Summary
Different models to investigate the prognosis of peripheral T cell lymphoma not otherwise specified (PTCL‐NOS) have been developed by means of retrospective analyses. Here we report on a new model designed on data from the prospective T Cell Project. Twelve covariates collected by the T Cell Project were analysed and a new model (T cell score), based on four covariates (serum albumin, performance status, stage and absolute neutrophil count) that maintained their prognostic value in multiple Cox proportional hazards regression analysis was proposed. Among patients registered in the T Cell Project, 311 PTCL‐NOS were retained for study. At a median follow‐up of 46 months, the median overall survival (OS) and progression‐free survival (PFS) was 20 and 10 months, respectively. Three groups were identified at low risk (LR, 48 patients, 15%, score 0), intermediate risk (IR, 189 patients, 61%, score 1–2), and high risk (HiR, 74 patients, 24%, score 3–4), having a 3‐year OS of 76% [95% confidence interval 61–88], 43% [35–51], and 11% [4–21], respectively (P < 0·001). Comparing the performance of the T cell score on OS to that of each of the previously developed models, it emerged that the new score had the best discriminant power. The new T cell score, based on clinical variables, identifies a group with very unfavourable outcomes.
Keywords: PTCL‐NOS, prognostic index, survival, risk factors, clinical variables
Peripheral T cell lymphomas (PTCLs) comprise a heterogeneous group of neoplasms reflecting the diverse cells of origin (post‐thymic lymphoid cells at different stages of differentiation) (Jones et al, 2000; Swerdlow et al, 2008), with different morphological patterns, phenotypes and clinical presentations, and account for 5–10% of all lymphoproliferative disorders in Western countries.
The overall incidence is 0·5–2 per 100 000 persons per year, with a striking epidemiological distribution (Vose et al, 2008; Bellei et al, 2012).
The 2008 World Health Organization (WHO) classification broadly divides PTCLs into four categories, into which subtypes can be further distinguished based on their clinical, immunophenotypical, morphological and biological features, overall characterized by aggressive clinical course and poor response to therapy (Swerdlow et al, 2008).
The most frequent subtype of PTCLs is PTCL, not otherwise specified (PTCL‐NOS) a basket term when features do not conform to known entities within the 2008 WHO classification.
The prognosis of patients with PTCL‐NOS is poor, and optimal therapy remains challenging. With standard anthracycline‐based therapy, the complete response rate ranges from 40% to 60%, with overall survival (OS) of 30–40% (Gallamini et al, 2004; Weisenburger et al, 2011).
The many studies performed to assess the contribution of clinical and biological factors in influencing the prognosis of PTCL‐NOS reported that poor Eastern Cooperative Oncology Group performance status (ECOG‐PS), advanced stage, presence of extranodal sites, bulky disease, high lactate dehydrogenase (LDH) levels and Ki67 rate were significantly correlated with shorter OS (Gallamini et al, 2004; Went et al, 2006; Weisenburger et al, 2011).
The usefulness of the International Prognostic Index (IPI), developed for diffuse large B cell lymphoma (DLBCL), has also been investigated and confirmed for PTCL‐NOS (Gallamini et al, 2004; Weisenburger et al, 2011).
To better define the clinical outcome of PTCL‐NOS, the Intergruppo Italiano Linfomi (now Fondazione Italiana linfomi, FIL) performed a large study on 385 patients diagnosed and treated in the 1990s and defined a prognostic model, the Prognostic Index for PTCL‐unspecified (PIT), in which age (<60 years), ECOG PS 2 or higher, LDH level above upper normal range, and bone‐marrow involvement were independent predictors of OS (Gallamini et al, 2004).
The PIT stratified the patients into four distinct groups with differing risk: low (no adverse factors), intermediate (1 adverse factor) intermediate‐high (2 adverse factors) and high (3–4 adverse factors), with a 5‐year OS of 62·3%, 52·9%, 32·9% and 18·3%, respectively (P < 0·0001).
The PIT was slightly more effective than the IPI in stratifying patients (Gallamini et al, 2004).
An updated version of the PIT (m‐PIT) was proposed, in which bone marrow involvement was replaced by Ki67 rate of expression, resulting in a more robust tool than the PIT (Went et al, 2006).
The most recent efforts to improve the understanding of clinical prognostic factors in PTCL‐NOS were undertaken by the International Peripheral T cell Lymphoma Project (IPTCLP) on a sample of 340 cases diagnosed between 1990 and 2002: both the PIT and the IPI remained highly significant for both OS and progression‐free survival (PFS) (P < 0·001) (Weisenburger et al, 2011). In univariate analysis, the presence of B‐symptoms, bulky disease ≥10 cm, elevated serum C‐reactive protein, a high number of transformed tumour cells, and platelet count less than 150 × 109/l adversely affected both OS and PFS; in multiple Cox proportional hazards (PH) regression analysis controlled for IPI, only bulky disease remained predictive for both OS and PFS, and thrombocytopenia for PFS (Weisenburger et al, 2011).
A common limitation of the latter studies is their retrospective nature.As a result, data have spanned several years, not been collected on consecutive cases, and do not account for changes in the classification systems (Gallamini et al, 2004; Weisenburger et al, 2011).
To evaluate prognosis prospectively, the IPTCLP established the T Cell Project, which collects an exhaustive set of clinical data and biological information. Herein we report on the analysis of prognostic factors performed on a cohort of 506 cases of PTCL‐NOS collected in the prospective T Cell Project.
Methods
Patients and methods
The T Cell Project (NCT01142674) was incepted in September 2006 as a prospective registry of patients with PTCL‐NOS, angioimmunoblastic T cell lymphoma (AITL), anaplastic large cell lymphoma (ALCL) and all of the rarer subtypes of nodal and extranodal aggressive histologies of PTCL.
Data collection was accomplished via electronic case report forms using a dedicated website (http://www.tcellproject.org) with adoption of the proper technology to ensure protection of the data of individual subjects in web communications.
The study was conducted in compliance with the Helsinki Declaration, was approved by the appropriate research Ethics Committees/Institutional Review Boards and required each patient to consent in written prior to registration.
