Abstract
Cord blood transplantation (CBT) is an effective option for treating hematological malignancies, but graft failure (GF) remains the primary cause of therapy failure. Thus, based on myeloablative conditioning (MAC) of busulfan with cyclophosphamide (Bu/Cy) or total body irradiation with Cy (TBI/Cy), fludarabine (Flu) was added to Bu/Cy and cytarabine (CA) to TBI/Cy for a modified myeloablative conditioning (MMAC). To compare the prognosis of MMAC with MAC, we conducted a retrospective study including 58 patients who underwent CBT with MAC or MMAC from 2000 to 2011. Neutrophil and platelet engraftment rate, overall survival (OS) and disease free survival (DFS) were significantly higher in the MMAC group (adjusted hazard ratio [HR], 2.58, 2.43, 0.36 and 0.37; p < 0.01, p = 0.01, p = 0.02 and p = 0.02, separately). Nonrelapse mortality (NRM) was comparable (p = 0.183). To validate the outcomes noted in the MMAC group, we conducted a prospective single‐arm clinical trial including 188 patients who underwent CBT with MMAC from 2011 to 2015. Engraftment rate, survival and NRM of the MMAC group in the prospective trail (MMAC‐P) were similar to the MMAC group in the retrospective study (MMAC‐R). This study is the first to demonstrate the superiority of MMAC to MAC in CBT for hematological malignancies.
Keywords: myeloablative conditioning, cord blood transplantation, antithymocyte globulin, hematological malignancies, engraftment
Short abstract
What's new?
Cord blood transplants can help patients with blood cancer, but too often, the transplant fails due to immune rejection or other problems. Typically, patients receive myeloablative conditioning (MAC) prior to CBT, but more intense regimen might improve transplant success. Here, the authors compared the success of modified myeloablative conditioning (MMAC) with conventional MAC by looking at 58 patients over an 11‐year period. They then followed up with a four‐year prospective study, including 188 patients who received cord blood transplant with MMAC. The modified conditioning regimen boosted graft success and improved survival of patients with hematological cancers.
Abbreviations
- ALL
acute lymphoblastic leukemia
- AML
acute myelogenous leukemia
- ATG
antithymocyte globulin
- Bu
busulfan
- CA
cytarabine
- CB
cord blood
- CBT
cord blood transplantation
- CSA
cyclosporine
- Cy
cyclophosphamide
- DFS
disease‐free survival
- DSA
donor‐specific anti‐HLA antibodies
- GF
graft failure
- GRFS
GVHD‐free/relapse‐free survival
- GVHD
graft‐vs.‐host disease
- GVL
graft versus leukemia
- HCT
hematopoietic stem cell transplantation
- HLA
human leukocyte antigen
- HR
hazard ratio
- MAC
myeloablative conditioning
- MDS
myelodysplastic syndrome
- MMAC
modified myeloablative conditioning
- MMF
mycophenolate mofeti
- MTX
methotrexate
- NRM
nonrelapse mortality
- OS
overall survival
- TBI
total body irradiation
Cord blood (CB) is an alternative option to standard graft sources for hematopoietic stem cell transplantation (HCT), and has been increasingly used in adults with hematological malignancies. There is considerable evidence that CB is a promising option for patients who lack a human leukocyte antigen (HLA)‐matched related or unrelated donor.1 Several studies comparing results of CBT and either bone marrow or peripheral blood stem cell transplantation showed similar results regarding OS and leukemia‐free survival, despite slower hematopoietic recovery and higher incidence of GF for CB transplant recipients.2, 3
GF is a life‐threatening complication of all kinds of HCT and occurs more frequently after CBT than after transplants using other standard graft sources.4 Some authors have reported that the overall incidence of GF after CBT is between 10% and 20%.3, 4, 5 GF can be due to immunological rejection of donor cells by residual host lymphocytes, the presence of donor‐specific anti‐HLA antibodies (DSA), or nonimmunologic mechanisms such as poor stem cell viability and viral infections.6, 7 Particularly, factors associated with GF in CBT can be inherently low stem cell doses, HLA mismatch, relative immaturity of CB lymphocytes and the increasing use of reduced intensity conditioning with CBT.4, 8, 9 Ioannis et al. studied the T‐cell immune reconstitution after CBT and found that in the early post‐transplant period, the predominant pathway of T‐cell reconstitution was mediated by the uniformly naïve T cells transferred from the cord blood or recipient T cells that had survived conditioning.10 Therefore, we speculate that the GF and delay of immune reconstitution of patients with MAC after CBT may be due to the deficiency of conditioning intensity, which will relatively increase the residual host lymphocytes. Patient lymphocytes will not only cause the immunological rejection but also compete with the naïve T cells for the space in bone marrow.
Furthermore, the use of antithymocyte globulin (ATG) and methotrexate (MTX) as part of graft‐versus‐host disease (GVHD) prophylaxis may also be the cause of GF and prolonged hematopoietic recovery. The use of ATG could impair the early development of donor‐derived T cells due to the persistence of these antibodies in the patient for several weeks after administration especially if given too close to graft infusion.11 Several studies have compared the outcomes between CBT with or without ATG, and the use of ATG was associated with lower OS and higher NRM, regardless of the lower incidence of GVHD.12, 13 Similarly, use of MTX‐containing regimen for GVHD prophylaxis has been associated with delayed engraftment and increased risk of graft failure in patients with hemoglobinopathies and malignant diseases transplanted with an HLA‐identical sibling CB unit.14, 15
Thus, we conducted a retrospective study to compare the outcomes of MMAC (Flu/Bu/Cy and CA/TBI/Cy) with MAC (Bu/Cy and TBI/Cy). The GVHD prophylaxis of MMAC included cyclosporine (CSA) and mycophenolate mofetil (MMF) while the GVHD prophylaxis of MAC also included ATG or MTX. We then conducted a prospective single‐arm clinical trial to validate the findings of the retrospective study.
