Abstract
The purpose of this study was to report the clinical features, laboratory findings, and cytomorphology, and prognosis of three patients with myelomatous pleural effusion (MPE). The literature pertaining to MPE was reviewed. The three cases and literature review suggest that MPE is rare and often associated with a poor prognosis. The correct diagnosis depends on the aggressive clinical characteristics, laboratory findings, and chromosomal abnormalities, but routine pathological examination of the pleural effusion has low sensitivity. Cell blocks stained with hematoxylin & eosin and by immunohistochemistry revealed that abnormal proliferation of plasma cells and light chain restrictive expression in MPE may be helpful for improving the detection rate of MPE.
Keywords: cell block, cytology, immunohistochemistry, multiple myeloma, pleural effusion
1. INTRODUCTION
Multiple myeloma (MM) is a malignant proliferation of plasma cells (PC) that usually invades the bone marrow, replacing the normal bone marrow and producing large amounts of light chain immunoglobulins (Ig).1 Accumulation of these proteins in vital organs, such as the kidneys and heart results in disease manifestation.
Pleural effusion (PE) may be a sign of thoracic involvement affecting about 6% of patients with MM.2, 3 It is particularly rare (<1%) for MM patients to present with myelomatous pleural effusion (MPE), especially for those with PE as an initial sign.3, 4 We describe three cases of MM, who presented with recurrent exudative PE. The purpose of this study was to report the clinical features, laboratory findings, cytomorphology, and prognosis in patients with MPE.
2. CASE REPORTS
2.1. Case 1
A 73‐year‐old woman complained of diffuse bone pain and fatigue for several months. Low back pain had started a few months before and progressed to severe disability. Laboratory findings showed an elevated erythrocyte sedimentation rate, gamma M component, and monoclonal IgG lambda light chain on serum immune electrophoresis. Serum IgG was 3430 mg/dL and lambda light chain was 2870 mg/dL. Radiographic examination showed osteolytic punched‐out lesions in the skull and pelvis. Computed tomography (CT) images of the chest indicated bilateral pleural effusion, especially on the left side, resulting in atelectasis (Figure 1A) and a distinctive pleural nodular‐like thickening. Multiple pathological rib fractures were seen (Figure 1B). Bone marrow aspiration revealed 90% of dystrophic PC. Thoracocentesis yielded exudative effusion with multiple atypical PC. PE smear showed numerous naive PC with a large nucleus, diffuse chromatin and, a moderate rim of basophilic cytoplasm. The ratio of the nucleus and cytoplasm (N/C ratio) was >0.6, and visible giant tumor cells were present (Figure 1C). Cell block of PE with hematoxylin and eosin (HE) staining and immunohistochemistry showed that PC had very prominent, centrally located (immunoblast‐like) nucleoli (Figure 1D) and were positive for CD138, CD38, and lambda light chains (Figure 1E,F), indicating that they were plasmablasts. The diagnosis of MM stage III group B (ISS criteria5) was made. She was treated with cyclophosphamide, thalidomide, and dexamethasone. The pleural effusion resolved completely, but the disease progressed systemically over the following month, and her performance status deteriorated. The effusions recurred bilaterally within 4 months. She was managed with palliative supportive care and died after 5 months.
Figure 1.

