Abstract
Aims
This analysis assessed the efficacy and safety of alirocumab, a proprotein convertase subtilisin/kexin type 9 inhibitor, in patients with or without metabolic syndrome (MetS) using pooled data from 10 phase 3 ODYSSEY trials.
Materials and Methods
Data from 4983 randomized patients (1940 with MetS; 1642 with diabetes excluded) were assessed in subgroups by MetS status. Efficacy data were analysed in 4 pools per study design: 2 placebo‐controlled pools (1 using alirocumab 150 mg every 2 weeks [Q2W], 1 using 75/150 mg Q2W) with background statin, and 2 ezetimibe‐controlled pools (both alirocumab 75/150 mg Q2W), 1 with and 1 without background statin. Alirocumab 75/150 mg indicates possible dose increase from 75 to 150 mg at Week 12 based on Week 8 LDL‐C.
Results
LDL‐C percentage reduction from baseline at Week 24 with alirocumab was 63.9% (MetS) and 56.8% (non‐MetS) in the pool of alirocumab 150 mg Q2W, and 42.2% to 52.2% (MetS) and 45.0% to 52.6% (non‐MetS) in 3 pools using 75/150 mg Q2W. Levels of other lipid and lipoprotein parameters were also improved with alirocumab treatment, including apolipoprotein B, non‐high‐density lipoprotein cholesterol (non‐HDL‐C), lipoprotein(a) and HDL‐C. Overall, the percentage change at Week 24 in LDL‐C and other lipids and lipoproteins did not vary by MetS status. Adverse event rates were generally similar between treatment groups, regardless of MetS status; injection‐site reactions occurred more frequently in alirocumab vs control groups.
Conclusions
Across study pools, alirocumab‐associated reductions in LDL‐C, apolipoprotein B, and non‐HDL‐C were significant vs control, and did not vary by MetS status.
Keywords: cardiovascular disease, clinical trial, dyslipidaemia, lipid‐lowering therapy
1. INTRODUCTION
Metabolic syndrome (MetS) is defined by a collection of related metabolic and physiological abnormalities, including central obesity, raised serum triglycerides (TGs), reduced high‐density lipoprotein cholesterol (HDL‐C), glucose intolerance and hypertension.1, 2, 3 MetS has been defined as the presence of 3 or more of the following: elevated waist circumference (which is assumed in the guidelines if body mass index [BMI] is >30 kg/m2); TGs ≥150 mg/dL or use of TG‐lowering medication; HDL‐C <40 mg/dL in men or <50 mg/dL in women; blood pressure ≥130/85 mm Hg or diagnosis of hypertension; and fasting plasma glucose (FPG) ≥100 mg/dL.1 MetS is a common syndrome with a rising prevalence worldwide, ranging from approximately 10% to 80% depending on regional variation and population demographics.1, 2 MetS is associated with an increased risk of cardiovascular (CV) disease (2‐fold), type 2 diabetes mellitus (5‐fold) and all‐cause mortality (1.5‐fold).1, 4, 5 However, multivariate analyses have shown that components typically associated with MetS (blood pressure, HDL‐C and blood glucose), but not MetS itself, are predictors of prevalent coronary heart disease.6
While statins are recommended as first‐line therapy for reducing levels of LDL‐C, not all patients achieve LDL‐C lowering with statin therapy sufficient to optimally reduce their CV risk. Additionally, statin intolerance can limit dosage and potency of the statin used, which is a significant factor in the reduced efficacy of statin therapy for some patients.7 In particular, patients with MetS may not achieve non‐HDL‐C goals following treatment with statins and other lipid‐lowering therapies (LLTs)8 due to elevated TG levels, which are indicators of very‐low‐density lipoprotein and remnant cholesterol levels.9 Individuals with MetS typically exhibit mixed dyslipidaemia, characterised by elevated TGs and lower HDL‐C, both of which are often associated with elevated apolipoprotein (Apo) B and non‐HDL‐C levels. In such cases, non‐HDL‐C or ApoB may give a better estimate of the concentration of atherogenic particles than low‐density lipoprotein cholesterol (LDL‐C), and they have been suggested as alternative treatment targets to LDL‐C for such individuals.4
Alirocumab, a proprotein convertase subtilisin/kexin type 9 inhibitor, has been approved in more than 40 countries, including the USA and the EU, for reducing elevated LDL‐C levels.10, 11 Individuals with metabolic syndrome have a range of clinical and metabolic characteristics that may impact the efficacy and/or safety of a PCSK9 inhibitor, including obesity, high triglycerides/low HDL‐C, high blood glucose and insulin resistance. For example, PCSK9 appears to have a role in glucose metabolism and PCSK9 levels have been shown to correlate with glycaemic parameters and insulin resistance, although evidence is conflicting among different studies.12, 13 Insulin signalling, which is known to influence LDL‐receptor expression (the target of PCSK9), is often dysregulated in metabolic syndrome.14 However, the use of PCSK9 inhibitors in individuals with metabolic syndrome is not well established. In the present study, we compared the efficacy and safety of alirocumab in patients with hypercholesterolaemia, with or without MetS, using data from 10 studies from the alirocumab ODYSSEY phase 3 clinical trial programme. This analysis contributes to a better understanding of the alirocumab target patient population.
2. METHODS
2.1. Study designs and pooling strategy
This analysis includes data from 10 phase 3 ODYSSEY studies (Figure 1), all of which have been described previously.15, 16, 17, 18, 19, 20, 21, 22, 23 Patients (n = 4983) were randomized to receive alirocumab or control (placebo or ezetimibe). In 8 of the 10 trials, patients were receiving background statin therapy, with or without other LLTs. The double‐blind treatment periods ranged between 24 and 104 weeks. In 8 studies, patients received an initial dose of alirocumab 75 mg every 2 weeks (Q2W), with a possible dose increase to 150 mg Q2W at Week 12, depending on LDL‐C levels at Week 8 (alirocumab 75/150 mg Q2W); 2 studies used alirocumab 150 mg Q2W throughout the study. Entry criteria for the ODYSSEY trials included baseline LDL‐C ≥70 mg/dL for those with prior CV events or ≥100 mg/dL for those without CV events but with other risk factors. Exceptions were the LONG TERM study (LDL‐C ≥70 mg/dL for all patients19), the MONO study (LDL‐C 100‐190 mg/dL for all patients21) and the HIGH FH study (LDL‐C ≥160 mg/dL for all patients23). All study protocols were approved by the appropriate review boards, and all patients provided written, informed consent.