Materials and Methods
Patient and donor selection
The retrospective study was conducted at the Department of Hematology, Affiliated Provincial Hospital of Anhui Medical University from April, 2000 to November, 2011. The trial enrolled 58 patients who underwent a single‐unit CBT as a first HCT. The MAC regimen consisted of Bu/Cy (Bu, 0.8 mg/kg per dose intravenously every 6 hr for 4 days; Cy, 60 mg/kg for 2 days) or TBI/Cy (TBI, 3 Gy twice daily for 2 days; Cy, 120 mg/kg). The MMAC regimen included the addition of Flu (30 mg/m2 for 4 days) to Bu/Cy or CA (2 g/m2 for 2 days) to TBI/Cy. The prospective study included data from 188 patients who underwent a single‐unit CBT as a first HCT following the MMAC regimen of Flu/Bu/Cy (n = 83) or CA/TBI/Cy (n = 105) from December, 2011 to December, 2015. All patients suffered from acute lymphoblastic leukemia (ALL), acute myelogenous leukemia (AML) or myelodysplastic syndrome (MDS) requiring allogeneic HCT. Patients who received a related HLA‐matched HCT or haploidentical transplantation or a double‐unit CBT were excluded. The choice of conditioning regimen was made according to the discussion of our transplantation team. Our protocol complied with the Declaration of Helsinki and was approved by the Anhui Medical University Institutional Review Board, and all patients included in the study provided informed consent. The validation trial was registered at http://www.chictr.org.cn as # ChiCTR‐ONRC‐11001430.
The CB unit selection was based primarily on the total nucleated cell number among 4/6 to 6/6 HLA‐matched units. HLA‐A and HLA‐B antigens were identified by serologic typing. HLA‐DRB1 alleles were determined by high‐resolution molecular typing. Anti‐HLA antibody screening was not performed. Cord blood units were obtained through the Chinese Cord Blood Bank Network.
GVHD prophylaxis and treatment and supportive care
All patients were given a combination of CsA and MMF for GVHD prophylaxis. CsA was started (2.5–3 mg/kg/day) on Day −1 and continued until patients were able to take CsA orally with target trough levels of 200–250 ng/mL for at least one month, and then CsA blood concentration was kept at about 150–250 ng/mL for at least 6 months. MMF (25–30 mg/kg/day, p.o. three times a day) was started on Day +1 until Day +28 or neutrophil recovery. Thirteen patients of the MAC group also received ATG (Fresenius, Germany) (7.5 mg/kg for 3 days) as part of the GVHD prophylaxis and the other 11 patients received MTX (7.5 mg/m2/day) on Day +1 and Day +3. The GVHD treatment and supportive care regimen including prophylaxis for infection, granulocyte colony‐stimulating factor use and blood product infusion was the same as previously reported.16
Definition
Neutrophil engraftment was defined as the achievement of an ANC ≥0.5 × 109/L for three consecutive days and platelet recovery was defined as the achievement of a platelet count ≥20 × 109/L unsupported by platelet transfusions for 7 days. Primary graft failure was defined as the failure to reach an ANC ≥0.5 × 109/L for at least three consecutive days or a mixed chimerism of donor cells ≥10% on Day 42 following transplantation based on chimerism analysis using polymorphic genetic markers.17 The assessment of pre‐engraftment syndrome, grading of acute GVHD (aGVHD) and grading of chronic GVHD (cGVHD) was performed according to standard criteria.18, 19, 20 Patients surviving in remission for least 100 days after transplantation were regularly evaluated for chronic GVHD. Relapse was defined based on morphological evidence of disease in the peripheral blood, marrow or extramedullary sites. NRM was defined as death after CBT without disease progression or relapse. The OS was measured from the time of CBT to the time of death from any cause or last recorded follow‐up. Post‐transplant disease‐free survival (DFS) was defined as the time interval from CBT to relapse, death or the last contact. GVHD‐free/relapse‐free survival (GRFS) was defined as the time interval from CBT to grade II to IV aGVHD, systemic therapy‐requiring cGVHD, relapse or death.21
Statistical analyses
Patient and transplantation characteristics from different groups were compared using the Mann–Whitney nonparametric U test for continuous variables and the χ 2 test or the Fisher‐exact test for the categorical variables. Engraftment, NRM, relapse, aGVHD and cGVHD were analyzed using Gray's method. NRM was calculated considering relapse as a competing risk. The Fine‐Gray proportional hazards model was used in multivariate analyses for engraftment, NRM, relapse, aGVHD and cGVHD. OS, DFS and GRFS rate were calculated with the Kaplan–Meier method and compared using log‐rank tests. Factors with significance or borderline significance (p < 0.2) in the univariate analysis were subjected to a multivariate analysis using the Cox proportional hazards model. Statistical analyses were performed using SPSS software, version 21.0. The α level of all tests and the p values was set at 0.05.
Results
Characteristics of patients and cord bloods
The characteristics of patients and transplants are illustrated in Tables 1 and 2 separately for retrospective study and prospective study. In the retrospective study, the median age was 11 years (2–42) and 41 (72.4%) of the patients were male, 34 (75.9%) were defined as a high risk and the median follow‐up period for surviving patients was ∼6.3 years. The patient's sex, diagnosis, disease status, extent of HLA match and cell dose showed no significant differences between the MAC group and the MMAC group. However, there were considerable differences with respect to patient's age, body weight, GVHD prophylaxis and conditioning types (Bu‐based or TBI‐based). Patients with MAC were younger and lighter. Thirteen patients in MAC group received ATG as part of GVHD prophylaxis, and the other 11 patients received MTX, while ATG and MTX were omitted in MMAC group (p < 0.05). Additionally, patients in MAC group received more Bu‐based conditioning (p < 0.05). In the prospective study, the median age was 13 years (2–47), 146 (65.8%) of the patients were male and 188 (84.7%) were defined as high risk and the median follow‐up period for surviving patients was ∼3.2 years. There were no statistical differences between MMAC‐R group and MMAC‐P group in patients' sex, age, body weight, disease status, nuclear cell count of grafts and extent of HLA match. However, there were more patients with ALL in MMAC‐P group and the median follow‐up period for surviving patients was shorter (p < 0.05 and p = 0.00, separately).
Table 1.