Most of the cases from the T Cell Project and the COMPLETE Registry (NCT01110733) underwent a central review of initial diagnosis, as per protocol.
Statistical methods
To determine the required PTCL‐NOS sample size, we initially assumed that each risk factor had a prevalence of at least 10%, the 5‐year survival rate of the entire study population was 45%, and the hazard ratio (HR) would be two with, as compared to without, the risk factor.
Under these conditions, there would be an 80% power to detect a statistically significant effect of the risk factor on outcome endpoint with a sample size of 460 patients with PTCL‐NOS, allowing an inter‐relationship between risk factors in multivariate analysis.
For the development of the prospective alternative prognostic model we included 12 variables selected from those reported in the literature to impact on survival of PTCL‐NOS, including clinical (ECOG PS, Ann Arbor stage, B‐symptoms, number of extra‐nodal sites), biological [LDH, albumin serum level, platelet count, haemoglobin level, lymphocyte/monocyte ratio (LMR), neutrophil/lymphocyte ratio (NLR)] and demographic (age, sex) factors.
The main endpoint of the study was OS, measured from the date of diagnosis until death from any cause or date of last know contact for living patients (Cheson et al, 1999). The secondary endpoint was PFS, defined as the time from diagnosis to progressive disease or death from any cause. OS and PFS were calculated using Kaplan–Meier estimators, comparison between categories performed by the log‐rank test and Cox PH regression, and the effect of the covariates reported as HR with 95% confidence interval (95 CI).
Continuous biological covariates were dichotomized according to usual clinical thresholds, except for NLR, that was modelled as a continuous covariate in an explorative Cox PH cubic spline analysis (Royston, 2000) adjusted by IPI; the degrees of freedom for NLR was selected on the basis of the minimum Akaike information criterion (AIC; Akaike, 1974).
The final model obtained from Cox PH regression included four covariates that showed negligible difference between log‐likelihood if compared with the full model including all 12 covariates.
The influence of measure for the effect of a single subject on the overall coefficient vector was checked by means of the likelihood displacement.
The proportionality of the hazard risk was graphically checked using the scaled Schoenfeld residuals method (Schoenfeld, 1982). All P values were two sided.
Performance of indices was compared by a measure of global fit (AIC; Akaike, 1974), and by a measure of concordance index (c‐Harrell) (Harrell, 2001), with low values of AIC indicating better fit and high c‐Harrell values indicating better discrimination.
An external validation sample of patients with newly diagnosed PTCL‐NOS registered in the COMPLETE Registry (NCT01110733) was used to further validate the predictive model.
The statistical analyses were performed using Stata version 14·0 or later (StataCorp. LLC, College Station, TX, USA), R version 3.3.0 or later (R Core Team 2016), and the ‘rms’ package for R (Harrell, 2016), version 4.4‐2 or later. P values <0·05 were considered statistically significant.
Results
Patient characteristics and treatment
Between September 2006 and October 2015, 506 cases of potentially assessable PTCL‐NOS were registered, and a total of 311 PTCL‐NOS patients (61%) were retained for developing the prognostic model (Fig 1); the list of the investigated covariates and their characteristics in the study cohort are summarized in Table 1.
Figure 1.
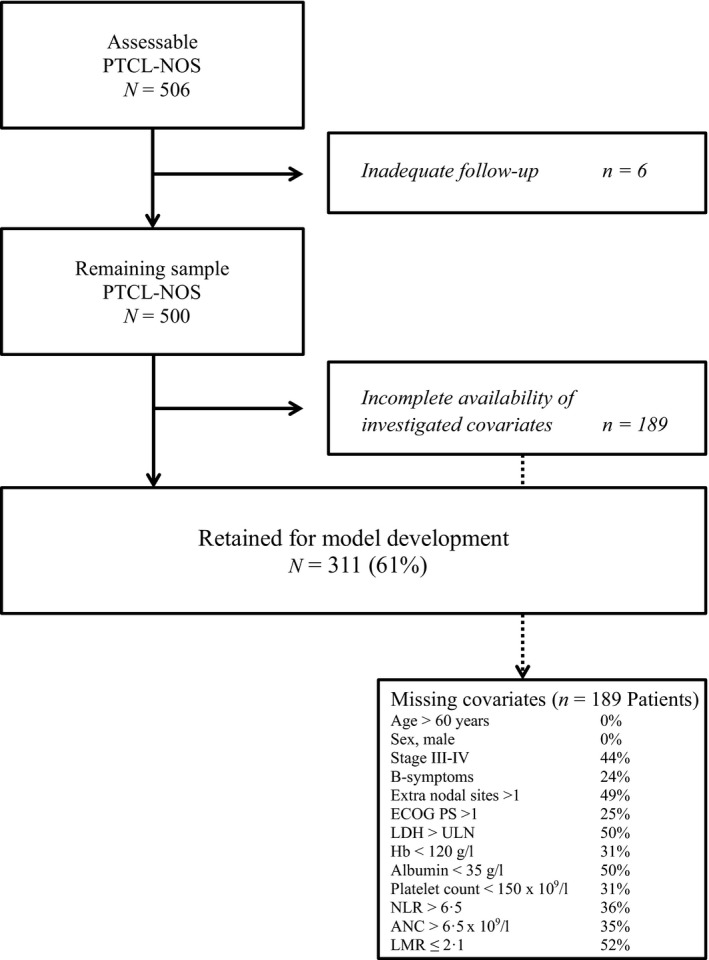
Flow chart of patients included in the analysis. ANC, absolute neutrophil count; ECOG‐PS, Eastern Cooperative Oncology Group performance status; Hb, haemoglobin; LMR, lymphocyte to monocyte ratio; NLR, neutrophil to lymphocyte ratio; PTCL‐NOS, peripheral t‐ cell lymphoma, not otherwise specified; ULN, upper limit of normality; LDH, lactated dehydrogenase.
Table 1.