(A) CT of the thorax showed bilateral sided pleural effusions especially in the left side resulting in atelectasis. (B) CT showed distinctive nodular‐like thickening of pleura (white arrow) and multiple pathological fractures of the rib (red arrows). (C) PE smear shows numerous naive PC and visible giant tumor cells (black arrow), (HE staining, ×40). (D) Cell block of PE showed: PC have a large nucleus, diffuse chromatin, a very prominent, centrally located (immunoblast‐like) nucleolus (black arrows), (HE staining, ×40). (E) Cell block of PE with immunohistochemistry showed: PC CD138 (+), (Envision, ×40). (F) Cell block of PE with immunohistochemistry showed: PC Lambda (+), (Envision, ×40) [Color figure can be viewed at http://wileyonlinelibrary.com]
2.2. Case 2
A 57‐year‐old female patient reported with a two‐month history of dull right lower chest pain that was not made worse by coughing or deep inspiration. The patient had been on a trial antibiotic treatment, which did not alleviate her symptoms. Therefore, she was diagnosed with “possible tuberculous pleuritis” at the local clinic, and was given antituberculous treatment, without improvement in her condition. No hemoptysis or fever was present. Physical examination revealed dullness and decreased breath sound at the base of the right lung. Magnetic resonance imaging (MRI) examination of the chest confirmed a huge soft tissue mass in the mediastinum, abdominal cavity, and retroperitoneum. The right lung showed multiple patchy masses (Figure 2A). The left side showed PE (Figure 2B). A thoracocentesis was performed and about 1000 mL of pleural fluid was drained from the right side. Analysis showed glucose at 5.17 mmol/L, proteins at 30.3 g/L, LDH at 3490 IU/L, and ADA at 78.6 U/L. The cytological examination revealed many abnormal PC that constituted about 80% of the total nucleated cells in the pleural aspirate. Immune fixation of proteins in the pleural fluid showed monoclonal IgD lambda light chains. Cell block indicated large abnormal proliferation of PC with varying degrees of atypia, including large cells with increased N/C ratios, coarse chromatin, and prominent nucleoli; these cells were identified to be plasmablasts (Figure 2C,D). Immunohistochemistry showed: CD38 (+), CD138 (+), CD56 (+), Kappa (‐), Lambda (+), and Ki67 (80%+) (Figure 2E‐H). The patient was diagnosed with light chain Lambda IgD type MM and underwent chemotherapy with carmustine, etoposide, ifosfamide, and dexamethasone. ISS criteria were stage III group B. The patient had a high extramedullary disease burden that could not be controlled with multiple chemotherapy regimens and she eventually died after 7 months.
Figure 2.

(A) MRI examination of the chest confirmed a huge soft tissue mass in the mediastinum, abdominal cavity, retroperitoneal (white arrow). The right‐side lung showed multiple patchy masses (red arrows). (B) MRI confirmed left‐sided pleural effusion and a soft tissue mass surrounding the vertebra (white arrow). (C) Cell block indicated large abnormal proliferation of PC with varying degrees of atypia, including large cells with increased N/C ratios (black arrow), (HE staining, ×40). (D) Cell block showed: PC have a large nucleus, diffuse chromatin, a very prominent, centrally located (immunoblast‐like) nucleolus (black arrows), (HE staining, ×40). (E) Cell block of PE with immunohistochemistry showed: PC CD38(+), (Envision, ×40). (F) Cell block of PE with immunohistochemistry showed: PC CD138 (+), (Envision, ×40) (G) Cell block of PE with immunohistochemistry showed: PC Lambda (+), (Envision, ×40). (H) Cell block of PE with immunohistochemistry showed: Ki67 index 80%, (Envision, ×40) [Color figure can be viewed at http://wileyonlinelibrary.com]
2.3. Case 3
A 64‐year‐old female presented with a 2‐month history of dry cough, mild fever, and night sweat. There was no chest pain, hemoptysis, or palpitation. Routine laboratory hematological tests revealed white blood cell count at 6.78 × 109/L, hemoglobin at 74 mg/L, platelet count at 60 × 109/L, total proteins at 96.0 g/L, albumin at 24.0 g/L, globulin at 72.0 g/L, and calcium at 1.86 mmol/L. The serum light chain kappa was 10 000 mg/dL and IgG was 7030 mg/dL. Chest high‐resolution computed tomography (HRCT) indicated bilateral PE, especially affecting the right side (Figure 3A) and centrums vermiform destruction (Figure 3B). The bone marrow aspiration biopsy showed hyperplasia of naive plasma cells (80%). Cytological examination of PE puncture and PE cell block indicated abnormal proliferation of PC, which looked like small lymphocytes, partly with plasmacytic differentiation and partly with high N/C ratio (Figure 3C,D). Immunohistochemistry showed: CD38 (+), CD138 (+), Ki67 (50%+), CD56 (+), Kappa (+), and Lambda (−) (Figure 3E,F). The patient was finally diagnosed as MM with pleural infiltration, IgG‐k type, stage III. The patient received chemotherapy (bortezomib and dexamethasone). After two cycles, the patient entered a coma. Blood gas analysis revealed carbon dioxide retention.
