Abstract
Objective
Because of its association with joint destruction, anti–citrullinated protein antibody (ACPA)–positive rheumatoid arthritis (RA) is considered to be more severe than ACPA‐negative RA. Clinically relevant joint destruction is now infrequent thanks to adequate disease suppression. According to patients, important outcomes are pain, fatigue, and independence. We evaluated whether ACPA‐positive RA patients diagnosed during or after 2000 have more severe self‐reported limitations and impairments, including restrictions at work, than ACPA‐negative RA patients.
Methods
A total of 492 ACPA‐positive and 450 ACPA‐negative RA patients who fulfilled the 2010 criteria and were included in the Leiden Early Arthritis Clinic cohort during or after 2000 were compared for self‐reported pain, fatigue, disease activity, general well‐being (measured by numerical rating scales), physical function (measured by the Health Assessment Questionnaire), and work restrictions, including absenteeism at baseline and during the 4‐year followup. Linear mixed models were used.
Results
At disease presentation, ACPA‐negative patients had more severe pain, fatigue, self‐reported disease activity scores, and functional disability (P < 0.05), although absolute differences were small. During followup, ACPA‐negative patients remained somewhat more fatigued (P = 0.002), whereas other patient‐reported impairments and limitations were similar. Thirty‐eight percent of ACPA‐negative and 48% of ACPA‐positive patients reported absenteeism (P = 0.30), with median 4 days missed in both groups in the last 3 months. Also, restrictions at work among employed patients and restrictions with household work were not statistically different at baseline and during followup.
Conclusion
In current rheumatology practice, ACPA‐positive RA is not more severe than ACPA‐negative RA in terms of patients’ relevant outcomes, including physical functioning and restrictions at work. This implies that efforts to further improve the disease course should be proportional to both disease subsets.
Introduction
Anti–citrullinated protein antibody (ACPA)–positive and ACPA‐negative rheumatoid arthritis (RA) patients are considered as having different disease entities with differences in etiopathology, as both subsets have differences in genetic and environmental risk factors 1. ACPA‐positive RA has always been considered as a more severe subset of RA, as the presence of ACPA is associated with more severe joint destruction and a higher mortality rate 2, 3, 4.
Significance & Innovations.
Anti–citrullinated protein antibody (ACPA)–positive rheumatoid arthritis (RA) is known for its more severe disease course, compared to ACPA‐negative RA.
With current treatment strategies, both disease subsets are equally severe in terms of patient‐reported outcomes, including physical functioning and restrictions at work.
This implies that further efforts to improve the disease course should be proportional to ACPA‐positive and ACPA‐negative RA.
During the last decade treatment strategies have improved, and earlier treatment initiation and treat‐to‐target approaches have resulted in better disease outcomes 5. Especially from the year 2000 onward, early treatment with methotrexate (MTX) has become key and, at present, clinically relevant joint destruction has become infrequent 6, 7, 8, 9, 10. In addition, RA patients no longer have an evidently increased mortality rate 11, 12, 13. Therefore, these traditional outcomes of RA have become less important. This leads to the consideration of what should be the current essential disease outcomes.
A recent study emphasized determining these outcomes, and according to patients, the important outcomes are pain, fatigue, and independence 14. Independence strongly relates to physical functioning and the ability to perform one's tasks at home and at work 15. It is still unknown if ACPA‐positive patients in current rheumatology practice have a worse disease than ACPA‐negative RA patients, as evaluated with the abovementioned patient‐reported outcomes (PROs). Therefore, this study assessed, in RA patients who were diagnosed from 2000 onward and were treated with up‐to‐date treatment strategies, whether ACPA‐positive patients have more severe PROs, including functional disability and work restrictions, than ACPA‐negative RA patients.
Patients and methods
Longitudinal cohort
Patients were included in the Leiden Early Arthritis Clinic (EAC) cohort, a population‐based inception cohort in The Netherlands that started in 1993. Inclusion required the presence of arthritis confirmed at physical examination and symptom duration <2 years. Baseline visit was at first presentation of arthritis at the outpatient clinic. Followup visits were performed yearly with questionnaires, 66 swollen (SJC66) and 68 tender joint counts (TJC68), and laboratory investigations (including C‐reactive protein [CRP] level, immunoglobulin M– rheumatoid factor [RF; positive if ≥3.5 IU/ml] and ACPA [anti–cyclic citrullinated peptide (anti‐CCP2), Eurodiagnostica, positive if ≥25 U/ml; from 2009 EliA CCP, Phadia, positive if ≥7 U/ml], as described in detail elsewhere 16). For the present study, RA patients included in the Leiden EAC cohort during or after 2000 were analyzed. Patients were treated according to routine care. According to local and national protocols, patients were treated initially with MTX; in case of failure a second conventional disease‐modifying antirheumatic drug (DMARD) was started or added, and in case of subsequent failure a biologic DMARD was allowed. The strategy of treatment adjustment changed over time, as in our hospital Disease Activity Score (DAS)–steered treatment adjustments became standard as of 2005 17, 18.
To measure experienced pain, fatigue, disease activity, and general well‐being, patients were asked by trained research nurses to indicate on single‐item numerical rating scales (NRS), ranging from 0 (no symptoms) to 10 (extreme symptoms), the grade that best reflected how they felt affected by arthritis during the last 24 hours. To measure limitations in physical functioning, the multi‐item Health Assessment Questionnaire (HAQ), expressed as a disability index (DI) from 0 (no disability) to 3 (severe disability), was used. A questionnaire on work ability was added to the study protocol in 2010. It contained questions from 1) the RA‐specific Work Productivity Survey, addressing work status and type of work; 2) the Work Productivity and Activity Impairment Rheumatoid Arthritis questionnaire, assessing influence of disease on productivity at a paid job (presenteeism) or during nonpaid work in the past 7 days, ranging from 0 (no restrictions) to 10 (severe restrictions); and 3) additional questions on the number of days patients had worked with these restrictions in the past 7 days, as well as work days absent in the past 3 months (see Supplementary Table 1, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.23497/abstract).
Among employed patients at baseline, we analyzed absenteeism and the number of patients that reported absenteeism; we also analyzed presenteeism (level of restrictions at work) and the number of days employed patients had worked with restrictions in the last week due to arthritis, as well as restrictions with household activities for all patients. Data were gathered at baseline and at the yearly followup visits. Written informed consent was obtained from all patients. The study was approved by the local medical ethics committee.
Patient selection
From all early arthritis patients included between 1993 and 2016 (n = 3,722), 2,615 were included during or after 2000; of these, 982 fulfilled the 2010 classification criteria for RA at baseline (19) (see Supplementary Figure 1, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.23497/abstract).