Baseline characteristics of the patients of the training sample (n = 311) including variables with possible impact on survival analysed
| Factor | N | % |
|---|---|---|
| Median age, years (range) | 63 (23–83) | |
| Age >60 years | 170 | 55 |
| Sex, male | 192 | 62 |
| Stage III–IV | 237 | 76 |
| B‐symptoms presence | 136 | 44 |
| Extra nodal sites >1 | 88 | 28 |
| ECOG PS >1 | 81 | 26 |
| LDH > ULN | 164 | 53 |
| Hb < 120 g/l | 122 | 39 |
| Albumin < 35 g/l | 118 | 38 |
| Platelet count < 150 × 109 cells/l | 65 | 21 |
| NLR > 6·5 | 64 | 21 |
| ANC > 6·5 × 109/l | 73 | 23 |
| LMR ≤ 2·1 | 129 | 41 |
ANC, absolute neutrophil count; ECOG‐PS, Eastern Cooperative Oncology Group performance status; Hb, haemoglobin; LDH, lactate dehydrogenase; LMR, lymphocyte to monocyte ratio; NLR, neutrophil to lymphocyte ratio.
The median age was 63 years (range 23–83), 62% of patients were male, and advanced stage disease was found in 76%.
Most patients of the study sample were from Europe (n = 157, 50%), followed by South America (n = 70, 23%), United States (n = 59, 19%) and Asia (n = 25, 8%).
The majority of patients were classified as low/low‐intermediate risk according to each of the indices previously reported that were applied: IPI 53%, PIT 53% (Gallamini et al, 2004), IPTCLP 75% (Weisenburger et al, 2011) and m‐PIT 88% Went et al, 2006), respectively.
Overall, 246 patients (79%) received systemic therapy with curative intent: of these 246 patients, 182 (74%) were treated with CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone)/CHOP‐like and 31 patients (13%) 43 (18%) with etoposide‐containing (CHOEP/CHOEP‐like) regimens; and 21 (9%) patients received other different regimens; Ten patients (4%) had a satisfactory initial response and were consolidated by stem cell transplant, with some geographical variations (Europe 4·6%, USA 8·9%, South America 1·8%, Asia 0·0%).
At a median follow‐up of 46 months (range 1–99), 170 deaths were recorded, most due to lymphoma (70%), followed by infection (9%), treatment toxicity (8%) or other (13%).
The 3‐year and 5‐year OS was 41% [95 CI 34–47] and 32% [95 CI 26–38], respectively, with a median OS of 20 months; the OS of the 189 cases excluded due to missing covariates was superimposable to that of study sample, suggesting a lack of selection bias (P = 0·431). The 3‐year and 5‐year PFS was 28% [95 CI 23–34] and 23% [95 CI 18–29], respectively, with a median PFS of 10 months (Fig 2).
Figure 2.
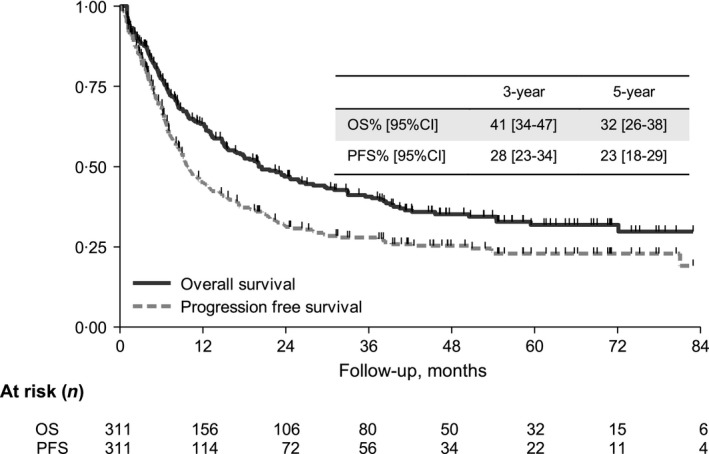
Kaplan–Meier curves of overall survival and progression‐free survival (for all patients in the training sample (n = 311). 95% CI, 95%confidence interval; OS, overall survival; PFS, progression‐free survival.
Prognostic model development
In univariate analysis, all the analysed variables had a statistically significant impact on OS (Table 2).
Table 2.
Univariate and multivariate Cox PH regression in the training sample (n = 311)
| 5‐year OS [95 CI] | Univariate | Multivariatea | |||
|---|---|---|---|---|---|
| Overall (n = 311) | 32 [26–38] | ||||
| Factor | Status | HR [95 CI] | HR [95 CI] | P‐value | |
| Stage | I–II | 52 [37–65] | 1·00 | 1·00 | |
| III–IV | 25 [18–33] | 2·16 [1·43–3·27] | 1·74 [1·14–2·65] | 0·010 | |
| ECOG PS | 0–1 | 38 [30–46] | 1·00 | 1·00 | |
| 2–4 | 15 [7–25] | 2·62 [1·91–3·61] | 2·12 [1·52–2·94] | <0·001 | |
| Albumin, g/l | ≥35 | 42 [34–52] | 1·00 | 1·00 | |
| <35 | 15 [8–24] | 2·66 [1·96–3·61] | 2·03 [1·47–2·81] | <0·001 | |
| ANC, ×109/l | ≤6·5 | 38 [30–45] | 1·00 | 1·00 | |
| >6·5 | 13 [5–26] | 2·05 [1·48–2·85] | 1·85 [1·33–2·58] | <0·001 | |
| NLR | ≤6·5 | 37 [30–45] | 1·00 | ||
| >6·5 | 13 [5–24] | 2·23 [1·60–3·12] | |||
| Age, years | ≤60 | 39 [30–48] | 1·00 | ||
| >60 | 26 [18–35] | 1·25 [0·92–1·70] | |||
| Sex | Female | 43 [31–54] | 1·00 | ||
| Male | 26 [19–34] | 1·54 [1·11–2·14] | |||
| B‐symptoms | No | 42 [33–51] | 1·00 | ||
| Yes | 18 [11–27] | 1·79 [1·32–2·43] | |||
| ENS, n | 0–1 | 32 [24–40] | 1·00 | ||
| >1 | 31 [21–42] | 1·19 [0·86–1·65] | |||
| LDH | ≤ULN | 44 [34–54] | 1·00 | ||
| >ULN | 21 [14–29] | 1·99 [1·46–2·73] | |||
| Hb, g/l | ≥120 | 37 [28–45] | 1·00 | ||
| <120 | 26 [17–35] | 1·43 [1·06–1·95] | |||
| Platelet count, ×109/l | ≥150 | 34 [27–42] | 1·00 | ||
| <150 | 23 [12–36] | 1·54 [1·08–2·20] | |||
| LMR | >2·1 | 37 [28–46] | 1·00 | ||
| ≤2·1 | 25 [16–34] | 1·53 [1·13–2·08] |
Slope shrinkage 0·955 (overfitting 0·045). c‐Harrell 0·706 (corrected 0·700). Log‐likelihood test final model versus full model, P = 0·273. Final model included 310 cases: one subject removed because of an influential point on the coefficient vector. Slope shrinkage and corrected c‐Harrell over 250 bootstrap replicates. 95 CI, 95% confidence interval; Cox PH, cox proportional hazard regression, Efron method for ties; ECOG‐PS, Eastern Cooperative Oncology Group performance status; ENS, extranodal sites; Hb, hemoglobin; HR, hazard ratio; LDH, lactated dehydrogenase; LMR, lymphocyte monocyte ratio; NLR, neutrophil lymphocyte ratio; PS, performance status; ULN, upper limit of normality.