Figure 1.
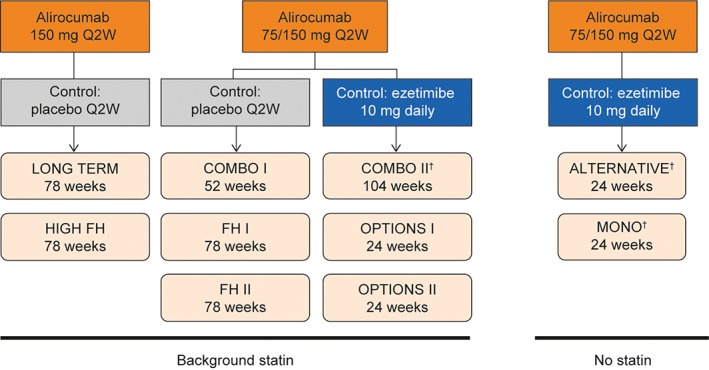
Overview of ODYSSEY studies included in this analysis. Abbreviations: LLT, lipid‐lowering therapy; Q2W, every 2 weeks. †Additional non‐statin LLTs were not allowed. http://clinicaltrials.gov identifiers: ALTERNATIVE, NCT01709513; COMBO I, NCT01644175; COMBO II, NCT01644188; FH I, NCT01623115; FH II, NCT01709500; HIGH FH, NCT01617655; LONG TERM, NCT01507831; MONO, NCT01644474; OPTIONS I, NCT01730040; OPTIONS II, NCT01730053
For the current analysis, efficacy data were analysed in 4 pools according to alirocumab starting dose, control (placebo or ezetimibe) and use of background statin therapy (yes/no), as shown in Figure 1. In the first pool (LONG TERM,19 HIGH FH23), patients received alirocumab 150 mg Q2W vs placebo (with statins); in the second (COMBO I,18 FH I & II15), alirocumab 75/150 mg Q2W vs placebo (with statins); in the third (COMBO II,17 OPTIONS I & II16, 20), alirocumab 75/150 mg Q2W vs ezetimibe (with statins); and in the fourth (ALTERNATIVE,22 MONO21), alirocumab 75/150 mg Q2W vs ezetimibe (without statins).
Patient baseline characteristics and safety and efficacy data were further analysed in subgroups, with and without MetS. MetS was defined in this analysis of the ODYSSEY trials using a definition similar to that proposed by the International Atherosclerosis Society,24 with 2 important differences. Firstly, waist circumference measurements were not performed in the alirocumab phase 3 program; instead, we used BMI as a proxy, as has been done previously.25 Second, although 1 of the criteria for the International Atherosclerosis Society definition was FPG ≥100 mg/dL, which would include individuals with type 2 diabetes, we introduced a cut‐off of 126 mg/dL for FPG so that individuals with type 2 diabetes would be excluded. This was to allow examination of the specific metabolic syndrome population known to be at risk of developing diabetes and cardiovascular disease. In this analysis, metabolic syndrome was defined as the presence of 3 or more of the following: BMI >30 kg/m2 for non‐Asians or >25 kg/m2 for Asians; TGs ≥150 mg/dL or use of TG‐lowering medication (which in this analysis included fibrates); HDL‐C <40 mg/dL in men or <50 mg/dL in women; blood pressure ≥130/85 mm Hg or diagnosis of hypertension; and FPG ≥100 to <126 mg/dL. Patients with type 1 or type 2 diabetes were excluded from the analysis (type 2 diabetes was defined based on medical history, or baseline glycated haemoglobin [HbA1c] ≥6.5%, or 2 FPG values ≥126 mg/dL at screening and randomization). All other patients (ie, those who did not have MetS or diabetes) were defined as non‐MetS for this analysis.
2.2. Endpoints
The current pooled analysis uses the same efficacy endpoints as the primary studies. The primary efficacy endpoint in all studies was the percentage change in LDL‐C from baseline to Week 24. LDL‐C levels were calculated using the Friedewald equation if TGs were <400 mg/dL; in instances above this threshold, LDL‐C was determined using the beta‐quantification method. Regardless, LDL‐C values derived by beta‐quantification were not included in the efficacy analysis. Secondary efficacy endpoints included the percentage change from baseline to Week 24 in other lipids, including non‐HDL‐C, ApoB, lipoprotein (a) (Lp[a]), TGs, HDL‐C and TG‐rich lipoprotein cholesterol (TRL‐C). Non‐HDL‐C was calculated by subtracting HDL‐C from total cholesterol. The concentration of TRL‐C was calculated by subtracting HDL‐C and calculated LDL‐C from total cholesterol, following the method of Nordestgaard et al.26 Lp(a) and ApoB levels in serum were measured from immunonephelometry by a central laboratory (Medpace Reference Laboratories, Cincinnati, Ohio and Leuven, Belgium, with the exception of the LONG TERM study,19 which used Covance Central Laboratory, Indianapolis, Indiana). Safety assessments included treatment‐emergent adverse events (TEAEs), defined as events occurring from the time of the first dose of study treatment to the last dose, plus 70 days. The change over time in glycaemic parameters (HbA1c and FPG) was also assessed.
2.3. Statistical analysis
Efficacy data were analysed using an intention‐to‐treat approach, as used in the primary trials, where all data were included regardless of adherence to treatment. The intention‐to‐treat population included all randomized participants with an evaluable primary efficacy endpoint (baseline calculated LDL‐C value and at least 1 post‐baseline calculated LDL‐C value up to Week 24). For most parameters, a mixed effects model with repeated measures (MMRM) was used to account for missing data. Least squares means and standard errors (SE) were taken from the MMRM analysis. For analysis of TGs and Lp(a), adjusted means and SEs were taken from multiple imputation followed by robust regression. Interaction P values were calculated using the same model as above for comparing the difference (between alirocumab and control) in percent change for lipid endpoints observed in both MetS and non‐MetS patients. The proportion of patients achieving lipid goals was estimated from multiple imputation using only lipid data from patients who were on‐treatment.
