Abstract
Background
Studies comparing upfront surgery with neoadjuvant treatment in pancreatic cancer may report only patients who underwent resection and so survival will be skewed. The aim of this study was to report survival by intention to treat in a comparison of upfront surgery versus neoadjuvant treatment in resectable or borderline resectable pancreatic cancer.
Methods
MEDLINE, Embase and the Cochrane Library were searched for studies reporting median overall survival by intention to treat in patients with resectable or borderline resectable pancreatic cancer treated with or without neoadjuvant treatment. Secondary outcomes included overall and R0 resection rate, pathological lymph node rate, reasons for unresectability and toxicity of neoadjuvant treatment.
Results
In total, 38 studies were included with 3484 patients, of whom 1738 (49·9 per cent) had neoadjuvant treatment. The weighted median overall survival by intention to treat was 18·8 months for neoadjuvant treatment and 14·8 months for upfront surgery; the difference was larger among patients whose tumours were resected (26·1 versus 15·0 months respectively). The overall resection rate was lower with neoadjuvant treatment than with upfront surgery (66·0 versus 81·3 per cent; P < 0·001), but the R0 rate was higher (86·8 (95 per cent c.i. 84·6 to 88·7) versus 66·9 (64·2 to 69·6) per cent; P < 0·001). Reported by intention to treat, the R0 rates were 58·0 and 54·9 per cent respectively (P = 0·088). The pathological lymph node rate was 43·8 per cent after neoadjuvant therapy and 64·8 per cent in the upfront surgery group (P < 0·001). Toxicity of at least grade III was reported in up to 64 per cent of the patients.
Conclusion
Neoadjuvant treatment appears to improve overall survival by intention to treat, despite lower overall resection rates for resectable or borderline resectable pancreatic cancer.
PROSPERO registration number: CRD42016049374.
Short abstract
Improved survival with neoadjuvant treatment
Introduction
Pancreatic cancer is recognized as having an overall poor prognosis and low resection rate. Long‐term survival remains limited even after tumour resection. Surgical resection with adjuvant chemotherapy is the current standard of care1. Recent trials1, 2 have reported improved median overall survival to 24·5–28 months with adjuvant treatment. However, these trials did not report how many eligible patients were fit enough to be randomized to receive adjuvant chemotherapy. Currently, the strongest predictors of survival include surgery with curative intent, early‐stage disease and complete (R0) resection3, 4. None of these predictors are influenced by adjuvant treatment.
In patients with resectable pancreatic cancer, a recent study5 of Surveillance, Epidemiology, and End Results (SEER) data from nearly 4000 patients suggested a survival benefit with neoadjuvant radiotherapy with or without chemotherapy over upfront surgery with or without adjuvant treatment. However, RCTs of neoadjuvant treatment compared with upfront surgery are lacking. Non‐randomized studies evaluating neoadjuvant treatment of patients with either borderline resectable or upfront resectable pancreatic cancer often suffer from selection bias because they report survival data only for patients who eventually underwent pancreatic resection. Patients with disease progression or severe toxicity who did not undergo resection are often excluded. Moreover, patients found to have metastatic or unresectable disease at exploratory surgery are also excluded5, 6.
The aim of this study was to perform a systematic review of studies comparing median overall survival of patients who underwent upfront surgery versus those who underwent neoadjuvant treatment in intention‐to‐treat analyses.
Methods
The systematic review was performed according to the PRISMA guidelines7. The review was registered at PROSPERO (registration number: CRD42016049374).
Search strategy
The literature was reviewed systematically by searching in MEDLINE, Embase and the Cochrane Library for studies published between 1 January 2000 and 6 December 2016. The search strategy included the following domains of Medical Subject Heading (MeSH) terms: ‘pancreatic neoplasm’, ‘survival’, ‘mortality’ and ‘survival analysis’; these were combined with ‘AND’ or ‘OR’. No language restrictions were used. For the MEDLINE and Embase searches, a McMaster specific prognosis filter was applied, completed with the authors' own terminology to cover the survival concept of the search strategy. A full description of the search is available in Appendix S1 (supporting information).
Eligibility
Studies including patients with resectable or borderline resectable pancreatic cancer, either treated by upfront surgery or with neoadjuvant treatment, and reporting median overall survival by intention to treat (based on the initial treatment assignment and not on the treatment eventually received) were included. No selection was made based on adjuvant treatment. Excluded were review articles, notes, letters, case reports (5 or fewer patients), animal studies, studies that did not report median overall survival by intention to treat, and studies that reported on only specific groups of patients (for example, those with renal impairment, older than 70 years, or with poor performance status). Studies that did not report median overall survival separately for resectable and borderline resectable pancreatic tumours were also excluded.
Study selection
Two authors screened the titles and abstracts independently for eligibility. After the first two rounds of screening, full‐text screening was carried out. Disagreements were resolved by discussion and consensus achieved. Primary and secondary outcomes were extracted from the full text. If studies had an overlapping cohort, the most recent study was included.
Methodological quality
All studies were assessed for risk of bias using a standard list of 11 potential risks of bias, based on the Oxford Centre for Evidence‐Based Medicine (CEBM) Critical Appraisal Skills Programme checklists for randomized trials and observational cohort studies, and the Cochrane Collaboration's tool for assessing risk of bias8, 9, 10, 11. All studies were graded according to the Oxford CEBM levels of evidence12.
Outcome measures
The primary outcome, median overall survival, was extracted from the included articles. Data on numbers of patients with (borderline) resectable pancreatic cancer, resectability criteria (for example, those of the National Comprehensive Cancer Network (NCCN) and American Hepato‐Pancreato‐Biliary Association (AHPBA)), and types of neoadjuvant treatment and adjuvant treatment were obtained. Secondary outcomes were: resection rate, completeness of resection (R0 resection rate, only for patients undergoing resection), pathological lymph node rate, reasons for unresectability, and toxicity of at least grade III after neoadjuvant treatment.
Statistical analyses
The weighted median overall survival was calculated for the studies reporting this information for groups with and without neoadjuvant treatment. The weighted estimate of median survival (m p) of both groups was derived by the formula used by Gillen and colleagues13 in a previous systematic review:
where m i denotes the median survival in a study population i (with i ranging from 1 to k, where k is the number of included studies) and w i refers to a study‐specific weight function. The number of study participants (divided by the total number of evaluable patients) was used as the weight.
The overall resection rate and the R0 rate for both groups were also calculated. The R0 rate was calculated for all patients and also for those who actually underwent resection of the pancreatic cancer. For both the overall resection rate and the R0 rate, the 95 per cent confidence interval was calculated using a proportion calculator14. The significance of differences in proportions was assessed by means of two‐tailed Fisher's exact test, with a significance level α = 0·050, using SPSS® version 22.0.0.2 (IBM, Armonk, New York, USA).
Results
A total of 18 828 records were identified, of which 122 screened were fully. Finally, 38 studies15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52 were included, with 3484 patients (Fig. 1). Study characteristics are summarized in Tables 1 and 2. Three RCTs, nine phase I or II trials, 12 prospective cohort studies and 14 retrospective cohort studies were included. The range of median age was 61·9–69·0 years in the upfront surgery group and 59–73 years in the neoadjuvant group (Tables 3 and 4). Overall, neoadjuvant treatment was administered to 1723 of 1738 patients (99·1 per cent). All studies used at least chemotherapy as neoadjuvant treatment, usually including gemcitabine (26 of 35 studies). Radiotherapy was given as part of the neoadjuvant treatment in 29 of 35 studies. No study used radiotherapy as the sole neoadjuvant treatment. The radiation dose ranged from 30 to 54 Gy.
Figure 1.