Figure 3.
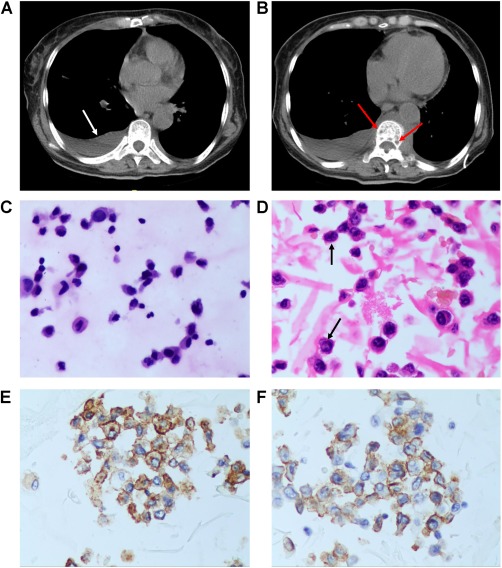
(A) HRCT indicated bilateral sided pleural effusions especially on the right side (white arrow). (B) HRCT indicated centrums vermiform destruction (red arrows). (C) PE smear indicated abnormal proliferation of PC look like small lymphocytes and with or without plasmacytic differentiation (HE staining, ×40). (D) Cell block of PE showed PC look like small lymphocytes partly show high NC ratio (black arrows), (HE staining, ×40). (E) Cell block of PE with immunohistochemistry showed: PC CD138(+), (Envision, ×40). (F) Cell block of PE with immunohistochemistry showed: PC CD56 (+), (Envision, ×40) [Color figure can be viewed at http://wileyonlinelibrary.com]
3. DISCUSSION
MM is a clonal B‐cell malignancy, characterized by proliferation of plasma cells and secretion of paraproteins. These plasma cells accumulate predominantly in the bone marrow; rarely, they invade other areas, especially the thorax. Pleural effusions are rarely associated with MM and most often signify a concurrent disease process. MPE are even more unusual, they occur in <1% of the cases, and carry a poor prognosis.3, 6 The first case of pleural effusion and involvement of serous cavities in MM was reported in 1994 by Rodriguez et al.7 Nevertheless, the incidence of PME could be higher than that reported by Kintzer et al.6, 8 and could depend on the method of detection. Indeed, modern methods such as flow cytometry could allow for the determination of the clonal nature of the disease.3, 9, 10, 11
Oudart et al.12 summarized the six etiologic factors that lead to pleural effusion in MM: congestive heart failure secondary to amyloidosis; chronic renal failure; nephritic syndrome secondary to renal tubular infiltration with paraprotein and development of glomerular damage; direct infiltration of pleural fluid from adjacent tissues; hypoalbuminemia; pulmonary embolism, secondary neoplasm, lymphatic drainage obstruction by tumor infiltration, infection and pleural myelomatous involvement. Here, myelomatous involvement of pleural and adjacent tissues were the most common reason for pleural exudates in our series.
A diagnostic criterion for MPE was suggested in 1994 by Rodriguez et al.7 Three parameters are defined to confirm MPE: (1) demonstration of a monoclonal protein in pleural fluid electrophoresis; (2) detection of atypical plasma cells in the pleural fluid; and (3) histological confirmation with a pleural biopsy or by autopsy.
