Abstract
Introduction
Bladder cancer is responsible for more than 130,000 deaths annually worldwide. Intravesical delivery of chemotherapeutic agents provides effective drug localization to the target area to reduce toxicity and increase efficacy. This study aimed to develop an intravesical delivery system of gemcitabine HCl (Gem-HCl) to provide a sustained-release profile, to prolong residence time, and to enhance its efficiency in the treatment of bladder cancer.
Materials and methods
For this purpose, bioadhesive microspheres were successfully prepared with average particle size, encapsulation efficiency, and loading capacity of 98.4 µm, 82.657%±5.817%, and 12.501±0.881 mg, respectively. For intravesical administration, bioadhesive microspheres were dispersed in mucoadhesive chitosan or in situ poloxamer gels and characterized in terms of gelation temperature, viscosity, mechanical, syringeability, and bioadhesive and rheological properties. The cytotoxic effects of Gem-HCl solution, Gem-HCl microspheres, and Gem-HCl microsphere-loaded gel formulations were evaluated in two different bladder cancer cell lines: T24 (ATCC HTB4TM) and RT4 (ATCC HTB2TM).
Results
According to cell-culture studies, Gem-HCl microsphere-loaded poloxamer gel was more cytotoxic than Gem-HCl microsphere-loaded chitosan gel. Antitumor efficacy of newly developed formulations were investigated by in vivo studies using bladder-tumor-induced rats.
Conclusion
According to in vivo studies, Gem-HCl microsphere-loaded poloxamer gel was found to be an effective and promising alternative for current intravesical delivery-system therapies.
Keywords: gemcitabine HCl, intravesical chemotherapy, superficial bladder cancer micro-spheres, mucoadhesive gel, in situ gel
Introduction
Although intensive research on cancer therapy, such as on surgery, radiotherapy, and chemotherapy, has been carried out, cancer still has high worldwide mortality.1–3 Bladder cancer is the ninth-most commonly diagnosed cancer in the world for both sexes and the second-most common malignancy of the urogenital tract.4 It is relatively common in more developed regions, and occurs among men more than women. The worldwide incidence rate for bladder cancer is 8.9 for men and 2.2 for women (sex ratio 4.04:1), but the reasons for this sex difference are still unclear.5 More than 70% of bladder cancers are noninvasive or superficially invasive at diagnosis. Transurethral resection of the tumor is the first-choice treatment for such patients, but it commonly results in tumor relapse, and thus more aggressive therapies are needed.6–8 Although a number of treatment strategies, including systemic immunotherapy/chemotherapy and radiotherapy, have been used recently, the overall survival rate has not improved, and bladder cancer is associated with serious morbidity and even mortality. Therefore, it is clear that alternative treatment approaches for bladder cancer are still needed.9,10
Intravesical therapy has the potential to be an alternative to the treatment of superficial bladder cancer. During this treatment, drugs are instilled directly into the bladder through a catheter. It ensures high drug concentrations in tumor-bearing bladder tissue while reducing systemic exposure and adverse effects. The most common agents for intravesical application for bladder cancer are immunotherapeutic (bacillus Calmette–Guérin) and chemotherapeutic (thiotepa, mitomycin C, doxorubicin, and epirubicin) agents. Unfortunately, these agents have been shown to achieve complete response of 34%–53%, and there is still a need for more effective chemotherapeutic agents for intravesical treatment of superficial bladder cancer.11
Gemcitabine hydrochloride (Gem-HCl; 2′,2′-difluorodeoxycytidine) is a water-soluble pyrimidine analogue with a broad spectrum of antitumor activity, and when given intravesically it has been shown to produce good response rates for the treatment of superficial bladder cancer.12,13 It is transported into the cell, phosphorylated, and incorporated into DNA and RNA, which causes inhibition of growth activity and mediates apoptosis.14 The success of intravesical chemotherapy with Gem-HCl depends on direct contact between the drug and the abnormal urothelium. Therefore, mechanisms that prolong exposure of the urothelium to the drug are expected to increase the efficacy of the treatment.15,16 For this purpose, several intravesical drug-delivery systems have been developed; however, these carriers are generally maintained intravesically for approximately 2 hours, due to washout by urine.17 This limitation can be avoided by the use of mucoadhesive drug-delivery systems, and mucoadhesion characteristics can be coupled with particulate systems, such as liposomes, nanoparticles, or microspheres (MSs).18 Among these systems, MSs have larger dimensions that ensure higher loading capacity. Also, when they are prepared with mucoadhesive polymers, they increase residence time, due to strong adhesion to the mucosa. However, to be administered intravesically, MSs need to be dispersed in liquids (ie, physiological serum) or in gel systems (ie, in situ or mucoadhesive gels). In situ gels are liquid formulations at storage conditions, but when administered in vivo, they transform into a gel at the target site with responses to various environmental conditions.19 Mucoadhesive gel systems can extend drug exposure in the bladder cavity beyond the voiding of urine and are capable of sustaining the release of active substances, ensuring highly desirable effects.20
The primary aim of this study was to develop bioadhesive Gem-HCl MSs prepared with Carbopol 2020 NF and Eudragit E100 (EE100) for the intravesical treatment of superficial bladder cancer. Afterward, MSs were dispersed in in situ poloxamer (Plx) gel or mucoadhesive chitosan (Chi) gel to prolong intravesical residence time, provide sustained release, and enhance efficiency. Finally, cytotoxic effects of Gem-HCl-loaded formulations were evaluated in T24 and RT4 bladder cancer cell lines and the efficacy of formulations histopathologically evaluated in male rats with an experimental non-muscle-invasive bladder cancer model.
Materials and methods
Gem-HCl was purchased from Sun Pharmaceutical Industries (Mumbai, India). Chi (high molecular weight) was purchased from Sigma-Aldrich (St Louis, MO, USA). Plx 188 and Plx 407 were kind gifts from BASF Chemicals (Ludwigshafen, Germany). EE100 and Carbopol 2020 NF were gifts from Evonik Industries (Essen, Germany) and Lubrizol (Wickliffe, OH, USA), respectively. Magnesium stearate was purchased from ZAG Laboratories (Istanbul, Turkey). All other chemicals were of analytical grade.
Fourier-transform infrared spectrum analysis
Gem-HCl, EE100, Carbopol 2020 NF, and their mixtures were homogeneously mixed with potassium bromide and the resulting powder mixture compressed under pressure for Fourier-transform infrared (FTIR) spectrum analysis. The disks obtained were scanned at 600–4,000 cm−1 wavelength and spectra obtained using IR spectrometry (Spectrum 100; PerkinElmer, Waltham, MA, USA).
Preparation of microspheres
MSs were prepared according to the solvent-evaporation method.15 Briefly, 1.5 g EE100 was dissolved in 8 mL acetone, and 1 g Carbopol 2020 NF was added as powder. Suspension of 0.3 g magnesium stearate and 0.5 g Gem-HCl in 4 mL acetone was prepared separately and added to the polymer dispersion. The homogeneous final dispersion was cooled to 5°C and poured slowly with stirring at 750 rpm into 80 mL liquid paraffin, which had previously also been cooled to 5°C. The obtained emulsion was stirred at 40°C for 50 minutes. After being cooled to room temperature, 10 mL n-hexane was added to the emulsion and stirring continued at 750 rpm for 10 minutes. The MS suspension in liquid paraffin was filtered and MSs washed with 50 mL n-hexane five times and dried at room temperature overnight. MSs were sieved and 125 µm used for further testing because of high production yield.15
Characterization of microspheres
Particle-size distribution
Mean diameters of MSs were determined with a Mastersizer 3000 (Hydro EV; Malvern Instruments, Malvern, UK). During measurements, they were dispersed in n-hexane and stirred at 2,000 rpm. All trials were run in triplicate.
Scanning electron microscopy
MSs were mounted onto an aluminum stub, sputter-coated with gold palladium (Au/Pd) using a vacuum evaporator, and surface morphology was examined by scanning electron microscopy (Phillips XL-30S FEG).
Encapsulation efficiency and drug loading of microspheres
Drug-loaded MSs (2 mg) were dissolved in 2 mg ammonium acetate buffer solution (pH 5.5) and mixed at 100 rpm for 24 hours at room temperature with incubator shaker (Lab-Line MaxQ 6000). The solution was filtered through a 0.22 µm syringe filter and the filtrate analyzed by ultra-performance liquid chromatography (UPLC) to determine the amount of Gem-HCl loaded in the MSs. Encapsulation efficiency and drug loading were calculated:
| (1) |
| (2) |
Gem-HCl was determined with a validated UPLC method on a Hypersil Gold (100×2.1 mL, 3 µm) column at 25°C. Acetonitril:ammonium acetate buffer solution (pH 5.5, 2.5:97.5 v:v) was used as the mobile phase at a flow rate of 400 µL/min. Retention of Gem-HCl was 1.78 minutes at 268 µm, while total analysis took 5 minutes. The calibration curve of Gem-HCl was linear (2–10 µg/mL, r2=0.9996). Limits of detection and quantification were 0.0625 µg/mL and 0.2083 µg/mL, respectively. All other validation data, such as intra- and interday precision, accuracy, selectivity, and stability, were within the limits.
Preparation of Chi and Plx gels
Mucoadhesive Chi gel was prepared by dissolving 2% (w:w) Chi in lactic acid solution.21 The gel was left at room temperature until the solution became clear. After 24 hours, microparticles (MPs) were suspended in the gel formulation with continuous stirring until a homogeneous mixture had been produced (10 mg MPs and 2 g gel). In situ Plx gel was prepared by adding Plx 407 (20% w:w) and Plx 188 (10% w:w) to distilled water at 4°C with continuous stirring (cold method). The gel was left at 4°C until it became clear. After 24 hours, MPs were suspended in the gel formulation with continuous stirring until a homogeneous mixture had been produced (10 mg MPs and 2 g of gel).22,23
Dilution of Chi and Plx gels with Tyrode solution
Mucoadhesive Chi gel and in situ Plx gel were diluted with Tyrode solution (used as artificial urine)24 at a 1:1 ratio to mimic in vivo conditions of the bladder. Subsequently, the diluted gels were characterized in detail to evaluate changes in their structure when they were mixed with urine.