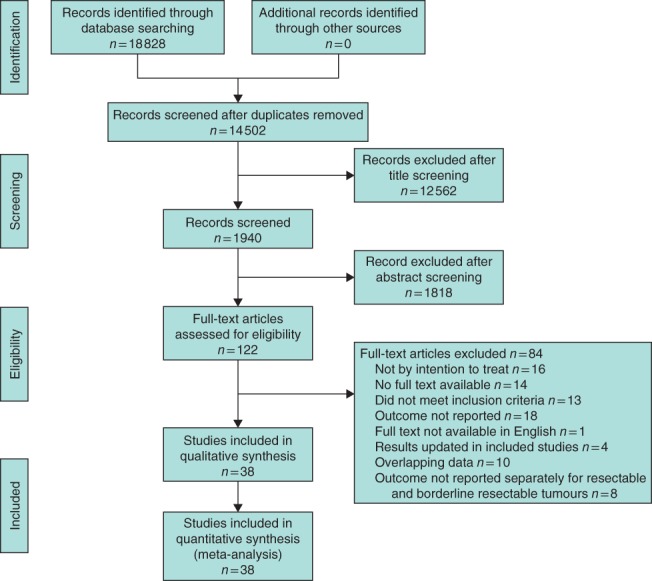
PRISMA flow chart showing selection of articles for review
Table 1.
Characteristics of 12 included studies that reported median overall survival after upfront surgery
| Reference | No. of patients | Country | Study design | Tumour | R0 criteria (mm)* | Adjuvant treatment initiated (%)† | Adjuvant treatment completed (%) |
|---|---|---|---|---|---|---|---|
| Casadei et al. 15 | 20 | Italy | RCT | R | > 1 | 22 | n.r. |
| Golcher et al. 16 | 33 | Germany | RCT | R | n.s. | 44 | n.r. |
| Bao et al. 17 | 78 | USA | Prospective | R | n.s. | 78 | n.r. |
| Raptis et al. 18 | 102 | UK | Prospective | R | n.r. | n.r. | n.r. |
| Tzeng et al. 19 | 52 | USA | Prospective | R | n.s. | n.r. | 60 |
| Fujii et al. 20 | 71 | Japan | Prospective | BR | > 1 | 100 | 42 |
| Fujii et al. 21 | 233 | Japan | Prospective | R | > 1 | 69 | 45·6 |
| Barbier et al. 22 | 85 | France | Retrospective | R | > 1 | 58 | n.r. |
| Papalevoza et al. 23 | 92 | USA | Retrospective | R | n.s. | Adjuvant CRT: 66 | n.r. |
| Kato et al. 24 | 624 | Japan | Retrospective | BR | n.s. | 78·7 | n.r. |
| Adjuvant CT only: 69·9 | |||||||
| Hirono et al. 25 | 331 | Japan | Retrospective | R + BR | 0 | BR‐A: 84·5 | 76 |
| Murakami et al. 26 | 25 | Japan | Retrospective | BR | n.s. | 48 | n.r. |
Definition of R0: > 1, more than 1 mm clearance from each margin; 0, no cancer cells along any margin.
Among patients who underwent resection of pancreatic cancer. R, resectable; n.r., not reported; n.s., not specified; prospective, prospective cohort study; BR, borderline resectable; retrospective, retrospective cohort study; CRT, chemoradiotherapy; CT, chemotherapy; BR‐A, borderline resectable with arterial involvement.
Table 2.
Characteristics of the 35 included studies that report median overall survival after neoadjuvant treatment
| Reference | No. of patients | Country | Study design | Tumour | R0 criteria (mm)* | Neoadjuvant treatment | Adjuvant treatment initiated (%)† | Adjuvant treatment completed (%) |
|---|---|---|---|---|---|---|---|---|
| Palmer et al. 27 | 50 | UK | RCT | R | n.s. | CT | n.r. | n.r. |
| Casadei et al. 15 | 18 | Italy | RCT | R | > 1 | CRT | 75 | n.r. |
| Golcher et al. 16 | 33 | Germany | RCT | R | n.s. | CRT | 37 | n.r. |
| Evans et al. 28 | 86 | USA | Phase II | R | 0 | CRT | n.r. | n.r. |
| Heinrich et al. 29 | 28 | Switzerland | Phase II | R | n.s. | CT | n.r. | n.r. |
| Le Scodan et al. 30 | 41 | France | Phase II | R | n.s. | CRT | n.r. | n.r. |
| Turrini et al. 31 | 34 | France | Phase II | R | 0 | CRT | n.r. | n.r. |
| Small et al. 32 | 17 | USA | Phase II | R + BR | n.s. | CRT | n.r. | n.r. |
| Esnaola et al. 33 | 13 | USA | Phase II | BR | n.s. | Mixed | n.r. | n.r. |
| Kim et al. 34 | 62 | USA | Phase II | R + BR | n.s. | CRT | 63 | 92 |
| O'Reilly et al. 35 | 38 | USA | Phase II | R | n.s. | CT | 96 | 89 |
| Shaib et al. 36 | 13 | USA | Phase I | BR | n.s. | CRT | n.r. | n.r. |
| Calvo et al. 37 | 15 | Spain | Prospective | R | n.s. | CRT | n.r. | n.r. |
| Ohigashi et al. 38 | 38 | Korea | Prospective | BR | n.s. | CRT | 100 | 100 |
| Katz et al. 39 | 22 | USA | Prospective | BR | 0 | CRT | 67 | 90 |
| Oh et al. 40 | 38 | Korea | Prospective | BR | n.s. | CRT | 61 | n.r. |
| Tzeng et al. 41 | 141 | USA | Prospective | BR | n.s. | CRT | n.r. | n.r. |
| Tzeng et al. 19 | 115 | USA | Prospective | R | n.s. | CRT | 7·8 | n.r. |
| Fujii et al. 20 | 21 | Japan | Prospective | BR | > 1 | CRT | 100 | 56 |
| Fujii et al. 21 | 40 | Japan | Prospective | R | > 1 | CRT | 83 | 56 |
| Ielpo et al. 42 | 11 | Spain | Prospective | BR | n.s. | CT | 100 | n.r. |
| Masui et al. 43 | 18 | Japan | Prospective | BR | > 1 | CT | 93 | n.r. |
| Takai et al. 44 | 32 | Japan | Retrospective | R | n.s. | CRT | n.r. | n.r. |
| Barbier et al. 22 | 88 | France | Retrospective | R | > 1 | CRT | n.r. | n.r. |
| Patel et al. 45 | 18 | USA | Retrospective | BR | 0 | CRT | n.r. | n.r. |
| Papalevoza et al. 23 | 144 | USA | Retrospective | R | n.s. | CRT | 32·9 | n.r. |
| Chuong et al. 46 | 57 | USA | Retrospective | BR | 0 | CRT | 84 | n.r. |
| Dholakia et al. 47 | 50 | USA | Retrospective | BR | 0 | CRT | 42 | n.r. |
| Boone et al. 48 | 61 | USA | Retrospective | R + BR | n.s. | Mixed | n.r. | n.r. |
| Rose et al. 49 | 64 | USA | Retrospective | BR | > 1 | CT/CRT | 90 | n.r. |
| Moningi et al. 50 | 14 | USA | Retrospective | BR | n.s. | CRT | n.r. | n.r. |
| Sho et al. 51 | 99 | Japan | Retrospective | R + BR | n.s. | CT/CRT | n.r. | R: 75 |
| BR‐V: 49 | ||||||||
| BR‐A: 31 | ||||||||
| Rashid et al. 52 | 121 | USA | Retrospective | BR | 0 | CRT | n.r. | n.r. |
| Hirono et al. 25 | 46 | Japan | Retrospective | BR | 0 | Mixed | 85 | 61 |
| Murakami et al. 26 | 52 | Japan | Retrospective | BR | n.s. | CT | 79 | n.r. |
Definition of R0: > 1, more than 1 mm clearance from each margin; 0, no cancer cells along any margin.
Among patients who underwent resection of pancreatic cancer. R, resectable; n.r., not reported; n.s., not specified; CT, chemotherapy; CRT, chemoradiotherapy; BR, borderline resectable; prospective, prospective cohort study; retrospective, retrospective cohort study; BR‐V, borderline resectable with venous involvement; BR‐A, borderline resectable with arterial involvement.
Table 3.