RA patients with missing ACPA status were excluded (n = 40); they did not differ in age, sex, SJC66, CRP level, RF positivity, or symptom duration from patients with available ACPA data (see Supplementary Table 2, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.23497/abstract). In total, 450 ACPA‐negative and 492 ACPA‐positive patients were studied. To evaluate whether the choice of classification criteria affected the results, analyses were repeated with RA defined as fulfilling the 1987 criteria 20. Of the 2,615 patients included from 2000 onward, 563 fulfilled the 1987 criteria at baseline (225 ACPA‐negative patients, 338 ACPA‐positive patients). A portion of the patients that fulfilled the 1987 criteria did not fulfill the 2010 criteria and vice versa.
Since the introduction of the questionnaire on work ability in 2010, 130 ACPA‐negative and 152 ACPA‐positive RA patients fulfilling the 2010 criteria were included (see Supplementary Figure 1, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.23497/abstract). Of these 282 patients, 24 ACPA‐negative and 36 ACPA‐positive patients did not fill in the work ability questionnaire at baseline. These 60 patients did not differ in age, sex, symptom duration, SJC66, CRP level, or RF positivity from those who did complete the work ability questionnaire (see Supplementary Table 3, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.23497/abstract). The addition of this questionnaire later in the study does not cause a bias, as missingness is completely at random.
Statistical analyses
We presented median levels and corresponding interquartile ranges. Baseline data were analyzed with a t‐test, the Mann‐Whitney U test, and chi‐square test as appropriate.
For longitudinal analyses between ACPA‐negative and ACPA‐positive patients, linear mixed models were used. Although PROs were non‐normally distributed, the residuals were normally distributed and thus fulfilled the requirement for linear mixed models. Patients were censored after 4 years of followup because the number of patients with followup longer than this period decreased. No random effects were added to the model 21, 22. This model has the advantage that all patient information, including those who had missing data of PROs, was used, as it assumes that missing outcomes can be estimated using available measurements. Also, to prevent bias due to selective dropout of patients, we did not apply a minimum followup duration for inclusion in the analyses. To determine the best‐fitting covariance matrix, the matrices available in SPSS were considered. Akaike information criterion was used to measure the goodness‐of‐fit, as this was best for the compound symmetry matrix. We obtained estimates of the main effect of ACPAs. Because the target variables are known to vary with age and sex, adjustments were made in all longitudinal analyses. For PROs of impairments and limitations, adjustments for the year of inclusion were also made 23, 24, 25. In analyses, median values of estimated coefficients of the longitudinal analyses are shown. IBM SPSS, version 23, was used, and values less than 0.05 were considered significant.
Sensitivity analyses
In sensitivity analyses, it was first evaluated whether results would be different when ACPA‐negative and RF‐negative patients were compared to patients with positive ACPA and/or RF, as part of the ACPA‐negative patients were RF positive. Second, analyses were repeated in patients that were included during or after 2005. This was done as this study aimed to evaluate patients who were treated according to current treatment strategies. Although an early start of MTX was common from 2000 onward, DAS‐steered treatment adjustments became fully integrated in daily practice in our hospital from 2005 onward 18.
Finally, as a reference showing that patients treated according to up‐to‐date treatment strategies were different from patients who were treated in the past, we performed similar analyses for patients who were included in the EAC between 1993 and 1999. In this era, DMARDs were initiated with delay, and/or mild DMARDs (such as hydroxychloroquine) were started as initial therapy, as described elsewhere 5.
Results
Patient characteristics
Table 1 presents baseline characteristics of included RA patients (fulfilling the 2010 criteria). ACPA‐negative patients were older (mean age 63 versus 54 years; P < 0.001), had more swollen joints than ACPA‐positive patients (median 9 versus 5; P < 0.001), more tender joints (median 16 versus 10; P < 0.001), and a shorter disease duration (median 103 versus 144 days; P < 0.001). Over time a similar proportion (70–80%) of patients achieved DAS‐remission (44 joints assessed, DAS < 2.4) (Figure 1), indicating that despite differences in characteristics between both groups the disease activity was equally suppressed in both groups.
Table 1.
Baseline characteristics of RA patients (fulfilling 2010 criteria) for analyses on patient‐reported impairments and limitations, and work restrictionsa
| Impairments and limitations | Work restrictions | |||||
|---|---|---|---|---|---|---|
| ACPA‐negative (n = 450)b | ACPA‐positive (n = 492)c | P | ACPA‐negative (n = 130)d | ACPA‐positive (n = 152)e | P | |
| Impairments and limitations | ||||||
| Age, mean ± SD years | 60 ± 16 | 54 ± 14 | < 0.001 | 59 ± 16 | 54 ± 14 | 0.009 |
| Female, no. (%) | 295 (66) | 333 (68) | 0.49 | 90 (69) | 99 (65) | 0.47 |
| TJC68, median (IQR) | 16 (10–24) | 10 (5–17) | < 0.001 | 13 (8–21) | 8 (4–12) | < 0.001 |
| SJC66, median (IQR) | 9 (4–14) | 5 (3–10) | < 0.001 | 7 (3–12) | 5 (2–8) | 0.005 |
| CRP (mg/liter), median (IQR) | 12 (3–32) | 11 (4–24) | 0.40 | 11 (3–32) | 8 (3–18) | 0.12 |
| RF positive, no. (%) | 154 (34) | 431 (88) | < 0.001 | 58 (45) | 129 (85) | < 0.001 |
| Symptom duration, median (IQR) days | 103 (58–194) | 144 (72–294) | < 0.001 | 94 (46–210) | 121 (68–280) | 0.035 |
P values for age obtained with t‐test, chi‐square test for dichotomous variables, and Mann‐Whitney U test for the other continuous variables. RA = rheumatoid arthritis; ACPA = anti–citrullinated peptide antibody; TJC = tender joint count (68 joints assessed); IQR = interquartile range; SJC = swollen joint count (66 joints assessed); CRP = C‐reactive protein; RF = rheumatoid factor.
TJC was missing in 2 patients, SJC in 1 patient, CRP level in 9 patients, RF in 1 patient, and symptom duration in 12 patients.
TJC was missing in 1 patient, CRP level in 6 patients, RF in 3 patients, and symptom duration in 27 patients.
Symptom duration was missing in 1 patient, and SJC in 1 patient.
RF was missing in 1 patient, CRP level in 1 patient, and symptom duration in 1 patient.
Figure 1.
