Abstract
Objective
Active surveillance (AS) offers a strategy to reduce overtreatment and now is a widely accepted treatment option for low-risk prostate cancer. An ideal tool for risk-stratification would detect aggressive cancers and exclude such men from taking up AS in the first place. We evaluate if a combination of transperineal template biopsy with magnetic resonance imaging (MRI)-targeted biopsy identifies significant prostate cancer amongst men initially diagnosed with low-risk prostate cancer.
Methods
This prospective, single-blinded study included men with low-risk prostate cancer (D'Amico's Criteria) diagnosed on conventional transrectal ultrasound-guided biopsy. Patients first underwent multiparametric MRI of the prostate ≥6 weeks after initial biopsy. Each suspicious lesion is mapped and assigned a Prostate Imaging Reporting and Data System (PIRADS) score. Template biopsy is first performed with the surgeon blinded to MRI findings followed by MRI-targeted biopsy using a robotic transperineal biopsy platform.
Results
The age of the 19 men included is 65.4 ± 4.9 years (mean ± SD). Prostate specific antigen (PSA) at diagnosis and at the time of transperineal biopsy were comparable (7.3 ± 1.7 ng/mL and 7.0 ± 1.8 ng/mL, p = 0.67), so were prostate volumes (34.2 ± 8.9 mL and 32.1 ± 13.4 mL, p = 0.28). MRI-targeted biopsy had a higher percentage of cancer detection per core compared to template biopsy (11.7% vs. 6.5%, p = 0.02), this was more than 3 times superior for Gleason 7 disease (5.9% vs. 1.6%, p < 0.01). Four of 18 (22.2%) patients with MRI lesions had significant disease with MRI-targeted biopsy alone. Three of 19 patients (15.8%) had significant disease with template biopsy alone. In combination, both techniques upclassified five patients (26.3%), all of whom underwent radical prostatectomy. Whole mount histology confirmed tumour location and grade. All six patients with PIRADS 5 lesions had cancer detected (66.6% significant disease).
Conclusion
A combination of MRI-targeted and template biopsy may optimally risk-classify “low-risk” patients diagnosed on initial conventional transrectal ultrasonography (TRUS) prostate biopsy.
Keywords: Active surveillance, Magnetic resonance imaging, Targeted biopsy, Transperineal prostate biopsy, Robotic biopsy, Low-risk prostate cancer
1. Introduction
Majority of low-risk prostate cancers are indolent and unlikely to significantly impact a man's lifespan. In the low-risk setting, active surveillance (AS) performs as well as curative treatment and in prospective cohort studies, has allowed up to 50%–60% of men to remain intervention-free at 10–15 years from diagnosis [1], [2], [3]. While inclusion criteria into AS trials are generally strict, at first rebiopsy, histological upgrading rates of up to 28% are still observed, resulting in an increase in risk category [4], [5]. This is thought to be largely due to shortcomings of the traditional 12-core random biopsy technique in adequately sampling the entire prostate gland resulting in under-detection of aggressive cancer. This may, in turn, contribute to patient anxiety and reduced uptake of AS as well as the need for fairly intensive regime of regular surveillance biopsies to subsequently identify those with more aggressive tumours. Indeed, among post-prostatectomy cohorts, men who initially fulfilled low-risk criteria had a 30%–50% chance of harboring higher-grade cancer upon complete examination of the prostate [6]. Efforts to address the weaknesses of conventional biopsy and better stratify patients for AS or treatment have now led to a proliferation of imaging, imaging-guided biopsy, mapping biopsy and biomarker tests [7].
An ideal tool for risk-stratification would detect aggressive cancers and exclude such men from taking up AS in the first place. Multiparametric magnetic resonance imaging (mpMRI) is a promising modality in the detection and localization of prostate cancer. MpMRI has a positive predictive value of up to 98% for clinically significant prostate cancer with especially good performance in higher-grade and larger tumours [8], [9]. Using mpMRI-targeted biopsy to provide histological confirmation of cancer grade could thus potentially be the risk-stratifying approach that is required, though this remains to be proven. We sought to evaluate the ability of a comprehensive biopsy comprising both transperineal mapping biopsy combined with mpMRI-targeted biopsy in identifying aggressive prostate cancer as a staging technique among men initially diagnosed with low-risk prostate cancer and deemed to be suitable candidates for AS. We report here, the findings from our pilot experience.