Characterization of gels
Gelation temperature and time of in situ Plx gels
Gelation temperature and time of in situ Plx gels were determined from oscillation measurements (Haake Mars rheometer, AR 2000; Thermo Fisher Scientific, Waltham, MA, USA) using a stainless-steel probe (plate/plate, 35 mm). Samples were heated at 7°C–70°C at a rate of 2°C/min. The temperature versus viscosity (η′) graph was plotted and the gel-transition point defined as halfway between the viscosity of the solution and the viscosity of the gel form (n=6).25
Mechanical, syringeability, and bioadhesive properties
Mechanical, syringeability, and bioadhesive properties of formulations were determined using a software-controlled penetrometer (TA-XT Plus texture analyzer; Stable Micro Systems, Godalming, UK) with a 0.5 kg load cell. For determination of mechanical properties, an analytical probe (10 mm diameter) was compressed twice into formulations to a defined depth at a constant rate (2 mm/s) at both 25°C and 37°C. Mechanical parameters (hardness, adhesiveness, compressibility, cohesiveness, and elasticity) were calculated from the obtained force–time curves (n=6).26 Syringeability was determined as the required work to expel formulations from a syringe, and was measured using a force transducer. Formulations were packed into plastic syringes connected to a catheter, and the plunger of the syringe was pushed at a constant force (0.5 N). Resistance to expressing the formulations through the catheter was derived from the area under the force–time curves at 25°C±0.1°C (n=6).27
Bioadhesive properties of formulations were determined using bovine mucosal bladder tissue obtained from a local slaughterhouse. Mucosal membrane sections were attached to the holder of the texture analyzer at 37°C. Gels were placed at the lower end of the probe and the probe lowered onto the bladder mucosa surface at a constant speed (1 mm/s). Contact force (0.05 N) was applied for 2 minutes, after which the probe was moved upward. The area under the curve (mucoadhesion) was determined from the resultant force–distance graph (n=6).16
Viscosity studies
The experimental setup for measuring the effective viscosity of formulations consists of a sine-wave viscometer (Vibro SV-10) with a measurement range of 10–10,000 mPa⋅s. The viscosity produced between the sensor plates of the viscometer and the sample fluid was measured at 25°C and 37°C±0.1°C (n=6).28
Rheological measurements
Rheological properties of formulations were characterized using a Haake Mars AR 2000 rheometer at 25°C and 37°C±0.1°C. Continuous-shear analysis of formulations were performed in flow mode with a parallel steel geometry plate (diameter 35 mm, 0.3 mm gap). Formulations were applied to the lower plate of the rheometer and flow curves measured over a range of shear rates (10–1,000/second).29 Oscillatory analysis of formulations was performed in the linear viscoelastic region to determine storage modulus (G′) and loss modulus (G″). Frequency-sweep analysis was performed at 1 Pa amplitude at a frequency range of 0.1–10 Hz (0.3 mm gap).30,31
In vitro release studies
Plx or Chi gels (2 g) containing 10 mg Gem-HCl-loaded MSs were put into dialysis membrane tubes (Spectra/Por regenerated cellulose, molecular weight cutoff 12,000–14,000 Da). Dialysis membrane tubes were placed into 100 mL pH 6.5 PBS to mimic slightly acidic conditions of urine and stirred at 300 rpm (37°C±0.1°C) At predetermined time intervals, samples were withdrawn and drug content analyzed using UPLC (n=6).
Determination of release mechanism
The data were fitted to Peppas’s equation and best-fit parameters calculated to determine the release mechanism of formulations:32
| (3) |
where Mt/M∞ is the fractional release, k the diffusional constant, and n the diffusional exponent that characterizes the drug-release mechanism.
Ex vivo permeation studies
Ex vivo permeation studies were performed on freshly excised bovine bladder mucosa with Franz-type diffusion cells. Briefly, mucosa was mounted on diffusion cells, formulations placed in a donor compartment, and the receptor compartment filled with pH 6.5 PBS (20 mL). Samples were taken from the receptor compartment at predetermined time intervals, and the permeated amount of Gem-HCl was determined with UPLC (n=6). At the end of ex vivo permeation study, the excess formulation was removed and the mucosa fixed in 4% neutral-buffered formalin. Standard procedures were applied to prepare 4 mm-thick tissue sections, specimens were stained with H&E, and mucosa samples were evaluated histopathologically with a microscope (Olympus BX50) to evaluate tissue damage to healthy mucosa.33
Cytotoxic assay of formulations
The cytotoxic effects of aqueous Gem-HCl, Gem-HCl-loaded MSs and Gem-HCl-loaded or blank MS containing and suspended in saline gels were evaluated by living cell counting. For this purpose, both RT4 (ATCC HTB2TM) and T24 (ATCC HTB4TM) cells were seeded in 12-well plates (1.5×105cells/well) and allowed to attach for 24 hours. Then, they were treated with different concentrations (0.1–100 µM) of saline containing Gem-HCl, saline containing Gem-HCl-loaded MSs, blank MSs suspended in saline, suspended in saline Plx or Chi gels containing Gem-HCl-loaded MSs and Plx or Chi gels containing blank MSs. Following 48 hours of exposure, the treatments were removed and cells washed with fresh McCoy’s 5A medium (Thermo Fisher Scientific). Then, cells were trypsinized with PBS disaggregated with a pipette. Harvested cells were stained with trypan blue dye and counted under microscopy (Olympus IX71) by two independent investigators using hemocytometry. Viable (unstained) cells have intact cell membranes that exclude trypan dyes, whereas unviable (stained) cells take up this dye. For each concentration of Gem-HCl or each formulation, the number of viable cells per milliliter was calculated.
Cells were also incubated in culture medium alone and served as a control for cell viability. Cell viability was calculated as a percentage of the absorbance reading of compounds compared to control readings. For statistical analysis, the cell-growth inhibition potency of formulations is expressed as IC50 values. IC50 was computed by nonlinear regression using GraphPad Prism 5.0 software. Cell death caused by the maximum concentration (100 µM) applied to cells was regarded as inhibition percentage. Data are shown as mean ± SD of six experiments.
In vivo studies
For in vivo studies, Sprague Dawley rats and N-butyl-N-(4-hydroxybutyl)nitrosamine (BBN), a chemical carcinogen, was used to generate the bladder cancer model. During the in vivo study, the animals were controlled regularly in terms of health status (ie, diet situation, weight loss). Rats with a median weight of 300–350 g were selected as the experimental animal model. They were maintained in cages at 22°C–24°C with a 12-hour/12-hour dark/light cycle and humidity of 55% with free access to water and food, fed ad libitum, and randomly divided into eleven experimental groups, with each group comprising seven rats (Table 1).
Table 1.
Experimental design of rat population for in vivo studies
| Animal groups | Rats, n |
|---|---|
| 1. Negative control (tumor−, treatment+) | 7 |
| 2. Positive control (tumor+, treatment−) | 7 |
| 3. Saline containing Gem-HCl | 7 |
| 4. Saline containing blank MSs | 7 |
| 5. Saline containing Gem-HCl-loaded MSs | 7 |
| 6. Plx gel containing Gem-HCl | 7 |
| 7. Plx gel containing blank MSs | 7 |
| 8. Plx gel containing Gem-HCl-loaded MSs | 7 |
| 9. Chi gel containing Gem-HCl | 7 |
| 10. Chi gel containing blank MSs | 7 |
| 11. Chi gel containing Gem-HCl-loaded MSs | 7 |
Abbreviations: Gem, gemcitabine; MSs, microspheres; Plx, poloxamer; Chi, chitosan.
Bladder tumors were induced by adding 0.05% BBN to freely available drinking water in dark bottles three times a week for 20 weeks. After 1 week without treatment, rats were given weekly intravesical instillations of formulations for 4 weeks. For intravesical administration, rats were anesthetized with weight-adjusted intraperitoneal doses of chloral hydrate (400 mg/kg). Bladders were emptied and washed with saline three times before drug instillation. Intravesical instillation was applied using a 17-gauge cannula. Separate groups were treated with the different formulations, and Gem-HCl doses were given as 4 mg in 0.5 mL formulations. Animals were observed daily and clinical signs noted. Antitumor efficacy was determined by survival rate and histopathology of tumor-induced bladders after treatment.
At the end of the study, animals were killed with an overdose of chloral hydrate and bladders removed. Bladder tissue samples were immediately inserted into 4% neutral buffered formalin. The samples were subsequently processed with conventional techniques. H&E-stained slides were reviewed under standard light microscopy. Histopathological alterations were assessed by an experienced pathologist blinded to the experimental groups and efficacy of treatment assessed. The “neoplasmic process” score in urothelial epithelium was assessed: normal was graded 0, urothelial hyperplasia (flat and/or papillary) was graded 1, urothelial dysplasia and hyperplasia were graded 2, urothelial neoplasm with low malignancy potency was graded 3, urothelial carcinoma papillary (low grade) was graded 4, urothelial carcinoma papillary (high grade) was graded 5, and urothelial carcinoma in situ/urothelial carcinoma (high grade, early invasive) was graded 6. In vivo studies were performed in accordance with the Turkish law for the protection of animals and were approved by the local ethical committee of the Kobay laboratory animals research and breeding unit (approval number 151, July 11, 2015, Ankara, Turkey).
