Abstract
Despite the well‐recognized importance of immunoglobulin therapy individualization during the treatment of chronic inflammatory demyelinating polyneuropathy (CIDP), the pathway to best achieve optimization is unknown. There are many pharmacokinetic and immunobiologic variables that can potentially influence the appropriateness of any individual therapy. Although identification of specific autoantibodies and their targets has only been accomplished in a minority of patients with CIDP, already the diagnostic and treatment implications of specific autoantibody detection are being realized. Individual variability in IgG pharmacokinetic properties including IgG catabolic rates and distribution, as well as the IgG level necessary for disease control also require consideration during the optimization process. For optimization to be successful there must be a measure of treatment response that has a clinically meaningful interpretation. There are currently available well‐defined and validated clinical assessment tools and outcome measures that are well suited for this purpose. While there remains much to learn on how best to manipulate immunopathology and immunoglobulin pharmacokinetics in the most favorable way, there currently exists an understanding of these principles to a degree sufficient to begin to develop rational and evidence‐based treatment optimization strategies.
Keywords: autoimmune neuromuscular diseases, chronic inflammatory demyelinating polyneuropathy, immune‐mediated neuropathies, intravenous immunoglobulin, pharmacokinetics
Introduction
Intravenous immunoglobulin (IVIG), plasmapheresis (PE), and corticosteroids (CS) are first line CIDP treatment options (Joint Task Force of the EFNS and the PNS, 2010 ). Existing trials have focused on the efficacy of immunotherapy in groups of patients with CIDP (Hughes et al., 2008 ), with less emphasis placed on understanding the variability of treatment responses in individual patients (Pestronk et al., 1988 ; Donaghy et al., 1994 ; Attarian et al., 2011 ; Eftimov et al., 2014 ; Léger, 2014 ). What does the existing literature tell us about the immunobiologic and immunoglobulin pharmacokinetic determinants of treatment response, and how might we manipulate these in our favor? This review discusses how our increasing knowledge of specific autoantibodies and individual variations in IgG metabolism can be utilized with disease‐specific clinical outcome assessments to individually tailor therapy.
The concept of optimizing immunotherapy for individual patients with inflammatory neuropathies is not new. For IVIG in CIDP, current guidelines recommend using the lowest effective maintenance dose and suggest that stable patients undergo periodic dose reduction or interval lengthening trials to establish the need for ongoing therapy (Joint Task Force of the EFNS and the PNS, 2010 ). The observation that in one clinical trial 44% of patients treated with placebo in addition to previous medications were able to reduce mean weekly doses of CS or IVIG by greater than 20% (RMC Trial Group, 2009 ) and in another trial 40% of placebo‐treated patients did not relapse even though an IVIG dependency test was required prior to randomization (van Schaik et al., 2018 ) indicates that overtreatment is common and not well addressed in clinical practice. The importance of treatment individualization as highlighted in current guidelines and supported by the findings in prior clinical trials is indisputable. What is less certain, however, is the best strategy by which to achieve that goal. There is much to be learned on how to escalate or de‐escalate IVIG therapy in such a way that maximizes clinical efficacy and minimizes over‐exposure and cost.
One important determinant of IVIG optimization is how best to manage and measure wear‐off. Wear‐off is the cyclic or periodic occurrence of clinical deterioration at an interval following an IVIG infusion. It is unknown if reducing wear‐off prevents permanent damage or if some degree of end of cycle deterioration is tolerated without accumulating disability. From a pathobiologic perspective, wear‐off implies that disability, at least to some extent, cannot be solely attributed to inflammatory damage and destruction of myelin or axon itself. There is a growing appreciation that dysfunction at the nodes of Ranvier plays an early pathologic role in some patients (Pollard and Armati, 2011 ; Berger et al., 2013 ; Querol et al., 2017 ) and that rapid clinical improvement following IVIG or PE may be driven by reversible nodal or paranodal immune‐mediated dysfunction (Pollard and Armati, 2011 ; Berger et al., 2013 ). Electrophysiologically, reversible conduction block and decreased compound muscle action potential amplitudes have been demonstrated within the context of autoantibodies that bind to nodal and paranodal proteins or ganglioside complexes (Harschnitz et al., 2014 ; Notturno et al., 2014 ; Delmont et al., 2017 ). These observations imply that although IVIG may temporarily reduce the effects of autoantibodies, as the concentration of therapeutic IgG dwindles the pathogenic process re‐emerges which in turn leads to the clinical occurrence of wear‐off (Berger et al., 2013 ; Rojavin et al., 2016 ).
In this review, we discuss how increasing knowledge of specific autoantibodies and individual variations in IgG metabolism, together with frequent quantitative strength and disability measurements, might be used to develop rational individualized treatment approaches. Although the term “immunoglobulin” when used herein refers to immunoglobulin administration intravenously (IVIG) or subcutaneously (SCIG), given the wealth of IVIG clinical data relative to SCIG, our focus is on optimization of IVIG during the treatment of CIDP.
Immunology of CIDP: Will Knowledge of Antibodies Change Treatment?
The diagnosis of CIDP relies on the combination of characteristic symptoms and signs, together with abnormalities on nerve conduction studies indicative of peripheral nerve demyelination or conduction block (Joint Task Force of the EFNS and the PNS, 2010 ). Albuminocytologic dissociation in cerebrospinal fluid, nerve enlargement or enhancement by magnetic resonance imaging (MRI), and characteristic histopathologic changes on nerve biopsy can add confidence in cases in which the electrophysiologic abnormalities are equivocal, but are not themselves diagnostic. The observation that almost half of the US patients that carry a diagnosis of CIDP are misdiagnosed (Allen and Lewis, 2015 ; Allen et al., 2018) underscores the challenges encountered in defining a disorder without a reliable biologic marker. In a tissue‐specific autoimmune disease like CIDP, an improved understanding of the characteristics of pathogenic antibodies and their target antigens has the capacity to not only improve CIDP diagnostic accuracy but may be critical when determining disease‐specific immunotherapy approaches.