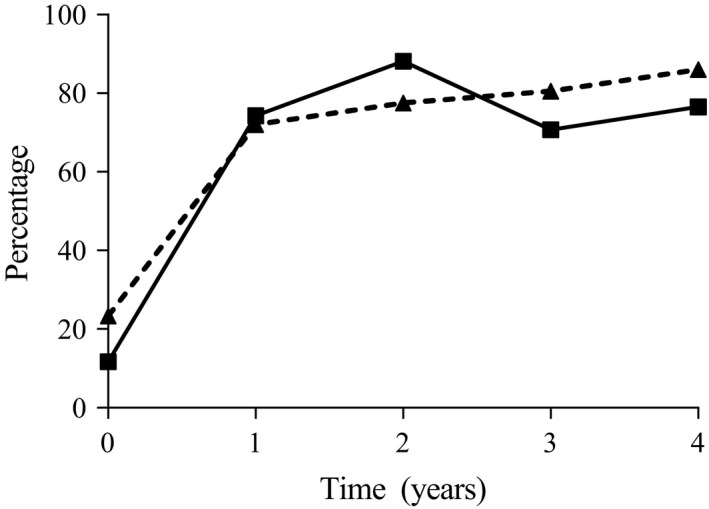
The percentages of anti–citrullinated protein antibody (ACPA)–negative (solid line, squares) and ACPA‐positive (broken line, triangles) rheumatoid arthritis patients (fulfilling 2010 criteria) achieving Disease Activity Score remission (<2.4) during 4 years of followup.
Patient‐reported impairments and limitations at baseline
ACPA‐negative patients reported statistically significant more pain than ACPA‐positive patients (median 5.8 versus 5.2; P = 0.045), more severe fatigue (median 5.5 versus 5.0; P = 0.003), more severe disease activity (median 6.1 versus 5.6; P = 0.006), and more functional disability (median 1.0 versus 0.9; P = 0.001), although absolute differences were small (Figure 2A). General well‐being was equal for both groups of patients (median 4.3 versus 4.0; P = 0.25).
Figure 2.
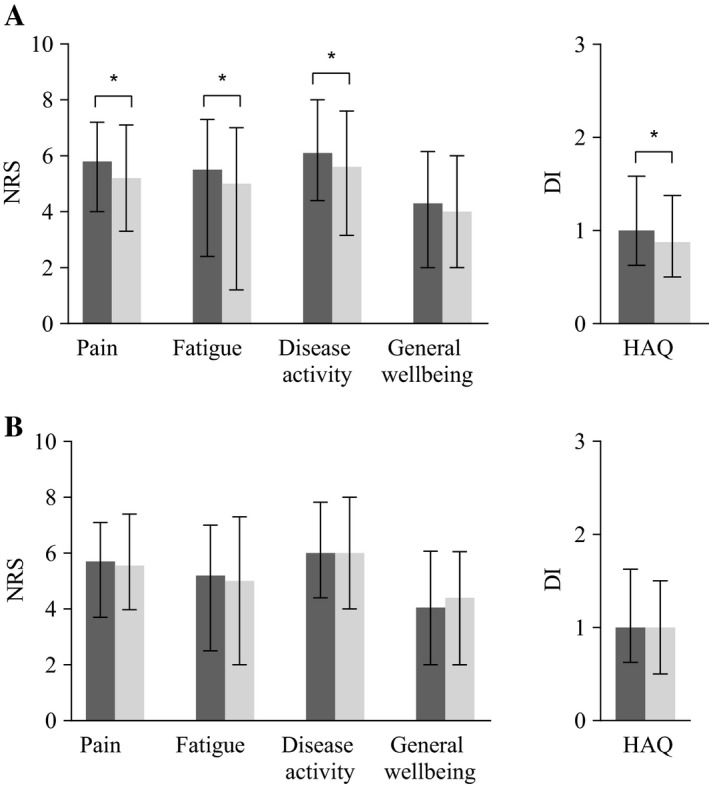
Patient‐reported outcomes of impairments and limitations of anti–citrullinated protein antibody (ACPA)–negative (black bars) and ACPA‐positive (gray bars) patients fulfilling the 2010 criteria (A) and the 1987 criteria (B) at baseline. Median values and corresponding interquartile ranges are shown for severity of self‐reported pain, fatigue, disease activity, and general well‐being, measured by numerical rating scale (NRS), ranging 0–10, and physical function by the Health Assessment Questionnaire (HAQ) disability index (DI), ranging 0–3, in the last 24 hours. * = P < 0.05 by Mann‐Whitney U test.
As, due to the composition of the 2010 criteria, ACPA‐negative patients can only fulfill the criteria in the case of >10 involved joints, and as ACPA‐negative patients indeed had more swollen joints, we hypothesized that the patient selection by the criteria used might explain the higher PROs in ACPA‐negative patients. Therefore analyses were repeated in the RA patients fulfilling the 1987 criteria. Baseline characteristics are shown in Supplementary Table 4, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.23497/abstract. Here, no significant differences were observed between ACPA‐negative and ACPA‐positive RA patients (Figure 2B).
Course of patient‐reported impairments and limitations during 4 years of disease
The 450 ACPA‐negative and 492 ACPA‐positive RA patients (fulfilling 2010 criteria) were studied during 4 years of followup, as shown in Figure 3, which shows the predicted values adjusted for age, sex, and year of inclusion. For all measured variables, the largest improvement was seen during the first year. Both patient groups had equal amounts of pain over time. ACPA‐negative patients remained more severely fatigued over time (P = 0.002, β = 0.53; this β indicates that on an NRS, ranging 0–10, ACPA‐negative patients were 0.5 more severely fatigued). The self‐reported disease activity and the HAQ were equal between both groups. We corrected for age in all analyses, and this had a significant effect in only the longitudinal analysis of the HAQ (β = 0.008, P < 0.001, on a scale ranging 0–3).
Figure 3.