2. Materials and methods
2.1. Patients
This is an institutional review board (IRB)-approved, prospective, single-blinded pilot study that included 19 men with low-risk prostate cancer according to D'Amico's Criteria (prostate specific antigen (PSA) ≤10 ng/mL, Gleason score ≤6, clinical stage ≤T2a) diagnosed on conventional transrectal ultrasonography (TRUS) biopsy. This clinical trial was conducted in accordance to the principles of the Helsinki Declaration and in adherence to the Standards of Reporting for MRI-targeted Biopsy Studies (START) recommendations [10].
2.2. MpMRI
All patients underwent a high field mpMRI examination. MRI images were obtained with a 3-T MRI imaging system (Magnetom Verio, Siemens, Erlangen, Germany) using a multi-channel pelvic phased array coil. The MRI protocols included high spatial resolution T2-weighted imaging in the axial, sagittal and coronal planes (turbo spin echo sequences), diffusion weighted imaging (DWI) in the axial plane (b values: 0–50, 500 and 1000 s/mm2) and dynamic contrast-enhanced (DCE) images. The acquisition parameters are summarised in Table 1. Apparent diffusion coefficient maps were generated from the diffusion weighted images by using the mono-exponential model on a voxel-wise basis, fitting the b-value data. For the DCE MRI, gadoterate meglumine (DOTAREM®, Guerbet LLC, Bloomington, IN, USA), was administered via an automatic power injector (Medrad, Indianola, PA, USA) at a dose of 0.1 mmol/kg body weight at a rate of 3 mL/s.
Table 1.
Acquisition parameters for multiparametric MRI of prostate.
| T2-Weighted | DWI | DCE | |
|---|---|---|---|
| Echo time/Repetition time (ms) | 82/5700 | 93/7700 | 1.76/4.86 |
| Slice thickness (mm) | 3 | 3 | 3 |
| Matrix | 384 × 384 | 144 × 160 | 154 × 192 |
| Field of view (mm) | 200 | 260 | 260 |
| Interslice gap (%) | 10 | 10 | 0 |
MRI, magnetic resonance imaging; DCE, dynamic contrast enhancement; DWI, diffusion weighted imaging.
A single dedicated genitourinary radiologist (with 7 years experience in prostate MRI) prospectively read and scored all the lesions. All assessments were made on a commercial Picture Archiving and Communication System (PACS) workstation (Carestream, Rochester, NY, USA). Each suspicious lesion detected was marked on an internationally standardised 24-sector prostate template grid [10] and assigned a Prostate Imaging Reporting and Data System (PIRADS) score. The studies performed prior to December 2014 were scored with the ESUR/PIRADS version 1.0 criteria. The studies performed from December 2014 were scored with the updated PIRADS version 2.0 criteria. The completed mapping template was then sealed in an envelope only to be opened on the day of biopsy.
2.3. Template biopsy followed by MRI-targeted biopsy
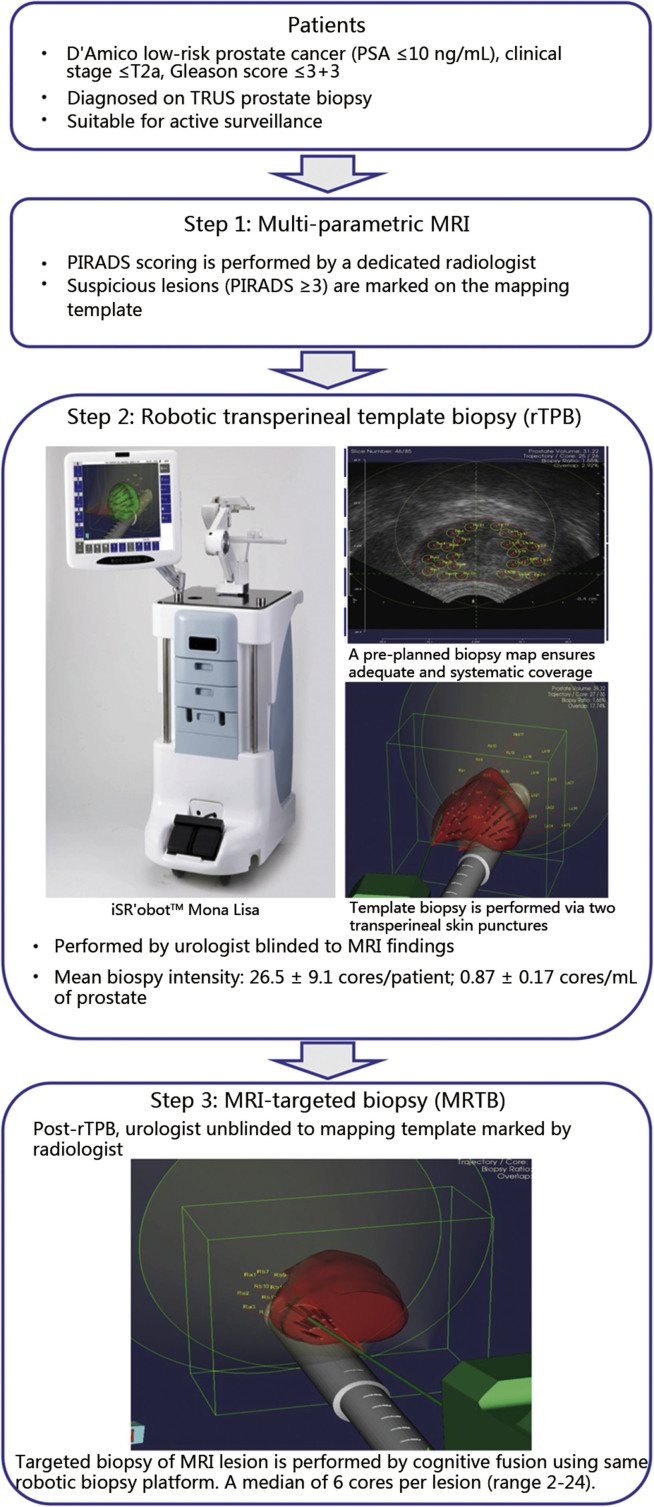