Stability studies
Formulation stability was studied by storing samples at three different temperatures and relative humidity (RH; 5°C±2°C, 25°C±5°C/60% RH, 40°C±5°C/75% RH). They were inspected visually for organoleptic properties, and Gem-HCl amounts were evaluated at days 15, 30, and 60.
Statistical analysis
Statistical data analysis was performed using Student’s t-test with P<0.05 as the minimal level of significance.
Results and discussion
Conventional bladder cancer treatment with systemic administration of chemotherapeutic drugs is highly ineffective, since only a small fraction of the drug reaches the affected site.34 Therefore, development of novel drug-delivery systems, especially for high-risk non-muscle-invasive bladder cancer, is an important topic considering the currently available options. Bladder is an especially appropriate organ for local application, because it is easily approached from outside the body. Therefore, among several proposed strategies, intravesical administration might be the most convenient clinical application to ensure maximal delivery of therapeutic agents at the site of the disease, to improve efficacy, and to minimize systemic side effects of antineoplastic agents. However, short dwelling time of drugs in the bladder and the low drug permeability of urothelium are the main factors that limit the success of the treatment. The efficacy of intravesical treatment might be increased by prolonging the residence time of the drug inside the bladder. By this way, drugs can attach to the urinary bladder wall and penetrate it for extended periods. For these reasons, the present study describes the preparation of mucoadhesive Gem-HCl-loaded MSs to provide a sustained-release profile, prolong residence time, and enhance efficiency for bladder cancer. In addition, MSs were dispersed in mucoadhesive Chi or in situ Plx gels for intravesical administration and comprehensive in vitro/ex vivo/in vivo evaluations performed.
FTIR analysis
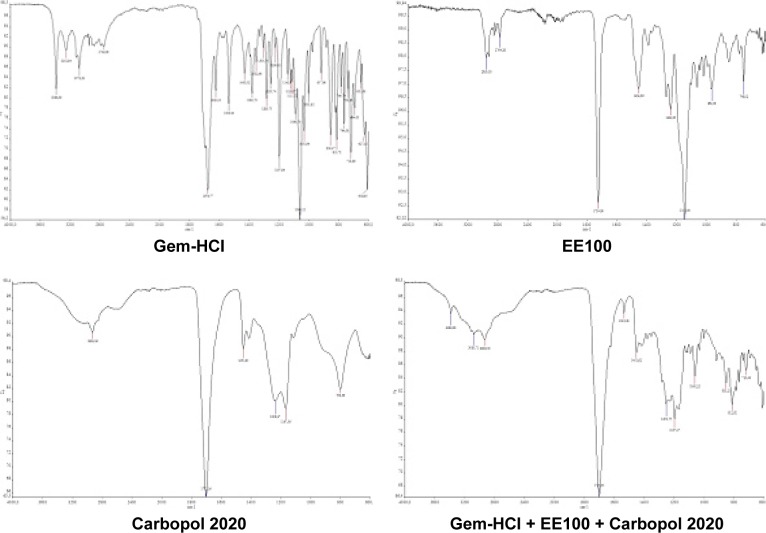
Data obtained from FTIR analysis were found to be compatible with the literature findings, and there was no interaction between the active substance and the polymers (Figure 1).
Figure 1.
Fourier-transform infrared spectra of Gem-HCl, EE100, Carbopol 2020, and their mixture.
Abbreviations: Gem, gemcitabine; EE100, Eudragit E100.
Preparation and characterization of microspheres
MSs were successfully prepared by solvent evaporation.15 For preformulation studies, different stirring rates and times were used for preparation of MSs (ie, 40 minutes at 1,000 rpm, 60 minutes at 1,000 rpm, 40 minutes at 750 rpm, and 60 minutes at 750 rpm). The results obtained showed that stirring rate and time were important parameters for this technique. For instance, the shapes of the particles were irregular at high stirring speed of 1,000 rpm. Also, 40 minutes of stirring time was not enough to disperse the inner phase in the outer phase at 750 rpm (data not shown). Similar results have been reported previously, and various manufacturing parameters (apparatus design, type of stirrer, stirring speed, viscosity of emulsion phases, and stabilizer concentration) were shown to affect particle characteristics.35,36 By keeping drug and polymer amounts constant, spherical particles with narrow size distribution and good surface characteristics were obtained when MPs were prepared for 60 minutes at 750 rpm, and so these parameters were selected as optimum for the preparation.
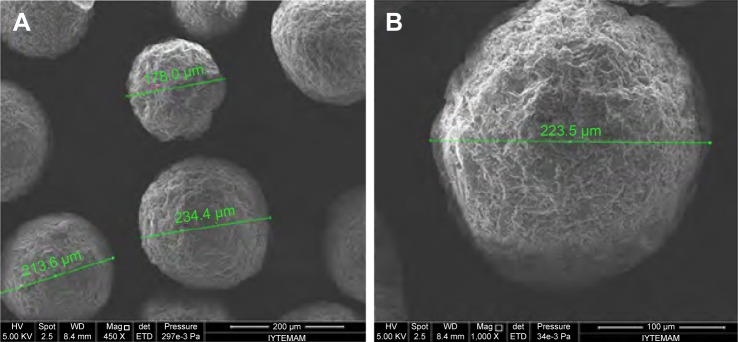
The particle size and distribution of MSs were determined by laser-light scattering. Particle size is expressed as mean volume diameter in micrometers and characterized by 10%, 50% and 90% undersized diameters: D10, D50, and D90. D50 was defined as the diameter where half the particles’ size was below this value, and was considered the mean diameter. The results obtained showed that 50% of the Gem-HCl-loaded MSs were <180 µm, whereas 50% of the blank MSs were <98.4 µm, with significantly narrower size distribution (Table 2). The surface morphology of the MSs was observed by scanning electron microscopy, and most MSs were shown to be spherical with rough surface morphology (Figure 2).
Table 2.
Particle-size distribution of formulations
| Blank MS formulation (sieved from 125 µm)
| ||||
|---|---|---|---|---|
| D10 (µm) | D50 (µm) | D90 (µm) | Span | |
| Mean | 49.100 | 98.400 | 156.000 | 1.086 |
| SD (%) | 0.300 | 0.849 | 3.320 | |
| RSD (%) | 0.610 | 0.863 | 2.130 | |
|
| ||||
|
Gem-HCl-loaded MS formulation (sieved from 125 µm)
| ||||
| Mean | 120.000 | 180.000 | 263.000 | 0.794 |
| SD (%) | 0.807 | 0.365 | 1.100 | |
| RSD (%) | 0.670 | 0.202 | 0.416 | |
Abbreviation: MS, microsphere.
Figure 2.
Scanning electron microscopy of Gem-HCl-loaded MSs.
Note: (A) Magnification 450×; (B) magnification 1,000×.
Abbreviations: Gem, gemcitabine; MSs, microspheres; Mag, magnification; HV, high voltage; WD, working distance; det, detector of secondary electrons; ETD, Everhart-Thornley detector.
Gem-HCl-loaded MSs were produced with high drug-encapsulation efficiency and loading capacity for a water-soluble active substance. Encapsulation efficiency and loading capacity were found to be 82.657%±5.817% and 12.501%±0.881%, respectively. Also, MPs were produced with high production yield (86.94% for total produced MSs and 47.615% for particles in 125 µm fraction). Considering the acceptable encapsulation efficiency, loading capacity, and high production yield, it can be concluded that solvent evaporation is a simple and suitable technique for producing Gem-HCl-loaded MSs.
Preparation of Chi and Plx gels
To modify the release profile of Gem-HCl, obtain sustained-release properties, prolong residence time, and increase therapeutic efficiency, MSs were suspended in two different gels for intravesical administration. The use of an in situ gel formulation allows for easy administration of the liquid formulation into the bladder. It also provides prolonged contact between the drug and the affected tissue, due to the in situ phase transition to a gel on the surface of the bladder. Another important advantage of in situ gel system is the formation of a uniformly thin layer of gel at the application site. This thin layer does not cause any disturbance in urine flow or affect bladder filling.37 In one of our previous studies, different Plx 407 and 188 combinations were evaluated for gelation temperature/time, mechanical, rheological, and bioadhesive properties. The results showed that a 20% Plx 407 and 10% Plx 188 mixture was an optimal in situ gelling system for mucosal applications.23 Therefore, in this study a 20% Plx 407 and 10% Plx 188 mixture was chosen as the in situ carrier for Gem-HCl MSs.
Designing intravesical formulations using bioadhesive biomaterials that are strongly adhered to urothelial cells prevents the carrier from being washed away during urine voiding and increases the residence time of the drug at the target site. Therefore, MSs were also suspended in bioadhesive GHI gel to prolong residence in the bladder. To this end, high-molecular-weight Chi at 2% concentration was chosen as the gel base because of its strong mucoadhesiveness, proper mechanical and rheological characteristics, and extended mucosal retention time.21
One of the important limitations of intravesical drug delivery is the dilution of instilled drug formulations with urine. The maximum urine volume in the bladder is generally 250–350 mL, and because of incomplete emptying, nearly 50 mL urine generally stays, even after voiding. Chemotherapeutic drugs are generally applied with intravesical installation of 20–60 mL formulation. Therefore, in vivo conditions in the bladder were simulated by dilution of formulations with artificial urine (Tyrode solution) at a 1:1 ratio, and changes in properties of in situ Plx gel and mucoadhesive Chi gel investigated in detail.16
Characterization of gels
The Chi and Plx gels containing Gem-HCl-loaded MSs were characterized in terms of their of gelation temperature, viscosity, mechanical, syringeability, bioadhesive, and rheological properties. Characterization studies were also performed with gel formulations without MSs and gel formulations diluted with Tyrode solution to see whether there were a change in the gel structure.