Generally, the most common isotype in myeloma is IgG (50%), followed by IgA (20%), light chain (20%), and IgD, IgE, IgM, and biclonal (<10%).13 Some MPE case reports have been published.14, 15, 16, 17, 18 A study of 23 patients with MM and PE by Zhong et al.19 represents the largest case series to date. In their study, 80% of PE cases were IgA subtype, whereas most of the remaining cases were classified as the IgG subtype.20 More cases of PE in the left side of the chest have been reported.21 Among our three cases, two presented with IgG type MM and the other was IgD. One important feature was that the two cases had bilateral pleural effusion. Due to plasma cell infiltration into the pleura.22
Cytological identification of malignant plasma cells within the pleural effusion has been considered as the best diagnostic method for MPE,23 especially for those patients who present with pleural effusion as one of the first signs. Sometimes, their routine blood and fluid biochemistry tests are nonspecific. In these cases, it is hard for a respiratory physician to take hematological malignancy into consideration and traumatic investigation is needed to give a final diagnosis. Due to the limited number of malignant plasma cells and their potential in vitro degeneration, diagnosis may fail in patients undergoing pleural effusion puncture examination.8 In our cases, case#2 had been misdiagnosed at their local clinic. We found diagnosis was assisted by taking advantage of the paraffin embedded cell block technique after centrifugation of the pleural effusion. The pathological sections easily revealed abnormal proliferation of plasmocytes and immunophenotyping. Therefore, cell blocks should be considered to assist with the diagnosis and differential diagnosis of serous cavity effusion. Compared to pleural biopsy, pleural effusion cell block is simpler and accurate, with less pain to the patient. Pleural biopsies are not always done in these patients and when done they are not always diagnostic or even helpful.24, 25
Morphology of plasma cells can be divided into three categories, mainly based on changes to the nucleus, such as diffuse chromatin pattern, prominent nucleolus, irregular contour of the nuclear membrane, and/or nuclear size larger than expected. These criteria correspond to “immaturity” or “aggressiveness” of the PC clone in MM.26 In about half of the cases (40%‐50% patients) with the so‐called “mature” PC subtype, N/C ratio asynchrony is observed, i.e., presence of one nucleolus, finely dispersed chromatin, and/or irregular nuclear contour contrast with a still large and blue (mature) cytoplasm. PC display condensed chromatin and the nucleolus is either absent or inconspicuous or <1 µm in size, as in normal PC.
One subtype is related to a low amount of cytoplasm, defined as lymphoplasmacytoid myeloma (10%‐15% patients)27 or as small cell type.28 If nearly all PC from this subtype display mature chromatin, at least 25%‐30% show high N/C ratio, usually >0.6.29 Another subtype, referred to as small lymphocyte‐like PC myeloma and representing 3.4% of all MM cases, was reported by Heerema‐McKenney et al.30 In this subtype, most PC look like small lymphocytes (at least 50% of the tumor cells) and may mimic mature B‐cell lymphoma with or without plasmacytic differentiation.30 This subtype may be also viewed as a peculiar variant of the lymphoplasmacytoid subtype.
A peculiar morphological change, corresponding to the presence of very immature plasmocytes named plasmablasts, is observed in 10%‐15% cases. Plasmablasts are characterized by a large nucleus (>10 µm in diameter), diffuse chromatin pattern, a very prominent, centrally located (immunoblast‐like) nucleolus >2 µm in diameter, and a moderate rim of basophilic cytoplasm (N/C ratio >0.6) with a faint or absent perinuclear clearing.31 They are considered as the most immature PC.32
Mature myeloma is related to favorable outcome, while immature myeloma, particularly plasmablastic myeloma, is related to dismal prognosis. Nevertheless, such classification is no longer included in current prognostic schemes.33 Among our three cases, two were of the lymphoplasmacytoid type and one was of the plasmablasts type. There was no mature PC.
The overall median survival for MPE has been reported as 4 months (ranging from 3 to 50 months), which is much lower than the median survival of 29 months observed in stage III MM.34 Among our patients, one died 5 months after diagnosis of MPE, one died after 7 months, and one was in a coma (the relatives asked for discharge and she was lost to follow‐up). All cases were stage III. The reason why these MPE patients showed short survival is relatively clear. At diagnosis, most patients were classified with advanced clinical stages using the ISS system. At the time of development of MPE, their laboratory characteristics already indicated an aggressive clinical course.
4. CONCLUSION
The present report and literature review suggest that MPE is rare and often associated with a poor prognosis. The correct diagnosis depends on the aggressive clinical characteristics, laboratory findings, and chromosomal abnormalities, but routine pathological examination of PE has low sensitivity. Cell blocks stained with HE and by immunohistochemistry revealed that abnormal proliferation of PC and light chain restrictive expression in MPE may be useful for improving the detection rate of MPE.