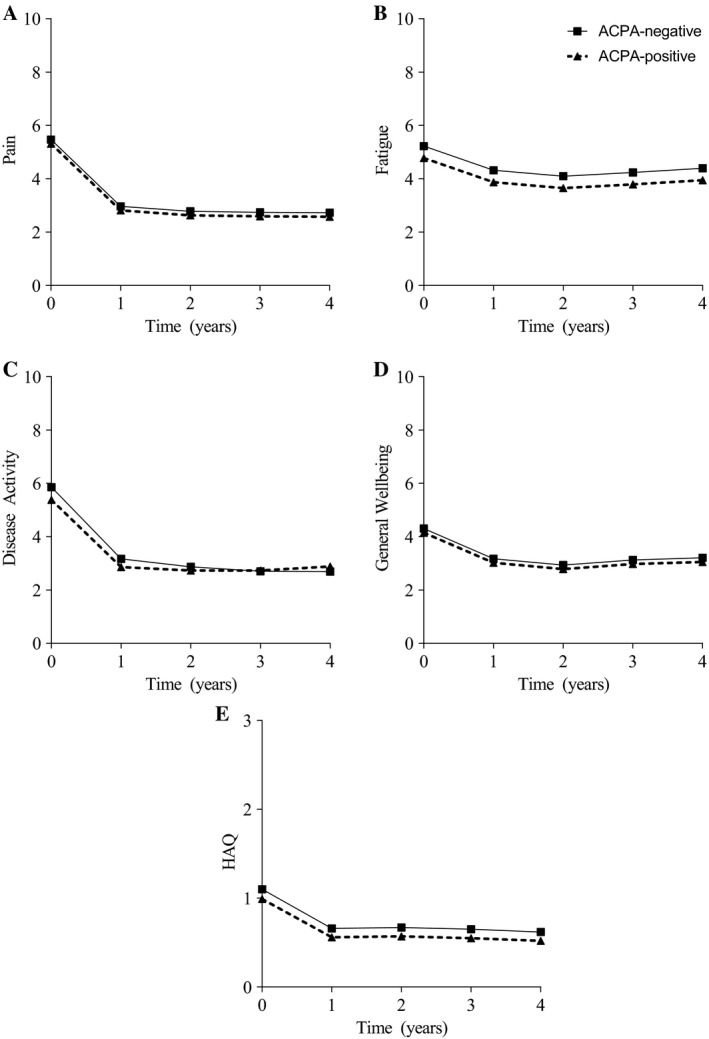
Patient‐reported outcomes of impairments and limitations of anti–citrullinated protein antibody (ACPA)–negative and ACPA‐positive rheumatoid arthritis (RA) patients fulfilling the 2010 criteria during 4 years of followup. Presented are median predicted values obtained by linear mixed models adjusted for age at inclusion, sex, and year of inclusion. In case of significance of the interaction between ACPA and time, this was added to the modeled figures. Pain, fatigue, disease activity, and general well‐being were measured by a numerical rating scale (range 0–10). Physical function was measured by the Health Assessment Questionnaire (HAQ) disability index (range 0–3). Both groups experienced equal pain; ACPA‐negative patients were more severely fatigued (P = 0.002, β = 0.53) than ACPA‐positive patients. Both groups had equal self‐reported disease activity, general well‐being, and HAQ. Following are the numbers of available data per followup year for ACPA‐positive patients for A, pain: 457, 316, 224, 240, 209; B, fatigue: 447, 276, 187, 202, 176; C, disease activity: 457, 315, 224, 239, 209; D, general well‐being: 449, 316, 223, 242, 210; and E, HAQ: 430, 268, 140, 221, 197. Following are the numbers of available data per followup year for ACPA‐negative patients for pain: 404, 257, 177, 165, 138; fatigue: 400, 232, 150, 143, 114; disease activity: 403, 257, 177, 166, 138; general well‐being: 401, 257, 175, 166, 139; and HAQ: 382, 224, 105, 147, 120.
When RA patients fulfilling the 1987 criteria were studied over time, no statistically significant differences were found for all variables (see Supplementary Figure 2, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.23497/abstract). Repeating the analyses in RA patients (both if fulfilling either the 2010 or the 1987 criteria), with additional correction for RF positivity, SJC66, and symptom duration, resulted in no significant differences between ACPA‐negative and ACPA‐positive patients.
Patient‐reported restrictions with work and household activities at baseline
Baseline characteristics of the 130 ACPA‐negative and 152 ACPA‐positive patients who completed questionnaires on work restrictions are presented in Table 1. Absenteeism and presenteeism among patients with paid work were not different at disease presentation, as shown in Table 2. Thirty‐eight percent of ACPA‐negative versus 48% of ACPA‐positive employed patients reported absenteeism in the last 3 months (P = 0.30), with median 4 days missed at work for both groups. Presenteeism due to arthritis was equal, median 3 versus 5, and the days worked with restrictions due to arthritis was median 4 versus 3 days for ACPA‐negative and ACPA‐positive patients, respectively. Also, restrictions due to arthritis at home were similar. The median level of restriction was 6 versus 7 in ACPA‐negative and ACPA‐positive patients, and median days restrictions due to arthritis was 7 versus 6, respectively. Statistically, differences were nonsignificant for all analyses.
Table 2.
Baseline data of ACPA‐negative and ACPA‐positive RA patients (fulfilling 2010 criteria) for restrictions with work and household activitiesa
| ACPA‐negative (n = 130) | ACPA‐positive (n = 152) | P | |
|---|---|---|---|
| Productivity at home | |||
| Level of restrictions in household work productivity past 7 days, median (IQR) | 6 (2–8) | 7 (3–8) | 0.84 |
| Days restricted in household productivity past 7 days, median (IQR) | 7 (3–7) | 6 (2–7) | 0.25 |
| Employed, no. (%) | 40 (31) | 69 (45) | 0.001 |
| Age, mean ± SD years | 47 ± 13 | 49 ± 11 | 0.31 |
| Type of work | 0.48 | ||
| Physical, no. (%) | 7 (18) | 16 (23) | |
| Physical and mental no. (%) | 19 (48) | 25 (36) | |
| Mental, no. (%) | 13 (33) | 27 (39) | |
| Productivity in the work placeb | |||
| Work hours per week, median (IQR) | 32 (20–38) | 28 (20–40) | 0.80 |
| Work days per week, median (IQR) | 5 (4–5) | 5 (3–5) | 0.31 |
| Missed any work in last 3 months, no. (%) | 15 (38) | 33 (48) | 0.30 |
| Days missed at work in last 3 months (absenteeism), median (IQR) | 4 (2–21) | 4 (2–12) | 0.92 |
| Level of restrictions in work productivity (presenteeism) past 7 days, median (IQR)c | 5 (2–8) | 3 (2–8) | 0.41 |
| Days restricted while at work past 7 days, median (IQR) | 4 (2–7) | 3 (0–5) | 0.20 |
ACPA = anti–citrullinated peptide antibody; RA = rheumatoid arthritis; IQR = interquartile range.
Analyzed in employed patients only.
On a 0–10 scale, where 0 means no restrictions and 10 means complete restrictions.
When the RA patients fulfilling the 1987 criteria were studied, no statistically significant differences were observed for all analyses (Supplementary Table 5, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.23497/abstract).
Course of patient‐reported restrictions with work and household activities during 4 years of disease
Presenteeism was assessed during 4 years of followup and was equal between both groups (P = 0.89). ACPA‐negative patients fulfilling the 2010 criteria had more days with restrictions at work (P = 0.02, β = 0.89; this β indicates that ACPA‐negative patients had 0.89 days more restrictions) than ACPA‐positive patients. Both restrictions at home (P = 0.17) and the days restrictions at home (P = 0.64) were equal, as illustrated by Figure 4. Evaluating the RA patients fulfilling the 1987 criteria over time revealed no significant differences in all these analyses (Supplementary Figure 3, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.23497/abstract).
Figure 4.