Gelation temperature and time of in situ Plx gels
The suitable temperature range for mucosal application has been reported to be 30°C–36°C.38 Also, the gelling temperature of the formulation should be >25°C to avoid difficulties in manufacturing, handling, and administration at room temperature.39 The bladder temperature is around 37°C,40 hence we aimed at preparing thermoreversible gel formulations that might be in a gel state at 30°C–36°C and a liquid state <25°C. In this way, the thermoreversibility of the in situ gel formulation would contribute to increased contact time in the bladder. The results showed that the gelation temperatures of Plx gel and Gem-HCl MS-loaded Plx gel were 34.09°C±1.54°C and 36.53°C±2.02°C, respectively, and these values indicated that the prepared formulations were in liquid state at room temperature and transformed into the gel state after instillation in the bladder. On the other hand, the gelation temperatures of Plx gel–Tyrode and MS-loaded Plx gel–Tyrode mixtures were significantly increased and found to be 51.73°C±0.99°C and 47.95°C±0.76°C, respectively. This unfavorable result showed that the presence of urine affected the gelation temperature of the formulation and the in situ gel formulation lost its gel structure in the bladder.
In addition, the gelation-time results support our findings, and dilution with Tyrode solution significantly extended the gelation time of the formulations. The gelation times of Plx gel, MS-loaded Plx gel, Plx gel–Tyrode solution, and MS-loaded Plx gel–Tyrode solution were found to be 327.67±25.88 seconds, 324.67±34.25 seconds, 457±4 seconds, and 419.45±18.49 seconds, respectively. To overcome these problems, emptying the bladder before drug instillation, suppression of the urine-production rate by the kidneys, or regulation of fluid intake before and after drug administration are highly recommended before the administration of in situ gels.15
Mechanical, syringeability, and bioadhesive properties
Intravesical formulations should have suitable mechanical properties for easy administration and patient compliance, such as easy removal from the catheter, high spreadability on the bladder mucosa, and strong mucoadhesion. Texture-profile analyses allow the gathering of information about the gel structure and evaluation of the mechanical properties of the formulations.27 In this study, the mechanical properties of the formulations were characterized in terms of hardness, compressibility, adhesiveness, elasticity, and cohesiveness. Hardness value should be low to allow easy administration and good spreadability. Compressibility value should be low to remove the formulation from the catheter easily. Adhesiveness can be related to mucoadhesive properties and should be high. Elasticity represents the return rate of the deformed sample to its beginning condition. Finally, cohesiveness shows the effect of repeated shearing stresses on the formulations.
As shown in Table 3, similar hardness values were obtained at 25°C for all formulations. However, due to gelation–temperature properties, Plx gel alone and Plx gel containing MSs showed 10.5-fold and 8.3-fold higher hardness values than Chi gel alone and Chi gel containing MSs at 37°C, respectively. Depending on increasing temperature, compressibility and adhesiveness values of Chi gels containing MSs decreased significantly, and this was thought to be associated with the thermal motion of the polymer molecules.41 No significant temperature-dependent change was observed with cohesiveness, hardness, or elasticity values of Chi gels. These results were in accordance with rheological evaluations. In contrast, Plx gels strengthened, and mechanical properties were improved by increasing the temperature from 25°C to 37°C. Blank and Plx gels containing MSs exhibited the highest hardness and compressibility values at 37°C, supporting the results obtained by oscillatory rheology (ie, increased elastic behavior represented by G″ in Figure 3). Higher adhesiveness could mean greater adhesion at the mucosal surface, which is a desirable characteristic required to increase drug retention in the bladder.42,43 Based on the results, it appeared that Plx gels containing MSs at 37°C could meet these desirable mechanical properties; nevertheless, further in vitro bioadhesion tests were performed to confirm initial results. The addition of MSs or the presence of Tyrode solution strongly weakened the mechanical properties of both mucoadhesive Chi and in situ Plx gels, but the most significant decrease was seen in Plx gels. These results showed that the presence of urine in the bladder is an important factor that may affect the retention of formulations.
Table 3.
Mechanical, syringeability, bioadhesion, and viscosity values of Chi and Plx gels (n=6)
| Formulation | Hardness, N | Compressibility, N⋅second | Adhesiveness, N⋅second | Cohesiveness | Elasticity | Syringeability, N⋅mm | Bioadhesion, mN⋅mm | Viscosity, cP |
|---|---|---|---|---|---|---|---|---|
| Chi gel | ||||||||
| 25°C | 0.014±0.001 | 0.042±0.007 | 0.121±0.028 | 1.008±0.024 | 0.896±0.112 | 130.140±2.857 | – | 1,200±0 |
| 37°C | 0.018±0.002 | 0.075±0.011 | 0.119±0.039 | 1.016±0.005 | 1.041±0.037 | – | 2,066.18±236.34 | 973.67±2.08 |
| Chi gel containing MSs | ||||||||
| 25°C | 0.016±0.00 | 0.120±0.004 | 0.085±0.013 | 1.029±0.015 | 1.061±0.052 | 131.340±7.564 | – | 836±1 |
| 37°C | 0.017±0.003 | 0.052±0.011 | 0.053±0.004 | 0.979±0.020 | 0.958±0.073 | – | 2,410.60±285.31 | 789.67±1.53 |
| Chi gel–Tyrode solution | ||||||||
| 37°C | 0.006±0.001 | 0.011±0.002 | 0.007±0.001 | 0.987±0.012 | 0.999±0.207 | – | 1,067.63±364.98 | 90.1±0.1 |
| Chi gel containing MS–Tyrode solution | ||||||||
| 37°C | 0.008±0 | 0.018±0.001 | 0.007±0 | 1.000±0 | 0.987±0.048 | – | 1,676.50±248.29 | 102.67±0.58 |
| Plx gel | ||||||||
| 25°C | 0.018±0 | 0.114±0.003 | 0.006±0.001 | 1.058±0.027 | 0.951±0.021 | 99.830±1.510 | – | 636±0 |
| 37°C | 0.189±0.009 | 0.355±0.019 | 0.297±0.017 | 1.008±0.005 | 0.905±0.017 | – | 1,951.74±186.17 | –* |
| Plx gel containing MSs | ||||||||
| 25°C | 0.010±0 | 0.026±0 | 0.005±0 | 1.000±0 | 0.978±0.042 | 120.584±4.184 | – | 722.67±1.53 |
| 37°C | 0.141±0.008 | 0.430±0.080 | 0.230±0.039 | 1.331±0.121 | 0.992±0.025 | – | 2,700.72±137.82 | –* |
| Plx gel–Tyrode solution | ||||||||
| 37°C | 0.005±0 | 0.006±0.001 | 0.004±0 | 1.025±0.051 | 0.974±0.084 | – | 1,072.62±51.76 | 27.2±0.1 |
| Plx gel containing MS–Tyrode solution | ||||||||
| 37°C | 0.008±0 | 0.028±0.001 | 0.006±0.002 | 1.013±0.033 | 1.034±0.145 | – | 1,408.53±87.46 | 38.23±0.15 |
Notes:
Could not be measured because of the high viscosity of the sample; bioadhesion studies and evaluation of formulations diluted with Tyrode solution studied only at 37°C to mimic in vivo conditions; syringeability studies conducted only at 25°C to mimic room temperature. Data presented as mean ± SD.
Abbreviations: Chi, chitosan; Plx, poloxamer; MS, microsphere.
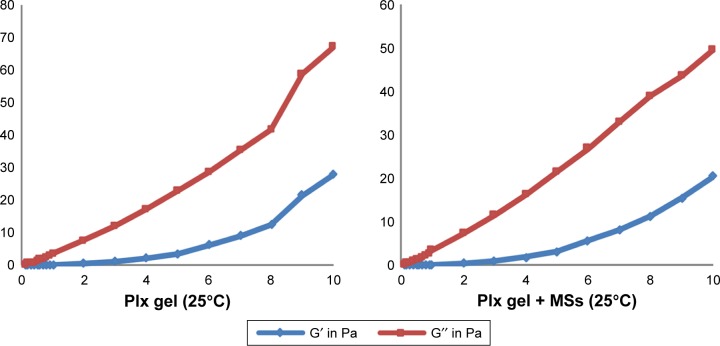
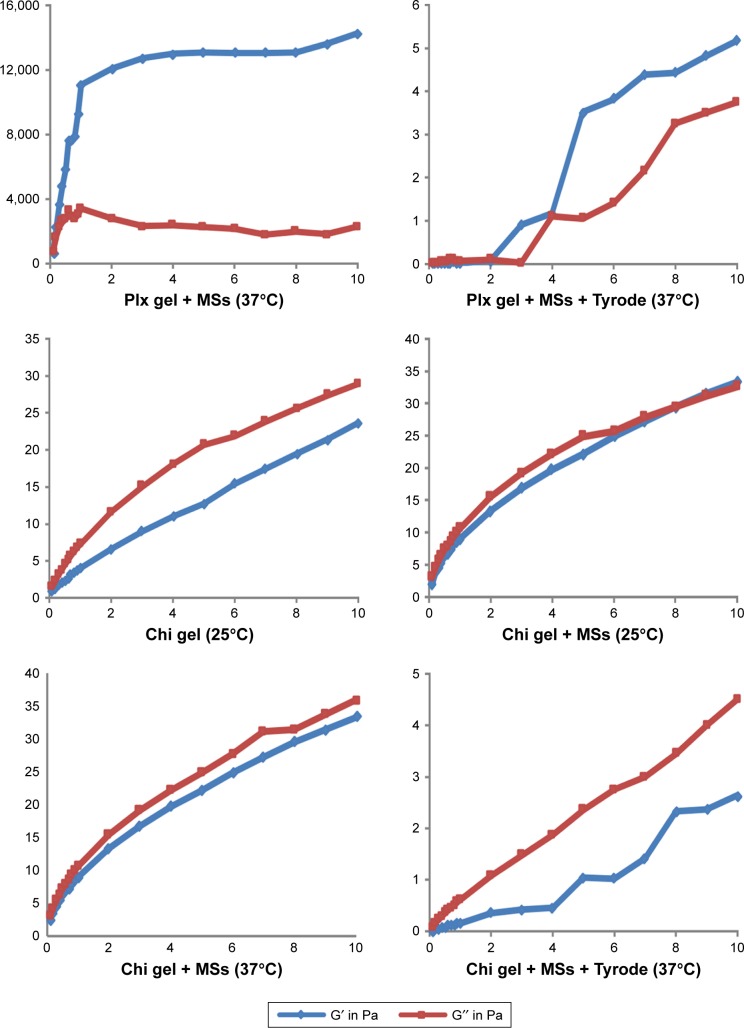
Figure 3.