Systematic template biopsy is first performed under general anaesthesia with the surgeon blinded to the MRI findings, using a robotic transperineal biopsy guidance platform (iSR'obot™ Mona Lisa, Biobot Surgical, Singapore), which was previously described [11], [12]. In brief, this system uses real-time 3D scanning technology coupled with the ultrasound image-guided system. The dual-cone concept with an innovative virtual pivot point allows maximal coverage of the prostate and multiple needle biopsies through only two perineal skin punctures to targeted locations in two fan-shaped trajectories. The positions of the needle biopsies are pinpointed with an accuracy of ±1 mm [12]. The number of cores taken in the template biopsy was based on a computer generated volume-dependent algorithm. This ensures uniform biopsy intensity for all patients. Upon completion of robotic transperineal template prostate biopsy (rTPB), the envelope containing the MRI targets was opened and the surgeon was then unblinded to the mapping template marked by the radiologist. Targeted biopsy was then planned and performed by cognitive fusion using the same robotic biopsy platform at the same setting. An average of 6 cores were taken per MRI lesion. Fig. 1 shows a flowchart that illustrates the sequence of events for patients in this trial.
Figure 1.
Flowchart of trial protocol. MRI, magnetic resonance imaging; PIRADS, Prostate Imaging Reporting and Data System; PSA, prostate specific antigen; TRUS, transrectal ultrasonography.
2.4. Outcome measures and statistical analysis
The rate of disease up-classification as well as cancer detection by rTPB and MRI-targeted prostate biopsy (MRTB) were analysed. Clinically significant disease was defined as disease containing Gleason component grade of 4 or higher grade. The association of PIRADS scoring [13], [14] of MRI lesion with significant disease was examined. Histological analysis was performed for five patients who eventually underwent radical prostatectomy. The tumour grades of biopsy cores and final histology were correlated and the correlations of tumour locations reported on MRI and whole mount histology were examined in these cases with the aid of a single dedicated pathologist (Aydin H). Further analysis including volume reclassification was done between combination biopsy and TRUS biopsy for cases with Gleason 6 detected on combination biopsy. Volume upclassification was defined in this study as a maximal core percentage of cancer greater than that of the original TRUS biopsy. Biopsy quality was also examined between MRTB and rTPB for cases with equivalent Gleason grade detection on both MRTB and rTPB. Continuous variables were reported as the mean ± SD or median. Fisher's exact test was used for comparison of detection rates between the two modalities. A p-value of less than 0.05 was considered significant. All statistical analysis was performed using IBM SPSS Statistics 23 (IBM, Armonk, NY, USA).