Median overall survival, resection rate and R0 rate after upfront surgery reported in 12 studies
| Reference | No. of patients | Median age (years) | Median OS (months) | Resection rate, ITT (%) | R0 rate* (%) | Patients with positive lymph nodes (%)* |
|---|---|---|---|---|---|---|
| Casadei et al. 15 | 20 | 67·5 | 19·5 | 75 | 33 | 87 |
| Golcher et al. 16 | 33 | 65·1 | 14·4 | 70 | 70 | 57 |
| Bao et al. 17 | 78 | 68† | 17·9 | 77 | 75 | 58 |
| Raptis et al. 18 | 102 | 64‡ | 12 | 32·7 | n.r. | n.r. |
| Tzeng et al. 19 | 52 | 61·9 | 25·3 | 92 | 81 | 81 |
| Fujii et al. 20 | 71 | 63 | 13·1 | 70 | 40 | 92 |
| Fujii et al. 21 | 233 | 67 | 23·5 | 87·6 | 70·1 | 71 |
| Barbier et al. 22 | 85 | 64 | 17 | 79 | 67 | 64 |
| Papalezova et al. 23 | 92 | 65† | 13 | 74 | 79 | 62 |
| Kato et al. 24 | 624 | 63·8 | 12·6 | 86·4 | 65·9 | 57 |
| Hirono et al. 25 | 331 | R: n.r. | R: 20·9 | R: 89·5 | R: n.r. | R: n.r. |
| BR‐V: n.r. | BR‐V: 16·3 | BR‐V: 92 | BR‐V: n.r. | BR‐V: n.r. | ||
| BR‐A: 69§ | BR‐A: 12·4 | BR‐A: 83·1 | BR‐A: 62·1 | BR‐A: 74·8 | ||
| Murakami et al. 26 | 25 | 67§ | 11·6 | 92 | 17 | 78 |
| Total | 1746 | Range 61·9–69 | 14·8 | 81·3 (79·4, 83·1) | 66·9 (64·2, 69·6) | 64·8 (62·0, 67·5) |
Values in parentheses are 95 per cent confidence intervals.
Among patients who underwent resection of pancreatic cancer.
Mean age.
Including patients with unresectable pancreatic tumours, who were not reported separately.
Including patients who received neoadjuvant treatment. OS, overall survival; ITT, intention to treat; R, resectable; n.r., not reported; BR‐V, borderline resectable with venous involvement; BR‐A, borderline resectable with arterial involvement.
Table 4.
Median overall survival, resection rate and R0 rate after neoadjuvant treatment reported in 35 studies
| Reference | No. of patients | Median age (years) | Median OS (months) | Resection rate ITT (%) | R0 rate (%)* | Patients with positive lymph nodes (%)* |
|---|---|---|---|---|---|---|
| Palmer et al. 27 | 50 | 66 | 13·6 | 54 | 74 | 56 |
| Casadei et al. 15 | 18 | 71·5 | 22·4 | 61 | 64 | 55 |
| Golcher et al. 16 | 33 | 62·5 | 17·4 | 58 | 90 | 32 |
| Evans et al. 28 | 86 | 65·8 | 22·7 | 74 | 89 | 38 |
| Heinrich et al. 29 | 28 | 59 | 26·5 | 89 | 80 | 64 |
| Le Scodan et al. 30 | 41 | 59·3 | 9·4 | 63 | 81 | 50 |
| Turrini et al. 31 | 34 | 61·5† | 15·5 | 50 | 100 | 24 |
| Small et al. 32 | 17 | 62‡ | R: 10·2 | R: 43 | n.r. | 0 |
| BR: 11·2 | BR: 30 | |||||
| Esnaola et al. 33 | 13 | 60 | 24·1 | 69 | 92 | n.r. |
| Kim et al.34 | 62 | 64‡ | R: 26·5 | R: 57 | 85 | 44 |
| BR: 18·4 | BR: 72 | |||||
| O'Reilly et al. 35 | 38 | 73 | 27·2 | 71 | 74 | 67 |
| Shaib et al. 36 | 13 | 64 | 11 | 62 | n.r. | 13 |
| Calvo et al. 37 | 15 | 61 | 10 | 60 | 78 | n.r. |
| Ohigashi et al. 38 | 38 | 66 | 32 | 82 | 97 | 10 |
| Katz et al. 39 | 22 | 64 | 21·7 | 68 | 93 | 33 |
| Oh et al. 40 | 38 | 59 | 21·2 | 61 | 78 | 4 |
| Tzeng et al. 41 | 141 | 63 | 19·1 | 59·6 | 91·7 | 48·8 |
| Tzeng et al. 19 | 115 | 65·5 | 28 | 82·6 | 89·5 | 51·5 |
| Fujii et al. 20 | 21 | 66 | 29·1 | 86 | 100 | 17 |
| Fujii et al. 21 | 40 | 65 | 24·9 | 90 | 86 | 39 |
| Ielpo et al. 42 | 11 | 61·8† | 20 | 73 | 100 | n.r. |
| Masui et al. 43 | 18 | 63 | 21·7 | 83 | 87 | 33 |
| Takai et al. 44 | 32 | 61·8 | 19·2 | 75 | n.r. | n.r. |
| Barbier et al. 22 | 88 | 65 | 15 | 43 | 92 | 29 |
| Patel et al. 45 | 18 | 67 | 15·6 | 50 | 89 | n.r. |
| Papalezova et al. 23 | 144 | 64 | 15 | 53·0 | 78·0 | 25 |
| Chuong et al. 46 | 57 | 64‡ | 16·4 | 56 | 97 | 34 |
| Dholakia et al. 47 | 50 | 63·5 | 17·2 | 58 | 93 | 28 |
| Boone et al. 48 | 61 | 64‡ | R: 20 | R: 95 | R: 86 | n.r. |
| BR: 22 | BR: 83 | BR: 70 | ||||
| Rose et al. 49 | 64 | 66 | 23·6 | 48 | 87 | 58 |
| Moningi et al. 50 | 14 | 67·2‡ | 14·4 | 29 | 100 | n.r. |
| Sho et al. 51 | 99 | R: 66·4† | R: 50·2 | R: 100 | R: 98 | n.r. |
| BR‐V: 66·3† | BR‐V: 26·6 | BR‐V: 97 | BR‐V: 97 | |||
| BR‐A: 66·0† | BR‐A: 18 | BR‐A: 84 | BR‐A: 81 | |||
| Rashid et al. 52 | 121 | 67 | 17 | 45·5 | 98·4 | 63·6 |
| Hirono et al. 25 | 46 | 69§ | 18 | 87 | 80 | 78 |
| Murakami et al. 26 | 52 | 67§ | 27·1 | 90 | 72 | 72 |
| Total | 1738 | Range 59–73 | 18·8 months | 66·0 (63·7, 68·2) | 86·8 (84·6, 88·7) | 43·8 (40·6, 47·1) |
Values in parentheses are 95 per cent confidence intervals.
Among patients who underwent resection of pancreatic cancer.
Mean age.
Including patients with unresectable pancreatic tumours, who were not reported separately.
Including patients who received upfront surgery. OS, overall survival; ITT, intention to treat; R, resectable; n.r., not reported; BR, borderline resectable; BR‐V, borderline resectable with venous involvement; BR‐A, borderline resectable with arterial involvement.
Adjuvant therapy was initiated in ten of 12 upfront surgery studies, and 68·6 per cent of patients who underwent resection started adjuvant treatment. In the neoadjuvant treatment group, adjuvant therapy was initiated in 18 of 35 studies, and 31 per cent of patients who had resection of the pancreatic tumour started adjuvant therapy. Fewer studies reported the numbers of patients who completed adjuvant therapy (Tables 1 and 2).
Methodological quality
Results of the methodological quality assessment of all studies are reported in Tables S1–S3 (supporting information). Most studies were retrospective (14) or prospective (12) cohort studies. The studies showed heterogeneity in treatment and potential bias in collecting data. A common risk of bias was the heterogeneity of neoadjuvant and adjuvant treatments within and between the studies. Furthermore, there was wide variation in the duration of follow‐up; in eight studies the follow‐up was shorter than 12 months. In addition, different criteria were used for resectability, although most studies used the NCCN guidelines.
