Abstract
Background and aims
Increasing the reach of smoking cessation services and/or including new but effective medications to the current provision may provide significant health and economic benefits; the scale of such benefits is currently unknown. The aim of this study was to estimate the cost‐effectiveness from a health‐care perspective of viable national level changes in smoking cessation provision in the Netherlands and England.
Methods
A Markov‐based state transition model [European study on Quantifying Utility of Investment in Protection from Tobacco model (EQUIPTMOD)] was used to estimate costs and benefits [expressed in quality‐adjusted life years (QALY)] of changing the current provision of smoking cessation programmes in the Netherlands and England. The changes included: (a) increasing the reach of top‐level services to increase potential quitters (e.g. brief physician advice); (b) increasing the reach of behavioural support (group‐based therapy and SMS text‐messaging support) to increase the success rates; (c) including a new but effective medication (cytisine); and (d) all changes implemented together (combined change). The costs and QALYs generated by those changes over 2, 5, 10 years and a life‐time were compared with that of the current practice in each country. Results were expressed as incremental net benefit (INB) and incremental cost‐effectiveness ratio (ICER). A sequential analysis from a life‐time perspective was conducted to identify the optimal change.
Results
The combined change was dominant (cost‐saving) over all alternative changes and over the current practice, in both countries. The combined change would generate an incremental net benefit of €11.47 (2 years) to €56.16 (life‐time) per smoker in the Netherlands and €9.96 (2 years) to €60.72 (life‐time) per smoker in England. The current practice was dominated by all alternative changes.
Conclusion
Current provision of smoking cessation services in the Netherlands and England can benefit economically from the inclusion of cytisine and increasing the reach of brief physician advice, text‐messaging support and group‐based therapy.
Keywords: Cost‐effectiveness, cytisine, economic evaluation, public health, return on investment, smoking cessation
Introduction
A decade after the Framework Convention on Tobacco Control (FCTC) the smoking prevalence among adults in Europe is still high, at 28% 1, 2. The FCTC introduced the MPOWER package, aiming to achieve progress in various aspects from effective monitoring of tobacco control efforts to offering help to people who want to stop smoking 2. However, the actual action taken after ratifying MPOWER has been inconsistent throughout Europe, which has led to some countries being more successful in decreasing their smoking prevalence 3. Nevertheless, the ‘O’ of the MPOWER—offer help to people who want to stop smoking—has been a key policy driving the fall in smoking prevalence 3.
A previous study describing a survey of tobacco control activity in 34 European countries suggested that the United Kingdom is leading in Europe with successful anti‐tobacco policies, including taxation on tobacco products, a smoking ban in public places, advertisement bans and treatment offers to stop smoking 3. This has contributed to a decrease in smoking prevalence from 25% in 2003 to 18% among people aged over 16 in 2013 4, 5. The Netherlands was ranked 13th in the same study 3. The smoking prevalence in the Netherlands was 30.8% in 2003 and decreased to 25.6% among people aged over 16 in 2013 6, 7. However, current services have experienced variation in effectiveness, and smoking prevalence thus remains high 1, 4, 6.
Good‐quality evidence around the effectiveness and cost‐effectiveness of interventions used in the current provision of smoking cessation services is available 8. However, it is less clear whether reorganizing the current provision, with an aim to achieving efficiency savings or improving reach, would deliver a better return on investment. As smoking cessation services are currently under threat from disinvestment due to funding cuts, the answers to the above policy questions are timely. Increasing the reach of smoking cessation services and/or including new but effective medications (e.g. cytisine) to the current provision may provide significant health and economic benefits. However, the scale of such benefits is currently unknown. Therefore, the aim of this study was to estimate the cost‐effectiveness from a health‐care perspective of viable national level changes in smoking cessation provision in the Netherlands and England. A European return‐on‐investment (ROI) model [European study on Quantifying Utility of Investment in Protection from Tobacco model (EQUIPTMOD)] was utilized for this study, with a view to guiding future investment decisions. This analysis was therefore restricted to two countries only (England and the Netherlands), as they have similar current provision of smoking cessation services compared to the other three EQUIPTMOD countries.
Methods
Study design
The EQUIPTMOD 9, a Markov‐based state transition economic model, was used to estimate the level of: (a) investment required to implement national level changes to smoking cessation services; and (b) potential benefits such changes would generate in the Netherlands and England. The EQUIPTMOD is based on an earlier version of a tobacco ROI tool developed for National Institute for Health and Care Excellence (NICE) 10. A cohort of current smokers are followed‐up for various time horizons (including life‐time) to capture costs and quality‐adjusted life years (QALY) gains, as receiving treatment (services) would alter their risk of developing smoking‐attributable diseases (lung cancer, coronary heart disease, chronic obstructive pulmonary diseases and stroke). In each cycle (year), the model takes in to account the balance of quitting and relapsing through a background quit rate. Full details about the model workings and assumptions are described elsewhere 9. Various time horizons (2, 5, 10 years and life‐time), discount rates of 3.5% for costs and effects (England) and 4% for costs and 1.5% for effects (Netherlands) 11 and health‐care perspectives were taken.
Population, intervention, comparator and outcomes
This study included national populations of England and the Netherlands. Table 1 summarizes the key population attributes, as captured from a review of various sources by the EQUIPT Study Group 12, 13.
Table 1.
Population size, smoking prevalence and potential quitters and current use of smoking cessation services 4, 6.
| the Netherlands | England | |
|---|---|---|
| Adult population (> 16) | 13 870 426 | 43 813 787 |
| Smoking prevalence (%) | 25.60% | 17.99% |
| Number of smokers making a quit attempt in the next 12 months (% of smokers) | 949 945 (26.75%) | 2 236 287 (28.37%) |
| Use pharmaceutical support (% of those smokers making a quit attempt in the next 12 months) | 39.95% | 34.70% |
| Use behavioural therapy (% of those smokers making a quit attempt in the next 12 months) | 8.92% | 8.61% |
| Combination pharmaceutical and behavioural therapy (% of those smokers making a quit attempt in the next 12 months) | 8.32% | 5.88% |
A number of smoking cessation interventions are implemented currently in the Netherlands and England. Table 2 summarizes the interventions included in the EQUIPTMOD. The selection of interventions was the result of best‐evidence review 14. The interventions were grouped into three broad categories: (a) top‐level interventions that encourage smokers to make quit attempts (e.g. brief physical advice); (b) pharmacological interventions that encourage those smokers already making quit attempts to succeed through use of a medication; and (c) behavioural interventions that encourage those smokers already making quit attempts to succeed through counselling, self‐help materials or mobile phone support.