3. RESULTS
3.1. Patient characteristics
The randomized population for this analysis included 3341 patients (1940 with MetS; 1401 non‐MetS); 1642 randomized individuals with diabetes were excluded. Baseline characteristics according to alirocumab starting dose, control type and MetS status for each of the 4 pools are shown in Table 1 and online in Table S1. Patients had a mean age of 50 to 63 years across the groups; there was a greater proportion of males than females; and patients were mostly white (>85%). As per the selection criteria for this analysis, MetS patient groups had higher BMI, higher systolic blood pressure, a higher percentage of individuals with hypertension, higher FPG levels, lower levels of HDL‐C, and higher levels of TGs compared to groups without MetS, for both placebo‐ and ezetimibe‐controlled studies (Table 1). A higher proportion of patients with MetS were receiving other LLTs in addition to statin (Table S1). Demographic and baseline characteristics were generally comparable between the alirocumab and control groups for each pool according to MetS status. However, for the ezetimibe‐controlled studies, patients without MetS who received alirocumab in the 75/150 mg Q2W (with statins) pool were more likely to have hypertension (57.8% vs 46.0%) and atherosclerotic CV disease (90.4% vs 82.0%) than those in the control group; patients without MetS who received alirocumab in the 75/150 mg Q2W (without statins) pool were more likely to have atherosclerotic CV disease (23.2% vs 15.7%) and less likely to be receiving other LLTs (10.0% vs 21.4%).
Table 1.
Patient baseline characteristics and baseline lipids (randomized population)
| ALI 150 vs PBO (with statins) | ALI 75/150 vs PBO (with statins) | ALI 75/150 vs EZE (with statins) | ALI 75/150 vs EZE (without statins) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ALI | PBO | ALI | PBO | ALI | EZE | ALI | EZE | ||
| Group, n | MetS | 599 | 334 | 294 | 137 | 254 | 168 | 75 | 79 |
| Non‐MetS | 429 | 184 | 261 | 131 | 166 | 100 | 60 | 70 | |
| Age, years, mean (SD) | MetS | 59.5 (10.5) | 60.4 (9.9) | 56.7 (12.2) | 55.2 (13.1) | 61.3 (9.5) | 61.9 (9.8) | 62.6 (8.2) | 63.2 (8.3) |
| Non‐MetS | 58.3 (12.1) | 58.6 (12.2) | 50.6 (13.6) | 52.6 (12.5) | 60.7 (9.7) | 60.3 (11.2) | 61.8 (7.3) | 59.9 (10.5) | |
| Male, % (n) | MetS | 65.8 (394) | 66.2 (221) | 60.9 (179) | 64.2 (88) | 74.0 (188) | 70.2 (118) | 58.7 (44) | 54.4 (43) |
| Non‐MetS | 64.3 (276) | 62.5 (115) | 52.5 (137) | 58.8 (77) | 74.1 (123) | 70.0 (70) | 51.7 (31) | 50.0 (35) | |
| Race, white, % (n) | MetS | 96.2 (576) | 96.4 (322) | 94.9 (279) | 91.2 (125) | 85.4 (217) | 91.7 (154) | 94.7 (71) | 87.3 (69) |
| Non‐MetS | 97.9 (420) | 96.7 (178) | 93.5 (244) | 94.7 (124) | 90.4 (150) | 88.0 (88) | 95.0 (57) | 98.6 (69) | |
| BMI, kg/m2, mean (SD) | MetS | 30.8 (5.3) | 30.6 (4.7) | 31.0 (4.4) | 30.9 (5.8) | 31.1 (5.2) | 30.7 (5.0) | 30.7 (5.1) | 30.3 (5.4) |
| Non‐MetS | 26.6 (3.7) | 26.5 (3.5) | 26.9 (4.0) | 27.0 (4.0) | 26.3 (3.2) | 26.8 (3.5) | 25.4 (3.3) | 25.1 (3.0) | |
| SBP, mm Hg, mean (SD) | MetS | 135.2 (15.7) | 135.2 (13.8) | 131.5 (13.7) | 129.3 (12.5) | 132.3 (14.0) | 131.9 (11.8) | 131.5 (12.6) | 130.4 (12.9) |
| Non‐MetS | 124.7 (15.1) | 125.5 (15.1) | 121.3 (12.9) | 122.4 (14.2) | 124.7 (14.1) | 121.9 (12.9) | 123.3 (13.4) | 124.4 (13.3) | |
| HbA1c, %, mean (SD) | MetS | 5.7 (0.4) | 5.6 (0.3) | 5.7 (0.4) | 5.6 (0.3) | 5.7 (0.3) | 5.7 (0.4) | 5.7 (0.3) | 5.6 (0.4) |
| Non‐MetS | 5.6 (0.3) | 5.6 (0.3) | 5.5 (0.3) | 5.5 (0.3) | 5.6 (0.3) | 5.6 (0.3) | 5.5 (0.3) | 5.5 (0.3) | |
| FPG, mg/dL, mean (SD) | MetS | 101.3 (11.5) | 100.0 (11.9) | 101.1 (12.3) | 100.4 (10.0) | 104.2 (11.4) | 101.7 (11.8) | 101.7 (15.4) | 98.6 (10.2) |
| Non‐MetS | 93.6 (9.7) | 94.0 (10.8) | 92.4 (9.3) | 93.3 (12.0) | 97.8 (15.2) | 96.9 (14.6) | 93.9 (11.7) | 93.0 (8.5) | |
| Hypertension, % (n) | MetS | 80.3 (481) | 82.9 (277) | 67.3 (198) | 65.0 (89) | 83.1 (211) | 88.1 (148) | 72.0 (54) | 72.2 (57) |
| Non‐MetS | 37.8 (162) | 39.7 (73) | 21.8 (57) | 22.9 (30) | 57.8 (96) | 46.0 (46) | 20.0 (12) | 18.6 (13) | |
| ASCVD,a % (n) | MetS | 85.8 (514) | 88.3 (295) | 61.6 (181) | 66.4 (91) | 91.3 (232) | 89.9 (151) | 44.0 (33) | 43.0 (34) |
| Non‐MetS | 76.5 (328) | 77.2 (142) | 42.9 (112) | 39.7 (52) | 90.4 (150) | 82.0 (82) | 23.3 (14) | 15.7 (11) | |
| Very high cardiovascular risk, % (n) | MetS | 87.5 (524) | 91.3 (305) | 63.6 (187) | 69.3 (95) | 92.5 (235) | 90.5 (152) | 44.0 (33) | 45.6 (36) |
| Non‐MetS | 78.8 (338) | 78.3 (144) | 44.4 (116) | 41.2 (54) | 91.0 (151) | 82.0 (82) | 25.0 (15) | 15.7 (11) | |
| LDL‐C, mg/dL, mean (SD) | MetS | 129.9 (50.6) | 128.9 (47.7) | 132.6 (48.7) | 134.0 (48.6) | 111.9 (37.5) | 106.1 (38.2) | 184.5 (69.3) | 174.2 (58.4) |