3. Results
The 19 men enrolled in the study have a mean age of 65.4 ± 4.9 years. The mean PSA at diagnosis by TRUS prostate biopsy was comparable to that at the time of rTPB and MRTB (7.3 ± 1.7 ng/mL v.s. 7.0 ± 1.8 ng/mL, p = 0.67) (Table 2). At TRUS biopsy, the mean prostate volume was 34.2 ± 8.9 mL and this did not differ much from the mean prostate volume at time of rTPB and MRTB (32.1 ± 13.4 mL, p = 0.28). The median interval from last PSA measurement to rTPB and MRTB was 8 weeks and the median interval from initial TRUS biopsy to mpMRI is 8.3 weeks (range 4.3–62.0). The mean biopsy intensity for rTPB was 0.87 ± 0.12 cores/mL prostate. The median operative time taken is 15 min (range 13–21).
Table 2.
Baseline demographics (n=19).
| Baseline characteristics | TRUS biopsy | rTPB | MRTB | p-Value |
|---|---|---|---|---|
| Age (year)a | 65.4 ± 4.9 | |||
| PSA at biopsy (ng/mL)a | 7.3 ± 1.7 | 7.0 ± 1.8 | 0.67 | |
| Interval of last PSA to biopsy (week)b | 4 (1–11) | 8 (4–57) | ||
| Prostate volume at biopsy (mL)a | 34.2 ± 8.9 | 32.1 ± 13.4 | 0.28 | |
MRTB, magnetic resonance imaging-targeted prostate biopsy; PSA, prostate specific antigen; rTPB, robotic transperineal template prostate biopsy; TRUS, transrectal ultrasonography.
Values are expressed as mean ± SD.
Values are expressed as median (range).
There were a total of 239 targeted cores of which 28 were positive for cancer, while 33 out of 511 template cores were positive for the 19 patients. This translated to a higher percentage of cancer detection per core from MRTB compared to rTPB (11.7% vs. 6.5%, p = 0.02). Specifically looking at percentage of Gleason score 7 (Gleason grade 3 + 4 or 4 + 3) detection per core, MRTB is more than 3 times superior to rTPB (5.9% for targeted core vs. 1.6% for template core, p < 0.01) (Table 3). The mean number of cores taken per patient for rTPB and MRTB was 26.9 ± 8.2 and 13.3 ± 5.8 respectively (p < 0.05) while the mean number of positive cores arising from rTPB and MRTB per patient was 1.74 ± 2.23 and 1.56 ± 2.79 respectively (p = 0.83) (Table 3). The mean maximum percentage core involvement was however significantly higher for MRTB compared to rTPB (25.6 ± 29.6 vs. 9.40 ± 9.15, p = 0.03).
Table 3.
Comparison of biopsy characteristics and outcomes of MRTB and rTPB.
| Variables | MRTB | rTPB | p-Value |
|---|---|---|---|
| Total no. of cores | 239 | 511 | |
| No. of positive cores | 28 | 33 | |
| No. of Gleason 7 cores | 14 | 8 | |
| Percentage of cancer detection per core (%) | 11.7 | 6.5 | 0.02 |
| Percentage of Gleason 7 detection per core (%) | 5.9 | 1.6 | <0.01 |
| Per patient analysis (mean ± SD) | |||
| No. of biopsy cores | 13.3 ± 5.8 | 26.9 ± 8.2 | <0.05 |
| Biopsy intensity (cores/mL) | 0.87 ± 0.12 | ||
| No. of positive cores | 1.56 ± 2.79 | 1.74 ± 2.23 | 0.83 |
| Max percentage core (%) | 25.6 ± 29.6 | 9.40 ± 9.15 | 0.03 |
MRTB, magnetic resonance imaging-targeted prostate biopsy; rTPB, robotic transperineal template prostate biopsy.