Table 2.
| Costs per person | Effect size | Reacha | ||
|---|---|---|---|---|
| Netherlands (€, 2015) | England (£, 2015) | |||
| Top‐level interventions | ||||
| Brief physician (GP) advice | 30.00 | 19.48 | 1.40 | 21% |
| Cut down to quit | Not applicable | 212.38 | 2.10 | 12% in England 0% in Netherlands |
| Pharmaceutical interventions | ||||
| Rx mono NRT | 225.05 | 106.44 | 1.60 | 5.00% |
| Rx combo NRT | 465.24 | 203.16 | 2.14 | 2.00% |
| Varenicline (SD) | 325.71 | 191.88 | 2.30 | 5.00% |
| Varenicline (extended duration) | 612.42 | 355.68 | 2.76 | 1.00% |
| Bupropion | 175.78 | 79.98 | 1.60 | 1.00% |
| Cytisineb | 24.29 | 17.63 | 3.98 | 0.00% |
| Behavioural interventions | ||||
| Specialist behavioural support: one‐to‐one | 465.00 | 120.64 | 1.40 | 2.00% |
| Specialist behavioural support: group‐based | 41.90 | 36.77 | 2.00 | 1.00% |
| Telephone support: proactive | 119.00 31 | 151.67 | 1.40 | 0.50% |
| SMS text messaging | 23.68a | 16.92 | 1.71 | 0.50% |
| Printed self‐help materials | 1.21 | 13.03 | 1.19 | 1.00% |
Figures from England where no corresponding figure for the Netherlands was available;
Not currently licensed for use in both countries. NRT = nicotine replacement therapy; SD = standard deviation; GP = general practitioner; SMS = short messaging service.
Although England and the Netherlands have similar smoking cessation services among the countries included in the EQUIPTMOD, it is important to note a few key differences in the way these interventions are delivered in England and the Netherlands. In England, the responsibility for public health services, including smoking cessation, is in the hands of local authorities 15. The Local Stop Smoking Services (LSSS) is free to use for all UK residents. The pharmacological and behavioural support options are available for the smokers to choose. In the Netherlands, the smoking cessation programme under the basic insurance package includes visit(s) to a general practitioner (GP) 16. The GP and the patient decide in consensus which help is needed. Pharmaceutical and/or behavioural support must be prescribed by a physician to be covered by insurance.
Behavioural support is offered both in the Netherlands and England. This can be one‐to‐one behavioural support, group‐based behavioural support, telephone support (proactive), short messaging service (SMS) text messaging or printed self‐help materials. Specialist behavioural support on a one‐to‐one basis consists of practical advice and emotional support and encouragement based on the Maudsley model by a health professional trained to the National Centre for Smoking Cessation and Training (NCSCT) standard or equivalent. Group‐based behavioural support is a group discussion based on the Maudsley model in a group of six to 30 smokers 17. This is also delivered by a health professional, as is the proactive telephone support. This telephone support entails practical advice and emotional support and encouragement according to principles set out in the National Health Service (NHS) and Monitoring Guidance or similar. SMS text messaging support is provided by an automated system that sends multiple texts per day containing practical advice and encouragement. Printed self‐help materials also give practical advice and encouragement; these are either one‐off book/booklets or multiple booklets.
Intervention attributes (costs, effects and reach) are described in Table 2. Reach refers to the proportion of smokers who currently make a quit attempt in a given year who are exposed to the intervention. Effect size refers to the ratio of the proportion of smokers exposed to the intervention who are estimated to achieve 12 months of smoking abstinence compared with not receiving the intervention, other things being equal. Effect sizes are point estimates and subject to both a margin of error because of sampling variation in the studies and also true variation as a function of variation in the delivery of the intervention. Relevant caveats of these estimates are discussed fully elsewhere 18. These data were derived from the literature or estimated, and costs were adjusted for inflation if they were from previous years. As data on reach of smoking cessation services in the Netherlands were not available, English estimates were used for Dutch intervention usage 10, 19.
For the purposes of this analysis, we defined five intervention packages that reflected alternative changes to the current provision of services (i.e. current practice). The current practice was the base comparator. The current practice included all interventions listed in Table 2 at their current levels of reach.
Alternative changes were identified as a prospective change in the current practice either by increasing or decreasing the reach of the interventions or including new but effective interventions. The size and feasibility of the change were tested with a Dutch policymaker and was also informed by previous stakeholder analyses 20. As shown in Table 3, changes A, B and C reflected increasing the reach of brief physician advice, the SMS text messaging support and group‐based therapy. In change D, a new but effective cessation pharmacotherapy—cytisine—was included. A recent review has found that although cytisine is not used widely in western countries, it has been shown to be effective 18. Cytisine is not currently licensed for use in both England and the Netherlands. In change E, we assumed that changes A–D would be implemented together (called, a combined change).
Table 3.