Patients and grafts characteristics for retrospective study
| MAC | MMAC | p | |
|---|---|---|---|
| Patient number | 24 | 34 | |
| Median age (range), years | 8 (2–42) | 14 (3–37) | 0.013a |
| ≤20 | 22 | 27 | |
| >20 | 2 | 7 | 0.367 |
| Median weight (range), kg | 26 (12–55) | 42 (12–66) | 0.005a |
| ≤45 | 22 | 23 | |
| >45 | 2 | 11 | 0.066 |
| Male sex, n (%) | 17 (71) | 25 (74) | 1 |
| Sex (donor/patient), n (%) | 0.354 | ||
| Male/male | 8 (33.3) | 8 (23.5) | |
| Male/female | 3 (12.5) | 7 (20.6) | |
| Female/male | 8 (33.3) | 16 (47.1) | |
| Female/female | 4 (16.7) | 2 (5.9) | |
| Missing data | 1 (4.2) | 1 (2.9) | |
| Diagnosis, n (%) | 0.371 | ||
| ALL | 15 (62.5) | 16 (47.1) | |
| AML or MDS | 9 (37.5) | 18 (52.9) | |
| Disease status, n (%) | 0.831 | ||
| CR1 | 15 (62.5) | 19 (55.9) | |
| CR2 | 6 (25) | 11 (34.5) | |
| ≥CR3 or NR | 3 (12.5) | 4 (11.8) | |
| Disease risk | 0.539 | ||
| Intermediate | 7 (29.2) | 7 (20.6) | |
| High | 17 (70.8) | 27 (79.4) | |
| Pretransplant therapy period | |||
| Median, days | 199 (98–1542) | 218 (80–2545) | 0.733 |
| ≤200 | 12 (50.0) | 16 (47.1) | |
| >200 | 11 (45.8) | 18 (52.9) | |
| Unknown | 1 (4.2) | 0 | 0.913 |
| No of HLA‐A, B, DR mismatched, n (%) | 0.090 | ||
| 0 | 1 (4.2) | 4 (11.8) | |
| 1 | 20 (83.3) | 19 (55.9) | |
| ≥2 | 3 (12.5) | 11 (34.5) | |
| Cell compositions in allograft | |||
| Infused nuclear cells 107/kg | 4.99 (2.70–16.24) | 4.03 (1.96–9.60) | 0.099 |
| ≤3.99 | 7 | 17 | |
| >3.99 | 17 | 17 | 0.188 |
| Infused CD34+ cells 105/kg | 2.45 (0.90–21.11) | 2.43 (1.04–5.24) | 0.548 |
| ≤2.38 | 10 | 16 | |
| >2.38 | 14 | 18 | 0.890 |
| Conditioning, n (%) | 0.002a | ||
| Bu based | 22 (91.7) | 17 (50.0) | |
| TBI based | 2 (83.3) | 17 (40.0) | |
| GVHD prophylaxis, n (%) | 0.000a | ||
| CSA/MMF/ATG | 13 (54.5) | 0 | |
| CSA/MMF/MTX | 11 (45.5) | 0 | |
| CSA/MMF | 0 | 34 (100) | |
| Follow‐up period (range), daysb | 3570 (2035–4864) | 2248 (1914–3088) | 0.039a |
Statistically significant.
Follow‐up period was for surviving patients.
Table 2.
Patients and grafts characteristics for prospective study
| MMAC‐R | MMAC‐P | p | |
|---|---|---|---|
| Patient number | 34 | 188 | |
| Median age, years (range) | 14 (3–37) | 13 (2–47) | 0.845 |
| ≤20 | 27 | 143 | |
| >20 | 7 | 45 | 0.838 |
| Median weight, kg (range) | 42 (12–66) | 42 (10–100) | 0.786 |
| ≤45 | 23 | 104 | |
| More than 45 | 11 | 84 | 0.251 |
| Male sex, n (%) | 25 (74) | 121 (64.4) | 0.401 |
| Sex (donor/patient), n (%) | 0.216 | ||
| Male/male | 8 (23.5) | 57 (30.3) | |
| Male/female | 7 (20.6) | 34 (18.1) | |
| Female/male | 16 (47.1) | 64 (34.0) | |
| Female/female | 2 (5.9) | 33 (17.6) | |
| Missing data | 1 (2.9) | 0 | |
| Diagnosis, n (%) | 0.021a | ||
| ALL | 16 (47.1) | 130 (69.1) | |
| AML or MDS | 18 (52.9) | 58 (30.9) | |
| Disease status, n (%) | 0.647 | ||
| CR1 | 19 (55.9) | 101 (53.7) | |
| CR2 | 11 (34.5) | 53 (28.2) | |
| ≥CR3 or NR | 4 (11.8) | 34 (18.1) | |
| Disease risk | 0.436 | ||
| Intermediate | 7 (20.6) | 27 (14.4) | |
| High or very high | 27 (79.4) | 161 (85.6) | |
| Pretransplant therapy period | |||
| Median, days | 218 (80–2545) | 227 (15–5449) | 0.367 |
| ≤200 | 16 | 80 | |
| >200 | 18 | 108 | 0.764 |
| No of HLA‐A, B, DR mismatched, n (%) | 0.889 | ||
| 0 | 4 (11.8) | 28 (14.9) | |
| 1 | 19 (55.9) | 100 (53.2) | |
| ≥2 | 11 (34.5) | 60 (31.9) | |
| Cell compositions in allografts (range) | |||
| Infused nuclear cells 107/kg | 4.03 (1.96‐9.60) | 3.91 (1.98‐17.27) | 0.744 |
| ≤4.89 | 17 | 99 | |
| >4.89 | 17 | 89 | 0.921 |
| Infused CD34+ cells 105/kg | 2.43 (1.04–5.24) | 2.31 (0.40‐10.55) | 0.735 |
| ≤2.82 | 16 | 97 | |
| >2.82 | 18 | 91 | 0.764 |
| Conditioning, n (%) | 0.657 | ||
| Bu based | 17 (50.0) | 105 (55.9) | |
| TBI based | 17 (40.0) | 83 (44.1) | |
| GVHD prophylaxis | 1 | ||
| CSA/MMF | 34 | 188 | |
| Follow‐up period (range), daysb | 2248 (1914–3088) | 824 (397–1237) | 0.000a |
Statistically significant.