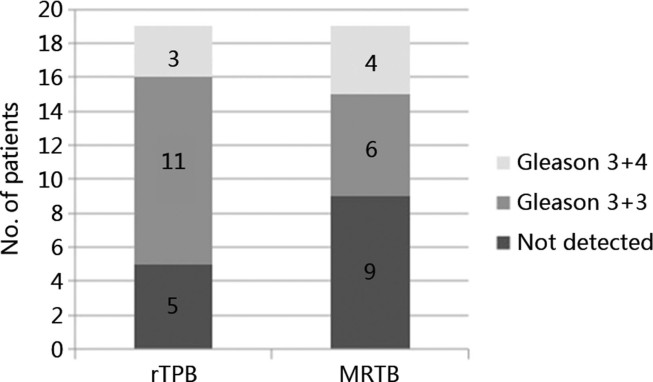
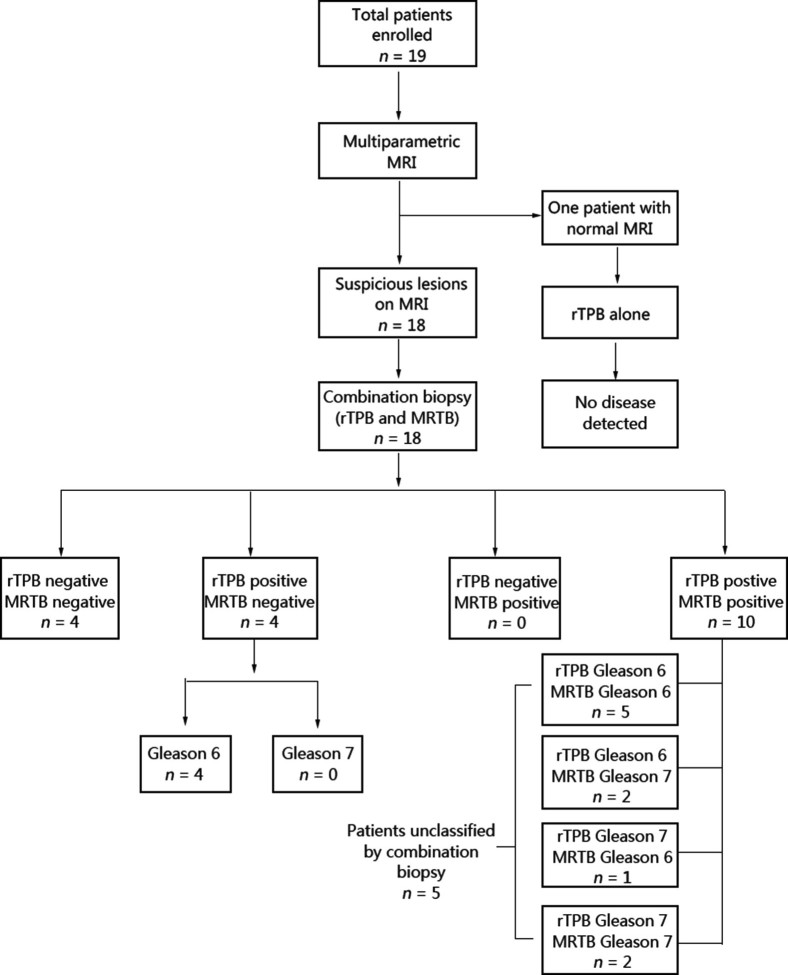
Of the 19 men, one did not have any MRI lesion detected. He underwent a 20-core template biopsy alone, which proved to be negative. Eighteen patients had lesions detected on MRI of which four (22.2%) had significant disease detected by MRTB alone. On the other hand, three out of 19 patients (15.8%) had significant disease detected by rTPB alone (Fig. 2). Two of the four patients with significant disease detected on MRTB also had significant disease detected in rTPB cores. This leaves two patients with significant disease detected solely by MRTB and one patient with significant disease detected solely by rTPB (Fig. 3). Table 4 summarises the parameters and biopsy outcome of all 19 men.
Figure 2.
Proportion of upclassification with rTPB and MRTB. MRTB, magnetic resonance imaging-targeted prostate biopsy; rTPB, robotic transperineal template prostate biopsy.
Figure 3.
Flow diagram of outcomes of all 19 men. MRI, magnetic resonance imaging; MRTB, MRI-targeted prostate biopsy; rTPB, robotic transperineal template prostate biopsy.
Table 4.
Age, PSA, prostate volume and biopsy outcomes of all 19 men.