Three RCTs were included, one27 of which randomized between neoadjuvant gemcitabine or gemcitabine combined with capecitabine in patients with resectable pancreatic cancer. The other two trials15, 16 randomized between neoadjuvant chemoradiotherapy and upfront surgery, but both were terminated early owing to poor accrual.
Primary outcome
The weighted median overall survival by intention to treat was 18·8 months in the neoadjuvant group and 14·8 months in the upfront surgery group.
Upfront surgery
Twelve studies15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 reported the median overall survival of 1746 patients undergoing upfront surgery for resectable or borderline resectable pancreatic cancer by intention to treat (Figs 2 and 3). Overall, 81·3 per cent of 1746 patients underwent resection, with an overall weighted median overall survival of 14·8 (range 11·6–25·3) months.
Figure 2.
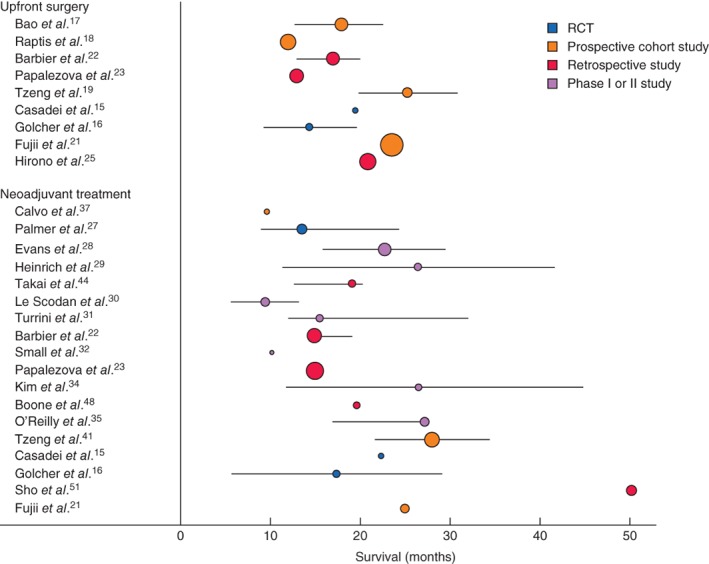
Median overall survival, with 95 per cent confidence intervals, for patients with resectable pancreatic cancer after upfront surgery and after neoadjuvant treatment. The square of radius of the spheres is related to number of patients in the study
Figure 3.
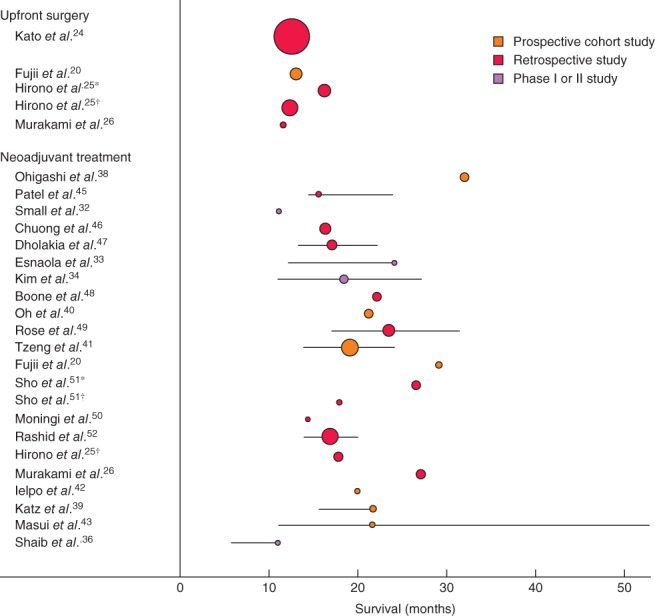
Median overall survival, with 95 per cent confidence intervals, for patients with borderline resectable pancreatic cancer after upfront surgery and after neoadjuvant treatment. The square of radius of the spheres is related to number of patients in the study. *Borderline resectable owing to venous involvement; †borderline resectable owing to arterial involvement
The weighted median overall survival of 819 patients with resectable pancreatic cancer was 17·7 (12–25·3) months15, 16, 17, 18, 19, 21, 22, 23, 25, compared with 12·8 (11·6–16·3) months for 927 patients with borderline resectable pancreatic cancer20, 24, 25, 26 (Figs 2 and 3). In the largest (retrospective) study of Kato and colleagues24, 63 of 624 patients (10·1 per cent) with borderline resectable pancreatic cancer also received neoadjuvant treatment and the median overall survival of these patients was not available separately. The outcome of the subgroup of patients who actually underwent resection was reported in seven16, 18, 22, 23, 24, 25, 26 of 12 studies; the weighted median overall survival was 15·0 months for these 1048 patients (not by intention to treat).
Neoadjuvant treatment
Thirty‐five studies15, 16, 19, 20, 21, 22, 23, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52 reported median overall survival after neoadjuvant treatment of 1738 patients with resectable or borderline resectable pancreatic cancer. The neoadjuvant regimens used are shown in Table 2. The weighted median overall survival was 18·8 (range 9·4–50·2) months after neoadjuvant treatment.
For the 18 studies15, 16, 19, 21, 22, 23, 27, 28, 29, 30, 31, 32, 34, 35, 37, 44, 48, 51 that reported the median overall survival of 857 patients with resectable pancreatic cancer, the weighted median overall survival was 18·2 (10–50·2) months (Fig. 2). In the 21 studies20, 25, 26, 32, 33, 34, 36, 38, 39, 40, 41, 42, 43, 45, 46, 47, 48, 49, 50, 51, 52 reporting the median overall survival after neoadjuvant treatment in 881 patients with borderline resectable cancer, the weighted median overall survival was 19·2 (11–32) months (Fig. 3).
The outcome for the subgroup of patients who actually underwent resection was reported in 19 studies16, 19, 22, 23, 25, 26, 27, 28, 29, 30, 31, 34, 37, 40, 41, 44, 46, 47, 52, and the weighted median overall survival was 26·1 months for these 764 patients (not by intention to treat).
Neoadjuvant chemotherapy versus chemoradiotherapy
Of all studies including patients who received neoadjuvant treatment, six used chemotherapy alone, 24 used chemoradiotherapy, and five used neoadjuvant chemotherapy in some patients and chemoradiotherapy in others. The weighted median overall survival was 20·9 (range 13·6–27·2) months for patients who received chemotherapy alone26, 27, 29, 35, 42, 43 and 17·8 (9·4–32) months15, 16, 19, 20, 21, 22, 23, 28, 30, 31, 32, 34, 36, 37, 38, 39, 40, 41, 44, 45, 46, 47, 50, 52 for chemoradiotherapy alone. Because of the heterogeneity between radiation dose and chemotherapy schedules, subset analyses should be interpreted with caution.
Secondary outcomes
Resection rate and R0 rate
The overall resection rate was lower in patients who had neoadjuvant treatment than in those who had upfront surgery (66·0 versus 81·3 per cent; P < 0·001).
After upfront surgery, the resection rate in all 1746 patients was 81·3 (95 per cent c.i. 79·4 to 81·3) (range 32·7–92) per cent. For patients with resectable pancreatic cancer, the resection rate was 76·8 (95 per cent c.i. 73·8 to 79·7) per cent, compared with 85·3 (82·9 to 87·5) per cent for those with borderline resectable pancreatic cancer (P < 0·001).
For patients who received neoadjuvant treatment, the resection rate was reported in 35 studies15, 16, 20, 21, 22, 23, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52 and was 66·0 (95 per cent c.i. 63·7 to 68·2) (range 29–100) per cent. For patients with resectable pancreatic cancer, the resection rate was 67·0 (95 per cent c.i. 63·7 to 70·1) per cent, compared with 65·0 (61·8 to 68·2) per cent for those with borderline resectable pancreatic cancer (P = 0·418). The resection rate for patients in the neoadjuvant group who underwent an exploratory laparotomy was 91·2 per cent.