Frequency-dependent changes of the viscoelastic properties of Chi versus Plx gels at 25°C and 37°C±0.1°C (n=6).
Notes: Frequency (Hz) on x-axis; moduli G′; G″ (Pa) on y-axis.
Abbreviations: Chi, chitosan; Plx, poloxamer; MSs, microspheres.
Syringeability can be considered the ability of a formulation to be easily administered by a catheter, and this property optimizes usability.27 Therefore, the work required to expel formulations from a catheter was evaluated at 25°C, and the results showed that consistently with the viscosity studies, the syringeability values of chitosan gels were higher than Plx gels. It can be concluded that the results obtained are in agreement with the viscosity and rheological studies and Plx gels are more easily applied with a catheter than Chi gels.
Intravesical drug-delivery systems could be made more effective by using bioadhesive materials that attach to the mucous membrane of the urothelium. This allows the formulation to be retained at the site of action for longer duration and ensures sustained and enhanced contact with the mucous layer.20,37,44 Bioadhesive formulations must fulfill three basic criteria: they should have rapid attachment or adhesion to the bladder wall after instillation in the bladder, they must not obstruct the flow of urine or any normal functions of the bladder, and they should be able to stay attached to the affected site, even after voiding of urine. In our study, the bioadhesive properties of the formulations were determined from the area of force–distance graphs obtained with the TA-TX Plus texture analyzer using freshly excised bovine-bladder mucosa. According to the results (Table 3), bioadhesive properties of our formulations were affected significantly by adding MSs or in the presence of Tyrode solution. At the beginning, Chi- and Plx-gel formulations had similar bioadhesive properties. Addition of MSs caused a significant increase in bioadhesion values of the gels, and this increase was more pronounced with Plx gel than Chi gel. On the other hand, when gel formulations were diluted with Tyrode solution, bioadhesive values of both gel formulations were significantly decreased. Plx and chitosan gels containing MSs showed 1.9 and 1.4 times higher bioadhesive values than their mixture with Tyrode solution. In addition, the results showed that MS-loaded Chi-gel formulations still had slightly higher bioadhesive properties than MS-loaded Plx gel. Finally, it can be concluded that both of the formulations maintained their bioadhesive properties on isolated urinary bladder tissue, even having been fully hydrated, and this may be promising for intravesical applications.
Viscosity studies
Viscosity affects mechanical properties of semisolid formulations and plays an important role in controlling drug release.45 In this study, we aimed to obtain suitable viscosity values to provide optimum fluidity at room temperature and for easy transduction through a catheter into the bladder.46 The viscosity of Chi gels decreased 1.23 times with increasing temperature (Table 3). The negative dependence of viscosity on Chi-gel temperature could be explained by enhanced chain flexibility and reduced root-mean-square unperturbed end-to-end distance of the polymer chains with increasing temperature.26,47 In addition, viscosity values of Chi gels were decreased with the incorporation of MSs at both 25°C and 37°C±0.1°C, and these results showed good agreement with literature findings.21,42
The viscosity of Plx gels with and without MSs increased at 37°C±0.1°C, due to the thermogelling property of Plx molecules, as expected. At 37°C±0.1°C, the viscosity of both Plx gels increased too much and could not be measured with a vibration viscometer. MS addition did not cause a significant change in Plx-gel viscosity. As mentioned previously, all formulations were diluted with Tyrode solution for simulation of in vivo conditions. This dilution caused significant loss of viscosity in all the gel formulations, and this decrease was more pronounced with Plx gels.
Rheological measurements
It is well known that rheological properties of formulations affect both ease of application and retention within an application site; therefore, they are very important parameters for choosing the optimum intravesical formulation.21 Therefore, detailed rheology studies were carried out on gel formulations and their dilutions with Tyrode solution at both room and body temperature.
First, flow properties of the formulations were determined with continuous shear analysis. The Plx formulations showed Newtonian flow at 25°C±1°C and non-Newtonian plastic flow at 37°C±1°C, as expected (data not shown). This confirmed that Plx-gel formulations were viscous liquid at room temperature and formed a semisolid gel at body temperature. At temperatures over the sol–gel transition temperature, this type of non-Newtonian flow is typical for Plx formulations.48 On the other hand, Chi-gel formulations had non-Newtonian plastic behavior with shear-thinning properties at both 25°C and 37°C (data not shown). This type of rheological behavior is desirable for intravesical drug administration, because when force is applied it will result in a decrease in viscosity and make the gel easy to be pushed through a catheter at room temperature.49
In addition, dilution with Tyrode solution caused a significant decrease in viscosity of all formulations. Plx gels continued the Newtonian-type flow even at 37°C when diluted with Tyrode solution, which could be explained by a significant decrease in viscosity. Since this may be unfavorable for intravesical application, it demonstrates the importance of evacuation of the bladder prior to in situ gel administration with regard to the formulation’s permanence. On the other hand, it was determined that dilution of Chi gels with Tyrode solution did not change their flow property, although it caused a marked decrease in viscosity. This was considered a positive feature for intravesical formulations prepared with Chi, as the pseudoplasticity demonstrates the continuity of gel formation.
Furthermore, to get comprehensive information on the rheological state of formulations, oscillation measurements were performed to yield information about viscous and elastic properties of the formulations. Oscillation tests are dynamic methods for determining the viscoelastic properties of the tested material in its rheological ground state. To get information about the viscous and elastic behavior of the system and the network structure formed by particle–particle interactions, a frequency-sweep test has to be performed. A frequency-sweep test is a dynamic test measuring the response of a system as a function of frequency at constant stress amplitude. It reveals the storage modulus G′ (elastic response) and the loss modulus G″ (viscous response).50
Figure 3 shows the plots of G′ and G″ as a function of frequency at two different temperature values. Formulations prepared with Chi exhibited viscoelastic properties and low gel strength. Mechanical spectra with frequency dependence of G′ and G″ and smaller separation between the two moduli indicated that Chi formulations could be considered weak gels at both temperatures.43 G′ and G″ moduli of Plx formulations were low at room temperature and rose significantly at body temperature. At 37°C, G′ dominated G″ for all frequencies and the gap between the two moduli became wider, indicating stronger gel strength. MS-loaded Plx gel was found to be frequency-independent, exhibiting an elastic structure (strong gel, G′>G″) at 37°C. Finally, the rheological behavior of Chi and Plx formulations diluted with Tyrode solution was evaluated, and liquid–solid behavior of macromolecular solutions was observed for all diluted gels. These results were in accordance with the reduced viscosity values and weak mechanical properties of Chi and Plx formulations diluted with Tyrode solution.
In vitro release studies
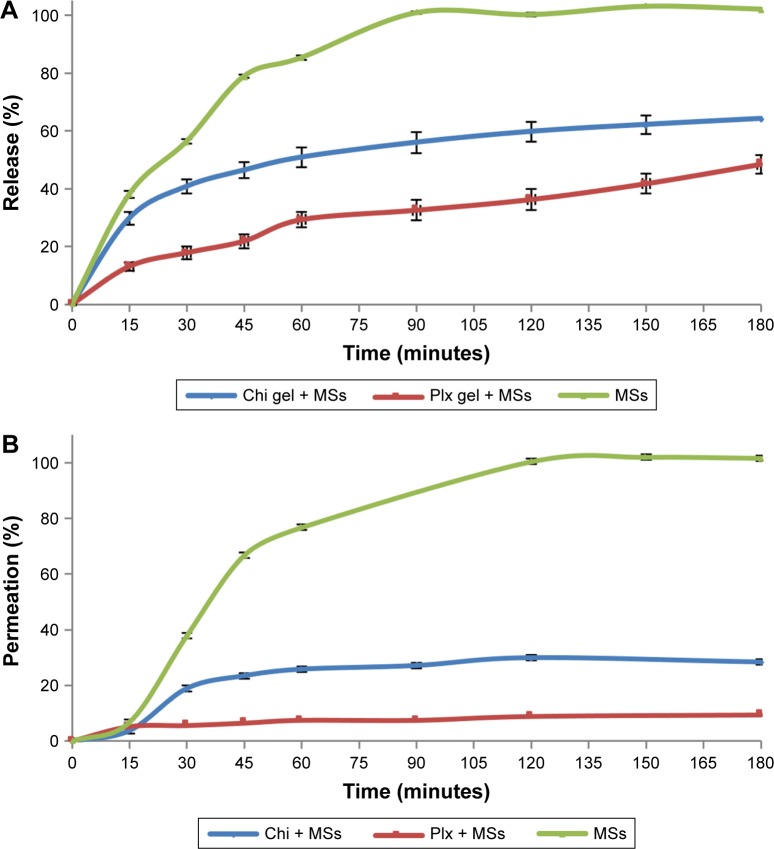
The in vitro release profile of Gem-HCl MSs and gel formulations containing Gem-HCl MSs were examined in PBS (pH 6.5) at 37°C±0.1°C, and results are displayed in Figure 4. According to results, Gem-HCl release from MSs can be defined as fast (nearly 85% within 60 minutes), which was probably the consequence of good swelling and permeability properties of EE100 above pH 5. This rapid release of Gem-HCl from MSs may result in drug loss with urinary excretion.
