected data and wrote the manuscript; R.M.S. analyzed data and reviewed and approved the manuscript; P. Kongtim. collected data, interpreted the results, and wrote the manuscript; J.C. and G.R. contributed with data collection and reviewed and approved the manuscript; W.W., A.A., S.A., C.M.H., S.P., M.Q., I.F.K., Q.B., B.O., U.P., E.J.S., D.M., K.R., P. Kebriaei, and R.E.C. contributed with treatment of patients and reviewed and approved the manuscript; and S.O.C. contributed with study design, data collection and interpretation, and manuscript writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stefan O. Ciurea, The University of Texas MD Anderson Cancer Center, Unit 423, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: sciurea@mdanderson.org.
References
- 1.Blood flashback: Thomas ED, Buckner CD, Banaji M, et al. . One hundred patients with acute leukemia treated by chemotherapy, total body irradiation, and allogeneic marrow transplantation. Blood. 1977;49(4):511-533. Blood. 2016;128(20):2373. [DOI] [PubMed] [Google Scholar]
- 2.Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood. 2016;127(1):62-70. [DOI] [PubMed] [Google Scholar]
- 3.Löwenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341(14):1051-1062. [DOI] [PubMed] [Google Scholar]
- 4.Grimwade D, Hills RK, Moorman AV, et al. ; National Cancer Research Institute Adult Leukaemia Working Group. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116(3):354-365. [DOI] [PubMed] [Google Scholar]
- 5.Petersdorf EW, Gooley TA, Anasetti C, et al. . Optimizing outcome after unrelated marrow transplantation by comprehensive matching of HLA class I and II alleles in the donor and recipient. Blood. 1998;92(10):3515-3520. [PubMed] [Google Scholar]
- 6.Sorror ML, Maris MB, Storb R, et al. . Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armand P, Gibson CJ, Cutler C, et al. . A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood. 2012;120(4):905-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slovak ML, Kopecky KJ, Cassileth PA, et al. . Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96(13):4075-4083. [PubMed] [Google Scholar]
- 9.Ciurea SO, Mulanovich V, Saliba RM, et al. . Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18(12):1835-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457-481. [Google Scholar]
- 11.Cox D. Regression models and life tables. J R Stat Soc Ser B. 1972;34:187-220. [Google Scholar]
- 12.van Putten W. CART: Stata module to perform classification and regression tree analysis. Chestnut Hill, MA: Statistical Software Components; 2006. [Google Scholar]