| Non‐MetS | 131.9 (48.3) | 129.4 (46.1) | 132.9 (47.0) | 131.6 (40.3) | 109.7 (38.4) | 110.3 (42.8) | 177.6 (73.0) | 182.7 (75.3) | |
| HDL‐C, mg/dL, mean (SD) | MetS | 47.7 (11.5) | 48.3 (12.2) | 47.8 (14.9) | 46.4 (13.7) | 46.7 (13.1) | 47.3 (12.6) | 46.9 (13.0) | 47.3 (10.1) |
| Non‐MetS | 55.2 (13.2) | 54.9 (11.2) | 56.4 (15.6) | 55.5 (15.0) | 55.0 (12.6) | 53.9 (13.5) | 60.1 (17.0) | 61.9 (18.8) | |
| TGs, mg/dL, median (Q1:Q3) | MetS | 145.1 (108.8:198.0) | 154.0 (108.8:197.3) | 141.0 (97.0:196.0) | 134.5 (101.0:174.0) | 148.5 (109.0:200.0) | 154.5 (112.5:207.0) | 174.0 (135.0:235.0) | 163.0 (115.0:225.0) |
| Non‐MetS | 93.8 (75.0:126.5) | 99.1 (73.7:124.9) | 93.0 (72.0:121.0) | 90.0 (72.0:109.0) | 98.0 (76.0:127.0) | 96.5 (78.0:118.5) | 100.0 (74.5:120.5) | 93.0 (77.0:128.0) | |
| TGs in patients on non‐statin LLTs | MetS | 130.5 (96.0: 177.9) | 145.1 (95.0: 196.0) | 125.0 (93.5: 180.5) | 123.0 (97.0: 170.0) | 131.0 (106.0: 208.0) | 142.0 (95.0: 191.5) | 207.0 (132.0: 240.0) | 197.0 (120.0: 232.5) |
| Non‐MetS | 88.0 (72.0: 111.5) | 94.7 (67.7: 126.5) | 89.0 (70.0: 109.0) | 90.0 (70.5: 104.5) | 89.0 (79.5: 106.0) | 111.0 (107.0: 113.0) | 122.0 (105.0: 125.0) | 108.0 (83.0: 129.0) | |
| TGs in patients not on non‐statin LLTs | MetS | 153.1 (115.9: 205.0) | 158.0 (115.9: 199.0) | 170.5 (118.0: 211.0) | 147.0 (113.0: 198.0) | 153.0 (110.0: 199.0) | 159.0 (115.0: 218.5) | 172.5 (153.0: 233.0) | 159.0 (112.0: 218.0) |
| Non‐MetS | 95.6 (75.0: 131.0) | 99.1 (78.8: 124.4) | 95.0 (72.0: 126.0) | 91.0 (73.0: 120.0) | 99.0 (76.0: 129.0) | 96.0 (78.0: 120.0) | 95.0 (74.0: 119.0) | 91.0 (73.0: 128.0) | |
Abbreviations: ALI, alirocumab; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; EZE, ezetimibe; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; LLTs, lipid‐lowering therapies; MetS, metabolic syndrome; PBO, placebo; SBP, systolic blood pressure; SD, standard deviation; TGs, triglycerides.
Including coronary heart disease, peripheral arterial disease and ischaemic stroke; transient ischaemic attack, carotid endarterectomy or carotid artery stent procedure, and renal artery stent procedure were also included in the definition used for the FH II, ALTERNATIVE and OPTIONS I & II trials.
3.2. Efficacy
LDL‐C percentage reductions from baseline at Week 24 with alirocumab were 63.9% (MetS) and 56.8% (non‐MetS) in the pool of alirocumab 150 mg Q2W (interaction P value <.05), 52.2% (MetS) and 45.0% (non‐MetS) in the pool of alirocumab 75/150 mg Q2W vs placebo (interaction P value >.05), 51.9% (MetS) and 52.6% (non‐MetS) in the pool of alirocumab 75/150 mg Q2W vs ezetimibe (with statins) (interaction P value >.05), and 42.2% (MetS) and 46.7% (non‐MetS) in the alirocumab 75/150 mg Q2W pool (without statins) (interaction P value >0.05) (Figure 2A).
Figure 2.

Percent change from baseline in A, calculated LDL‐C; B, ApoB; C, non‐HDL‐C; D, TGs; E, Lp(a); F, HDL‐C and G, TRL‐C at Week 24: Subgroup analysis according to MetS status at baseline (intention‐to‐treat analysis). Abbreviations: Apo, apolipoprotein; CI, confidence interval; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; Lp(a), lipoprotein(a); LS, least squares; MetS, metabolic syndrome; SE, standard error; TG, triglyceride; TRL‐C, triglyceride‐rich lipoprotein cholesterol. Non‐HDL‐C was calculated by subtracting HDL‐C from total cholesterol. TRL‐C was calculated by subtracting (HDL‐C and calculated LDL‐C) from total cholesterol
Across the 4 efficacy pools, by Week 24, alirocumab reduced ApoB by 35.0% to 55.1% (MetS) and 35.9% to 51.3% (non‐MetS) (Figure 2B), and non‐HDL‐C by 38.2% to 54.2% (MetS) and 40.0% to 50.0% (non‐MetS) (Figure 2C). ApoB was reduced to a larger extent in the patients with MetS compared with non‐MetS subjects in the alirocumab 75/150 vs placebo pool only at Week 24 (interaction P values <.05) (Figure 2B). For non‐HDL‐C, there was no difference in percent reduction from baseline at Week 24 for those with vs without MetS (Figure 2C). Apart from LDL‐C percent reductions in the alirocumab 150 mg Q2W pool (interaction P value <.05), there were no differences in LDL‐C, ApoB or non‐HDL‐C reductions between MetS and non‐MetS groups at Week 12 (interaction P values >.05) (Figure S1A‐C).
A greater proportion of patients receiving alirocumab achieved LDL‐C and non‐HDL‐C goals compared with controls at Week 24, regardless of MetS status (Tables S2 and S3).
Across the pools, by Week 24, TGs were reduced by 7.1% to 15.9% (MetS) and 6.5% to 14.9% (non‐MetS) (Figure 2D), with similar results observed at Week 12 (Figure S1D). Lp(a) was reduced by 21.3% to 29.5% (MetS) and 22.7% to 28.8% (non‐MetS) at Week 24 (Figure 2E). Reductions in each of these parameters were similar regardless of MetS status at Week 24 (interaction P values >.05).