The R0 resection rate (only for patients who underwent resection) was higher in patients who had neoadjuvant treatment (86·8 versus 66·9 per cent; P < 0·001). The R0 resection rate was also higher with neoadjuvant treatment when the results were reported by intention to treat (58·0 versus 54·9 per cent; P = 0·088). This difference is obviously smaller, because it is the resection rate multiplied by the R0 rate.
The R0 resection rate was reported in 11 studies15, 16, 17, 19, 20, 21, 22, 23, 24, 25, 26 after upfront surgery and was 66·9 (95 per cent c.i. 64·2 to 69·6) (range 17–81) per cent. After upfront surgery, the R0 resection rate was 71·4 per cent for patients with resectable pancreatic cancer, and 63·9 per cent for those with borderline resectable pancreatic cancer. For patients treated with neoadjuvant therapy who underwent exploratory laparotomy followed by resection, the R0 resection rate was 86·8 (95 per cent c.i. 84·6 to 88·7) (range 38·9–100) per cent. After neoadjuvant treatment, the R0 resection rate was 85·0 per cent among patients with resectable pancreatic cancer and 88·6 per cent for those with borderline resectable cancer.
Pathological lymph node rate
The pathological lymph node rate was reported in 11 studies15, 16, 17, 19, 20, 21, 22, 23, 24, 25, 26 after upfront surgery and was 64·8 (95 per cent c.i. 62·0 to 67·5) per cent, compared with 43·8 (40·6 to 47·1) per cent after neoadjuvant treatment in 27 studies15, 16, 19, 20, 21, 22, 23, 25, 26, 27, 28, 29, 30, 31, 32, 34, 35, 36, 38, 39, 40, 41, 43, 46, 47, 49, 52. This difference in pathological lymph node rates between the two groups was significant (P < 0·001).
Reasons for not performing surgery
Of the 35 neoadjuvant therapy studies, 29 reported the reason for not performing exploratory surgery. In total, 306 patients (17·8 per cent) did not proceed to exploratory surgery. Progression of disease (locally advanced or metastasis) was the most common reason for not undertaking exploratory surgery in 64·4 per cent of these patients. In total, 55 patients (18·0 per cent) could not undergo surgery because of severe side‐effects or deterioration of performance after neoadjuvant treatment, representing 3·2 per cent of all patients starting neoadjuvant treatment. For the remaining patients there were other reasons, or the reason was not known. The reasons for not performing tumour resection during exploratory surgery were reported in 23 of the 35 studies (Table S4, supporting information). Resection was not undertaken in at least 532 patients (15·3 per cent of all 3484 included patients). The most common reason for this was distant metastasis in 42·5 per cent of these patients. Disease progression was the reason for not resecting the tumour in 25·6 per cent.
Toxicity
There was a wide range of reported toxicity of neoadjuvant treatment across studies. The most common reported adverse events were gastrointestinal (emesis, nausea and diarrhoea) and haematological (thrombopenia, leucopenia). Toxicity of at least grade III was reported in 21 studies15, 16, 20, 25, 27, 28, 29, 30, 31, 32, 33, 34, 36, 37, 38, 39, 42, 43, 44, 46, 50, with a rate of up to 64 per cent, involving mostly leucopenia, thrombocytopenia, nausea and fatigue. Katz and colleagues39 reported a grade III toxicity rate of 64 per cent, in a study in which FOLFIRINOX (leucovorin, 5‐fluorouracil, irinotecan and oxaliplatin) chemotherapy was combined with radiotherapy at a dose of 50·4 Gy. Grade IV toxicity was reported in 13 studies, and consisted mostly of haematological adverse events.
Discussion
In this systematic review, median overall survival was 18·8 months after neoadjuvant treatment versus 14·8 months after upfront surgery of resectable or borderline pancreatic cancer in intention‐to‐treat analysis. The R0 resection rate and pathological lymph node rate were also improved in the neoadjuvant group. These results suggest the superiority of neoadjuvant treatment over upfront surgery. Previous studies13, 53 reported outcomes of patients who actually underwent resection, rather than reporting by intention to treat, thus introducing a survival bias.
Median survival times for patients who actually underwent resection were 26·1 months in the neoadjuvant group and 15·0 months for upfront surgery in this review. This difference in median overall survival between the groups (11·1 months) is much bigger than the difference in the intention‐to‐treat analysis (4·0 months). Reporting by intention to treat reduces potential bias in treatment effect as not all patients proceed to surgery, and a large proportion of patients do not receive adjuvant chemotherapy owing to postoperative complications. Prospective phase II studies investigating the role of neoadjuvant treatment have to report on all patients included in the trial by intention to treat54. Therefore, for a fair comparison, upfront surgery studies and observational studies of neoadjuvant treatment should also report by intention to treat.
In the present review, 17·8 per cent of patients who had neoadjuvant treatment did not undergo exploratory surgery. This selects out patients with an aggressive pancreatic cancer that would probably have progressed in a short time after surgery anyway, thus avoiding a potentially harmful operation. In the upfront surgery group, the resection rate for patients with borderline resectable pancreatic cancer was significantly higher than that for patients with resectable tumours (85·3 versus 76·8 per cent respectively). This is a counterintuitive finding, as one would expect the resection rate to be higher for resectable pancreatic cancer. There is no good explanation for this finding, but the different criteria being used worldwide for assessing resectability or suboptimal preoperative assessment on CT may play a role. Centralization of pancreatic surgery has led to increased resection rates55, but this was not investigated here.
The R0 resection rate among patients actually undergoing tumour resection was significantly better in the neoadjuvant treatment group, which is in line with the hypothesis that neoadjuvant treatment provides higher R0 rates than surgery alone56. The R0 resection rate after upfront surgery is comparable to rates of 29–81 per cent, depending on the R0 criteria being used, in recent large series of pancreatic cancer resection1, 57, 58. The pathological lymph node rate was also significantly different between the upfront surgery and neoadjuvant treatment groups, which may be the result of the neoadjuvant treatment causing regression of lymph node metastases59.
No difference in surgical morbidity and mortality has been reported in studies comparing neoadjuvant treatment with upfront surgery60, 61, 62. A possible advantage of neoadjuvant radiation is the development of pancreatic fibrosis, which may be associated with reduced occurrence of pancreas fistula after resection60, 61, 63. Adjuvant chemotherapy is the current standard of care after resection of pancreatic cancer1, but this treatment is often not given, or not completed, owing to a prolonged complicated postoperative course, or the preference of the patient or doctor. Data from the Netherlands Cancer Registry64 revealed that only 54 per cent of all patients undergoing pancreatoduodenectomy received adjuvant chemotherapy, because of toxicity, age and other factors. In the present review, the toxicity reported most frequently consisted of adverse gastrointestinal and haematological events. Overall, treatment‐related toxicity was given as the reason for not proceeding to exploratory surgery in only 3·2 per cent of the 1723 patients who started neoadjuvant treatment.
Median overall survival varied widely across the studies, which may be explained by the different criteria used for resectability. Most studies used the NCCN or MD Anderson Cancer Center criteria for resectability65, 66, but some studies used neither of these. Objective definitions of resectability are critical for the conduct of clinical trials of neoadjuvant treatment. Another explanation for the heterogeneity may be the variation in neoadjuvant treatment regimens across studies. The difference in receipt of postoperative adjuvant treatment (68·6 per cent in the upfront surgery group versus 31 per cent in the neoadjuvant group) may in part be explained by the fact that these patients had already received part or all of their systemic therapy before surgery.