Alternative changes to the current provision examined in the study.
| Change | Description | Implications |
|---|---|---|
| A |
Increase reach: brief physician advice Rationale: with brief physician advice there is a relative increase of 1.4 in the percentage of smokers making a quit attempt in the next 12 months compared to the percentage making a quit attempt in the next 12 months at baseline 32. The costs of a brief physician advice are €30.00 per smoker in the Netherlands and £19.48 in England 31. Aim: increase the percentage of smokers receiving a brief physician advice from 21 to 22% |
Number of smokers making quit attempts may increase |
| B |
Increase reach: specialist group‐based behavioural therapy Rationale: group‐based therapy has a relative effectiveness of 2.0, whereas one‐to‐one therapy has a relative effectiveness of 1.4 33. Group‐based therapy costs €41.90 in the Netherlands and £36.77 in England 10, 21, 22, one‐to‐one therapy costs €465.00 in the Netherlands and £121.64 in England 31, 34 Aim: decrease the number of people receiving one‐to‐one specialist behavioural therapy by 25%, and assume that these people use group‐based specialist behavioural therapy instead |
Success rate of the quit attempts in specialist behavioural therapy may rise, while the costs may decrease |
| C |
Increase reach: SMS text‐messaging support Rationale: SMS text‐messaging support is €23.68 per person in the Netherlands and £16.92 in England 35, and has a relative effectiveness of 1.71 36 Aim: increase the number of people receiving SMS text‐messaging support by 25% |
Success rate following quit‐attempts by smokers on text messaging support may increase |
| D |
Include new but effective pharmacotherapy (cytisine) Rationale: nicotine receptor partial agonists such as varenicline or cytisine aim to reduce withdrawal symptoms and thus increase the chance of success in quit attempts. Cytisine, a similar medication to varenicline, is not yet licensed for use in the Netherlands and UK but has been found to be effective (RR = 3.98) in trials 37 and potentially cost‐effective in modelling studies 28. Including cytisine, which is significantly cheaper than varenicline, to the current provision of smoking cessation services may therefore provide better value for money (case for license and use) Aim: assume 50% of smokers currently using varenicline (standard duration) will use cytisine instead |
Success rate of quit‐attempts by smokers on cytisine may rise, while the costs may decrease |
| E |
Combined change Rationale: encouraging more smokers to make quit attempts, improving the reach of services for those who have made quit attempts, and including new but effective cessation medications, together may lead to more successful quitters than the current provision of smoking cessation services Aim: combine changes A with B, C and D |
Implementation costs may be higher but could be offset by the benefits from increased number of quitters |
RR = relative risk; SMS = short messaging service.
The final outcomes included QALYs generated by each change and the current practice, as recommended by the National Institute for Health and Care Excellence (NICE) and also recommended by the Dutch guideline for economic evaluations 21. In this paper, ‘costs’ refers to the sum of two cost components: (i) costs required to deliver an intervention package; and (ii) costs associated with treating the smoking‐attributable diseases.
Analyses
Two levels of analyses were conducted. First, we estimated QALYs gained per 1000 smokers and incremental net benefit (INB) per smoker over four time horizons (2, 5, 10 years and the life‐time of current smokers). To estimate the INB, a QALY gain was converted to € using the cost‐effectiveness threshold of GB£ 20 000 as recommended by NICE in England 22 and €25 000 in the Netherlands 23. This is a standard approach taken in health economic evaluations, but is subject to debate because a threshold value also reflects political will and financial constraints in a country. Nevertheless, INBs are easy to interpret and thus may be useful in decision making. A positive value of INB demonstrates that the benefits exceed the costs, and hence a positive ROI is expected. Costs were expressed in £ for England, and were then converted to € for comparison purposes (£0.72584 = €1) 12.
The QALY gains and corresponding INB estimates provided the first level analyses showing the extent of net benefit that would be generated over time by respective changes A–E, compared to current practice. Next, a sequential analysis was conducted to determine which alternative change was optimal (i.e. provided the best value for money). This was performed by identifying ‘dominance’ and ‘extended dominance’, the terms used by health economists to refer to the process of eliminating less cost‐effective options from the choice set 24. For this, current practice (base comparator) and alternative changes A–E were first ranked by outcomes (QALYs per smoker) from low to high. A ‘dominated’ alternative had either higher expected costs and lower expected outcomes than another comparator; or higher expected costs and the same expected outcomes as another comparator; or the same expected costs and lower expected outcomes as another comparator.
An alternative was subject to ‘extended dominance’ when it could not be considered cost‐effective regardless of a decision maker's threshold value for an outcome. For this, once all dominated alternatives were eliminated and remaining alternatives were placed in ascending order of outcomes, the incremental cost‐effectiveness ratio (ICER) was calculated for each alternative versus the preceding alternative. If the ICER for, for instance, an alternative X was lower than that for another alternative, for instance, Y, and X had greater effectiveness than Y, Y was subject to ‘extended dominance’ through both X and the base comparator. This process led to the identification of the most cost‐effective alternative among the choice set.
As the confidence interval for the relative risk showing effectiveness of cytisine is wide [2.01, 7.87], a deterministic (univariate) sensitivity analysis was conducted to ascertain whether the QALY gains and INB were sensitive to cytisine's effectiveness values. The results were depicted in a tornado diagram. No probabilistic sensitivity analysis was conducted, but the implication of this for the study's conclusions is considered in the Discussion section.
Results
Table 4 presents the QALY gains per 1000 smokers and corresponding incremental net benefit values (per smoker) for all time horizons included in the study. As expected, the benefits of the changes A–E compared with current practice increased over time. For example, change A in the Netherlands led to QALY gains of 0.0043 per 1000 smokers in 2 years but it increased to 0.1049 over the life‐time. The corresponding incremental net benefit values were –€0.38 per smoker in 2 years (a net loss) but €2.53 per smoker during the life‐time (a net gain). A similar trend was observed for England, although QALY gains per 1000 smokers were generally higher in England than in the Netherlands. Change A delivered the least benefit while change E delivered the most benefits on all time horizons. Changes B–D led to a positive return on investment from the second year onwards, while change A did so from the 5th year onwards only in England and during the life‐time in the Netherlands (Table 4).
Table 4.