At Week 24, the use of other non‐statin LLTs did not influence the percent change from baseline in TG levels observed with alirocumab (Figure S2); for patients with MetS, the reduction from baseline was 13.3% to 22.6% (with LLT) vs 6.3% to 19.0% (without LLT) and for non‐MetS subjects, the reduction from baseline was 3.2% to 14.7% (with LLT) vs 0% to 10.0% (without LLT). Non‐statin LLTs were examined in this analysis because of their potential effects on TG levels.
An increase in HDL‐C levels of 4.0% to 10.1% was also observed across the 4 pools following alirocumab therapy at Week 24 (Figure 2F). HDL‐C was increased to a larger extent in the patients with MetS compared with the non‐MetS subjects in the alirocumab 75/150 mg vs placebo pool only (interaction P value <.05); however, no significant difference between the MetS and non‐MetS groups was observed at Week 12 (interaction P value >.05) (Figure S1E). For patients with MetS or non‐MetS subjects, the use of non‐statin LLTs at Week 24 did not influence the percent change from baseline in HDL‐C levels observed with alirocumab (Figure S3).
Across the 3 pools with patients on background statin therapy, alirocumab reduced TRL‐C by 2.9% to 12.7% at Week 24, regardless of MetS status (Figure 2G). In contrast, for alirocumab‐treated patients in the alirocumab 75/150 vs ezetimibe (without statins) pool, TRL‐C was decreased (−4.8%) in patients with MetS and increased (+1.9%) in subjects without MetS (Figure 2G).
The percentage of patients for whom the alirocumab dose was increased from 75 to 150 mg Q2W at Week 12 was 36.1% (MetS) and 32.6% (non‐MetS) in the 75/150 mg vs placebo pool, was 17.7% (MetS) and 15.1% (non‐MetS) in the 75/150 mg vs ezetimibe with statin pool, and was 41.3% (MetS) and 35.0% (non‐MetS) in the 75/150 mg vs ezetimibe without statin pool.
3.3. Safety of alirocumab with respect to patients by MetS status
Time profiles of mean HbA1c and FPG levels over the treatment period showed that alirocumab had no clinically meaningful effect on these parameters compared to control treatments, for patients both with and without MetS (Figure 3). TEAE rates were generally similar across all treatment groups, irrespective of MetS status (Table 2). The most common TEAEs were nasopharyngitis, injection‐site reaction, myalgia and upper respiratory tract infection. Injection‐site reactions occurred more frequently in the alirocumab group vs the control group, for both placebo‐controlled (6.5% vs 6.4% [MetS] and 11.1% vs 6.1% [non‐MetS]) and ezetimibe‐controlled (2.7% vs 2.0% [MetS] and 4.0% vs 1.8% [non‐MetS]) trials (Table 2).
Figure 3.
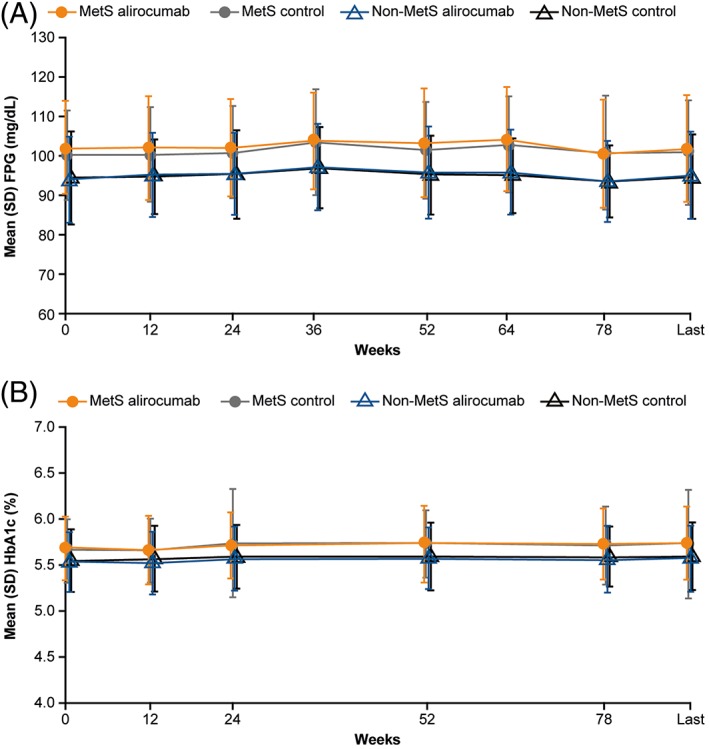
Time profile of A, mean FPG and B, mean HbA1c levels: Subgroup analysis according to MetS status for pool of phase 3 studies (safety population). Abbreviations: FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; MetS, metabolic syndrome; SD, standard deviation. Figures show combined pools for ezetimibe and placebo control pools, as differences between alirocumab and control were similar in both pools. Last value defined as the last value collected up to 21 days after the last double‐blind study treatment
Table 2.