Figure 4.
In vitro release (A) and ex vivo permeation (B) profiles of Gem-HCl from MS-loaded Chi gel, Plx gel, and MSs in PBS of pH 6.5.
Abbreviations: Gem, gemcitabine; MS, microsphere; Chi, chitosan; Plx, poloxamer.
The release profile of MS-loaded gel formulations indicated that nearly 30% of Gem-HCl was released from the Plx gel containing MSs and 50% of Gem-HCl released from the Chi gel containing MSs within 60 minutes. These results showed that Chi or Plx gels significantly slowed the release rate of Gem-HCl. Sustained release was obtained for Gem-HCl with gel formulations during 3 hours, and both of the gel formulations can be defined as effective for modifying release properties of Gem-HCl. Plx gel slowed down drug release further than Chi gel because of the higher viscosity values at 37°C. The increase in gel viscosity reduced the Gem-HCl release rate, because the gel-dissolution time extended and drug diffusion through gel matrix was prolonged. This sustained and slow release might be an important advantage of Plx gels, because slower release of intravesically administered drugs ensures extended drug presence in the bladder without the need for intermittent catheterization, increases drug efficacy, and reduces or eliminates harmful drug side effects.51,52 For determination of release mechanism, n, k and r2 values calculated with Peppas’s equation are listed in Table 4.
Table 4.
Release parameters of Gem-HCl from formulations
| Release component (n) | Kinetic constant (log k) | r2 | |
|---|---|---|---|
| MSs | 0.565 | 0.916 | 0.999 |
| Plx gel containing MSs | 0.462 | 0.573 | 0.999 |
| Chi gel containing MSs | 0.545 | 0.832 | 0.920 |
Abbreviations: Gem, gemcitabine; MSs, microspheres; Plx, poloxamer; Chi, chitosan.
Ex vivo permeation studies
The potential permeation profiles of the formulations were determined on freshly excised bovine-bladder mucosa, and the results are shown in Figure 4. When the permeation profiles were investigated, it was observed that the permeated ratio of Gem-HCl from MSs (76.74%) was higher than MS-loaded Plx and Chi gels, similarly to in vitro release profiles. This result was again probably due to very good swelling and permeability of EE100. The permeated percentage of Gem-HCl within 60 minutes from MS-loaded Chi and Plx gel reached 25.77% and 7.54%, respectively. This low ratio of permeated Gem-HCl from gels can be explained by the interaction of gels with bladder mucosa. This result can indicate retention of Gem-HCl in the mucosa layer and probably cause a local effect of MS-loaded gel formulations.53
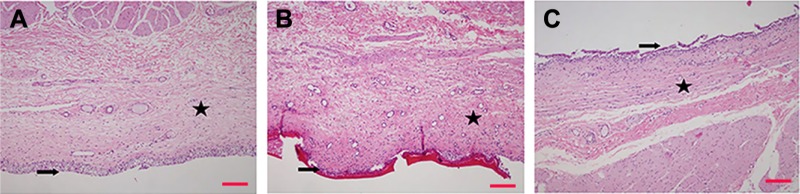
Histopathological evaluation
Ex vivo histopathological examinations were performed to observe the effect of the formulations on the bladder mucosa. The results of the histopathological evaluation of the bladder mucosa are shown in Figure 5. Firstly, it was determined that no pathological features were recorded in the urothelial epithelium, lamina propria, or muscularis propria of untreated empty mucosa which was placed directly into formalin and the mucosa treated with the buffer solution instead of the formulation for control purposes. A layer covering the surface of the urothelial epithelium was observed at urothelia with Chi-gel formulation containing MPs, and this layer was associated with the applied formulation. No pathological features were noted with this formulation in the lamina propria or muscularis propria.
Figure 5.
Microscopic images of bovine bladder mucosa.
Notes: (A) Healthy bladder mucosa; (B) bladder mucosa treated with MS-loaded Chi gel; (C) bladder mucosa treated with MS-loaded Plx gel. H&E staining, magnification 40×; ➔ urothelium, ★ lamina propria. Scale bar 200 µm.
Abbreviations: MS, microsphere; Chi, chitosan; Plx, poloxamer.
Urothelia with Plx gel containing MPs showed a tendency of refraction and spillage of urothelial epithelium at the light-microscopy level. No pathological features were noted in the lamina propria or muscularis propria. Finally, the results obtained suggest that Gem-HCl-loaded MSs and Gem-HCl MSs containing Chi and Plx gels did not cause any damage to healthy bladder mucosa, and these formulations might thus be regarded as safe.
Cytotoxic assay for formulations
Gem-HCl solution and Gem-HCl MS- and Gem-HCl MS-loaded gel formulations prepared for intravesical administration were evaluated in two different bladder cancer cell lines–T24 (ATCC HTB-4TM) and RT4 (ATCC HTB-2TM)–to assess cytotoxic effects. These cell lines are used widely as respective superficial and invasive models of human bladder cancer. RT4 is representative of noninvasive superficial cancer and T24 representative of invasive bladder tumor with a metastatic profile.54 Firstly, the potencies of cell-growth inhibition of the formulations (IC50 values) were determined for RT4 and T24 cell lines, and results are shown in Table 5. Gem-HCl solution was found to be more potent on RT4 cells than T24 cells in terms of cytotoxicity (IC50 12.55±0.62 and 24.35±1.05 µM, respectively). The difference in cytotoxic effects of Gem-HCl solution in the two cell types could have been caused by genetic differences between the cell lines.55 T24 has much faster metabolism and divides more readily than RT4.56 T24 cells are also more malignant than RT4 cells. However, the Gem-HCl-loaded MSs were found to be more potent than aqueous Gem-HCl solution in both cell lines. It can be conceivable that MS formulations have high potency at the same dose compared with a commercial aqueous formulation. Empty MS-loaded gel formulations had no cytotoxic effects on either cell line (data not shown). In addition, Gem-HCl-loaded MSs exhibited greater cytotoxicity against T24 cells (24.35–15.88 µM), which represented more malignancy than in RT4 cells (12.55–10.16 µM). This suggested that the Gem-HCl-loaded MS formulation was more selective for T24 malignant bladder cells. The Gem-HCl MS-loaded Plx-gel formulation was more potent than the Chi-gel formulation in both cell lines. Plx-loaded gemcitabine gels were almost as potent as the Gem-HCl solution. It should be noted that a gel formulation will be in contact with tumor cells for a longer time than an aqueous solution of gemcitabine in the bladder. It is expected that prolonged exposure of the urothelium to a drug increases the efficacy of the treatment. Farr et al found a 10,000–100,000-fold selectivity of gemcitabine treatment on cancer (RT4, T24) relative to noncancer (UROTSA) urothelial cell lines.57 Therefore, it could be expected that in vivo application of MSs and their gel formulations would be comparable to conventionally used Gem-HCl in patients suffering from bladder cancer.
Table 5.
IC50 values (µM) of Gem-HCl solution, Gem-HCl MS-, and Gem-HCl MS-loaded Chi and Plx gel formulations on RT4 and T24 cells
| RT4 cells | T24 cells | |
|---|---|---|
| Gem-HCl | 12.55±0.62 | 24.35±1.05 |
| Gem-HCl MSs | 10.16±1.10 | 15.88±1.21 |
| Gem-HCl MS-loaded Chi gel | 13.11±2.81 | 71.75±52.77 |
| Gem-HCl MS-loaded Plx gel | 11.73±1.97 | 26.84±3.98 |
Note: Data presented as mean ± SD.
Abbreviations: Gem, gemcitabine; MS, microsphere; Chi, chitosan; Plx, poloxamer.
In vivo studies
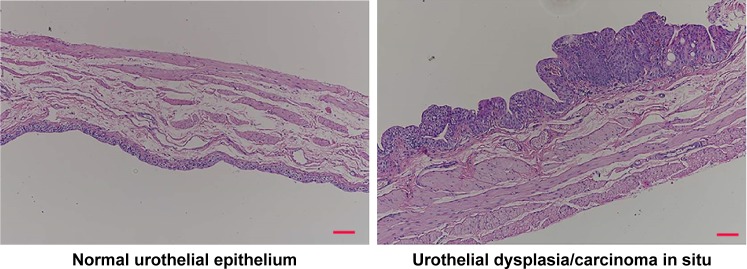
In vivo animal studies for intravesical formulations of Gem-HCl were started after cytotoxic assays. At the end of 20 weeks, randomly selected rats were killed using an overdose of chloral hydrate to see if they had carcinoma in situ, and bladders removed and fixed in 4% neutral buffered formalin. After histopathological procedures had been applied, the degree of tissue damage was determined using light microscopy. Although different lesions were observed with varied characteristics, disease symptoms were observed at the base of the bladder of each rat (Figure 6). As a result of this evaluation, the second stage for in vivo studies (the treatment process) was started.
Figure 6.
Microscopic images of normal urothelial epithelium and urothelial dysplasia/carcinoma in situ. Scale bar 200 µm.
During the treatment period, 4 mg Gem-HCl in 0.5 mL formulation were administered to rats once weekly during 4 weeks. After the last administration, one week was spent empty, and at the end of the fifth week the rats were killed. Following routine histopathology procedure, bladders were examined under light microscopy. Histopathologically, the changes in the bladder mucosa were scored as normal urothelial epithelium, hyperplasia, dysplasia/carcinoma in situ, or urothelial carcinoma. Developmental characteristics (papillary–nodular), invasiveness, and squamous features were evaluated in urothelial carcinomas. The term “urothelial carcinoma” was used for all cases with and without glandular squamous areas.
Urothelial carcinomas including squamous areas were seen with histopathological examination of the control group (group 2). The development of multifocal papillary lesions was anterior, and invasion was not evaluated for this group. For two of the rats on which Gem-HCl-containing Plx-gel formulations were administered, dysplasia findings were more pronounced (group 6). In addition, lesions accompanied by peripheral mucosa were also detected in animals with marked dysplasia findings.