Follow‐up period was for surviving patients.
Neutrophil and platelet engraftment
In the retrospective study, neutrophil engraftment rate by 30 days was significantly higher in MMAC group (Fig. 1 a; 97.1% vs. 62.5%, p < 0.01). This difference was also significant in univariate analysis (Supporting Information, Table S1; HR, 2.39; 95%CI, 1.29–4.45; p < 0.01). Besides, more loci of HLA mismatch and shorter pretransplant therapy period were associated with lower neutrophil engraftment rate with borderline significance (p < 0.2). With the extent of HLA mismatch and pretransplant therapy period as the confounding factors, MMAC showed remarkable association with higher incidence of neutrophil engraftment (Supporting Information, Table S1; adjusted HR, 2.58; 95%CI, 1.39–4.79; p < 0.01) in the multivariate analysis.
Figure 1.
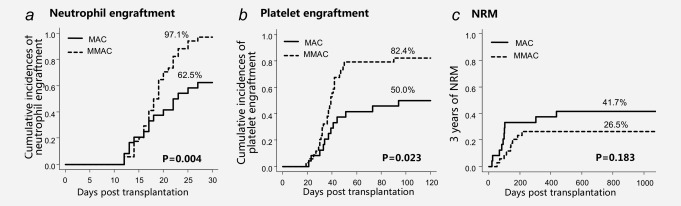
Engraftment and NRM after CBT in MAC group and MMAC group.
Platelet engraftment rate by 120 days was superior in the MMAC group (Fig. 1 b; 82.4% vs. 50.0%, p < 0.05). In the univariate analysis, MMAC, heavier weight (weighted 45 kg or heavier) and more CD34+ cells were associated with higher platelet engraftment rate with borderline significance (p < 0.2) (p = 0.01, p = 0.19 and p = 0.18, separately). In the multivariate analysis including these three factors, MMAC showed significant association with the increase of platelet engraftment (Supporting Information, Table S1; adjusted HR, 2.43; 95%CI, 1.23–4.79; p = 0.01).
In the validation study, there were no statistical differences between MMAC‐P group and MMAC‐R group in neither neutrophil engraftment rate nor platelet engraftment rate (96.3% vs. 97.1% and 88.8% vs. 82.4%, p = 0.28 and p = 0.56, separately), which demonstrated the efficacy and constancy of MMAC in improving engraftment.
Nonrelapse Mortality
NRM of the MMAC group was lower than the MAC group in the retrospective study (Fig. 1 c; 26.5% vs. 41.7%, p = 0.18). While in the prospective study, the rate of NRM was further reduced in the MMAC‐P group (Fig. 3 c; 17.8% vs. 26.5%, p = 0.22).
Figure 3.
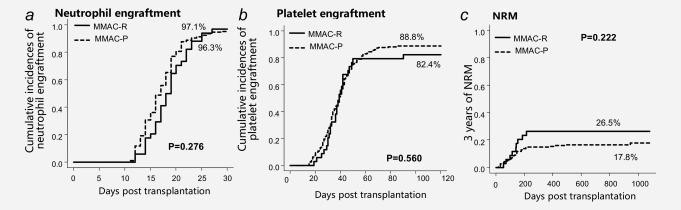
Engraftment and NRM after CBT in MMAC‐R group and MMAC‐P group in validation study.
We also analyzed the causes of NRM. Ten patients in MAC group, 9 in MMAC‐R group and 38 in MMAC‐P group died without relapse. The main factors leading to NRM were infection and GVHD. There were no statistical differences between these three groups in each cause.
Relapse and GVHD
The cumulative incidence of relapse by 3 years was lower in MMAC Group (5.9% vs. 12.5%, p = 0.37). However, in the validation study, relapse rate was higher in the MMAC‐P Group (23.4% vs. 5.9%, p = 0.03). Univariate analysis showed that MMAC‐P, more patients with ALL, younger age (aged 20 years or younger), worse disease status (CR3 or NR), male and more infused nuclear cells (infused 3.99 × 107/kg or more) were associated with higher rate of relapse. One locus of HLA mismatch was associated with lower relapse rate (Supporting Information, Table S4). In the multivariate analysis, more patients with AML/MDS and one locus of HLA mismatch were related to lower relapse rate (Supporting Information, Table S4; adjusted HR, 0.33 and 0.42; p = 0.03 and 0.04, separately). While male showed significant relation to higher relapse rate (Supporting Information, Table S4; adjusted HR, 2.83; p = 0.02).
Grade II to IV aGVHD, Grade III to IV aGVHD and cGVHD showed no statistical differences in both retrospective study and validation study.
Survival
In the retrospective study, 3 years of OS and DFS were significantly improved in the MMAC group (Figs. 2 a and 2 b; 67.6% vs. 45.8% and 67.6% vs. 45.8%, p < 0.05 and p < 0.05, separately). These differences were also significant in univariate analysis (Supporting Information, Table S2; HR, 0.45 and 0.45; 95%CI, 0.20–1.02 and 0.20–1.00; p = 0.06 and 0.05, separately) with borderline significance (p < 0.2). Besides, grade III to IV aGVHD was associated with the reduction of OS and DFS (Supporting Information, Table S2; HR, 5.89 and 5.58; 95%CI, 2.23–15.58 and 2.10–14.79; p = 0.00 and 0.00, separately). With grade III to IV aGVHD as a confounding factor, MMAC showed a remarkable relation with the increase of OS (Supporting Information, Table S2; HR, 0.36 and 0.37; 95%CI, 0.16–0.84 and 0.16–0.84; p = 0.02 and 0.02, separately). In the validation study, 3 years of OS and DFS were almost the same between the MMAC‐P group and MMAC‐R group (Figs. 4 a and 4 b; 68.3% vs. 67.6% and 68.3% vs. 58.9%, p = 0.52, separately).
Figure 2.