Safety analysis (safety population)
| % (n) | Placebo‐controlled studies | Ezetimibe‐controlled studies | ||||||
|---|---|---|---|---|---|---|---|---|
| MetS (n = 1364) | Non‐MetS (n = 1001) | MetS (n = 576) | Non‐MetS (n = 395) | |||||
| ALI(n = 893) | PBO(n = 471) | ALI(n = 687) | PBO(n = 314) | ALI(n = 329) | EZE(n = 247) | ALI(n = 226) | EZE(n = 169) | |
| TEAEs | 77.4 (691) | 80.5 (379) | 74.8 (514) | 75.5 (237) | 72.3 (238) | 70.4 (174) | 69.0 (156) | 69.8 (118) |
| Treatment‐emergent SAEs | 13.7 (122) | 13.6 (64) | 11.8 (81) | 9.9 (31) | 12.2 (40) | 12.6 (31) | 13.7 (31) | 8.9 (15) |
| TEAEs leading to death | 0.3 (3) | 0.4 (2) | 0.7 (5) | 1.3 (4) | 0 | 0.8 (2) | 0.4 (1) | 1.8 (3) |
| TEAEs leading to discontinuations | 4.5 (40) | 5.3 (25) | 4.9 (34) | 4.1 (13) | 9.7 (32) | 8.1 (20) | 8.0 (18) | 11.2 (19) |
| TEAEs by preferred term in ≥5% individuals | ||||||||
| Nasopharyngitis | 12.5 (112) | 11.0 (52) | 11.9 (82) | 14.0 (44) | 6.4 (21) | 4.5 (11) | 6.6 (15) | 10.1 (17) |
| Injection‐site reaction | 6.5 (58) | 6.4 (30) | 11.1 (76) | 6.1 (19) | 2.7 (9) | 2.0 (5) | 4.0 (9) | 1.8 (3) |
| Myalgia | 5.5 (49) | 3.8 (18) | 4.7 (32) | 4.8 (15) | 8.5 (28) | 8.9 (22) | 6.6 (15) | 7.7 (13) |
| Upper respiratory tract infection | 5.4 (48) | 6.2 (29) | 6.3 (43) | 7.0 (22) | 7.3 (24) | 6.9 (17) | 4.9 (11) | 3.6 (6) |
| Influenza | 6.9 (62) | 4.2 (20) | 5.8 (40) | 6.4 (20) | 3.0 (10) | 2.0 (5) | 3.5 (8) | 2.4 (4) |
| Headache | 4.6 (41) | 5.9 (28) | 6.4 (44) | 5.7 (18) | 5.8 (19) | 2.8 (7) | 3.5 (8) | 4.1 (7) |
| Arthralgia | 5.3 (47) | 5.9 (28) | 4.2 (29) | 5.7 (18) | 4.6 (15) | 4.9 (12) | 4.9 (11) | 3.0 (5) |
| Back pain | 4.6 (41) | 5.9 (28) | 4.7 (32) | 5.4 (17) | 1.8 (6) | 3.2 (8) | 3.5 (8) | 4.7 (8) |
| Diarrhoea | 5.6 (50) | 5.7 (27) | 4.9 (34) | 1.6 (5) | 2.7 (9) | 1.6 (4) | 4.4 (10) | 4.7 (8) |
| Dizziness | 3.0 (27) | 4.9 (23) | 2.6 (18) | 2.5 (8) | 2.7 (9) | 5.7 (14) | 4.4 (10) | 3.0 (5) |
| Urinary tract infection | 4.8 (43) | 3.8 (18) | 4.8 (33) | 5.1 (16) | 2.1 (7) | 4.0 (10) | 0.9 (2) | 2.4 (4) |
Abbreviations: ALI, alirocumab; EZE, ezetimibe; PBO, placebo; MetS, metabolic syndrome; SAE, serious adverse event; TEAE, treatment‐emergent adverse event.
4. DISCUSSION
In this pooled analysis of 10 phase 3 ODYSSEY trials, alirocumab reduced levels of LDL‐C, ApoB, Lp(a), TGs and non‐HDL‐C in patients with hypercholesterolaemia, regardless of MetS status. These findings are important because more than one‐third of patients in the ODYSSEY trials had MetS (39%). This analysis demonstrated that the typical characteristics of these patients (mixed dyslipidaemia together with obesity, glucose intolerance and hypertension, most likely with additional concomitant medications) did not seem to have a major impact on the efficacy and safety of alirocumab. A slightly larger reduction in LDL‐C was observed in individuals with MetS (vs no MetS) following alirocumab treatment in 1 of the study pools; however, as this was not observed in the other pools, it is unclear whether observed differences are real effects or the result of pooling the post‐randomization groups with relatively small numbers of individuals.
The observed reductions in ApoB and non‐HDL‐C are of particular importance, as they may give a better estimate of the concentration of atherogenic particles than calculated LDL‐C for patients with MetS because of the mixed dyslipidaemia profile.4, 24 It is interesting to note that alirocumab can increase the fractional clearance rates of non‐LDL ApoB 100‐containing particles such as intermediate density lipoprotein and, possibly, Lp(a).27 The moderate reductions in TGs and moderate increases in HDL‐C observed following alirocumab treatment also demonstrate an improvement in the lipid profile.
Patients with MetS had a higher rate of other LLT use, including increased fenofibrate, which is often prescribed to reduce TG levels.28 However, use of non‐statin LLTs in combination with alirocumab did not affect the moderate reductions in TGs seen with alirocumab treatment. In addition, the use of non‐statin LLTs did not affect the modest increases in HDL‐C levels observed with alirocumab treatment.
Previous studies have shown that patients with MetS may not achieve their non‐HDL‐C goal following treatment with statins and other LLTs; in particular, the presence of MetS was shown to be a factor contributing to a greater difference between recommended vs attained non‐HDL‐C levels.8 In the present analysis, a higher proportion of patients receiving alirocumab achieved both LDL‐C and non‐HDL‐C goals compared with patients receiving placebo or ezetimibe, irrespective of MetS status.
With regards to safety, MetS status did not affect the incidence of TEAEs, with similar rates observed between the alirocumab and control groups. In this analysis, HbA1c and FPG levels were found to be unaffected by alirocumab in individuals both with and without MetS. Some studies have suggested that statin therapy is associated with an increased incidence of type 2 diabetes.29, 30, 31 In addition, a Mendelian randomization study found that PCSK9 and 3‐hydroxy‐3‐methylglutaryl‐coenzyme A reductase (HMGCR, the target of statin therapy) genetic variants associated with lower LDL‐C were correlated with a reduced risk of CV events but also an increased risk of diabetes.32 However, the authors emphasize that as PCSK9 antibodies bind extracellular PCSK9, their biological effects may not be the same as those observed with PCSK9 genetic variants which lower LDL‐C levels over the course of a lifetime.32 Furthermore, a pooled analysis of 10 ODYSSEY phase 3 studies, with follow‐up periods of between 24 and 104 weeks, indicated that there was no evidence of an effect of alirocumab on the transition to new‐onset diabetes in those individuals without diabetes or with pre‐diabetes at baseline.33 Also of note, subgroup analyses of ODYSSEY trials have shown no effect of diabetes on the efficacy of alirocumab in terms of LDL‐C reduction from baseline up to Week 104.19, 34 Similarly, no effect of pre‐diabetes on the efficacy and safety of alirocumab was observed.35 Lastly, a prespecified analysis of the FOURIER clinical outcomes study with another PCSK9 inhibitor, evolocumab, showed no effect of diabetic status on efficacy and safety with follow‐up to 2.2 years, and no effect of evolocumab on the incidence of new‐onset diabetes.36
Limitations of this analysis include the relatively short treatment periods of the ODYSSEY trials and the small number of patients in some subgroups. Waist circumference, a usual parameter for assessing obesity/MetS, was not measured in the trials. In addition, the analysis was performed on post‐randomization groups, and some differences in baseline characteristics between the resulting alirocumab and control subgroups were noted. In order to provide sufficient numbers of patients for analysis, data were pooled from studies including patients with different clinical characteristics (eg, heterozygous familial hypercholesterolaemia,15, 23 non‐familial hypercholesterolaemia with prior cardiovascular disease or other risk factors,17, 18 or statin intolerance22), which may limit interpretation of results.