It was observed that urothelial mucosa was normal in all rats that had been treated with Plx gel containing Gem-HCl-loaded MSs (group 8). When the results obtained from the group administered Chi gel containing Gem-HCl-loaded MPs were examined (group 11), findings in favor of focal minimal urothelial dysplasia were observed in the mucosa. When the results obtained from the groups of Chi and Plx gels containing empty MSs were examined (groups 7 and 10), no treatment finding was found. Also, two of the rats administered saline containing Gem-HCl (group 3) had evidence of hyperplasia, with thickening of mucosal epithelium in one and findings of dysplasia were observed in the other. In the remaining animals, carcinoma findings and lesions accompanying the surrounding mucosa were detected.
Among the rats to which empty MSs had been administered in saline (group 4), there were findings in favor of hyperplasia due to thickening of the mucosal epithelium in the dysplasia findings and accompanying lesions in the surrounding mucosa. Among the rats to which Gem-HCl-loaded MSs had been applied in saline (group 5), mucosa was common in two, whereas epithelial thickening was observed in favor of hyperplasia in one. The findings of dysplasia were also observed in both. One of them draws attention, with widespread lesions that accompanied the surrounding mucosa. Summarizing the results of in vivo studies, intravesical treatment with once-weekly Plx gel with Gem-HCl-loaded MSs was found to be more effective than the others.
Stability studies
Stability tests were performed with all the formulations at 5°C±2°C, 25°C±5°C/60% RH, and 40°C±5°C/75% RH. General appearance and organoleptic properties of MSs did not change significantly in 2 months during stability studies. The proportion of active substance in the MPs kept at 5°C±2°C, 25°C±5°C/60% RH, and 40°C±5°C/75% RH was found to be 72.27%, 72.92%, and 65.20% at the end of the 2 months, respectively (Table 6). Stability studies were also performed with gel formulations containing MPs (data not shown). Firstly, gels were evaluated in terms of their pH values. There was no significant change in pH values of the formulations at these temperatures during this time period. Gel formulations were also evaluated in terms of their viscosity and Plx gels examined in terms of gelation temperature and gelation time. According to the obtained results, a marked decrease in viscosity values was observed in formulations kept at 40°C±5°C and 75% RH. In addition, it was observed that the gelation temperature and time of Plx gels increased significantly. For these reasons, it was concluded that it would be appropriate to keep all formulations in the refrigerator and protected against high temperature and humidity.
Table 6.
Gem-HCl amounts in MSs (µg)
| 5°C±2°C | 25°C±5°C, 60% RH | 40°C±5°C, 75% RH | |
|---|---|---|---|
| Beginning | 9.042±1.323 | 9.042±1.323 | 9.042±1.323 |
| Day 15 | 8.991±1.664 | 8.915±1.080 | 8.162±1.546 |
| Day 30 | 8.967±0.940 | 8.808±1.666 | 7.826±1.944 |
| Day 60 | 6.535±0.864 | 6.594±1.236 | 5.896±0.856 |
Abbreviations: Gem, gemcitabine; MSs, microspheres; RH, relative humidity.
Conclusion
Direct instillation of drugs into the bladder is an efficient alternative to systemic delivery, since it reduces side effects, prevents first-pass effects, and consequently allows a more effective treatment. However, this method is limited by excessive drug loss during voiding. To increase the residence time of the drug in the bladder and achieve a more effective intravesical treatment, Gem-HCl MSs were prepared and loaded in gel formulations in the present study. The results obtained revealed that MS-loaded gel formulations exhibited suitable properties for intravesical administration of Gem-HCl. According to the results, Chi and Plx gels might be alternative carriers for intravesical administration of Gem-HCl-loaded MSs. However, with their strong gel structure, desirable mechanical, bioadhesive, and in vitro and ex vivo sustained-release properties, Plx-gel formulations came forward as the better carrier for Gem-HCl MSs. In addition, according to the cell-culture studies, Gem-HCl MS-loaded Plx gel showed greater cytotoxicity than Chi-gel formulations in both cell types. However, when diluted with artificial urine to mimic the conditions in bladder, Plx gels lost their in situ gelling properties at body temperature. To overcome this problem, such strategies as emptying the bladder before drug instillation, suppression of urine-production rate by the kidneys, and regulation of fluid intake before and after drug administration are recommended.
Acknowledgments
This study was supported by the Scientific and Technological Research Council of Turkey (grant TUBITAK-112/S/293). The authors would also like to thank the TR Prime Ministry State Planning Organization Foundation (project 09/DPT/001).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wang S, Zhao J, Hu J, et al. Phase-changeable and bubble-releasing implants for highly efficient HIFU-responsive tumor surgery and chemotherapy. J Mater Chem B. 2016;4(46):7368–7378. doi: 10.1039/c6tb01861k. [DOI] [PubMed] [Google Scholar]
- 2.Wang S, Zhao J, Yang H, et al. Bottom-up synthesis of WS2 nanosheets with synchronous surface modification for imaging guided tumor regression. Acta Biomater. 2017;58:442–454. doi: 10.1016/j.actbio.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Wu C, Zhao J, Hu F, et al. Design of injectable agar-based composite hydrogel for multi-mode tumor therapy. Carbohydr Polym. 2018;180:112–121. doi: 10.1016/j.carbpol.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 4.Moreira JM, Ohlsson G, Gromov P, et al. Bladder cancer-associated protein, a potential prognostic biomarker in human bladder cancer. Mol Cell Proteomics. 2010;9(1):161–177. doi: 10.1074/mcp.M900294-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 6.Mattioli F, Curotto A, Manfredi V, et al. Intravesical gemcitabine in superficial bladder cancer: a phase II safety, efficacy and pharmacokinetic study. Anticancer Res. 2005;25(3C):2493–2496. [PubMed] [Google Scholar]
- 7.Kondylis FI, Demirci S, Ladaga L, et al. Outcomes after intravesical bacillus Calmette-Guérin are not affected by substaging of high grade T1 transitional cell carcinoma. Int J Urol. 2000;163(4):1120–1123. [PubMed] [Google Scholar]
- 8.Erdoğar N, Iskit AB, Eroğlu H, Sargon MF, Mungan NA, Bilensoy E. Antitumor efficacy of bacillus Calmette-Guérin loaded cationic nanoparticles for intravesical immunotherapy of bladder tumor induced rat model. J Nanosci Nanotechnol. 2015;15(12):10156–10164. doi: 10.1166/jnn.2015.11690. [DOI] [PubMed] [Google Scholar]
- 9.Herr HW, Bajorin DF, Scher HI. Neoadjuvant chemotherapy and bladder-sparing surgery for invasive bladder cancer: ten-year outcome. J Clin Oncol. 1998;16(4):1298–1301. doi: 10.1200/JCO.1998.16.4.1298. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu H, Akasaka S, Suzuki S, Akimoto M, Shimada T. Preferential gene transfer to BBN-induced rat bladder tumor by simple instillation of adenoviral vector. Urology. 2001;57(3):579–584. doi: 10.1016/s0090-4295(00)01008-6. [DOI] [PubMed] [Google Scholar]
- 11.Cozzi PJ, Bajorin DF, Tong W, et al. Toxicology and pharmacokinetics of intravesical gemcitabine: a preclinical study in dogs. Clin Cancer Res. 1999;5(9):2629–2637. [PubMed] [Google Scholar]
- 12.Witjes JA, van der Heijden AG, Vriesema JL, Peters GJ, Laan A, Schalken JA. Intravesical gemcitabine: a phase 1 and pharmacokinetic study. Eur Urol. 2004;45(2):182–186. doi: 10.1016/j.eururo.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Gontero P, Tizzani A. Intravesical gemcitabine: state of the art. Eur Urol Suppl. 2007;6(14):809–815. [Google Scholar]
- 14.Bergman AM, Pinedo HM, Peters GJ. Determinants of resistance to 2′,2′-difluorodeoxycytidine (gemcitabine) Drug Resist Updat. 2002;5(1):19–33. doi: 10.1016/s1368-7646(02)00002-x. [DOI] [PubMed] [Google Scholar]
- 15.Burjak M, Bogataj M, Velnar M, Grabnar I, Mrhar A. The study of drug release from microspheres adhered on pig vesical mucosa. Int J Pharm. 2001;224(1–2):123–130. doi: 10.1016/s0378-5173(01)00748-7. [DOI] [PubMed] [Google Scholar]
- 16.Şenyiğit ZA, Karavana SY, İlem-Özdemir D, et al. Design and evaluation of an intravesical delivery system for superficial bladder cancer: preparation of gemcitabine HCl-loaded chitosan-thioglycolic acid nanoparticles and comparison of chitosan/poloxamer gels as carriers. Int J Nanomedicine. 2015;10:6493–6507. doi: 10.2147/IJN.S93750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Au JL, Jang SH, Wientjes MG. Clinical aspects of drug delivery to tumors. J Control Release. 2002;78(1–3):81–95. doi: 10.1016/s0168-3659(01)00488-6. [DOI] [PubMed] [Google Scholar]
- 18.Shaikh R, Singh TR, Garland MJ, Woolfson AD, Donnelly RF. Mucoadhesive drug delivery systems. J Pharm Bioallied Sci. 2011;3(1):89–100. doi: 10.4103/0975-7406.76478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoare TR, Kohane DS. Hydrogels in drug delivery: progress and challenges. Polymer. 2008;49(8):1993–2007. [Google Scholar]