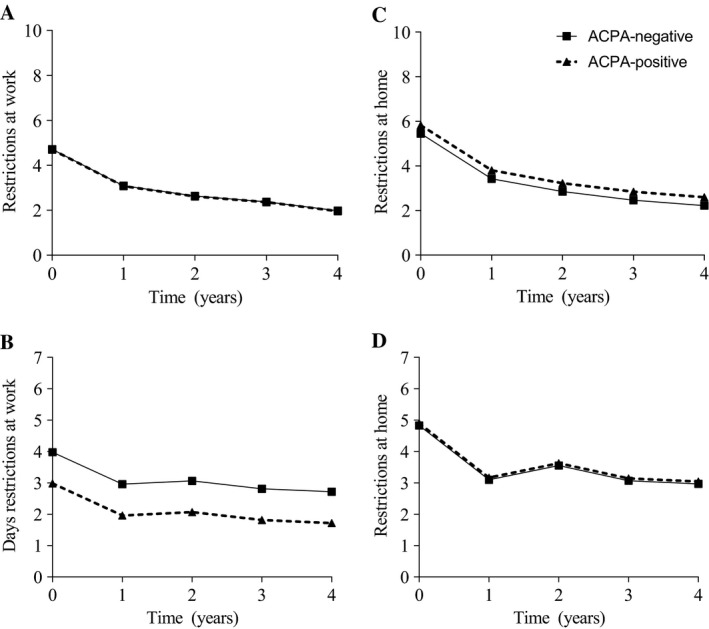
Restrictions with work and household activities in anti–citrullinated protein antibody (ACPA)–negative and ACPA‐positive rheumatoid arthritis (RA) patients fulfilling the 2010 criteria. Presented are median predicted values obtained by linear mixed models adjusted for age at inclusion and sex of ACPA‐negative and ACPA‐positive RA patients according to the 2010 criteria. A, Presenteeism (level of restrictions at work), B, number of days restrictions at work, C, level of restrictions, and D, days restrictions with household activities (at home) over 4 years of followup. Level of restrictions was measured on a scale of 0–10. Days restrictions due to arthritis ranged from 0–7 days. Presenteeism was equal (P = 0.89). ACPA‐negative patients had a significantly higher number of days restrictions at work due to arthritis (P = 0.02, β = 0.89). Level of restrictions (P = 0.17) and number of days they had restrictions at home (P = 0.64) were equal. Following are the numbers of available data per followup year for ACPA‐positive patients for level of restrictions at work: 63, 52, 44, 31, 19; days restrictions at work: 53, 50, 36, 27, 17; level of restrictions at home: 103, 83, 75, 59, 37; and days restrictions at home: 89, 69, 63, 51, 28. Following are the numbers of available data per followup year for ACPA‐negative patients for level of restrictions at work: 38, 20, 12, 9, 5; days restrictions at work: 36, 17, 10, 9, 4; level of restrictions at home: 82, 58, 39, 24, 12; and days restrictions at home: 71, 44, 26, 16, 8.
Results of sensitivity analyses
Because 33% of the ACPA‐negative RA patients fulfilling the 1987 criteria were RF positive, patients without ACPA or RF (n = 296) were compared to patients with ACPA and/or RF (n = 646). At baseline, patients without ACPA and/or RF had more self‐reported pain (P = 0.003), were more severely fatigued (P = 0.045), had a more severe disease activity (P < 0.001), and more severe functional disability (P = 0.001). General well‐being was equal (P = 0.17). Thus, these findings were similar to the results of the main analyses. Over 4 years of followup, patients without ACPA and/or RF had more severe pain (P = 0.007, β = 0.37), were more severely fatigued (P = 0.001, β = 0.60), had more severe disease activity (P = 0.001, β = 0.44), and more severe general well‐being (P = 0.026, β = 0.29). The HAQ over time was not statistically different between both groups (P = 0.08).
RA patients (fulfilling 2010 criteria) without ACPA or RF (n = 72) and patients with ACPA and/or RF (n = 210) were evaluated for restrictions at work and at home. At baseline, patients with and without ACPA and/or RF had equal absenteeism (P = 0.21), presenteeism (P = 0.75), number of days restrictions at work (P = 0.31), level of restrictions at home (P = 0.91), and number of days restrictions at home (P = 0.97). Over 4 years of followup, both groups had equal presenteeism (P = 0.78). ACPA‐ and RF‐negative patients had more days restrictions at work due to arthritis (P = 0.043, β = 1.2). The level of restrictions at home (P = 0.77) and days restrictions at home (P = 0.50) were equal between the groups.
Because DAS‐steered treatment became regular as of 2005, analyses were repeated for RA patients fulfilling the 2010 criteria included during or after 2005. This showed similar results as that of the total group. At baseline, ACPA‐negative patients reported more severe pain than ACPA‐positive patients (P = 0.016, β = 0.20), more severe fatigue (P = 0.003, β = 0.45), more severe disease activity (P < 0.001, β = 0.39), more severe general well‐being (P = 0.029, β = 0.14), and more functional disability (P = 0.001, β = 0.08, on a scale ranging 0–3). Also, followup data showed similar results as that of the total group, as shown in Supplementary Figure 4, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.23497/abstract.
Finally, to compare the main findings with those obtained on RA patients that were treated in earlier time periods and thus with different treatment strategies, the analyses of patient‐reported impairments and limitations in ACPA‐positive and ACPA‐negative patients were also performed on RA patients included in the EAC between 1993 and 1999 (n = 335). As shown in Supplementary Figure 5 (available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.23497/abstract), several PROs were more severe in ACPA‐positive RA patients during 4 years of followup; statistical significance was reached for general well‐being (P = 0.020, β = 0.10) and a tendency toward significance for patient‐reported disease activity (P = 0.06, β = 0.05).
Discussion
This large longitudinal study assessed whether, at present, ACPA‐positive RA patients are still more severely affected than ACPA‐negative RA patients, using self‐reported impairments and limitations, including functional disability and restrictions at work as outcomes. The current availability of treatment strategies to suppress inflammation drastically reduced the frequency and degree of joint damage, causing the prospects of RA patients to change substantially 10. Consequently, other disease outcomes have become central, and patients have rated pain, fatigue, well‐being, and independence, factors which have been studied here, as most important 14. In addition, physical functioning and work ability, the key components of independence in RA, are important from a socioeconomic perspective. We did not observe a more severe disease patient burden in ACPA‐positive RA. Obviously, the present data require validation in an independent cohort. Nonetheless, the assumption that ACPA‐positive RA is a more severe disease seems no longer true in current rheumatology practice when the mentioned PROs are considered.
Contrary to the hypothesis tested, we actually observed some differences to the detriment of ACPA‐negative patients. However, these differences were small and clinically irrelevant. As ACPA‐negative patients were older, which was also shown in previously performed studies, we corrected for age in all longitudinal analyses 26. Also, when analyses were additionally adjusted for comorbidities, similar results were obtained (data not shown). We hypothesized that the small differences found were most likely caused by the fact that RA patients without ACPA or RF need >10 joints involved to fulfill the criteria, and ACPA‐negative patients with positive RF required 4–10 joints involved, as reflected by the patient characteristics, which showed that ACPA‐negative RA patients (according to the 2010 criteria) had a higher TJC and SJC than ACPA‐positive patients. This effect has been observed before 27, 28. For this reason we repeated the analyses in patients classified according to the 1987 criteria, with a more similar joint count between ACPA‐negative and ACPA‐positive patients. Then, we did not observe the somewhat higher disease burden for ACPA‐negative RA, when treated with current treatment strategies and considering patient‐reported impairments and limitations. Thus, although this study was not set up to compare the 2010 and 1987 criteria, the presented data do confirm previously reported observations that ACPA‐positive RA by the 2010 criteria consists of a less severe subset of patients than ACPA‐positive RA by the 1987 criteria, and that ACPA‐negative RA by the 2010 criteria consists of a more severe subset of patients compared to ACPA‐negative RA by the 1987 criteria 27, 28.