In order to understand how specific autoantibodies influence immunoglobulin treatment it is useful to understand what is known about IgG's mechanism of action. IgG therapy is thought to act by reducing the amounts and/or effector functions of pathologic autoantibodies (Berger et al., 2013 ). Mechanisms dependent on competition between therapeutic (normal) and pathogenic IgG include saturating neonatal Fc receptors (FcRn), anti‐idiotype neutralization, inhibition of complement deposition, and possibly feedback inhibition of B‐cell antibody production (Kuitwaard et al., 2009 ; Berger et al., 2013 ; Schwab and Nimmerjahn, 2013 ; Espéli et al., 2016 ). Each of these mechanisms has the potential for manipulation, which may be influenced by the specific autoantibody. For example, autoantibody Fc domain structure is defined by class (IgG, IgM, or IgA) and subclass (IgG1‐4). If this structure was known for any particular autoantibody, it might help predict the likelihood that FcRn blocking or saturating agents will be effective. Knowing the different Fc domain structures and the effector mechanisms they recruit (Table 1) may also help determine which immunotherapy is most likely to be effective in any given disease subset. There is much to learn on how specific autoantibodies influence immunoglobulin treatment in any specific patient. Given the observations that some patients with CIDP carry on for years with cyclic IVIG treatment‐related fluctuations, one underlying principal of IVIG therapy is that it competes with but does not fundamentally alter autoantibody production or function (Dacci et al., 2010 ; Pollard and Armati, 2011 ; Kokubun et al., 2013 ; Debs et al., 2017 ).
Table 1.
Properties of major serum immunoglobulin classes and subclasses.
| Ig type | % of serum IgG | Half‐life (days) | Complement classical activation pathway | Binding to FcRn | Binding to myeloid FcRs |
|---|---|---|---|---|---|
| IgA | (0.60–3.50)* | 4–6 | − | − | (FcαRs) |
| IgM | (0.40–2.30)* | 5–6 | ++++ | − | − |
| IgG1 | 67–75% | 23–28 | +++ | ++++ | +++ |
| IgG2 | 22–25% | 23–28 | + | ++ | ++ |
| IgG3 | 7–10% | 5–10 | +++ | ++ | +++ |
| IgG4 | 3–5% | 23–28 | − | +++ | + |
IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; FcαR, IgA Fc receptor; FcR, receptors specific for the Fc region of immunoglobulins; FcRn, neonatal Fc receptor.
For IgA and IgM, serum concentration in g/l is given. For IgG subclasses, % of total IgG is given. Normal total IgG concentrations are 7.25–12.50 g/l in most laboratories.
There are scenarios in which autoantibody recognition may already directly influence individualization of immunotherapy. Anti‐neurofascin 155 (NF155) antibodies have been detected in 3.3%–7.1% of patients with chronic inflammatory polyneuropathies (Ng et al., 2012 ; Querol et al., 2014 ; Querol and Illa, 2015 ; Devaux et al., 2016 ) and between 2.4% and 7.5% of patients have been found to have anti‐contactin 1 (CNTN1) antibodies (Querol et al., 2013 ; Yan et al., 2014 ; Miura et al., 2015 ). NF155 and CNTN1 are localized to the paranodal region and both antibodies are of the IgG4 isotype. One emerging feature in patients that harbor these antibodies is a reportedly poor response to IVIG but more favorable responses to CS and B‐cell depletion therapy. One potential explanation for why IVIG may be less effective in these subsets of CIDP patients is that the IgG4 isotype does not efficiently activate complement and has a low affinity for Fc receptors on effector cells (Labasque et al., 2014 ; Vidarsson et al., 2014 ). Although these anecdotal treatment observations currently based on only small case series need confirmation in larger groups of patients, detection of NF155 and CNTN1 autoantibodies might shift treatment paradigms for autoantibody‐defined subsets of patients in consideration of their specific pathophysiology. Future treatment strategies may take earlier advantage of CS and anti‐B‐cell therapies such as rituximab, with a deemphasized role of IVIG (Huijbers et al., 2015 ; Halder et al., 2016 ). Yet‐to‐be identified autoantibodies and target antigens might provide similar insight into other groups of CIDP patients. Furthermore, knowledge of the specificity and affinity of autoantibodies and recognition of the target antigen will likely facilitate utilization of new and improved therapies. Agents in clinical development include FcRn blockers (Zuercher et al., 2016 ; Kiessling et al., 2017 ), complement inhibitors (Fitzpatrick et al., 2011 ), antigen‐specific adsorbents/binding site blockers (Herrendorff et al., 2017 ), and blockers of pro‐inflammatory Fc receptors (Schwab and Nimmerjahn, 2013 ).
IgG Metabolism and its Impact on Individualized Therapy
When considering strategies for optimization of immunoglobulin therapy, an understanding of the individual variations in the distribution and metabolism of therapeutic IgG may be equal in importance to the characteristics of pathological autoantibodies. The speed with which serum IgG levels rise depends on the method of IgG delivery. When immunoglobulin is administered intravascularly, serum IgG levels peak immediately (Fig. 1) and then drop by about 50% over 2–4 days as IgG is distributed into the total extracellular fluid volume (Bonilla, 2008 ). In contrast to IV administration, following subcutaneous immunoglobulin administration, serum IgG levels rise more slowly, peaking at 48–72 h (Fig. 2) (Berger, 2004 ; Bonilla, 2008 ). Regardless of the route of delivery, once IgG reaches the intravascular space, catabolism proceeds as a first‐order process with a half‐life of 21–30 days (Bonilla, 2008 ). The relatively slow catabolism of IgG, as compared to other plasma proteins, is due to a saturable endothelial cell receptor, FcRn, which protects endocytosed IgG from lysosomal degradation (Fig. 2).
Figure 1.