In summary, this pooled analysis showed that alirocumab produced significant reductions in both LDL‐C and non‐HDL‐C, of similar magnitudes in individuals both with and without MetS, and was generally well tolerated, with no apparent effect on measures of glycaemic control.
Supporting information
Table S1. Further baseline characteristics (randomized population).
Table S2. Percentage of patients who achieved risk‐based LDL‐C goalsa at Week 24 according to MetS status (on‐treatment analysis).
Table S3. Percentage of patients who achieved non‐HDL‐C goal (<100 mg/dL) at Week 24 according to MetS status (on‐treatment analysis).
Figure S1. Percent change from baseline in A, calculated LDL‐C; B, ApoB; C, non‐HDL‐C; D, TGs and E, HDL‐C at Week 12: Subgroup analysis according to MetS status at baseline (ITT analysis).
Figure S2. Percent change from baseline in TGs at Week 24 in participants A, with MetS and B, without MetS: Subgroup analysis according to non‐statin LLT status at randomization (ITT analysis).
Figure S3. Percent change from baseline in HDL‐C at Week 24 in participants A, with MetS and B, without MetS: Subgroup analysis according to non‐statin LLT status at randomization (ITT analysis).
ACKNOWLEDGMENTS
The authors would like to thank the patients, their families, and all investigators involved in the ODYSSEY studies. In addition, the authors would like to thank Desmond Thompson for providing further statistical analyses. Catherine Domenger, of Sanofi, reviewed and provided editorial comments on the manuscript. Medical writing assistance and editorial support, under the direction of the authors, was provided by Rachel Dunn PhD and Rob Campbell PhD, both of Prime (Knutsford, UK) and funded by Sanofi and Regeneron Pharmaceuticals, Inc. according to Good Publication Practice guidelines (Link).
Conflict of interest
R. R. H. has received research funding from Abbott, AstaMed, Eli Lilly, Hitachi, Novo Nordisk, Sanofi‐Lexicon and Viacyte; and has been a consultant/advisory panel member for Alere, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eisai, Elcelyx, Intarcia, Ionis, Janssen/Johnson & Johnson, Merck and Sanofi‐Aventis. D. M.‐W. has participated in speakers' bureaux for Amgen, Boehringer Ingelheim, Lilly, Sanofi, Merck (MSD), AstraZeneca and Novartis; and has been a consultant/advisory panel member for Sanofi, Boehringer Ingelheim, Merck, AstraZeneca and Novartis. P. R. T. has been a consultant/advisory panel member for Roche; and has participated in speaker's bureaux for Amgen, Regeneron Pharmaceuticals, Inc., Sanofi and Boehringer Ingelheim. M. B.‐B. and A. L. are employees of/stockholders in Sanofi. M. J. L. is an employee of/stockholder in Regeneron Pharmaceuticals, Inc. H. N. G. has received research funding from Genzyme (Sanofi), Merck, Sanofi/Regeneron Pharmaceuticals, Inc. and Amgen; and has been a consultant/advisory panel member for Amarin, Amgen, AstraZeneca, Bristol Myers Squibb, GlaxoSmithKline, Ionis, Janssen, Kowa, Merck, Novartis, Sanofi/Regeneron Pharmaceuticals, Inc. and Pfizer.
Author contributions
The sponsors were involved in the study design, collection, analysis and interpretation of data. R. R. H., D. M.‐W., P. R. T., M. B.‐B., M. J. L., A. L., and H. N. G. contributed to the data analysis and interpretation of the data, and critically reviewed and edited the manuscript. In addition, R. R. H., M. B.‐B., M. J. L. and A. L. contributed to the concept or study design; and H. N. G. contributed to the data acquisition. All authors approved the final version. The authors had unrestricted access to study data, were responsible for all content and editorial decisions, and received no honoraria related to the development of this publication.
Henry RR, Müller‐Wieland D, Taub PR, et al. Effect of alirocumab on lipids and lipoproteins in individuals with metabolic syndrome without diabetes: Pooled data from 10 phase 3 trials. Diabetes Obes Metab. 2018;20:1632–1641. https://doi.org/10.1111/dom.13273
Funding information This analysis was funded by Sanofi and Regeneron Pharmaceuticals, Inc.
REFERENCES
- 1. Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640‐1645. [DOI] [PubMed] [Google Scholar]
- 2. Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3. Bays H, Jones PH, Orringer CE, Brown WV, Jacobson TA. National Lipid Association Annual Summary of Clinical Lipidology 2016. J Clin Lipidol. 2016;10(suppl):S1‐S43. [DOI] [PubMed] [Google Scholar]
- 4. Jacobson TA, Ito MK, Maki KC, et al. National Lipid Association recommendations for patient‐centered management of dyslipidemia: part 1‐‐full report. J Clin Lipidol. 2015;9:129‐169. [DOI] [PubMed] [Google Scholar]
- 5. Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;56:1113‐1132. [DOI] [PubMed] [Google Scholar]
- 6. Alexander CM, Landsman PB, Teutsch SM, Haffner SM, Third National Health and Nutrition Examination Survey (NHANES III) , National Cholesterol Education Program (NCEP) . NCEP‐defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52:1210‐1214. [DOI] [PubMed] [Google Scholar]
- 7. Stulc T, Ceška R, Gotto AM Jr. Statin intolerance: the clinician's perspective. Curr Atheroscler Rep. 2015;17:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Santos RD, Waters DD, Tarasenko L, et al. A comparison of non‐HDL and LDL cholesterol goal attainment in a large, multinational patient population: the Lipid Treatment Assessment Project 2. Atherosclerosis. 2012;224:150‐153. [DOI] [PubMed] [Google Scholar]
- 9. Mark L, Vallejo‐Vaz AJ, Reiber I, Paragh G, Kondapally Seshasai SR, Ray KK. Non‐HDL cholesterol goal attainment and its relationship with triglyceride concentrations among diabetic subjects with cardiovascular disease: a nationwide survey of 2674 individuals in Hungary. Atherosclerosis. 2015;241:62‐68. [DOI] [PubMed] [Google Scholar]
- 10. Sanofi . Praluent prescribing information (USA). http://products.sanofi.us/praluent/praluent.pdf. Accessed April 25, 2017.