Final model estimated in sample of 310 patients, one excluded because it was an outlier. Median follow‐up 46 months (range 1–99 months).
The 170 reported events correspond to an event/variable ratio of 14/1, which was acceptable to perform the multivariate analysis (Harrell et al, 1984; Smith et al, 1992); risk groups were defined by comparing the relative risk of death in patients with each possible number of presenting risk factors and combining the categories with a similar relative risk.
From multiple Cox PH regression analysis four factors were predictive of OS: stage, ECOG‐PS, serum albumin level and absolute neutrophil count (ANC) (Table 2).
The prognostic model (T cell score) was developed considering each adverse factor as having weight = 1, and identified three groups at different risk: low‐risk (LR, 48 patients, 15%, score zero), intermediate risk (IR, 189 patients, 61%, score one or two), and high‐risk (HiR, 74 patients, 24%, score three or four).
The three risk groups had a 3‐year and 5‐year OS of 76% [95 CI 61–88] and 69% [95 CI 49–83], 43% [95 CI 35–51] and 31% [95 CI 23–40], 11% [95 CI 4–21] and 8% [95 CI 2–18] for patients at LR, IR and HiR respectively (P < 0·001) (Table 3 and Fig 3A).
Table 3.
Distribution of patients with PTCL‐NOS by risk score in the training sample (n = 311) and in the external validation sample (n = 98)
| Training sample | ||||||
|---|---|---|---|---|---|---|
| Risk (score) | N | % | Events | 3‐year OS [95 CI] | HR [95 CI] | P‐value |
| Low (0) | 48 | 15 | 12 | 76 [61–88] | 1·00 | |
| Intermediate (1–2) | 189 | 61 | 100 | 43 [35–51] | 3·08 [1·65–5·76] | <0·001 |
| High (3–4) | 74 | 24 | 58 | 11 [4–21] | 8·88 [4·62–17·1] | <0·001 |
| High versus Intermediate | 2·88 [2·07–4·00] | <0·001 | ||||
|
c‐Harrell 0·674 Median follow‐up: 46 months (range 1–99 months) | ||||||
| External validation sample | ||||||
| Low (0) | 18 | 18 | 5 | 69 [46–100] | 1·00 | |
| Intermediate (1–2) | 54 | 55 | 30 | 41 [29–57] | 2·27 [0·88–5·85] | 0·091 |
| High (3–4) | 26 | 27 | 17 | 31 [17–57] | 3·80 [1·40–10·3] | 0·009 |
|
c‐Harrell 0·631 Median follow‐up: 18 months (range 0–68 months) | ||||||
95 CI, 95% confidence interval; HR, hazard ratio; OS, overall survival; PTCL‐NOS, peripheral t‐ cell lymphoma, not otherwise specified.
Figure 3.
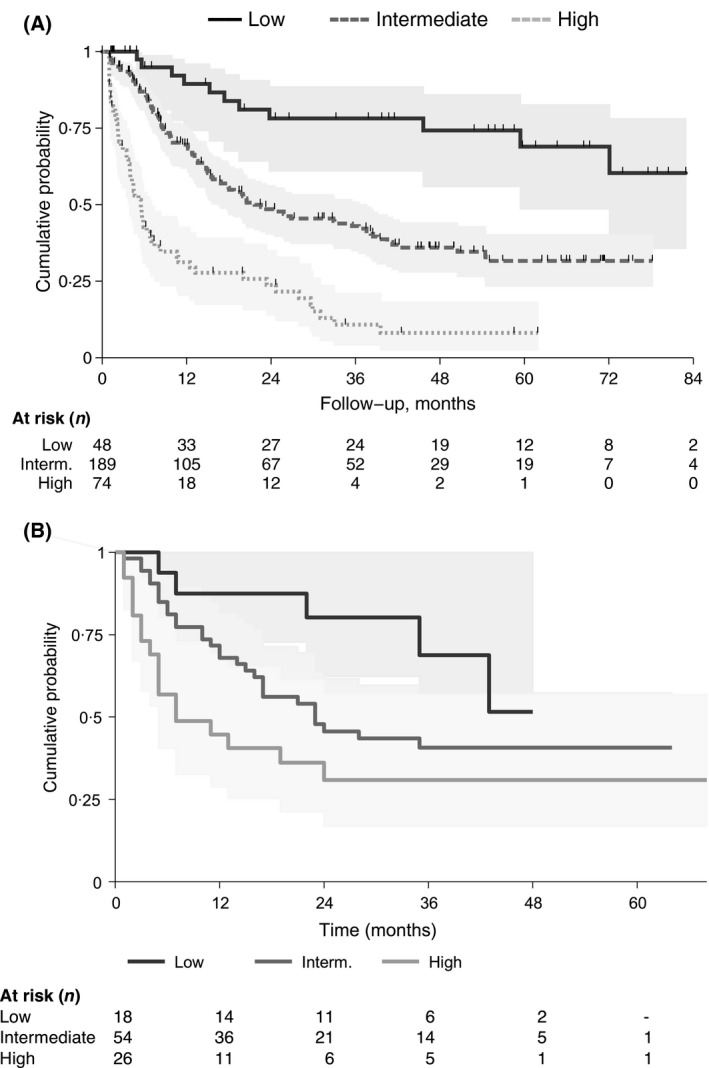
Kaplan–Meier curves of overall survival by risk groups identified by the model in the training sample (n = 311) (Panel A) and in the validation sample (n = 98) (Panel B). Interm., intermediate.