| Patient code | Age (year) | PSA at biopsy (ng/mL) | Prostate volume (mL) | MRI highest PIRADS | rTPB |
MRTB |
||
|---|---|---|---|---|---|---|---|---|
| No. positive cores/total cores taken | Gleason score | No. positive cores/total cores taken | Gleason score | |||||
| 1 | 75 | 6.2 | 33.3 | 4 | 0/22 | NA | 0/8 | NA |
| 2 | 65 | 8.3 | 40.0 | 4 | 1/28 | 6 | 0/10 | NA |
| 3a | 61 | 5.6 | 31.0 | 5 | 10/26 | 7 | 2/18 | 7 |
| 4 | 71 | 7.0 | 37.0 | 4 | 1/26 | 6 | 0/23 | NA |
| 5 | 59 | 9.7 | 17.5 | 5 | 1/18 | 6 | 2/18 | 6 |
| 6 | 63 | 7.1 | 22.0 | NA | 0/20 | NA | NA | NA |
| 7 | 66 | 8.2 | 37.0 | 4 | 1/27 | 6 | 0/18 | NA |
| 8b | 58 | 5.8 | 21.0 | 4 | 2/24 | 7 | 1/12 | 6 |
| 9 | 68 | 7.6 | 36.6 | 4 | 2/28 | 6 | 0/8 | NA |
| 10c | 68 | 9.9 | 14.0 | 5 | 3/17 | 6 | 12/12 | 7 |
| 11c | 68 | 5.8 | 20.0 | 5 | 2/20 | 6 | 3/6 | 7 |
| 12 | 69 | 10.0 | 49.0 | 3 | 0/32 | NA | 0/6 | NA |
| 13 | 72 | 6.1 | 60.0 | 4 | 3/52 | 6 | 1/19 | 6 |
| 14 | 58 | 4.7 | 58.0 | 3 | 0/38 | NA | 0/24 | NA |
| 15 | 71 | 7.8 | 28.7 | 5 | 2/23 | 6 | 2/17 | 6 |
| 16 | 64 | 4.0 | 32.0 | 3 | 1/25 | 6 | 2/12 | 6 |
| 17a | 61 | 7.0 | 26.0 | 5 | 2/22 | 7 | 2/6 | 7 |
| 18 | 63 | 8.2 | 31.0 | 3 | 0/28 | NA | 0/10 | NA |
| 19 | 63 | 4.7 | 39.0 | 3 | 2/35 | 6 | 1/12 | 6 |
MRTB, magnetic resonance imaging-targeted prostate biopsy; NA, not applicable; PIRADS, Prostate Imaging Reporting and Data System; PSA, prostate specific antigen; rTPB, robotic transperineal template prostate biopsy.
Patients upclassified by both MRTB and rTPB.
Patients upclassified by rTPB alone.
Patients upclassified by MRTB alone.
Combination biopsy detected Gleason 6 disease (equivalent biopsy outcome as TRUS biopsy) in nine patients of which five patients (55.6%) had volume upclassification and greater number of positive cores detected compared to TRUS biopsy (Table 5). Biopsy Gleason grade was similar on MRTB and rTPB in seven patients (five patients with Gleason 6, two patients with Gleason 7) and analysis of the biopsy quality between the two shows a higher percentage cancer detection per core (11.8% vs. 10.4%) and mean maximum core percentage of positive cores (27.5% vs. 16.1%) for MRTB (Table 6).
Table 5.
Comparison of No. of positive cores and maximum core percentage of cancer of combination biopsy with TRUS biopsy for all cases of Gleason 6 and 7 disease detected by combination biopsy.
| Parameters of biopsy quality | Combination biopsy |
|
|---|---|---|
| Gleason 6 (n = 9) | Gleason 7 (n = 5) | |
| No. of positive cores compared to TRUS Biopsy | ||
| Greater Equal Less |
5 3 1 |
5 0 0 |
| Volume reclassification (Max core percentage) compared to TRUS Biopsy | ||
| Yes No |
5 4 |
5 0 |
TRUS, transrectal ultrasonography.
Table 6.
Comparison between MRTB and rTPB for cases of equivalent grade detection (Gleason 6 and 7) with both modalities.
| Variables | MRTB | rTPB | p-Value |
|---|---|---|---|
| Total No. of cores | 102 | 201 | |
| No. of positive cores | 12 | 21 | |
| Mean maximum core percentage of positive cores (%) | 27.5 | 16.1 | 0.21 |
| Percentage of cancer detection per core (%) | 11.8 | 10.4 | 0.71 |
MRTB, MRI-targeted prostate biopsy; rTPB, robotic transperineal template prostate biopsy.