Short‐, medium‐ and long‐term benefits of alternative changes A–E compared with the current provision.
| Change | Time horizon | Netherlands | England | |||
|---|---|---|---|---|---|---|
| QALYs gained per 1000 smokers | Incremental net benefit (€ per smoker) | QALYs gained per 1000 smokers | Incremental net benefit (£ per smoker) | Incremental net benefit (€ per smoker) (£0.72584 = €1) | ||
| A. Increase reach: GP brief advice | 2 years | 0.0043 | −0.38 | 0.0050 | −0.15 | −0.21 |
| 5 years | 0.0113 | −0.14 | 0.0129 | 0.05 | 0.07 | |
| 10 years | 0.0240 | −0.27 | 0.0259 | 0.37 | 0.51 | |
| Life‐time | 0.1049 | 2.53 | 0.0803 | 1.62 | 2.25 | |
| B. Increase reach: group‐based behavioural therapy | 2 years | 0.0110 | 2.51 | 0.0125 | 0.75 | 1.03 |
| 5 years | 0.0290 | 3.13 | 0.0320 | 1.24 | 1.71 | |
| 10 years | 0.0620 | 4.19 | 0.0643 | 2.03 | 2.80 | |
| Life‐time | 0.2704 | 10.02 | 0.1997 | 5.15 | 7.10 | |
| C. Increase reach: SMS text‐messaging support | 2 years | 0.0032 | 0.09 | 0.0037 | 0.08 | 0.11 |
| 5 years | 0.0086 | 0.27 | 0.0095 | 0.22 | 0.30 | |
| 10 years | 0.0183 | 0.58 | 0.0190 | 0.46 | 0.63 | |
| Life‐time | 0.0800 | 2.31 | 0.0591 | 1.38 | 1.90 | |
| D. Include new but effective pharmacotherapy (cytisine) | 2 years | 0.0461 | 9.19 | 0.0824 | 6.51 | 8.97 |
| 5 years | 0.1218 | 11.78 | 0.2102 | 9.75 | 13.43 | |
| 10 years | 0.2602 | 16.23 | 0.4229 | 14.95 | 20.60 | |
| Life‐time | 1.1351 | 40.70 | 1.3137 | 35.45 | 48.83 | |
| E. Combined change | 2 years | 0.0653 | 11.47 | 0.1049 | 7.23 | 9.96 |
| 5 years | 0.1727 | 15.15 | 0.2677 | 11.35 | 15.64 | |
| 10 years | 0.3690 | 21.45 | 0.5384 | 17.98 | 24.77 | |
| Life‐time | 1.6098 | 56.16 | 1.6726 | 44.07 | 60.72 | |
GP = general practice; SMS = short messaging service.
Table 5 presents the modelling results (life‐time costs and QALYs per smoker) to aid the sequential analysis. The current practice was dominated by all scenarios. Changes A and C were dominated by changes D, B and E; change B was dominated by D and E; and change D was dominated by E. Change E was dominant overall.
Table 5.
Sequential analysis of changes A–E for the Netherlands and England.
| Changea | QALYs per smoker (Netherlands) | Costs (€) per smoker (Netherlands) | QALYs per smoker (England) | Costs (£) per smoker (England) | Costs (€) per smokerb (England) | Both the Netherlands and England |
|---|---|---|---|---|---|---|
| Current practice | 20.9537 | 18301.80 | 14.7909 | 11717.49 | 16143.35 | Dominated by all |
| C. Increase reach: SMS text‐messaging support | 20.9538 | 18301.49 | 14.7910 | 11717.30 | 16143.08 | Dominated by D, B and E |
| A. Increase reach: GP brief advice | 20.9538 | 18301.89 | 14.7910 | 11717.48 | 16143.33 | Dominated by D, B and E |
| B. Increase reach: group‐based behavioural therapy | 20.9540 | 18298.54 | 14.7911 | 11716.34 | 16141.77 | Dominated by D and E |
| D. Include new but effective pharmacotherapy (cytisine) | 20.9548 | 18289.47 | 14.7923 | 11708.32 | 16130.72 | Dominated by E |
| E. Combined change | 20.9553 | 18.285.88 | 14.7926 | 11706.87 | 16128.73 | Dominant over all |
Changes A–E are ranked by ascending QALYs per smoker values. Costs and effects are discounted.
Conversion rate £0.72584 = €1. GP = general practice; SMS = short messaging service.
Figure 1 presents a tornado diagram showing the sensitivity of INB estimates to cytisine effect size values over all time horizons for England and the Netherlands. For this, lower (=2.01) and upper values (=7.87) of relative risk (RR) were used. With the lower value of RR, the INB estimates varied by between −21% (2 years) and −96% (life‐time) for the Netherlands and between −39% (2 years) and −103% (life‐time) for England, compared with the base case value as reported in Table 4. With the upper value of RR, the INB estimates varied by between +42% (2 years) and +189% (life‐time) for the Netherlands and between +77% (2 years) and +203% (life‐time) for England. Both values nevertheless provided positive INB values overall, except for one (the life‐time INB with lower RR value in England was negative).
Figure 1.
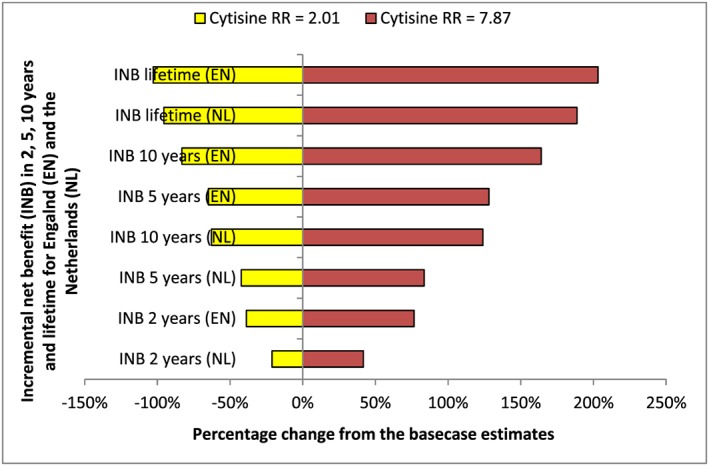
Tornado diagram showing the sensitivity of incremental net benefit to cytisine's effect size values for various time horizons in England and the Netherlands
Discussion
Summary of the key findings
Our analysis suggested that certain changes to the current provision of the smoking cessation services may provide a better ROI compared to the current practice. This was observed in both the Netherlands and England, although the actual ROI was slightly different between the countries. In particular, increasing the reach of brief GP advice, SMS text messaging support and group‐based behavioural therapy as well as including cytisine (which is not currently licensed for use in both countries) as a cessation medication could be cost‐effective policy changes. Implementing all those changes together would be the most cost‐effective policy option.