The model also proved to be a robust tool for PFS: the 5‐year PFS was 52% [95 CI 33–67], 22% [95 CI 16–30] and 7% [95 CI 2–16] in LR, IR and HiR, respectively (P < 0·001; data not shown).
External validation
In view of the fact that some US Institutions participated in both the T Cell Project and the COMPLETE registry, a preliminary crosscheck was performed to exclude the COMPLETE registry cases that were used for the T cell score development from the validation sample: 98 patients remained available for the validation, with a median age of 61 years (range 24–90), 65% male, 76% presented with advanced stage.
The median follow‐up of the validation cohort was 18 months (range 0–68), and 52 events for survival (53% of patients) were recorded: due to the shorter follow‐up of the validation sample, 3‐year OS is presented, which was 43% [95 CI 33–55] for the entire cohort, with a median OS of 23 months.
Applying the model in the validation sample, multiple Cox PH regression analysis stratified the patients as follows: LR, 18 patients (18%); IR, 54 patients (55%); HiR, 26 patients (27%): the 3‐year OS of the three groups was 69% [95 CI 46–100], 41% [95 CI 29–57] and 31% [95 CI 17–57] for the LR, IR and HiR, respectively (P = 0·02) (Table 3 and Fig 3B).
Notably, the distribution of the different risk groups was superimposable in the training and validation samples, being 15% and 18% in LR, 61% and 55% in IR, and 24% and 27% in HiR, respectively, and the discriminant power between the two groups was also comparable, with c‐Harrell 0·674 and 0·631 in the training and validation samples, respectively.
The Kaplan‐Meier curves for LR and IR were similar in the two cohorts. However, HiR patients had an apparent better survival in the external validation cohort (3‐year OS 27% vs. 11% and HR = 3·80 vs. 8·88) (Fig 3).
Discussion
The prognosis of PTCL‐NOS is poor, for both the first line and salvage settings. There is consequently an urgent need to risk stratify the affected patients through accurate prognostic models. Among all the previously reported indices, IPI and PIT are the most commonly used. Notably, there is a considerable overlap in parameters used to build all the various models, all developed based on retrospective data collections.
The present study proposes a new model (T cell score) that is able to stratify patients into three groups with differing risk, which was developed in a subset of 311 patients with PTCL‐NOS prospectively registered in the T Cell Project starting from 12 covariates with a significant impact on OS in univariate analysis, and based on the four covariates that maintained their impact in multivariate analysis (serum albumin level, (ANC), ECOG‐PS and stage).
ECOG‐PS and stage were previously well recognized as having a prognostic impact in PTCL by a series of authors (López‐Guillermo et al, 1998; Savage et al, 2004; Lee et al, 2009), and indeed they were already included in previously reported indices (Gallamini et al, 2004; Went et al, 2006; Weisenburger et al, 2011), showing them to be highly significant predictors for both OS and PFS.
In recent years, low serum albumin was reported to have an adverse prognostic impact on OS in PTCL, both in univariate analysis (Watanabe et al, 2010) and as an independent predictor (Chihara et al, 2009; Raina et al, 2010).
There is increasing and consistent evidence in recent literature that cancer‐associated inflammation is a key determinant of outcome in patients with cancer (Mantovani et al, 2008; Grivennikov et al, 2010; Gu et al, 2016). One routinely available marker of the systemic inflammatory response is the NLR. A single institution experience reported on 119 mycosis fungoides (MF) patients having a NLR of 2·07 ± 1·17 compared to 1·76 ± 0·53 for the control group (P < 0·05), confirming that a high NLR at MF diagnosis represents a simple, poor prognostic factor for identifying high‐risk patients with MF (Cengiz et al, 2017). Recently, Beltran et al (2016) retrospectively evaluated 83 PTCL‐unspecified patients in terms of NLR, and reported that in multivariate analyses, a NLR ≥ 4 was independently associated with worse OS after adjustment for the IPI and the PIT scores.
Chen et al (2014) correlated elevated ANC levels (>7·3 × 109/l) to an inferior OS (P = 0·017, HR 1·56) in multivariate analysis on 817 treatment‐naïve DLBCL registered in the MD Anderson Cancer Center lymphoma database; additionally, Spassov et al (2015) retrospectively reviewed the clinical outcome of 174 R‐CHOP treated primary nodal DLBCL, and they found an inferior OS was significantly associated with decreased albumin (≤39·4 g/l, P < 0·001) and elevated ANC (>5·19 × 109/l, P = 0·011) which was confirmed in multivariate analysis.
Our analyses demonstrated that ANC strongly correlated with NLR, with 92% of the cases in our cohort classified into the same risk group (K statistics 0·854; P < 0·001) when using either the NLR or ANC; thus, ANC was retained instead of NLR in the analysis (Table 2).
To date, the innate mechanisms of tumour pathogenesis and progression remains unclear. However, several studies have indicated that tumour pathogenesis and progression are closely associated with the tumour microenvironment. Recent studies have suggested that a systemic inflammatory state is associated with the malignant biological behaviour of the tumour. In particular, elevated ANC has been found as a predictor of poor prognosis in various types of tumours, including gastric, colorectal, pancreatic, breast and lung cancers and Hodgkin Lymphoma.