- 11. Sanofi . Praluent summary of product characteristics (EC). http://ec.europa.eu/health/documents/community-register/2015/20150923132812/anx_132812_en.pdf. Accessed April 25, 2017.
- 12. Ferri N, Ruscica M. Proprotein convertase subtilisin/kexin type 9 (PCSK9) and metabolic syndrome: insights on insulin resistance, inflammation, and atherogenic dyslipidemia. Endocrine. 2016;54:588‐601. [DOI] [PubMed] [Google Scholar]
- 13. Filippatos TD, Filippas‐Ntekouan S, Pappa E, Panagiotopoulou T, Tsimihodimos V, Elisaf MS. PCSK9 and carbohydrate metabolism: a double‐edged sword. World J Diabetes. 2017;8:311‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rask‐Madsen C, Kahn CR. Tissue‐specific insulin signaling, metabolic syndrome, and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2012;32:2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kastelein JJ, Ginsberg HN, Langslet G, et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J. 2015;36:2996‐3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bays H, Gaudet D, Weiss R, et al. Alirocumab as add‐on to atorvastatin versus other lipid treatment strategies: ODYSSEY OPTIONS I randomized trial. J Clin Endocrinol Metab. 2015;100:3140‐3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cannon CP, Cariou B, Blom D, et al. Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur Heart J. 2015;36:1186‐1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kereiakes DJ, Robinson JG, Cannon CP, et al. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: the ODYSSEY COMBO I study. Am Heart J. 2015;169:906‐915. e913. [DOI] [PubMed] [Google Scholar]
- 19. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489‐1499. [DOI] [PubMed] [Google Scholar]
- 20. Farnier M, Jones P, Severance R, et al. Efficacy and safety of adding alirocumab to rosuvastatin versus adding ezetimibe or doubling the rosuvastatin dose in high cardiovascular‐risk patients: the ODYSSEY OPTIONS II randomized trial. Atherosclerosis. 2016;244:138‐146. [DOI] [PubMed] [Google Scholar]
- 21. Roth EM, Taskinen MR, Ginsberg HN, et al. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: results of a 24 week, double‐blind, randomized Phase 3 trial. Int J Cardiol. 2014;176:55‐61. [DOI] [PubMed] [Google Scholar]
- 22. Moriarty PM, Thompson PD, Cannon CP, et al. Efficacy and safety of alirocumab vs ezetimibe in statin‐intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol. 2015;9:758‐769. [DOI] [PubMed] [Google Scholar]
- 23. Ginsberg HN, Rader DJ, Raal FJ, et al. Efficacy and safety of alirocumab in patients with heterozygous familial hypercholesterolemia and LDL‐C of 160 mg/dl or higher. Cardiovasc Drugs Ther. 2016;30:473‐483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Expert Dyslipidemia Panel of the International Atherosclerosis Society Panel Members . An International Atherosclerosis Society Position Paper: global recommendations for the management of dyslipidemia‐‐full report. J Clin Lipidol. 2014;8:29‐60. [DOI] [PubMed] [Google Scholar]
- 25. Deedwania P, Barter P, Carmena R, et al. Reduction of low‐density lipoprotein cholesterol in patients with coronary heart disease and metabolic syndrome: analysis of the Treating to New Targets study. Lancet. 2006;368:919‐928. [DOI] [PubMed] [Google Scholar]
- 26. Nordestgaard BG, Benn M, Schnohr P, Tybjaerg‐Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299‐308. [DOI] [PubMed] [Google Scholar]
- 27. Reyes‐Soffer G, Pavlyha M, Ngai C, et al. Effects of PCSK9 inhibition with alirocumab on lipoprotein metabolism in healthy humans. Circulation. 2016;135:352‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Santarus . Fenoglide prescribing information (USA). https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022118s005lbl.pdf. Accessed April 27, 2017.
- 29. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta‐analysis of randomised statin trials. Lancet. 2010;375:735‐742. [DOI] [PubMed] [Google Scholar]
- 30. Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive‐dose compared with moderate‐dose statin therapy: a meta‐analysis. JAMA. 2011;305:2556‐2564. [DOI] [PubMed] [Google Scholar]
- 31. Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 2012;380:565‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ference BA, Robinson JG, Brook RD, et al. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med. 2016;375:2144‐2153. [DOI] [PubMed] [Google Scholar]
- 33. Colhoun HM, Ginsberg HN, Robinson JG, et al. No effect of PCSK9 inhibitor alirocumab on the incidence of diabetes in a pooled analysis from 10 ODYSSEY Phase 3 studies. Eur Heart J. 2016;37:2981‐2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leiter LA, Zamorano JL, Bujas‐Bobanovic M, et al. Lipid‐lowering efficacy and safety of alirocumab in patients with or without diabetes: a sub‐analysis of ODYSSEY COMBO II. Diabetes Obes Metab. 2017;19:989‐996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leiter LA, Muller‐Wieland D, Baccara‐Dinet MT, Letierce A, Samuel R, Cariou B. Efficacy and safety of alirocumab in people with prediabetes vs those with normoglycaemia at baseline: a pooled analysis of 10 phase III ODYSSEY clinical trials. Diabet Med. 2018;35:121‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sabatine MS, Leiter LA, Wiviott SD, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new‐onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:941‐950. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Further baseline characteristics (randomized population).
Table S2. Percentage of patients who achieved risk‐based LDL‐C goalsa at Week 24 according to MetS status (on‐treatment analysis).
Table S3. Percentage of patients who achieved non‐HDL‐C goal (<100 mg/dL) at Week 24 according to MetS status (on‐treatment analysis).
Figure S1. Percent change from baseline in A, calculated LDL‐C; B, ApoB; C, non‐HDL‐C; D, TGs and E, HDL‐C at Week 12: Subgroup analysis according to MetS status at baseline (ITT analysis).
Figure S2. Percent change from baseline in TGs at Week 24 in participants A, with MetS and B, without MetS: Subgroup analysis according to non‐statin LLT status at randomization (ITT analysis).
Figure S3. Percent change from baseline in HDL‐C at Week 24 in participants A, with MetS and B, without MetS: Subgroup analysis according to non‐statin LLT status at randomization (ITT analysis).