To appreciate the results fully, it may be worth comparing the differences in smoking‐related data in the two countries. The smoking prevalence in older age groups (Supporting information, Appendix Supplementary information) is higher in the Netherlands than in England, which may have resulted in fewer QALY gains 4, 25. The costs of treating smoking‐attributable diseases in the Netherlands are much higher than in England (Supporting information, Appendix Supplementary information). Together, these may have resulted in the intervention effects being more favourable in England than in the Netherlands.
Implications of the findings
This study provides the English and the Dutch decision‐makers with financial justifications for considering potential changes that could be made to improve the ROI from existing smoking cessation services. If the current smoking cessation services can be reorganized, as suggested in this study, more smokers would make a successful quit attempt. Health gains and cost savings would thus go hand in hand. In particular, this study makes a business case for licensing cytisine, a less costly alternative to varenicline, for use in the current stop smoking services. Decision‐makers now have this important information, as they aim to implement the FCTC more effectively 2.
Comparison with the wider literature
A systematic review of effectiveness studies on the NHS smoking cessation services by Bauld et al. 26 showed that the services were effective in successfully people to stop smoking supporting in the short‐ and long terms. Another systematic review by West and colleagues 18 suggested that smoking cessation services are also effective and affordable in middle‐ and high‐income countries 8. However, it was less clear whether these interventions when implemented collectively (current practice) would be as cost‐effective as when the current provision is reorganized to improve the reach and cut costs. Our study investigated this important policy question by quantifying the net benefit from implementing certain changes to the current provision. The changes analysed in this study are viable policy options (but subject to licensing regulations in the case of cytisine). Therefore, our analysis is expected to encourage similar future studies, e.g. evaluation of potential cost‐effective changes by including computer‐tailored eHealth programmes in the Netherlands 27. This analysis also supports the findings from a previous modelling exercise that looked at the cost‐effectiveness of cytisine versus varenicline 28. However, we extend this knowledge by estimating the probable return on investment if cytisine was included to the current provision of stop smoking services.
Limitations of the study
This study has a few limitations. First, the analyses presented in this study are based on the EQUIPTMOD, and this has meant that the results presented here are subject to the limitations of the model itself 9. Secondly, although the costs internal to the model (e.g. increased cost of group‐based behavioural therapy when its reach is increased) are taken into account in generating ROI metrics, we acknowledge that there may be other costs external to the model (e.g. cost of designing a policy change) which were not considered in the analysis. Given the unavailability of data, we could not be certain to what extent adding such costs would change our conclusions; our best guess is that it would not, given the observed size of the incremental net benefits before such costs were considered. Thirdly, we were not able to assess the extent of practicality of the recommended policy changes; rather, the aim of this analysis is to generate this debate in the policymaking circle. Fourthly, our analysis considered the cost of a full implementation of interventions (e.g. a full course of cytisine was assumed). In real‐world practice, however, most smokers relapse/fail to quit and hence stop using medication, and even those who quit successfully do not use a full course. Our study might therefore have overestimated the implementation costs. However, for the purpose of ROI modelling here, we assumed that a full course of treatment was necessary in order for the interventions to be as effective as in the original trials 18.
Finally, we could not assess the uncertainty concerning point estimates presented in this analysis due to the design of the EQUIPTMOD (i.e. the user interface currently does not support uncertainty estimates for sequential analysis). Given that uncertainty in point estimates from the model has been tested elsewhere, both deterministically and probabilistically 29, 30, the overall estimates presented here may still be robust. With regard to cytisine, the univariate sensitivity analysis presented here suggested that the ROI estimates were sensitive to the uncertainty concerning the effect size of the medication. However, even with the lower value of RR, one could expect a positive incremental net benefit from the inclusion of cytisine to the current provision of stop smoking services.
Conclusion
Current provision of smoking cessation services in the Netherlands and England can benefit from some viable national level changes such as the inclusion of cytisine as a cessation treatment as well as increasing the reach of brief physician advice, text messaging support and group‐based therapy.
Ethical approval
Not applicable in this analysis. However, the main study EQUIPT (http://equipt.eu), of which the current analysis is a part, received full ethical clearance from Brunel University London Research Ethics Committee.
Declaration of interests
None declared, except that R.W. R.W has undertaken paid consultancy and received research funding and hospitality from companies that develop and manufacture smoking cessation medications (Pfizer, GSK, J&J). He is an unpaid adviser to the UK's National Centre for Smoking Cessation and Training.
Supporting information
Supplementary information
Appendix S1 Data used in the European study on Quantifying Utility of Investment in Protection from Tobacco model (EQUIPTMOD).
Table S1 Smoking prevalence for England by age and sex (4).
Table S2 Smoking prevalence for the Netherlands by age and sex (6).
Table S3 Relative risks of smoking attributable diseases by sex and age.
Table S4 Treatment costs for smoking attributable diseases included in the model.
Table S5 Productivity losses, average wage and background quit rates for the Netherlands and England
Table S6 Passive smoking.
Acknowledgements
The EQUIPT project has received funding from the European Union's Seventh Framework Programme for research, technological development and demonstration under grant agreement no. 602 270(EQUIPT). We would like to thank all stakeholders who provided inputs to this study. Thanks are also due to Adam Lester‐George and the anonymous reviewers, for their helpful comments on the earlier draft of this manuscript.