Considering the OS of the different risk groups identified by the T cell score, patients with a score zero, i.e. none of the four adverse prognostic factors (15% of the cohort) had a 5‐year OS of 69%, i.e. a better outcome with respect to patients reported at low‐risk by previous indices. The T cell score also identified a group with very unfavourable risk, with a score of 3–4 (24% of the patients), and a 5‐year OS of 8%.
The external validation was performed on 98 patients with PTCL‐NOS registered in the COMPLETE registry: the validation and training samples had similar patient characteristics, showed a homogeneous distribution of risk groups, and had a superimposable discriminant power, as suggested by the c‐Harrell values and by a P = 0·02.
Outcomes for the LR and the IR groups were superimposable in the training and the external validation sample, while in the latter a better OS for HiR group was recorded; this might be due to the large differences between the training and the external validation sample in median follow‐up (49 vs. 18 months, respectively) and the numeric difference (331 vs. 98 patients, respectively).
Frontline therapy was homogeneous in our cohort with >80% receiving anthracycline‐based therapy; however, given that therapy influences significantly prognostic factors, the score will need to be validated as new therapies evolve.
In the analysis conducted by the IPTCLP (Weisenburger et al, 2011), Ki67 proliferation index, transformed tumour cells, Epstein–Barr virus (EBV)‐encoded small RNA‐positive T cells, CD56 and CD30 expression were also found to be adverse prognostic factors both for OS and PFS in univariate analysis, while multivariate analysis controlling for the IPI, only transformed cells >70% was predictive for OS, and no pathological feature was predictive for PFS; in the 311 patients used as training sample for the T cell score, CD30 expression was available in 43% of our patients, thus precluding its incorporation in the model development; however, the analysis performed regarding the impact of CD30 expression on OS in the available cases did not lead to a significant difference (P = 0·428).
The pattern of expression of T cell helper type 1 (Th1)‐ or type 2 (Th2)‐associated antigens or activated T‐cell receptor evaluated in a series of T cell Non‐Hodgkin lymphoma patients allowed the identification of subgroups of PTCL‐NOS patients with different probabilities of survival: in particular, patients with PTCL‐NOS expressing one of Th1 or Th2 antigens tended to show favourable prognosis as compared with cases not expressing Th1 or Th2 antigens (Tsuchiya et al, 2004); moreover, the recently revised 2016 WHO classification (Swerdlow et al, 2016) recognizes a category with a T follicular helper type phenotype that includes prior cases of PTCL‐NOS as defined by the WHO 2008 clasification (Swerdlow et al, 2008).
Finally, gene expression profiling studies have reported reclassification of 37% morphologically diagnosed PTCL‐NOS cases into other subtypes (Iqbal et al, 2014); in the remainder of the cases two major subgroups were identified by either high expression of GATA3 or TBX21 with the former associated with a poor OS, and high expression of a cytotoxic gene‐signature within the TBX21 subgroup showing poor clinical outcome.
Like previous models, the new T cell score it is also based only on clinical variables, and does not account for differences bought about by the new molecular and genotypic findings, which could have potential clinical relevance (Table SI).
In conclusion, although the IPI, PIT, IPTCLP and m‐PIT still remain useful in defining risk for PTCL‐NOS, the T cell score, developed on a prospectively collected data set, better stratifies patients and has the best performance compared to the other indices; further studies implementing some of the emerging biological variables to clinical factors, need to be performed to determine if clinical risk can be further refined and allow for better risk stratification.
Author Contributions
Massimo Federico designed the study. Monica Bellei, Luigi Marcheselli, Martina Manni and Vittoria Tarantino performed the analysis. Marc Schwartz, Young‐Hyeh Ko, Maria E. Cabrera, Steven Horwitz, Won Seog Kim, Andrei Shustov, Francine M. Foss, Arnon Nagler, Kenneth Carson, Lauren C. Pinter‐Brown, Silvia Montoto, Michele Spina, Tatyana A. Feldman, Mary Jo Lechowicz, Sonali M. Smith, Frederick Lansigan, Raul Gabus, Julie M. Vose and Ranjana H. Advani provided patients and study material. Stefano Pileri performed the centralized review. All authors read and approved the manuscript.
Supporting information
Table SI. Distribution, overall survival (OS) and Progression‐free survival (PFS) of patients of the Training sample according to the previously proposed risk scores (IPI, PIT, IPTCLP, m‐PIT).
Acknowledgements
This work was supported by a grant from: Fondazione Cassa di Risparmio di Modena, Associazione Angela Serra per la Ricerca sul Cancro, Fondazione Italiana Linfomi, Allos Therapeutics, and Spectrum Pharmaceuticals, AIRC 5x1000 (grant n. 10007 to Stefano Pileri), the NIH/NCI CCSG P30 CA008748 (grant to Steven Horwitz). The authors wish to thank Lisa Bellm for her valuable help in the check of the patients included in both the T Cell Project and the COMPLETE Registry, a mandatory job to avoid validation of the model with cases already used for model development, and for her kindly supporting and favoring communications between the two networks.