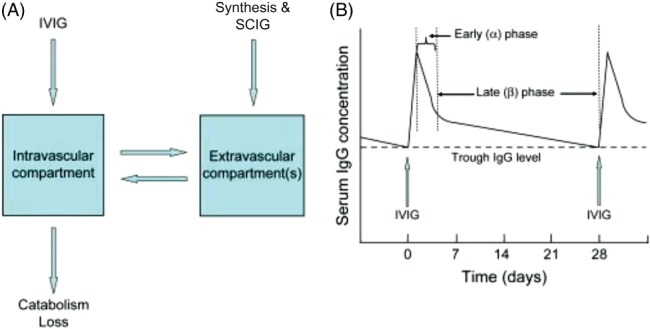
(A) Schematic model for distribution and metabolism of IgG illustrating a “two compartment model.” Note that newly synthesized IgG as well as subcutaneously administered IgG (SCIG) are initially in the extravascular compartment and then move into the intravascular compartment by diffusion and lymphatic circulation. (B) Pharmacokinetics of serum IgG after a dose of IVIG. IVIG, intravenous immunoglobulin; SCIG, subcutaneous immunoglobulin (Bonilla, 2008 ). Copyright 2008. Reprinted with permission from Elsevier.
Figure 2.
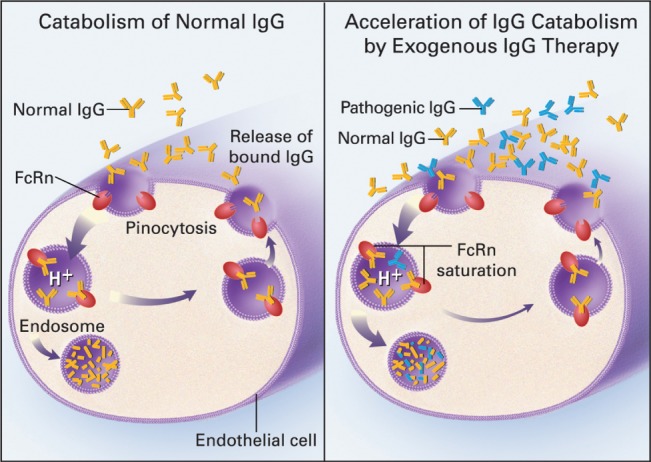
Role of FcRn in determining survival of IgG in the circulation. Left panel: under normal conditions, IgG from the serum binds to FcRn and goes through an endosomal pathway which avoids lysosomal catabolism. The IgG is thus returned intact to the circulation. Right panel: If the serum IgG level is raised by exogenous IgG, FcRn becomes saturated and much of the IgG goes through the default endosomal pathway which results in lysosomal catabolism. Because of the high proportion of exogenous normal IgG, endogenous pathogenic IgG is preferentially degraded (Yu and Lennon, 1999 ). Copyright 1999 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
The principle of first‐order kinetics is one fundamental concept that requires consideration when attempting to optimize immunoglobulin therapy. First‐order kinetics means that the higher the serum IgG level, the faster it will be catabolized (Fig. 3) (Waldmann and Strober, 1969 ). As illustrated in the figure, if the IgG concentration is raised from 8 g/l to 30 g/l the half‐life drops from 28 to 11 days. High‐dose IVIG infusions can raise serum IgG concentrations to peaks of 30–50 g/l or higher (Reinhart and Berchtold, 1992 ; Dalakas, 1994 ). In contrast, the peak serum concentration achieved with SCIG is on average only 61% of the peak achieved with IV infusions of the same dose (Berger et al., 2011 ). Whereas IVIG bolus infusions may result in trough‐to‐peak serum IgG differences greater than 100% of the overall mean, frequent SCIG administration may result in peak‐trough differences of only about 5% of the overall mean (Berger, 2004 ; Bonilla, 2008; Berger et al., 2011 ). Regardless of whether the route of admistration is by IV or SC, with more frequent administration of fractional doses, the peaks and troughs will approach each other and ultimately near “steady‐state” concentrations will be reached (Berger, 2004 ; Buclin et al., 2009 ). The impact that these findings have on immunoglubulin optimization becomes apperent when considering that in Guillian–Barre Syndrome (Kuitwaard et al., 2009 ) and perhaps CIDP (Debs et al., 2017 ) there appears to be a serum IgG level which must be reached in order to achieve a desirable clinical outcome. These findings indicate that high and stable trough levels may have particular importance in immunoglobulin optimization such that a satisfactory minimum IgG level is achieved while avoiding the accelerated catabolism that comes with high immunoglobulin peak levels. A clinical study exploring IgG levels and clinical efficacy in patients subjected to frequent low dose vs. less frequent higher dose IVIG is currently underway (Kuitwaard, personal communication, 2017).
Figure 3.
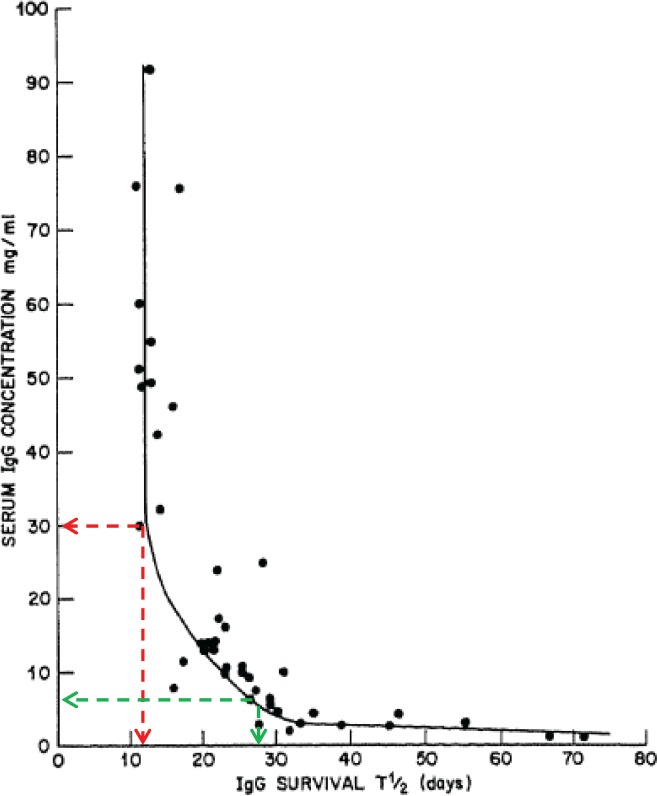
High concentrations of IgG (ie, greater than 20 g/l) lead to rapid catabolism and shorter survival in the circulation (half‐life). At an IgG concentration of 30 g/l (30 mg/ml as shown here) for example, shortly after an IVIG infusion the half‐life would be 11 days (red dashed line). In contrast, at a normal serum IgG concentration (8 g/l) the half‐life is 28 days (Waldmann and Strober, 1969 ). Copyright 2016 Karger Publishers, Basel, Switzerland. Reprinted with permission.