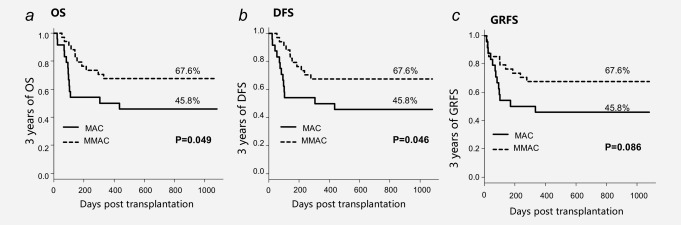
Survival after CBT in MAC group and MMAC group.
Figure 4.
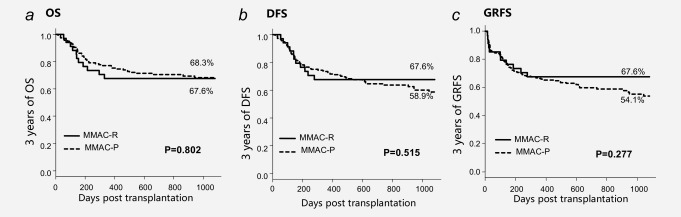
Survival after CBT in MMAC‐R group and MMAC‐P group in validation study.
Three years of GRFS was lower in the MAC group than MMAC group (Fig. 2 c; 45.8% vs. 67.6%; p = 0.09) in the retrospective study. In the prospective study, three years of GRFS was almost the same between MMAC‐P group and MMAC‐R group (Fig. 4 c; 54.1% vs. 67.6%, p = 0.28).
Immune reconstitution
In this study, we also analyzed the immune reconstitution of T cells and NK cells one month after transplantation. In the retrospective study, the proportion of CD3+ cells and CD8+ T cells accounting for lymphocytes was slightly higher in the MMAC group than MAC Group (57.7% vs. 35.2% and 40.0% vs. 20.8%, p = 0.16 and p = 0.25, separately). And there were significant differences in the proportion of CD4+ T cells and NK cells to lymphocytes between MMAC group and MAC Group (17.9% vs. 5.4% and 33.9% vs. 14.2, p = 0.01 and p < 0.05, separately).
In the validation study, there were no statistical differences between MMAC‐P group and MMAC‐R group in the proportion of CD3+ cells, CD4+ T cells, CD8+ T cells and NK cells accounting for lymphocytes (49.2% vs. 57.7%, 13.9% vs. 17.9%, 21.4% vs. 40.0% and 41.6% vs. 33.9%; p = 0.71, p = 0.25, p = 0.16 and p = 0.84, separately).
Discussion
In this study, we compare the prognosis of MMAC (Flu/Bu/Cy or CA/TBI/Cy) with MAC (Bu/Cy or TBI/Cy) in CBT for hematological malignancies (ALL/AML/MDS), and there are three main findings: MMAC significantly improved engraftment rate and overall survival and at the same time avoided the increase of NRM.
We found that MMAC without ATG or MTX for GVHD prophylaxis showed remarkable association with the increase of engraftment rates for both neutrophil and platelet (adjusted HR, 2.58 and 2.43; p < 0.01 and p = 0.01, separately). Similar to our results, some authors have reported that lower conditioning intensity is associated with delayed immune reconstitution and a higher incidence of GF.8, 9 Notably, conditioning type (TBI‐based or Bu‐based) was significantly different between the MAC group and MMAC group in the retrospective study, and TBI has always been a favorable factor in improving neutrophil engraftment in CBT.22, 23 To identify the relationship between conditioning type and neutrophil engraftment rate in this study, we did a univariate analysis, and there was no statistical difference (p = 0.77). The different proportion of TBI‐based conditioning in the two groups was not relatively related to the difference of neutrophil engraftment rate.
The improvement of engraftment may be attributed to the pharmacological function of Flu and CA. Flu is a nucleoside analog with significant immunosuppressive activity and has been substituted for Cy to develop high‐intensity conditioning with low toxicity.24 Besides, Flu inhibits DNA repair and thus is synergistic with alkylators that cause breaks in the DNA strand. The use of Flu in MAC for the CBT recipients, especially those with lower TNC does show improved neutrophil and platelet recovery.15 CA is a cell cycle‐specific antineoplastic agent that affects cells only in S‐phase. Although its mechanism of action is not entirely understood, its metabolite cytarabine triphosphate is believed to inhibit the action of DNA polymerase. It may also function as a false substrate when incorporated into DNA. Numbers of studies have demonstrated the superiority of CA added to TBI/Cy.25, 26, 27, 28 Furthermore, granulocyte colony‐stimulating factor is believed to increase the susceptibility of myeloid leukemic cells to CA, thereby contributing to decreased relapse rate.27, 28 The addition of Flu and CA to the conventional MAC enhances the intensity of the conditioning program and further eradicates the residual host lymphocytes, which may not only “create enough space” for the engraftment of CB‐derived T cells but also weaken the immunological rejection induced by the host T cells surviving conditioning.
The omission of ATG and MTX may protect the naïve T cells. Predominant T‐cell reconstitution in the early post‐transplant period is mediated by the CB‐derived naïve T cells which are at a small number and easily rejected.10 The use of ATG could impair the early development of donor‐derived T cells due to the persistence of these antibodies in the patient for several weeks after administration especially if given too close to graft infusion.11 Similarly, use of MTX is also associated with delayed engraftment and increased risk of graft failure.14, 15 Reducing the intensity of the GVHD prophylaxis regimen by avoiding the use of ATG and MTX will relatively “protect” the naïve T cells from the toxicity of these two drugs and achieved a higher rate of engraftment.29 In this study, we also found that CD4+ T cells and NK cells of MMAC group grew faster than MAC group at one month after transplantation, which may be related with the omission of ATG in MMAC group. T‐cell immune reconstitution has been associated with the use of ATG shortly before or after infusion of CB as ATG will increase the depletion of T cells and prohibit the proliferation of CB.30 The reduction of ATG after CBT could achieve an early CD4+ T‐cell immune reconstitution and no exposure to ATG results in even exceptional immune reconstitution potential, as recently shown.31 NK cells typically recover within the first month after transplant and are therefore one of the first lymphocytes to recover after transplant, regardless of stem‐cell source.32 Due to the delay of T‐cell immune recovery, NK cells are a vital lymphocyte subset exerting GVL effects and have been associated with superior OS, less CMV infection and reduced relapse rate.33 Zhao et al. have reported that NK‐cell immune reconstitution after haplo‐identical HCT was affected by conditioning regimen, immune suppression therapy and level of T‐cell depletion.34 However, factors influencing NK‐cell recovery after CBT remain unclear, and more studies are needed to elucidate the rapid growth of NK cells in MMAC group.