CONFLICT OF INTEREST
All authors declare that they have no any conflict of interests.
Disclosure of Grants
None.
ACKNOWLEDGMENTS
None.
Chen H, Li P, Xie Y, Jin M. Cytology and clinical features of myelomatous pleural effusion: Three case reports and a review of the literature. Diagnostic Cytopathology. 2018;46:604–609. https://doi.org/10.1002/dc.23894
REFERENCES
- 1. Kyle RA, Rajkumar SV. Plasma cell disorders In: Goldman L, Ausiello DA, eds. Cecil Textbook of Medicine. Philadelphia: WB Saunders; 2004. [Google Scholar]
- 2. Uskul BT, Turker H, Emre Turan F, et al. Pleural effusion as the first sign of multiple myeloma. Tuberk Toraks. 2008;56:439–442. [PubMed] [Google Scholar]
- 3. Harbhajanka A, Brickman A, Park JW, Reddy VB, Bitterman P, Gattuso P. Cytomorphology, clinicopathologic, and cytogenetics correlation of myelomatous effusion of serous cavities: a retrospective review. Diagn Cytopathol. 2016;44:742–747. [DOI] [PubMed] [Google Scholar]
- 4. Xu XL, Shen YH, Shen Q, Zhou JY. A case of bilateral pleural effusion as the first sign of multiple myeloma. Eur J Med Res. 2013;18:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–3420. [DOI] [PubMed] [Google Scholar]
- 6. Kintzer JS, Jr. , Rosenow EC, 3rd , Kyle RA. Thoracic and pulmonary abnormalities in multiple myeloma. A review of 958 cases. Arch Intern Med. 1978;138:727–730. [PubMed] [Google Scholar]
- 7. Rodriguez JN, Pereira A, Martinez JC, Conde J, Pujol E. Pleural effusion in multiple myeloma. Chest. 1994;105:622–624. [DOI] [PubMed] [Google Scholar]
- 8. Wang Z, Xia G, Lan L, et al. Pleural effusion in multiple myeloma. Intern Med. 2016;55:339–345. [DOI] [PubMed] [Google Scholar]
- 9. Bode‐Lesniewska B. Flow cytometry and effusions in lymphoproliferative processes and other hematologic neoplasias. Acta Cytol. 2016;60:354–364. [DOI] [PubMed] [Google Scholar]
- 10. Tong LC, Ko HM, Saieg MA, Boerner S, Geddie WR, da Cunha Santos G. Subclassification of lymphoproliferative disorders in serous effusions: a 10‐year experience. Cancer Cytopathol. 2013;121:261–270. [DOI] [PubMed] [Google Scholar]
- 11. Arora P, Gupta SK, Mallik N, Mittal R, Sharma OD, Kumar L. Flow cytometry in diagnosis of myelomatous pleural effusion: a case report. Indian J Hematol Blood Transfus. 2016;32:138–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oudart JB, Maquart FX, Semouma O, Lauer M, Arthuis‐Demoulin P, Ramont L. Pleural effusion in a patient with multiple myeloma. Clin Chem. 2012;58:672–674. [DOI] [PubMed] [Google Scholar]
- 13. McKenna RW, Kyle RA, Kuehl WM. Plasma cell neoplasms In: Swerdlow SH, Campo E, Harris NL, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2008. [Google Scholar]
- 14. Amaraneni A, Saeed U, Malik D, Brown M, Chandana SR. Bilateral myelomatous pleural effusion: presentation of two cases. Blood Res. 2016;51:142–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghorbel IB, Feki NB, Lamloum M, et al. Pleural myelomatous involvement in multiple myeloma: five cases. Ann Saudi Med. 2015;35:327–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang LL, Li YY, Hu CP, Yang HP. Myelomatous pleural effusion as an initial sign of multiple myeloma‐a case report and review of literature. J Thorac Dis. 2014;6:E152–E159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dharan M. Unilateral pleural effusion as a presenting manifestation of plasma cell myeloma (multiple myeloma): a case report. Acta Cytol. 2010;54:780–782. [PubMed] [Google Scholar]