Beyond the normal physiological characteristics of IgG catabolism that may be manipulated to achieve treatment optimization, there are also patient to patient differences that influence optimization. Different patients vary in the expression and function of FcRn, which can also be affected by cytokines such as tumor necrosis factor (Liu et al., 2007 ). The variability in FcRn is one explanation for why the half‐life of the catabolic phase has a fairly broad distribution of 21–30 days. The reasons for this variability are currently unknown. Genetic polymorphisms in the FcRn receptor structural gene have been studied in only relatively few patients, but have not yet been shown to correlate with serum IgG levels or the response to IVIG (Fokkink et al., 2016 ; Vlam et al., 2014 ).
Wear‐Off with IVIG and What it Means in Clinical Practice
As might be predicted based upon patient to patient immunoglobulin pharmacokinetic heterogeneity and immunopathogenic diversity, treatment responses to IVIG can be variable between patients and even in the same patient at different stages of disease. One challenge that arises during IVIG treatment optimization is how best to manage wear‐off and other treatment‐related fluctuations, a rather dramatic example of which is shown in Fig. 4. In clinical practice the extent of wear‐off can be highly variable. In order to continuously maintain strength and disability, one study found that 60% of subjects needed IVIG at intervals ≤14 days (Kuitwaard et al., 2013 ). Several other studies (Table 2) have shown that many patients do better when the IVIG dosing interval is less than the expected half‐life of IgG (Broyles et al., 2013 ; Rajabally et al., 2013 ). Clinical experience tells us that other patients do not have clinically meaningful treatment‐related fluctuations and may maintain stability with less frequent IVIG dosing at 4 to 6‐week intervals. If different IgG levels are required to counter different autoantibodies and IgG pharmacokinetic variability influences speed of IgG catabolism in different patients then it becomes clear that dose and interval of immunoglobulin therapy should be individualized and one important determinant of optimization is quantification of treatment‐related fluctuations.
Figure 4.
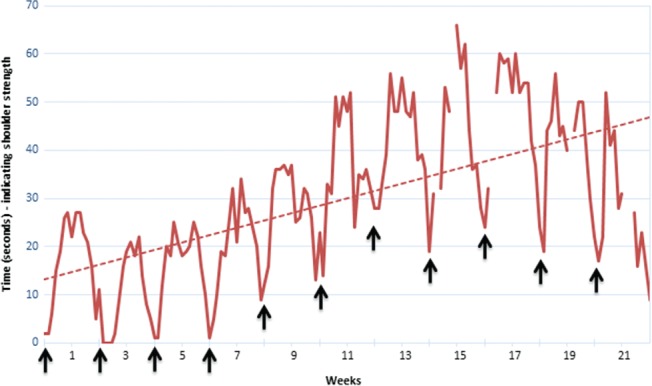
Wear‐off in one patient with CIDP. Self‐recorded daily measurement of maximum time that left upper limb can be held outstretched (patient seated with shoulder 90° flexed and elbow extended). This non‐standard outcome measure was chosen by the patient as the most practical measure of disability in his left upper limb. IVIG was given at 1.86 g/kg every two weeks (↑). The troughs (minimum time outstretched) around the day of each IVIG treatment show that his shoulder strength weakened as IVIG wore off. The rising baseline shows gradually increasing strength over several months indicating cumulative benefit following increased treatment frequency from 1.86 g/kg/2.5 weeks to 1.86 g/kg/2 weeks at the start of the measurement period (Hadden, personal communication, 2017).
Table 2.
IgG doses and intervals.
| Reference | Patients (n) | Mean Ig dose (g/kg) | Mean interval (weeks) | Patients (%) receiving IgG at interval ≤ 14 days |
|---|---|---|---|---|
| Lunn et al., 2016 | CIDP 39 | 1.4 | 4.3 | 17 |
| MMN 24 | ||||
| Kuitwaard et al., 2013 | CIDP 25 | 0.43 | 2.4 | 60 |
| Rajabally et al., 2006, * | CIDP 15 | 0.69* | 6.9 | 13 |
| Broyles et al., 2013 | CIDP 31 | 0.87 | 4.0 | 25 |
CIDP, chronic inflammatory demyelinating polyneuropathy; IgG, immunoglobulin G; MMN, multifocal motor neuropathy; SEM, standard error of the mean.
Note that common criteria for defining endpoints and/or selecting maintenance doses were not employed across the different studies.
Final or lowest dose per course.
One of the major aims of the Peripheral Neuropathy Outcome Measures Standardization (PeriNomS) study was to better define the metrics used to assess and follow patients with inflammatory neuropathies (Draak et al., 2014 ). The authors emphasized the importance of measuring disability (i.e., activities and participation), strength and sensory impairment, and quality of life in patients with CIDP. The Inflammatory Rasch‐built Overall Disability Scale (I‐RODS), INCAT disability score, and grip strength measurements using the Martin Vigorimeter® (Martin GmbH & Co., Tutlingen, Germany) have emerged as strongly validated and valuable assessment tools. Grip strength in particular provides a quantitative, immediately available result that can be easily performed during physician office visits (Berger and Allen, 2015 ). In a randomized controlled trial, grip strength was shown to provide objective documentation of global neurologic status in patients with CIDP, not just limited to the upper limb or exclusively motor function (Vanhoutte et al., 2013 ). Grip strength and I‐RODS have also been shown to correlate with each other (Draak et al., 2016 ). The observation that daily grip strength measurements can vary dramatically during a single IVIG treatment cycle with some patients showing maximum strength more than 50% higher than minimal strength and other patients showing no significant changes (Hadden, personal communication, 2017) highlights the potential for grip strength monitoring to be a useful adjunct assessment when determining the IgG dose and interval most appropriate for any individual patient. Further research is needed to better understand what magnitude of intracycle grip strength and disability fluctuations are acceptable, and what magnitude should trigger therapy changes to maximize short‐term function and minimize long‐term disability. Such questions are currently being explored in a study which records daily grip strength and weekly disability outcomes over a 6 month period in a cohort of IVIG‐treated CIDP patients (Allen, personal communication, 2017).