In current research, there is a tendency to concentrate more on ACPA‐positive than on ACPA‐negative RA. For example, many more whole genome genetic studies were performed on ACPA‐positive RA 29. This focus in etiopathologic studies is possibly explained by the paradigm that ACPA‐negative RA might represent a more heterogeneous subset of patients, and that current research has revealed fewer clues on the possible causes or mediators of ACPA‐negative RA. This could have resulted in ACPA having conquered a more prominent position in the identification of RA within the 2010 criteria. This study, however, does not intend to address the issue on the classification criteria. The data presented clearly demonstrate that, at present, ACPA‐negative RA is equally severe as ACPA‐positive RA when patient‐reported impairments and limitations are studied as outcomes. This has implications for future research, both for etiopathophysiologic and clinical studies. The present data highlight the importance of keeping the scope set on ACPA‐negative RA as well, because it has become an equally severe disease. Moreover, the prevalence of ACPA‐negative RA, as measured in early arthritis cohorts, concerns up to half of the total RA‐population 26, 30.
The risk of misdiagnosis is often estimated higher for ACPA‐negative RA than for ACPA‐positive RA. In this study, patients were diagnosed with RA according to the treating rheumatologist, and this clinical diagnosis was verified after 1 year of disease in the medical files of all patients. Hence, patients who developed other diseases were no longer in the data set. Thereafter, patients were checked as to whether they fulfilled the 2010 criteria (or 1987 criteria for subanalyses). When all these conditions were met, patients were included. Because of this stringent selection, we think that the risk of misdiagnosis of ACPA‐negative RA is low.
Secondary comorbidities like fibromyalgia (FM) could also influence the PROs, like pain and fatigue 31. Data on secondary FM were not collected in our cohort. However, we have no reason to believe that secondary FM would have influenced our comparisons between autoantibody‐positive and autoantibody‐negative patients, as previous studies have demonstrated that RA patients with and without concomitant FM have an equal prevalence of RF positivity 31, 32, 33, 34.
Measuring patients’ perceptions of health is a standard approach in observational studies and epidemiologic research. Measurements of PROs have proven to be valid and responsive and are sensitive to detect differences between patient groups 28, 35, 36, 37, 38. This is the first study to extensively compare several PROs and work ability among ACPA‐positive and ACPA‐negative RA patients during 4 years of followup. Other studies have included the HAQ or DAS in their analyses and sometimes other PROs 28, 36, 37, 38, 39. However, these measurements were mainly performed at baseline and were not conducted to find differences between ACPA‐positive and ‐negative patients over time. Further, it was shown that patients with RA in countries with higher welfare score worse on PROs, despite lower levels of objectively measured disease outcomes 8, 25, 40. However, this study was conducted in only 1 country.
PROs may be influenced by secular trends 23, 24, 25. Patients studied were included between 2000 and 2014. To prevent confounding effects, we corrected the analyses for the year of inclusion. There is no reason to believe that personal contextual factors such as education or self‐efficacy are different between ACPA‐positive and ACPA‐negative patients.
A limitation of PROs could be that reproducibility is sometimes not very satisfactory 41, and the difficulty of any study using self‐reported outcomes can be that they may be susceptible to nonresponse and recall bias. We do not expect this to cause a difference for the comparison made. Also, we are aware that absenteeism is calculated with a recall period of 3 months, and some patients present with symptom durations shorter than this. However, if this had any effect, it would have led only to an underestimation of absenteeism in ACPA‐negative patients, as they more often had a shorter symptom duration at presentation.
Our frequencies of employment, absenteeism, and presenteeism are in accordance with previous studies in RA that show the considerable interference experienced by RA patients by means of work restrictions, even despite improved treatment strategies 42, 43, 44. Data of the Dutch reference population were obtained from the Dutch Centraal Bureau voor Statistiek 45. Here, 45% of the people ages 45–55 years (this was the most prevalent age category in the patient cohort) had missed days at work due to sickness during the 12 months of 2016. The average sick leave was 8 days per year. In comparison, 38–48% of the employed ACPA‐negative and ACPA‐positive RA patients missed days at work during a 3‐month period. In both ACPA groups the median days missed at work were 4 per 3 months; extrapolation to a 12‐month period would result in an estimated sick leave of 16 days per year. This is evidently more than the sick leave in the reference population, and these data confirm that RA patients currently still have increased work restrictions. Furthermore, this study adds that no differences were found between ACPA‐positive and ACPA‐negative patients. Notably, the number of working patients was relatively small in our data set, but findings that the results on restrictions at work were similar to those of the patient‐reported impairments and limitations show face validity.
This study was conducted to evaluate patients who were diagnosed early and were treated using up‐to‐date treatment strategies consisting of early initiation of MTX and DAS‐steered treatment adjustments. We cannot compare the actual DMARDs used over time in both groups, as these data were not collected in a sufficiently accurate manner. According to local guidelines, initial treatment of ACPA‐positive and ACPA‐negative RA was similar: the treatment regimen consisted of initial treatment with a DMARD (preferably MTX); in case of failure a second conventional DMARD was started, and in case of subsequent failure a biologic DMARD was allowed. From 2005 onward, in our hospital, DAS‐steered treatment became standard 18, meaning that treatment regimens were adjusted based on the individual's disease activity. Analysis of biologic DMARDs used after 2 years of followup revealed that these were used by 9% of ACPA‐positive patients and 1% of ACPA‐negative patients. Furthermore, the disease activity measured during followup was similar in both groups. Hence, it is possible that the ACPA‐positive patients required more, or more aggressive, DMARDs to achieve a similar DAS. Our results could therefore be considered as the consequence of improved treatment strategies. In line with this idea, we evaluated whether PROs were different between ACPA‐positive and ACPA‐negative patients who were treated in earlier periods with treatment strategies that are now considered outdated. Although the number of patients in this group was smaller, ACPA‐positive patients indeed had some PROs that were worse than those of ACPA‐negative patients. Results of the present study therefore imply that, thanks to improved treatment strategies, not only have differences between ACPA‐positive and ACPA‐negative RA in outcomes, such as joint damage severity, diminished or disappeared, but that the same can be seen for differences in PROs.