Anraad, C. , Cheung, K.‐L. , Hiligsmann, M. , Coyle, K. , Coyle, D. , Owen, L. , West, R. , de Vries, H. , Evers, S. M. , and Pokhrel, S. (2018) Assessment of cost‐effective changes to the current and potential provision of smoking cessation services: an analysis based on the EQUIPTMOD. Addiction, 113: 96–105. https://doi.org/10.1111/add.14093.
References
- 1. World Health Organization (WHO) report on the global tobacco epidemic, 2013 enforcing bans on tobacco advertising, promotion and sponsorship [internet]. Geneva: World Health Organization; 2013. [cited 2016 April 20]. Available at: http://site.ebrary.com/id/10931319 (accessed 30 June 2016) (Archived at http://www.webcitation.org/6u5iQDpfP on 9 October 2017).
- 2. World Health Organization (WHO) framework convention on tobacco control [internet]. Geneva, Switzerland: World Health Organization; 2003. Available at: http://apps.who.int/iris/bitstream/10665/42811/1/9241591013.pdf (accessed 30 June 2016) (Archived at http://www.webcitation.org/6u5iTZLnI on 9 October 2017). [Google Scholar]
- 3. Joossens L, Raw M. The tobacco control scale 2013 in Europe [internet]. Association of European Cancer Leagues; 2014. Available at: http://www.europeancancerleagues.org/images/TobaccoControl/TCS_2013_in_Europe_13-03-14_final_1.pdf (accessed on 30 June 2016) (Archived at http://www.webcitation.org/6u5iX19bB on 9 October 2017).
- 4. Health Survey England 2013 [internet]. Health and Social Care Information Centre; 2014. December. Available at: http://www.hscic.gov.uk/catalogue/PUB16076 (accessed 30 June 2016) (Archived at http://www.webcitation.org/6u5iZpnRC on 9 October 2017).
- 5. Statistics on Smoking, England [internet]. Health and Social Care Information Centre; 2006. Available at: https://data.gov.uk/dataset/statistics_on_smoking_england (accessed 30 June 2016) (Archived at http://www.webcitation.org/6u5idnVXR on 9 October 2017).
- 6. German: Leefstijl, preventief onderzoek; persoonskenmerken 2010–2013 [internet]. Centraal Bureau voor de Statistiek; 2015. English: [Lifestyle, preventive research; personal characteristics 2010–2013 [internet] — Statistics Netherlands, Central Agency for Statistics]. Available at: http://statline.cbs.nl/Stpublication/publication/?DM=SLNL&PA=81177ned&D1=0,3,10‐11,39‐40,42‐44&D2=0‐12,26‐38&D3=0&D4=l&VW=T (accessed 30 June 2016) (Archived at http://www.webcitation.org/6u5iqOcNK on 9 October 2017).
- 7. German: Gezondheid, leefstijl, zorggebruik; t/m 2009 [Internet]. Den Haag/Heerlen: Centraal Bureau voor de Statistiek; 2010 March [cited 2016 June 30] English: Health, lifestyle, use of healthcare; up until 2009 [Internet] The Hague/Heerlen: Central Bureau for Statistics Gezondheid, leefstijl, zorggebruik; t/m 2009 [Internet]. Den Haag/Heerlen: Statistics Netherlands (Central Agency for Statistics); 2010 March [cited 2016 June 30]. Available at: http://statline.cbs.nl/Statweb/publication/?VW=T&DM=SLNL&PA=03799&D1=202-209&D2=0&D3=0&D4=(l-11)-l&HD=160305-0019 (accessed 30 June 2016) (Archived at http://www.webcitation.org/6u5iqOcNK on 9 October 2017).
- 8. West R., Raw M., McNeill A., Stead L., Aveyard P., Bitton J. et al Health‐care interventions to promote and assist tobacco cessation: a review of efficacy, effectiveness and affordability for use in national guideline development: health‐care interventions: tobacco cessation. Addiction 2015; 110: 1388–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coyle K., Coyle D., Lester‐George A., West R., Kalo Z., Hiligsmann M. et al Development and application of an economic model (EQUIPTMOD) to assess the impact of smoking cessation. Addiction 2017; https://doi.org/10.1111/add.14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pokhrel S., Coyle K., Coyle D., Lester‐George A., West R., Trapero‐Bertran M. et al Estimating return on Investment of Tobacco Control: NICE tobacco ROI tool. London: Health Economics Research Group, Brunel University, National Institure of Health and Care Excellence; 2014. [Google Scholar]
- 11. Hakkaart‐van Roijen L., van der Linden N., Bouwmans C.A.M., Kanters T., Tan S. S. German: Kostenhandleiding, Methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg. English: Costing manual: Methodology of costing research and reference prices for economic evaluations in healthcare. 2015. Available at: http://docplayer.nl/12082781-Kostenhandleiding-methodologie-van-kostenonderzoek-en-referentieprijzen-voor-economische-evaluaties-in-de-gezondheidszorg.html (accessed 16 June 2016) (Archived at http://www.webcitation.org/6vmpKdlh0 on 17 December 2017).
- 12. The EQUIPT Study Group . EQUIPTMOD technical manual Appendix—England [internet]. 2016. Available at: http://equipt.eu/deliverables (accessed 30 June 2016) (Archived at http://www.webcitation.org/6u5iwgrpo on 9 October 2017).
- 13. The EQUIPT Study Group . EQUIPTMOD technical manual Appendix—the Netherlands [internet]. 2016. Available at: http://equipt.eu/deliverables(accessed 30 June 2016) (Archived at http://www.webcitation.org/6u5iwgrpo on 9 October 2017).
- 14. The EQUIPT Study Group . EQUIPTMOD technical manual [internet]. 2016. Available at: http://www.equipt.eu/deliverables/ (accessed 30 June 2016) (Archived at http://www.webcitation.org/6u5iwgrpo on 9 October 2017).
- 15. Public Health England . About us [internet]. 2016. [cited 2016 April 28]. Available at: https://www.gov.uk/government/organisations/public-health-england/about (accessed 30 June 2016) (Archived at http://www.webcitation.org/6u5izGkUc on 9 October 2017).