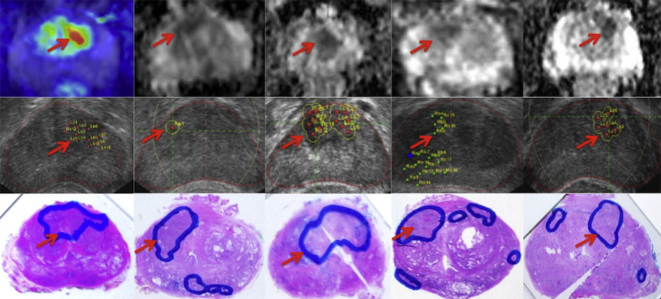
The combination biopsy upclassified a total of five patients (26.3%), of which two were detected solely by MRTB, two by both techniques and one by rTPB alone. All five patients had greater number of positive cores as well as greater maximal core percentage of cancer on combination biopsy compared to original TRUS biopsy (Table 5). All five patients subsequently underwent radical prostatectomy. Further analysis of the whole mount histology confirmed tumour grade and location in all five patients who were upclassified. Table 7 shows the comparison of disease location detected on initial TRUS biopsy and on combination biopsy for the 14 men who had cancer detected on combination biopsy, as well as with whole mount histology for six patients who eventually underwent robotic radical prostatectomy. Fig. 4 illustrates the correlation between MRI detected lesions with targeted biopsy locations and ultimately with the whole mount analysis. All six patients with PIRADS 5 lesion had cancer detected of which two thirds (4 patients) were significant disease. This translates to a positive predictive value of 100% for cancer and 66.6% for significant disease in PIRADS 5 lesions in our series. All Gleason 7 disease detected by MRTB correlated with PIRADS 5 lesions in the prostate. There were no complications observed, such as gross haematuria requiring manual bladder washout, acute urinary retention and sepsis, arising from rTPB or MRTB and all patients were discharged on the same day.
Table 7.
Comparison of location and Gleason score of positive cores between initial TRUS biopsy, combination biopsy and final histology of prostatectomy.
| Patient | TRUS |
rTPB |
MRTB |
RRP |
||||
|---|---|---|---|---|---|---|---|---|
| Location of positive cores | Gleason | Location of positive cores | Gleason | Location of positive cores | Gleason | Location of tumour | Gleason | |
| 1 | Unknown | 6 | Mid gland AZ | 6 | Not detected | Not done | ||
| 2 | Unknown | Mid gland AZ | 6 | Not detected | Not done | |||
| 3 | Right mid gland PZ | 6 | Right mid gland TZ | 6 | Not detected | Not done | ||
| 4 | Right mid gland PZ | 6 | Left mid gland PZ | 6 | Right mid gland PZ | 6 | Not done | |
| 5 | Right mid gland PZ | 6 | Midline mid gland PZ | 6 | Right mid gland PZ | 6 | Not done | |
| 6 | Right mid gland AZ | 6 | Left mid gland AZ | 6 | Left mid gland AZ | 6 | Not done | |
| 7 | Right mid gland AZ | 6 | Right mid gland TZ AZ | 6 | Right mid gland AZ | 6 | Not done | |
| 8 | Right mid gland PZ | 6 | Left apex to mid gland TZ PZ | 6 | Not detected | Left apex to mid gland PZ Right mid gland PZ |
6 6 |
|
| 9 | Left mid gland AZ | 6 | Right apex AZ | 7 | Left apex to mid gland AZ | 6 | Bilateral AZ | 7 |
| 10 | Unknown | 6 | Left base to mid gland AZ | 6 | Left base to mid gland AZ | 6 | Not done | |
| 11 | Left mid gland AZ | 6 | Right mid gland TZ Left mid gland AZ |
7 | Left mid gland AZ | 7 | Left AZ | 7 |
| 12 | Right mid gland AZ | 6 | Right base to mid gland TZ | 6 | Right base to mid gland TZ | 7 | Bilateral AZ | 7 |
| 13 | Unknown | 6 | Midline apex AZ | 6 | Midline apex AZ | 7 | Bilateral apex AZ | 7 |
| 14 | Bilateral mid gland PZ | 6 | Right apex to mid gland TZ PZ | 7 | Right apex to mid gland TZ PZ | 7 | Right apex to mid gland PZ | 7 |
AZ, anterior zone; MRTB, MRI-targeted prostate biopsy; PZ, peripheral zone; RRP, robotic radical prostatectomy; rTPB, robotic transperineal template prostate biopsy; TRUS, transrectal ultrasonography, TZ, transitional zone.
*Shaded cells highlight correlation of disease for that patient.
Figure 4.
Correlation of multiparametric MRI detected lesions with targeted biopsy plan and whole mount histology. Red arrows point to corresponding lesions and each column represents an individual case. First row shows multiparametric magnetic resonance imaging detected lesions, second row shows the biopsy planning and location of cores, and third row shows the whole mount histology slide.