In conclusion, this study thoroughly compared various PROs and restrictions with work during followup in ACPA‐positive and ACPA‐negative RA patients. The study demonstrated that ACPA‐positive and ACPA‐negative RA managed with current treatment strategies represent an equally severe subset of disease. We do not know if rheumatologists take PROs into account when making treatment decisions. However, as joint damage becomes less relevant as an outcome, in the future we should explore whether PROs can be considered. Further research is required, but the important personal health impact, as well as the socioeconomic burden highlighted by the present study, implies that efforts to further improve the disease course should be proportional to ACPA‐positive and ACPA‐negative RA.
Author contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Boer had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Boer, van der Helm van Mil.
Acquisition of data
Boer, van der Helm van Mil.
Analysis and interpretation of data
Boer, Boonen, van der Helm van Mil.
Supporting information
Supported by a Vidi‐grant of the Netherlands Organization for Health Research and Development, by the European Research Council under the European Union's Horizon 2020 Research and Innovation Programme (grant 714312), and by the Dutch Arthritis Foundation.
References
- 1. Ajeganova S, Humphreys JH, Verheul MK, van Steenbergen HW, van Nies JA, Hafstrom I, et al. Anticitrullinated protein antibodies and rheumatoid factor are associated with increased mortality but with different causes of death in patients with rheumatoid arthritis: a longitudinal study in three European cohorts. Ann Rheum Dis 2016;75:1924–32. [DOI] [PubMed] [Google Scholar]
- 2. Akdemir G, Verheul MK, Heimans L, Wevers de Boer KV, Goekoop‐Ruiterman YP, van Oosterhout M, et al. Predictive factors of radiological progression after 2 years of remission‐steered treatment in early arthritis patients: a post hoc analysis of the IMPROVED study. RMD Open 2016;2:e000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Farragher TM, Goodson NJ, Naseem H, Silman AJ, Thomson W, Symmons D, et al. Association of the HLA–DRB1 gene with premature death, particularly from cardiovascular disease, in patients with rheumatoid arthritis and inflammatory polyarthritis. Arthritis Rheum 2008;58:359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dadoun S, Zeboulon‐Ktorza N, Combescure C, Elhai M, Rozenberg S, Gossec L, et al. Mortality in rheumatoid arthritis over the last fifty years: systematic review and meta‐analysis. Joint Bone Spine 2013;80:29–33. [DOI] [PubMed] [Google Scholar]
- 5. Ajeganova S, van Steenbergen HW, van Nies JA, Burgers LE, Huizinga TW, van der Helm‐van Mil AH. Disease‐modifying antirheumatic drug‐free sustained remission in rheumatoid arthritis: an increasingly achievable outcome with subsidence of disease symptoms. Ann Rheum Dis 2016;75:867–73. [DOI] [PubMed] [Google Scholar]
- 6. Van Steenbergen HW, Tsonaka R, Huizinga TW, Boonen A, van der Helm‐van Mil AH. Fatigue in rheumatoid arthritis; a persistent problem: a large longitudinal study. RMD Open 2015;1:e000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Minichiello EL, Boissier MC. Time trends in the incidence, prevalence, and severity of rheumatoid arthritis: a systematic literature review. Joint Bone Spine 2016;83:625–30. [DOI] [PubMed] [Google Scholar]
- 8. Pincus T, Sokka T, Kautiainen H. Patients seen for standard rheumatoid arthritis care have significantly better articular, radiographic, laboratory, and functional status in 2000 than in 1985. Arthritis Rheum 2005;52:1009–19. [DOI] [PubMed] [Google Scholar]
- 9. Sokka T, Kautiainen H, Hakkinen A, Hannonen P. Radiographic progression is getting milder in patients with early rheumatoid arthritis: results of 3 cohorts over 5 years. J Rheumatol 2004;31:1073–82. [PubMed] [Google Scholar]
- 10. Heimans L, Wevers‐deBoer KV, Ronday HK, Collee G, de Sonnaville PB, Grillet BA, et al. Can we prevent rapid radiological progression in patients with early rheumatoid arthritis? Clin Rheumatol 2015;34:163–6. [DOI] [PubMed] [Google Scholar]
- 11. Van Nies JA, van der Helm‐van Mil AH. Is early remission associated with improved survival or is arthritis persistency associated with increased mortality in early arthritis? Comparisons with the general population. Ann Rheum Dis 2013;72:e25. [DOI] [PubMed] [Google Scholar]
- 12. Lacaille D, Avina‐Zubieta JA, Sayre EC, Abrahamowicz M. Improvement in 5‐year mortality in incident rheumatoid arthritis compared with the general population‐closing the mortality gap. Ann Rheum Dis 2017;76:1057–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Myasoedova E, Gabriel S, Matteson EL, Davis JM III, Therneau TM, Crowson CS. Decreased cardiovascular mortality in patients with incident rheumatoid arthritis (RA) in recent years: dawn of a new era in cardiovascular disease in RA? J Rheumatol 2017;44:732–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Tuyl LH, Sadlonova M, Hewlett S, Davis B, Flurey C, Goel N, et al. The patient perspective on absence of disease activity in rheumatoid arthritis: a survey to identify key domains of patient‐perceived remission. Ann Rheum Dis 2017;76:855–61. [DOI] [PubMed] [Google Scholar]
- 15. Lenssinck ML, Burdorf A, Boonen A, Gignac MA, Hazes JM, Luime JJ. Consequences of inflammatory arthritis for workplace productivity loss and sick leave: a systematic review. Ann Rheum Dis 2013;72:493–505. [DOI] [PubMed] [Google Scholar]
- 16. De Rooy DP, van der Linden MP, Knevel R, Huizinga TW, van der Helm‐van Mil AH. Predicting arthritis outcomes: what can be learned from the Leiden Early Arthritis Clinic? Rheumatology (Oxford) 2011;50:93–100. [DOI] [PubMed] [Google Scholar]
- 17. Combe B, Landewe R, Lukas C, Bolosiu HD, Breedveld F, Dougados M, et al. EULAR recommendations for the management of early arthritis: report of a task force of the European Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2007;66:34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goekoop‐Ruiterman YP, de Vries‐Bouwstra JK, Allaart CF, van Zeben D, Kerstens PJ, Hazes JM, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum 2005;52:3381–90. [DOI] [PubMed] [Google Scholar]
- 19. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO III, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 20. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 21. Knevel R, Tsonaka R, le Cessie S, van der Linden MP, Huizinga TW, van der Heijde DM, et al. Comparison of methodologies for analysing the progression of joint destruction in rheumatoid arthritis. Scand J Rheumatol 2013;42:182–9. [DOI] [PubMed] [Google Scholar]