The expert consensus statement of the AHPBA67 indicates that neoadjuvant therapy provides a rational alternative to an upfront surgery approach and could be considered in all patients with resectable pancreatic cancer. Evidence from RCTs is still lacking. The Dutch Pancreatic Cancer Group has just finished accrual of the multicentre randomized PREOPANC trial (EU Clinical Trials Register: 2012‐003181‐40) of neoadjuvant chemoradiotherapy versus upfront surgery68. The hypothesis is that neoadjuvant chemoradiotherapy may result in an increase in R0 resection rate and overall survival in patients with resectable or borderline resectable pancreatic cancer68. The trial has randomized the required 248 patients during a 4‐year interval and the first results are expected in 2018. Five other randomized trials69, 70, 71, 72, 73 are ongoing in Germany, Switzerland and Norway to investigate the role of neoadjuvant treatment in resectable pancreatic cancer. Two previous RCTs15, 16 from Italy and Germany were terminated early because of poor accrual.
Some limitations of the present systematic review must be taken into account. First, the quality of the included studies is moderate; the majority are retrospective studies, with high suspicion of bias. Only three studies were RCTs, and only two of these, with a total of 104 patients, randomized between upfront surgery and neoadjuvant treatment followed by surgery. Both these studies were terminated early. Owing to the clinical and methodological heterogeneity, no network analysis could be performed. Despite the limitations, the results provide the most reliable survival data, reported by intention to treat, in patients with resectable or borderline resectable pancreatic cancer.
Editor's comments

Supporting information
Appendix S1 The final update of the search was done on December 6th 2016.
Table S1 Critical appraisal of studies reporting on median overall survival of patients with resectable or borderline resectable pancreatic cancer after upfront surgery
Table S2 Critical appraisal of studies reporting on median overall survival of patients with resectable or borderline resectable pancreatic cancer after upfront surgery or neoadjuvant treatment followed by surgery
Table S3 Critical appraisal of studies reporting on median overall survival of patients with resectable or borderline resectable pancreatic cancer after neoadjuvant treatment followed by surgery
Table S4 Reasons for not performing exploratory laparotomy or resection, reported in the 38 studies
Acknowledgements
The authors thank L. Barbier, R. Casadei, H. Golcher, S. Helton, H. Kato, P. A. Lind, T. Masui, R. Neale, J. B. Rose and C. R. Shubert for providing more detailed information about their articles; and R. Hollman for providing the median OS figures. J.A.V. and M.G.B. received a grant (no. 2014‐7244) from the Dutch Cancer Society for studies on pancreatic cancer.
Disclosure: The authors declare no conflict of interest.
References
- 1. Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM et al; European Study Group for Pancreatic Cancer. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC‐4): a multicentre, open‐label, randomised, phase 3 trial. Lancet 2017; 389: 1011–1024. [DOI] [PubMed] [Google Scholar]
- 2. Sinn M, Bahra M, Liersch T, Gellert K, Messmann H, Bechstein W et al CONKO‐005: adjuvant chemotherapy with gemcitabine plus erlotinib versus gemcitabine alone in patients after R0 resection of pancreatic cancer: a multicenter randomized phase III trial. J Clin Oncol 2017; 35: 3330–3337. [DOI] [PubMed] [Google Scholar]
- 3. Shaib Y, Davila J, Naumann C, El‐Serag H. The impact of curative intent surgery on the survival of pancreatic cancer patients: a U.S. population‐based study. Am J Gastroenterol 2007; 102: 1377–1382. [DOI] [PubMed] [Google Scholar]
- 4. Shrikhande SV, Barreto SG. Surgery for pancreatic carcinoma: state of the art. Indian J Surg 2012; 74: 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stessin AM, Meyer JE, Sherr DL. Neoadjuvant radiation is associated with improved survival in patients with resectable pancreatic cancer: an analysis of data from the surveillance, epidemiology, and end results (SEER) registry. Int J Radiat Oncol Biol Phys 2008; 72: 1128–1133. [DOI] [PubMed] [Google Scholar]
- 6. Dutch Institute for Clinical Auditing (DICA) . Jaarrapportage 2014 http://www.clinicalaudit.nl [accessed 1 September 2017].
- 7. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP et al The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oxford Centre for Evidence‐Based Medicine . Critical Appraisal for Therapy Articles, 2005 http://www.cebm.net/critical-appraisal [accessed 1 September 2017].
- 9. Critical Appraisal Skills Programme . 11 Questions to Help You Make Sense of a Trial. Randomised Controlled Trials Checklist; 2013. http://www.casp-uk.net/#!casp-tools-checklists/c18f8 [accessed 1 September 2017].
- 10. Critical Appraisal Skills Programme . 12 Questions to Help You Make Sense of Cohort Study. Cohort Study Checklist; 2013. http://www.casp-uk.net/#!casp-tools-checklists/c18f8 [accessed 1 September 2017].
- 11. Higgins JP, Altman DG, Gøtzsche PC, Juni P, Moher D, Oxman AD et al; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oxford Centre for Evidence‐Based Medicine, Levels of Evidence Working Group . The Oxford 2011 Levels of Evidence. http://www.cebm.net/wp-content/uploads/2014/06/CEBM-Levels-of-Evidence-2.1.pdf [accessed 1 September 2017]. [Google Scholar]
- 13. Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta‐analysis of response and resection percentages. PLoS Med 2010; 7: e1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. UCSF Clinical & Translational Science Institute . Sample Size Calculator: Confidence Interval for a Proportion http://www.sample-size.net/confidence-interval-proportion [accessed 1 September 2017].
- 15. Casadei R, Di Marco M, Ricci C, Santini D, Serra C, Calculli L et al Neoadjuvant chemoradiotherapy and surgery versus surgery alone in resectable pancreatic cancer: a single‐center prospective, randomized, controlled trial which failed to achieve accrual targets. J Gastrointest Surg 2015; 19: 1802–1812. [DOI] [PubMed] [Google Scholar]
- 16. Golcher H, Brunner TB, Witzigmann H, Marti L, Bechstein WO, Bruns C et al Neoadjuvant chemoradiation therapy with gemcitabine/cisplatin and surgery versus immediate surgery in resectable pancreatic cancer: results of the first prospective randomized phase II trial. Strahlenther Onkol 2015; 191: 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bao P, Potter D, Eisenberg DP, Lenzner D, Zeh HJ, Lee lii KK et al Validation of a prediction rule to maximize curative (R0) resection of early‐stage pancreatic adenocarcinoma. HPB (Oxford) 2009; 11: 606–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Raptis DA, Fessas C, Belasyse‐Smith P, Kurzawinski TR. Clinical presentation and waiting time targets do not affect prognosis in patients with pancreatic cancer. Surgeon 2010; 8: 239–246. [DOI] [PubMed] [Google Scholar]
- 19. Tzeng CW, Tran Cao HS, Lee JE, Pisters PW, Varadhachary GR, Wolff RA et al Treatment sequencing for resectable pancreatic cancer: influence of early metastases and surgical complications on multimodality therapy completion and survival. J Gastrointest Surg 2014; 18: 16–24. [DOI] [PubMed] [Google Scholar]
- 20. Fujii T, Yamada S, Murotani K, Kanda M, Sugimoto H, Nakao A et al Inverse probability of treatment weighting analysis of upfront surgery versus neoadjuvant chemoradiotherapy followed by surgery for pancreatic adenocarcinoma with arterial abutment. Medicine (Baltimore) 2015; 94: e1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fujii T, Satoi S, Yamada S, Murotani K, Yanagimoto H, Takami H et al Clinical benefits of neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreatic head: an observational study using inverse probability of treatment weighting. J Gastroenterol 2017; 52: 81–93. [DOI] [PubMed] [Google Scholar]