In recent years, increased attention has been paid to the presence of DSA. Recent studies have shown the close relationship between DSA in the recipient and graft failure in HCT.35, 36 Owing to the lack of related data in our database, we did not perform analysis of the role of DSA in neither the MAC group nor the MMAC group. A large‐scale prospective multicenter study including the analysis of DSA is needed.
We showed that MMAC was significantly associated with the improvement of OS and DFS, which may be attributed to the low incidence of relapse and NRM of the MMAC group. It is widely acknowledged that relapse generally results from residual malignant cells that survived the preparative regimen and are not eliminated by the graft‐versus‐leukemia (GVL) effect. The reduction of relapse rate in the MMAC group was relatively the result of the antileukemia effects of both the conditioning regimens and the cord blood. On the one hand, with the addition of Flu and CA, MMAC can eradicate the residual host lymphocytes more thoroughly, which make it difficult for the host malignant cells to survive from the conditioning regimen. On the other hand, CB has a powerful GVL effect which is crucial in eliminating the drug‐resistant malignant cells. Milano et al.37 reported that among patients with pretransplantation minimal residual disease, OS after CBT was as favorable as HCT with HLA‐matched unrelated donor and much higher than HCT with HLA‐mismatched unrelated donor, and the probability of relapse was lower in the cord‐blood group than in either of the other groups, which indicated that cord blood may have a stronger GVL effect than other graft sources. In this study, we found that both relapse rate and cGVHD rate were lower in the MMAC group, which seemed to be inconsistent with the previous reports as some authors believed that GVL effect was closely related to cGVHD.38, 39, 40 Nevertheless, most of these studies were designed for HCT with matched sibling donors, haploidentical‐related donors and unrelated donors, the GVL effects of CBT may work in a different way. Despite the possible GVL effect, cGVHD was reported to have an adverse effect on NRM41 and was the main cause of poor quality of life after transplantation.42 However, low probability of cGVHD has always been one of the advantages of CB,43 and many studies reported that the relapse rate in CBT was similar to or lower than in other kinds of HCT,44, 45, 46 which reflected the clinical separation of cGVHD and the GVL effect in CB. Furthermore, our previous study showed that long‐term survivors with CBT had less cGVHD and higher quality of life compared to HCT in HLA‐identical sibling's donors.47 Thus, our protocol of MMAC without ATG not only makes full use of the GVL effects of CBT but also keeps its advantage of low probability of cGVHD.
In terms of the complications, MMAC was not associated with an increase in the cumulative incidence of 3‐year NRM when compared with MAC (Supporting Information, Table S3; 26.5% vs. 41.7%, p = 0.18) and causes of NRM were comparable between the two groups. Also, in the validation study, NRM of the MMAC‐P group was similar to that of the MMAC‐R group (Supporting Information, Table S3; 17.8% vs. 26.5%, p = 0.22). There are no more fatal complications induced by the intensification of the conditioning regimen. The cumulative incidence of grade II to IV aGVHD and grade III to IV aGVHD were identical between the MAC group, the MMAC‐R group and the MMAC‐P group, which indicated that the complications of MMAC were comparable to MAC.
The relapse rate of the MMAC group was lower than the MAC Group (5.9% vs. 12.5%, p = 0.37). However, in the validation study, relapse rate was higher in the MMAC‐P Group (23.4% vs. 5.9%, p = 0.03), which may be related to the higher proportion of patients with ALL in the MMAC‐P Group (69.1% vs. 47.1%, p = 0.02). Some authors have reported that compared to patients with AML/MDS, those with ALL have a higher incidence of extramedullary relapse.48, 49 In this study, we also found that patients with AML/MDS had a lower relapse rate than patients with ALL (adjusted HR, 0.33; p = 0.03), which indicated that diagnosis of ALL was an independent risk factor for relapse in CBT. However, our center is the largest and most experienced center for CBT in China, different methods have been developed to prevent and treat post‐CBT relapse, such as monitoring WT1 in the early phase after CBT and injecting interferon instantly when the value of WT1 is beyond the upper level set in our center. More works are needed to further modify our CBT protocol and lower down the relapse rate of patients with ALL.
In conclusion, MMAC significantly improves the neutrophil engraftment rate, the platelet engraftment rate, 3 years of OS and DFS without increasing NRM in single‐unit CBT for hematological malignancies (ALL/AML/MDS). However, the retrospective study has all the inherent biases and the sample size is small, and the prospective study is nonrandomized in consideration of ethical principles. A larger prospective multicenter study is needed to verify the outcomes shown in this study.
Supporting information
Supporting Information Table 1
Supporting Information Table 2
Supporting Information Table 3
Supporting Information Table 4
Acknowledgement
The authors thank all the physicians and staff at the hospitals and the cord blood banks in China for their help in this study and Joseph Rosenthal, MD for assistance in editing this article.
Conflict of Interest: The authors have nothing to disclose.