One practical consideration that commonly arises during IVIG optimization is whether it is preferable to adjust IVIG dose or interval. There is no best answer to this question, and ultimately the approach to IVIG optimization should blend individual patient treatment responses, tolerability, and convenience of administration. However, when optimizing IVIG, it is important to recall that (1) IVIG half‐life can vary between 21 and 30 days, (2) there is likely a minimum serum IgG threshold that must be reached in order to achieve efficacy, and (3) IgG catabolism accelerates with very high serum IgG levels. With these points in mind, consider three scenarios. In the first, clinically meaningful treatment‐related fluctuations are present, necessitating escalated immunoglobulin therapy. Increasing IVIG dose while keeping the interval constant may keep the serum IgG level above the necessary therapeutic minimum to achieve the desired clinical effect, but at the cost of accelerated IgG catabolism which in turn may result in an overall higher total amount of IgG needed (Gouilleux‐Gruart et al., 2013 ). Alternatively, fractionating the treatment into smaller doses at shorter intervals may minimize clinical fluctuations and immunoglobulin exposure as the serum IgG level reaches a stable steady state with higher trough level (Berger, 2004 ; Buclin et al., 2009 ). In the second scenario, treatment‐related fluctuations are unequivocally present but are minimal without an impact on disability. In this setting no treatment modification may be needed, since a minor degree of wear‐off may provide assurance that treatment is needed and not over‐utilized. In the third scenario, no treatment‐related fluctuations are present. In that situation, immunoglobulin de‐escalation is encouraged. The optimal strategy to wean IVIG is unknown. In cases in which one important determinant of optimization is maintaining a stable IgG steady‐state trough‐level, then periodic IVIG dose reduction with stable infusion frequency may be preferable to interval lengthening with stable dosing. Regardless of whether treatment is escalated or de‐escalated, or which optimization approach is taken, collection of validated outcomes during optimizing is of critical importance when interpreting the response to treatment changes.
As opposed to the general optimization comments above that might be applied to patients already on maintenance therapy, a useful treatment optimization protocol designed for IVIG‐naïve patients was developed by Lunn et al. ( 2016 ) (Fig. 5). In this approach the patient with CIDP is given a loading dose of 2 g/kg IVIG, which is repeated 6 weeks later if the patient is not fully normalized. No further IVIG is given unless deterioration occurs, in which case regularly scheduled IVIG is started. The time interval from IVIG treatment to deterioration is used as the individualized interval for all subsequent IVIG. After two further 2 g/kg loading doses, the dose is reduced by 20% per course. When relapse or wear‐off emerges, that dose is set as the lowest dose without wear‐off. In a cohort of 71 patients (including 39 with CIDP and 24 with MMN) utilization of this protocol resulted in a mean (± standard error) dose of 1.4 ± 0.6 g/kg at a mean interval of 4.3 weeks (range 0.5 to 10 weeks) (Lunn et al., 2016 ). A similar, but not identical, approach has been proposed by the Department of Neurology Erasmus MC (Kuitwaard et al., 2017 ). Neither the Lunn nor the Erasmus MC approaches were designed to be dogmatic, but both highlight important principles that should be considered in any optimization approach. These principles include structured tapering protocols to determine IgG dependency, close clinical monitoring for wear‐off, and an appreciation for treatment response variability. The presence or absence of any specific autoantibody will also provide rational for further refinement of the optimization approach or immunotherapy of choice.
Figure 5.
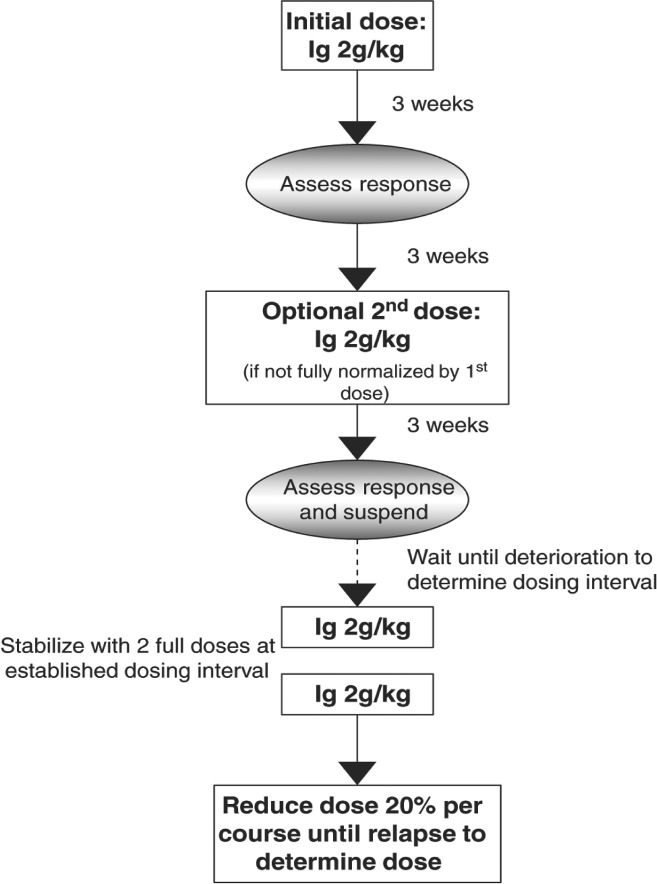
One possible treatment algorithm to optimize dose and dosing interval of immunoglobulin. (Ig = IVIG) (Lunn et al., 2016 ). Reprinted with permission.
Conclusion
Presently there is no “best” approach to treatment optimization, and there is much to be considered when attempting to manipulate immunotherapy and immunoglobulin pharmacokinetics in the most favorable way (Table 3). Differences in IgG catabolic rates and IgG level necessary for disease control are major determinants that require consideration in every individual patient. An evolving understanding of the heterogeneity of FcRn expression and function may further facilitate personalized treatment approaches. Ultimately an improved understanding of the specificity and affinity of pathogenic autoantibodies in what is now broadly considered to be idiopathic CIDP, combined with what is known about immunoglobulin pharmacokinetics, may provide clinicians the most precise pathway to optimal therapy selection and the roadmap to maximize the effects of that therapy. Utilization of validated outcome measures during routine clinical care is of great importance in order to objectify the response to treatment, quantify treatment‐related fluctuations, and detect relapse in patients undergoing therapy weaning or suspension. As our ability to rationally design and assess personalized therapeutic regimens improves so too does our capacity to explore other yet‐to‐be answered questions, including understanding the long‐term impact of short‐term optimization and understanding which optimized interventions are not only the most efficacious but also the most cost‐effective.