- 22. Barbier L, Turrini O, Grégoire E, Viret F, Le Treut YP, Delpero JR. Pancreatic head resectable adenocarcinoma: preoperative chemoradiation improves local control but does not affect survival. HPB (Oxford) 2011; 13: 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Papalezova KT, Tyler DS, Blazer DG III, Clary BM, Czito BG, Hurwitz HI et al Does preoperative therapy optimize outcomes in patients with resectable pancreatic cancer? J Surg Oncol 2012; 106: 111–118. [DOI] [PubMed] [Google Scholar]
- 24. Kato H, Usui M, Isaji S, Nagakawa T, Wada K, Unno M et al Clinical features and treatment outcome of borderline resectable pancreatic head/body cancer: a multi‐institutional survey by the Japanese Society of Pancreatic Surgery. J Hepatobiliary Pancreat Sci 2013; 20: 601–610. [DOI] [PubMed] [Google Scholar]
- 25. Hirono S, Kawai M, Okada KI, Miyazawa M, Shimizu A, Kitahata Y et al Treatment strategy for borderline resectable pancreatic cancer with radiographic artery involvement. Pancreas 2016; 45: 1438–1446. [DOI] [PubMed] [Google Scholar]
- 26. Murakami Y, Uemura K, Sudo T, Hashimoto Y, Kondo N, Nakagawa N et al Survival impact of neoadjuvant gemcitabine plus S‐1 chemotherapy for patients with borderline resectable pancreatic carcinoma with arterial contact. Cancer Chemother Pharmacol 2017; 79: 37–47. [DOI] [PubMed] [Google Scholar]
- 27. Palmer DH, Stocken DD, Hewitt H, Markham CE, Hassan AB, Johnson PJ et al A randomized phase 2 trial of neoadjuvant chemotherapy in resectable pancreatic cancer: gemcitabine alone versus gemcitabine combined with cisplatin. Ann Surg Oncol 2007; 14: 2088–2096. [DOI] [PubMed] [Google Scholar]
- 28. Evans DB, Varadhachary GR, Crane CH, Sun CC, Lee JE, Pisters PW et al Preoperative gemcitabine‐based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008; 26: 3496–3502. [DOI] [PubMed] [Google Scholar]
- 29. Heinrich S, Pestalozzi BC, Schäfer M, Weber A, Bauerfeind P, Knuth A et al Prospective phase II trial of neoadjuvant chemotherapy with gemcitabine and cisplatin for resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008; 26: 2526–2531. [DOI] [PubMed] [Google Scholar]
- 30. Le Scodan R, Mornex F, Girard N, Mercier C, Valette PJ, Ychou M et al Preoperative chemoradiation in potentially resectable pancreatic adenocarcinoma: feasibility, treatment effect evaluation and prognostic factors, analysis of the SFRO‐FFCD 9704 trial and literature review. Ann Oncol 2009; 20: 1387–1396. [DOI] [PubMed] [Google Scholar]
- 31. Turrini O, Ychou M, Moureau‐Zabotto L, Rouanet P, Giovannini M, Moutardier V et al Neoadjuvant docetaxel‐based chemoradiation for resectable adenocarcinoma of the pancreas: new neoadjuvant regimen was safe and provided an interesting pathologic response. Eur J Surg Oncol 2010; 36: 987–992. [DOI] [PubMed] [Google Scholar]
- 32. Small W Jr, Mulcahy MF, Rademaker A, Bentrem DJ, Benson AB, Weitner BB et al Phase II trial of full‐dose gemcitabine and bevacizumab in combination with attenuated three‐dimensional conformal radiotherapy in patients with localized pancreatic cancer. Int J Radiat Oncol Biol Phys 2011; 80: 476–482. [DOI] [PubMed] [Google Scholar]
- 33. Esnaola NF, Chaudhary UB, O'Brien P, Garrett‐Mayer E, Camp ER, Thomas MB et al Phase 2 trial of induction gemcitabine, oxaliplatin, and cetuximab followed by selective capecitabine‐based chemoradiation in patients with borderline resectable or unresectable locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2014; 88: 837–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim EJ, Ben‐Josef E, Herman JM, Bekaii‐Saab T, Dawson LA, Griffith KA et al A multi‐institutional phase 2 study of neoadjuvant gemcitabine and oxaliplatin with radiation therapy in patients with pancreatic cancer. Cancer 2013; 119: 2692–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. O'Reilly EM, Perelshteyn A, Jarnagin WR, Schattner M, Gerdes H, Capanu M et al A single‐arm, nonrandomized phase II trial of neoadjuvant gemcitabine and oxaliplatin in patients with resectable pancreas adenocarcinoma. Ann Surg 2014; 260: 142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shaib WL, Hawk N, Cassidy RJ, Chen Z, Zhang C, Brutcher E et al A phase 1 study of stereotactic body radiation therapy dose escalation for borderline resectable pancreatic cancer after modified FOLFIRINOX (NCT01446458). Int J Radiat Oncol Biol Phys 2016; 96: 296–303. [DOI] [PubMed] [Google Scholar]
- 37. Calvo FA, Matute R, García‐Sabrido JL, Gómez‐Espí M, Martínez NE, Lozano MA et al Neoadjuvant chemoradiation with tegafur in cancer of the pancreas: initial analysis of clinical tolerance and outcome. Am J Clin Oncol 2004; 27: 343–349. [DOI] [PubMed] [Google Scholar]
- 38. Ohigashi H, Ishikawa O, Eguchi H, Takahashi H, Gotoh K, Yamada T et al Feasibility and efficacy of combination therapy with preoperative full‐dose gemcitabine, concurrent three‐dimensional conformal radiation, surgery, and postoperative liver perfusion chemotherapy for T3‐pancreatic cancer. Ann Surg 2009; 250: 88–95. [DOI] [PubMed] [Google Scholar]
- 39. Katz MH, Shi Q, Ahmad SA, Herman JM, Marsh Rde W, Collisson E et al Preoperative modified FOLFIRINOX treatment followed by capecitabine‐based chemoradiation for borderline resectable pancreatic cancer: Alliance for Clinical Trials in Oncology Trial A021101. JAMA Surg 2016; 151: e161137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Oh TG, Chung MJ, Bang S, Park SW, Chung JB, Song SY et al Validation of group B borderline resectable pancreatic cancer: retrospective analysis. Gut Liver 2014; 8: 557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tzeng CWD, Balachandran A, Ahmad M, Lee JE, Krishnan S, Wang H et al Serum carbohydrate antigen 19‐9 represents a marker of response to neoadjuvant therapy in patients with borderline resectable pancreatic cancer. HPB (Oxford) 2014; 16: 430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ielpo B, Duran H, Diaz E, Fabra I, Caruso R, Ferri V et al Preoperative treatment with gemcitabine plus nab‐paclitaxel is a safe and effective chemotherapy for pancreatic adenocarcinoma. Eur J Surg Oncol 2016; 42: 1394–1400. [DOI] [PubMed] [Google Scholar]
- 43. Masui T, Doi R, Kawaguchi Y, Sato A, Nakano K, Ito T et al Concurrent gemcitabine+S‐1 neoadjuvant chemotherapy contributes to the improved survival of patients with small borderline‐resectable pancreatic cancer tumors. Surg Today 2016; 46: 1282–1289. [DOI] [PubMed] [Google Scholar]
- 44. Takai S, Satoi S, Yanagimoto H, Toyokawa H, Takahashi K, Terakawa N et al Neoadjuvant chemoradiation in patients with potentially resectable pancreatic cancer. Pancreas 2008; 36: e26–e32. [DOI] [PubMed] [Google Scholar]
- 45. Patel M, Hoffe S, Malafa M, Hodul P, Klapman J, Centeno B et al Neoadjuvant GTX chemotherapy and IMRT‐based chemoradiation for borderline resectable pancreatic cancer. J Surg Oncol 2011; 104: 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chuong MD, Springett GM, Freilich JM, Park CK, Weber JM, Mellon EA et al Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Biol Phys 2013; 86: 516–522. [DOI] [PubMed] [Google Scholar]