References
- Akaike, H. (1974) A new look at the statistical model identification. IEEE Transactions on Automatic Control, 16, 716–723. [Google Scholar]
- Bellei, M. , Chiattone, C.S. , Luminari, S. , Pesce, E.A. , Cabrera, M.E. , de Souza, A.C. , Gabùs, R. , Zoppegno, L. , Milone, J. , Pavlovsky, A. , Connors, J.M. , Foss, F.M. , Horwitz, S.M. , Liang, R. , Montolo, S. , Pileri, S.A. , Polliak, A. , Vose, J.M. , Zinzani, P.L. , Zucca, E. & Federico, M. (2012) T‐cell lymphoma in South America and Europe. Revista Brasileira de Hematologia e Hemoterapia, 34, 42–47. ISSN: 1516‐8484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran, B.E. , Aguilar, C. , Quiñones, P. , Morales, D. , Chavez, J.C. , Sotomayor, E.M. & Castillo, J.J. (2016) The neutrophil‐to‐lymphocyte ratio is an independent prognostic factor in patients with peripheral T‐cell lymphoma, unspecified. Leukemia & Lymphoma, 57, 58–62. [DOI] [PubMed] [Google Scholar]
- Cengiz, F.P. , Emiroglu, N. , Ozkaya, D.B. , Bahali, A.G. , Su, O. & Onsun, N. (2017) Prognostic evaluation of neutrophil/lymphocyte ratio in patients with mycosis fungoides. Annals of Clinical and Laboratory Science, 47, 25–28. [PubMed] [Google Scholar]
- Chen, Y. , Rodriguez, M.A. , Feng, L. , Bi, W. , Zhang, L. & Wang, M. (2014) Peripheral absolute neutrophil, mmonocyte and lymphocyte counts and clinical ooutcome in diffuse large B‐cell lymphoma. Blood, 124, 4416. [Google Scholar]
- Cheson, B.D. , Horning, S.J. , Coiffier, B. , Shipp, M.A. , Fisher, R.I. , Connors, J.M. , Lister, T.A. , Vose, J. , Grillo‐López, A. , Hagenbeek, A. , Cabanillas, F. , Klippensten, D. , Hiddemann, W. , Castellino, R. , Harris, N.L. , Armitage, J.O. , Carter, W. , Hoppe, R. & Canellos, G.P. (1999) Report of an international workshop to standardize response criteria for non‐Hodgkin's lymphomas. NCI Sponsored International Working Group. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 17, 1244. [DOI] [PubMed] [Google Scholar]
- Chihara, D. , Oki, Y. , Ine, S. , Yamamoto, K. , Kato, H. , Taji, H. , Kagami, Y. , Yatabe, Y. , Nakamura, S. & Morishima, Y. (2009) Analysis of prognostic factors in peripheral T‐cell lymphoma: prognostic value of serum albumin and mediastinal lymphadenopathy. Leukemia & Lymphoma, 50, 1999–2004. [DOI] [PubMed] [Google Scholar]
- Gallamini, A. , Stelitano, C. , Calvi, R. , Bellei, M. , Mattei, D. , Vitolo, U. , Morabito, F. , Martelli, M. , Brusamolino, E. , Iannitto, E. , Zaja, F. , Cortelazzo, S. , Rigacci, L. , Devizzi, L. , Todeschini, G. , Santini, G. , Brugiatelli, M. & Federico, M. (2004) Peripheral T‐cell lymphoma unspecified (PTCL‐U): a new prognostic model from a retrospective multicentric clinical study. Blood, 103, 2474–2479. [DOI] [PubMed] [Google Scholar]
- Grivennikov, S.I. , Greten, F.R. & Karin, M. (2010) Immunity, inflammation, and cancer. Cell, 140, 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, L. , Li, H. , Chen, L. , Ma, X. , Li, X. , Gao, Y. , Zhang, Y. , Xie, Y. & Zhang, X. (2016) Prognostic role of lymphocyte to monocyte ratio for patients with cancer: evidence from a systematic review and meta‐analysis. Oncotarget, 7, 31926–31942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell, F.E. (2001) Regression Modeling Strategies New York. Springer, New York, NY. [Google Scholar]
- Harrell, F.E. (2016) rms: Regression modeling strategies. R package. Available at: http://biostat.mc.vanderbilt.edu/rms.
- Harrell, F.E. , Lee, K.L. , Califf, R.M. , Pryor, D.B. & Rosati, R.A. (1984) Regression modelling strategies for improved prognostic prediction. Statistics in Medicine, 3, 143–152. [DOI] [PubMed] [Google Scholar]
- Iqbal, J. , Wright, G. , Wang, C. , Rosenwald, A. , Gascoyne, R.D. , Weisenburger, D.D. , Greiner, T.C. , Smith, L. , Guo, S. , Wilcox, R.A. , Teh, B.T. , Lim, S.T. , Tan, S.Y. , Rimsza, L.M. , Jaffe, E.S. , Campo, E. , Martinez, A. , Delabie, J. , Braziel, R.M. , Cook, J.R. , Iqbal, J. , Wright, G. , Wang, C. , Rosenwald, A. , Gascoyne, R.D. , Weisenburger, D.D. , Greiner, T.C. , Smith, L. , Guo, S. , Wilcox, R.A. , Teh, B.T. , Lim, S.T. , Tan, S.Y. , Rimsza, L.M. , Jaffe, E.S. , Campo, E. , Martinez, A. , Delabie, J. , Braziel, R.M. , Cook, J.R. , Tubbs, R.R. , Ott, G. , Geissinger, E. , Gaulard, P. , Piccaluga, P.P. , Pileri, S.A. , Au, W.Y. , Nakamura, S. , Seto, M. , Berger, F. , de Leval, L. , Connors, J.M. , Armitage, J. , Vose, J. , Chan, W.C. & Staudt, L.M. ; Lymphoma Leukemia Molecular Profiling Project and the International Peripheral T‐cell Lymphoma Project (2014) Gene expression signatures delineate biological and prognostic subgroups in peripheral T‐cell lymphoma. Blood, 123, 2915–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, D. , O'Hara, C. , Kraus, M.D. , Perez‐Atayde, A.R. , Shahsafaei, A. , Wu, L. & Dorfman, D.M. (2000) Expression pattern of T‐cell‐associated chemokine receptors and their chemokines correlates with specific subtypes of T‐cell non‐Hodgkin lymphoma. Blood, 96, 685–690. [PubMed] [Google Scholar]
- Lee, Y. , Uhm, J.E. , Lee, H.‐Y. , Park, M.J. , Kim, H. , Oh, S.J. , Jang, J.H. , Kim, K. , Jung, C.W. , Ahn, Y.C. , Park, K. , Ko, Y.H. & Kim, W.S. (2009) Clinical features and prognostic factors of patients with ‘peripheral T cell lymphoma, unspecified’. Annals of Hematology, 88, 111–119. [DOI] [PubMed] [Google Scholar]