Table 3.
Considerations for treatment optimization during treatment of CIDP.
|
CIDP, chronic inflammatory demyelinating polyneuropathy; CS, corticosteroid; I‐RODS, Inflammatory Rasch‐built Overall Disability Scale; IgG4, immunoglobulin G4; IVIG, intravenous immunoglobulin; PE, plasmapheresis.
Acknowledgements
The authors wish to thank Jason Heckler of Meridian HealthComms, Cheshire, UK for editorial assistance, formatting, and processing the manuscript.
References
- Allen JA, Lewis RA (2015). CIDP diagnostic pitfalls and perception of treatment benefit. Neurology 85:498–504. [DOI] [PubMed] [Google Scholar]
- Allen JA, Ney J, Lewis RA (2018). Electrodiagnostic errors contribute to chronic inflammatory demyelinating polyneuropathy misdiagnosis. Muscle Nerve 57:542–549. [DOI] [PubMed] [Google Scholar]
- Attarian S, Verschueren A, Franques J, Salort‐Campana E, Jouve E, Pouget J (2011). Response to treatment in patients with Lewis‐Sumner syndrome. Muscle Nerve 44:179–184. [DOI] [PubMed] [Google Scholar]
- Berger M (2004). Subcutaneous immunoglobulin replacement in primary immunodeficiencies. Clin Immunol 112:1–7. [DOI] [PubMed] [Google Scholar]
- Berger M, Allen JA (2015). Optimizing IgG therapy in chronic autoimmune neuropathies: a hypothesis driven approach. Muscle Nerve 51:315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M, Rojavin M, Kiessling P, Zenker O (2011). Pharmacokinetics of subcutaneous immunoglobulin and their use in dosing of replacement therapy in patients with primary immunodeficiencies. Clin Immunol 139:133–141. [DOI] [PubMed] [Google Scholar]
- Berger M, McCallus DE, Lin CS (2013). Rapid and reversible responses to IVIG in autoimmune neuromuscular diseases suggest mechanisms of action involving competition with functionally important autoantibodies. J Peripher Nerv Syst 18:275–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla FA (2008). Pharmacokinetics of immunoglobulin administered via intravenous or subcutaneous routes. Immunol Allergy Clin N Am 28:803–819. [DOI] [PubMed] [Google Scholar]
- Broyles R, Rodden L, Riley P, Berger M (2013). Variability in intravenous immunoglobulin G regimens for autoimmune neuromuscular disorders. Postgrad Med 125:65–72. [DOI] [PubMed] [Google Scholar]
- Buclin T, Nicod M, Kellenberger S (2009). Pharmacokinetics: Repeated Administration. Available at: https://sepia.unil.ch/pharmacology/index.php?id=70. Accessed Nov 16, 2017.
- Dacci P, Riva N, Scarlato M, Andresen I, Schmidt D, Comi G, Fazio R (2010). Subcutaneous immunoglobulin therapy for the treatment of multifocal motor neuropathy: a case report. Neurol Sci 31:829–831. [DOI] [PubMed] [Google Scholar]
- Dalakas MC (1994). High‐dose intravenous immunoglobulin and serum viscosity: risk of precipitating thromboembolic events. Neurology 44:223–226. [DOI] [PubMed] [Google Scholar]
- Debs R, Reach P, Cret C, Demeret S, Saheb S, Maisonobe T, Viala K (2017). A new treatment regimen with high‐dose and fractioned immunoglobulin in a special subgroup of severe and dependent CIDP patients. Int J Neurosci 127:864–872. [DOI] [PubMed] [Google Scholar]
- Delmont E, Manso C, Querol L, Cortese A, Berardinelli A, Lozza A, Belghazi M, Malissart P, Labauge P, Taieb G, Yuki N, Illa I, Attarian S, Devaux JJ (2017). Autoantibodies to nodal isoforms of neurofascin in chronic inflammatory demyelinating polyneuropathy. Brain 140:1851–1858. [DOI] [PubMed] [Google Scholar]
- Devaux JJ, Miura Y, Fukami Y, Inoue T, Manso C, Belghazi M, Sekiguchi K, Kokubun N, Ichikawa H, Wong AH, Yuki N (2016). Neurofascin‐155 IgG4 in chronic inflammatory demyelinating polyneuropathy. Neurology 86:800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaghy M, Mills KR, Boniface SJ, Simmons J, Wright I, Gregson N, Jacobs J (1994). Pure motor demyelinating neuropathy: deterioration after steroid treatment and improvement with intravenous immunoglobulin. J Neurol Neurosurg Psychiatry 57:778–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draak TH, Vanhoutte EK, van Nes SI, Gorson KC, Van der Pol WL, Notermans NC, Nobile‐Orazio E, Leger JM, Van den Bergh PY, Lauria G, Bril V, Katzberg H, Lunn MP, Pouget J, van der Kooi AJ, Hahn AF, Doorn PA, Cornblath DR, van den Berg LH, Faber CG, Merkies IS, PeriNomS Study Group (2014). Changing outcome in inflammatory neuropathies: Rasch‐comparative responsiveness. Neurology 83:2124–2132. [DOI] [PubMed] [Google Scholar]
- Draak TH, Gorson KC, Vanhoutte EK, van Nes SI, van Doorn PA, Cornblath DR, van den Berg LH, Faber CG, Merkies IS, PeriNomS Study Group (2016). Correlation of the patient's reported outcome inflammatory‐RODS with an objective metric in immune‐mediated neuropathies. Eur J Neurol 23:1248–1253. [DOI] [PubMed] [Google Scholar]
- Eftimov F, Liesdek MH, Verhamme C, van Schaik IN, PREDICT Study Group (2014). Deterioration after corticosteroids in CIDP may be associated with pure focal demyelination pattern. BMC Neurol 14:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espéli M, Smith KG, Clatworthy MR (2016). FcgammaRIIB and autoimmunity. Immunol Rev 269:194–211. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick AM, Mann CA, Barry S, Brennan K, Overell JR, Willison HJ (2011). An open label clinical trial of complement inhibition in multifocal motor neuropathy. J Peripher Nerv Syst 16:84–91. [DOI] [PubMed] [Google Scholar]