- 47. Dholakia AS, Hacker‐Prietz A, Wild AT, Raman SP, Wood LD, Huang P et al Resection of borderline resectable pancreatic cancer after neoadjuvant chemoradiation does not depend on improved radiographic appearance of tumor–vessel relationships. J Radiat Oncol 2013; 2: 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Boone BA, Steve J, Zenati MS, Hogg ME, Singhi AD, Bartlett DL et al Serum CA 19‐9 response to neoadjuvant therapy is associated with outcome in pancreatic adenocarcinoma. Ann Surg Oncol 2014; 21: 4351–4358. [DOI] [PubMed] [Google Scholar]
- 49. Rose JB, Rocha FG, Alseidi A, Biehl T, Moonka R, Ryan JA et al Extended neoadjuvant chemotherapy for borderline resectable pancreatic cancer demonstrates promising postoperative outcomes and survival. Ann Surg Oncol 2014; 21: 1530–1537. [DOI] [PubMed] [Google Scholar]
- 50. Moningi S, Dholakia AS, Raman SP, Blackford A, Cameron JL, Le DT et al The role of stereotactic body radiation therapy for pancreatic cancer: a single‐institution experience. Ann Surg Oncol 2015; 22: 2352–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sho M, Akahori T, Tanaka T, Kinoshita S, Nagai M, Tamamoto T et al Importance of resectability status in neoadjuvant treatment for pancreatic cancer. J Hepatobiliary Pancreat Sci 2015; 22: 563–570. [DOI] [PubMed] [Google Scholar]
- 52. Rashid OM, Pimiento JM, Gamenthaler AW, Nguyen P, Ha TT, Hutchinson T et al Outcomes of a clinical pathway for borderline resectable pancreatic cancer. Ann Surg Oncol 2015; 23: 1371–1379. [DOI] [PubMed] [Google Scholar]
- 53. Laurence JM, Tran PD, Morarji K, Eslick GD, Lam VW, Sandroussi C. A systematic review and meta‐analysis of survival and surgical outcomes following neoadjuvant chemoradiotherapy for pancreatic cancer. J Gastrointest Surg 2011; 15: 2059–2069. [DOI] [PubMed] [Google Scholar]
- 54. Gupta SK. Intention‐to‐treat concept: a review. Perspect Clin Res 2011; 2: 109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gooiker GA, Lemmens VE, Besselink MG, Busch OR, Bonsing BA, Molenaar IQ et al Impact of centralization of pancreatic cancer surgery on resection rates and survival. Br J Surg 2014; 101: 1000–1005. [DOI] [PubMed] [Google Scholar]
- 56. Chua TC, Saxena A. Preoperative chemoradiation followed by surgical resection for resectable pancreatic cancer: a review of current results. Surg Oncol 2011; 20: e161–e168. [DOI] [PubMed] [Google Scholar]
- 57. Howard TJ, Krug JE, Yu J, Zyromski NJ, Schmidt CM, Jacobson LE et al A margin‐negative R0 resection accomplished with minimal postoperative complications is the surgeon's contribution to long‐term survival in pancreatic cancer. J Gastrointest Surg 2006; 10: 1338–1345. [DOI] [PubMed] [Google Scholar]
- 58. Chandrasegaram MD, Goldstein D, Simes J, Gebski V, Kench JG, Gill AJ et al Meta‐analysis of radical resection rates and margin assessment in pancreatic cancer. Br J Surg 2015; 102: 1459–1472. [DOI] [PubMed] [Google Scholar]
- 59. Roland CL, Yang AD, Katz MH, Chatterjee D, Wang H, Lin H et al Neoadjuvant therapy is associated with a reduced lymph node ratio in patients with potentially resectable pancreatic cancer. Ann Surg Oncol 2015; 22: 1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cooper AB, Parmar AD, Riall TS, Hall BL, Katz MH, Aloia TA et al Does the use of neoadjuvant therapy for pancreatic adenocarcinoma increase postoperative morbidity and mortality rates? J Gastrointest Surg 2015; 19: 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Denbo JW, Bruno ML, Cloyd JM, Prakash L, Lee JE, Kim M et al Preoperative chemoradiation for pancreatic adenocarcinoma does not increase 90‐day postoperative morbidity or mortality. J Gastrointest Surg 2016; 20: 1975–1985. [DOI] [PubMed] [Google Scholar]
- 62. Araujo RL, Gaujoux S, Huguet F, Gonen M, D'Angelica MI, DeMatteo RP et al Does pre‐operative chemoradiation for initially unresectable or borderline resectable pancreatic adenocarcinoma increase post‐operative morbidity? A case‐matched analysis. HPB (Oxford) 2013; 15: 574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ishikawa O, Ohigashi H, Imaoka S, Teshima T, Inoue T, Sasaki Y et al Concomitant benefit of preoperative irradiation in preventing pancreas fistula formation after pancreatoduodenectomy. Arch Surg 1991; 126: 885–889. [DOI] [PubMed] [Google Scholar]
- 64. Bakens MJ, van der Geest LG, van Putten M, van Laarhoven HW, Creemers GJ, Besselink MG et al The use of adjuvant chemotherapy for pancreatic cancer varies widely between hospitals: a nationwide population‐based analysis. Cancer Med 2016; 5: 2825–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. National Comprehensive Cancer Network . NCCN Guideline: Pancreatic Adenocarcinoma. Version 2.2016 http://www.tri-kobe.org/nccn/guideline/pancreas/english/pancreatic.pdf [accessed 1 September 2017].
- 66. Katz MH, Marsh R, Herman JM, Shi Q, Collison E, Venook AP et al Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol 2013; 20: 2787–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Abrams RA, Lowy AM, O'Reilly EM, Wolff RA, Picozzi VJ, Pisters PW. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol 2009; 16: 1751–1756. [DOI] [PubMed] [Google Scholar]
- 68. Versteijne E, van Eijck CH, Punt CJ, Suker M, Zwinderman AH, Dohmen MA et al; Dutch Pancreatic Cancer Group (DPCG). Preoperative radiochemotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC trial): study protocol for a multicentre randomized controlled trial. Trials 2016; 17: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tachezy M, Gebauer F, Petersen C, Arnold D, Trepel M, Wegscheider K et al Sequential neoadjuvant chemoradiotherapy (CRT) followed by curative surgery vs. primary surgery alone for resectable, non‐metastasized pancreatic adenocarcinoma: NEOPA – a randomized multicenter phase III study (NCT01900327, DRKS00003893, ISRCTN82191749). BMC Cancer 2014; 14: 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Heinrich S, Pestalozzi B, Lesurtel M, Berrevoet F, Laurent S, Delpero JR et al Adjuvant gemcitabine versus NEOadjuvant gemcitabine/oxaliplatin plus adjuvant gemcitabine in resectable pancreatic cancer: a randomized multicenter phase III study (NEOPAC study). BMC Cancer 2011; 11: 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ettrich TJ, Berger AW, Muche R, Lutz MP, Prasnikar N, Uhl W et al NEONAX: neoadjuvant plus adjuvant or only adjuvant nab‐paclitaxel plus gemcitabine for resectable pancreatic cancer – a phase II study of the AIO Pancreatic Cancer Group. J Clin Oncol 2014; 32(Suppl 15): tps4158. [Google Scholar]
- 72. Hozaeel W, Pauligk C, Homann N, Luley K, Kraus TW, Trojan J et al Randomized multicenter phase II/III study with adjuvant gemcitabine versus neoadjuvant/adjuvant FOLFIRINOX in resectable pancreatic cancer: the NEPAFOX trial. J Clin Oncol 2015; 33(Suppl 15): tps4152. [Google Scholar]
- 73. Labori KJ, Lassen K, Hoem D, Grønbech JE, Søreide JA, Mortensen K et al Neoadjuvant chemotherapy versus surgery first for resectable pancreatic cancer (Norwegian Pancreatic Cancer Trial – 1 (NorPACT‐1)) – study protocol for a national multicentre randomized controlled trial. BMC Surg 2017; 17: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 The final update of the search was done on December 6th 2016.
Table S1 Critical appraisal of studies reporting on median overall survival of patients with resectable or borderline resectable pancreatic cancer after upfront surgery
Table S2 Critical appraisal of studies reporting on median overall survival of patients with resectable or borderline resectable pancreatic cancer after upfront surgery or neoadjuvant treatment followed by surgery
Table S3 Critical appraisal of studies reporting on median overall survival of patients with resectable or borderline resectable pancreatic cancer after neoadjuvant treatment followed by surgery
Table S4 Reasons for not performing exploratory laparotomy or resection, reported in the 38 studies