- 22. Knevel R, de Rooy DP, Zhernakova A, Grondal G, Krabben A, Steinsson K, et al. Association of variants in IL2RA with progression of joint destruction in rheumatoid arthritis. Arthritis Rheum 2013;65:1684–93. [DOI] [PubMed] [Google Scholar]
- 23. Sokka T, Makinen H, Hannonen P, Pincus T. Most people over age 50 in the general population do not meet ACR remission criteria or OMERACT minimal disease activity criteria for rheumatoid arthritis. Rheumatology (Oxford) 2007;46:1020–3. [DOI] [PubMed] [Google Scholar]
- 24. Sokka T, Toloza S, Cutolo M, Kautiainen H, Makinen H, Gogus F, et al. Women, men, and rheumatoid arthritis: analyses of disease activity, disease characteristics, and treatments in the QUEST‐RA study. Arthritis Res Ther 2009;11:R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nieuwenhuis WP, de Wit MP, Boonen A, van der Helm‐van Mil AH. Changes in the clinical presentation of patients with rheumatoid arthritis from the early 1990s to the year 2010: earlier identification but more severe patient reported outcomes. Ann Rheum Dis 2016;75:2054–6. [DOI] [PubMed] [Google Scholar]
- 26. Boeters DM, Mangnus L, Ajeganova S, Lindqvist E, Svensson B, Toes RE, et al. The prevalence of ACPA is lower in rheumatoid arthritis patients with an older age of onset but the composition of the ACPA response appears identical. Arthritis Res Ther 2017;19:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Van der Helm‐van Mil AH, Zink A. What is rheumatoid arthritis? Considering consequences of changed classification criteria. Ann Rheum Dis 2017;76:315–31. [DOI] [PubMed] [Google Scholar]
- 28. Nordberg LB, Lillegraven S, Lie E, Aga AB, Olsen IC, Hammer HB, et al. Patients with seronegative RA have more inflammatory activity compared with patients with seropositive RA in an inception cohort of DMARD‐naive patients classified according to the 2010 ACR/EULAR criteria. Ann Rheum Dis 2017;76:341–5. [DOI] [PubMed] [Google Scholar]
- 29. Viatte S, Plant D, Raychaudhuri S. Genetics and epigenetics of rheumatoid arthritis. Nat Rev Rheumatol 2013;9:141–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lukas C, Combe B, Ravaud P, Sibilia J, Landew R, van der Heijde D. Favorable effect of very early disease‐modifying antirheumatic drug treatment on radiographic progression in early inflammatory arthritis: data from the Etude et Suivi des Polyarthrites Indifferenciees Recentes (study and followup of early undifferentiated polyarthritis). Arthritis Rheum 2011;63:1804–11. [DOI] [PubMed] [Google Scholar]
- 31. Ranzolin A, Brenol JC, Bredemeier M, Guarienti J, Rizzatti M, Feldman D, et al. Association of concomitant fibromyalgia with worse disease activity score in 28 joints, health assessment questionnaire, and short form 36 scores in patients with rheumatoid arthritis. Arthritis Rheum 2009;61:794–800. [DOI] [PubMed] [Google Scholar]
- 32. Chakr R, Brenol C, Ranzolin A, Bernardes A, Dalosto AP, Ferrari G, et al. Rheumatoid arthritis seems to have DMARD treatment decision influenced by fibromyalgia. Rev Bras Reumatol Engl Ed 2017;57:403–11. [DOI] [PubMed] [Google Scholar]
- 33. Naranjo A, Ojeda S, Francisco F, Erausquin C, Rua‐Figueroa I, Rodriguez‐Lozano C. Fibromyalgia in patients with rheumatoid arthritis is associated with higher scores of disability [letter]. Ann Rheum Dis 2002;61:660–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wolfe F, Michaud K. Severe rheumatoid arthritis (RA), worse outcomes, comorbid illness, and sociodemographic disadvantage characterize RA patients with fibromyalgia. J Rheumatol 2004;31:695–700. [PubMed] [Google Scholar]
- 35. Strand V, Cohen S, Crawford B, Smolen JS, Scott DL, and Leflunomide Investigators Groups . Patient‐reported outcomes better discriminate active treatment from placebo in randomized controlled trials in rheumatoid arthritis. Rheumatology (Oxford) 2004;43:640–7. [DOI] [PubMed] [Google Scholar]
- 36. Barra L, Pope JE, Orav JE, Boire G, Haraoui B, Hitchon C, et al. Prognosis of seronegative patients in a large prospective cohort of patients with early inflammatory arthritis. J Rheumatol 2014;41:2361–9. [DOI] [PubMed] [Google Scholar]
- 37. Van den Broek M, Dirven L, Klarenbeek NB, Molenaar TH, Han KH, Kerstens PJ, et al. The association of treatment response and joint damage with ACPA‐status in recent‐onset RA: a subanalysis of the 8‐year follow‐up of the BeSt study. Ann Rheum Dis 2012;71:245–8. [DOI] [PubMed] [Google Scholar]
- 38. Aletaha D, Alasti F, Smolen JS. Rheumatoid factor, not antibodies against citrullinated proteins, is associated with baseline disease activity in rheumatoid arthritis clinical trials. Arthritis Res Ther 2015;17:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Andersson ML, Forslind K, Hafstrom I, and the BS Group . Patients with early rheumatoid arthritis in the 2000s have equal disability and pain despite less disease activity compared with the 1990s: data from the BARFOT Study over 8 Years. J Rheumatol 2017;44:723–31. [DOI] [PubMed] [Google Scholar]
- 40. Putrik P, Ramiro S, Keszei AP, Hmamouchi I, Dougados M, Uhlig T, et al. Lower education and living in countries with lower wealth are associated with higher disease activity in rheumatoid arthritis: results from the multinational COMORA study. Ann Rheum Dis 2016;75:540–6. [DOI] [PubMed] [Google Scholar]
- 41. Kvien TK, Heibert T. Patient perspective in outcome assessments: perceptions or something more? J Rheumatol 2003;30:873–6. [PubMed] [Google Scholar]
- 42. Zirkzee EJ, Sneep AC, de Buck PD, Allaart CF, Peeters AJ, Ronday HK, et al. Sick leave and work disability in patients with early arthritis. Clin Rheumatol 2008;27:11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smolen JS, Han C, van der Heijde D, Emery P, Bathon JM, Keystone E, et al. Infliximab treatment maintains employability in patients with early rheumatoid arthritis. Arthritis Rheum 2006;54:716–22. [DOI] [PubMed] [Google Scholar]
- 44. Smolen JS, Kremer JM, Gaich CL, DeLozier AM, Schlichting DE, Xie L, et al. Patient‐reported outcomes from a randomised phase III study of baricitinib in patients with rheumatoid arthritis and an inadequate response to biological agents (RA‐BEACON). Ann Rheum Dis 2016;76:694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Centraal Bureau voor de Statistiek . Sickness absence according to employees; sex and age. 2017. URL: http://statline.cbs.nl/StatWeb/publication/?DM=SLNL&PA=83056ned.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