- 16. German: Het Nederlandse Zorgstelsel. Den Haag: Ministerie van Volksgezondheid, Welzijn en Sport; 2016. English: The Dutch Healthcaresystem. The Hague: Ministry of Health, Welfare and Sport; 2016. [Google Scholar]
- 17. Hajek P. Withdrawal‐oriented therapy for smokers. Addiction 1989; 84: 591–598. [DOI] [PubMed] [Google Scholar]
- 18. West R., Coyle K., Owen L., Coyle D., Pokhrel S., Estimates of effectiveness and reach for ‘return on investment’ modelling of smoking cessation interventions using data from England. Addiction 2017; https://doi.org/10.1111/add.14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pharmacotherapeutic compass. Diemen: Dutch Health Care Insurance Board; 2006. [Google Scholar]
- 20. Vokó Z., Cheung K. L., Józwiak‐Hagymásy J., Wolfenstetter S., Jones T. et al Similarities and differences between stakeholders’ opinions on using health technology assessment (HTA) information across five European countries: results from the EQUIPT survey. Health Res Policy Syst [internet] 2016. https://doi.org/10.1186/s12961-016-0110-7 [cited 2017 April 12]. Available at: 38 http://health-policy-systems.barticles/articles/10.1186/s12961-016-0110-7, 38, 38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Edwards R. T., Charles J. M., Lloyd‐Williams H. Public health economics: a systematic review of guidance for the economic evaluation of public health interventions and discussion of key methodological issues. BMC Public Health 2013; 13: 1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guide to the Methods of Technology Appraisal 2013 [internet]. National Institute for Health and Care Excellence; 2013 April. Available at: https://www.nice.org.uk/article/PMG9/chapter/5-The-reference-case (accessed 30 June 2016) (Archived at http://www.webcitation.org/6u5jB6cSO on 9 October 2017). [PubMed]
- 23. German: Zinnige en duurzame zorg [internet]. Raad voor Volksgezondheid en Samenleving; 2006 June. English: Meaningful and sustainable care [internet]. Council for Public Health and Society; 2006 June. Available at: https://www.raadrvs.nl/publicaties/item/zinnige-en-duurzame-zorg (accessed June 2016) (Archived at http://www.webcitation.org/6u5jD3Nrf on 9 October 2017).
- 24. Morris S., Devlin N., Parkin D. Economic Analysis in Health Care, 2nd edn. Chichester: John Wiley & Sons; 2012. [Google Scholar]
- 25. German: Gezondheidsenquête/Leefstijlmonitor [internet]. Centraal Bureau voor de Statistiek and Rijksinstituut voor Volksgezondheid en Milieu. English: Health survey/Lifestyle monitor. Statistics Netherlands (Central Agency for Statistics) and the National Institute for Public Health and the Environment (RIVM).Available at: https://www.volksgezondheidenzorg.info/content/gezondheidsenqu%C3%AAte-ge-leefstijlmonitor-lsm#overlay-context=onderwerp/overgewicht/cijfers-context/trends (accessed 30 June 2016) (Archived at http://www.webcitation.org/6u5jGUSSw on 9 October 2017).
- 26. Bauld L., Bell K., McCullough L., Richardson L., Greaves L. The effectiveness of NHS smoking cessation services: a systematic review. J Public Health 2010; 32: 71–82. [DOI] [PubMed] [Google Scholar]
- 27. Cheung K. L., Wijnen B., de Vries H. A review of the theoretical basis, effects, and cost effectiveness of online smoking cessation interventions in the Netherlands: a mixed‐methods approach. J Med Internet Res 2017; 19: e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leaviss J., Sullivan W., Ren S., Everson‐Hock E., Stevenson M., Stevens J. W. et al What is the clinical effectiveness and cost‐effectiveness of cytisine compared with varenicline for smoking cessation? A systematic review and economic evaluation. Health Technol Assess [internet] 2014; 18; May [cited 2016 May 5]. Available at: http://www.journalslibrary.nihr.ac.uk/hta/volume-18/issue-33 (accessed 30 June 2016) (Archived at http://www.webcitation.org/6u5jIMXIs on 9 October 2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huber M. B., Präger M., Coyle K., Coyle D., Lester‐George A., Trapero‐Bertran M. et al Cost‐effectiveness of increasing the reach of smoking cessation interventions in Germany: results from the EQUIPTMOD. Addiction 2017; https://doi.org/10.1111/add.14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Trapero‐Bertran M., Muñoz C., Coyle K., Coyle D., Lester‐George A., Pokhrel S. et al Cost‐effectiveness of alternative smoking cessation scenarios in Spain: results from the EQUIPTMOD. Addiction 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hakkaart‐ van Roijen L., Tan S. S., Bouwmans C. A. M. German: Handleiding voor kostenonderzoek, geactualiseerde versie 2010 [internet]. Instituut for Medical Technology Assessment Erasmus Universiteit Rotterdam, College voor Zorgverzekeringen; 2010. English: Manual for costing studies; updated version. Available at: http://docplayer.nl/6361834-Handleiding-voor-kostenonderzoek-methoden-en-standaard-kostprijzen-voor-economische-evaluaties-in-de-gezondheidszorg.html (accessed 30 June 2016) (Archived at http://www.webcitation.org/6u5jKCxND on 9 October 2017).