- Fokkink WJ, Haarman AE, Tio‐Gillen AP, van Rijs W, Huizinga R, van Doorn PA, Jacobs BC (2016). Neonatal fc receptor promoter gene polymorphism does not predict pharmacokinetics of IVIg or the clinical course of GBS. Ann Clin Transl Neurol 3:547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouilleux‐Gruart V, Chapel H, Chevret S, Lucas M, Malphettes M, Fieschi C, Patel S, Boutboul D, Marson MN, Gerard L, Lee M, Watier H, Oksenhendler E, Defi Study Group (2013). Efficiency of immunoglobulin G replacement therapy in common variable immunodeficiency: correlations with clinical phenotype and polymorphism of the neonatal fc receptor. Clin Exp Immunol 171:186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder D, Cockwell P, Richter AG, Roberts KJ, Hirschfield GM (2016). An overview of the diagnosis and management of immunoglobulin G4‐related disease. Can Med Assoc J 188:953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harschnitz O, Jongbloed BA, Franssen H, Straver DC, van der Pol WL, van den Berg LH (2014). MMN: from immunological cross‐talk to conduction block. J Clin Immunol 34:S112–S119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann MA, Mosberg‐Galili R, Lotan I, Steiner I (2014). Maintenance IVIg therapy in myasthenia gravis does not affect disease activity. J Neurol Sci 338:39–42. [DOI] [PubMed] [Google Scholar]
- Herrendorff R, Hänggi P, Pfister H, Yang F, Demeestere D, Hunziker F, Frey S, Schaeren‐Wiemers N, Steck AJ, Ernst B (2017). Selective in vivo removal of pathogenic anti‐MAG autoantibodies ‐ a novel treatment option for anti‐MAG neuropathy. J Periph Nerv Syst 22:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RA, Donofrio P, Bril V, Dalakas MC, Deng C, Hanna K, Hartung HP, Latov N, Merkies IS, van Doorn PA, ICE Study Group (2008). Intravenous immune globulin (10% caprylate‐chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomised placebo‐controlled trial. Lancet Neurol 7:136–144. [DOI] [PubMed] [Google Scholar]
- Huijbers MG, Querol LA, Niks EH, Plomp JJ, van der Maarel SM, Graus F, Dalmau J, Illa I, Verschuuren JJ (2015). The expanding field of IgG4‐mediated neurological autoimmune disorders. Eur J Neurol 22:1151–1161. [DOI] [PubMed] [Google Scholar]
- Joint Task Force of the EFNS and the PNS (2010). European Federation of Neurological Societies/peripheral nerve society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the peripheral nerve society‐‐first revision. J Peripher Nerv Syst 15:1–9. [DOI] [PubMed] [Google Scholar]
- Kiessling P, Lledo‐Garcia R, Watanabe S, Langdon G, Tran D, Bari M, Christodoulou L, Jones E, Price G, Smith B, Brennan F, White I, Jolles S (2017). The FcRn inhibitor rozanolixizumab reduces human serum IgG concentration: a randomized phase 1 study. Sci Transl Med 9:414–420. [DOI] [PubMed] [Google Scholar]
- Kokubun N, Sada T, Yuki N, Okabe M, Hirata K (2013). Optimization of intravenous immunoglobulin in chronic inflammatory demyelinating polyneuropathy evaluated by grip strength measurement. Eur Neurol 70:65–69. [DOI] [PubMed] [Google Scholar]
- Kuitwaard K, de Gelder J, Tio‐Gillen AP, Hop WC, van Gelder T, van Toorenenbergen AW, van Doorn PA, Jacobs BC (2009). Pharmacokinetics of intravenous immunoglobulin and outcome in Guillain‐Barre syndrome. Ann Neurol 66:597–603. [DOI] [PubMed] [Google Scholar]
- Kuitwaard K, van Doorn PA, Vermeulen M, van den Berg LH, Brusse E, van der Kooi AJ, van der Pol WL, van Schaik IN, Notermans N, Tio‐Gillen AP, van Rijs W, van Gelder T, Jacobs BC (2013). Serum IgG levels in IV immunoglobulin treated chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry 84:859–861. [DOI] [PubMed] [Google Scholar]
- Kuitwaard K, Fokkink WR, Brusse E, Vrancken AFJE, Eftimov F, Notermans NC, van der Kooi AJ, Merkies ISJ, Jacobs BC, van Doorn PA (2017). Maintenance IV immunoglobulin treatment in chronic inflammatory demylenating polyradiculoneuropathy. J Peripher Nerv Syst 22:425–432. [DOI] [PubMed] [Google Scholar]
- Labasque M, Hivert B, Nogales‐Gadea G, Querol L, Illa I, Faivre‐Sarrailh C (2014). Specific contactin N‐glycans are implicated in neurofascin binding and autoimmune targeting in peripheral neuropathies. J Biol Chem 289:7907–7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léger J‐M (2014). Immunoglobulin (Ig) in multifocal motor neuropathy (MMN): update on evidence for Ig treatment in MMN. Clin Exp Immunol 178:42–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ye L, Christianson GJ, Yang JQ, Roopenian DC, Zhu X (2007). NF‐kappaB signaling regulates functional expression of the MHC class I‐related neonatal fc receptor for IgG via intronic binding sequences. J Immunol 179:2999–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn MP, Ellis L, Hadden RD, Rajabally YA, Winer JB, Reilly MM (2016). A proposed dosing algorithm for the individualized dosing of human immunoglobulin in chronic inflammatory neuropathies. J Peripher Nerv Syst 21:33–37. [DOI] [PubMed] [Google Scholar]