References
- 1. Munoz J, Shah N, Rezvani K, et al. Concise review: umbilical cord blood transplantation: past, present, and future. Stem Cell Transl Med 2014; 3:1435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rocha V, Labopin M, Sanz G, et al. Transplants of umbilical‐cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med 2004; 351:2276–85. [DOI] [PubMed] [Google Scholar]
- 3. Eapen M, Rocha V, Sanz G, et al. Effect of graft source on unrelated donor haemopoietic stem‐ cell transplantation in adults with acute leukemia: a retrospective. Lancet Oncol 2010; 11:653–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singh H, Nikiforow S, Li S, et al. Outcomes and management strategies for graft failure after umbilical cord blood transplantation. Am J Hematol 2014; 89:1097–101. [DOI] [PubMed] [Google Scholar]
- 5. Barker JN, Scaradavou A, Stevens CE. Combined effect of total nucleated cell dose and HLA match on transplantation outcome in 1061 cord blood recipients with hematologic malignancies. Blood 2010; 115:1843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cutler C, Kim HT, Sun L, et al. Donor‐specific anti‐HLA antibodies predict outcome in double umbilical cord blood transplantation. Blood 2011;118:6691–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Olsson R, Remberger M, Schaffer M, et al. Graft failure in the modern era of allogeneic hematopoietic SCT. Bone Marrow Transplant 2013; 48:537–43. [DOI] [PubMed] [Google Scholar]
- 8. Satwani P, Jin ZZ, Duffy D, et al. Transplantation‐related mortality, graft failure, and survival after reduced‐toxicity conditioning and allogeneic hematopoietic stem cell transplantation in 100 consecutive pediatric recipients. Biol Blood Marrow Transplant. 2013; 19:552–61. [DOI] [PubMed] [Google Scholar]
- 9. Narimatsu H, Watanabe M, Kohno A, et al. High incidence of graft failure in unrelated cord blood transplantation using a reduced‐intensity preparative regimen consisting of fludarabine and melphalan. Bone Marrow Transplant 2008;41:753–6. [DOI] [PubMed] [Google Scholar]
- 10. Politikos I, Boussiotis VA. The role of the thymus in T‐cell immune reconstitution after umbilical cord blood transplantation. Blood 2014; 124:3201–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pascal L, Tucunduva L, Ruggeri A, et al. Impact of ATG‐containing reduced‐intensity conditioning after single‐ or double‐unit allogeneic cord blood transplantation. Blood 2015; 126:1027–32. [DOI] [PubMed] [Google Scholar]
- 12. Pascal L, Mohty M, Ruggeri A, et al. Impact of rabbit ATG‐containing myeloablative conditioning s on the outcome of patients undergoing unrelated single‐unit cord blood transplantation for hematological malignancies. Bone Marrow Transplant 2015; 50:45–50. [DOI] [PubMed] [Google Scholar]
- 13. Sauter C, Abboud M, Jia XY, et al. Serious infection risk and immune recovery after double unit cord blood transplantation without anti‐thymocyte globulin. Biol Blood Marrow Transplant 2011; 17:1460–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hamilton BK, Rybicki L, Dean R, et al. Cyclosporine in combination with mycophenolate mofetil versus methotrexate for graft versus host disease prevention in myeloablative HLA‐identical sibling donor allogeneic hematopoietic cell transplantation. Am J Hematol 2015; 90:144–8. [DOI] [PubMed] [Google Scholar]
- 15. Petropoulou AD, Rocha V. Risk factors and options to improve engraftment in unrelated cord blood transplantation. Stem Cells Int. 2011;2011:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu HL, Sun ZM, Geng LQ, et al. Unrelated cord blood transplantation for newly diagnosed patients with severe acquired aplastic anemia using a reduced intensity conditioning: high graft rejection, but good survival. Bone Marrow Transplant 2012; 47:1186–90. [DOI] [PubMed] [Google Scholar]
- 17. Ruggeri A, Labopin M, Sormani MP, et al. Engraftment kinetics and graft failure after single umbilical cord blood transplantation using a myeloablative conditioning regimen. Haematologica 2014; 99:1509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft‐versus‐host disease in human recipients of marrow from HLA‐matched sibling donors. Transplantation 1974; 18:295–304. [DOI] [PubMed] [Google Scholar]
- 19. Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15:825–8. [PubMed] [Google Scholar]
- 20. Filipovich AH, Weisdorf D, Pavletic S, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft‐versus‐host disease: I. diagnosis and staging working group report. Biol Blood Marrow Transplant 2005;11:945–56. [DOI] [PubMed] [Google Scholar]
- 21. Holtan SG, Defor TE, Lazaryan A, et al. Composite endpoint of graft‐versus‐host disease‐free, relapse‐free survival after allogeneic hematopoietic cell transplantation. Blood 2015; 125:1333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nakason H, Fuji S, Yakushijin K, et al. Impact of total body irradiation on successful neutrophil engraftment in unrelated bone marrow or cord blood transplantation. Am J Hematol 2017; 92:171–8. [DOI] [PubMed] [Google Scholar]
- 23. Madureira AB, Eapen M, Locatelli F, et al. Analysis of risk factors influencing outcome in children with myelodysplastic syndrome after unrelated cord blood transplantation. Leukemia 2011; 25:449–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu H, Zhai X, Song ZY, et al. Busulfan plus fludarabine as a myeloablative conditioning regimen compared with busulfan plus cyclophosphamide for acute myeloid leukemia in first complete remission undergoing allogeneic hematopoietic stem cell transplantation: a prospective and multicenter study. J Hematol Oncol 2013; 6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arai Y, Takeda J, Aoki K, et al. Efficiency of high‐dose cytarabine added to CY/TBI in cord blood transplantation for myeloid malignancy. Blood 2015; 126:415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mori T, Aisa Y, Kato J, et al. Safety and efficacy of total body irradiation, cyclophosphamide, and cytarabine as a conditioning regimen for allogeneic hematopoietic stem cell transplantation in patients with acute lymphoblastic leukemia. Am J Hematol 2012; 87:349–53. [DOI] [PubMed] [Google Scholar]