- López‐Guillermo, A. , Cid, J. , Salar, A. , López, A. , Montalbán, C. , Castrillo, J.M. , González, M. , Ribera, J.M. , Brunet, S. , García‐Conde, J. , Fernández de Sevilla, A. , Bosch, F. & Montserrat, E. (1998) Peripheral T‐cell lymphomas: initial features, natural history, and prognostic factors in a series of 174 patients diagnosed according to the R.E.A.L. Classification. Annals of Oncology: Official Journal of the European Society for Medical Oncology, 9, 849–855. [DOI] [PubMed] [Google Scholar]
- Mantovani, A. , Allavena, P. , Sica, A. & Balkwill, F. (2008) Cancer‐related inflammation. Nature, 454, 436–444. [DOI] [PubMed] [Google Scholar]
- R Core Team . (2016) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Raina, V. , Singhal, M.K. , Sharma, A. , Kumar, L. , Kumar, R. , Duttagupta, S. , Kumar, B. & Das, P. (2010) Clinical characteristics, prognostic factors, and treatment outcomes of 139 patients of peripheral T‐cell lymphomas from AIIMS, New Delhi, India. Journal of Clinical Oncology, 28, e18549–e18549. [Google Scholar]
- Royston, P. (2000) A strategy for modelling the effect of a continuous covariate in medicine and epidemiology. Statistics in Medicine, 19, 1831–1847. [DOI] [PubMed] [Google Scholar]
- Savage, K.J. , Chhanabhai, M. , Gascoyne, R.D. & Connors, J.M. (2004) Characterization of peripheral T‐cell lymphomas in a single North American institution by the WHO classification. Annals of Oncology: Official Journal of the European Society for Medical Oncology, 15, 1467–1475. [DOI] [PubMed] [Google Scholar]
- Schoenfeld, D. (1982) Partial residuals for the proportional hazards regression model. Biometrika, 69, 239–241. [Google Scholar]
- Smith, L.R. , Harrell, F.E. & Muhlbaier, L.H. (1992) Problems and potentials in modelling survival In: Medical Effectiveness Research Data Methods Summary Report (eds. by Grady M.L. & Schwartz H.A.), pp. 151–159. AHCPR, Rockville, MD: Pub. No. 92‐0056 US Dept. of Health and Human Services, Agency for Health Care Policy and Research. [Google Scholar]
- Spassov, B. , Vassileva, D. , Michaylov, G. , Balatzenko, G. & Guenova, M. (2015) Serum albumin and peripheral blood lymphocyte/monocyte ratio at diagnosis may provide additional prognostic information in R‐IPI good risk diffuse large‐B‐cell lymphoma patients treated with R‐CHOP. Blood, 126, 2658.26679542 [Google Scholar]
- Swerdlow, S. , Campo, E. , Harris, N. , Jaffe, E.S. , Pileri, S.A. , Stein, H. , Thiele, J. & Wardiman, J.W. (2008) WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th edn IARC Press, Lyon, France. [Google Scholar]
- Swerdlow, S.H. , Campo, E. , Pileri, S.A. , Harris, N.L. , Stein, H. , Siebert, R. , Advani, R. , Ghielmini, M. , Salles, G.A. , Zelenetz, A.D. & Jaffe, E.S. (2016) The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood, 127, 2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya, T. , Ohshima, K. , Karube, K. , Yamaguchi, T. , Suefuji, H. , Hamasaki, M. , Kawasaki, C. , Suzumiya, J. , Tomonaga, M. & Kikuchi, M. (2004) Th1, Th2, and activated T‐cell marker and clinical prognosis in peripheral T‐cell lymphoma, unspecified: comparison with AILD, ALCL, lymphoblastic lymphoma, and ATLL. Blood, 103, 236–241. [DOI] [PubMed] [Google Scholar]
- Vose, J. , Armitage, J. & Weisenburger, D. (2008) International peripheral T‐cell and natural killer/T‐cell lymphoma study: pathology findings and clinical outcomes. Journal of Clinical Oncology, 26, 4124–4130. [DOI] [PubMed] [Google Scholar]
- Watanabe, T. , Kinoshita, T. , Itoh, K. , Yoshimura, K. , Ogura, M. , Kagami, Y. , Yamaguchi, M. , Kurosawa, M. , Tsukasaki, K. , Kasai, M. , Tobinai, K. , Kaba, H. , Mukai, K. , Nakamura, S. , Ohshima, K. , Hotta, T. & Shimoyama, M. (2010) Pretreatment total serum protein is a significant prognostic factor for the outcome of patients with peripheral T/natural killer‐cell lymphomas. Leukemia & Lymphoma, 51, 813–821. [DOI] [PubMed] [Google Scholar]
- Weisenburger, D.D. , Savage, K.J. , Harris, N.L. , Gascoyne, R.D. , Jaffe, E.S. , MacLennan, K.A. , Rudiger, T. , Pileri, S. , Nakamura, S. , Nathwani, B. , Campo, E. , Berger, F. , Coiffier, B. , Kim, W.‐S. , Holte, H. , Federico, M. , Au, W.Y. , Tobinai, K. , Armitage, J.O. & Vose, J.M. (2011) Peripheral T‐cell lymphoma, not otherwise specified: a report of 340 cases from the International Peripheral T‐cell Lymphoma Project. Blood, 117, 3402–3408. [DOI] [PubMed] [Google Scholar]
- Went, P. , Agostinelli, C. , Gallamini, A. , Piccaluga, P.P. , Ascani, S. , Sabattini, E. , Bacci, F. , Falini, B. , Motta, T. , Paulli, M. , Artusi, T. , Piccioli, M. , Zinzani, P.L. & Pileri, S.A. (2006) Marker expression in peripheral T‐cell lymphoma: a proposed clinical‐pathologic prognostic score. Journal of Clinical Oncology, 24, 2472–2479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Distribution, overall survival (OS) and Progression‐free survival (PFS) of patients of the Training sample according to the previously proposed risk scores (IPI, PIT, IPTCLP, m‐PIT).