- 18. Chang H, Chou WC, Lee SY, Huang JY, Hung YH. Myelomatous pleural effusion in a patient with plasmablastic myeloma: a case report. Diagn Cytopathol. 2009;37:205–207. [DOI] [PubMed] [Google Scholar]
- 19. Zhong Y, Zhang J, Wang H. Myelomatous pleural effusion involvement in 23 patients with multiple myeloma: a single‐center clinical analysis. Thorac Cancer. 2015;6:359–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim YM, Lee KK, Oh HS, et al. Myelomatous effusion with poor response to chemotherapy. J Korean Med Sci. 2000;15:243–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim YJ, Kim SJ, Min K, et al. Multiple myeloma with myelomatous pleural effusion: a case report and review of the literature. Acta Haematol. 2008;120:108–111. [DOI] [PubMed] [Google Scholar]
- 22. Raci‐Wetherbee E, Dincer HE. IgG myeloma presenting as a large mediastinal mass and pleural effusion. J Bronchology Interv Pulmonol. 2012;19:65–67. [DOI] [PubMed] [Google Scholar]
- 23. Safa AM, Van Ordstrand HS. Pleural effusion due to multiple myeloma. Chest. 1973;64:246–248. [DOI] [PubMed] [Google Scholar]
- 24. Lau LG, Chng WJ, Tan LH, Liu TC. Malignant pleural effusion in a patient with multiple myeloma. Diagn Cytopathol. 2005;32:171–172. [DOI] [PubMed] [Google Scholar]
- 25. Natori K, Izumi H, Nagase D, et al. IgD myeloma indicated by plasma cells in the peripheral blood and massive pleural effusion. Ann Hematol. 2008;87:587–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goasguen JE, Zandecki M, Mathiot C, et al. Mature plasma cells as indicator of better prognosis in multiple myeloma. New methodology for the assessment of plasma cell morphology. Leuk Res. 1999;23:1133–1140. [DOI] [PubMed] [Google Scholar]
- 27. Fonseca R, Blood EA, Oken MM, et al. Myeloma and the t(11;14)(q13;q32); evidence for a biologically defined unique subset of patients. Blood. 2002;99:3735–3741. [DOI] [PubMed] [Google Scholar]
- 28. Bartl R, Frisch B, Fateh‐Moghadam A, Kettner G, Jaeger K, Sommerfeld W. Histologic classification and staging of multiple myeloma. A retrospective and prospective study of 674 cases. Am J Clin Pathol. 1987;87:342–355. [DOI] [PubMed] [Google Scholar]
- 29. Garand R, Avet‐Loiseau H, Accard F, Moreau P, Harousseau JL, Bataille R. t(11;14) and t(4;14) translocations correlated with mature lymphoplasmacytoid and immature morphology, respectively, in multiple myeloma. Leukemia. 2003;17:2032–2035. [DOI] [PubMed] [Google Scholar]
- 30. Heerema‐McKenney A, Waldron J, Hughes S, et al. Clinical, immunophenotypic, and genetic characterization of small lymphocyte‐like plasma cell myeloma: a potential mimic of mature B‐cell lymphoma. Am J Clin Pathol. 2010;133:265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bartl R, Frisch B, Burkhardt R, et al. Bone marrow histology in myeloma: its importance in diagnosis, prognosis, classification and staging. Br J Haematol. 1982;51:361–375. [DOI] [PubMed] [Google Scholar]
- 32. Fritz E, Ludwig H, Kundi M. Prognostic relevance of cellular morphology in multiple myeloma. Blood. 1984;63:1072–1079. [PubMed] [Google Scholar]
- 33. Ribourtout B, Zandecki M. Plasma cell morphology in multiple myeloma and related disorders. Morphologie. 2015;99:38–62. [DOI] [PubMed] [Google Scholar]
- 34. Kamble R, Wilson CS, Fassas A, et al. Malignant pleural effusion of multiple myeloma: prognostic factors and outcome. Leuk Lymphoma. 2005;46:1137–1142. [DOI] [PubMed] [Google Scholar]