- 27. Mori T, Tanaka M, Kobayashi T, et al. Prospective multicenter study of single‐unit cord blood transplantation with myeloablative conditioning for adult patients with high‐risk hematologic malignancies. Biol Blood Marrow Transplant 2013; 19:486–91. [DOI] [PubMed] [Google Scholar]
- 28. Konuma T, Takahashi S, Uchida N, et al. Effect of granulocyte colony‐ stimulating factor‐combined conditioning in cord blood transplantation for myelodysplastic syndrome and secondary acute myeloid leukemia: a retrospective study in japan. Biol Blood Marrow Transplant 2015; 21:1632–40. [DOI] [PubMed] [Google Scholar]
- 29. Danby R, Rocha V. Improving engraftment and immune reconstitution in umbilical cord blood transplantation. Front Immunol 2014; 5:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Admiraal R, van Kesteren C, Jol‐van der Zijde CM, et al. Association between anti‐thymocyte globulin exposure and CD4+ immune reconstitution in paediatric haemopoietic cell transplantation: a multicentre, retrospective pharmacodynamic cohort analysis. Lancet Haematol 2015;2:e194. [DOI] [PubMed] [Google Scholar]
- 31. Admiraal R, Lindemans CA, Van KC, et al. Excellent T‐cell reconstitution and survival provided ATG exposure is low or absent after pediatric cord blood transplantation. Blood 2016; 128:2734–41. [DOI] [PubMed] [Google Scholar]
- 32. Rénard C, Barlogis V, Mialou V, et al. Lymphocyte subset reconstitution after unrelated cord blood or bone marrow transplantation in children. Br J Haematol 2011; 152:322–30. [DOI] [PubMed] [Google Scholar]
- 33. Dickinson AM, Norden J, Li S, et al. Graft‐versus‐leukemia effect following hematopoietic stem cell transplantation for leukemia. Front Immunol. 2017; 8:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao XY, Chang YJ, Huang XJ. Conflicting impact of alloreactive NK cells on transplantation outcomes after haploidentical transplantation: do the reconstitution kinetics of natural killer cells create these differences? Biol Blood Marrow Transplant 2011;17:1436–42. [DOI] [PubMed] [Google Scholar]
- 35. Ruggeri A, Rocha V, Masson E, et al. Impact of donor‐specific anti‐HLA antibodies on graft failure and survival after reduced intensity conditioning‐unrelated cord blood transplantation: a Eurocord, Société Francophone d'Histocompatibilité et d'Immunogénétique (SFHI) and Société Française de Greffe de Moelle et de Thérapie Cellulaire (SFGM‐TC) analysis. Haematologica 2013;98:1154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sarah MZ, Pascale L, Jean‐Luc T, et al. Donor‐specific anti‐HLA antibodies in allogeneic hematopoietic stem cell transplantation. Front Immunol 2016; 7:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Filippo M, Ted G, Brent W, et al. Cord‐blood transplantation in patients with minimal residual disease. N Engl J Med 2016; 375:944–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baron F, Labopin M, Niederwieser D, et al. Impact of graft‐versus‐host disease after reduced‐intensity conditioning allogeneic stem cell trans‐ plantation for acute myeloid leukemia: a report from the Acute Leukemia Working Party of the European group for blood and marrow transplantation. Leukemia. 2012;26:2462–8. [DOI] [PubMed] [Google Scholar]
- 39. Signori A, Crocchiolo R, Oneto R, et al. Chronic GVHD is associated with lower relapse risk irrespective of stem cell source among patients receiving transplantation from unrelated donors. Bone Marrow Transplant 2012; 47:1474–8. [DOI] [PubMed] [Google Scholar]
- 40. Saillard C, Crocchiolo R, Furst S, et al. National Institutes of Health classification for chronic graft‐versus‐host disease predicts outcome of allo‐hematopoietic stem cell transplant after fludarabine‐busulfan‐antithymocyte globulin conditioning regimen. Leuk Lymphoma 2014; 55:1106–12. [DOI] [PubMed] [Google Scholar]
- 41. Weisdorf D, Zhang MJ, Arora M, et al. Graft‐versus‐host disease induced graft‐versus‐leukemia effect: greater impact on relapse and disease‐free survival after reduced intensity conditioning. Biol Blood Marrow Transplant 2012; 18:1727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Richard JS, Manuel R, Alejandro L, et al. Physical function and quality of life in patients with chronic graft‐versus‐host‐disease: a summary of preclinical and clinical studies and a call for exercise intervention trials in patients. Bone Marrow Transplant 2016; 51:13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sarvaria A, Basar R, Mehta RS, et al. IL‐10+ regulatory B cells are enriched in cord blood and may protect against cGVHD after cord blood transplantation. Blood 2016; 128:1346–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mo XD, Tang BL, Zhang XH, et al. Comparison of outcomes after umbilical cord blood and unmanipulated haploidentical hematopoietic stem cell transplantation in children with high‐risk acute lymphoblastic leukemia. Int J Cancer 2016; 139:2106–15. [DOI] [PubMed] [Google Scholar]
- 45. Zheng CC, Zhu XY, Tang BL, et al. Clinical separation of cGvHD and GvL and better GvHD‐free/relapse‐free survival (GRFS) after unrelated cord blood transplantation for AML. Bone Marrow Transplant 2017; 52:88–94. [DOI] [PubMed] [Google Scholar]
- 46. Ruggeri A, Labopin M, Sanz G, et al. Comparison of outcomes after unrelated cord blood and unmanipulated haploidentical stem cell transplantation in adults with acute leukemia. Leukemia 2015; 29:1891–900. [DOI] [PubMed] [Google Scholar]
- 47. Liu HL, Sun ZM, Geng LQ, et al. Similar survival, but better quality of life after myeloablative transplantation using unrelated cord blood vs matched sibling donors in adults with hematologic malignancies. Bone Marrow Transplant 2016; 49:1063–9. [DOI] [PubMed] [Google Scholar]
- 48. Ge L, Ye F, Mao XL, et al. Extramedullary relapse of acute leukemia after allogeneic hematopoietic stem cell transplantation: different characteristics between acute myelogenous leukemia and acute lymphoblastic leukemia. Biol Blood Marrow Transplant 2014; 20:1040–7. [DOI] [PubMed] [Google Scholar]
- 49. Shem‐Tov N, Saraceni F, Danylesko I, et al. Isolated extramedullary relapse of acute leukemia after allogeneic stem‐cell transplantation: different kinetics and better prognosis than systemic relapse. Biol Blood Marrow Transplant 2017; 15:59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table 1
Supporting Information Table 2
Supporting Information Table 3
Supporting Information Table 4