- 20.Lu S, Neoh KG, Kang ET, Mahendran R, Chiong E. Mucoadhesive polyacrylamide nanogel as a potential hydrophobic drug carrier for intravesical bladder cancer therapy. Eur J Pharm Sci. 2015;72:57–68. doi: 10.1016/j.ejps.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Senyiğit ZA, Karavana SY, Eraç B, Gürsel O, Limoncu MH, Baloğlu E. Evaluation of chitosan based vaginal bioadhesive gel formulations for antifungal drugs. Acta Pharm. 2014;64(2):139–156. doi: 10.2478/acph-2014-0013. [DOI] [PubMed] [Google Scholar]
- 22.Choi HG, Oh YK, Kim CK. In situ gelling and mucoadhesive liquid suppository containing acetaminophen: enhanced bioavailability. Int J Pharm. 1998;165(1):23–32. [Google Scholar]
- 23.Baloglu E, Karavana SY, Senyigit ZA, Guneri T. Rheological and mechanical properties of poloxamer mixtures as a mucoadhesive gel base. Pharm Dev Technol. 2011;16(6):627–636. doi: 10.3109/10837450.2010.508074. [DOI] [PubMed] [Google Scholar]
- 24.Chutipongtanate S, Thongboonkerd V. Systematic comparisons of artificial urine formulas for in vitro cellular study. Anal Biochem. 2010;402(1):110–112. doi: 10.1016/j.ab.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 25.Karavana SY, Rençber S, Şenyiğit ZA, Baloğlu E. A new in-situ gel formulation of itraconazole for vaginal administration. Pharmacol Pharm. 2012;3(4):417–426. [Google Scholar]
- 26.Jones DS, Woolfson AD, Djokic J. Texture profile analysis of bioadhesive polymeric semisolids: mechanical characterization and investigation of interactions between formulation components. J Appl Polym Sci. 1996;61(12):2229–2234. [Google Scholar]
- 27.Jones DS, Woolfson AD, Brown AF, Coulter WA, McClelland C, Irwin CR. Design, characterisation and preliminary clinical evaluation of a novel mucoadhesive topical formulation containing tetracycline for the treatment of periodontal disease. J Control Release. 2000;67(2–3):357–368. doi: 10.1016/s0168-3659(00)00231-5. [DOI] [PubMed] [Google Scholar]
- 28.Turgut A, Tavman I, Chirtoc M, Schuchmann HP, Sauter C, Tavman S. Thermal conductivity and viscosity measurements of water-based TiO2 nanofluids. Int J Thermophys. 2009;30(4):1213–1226. [Google Scholar]
- 29.Jones DS, Woolfson AD, Brown AF. Textural, viscoelastic and mucoadhesive properties of pharmaceutical gels composed of cellulose polymers. Int J Pharm. 1997;151(2):223–233. [Google Scholar]
- 30.Andrews GP, Gorman SP, Jones DS. Rheological characterisation of primary and binary interactive bioadhesive gels composed of cellulose derivatives designed as ophthalmic viscosurgical devices. Biomaterials. 2005;26(5):571–580. doi: 10.1016/j.biomaterials.2004.02.062. [DOI] [PubMed] [Google Scholar]
- 31.Andrews GP, Jones DS. Rheological characterization of bioadhesive binary polymeric systems designed as platforms for drug delivery implants. Biomacromolecules. 2006;7(3):899–906. doi: 10.1021/bm050620y. [DOI] [PubMed] [Google Scholar]
- 32.Peppas NA. Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helv. 1985;60(4):110–111. [PubMed] [Google Scholar]
- 33.Gomez P, Gil ES, Lovett ML, et al. The effect of manipulation of silk scaffold fabrication parameters on matrix performance in a murine model of bladder augmentation. Biomaterials. 2011;32(30):7562–7570. doi: 10.1016/j.biomaterials.2011.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nirmal J, Chuang YC, Tyagi P, Chancellor MB. Intravesical therapy for lower urinary tract symptoms. Urol Sci. 2012;23:70–77. [Google Scholar]
- 35.Pongbaibul Y, Maruyama K, Iwatsuru M. Formation and in-vitro evaluation of theophylline-loaded poly(methyl methacrylate) microspheres. J Pharm Pharmacol. 1988;40(8):530–533. doi: 10.1111/j.2042-7158.1988.tb05296.x. [DOI] [PubMed] [Google Scholar]
- 36.Haznedar S, Dortunç B. Preparation and in vitro evaluation of Eudragit microspheres containing acetazolamide. Int J Pharm. 2004;269(1):131–140. doi: 10.1016/j.ijpharm.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Guhasarkar S, Banerjee R. Intravesical drug delivery: challenges, current status, opportunities and novel strategies. J Control Release. 2010;148(2):147–159. doi: 10.1016/j.jconrel.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 38.Almomen A, Cho S, Yang CH, et al. Thermosensitive progesterone hydrogel: a safe and effective new formulation for vaginal application. Pharm Res. 2015;32(7):2266–2279. doi: 10.1007/s11095-014-1616-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cho E, Gwak H, Chun I. Formulation and evaluation of ondansetron nasal delivery systems. Int J Pharm. 2008;349(1–2):101–107. doi: 10.1016/j.ijpharm.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 40.Fallis WM. Monitoring urinary bladder temperature in the intensive care unit: state of the science. Am J Crit Care. 2002;11(1):38–45. [PubMed] [Google Scholar]
- 41.el-Hefian EA, Elgannoudi ES, Mainal A, Yahaya AH. Characterization of chitosan in acetic acid: rheological and thermal studies. Turk J Chem. 2010;34:47–59. [Google Scholar]
- 42.Cevher E, Sensoy D, Taha MA, Araman A. Effect of thiolated polymers to textural and mucoadhesive properties of vaginal gel formulations prepared with polycarbophil and chitosan. AAPS PharmSciTech. 2008;9(3):953–965. doi: 10.1208/s12249-008-9132-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ström A, Schuster E, Goh SM. Rheological characterization of acid pectin samples in the absence and presence of monovalent ions. Carbohydr Polym. 2014;113:336–343. doi: 10.1016/j.carbpol.2014.06.090. [DOI] [PubMed] [Google Scholar]
- 44.Tuğcu-Demiröz F, Acartürk F, Özkul A. Preparation and characterization of bioadhesive controlled-release gels of cidofovir for vaginal delivery. J Biomater Sci Polym Ed. 2015;26(17):1237–1255. doi: 10.1080/09205063.2015.1082808. [DOI] [PubMed] [Google Scholar]
- 45.Hwang TL, Fang CL, Chen CH, Fang JY. Permeation enhancer-containing water-in-oil nanoemulsions as carriers for intravesical cisplatin delivery. Pharm Res. 2009;26(10):2314–2323. doi: 10.1007/s11095-009-9947-6. [DOI] [PubMed] [Google Scholar]
- 46.Liu CW, Chang LC, Lin KJ, et al. Preparation and characterization of gelatin-based mucoadhesive nanocomposites as intravesical gene delivery scaffolds. Biomed Res Int. 2014;2014:473823. doi: 10.1155/2014/473823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dumitriu S. Polymeric Biomaterials. 2nd ed. New York: Marcel Dekker; 2001. [Google Scholar]
- 48.Baloglu E, Karavana SY, Senyigit ZA, et al. In-situ gel formulations of econazole nitrate: preparation and in-vitro and in-vivo evaluation. J Pharm Pharmacol. 2011;63(10):1274–1282. doi: 10.1111/j.2042-7158.2011.01315.x. [DOI] [PubMed] [Google Scholar]
- 49.Chatta D, Cottrell L, Burnett B, Laverty G, McConville C. The use of water-soluble mucoadhesive gels for the intravesical delivery of epirubicin to the bladder for the treatment of non-muscle-invasive bladder cancer. J Pharm Pharmacol. 2015;67(10):1355–1362. doi: 10.1111/jphp.12441. [DOI] [PubMed] [Google Scholar]
- 50.Lippacher A, Müller RH, Mäder K. Semisolid SLN dispersions for topical application: influence of formulation and production parameters on viscoelastic properties. Eur J Pharm Biopharm. 2002;53(2):155–160. doi: 10.1016/s0939-6411(01)00233-8. [DOI] [PubMed] [Google Scholar]
- 51.Ricci EJ, Lunardi LO, Nanclares DM, Marchetti JM. Sustained release of lidocaine from poloxamer 407 gels. Int J Pharm. 2005;288(2):235–244. doi: 10.1016/j.ijpharm.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 52.Betsiou M, Bantsis G, Zoi I, Sikalidis C. Adsorption and release of gemcitabine hydrochloride and oxaliplatin by hydroxyapatite. Ceram Int. 2012;38(4):2719–2724. [Google Scholar]
- 53.Nava G, Piñón E, Mendoza L, Mendoza N, Quintanar D, Ganem A. Formulation and in vitro, ex vivo and in vivo evaluation of elastic liposomes for transdermal delivery of ketorolac tromethamine. Pharmaceutics. 2011;3(4):954–970. doi: 10.3390/pharmaceutics3040954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vianna DR, Ruschel L, Dietrich F, et al. 4-Methylcoumarins with cytotoxic activity against T24 and RT4 human bladder cancer cell lines. MedChemComm. 2015;6(5):c5md00039d. [Google Scholar]
- 55.O’Toole CM, Povey S, Hepburn P, Franks LM. Identity of some human bladder cancer cell lines. Nature. 1983;301(5899):429–430. doi: 10.1038/301429a0. [DOI] [PubMed] [Google Scholar]
- 56.Zhao ZF, Wang K, Guo FF, Lu H. Inhibition of T24 and RT4 human bladder cancer cell lines by heterocyclic molecules. Med Sci Monit. 2017;23:1156–1164. doi: 10.12659/MSM.898265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Farr ES, Chess-Williams R, McDermott CM. Gemcitabine: selective cytotoxicity, induction of inflammation and effects on urothelial function. Toxicol Appl Pharmacol. 2017;316:1–9. doi: 10.1016/j.taap.2016.12.011. [DOI] [PubMed] [Google Scholar]