- 32. Aveyard P., Begh R., Parsons A., West R. Brief opportunistic smoking cessation interventions: a systematic review and meta‐analysis to compare advice to quit and offer of assistance: brief interventions for smoking cessation. Addiction 2012; 107: 1066–1073. [DOI] [PubMed] [Google Scholar]
- 33. Lancaster T., Stead L. F. Individual behavioural counselling for smoking cessation In: The Cochrane Collaboration , editor. Cochrane Database of Systematic Reviews [internet]. Chichester, UK: John Wiley & Sons, Ltd; 2005. [cited 2016 April 25]. https://doi.org/10.1002/14651858.CD001292.pub2. [Google Scholar]
- 34. National Institute for Health and Care Excellence (NICE) Smoking cessation: NICE public health guidance 10. London: NICE; 2008. [Google Scholar]
- 35. Guerriero C., Cairns J., Roberts I., Rodgers A., Whittaker R., Free C. The cost‐effectiveness of smoking cessation support delivered by mobile phone text messaging: Txt2stop. Eur J Health Econ 2013; 14: 789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Whittaker R., McRobbie H., Bullen C., Borland R., Rodgers A., Gu Y. Mobile phone‐based interventions for smoking cessation In: The Cochrane Collaboration , editor. Cochrane Database of Systematic Reviews [internet]. Chichester, UK: John Wiley & Sons, Ltd; 2012. [cited 2016 April 25]. https://doi.org/10.1002/14651858.CD006611.pub3. [Google Scholar]
- 37. Cahill K., Lindson‐Hawley N., Thomas K. H., Fanshawe T. R., Lancaster T. Nicotine receptor partial agonists for smoking cessation In: The Cochrane Collaboration , editor. Cochrane Database of Systematic Reviews [internet]. Chichester, UK: John Wiley & Sons, Ltd; 2016. [cited 2017 Oct 9]. https://doi.org/10.1002/14651858.CD006103.pub7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. US Department of Health and Human Services (USDHHS) . The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: USDHHS; 2014. [Google Scholar]
- 39. Thun M. J., Carter B. D., Feskanich D., Freedman N. D., Prentice R., Lopez A. D. et al 50‐year trends in smoking‐related mortality in the United States. N Engl J Med 2013; 368: 351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kosten van Ziekten database [internet]. Rijksinstituut voor Volksgezondheid en Milieu; 2013. [cited 2016 April 20]. Available at: https://www.volksgezondheidenzorg.info/kosten-van-ziekten#node-zorgkosten-nederland
- 41. Doodsoorzakenstatistiek [internet]. Centraal Bureau voor de Statistiek; n.d. Available at: https://bronnen.zorggegevens.nl/Bron?naam=Doodsoorzakenstatistiek
- 42. Nationaal Kompas Volksgezondheid: Volksgezondheid Toekomstverkenning. versie 4.17 [internet]. Bilthoven: Rijksinstituut voor Volksgezondheid en Milieu; 2014. Available at: http://www.rivm.nl/dsresource?objectid=c71fdd51-029a-4411-8d6f-a79f9a574c11&type=org&disposition=inline
- 43. Suijkerbuijk A. W. M., de Wit G. A., Wijga A., Heijmans M. J. W. M., Hoogendoorn M., Rutten‐van Mölken M. P. M. H. et al Maatschappelijke kosten van astma, COPD en respiratoire allergie. Ned Tijdschr Geneeskd [internet] 2013 November 12;157. Available at: https://www.ntvg.nl/artikelen/maatschappelijke-kosten-van-astma-copd-en-respiratoire-allergie [PubMed]
- 44. Gezondheid en zorg in cijfers, 2007 [internet]. Centraal Bureau voor de Statistiek; 2007. Available at: https://www.cbs.nl/nl-nl/publicatie/2007/47/gezondheid-en-zorg-in-cijfers-2007
- 45. Weng S. F., Ali S., Leonardi‐Bee J. Smoking and absence from work: systematic review and meta‐analysis of occupational studies. Addiction 2013; 108: 307–319. [DOI] [PubMed] [Google Scholar]
- 46. Office for National Statistics (ONS) . Annual Survey Of Hours and Earnings: 2014 Provisional Results. London: ONS; 2014. [Google Scholar]
- 47. West R. Background smoking cessation rates in England [internet]. 2006. Available at: http://www.smokinginengland.info/downloadfile/?type=sts-documents&src=6
- 48. Öberg M., Jaakkola M. S., Prüss‐Üstün A., Schweizer C., Woodward A. Second‐hand smoke: assessing the burden of disease at national and local levels, WHO Environ Burd Dis Ser Geneva: World Health Organization; 2010, p. 18. [Google Scholar]
- 49. Shepherd J., Rogers G., Anderson R., Main C., Thompson‐Coon J., Hartwell D. et al Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long‐acting beta2 agonists for the treatment of chronic asthma in adults and children aged 12 years and over. Health Technol Assess (Winchester) 2008; 12:iii–iv: 1–360. [DOI] [PubMed] [Google Scholar]
- 50. NIVEL . LINH‐zorgregistratie huisartsen. Incidentie‐ en prevalentiecijfers in de huisartsenpraktijk (2011) [internet]. NIVEL; 2011. Available at: http://www.nivel.nl/incidentie-en-prevalentiecijfers-in-de-huisartsenpraktijk
- 51. Wolleswinkel‐van den Bosch J. H., Stolk E. A., Francois M., Gasparini R., Brosa M. The health care burden and societal impact of acute otitis media in seven European countries: results of an internet survey. Vaccine 2010; 28: G39–G52. [DOI] [PubMed] [Google Scholar]
- 52. Office for National Statistics (ONS) . Annual mid‐year population estimates: 2014. London: ONS; 2015. [Google Scholar]
- 53. Royal College of Physicians of London , Tobacco Advisory Group , Royal College of Physicians of London . Passive smoking and children [internet]. London: Royal College of Physicians; 2010. [cited 2016 June 23]. Available at: http://www.dawsonera.com/depp/reader/protected/external/AbstractView/S9781860163760 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information
Appendix S1 Data used in the European study on Quantifying Utility of Investment in Protection from Tobacco model (EQUIPTMOD).
Table S1 Smoking prevalence for England by age and sex (4).
Table S2 Smoking prevalence for the Netherlands by age and sex (6).
Table S3 Relative risks of smoking attributable diseases by sex and age.
Table S4 Treatment costs for smoking attributable diseases included in the model.
Table S5 Productivity losses, average wage and background quit rates for the Netherlands and England
Table S6 Passive smoking.