- Miura Y, Devaux JJ, Fukami Y, Manso C, Belghazi M, Wong AH, Yuki N, CNTN1‐CIDP Study Group (2015). Contactin 1 IgG4 associates to chronic inflammatory demyelinating polyneuropathy with sensory ataxia. Brain 138:1484–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng JK, Malotka J, Kawakami N, Derfuss T, Khademi M, Olsson T, Linington C, Odaka M, Tackenberg B, Prüss H, Schwab JM, Harms L, Harms H, Sommer C, Rasband MN, Eshed‐Eisenbach Y, Peles E, Hohlfeld R, Yuki N, Dornmair K, Meinl E (2012). Neurofascin as a target for autoantibodies in peripheral neuropathies. Neurology 7:2241–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notturno F, Di Febo T, Yuki N, Fernandez Rodriguez BM, Corti D, Nobile‐Orazio E, Carpo M, De Lauretis A, Uncini A (2014). Autoantibodies to neurofascin‐186 and gliomedin in multifocal motor neuropathy. J Neuroimmunol 276:207–212. [DOI] [PubMed] [Google Scholar]
- Pestronk A, Cornblath DR, Ilyas AA, Baba H, Quarles RH, Griffin JW, Alderson K, Admas RN (1988). A treatable multifocal motor neuropathy with antibodies to GM1 ganglioside. Ann Neurol 24:73–78. [DOI] [PubMed] [Google Scholar]
- Pollard JD, Armati PJ (2011). CIDP ‐ the relevance of recent advances in Schwann cell/axonal neurobiology. J Peripher Nerv Syst 16:15–23. [DOI] [PubMed] [Google Scholar]
- Querol L, Illa I (2015). Paranodal and other autoantibodies in chronic inflammatory neuropathies. Curr Opin Neurol 28:474–479. [DOI] [PubMed] [Google Scholar]
- Querol L, Nogales‐Gadea G, Rojas‐Garcia R, Martinez‐Hernandez E, Diaz‐Manera J, Suárez‐Calvet X, Navas M, Araque J, Gallardo E, Illa I (2013). Antibodies to contactin‐1 in chronic inflammatory demyelinating polyneuropathy. Ann Neurol 73:370–380. [DOI] [PubMed] [Google Scholar]
- Querol L, Nogales‐Gadea G, Rojas‐Garcia R, Diaz‐Manera J, Pardo J, Ortega‐Moreno A, Sedano MJ, Gallardo E, Berciano J, Blesa R, Dalmau J, Illa I (2014). NF IgG4 antibodies in CIDP associate with disabling tremor and poor response to IVIg. Neurology 82:879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querol L, Devaux J, Rojas‐Garcia R, Illa I (2017). Autoantibodies in chronic inflammatory neuropathies: diagnostic and therapeutic implications. Nat Rev Neurol 13:533–547. [DOI] [PubMed] [Google Scholar]
- Rajabally YA, Seow H, Wilson P (2006). Dose of intravenous immunoglobulins in chronic inflammatory demyelinating polyneuropathy. J Peripher Nerv Syst 11:325–329. [DOI] [PubMed] [Google Scholar]
- Rajabally YA, Wong SL, Kearney DA (2013). Immunoglobulin G level variations in treated chronic inflammatory demyelinating polyneuropathy: clues for future treatment regimens? J Neurol 260:2052–2056. [DOI] [PubMed] [Google Scholar]
- Reinhart WH, Berchtold PE (1992). Effect of high‐dose intravenous immunoglobulin therapy on blood rheology. Lancet 339:662–664. [DOI] [PubMed] [Google Scholar]
- RMC Trial Group (2009). Randomised controlled trial of methotrexate for chronic inflammatory demyelinating polyradiculoneuropathy (RMC trial): a pilot, multicentre study. Lancet Neurol 8:158–164. [DOI] [PubMed] [Google Scholar]
- Rojavin MA, Hubsch A, Lawo JP (2016). Quantitative evidence of wear‐off effect at the end of the intravenous IgG (IVIG) dosing cycle in primary immunodeficiency. J Clin Immunol 36:210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schaik IN, Bril V, van Geloven N, Hartung HP, Lewis RA, Sobue G, Lawo JP, Praus M, Mielke O, Durn BL, Cornblath DR, ISJ M, PATH Study Group (2018). Subcutaneous immunoglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy (PATH): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Neurol 17:35–46. [DOI] [PubMed] [Google Scholar]
- Schwab I, Nimmerjahn F (2013). Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat Rev Immunol 13:176–189. [DOI] [PubMed] [Google Scholar]
- Vanhoutte EK, Faber CG, Merkies IS, PeriNomS Study Group (2013). 196th ENMC International Workshop: outcome measures in inflammatory peripheral neuropathies 8‐10 February 2013, Naarden, the Netherlands. Neuromuscul Disord 23:924–933. [DOI] [PubMed] [Google Scholar]
- Vidarsson G, Dekkers G, Rispens T (2014). IgG subclasses and allotypes: from structure to effector functions. Front Immunol 5:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlam L, Cats EA, Willemse E, Franssen H, Medic J, Piepers S, Veldnik JH, van den Berg LH, van der Pol WL (2014). Pharmacokinetics of intravenous immunoglobulin in multifocal motor neuropathy. J Neurol Neurosurg Psych 85:1145–1148. [DOI] [PubMed] [Google Scholar]
- Waldmann TA, Strober W (1969). Metabolism of immunoglobulins. Prog Allergy 13:1–110. [DOI] [PubMed] [Google Scholar]
- Yan W, Nguyen T, Yuki N, Ji Q, Yiannikas C, Pollard JD, Mathey EK (2014). Antibodies to to neurofascin exacerbate adoptive transfer experimental autoimmune neuritis. J Neuroimmunol 277:13–17. [DOI] [PubMed] [Google Scholar]
- Yu Z, Lennon VA (1999) Mechanism of intravenous immune globulin therapy in antibody‐mediated autoimmune diseases. New Engl J Med 340:227–228. [DOI] [PubMed] [Google Scholar]
- Zuercher AW, Spirig R, Baz Morelli A, Kasermann F (2016). IVIG in autoimmune disease ‐ potential next generation biologics. Autoimmun Rev 15:781–785. [DOI] [PubMed] [Google Scholar]