4. Discussion
The use of AS in men with low-risk prostate cancer has been successful in reducing overtreatment. However, patient selection remains a point of concern. Studies of men thought to be suitable for AS among prostatectomy cohorts have found a 30%–50% disqualification rate upon pathological examination of the prostate. Mufarrij et al. [15] reported that 46% of cases considered to be low-risk and candidates for AS based on preoperative TRUS systematic biopsy had disease upgraded to a Gleason score of 7 or greater at final histopathology. The random nature of the biopsy technique relies on sampling efficiency for cancer detection and thus, is subject to sampling error that can result in up to 34% of cases with clinically significant tumours being missed on initial biopsy [16], [17]. Furthermore, TRUS guided prostate biopsy primarily targets the posterior aspect of the peripheral zone, missing 30%–40% of the prostate cancer located in the anterolateral portion of the peripheral zone, transitional zone and midline pre-urethral anterior fibromuscular stroma (AFMS) [17]. This deficiency of TRUS guided biopsy is overcome by transperineal biopsy techniques, which have been shown to improve cancer detection in the anterior zone, contributing in part to the increased detection rate of this modality [18].
The need to better characterize men for appropriate risk-stratification to AS or intervention has led to much interest in the rapidly evolving fields of prostate imaging and biopsy. Transperineal biopsy techniques allow for saturation biopsies of the prostate with less fears of rectal complications and, when performed as a mapping biopsy at every 5 mm interval, may be considered an “exhaustive” biopsy of the prostate [19]. Transperineal mapping biopsies in the setting of risk-stratifying men for active surveillance has been found to detect more aggressive disease 30% of the time [20]. Though attaining such a high core density leads to concerns about haematuria, erectile dysfunction or urinary retention, Valerio et al. [21] have reported that decreasing the core density results in decreased detection of clinically significant cancer.
Non-invasive imaging offers a means of selective disease localisation as we face the increasing challenge to preferentially detect higher grade cancer while avoiding lower grade tumours. There is a growing body of evidence that suggests an improved performance of MRI for the detection of early prostate cancer, when both anatomical (T1- and T2-weighted images) and functional sequences which include dynamic contrast, diffusion weighting, and/or spectroscopy are used in combination [22], [23]. More importantly, evidence points towards a heightened detection of clinically significant disease with mpMRI. Turkbey et al. [8] described a 98% positive predictive value for prostate MRI, and found improved sensitivity for higher grade tumours and those larger than 5 mm in diameter. Le et al. [9] recently reported the overall mpMRI sensitivity for tumour detection to be 47% with increased sensitivity for tumours larger than 1.0 cm (72%), higher-grade tumours (72% for Gleason ≥7), and index tumours (80%). Such preferential non-invasive diagnosis of clinically significant tumours constitutes a major potential advantage of MRI and has paved the way for the role of targeted biopsies of the prostate [11], [24], [25].
Our study explores the role of MRI-targeted biopsy using a transperineal approach via a robotic biopsy device (iSR'obot™ Mona Lisa). The advantages of the transperineal dual-cone trajectories, safety and feasibility of which had previously been reported by our group [11], [12]. The unique features of this transperineal biopsy system lies in the fact that it is a 7-axis robotic system that harnesses ultrasound imaging technology combined with innovative virtual reality registration to enable real-time image-guided biopsies of the prostate. As a robotically guided platform, errors in biopsy trajectory are potentially reduced compared to a free-hand biopsy and as a transperineal approach, there may be less risk of sepsis compared to a transrectal approach. However, it has not been previously evaluated as a platform for both robotic transperineal template saturation biopsy and MRI-targeted biopsy.
The results from our study have shown that on systematic template biopsy alone, we were able to pick up clinically significant disease in 15.8% of the low-risk cohort. With comparable prostate volumes at both TRUS biopsy and rTPB, the increased detection and upclassification could be explained by a function of increased biopsy intensity. Systematic template biopsy by virtue of having a higher biopsy intensity may still detect clinically significant disease in areas not considered suspicious on mpMRI [26], [27]. This was demonstrated in our study with one patient having clinically significant disease detected solely by rTPB. Nevertheless, in this study it appeared that MRTB had a higher detection rate of clinically significant disease and a better percentage cancer detection per core biopsy (11.7% vs. 6.5%, p = 0.02) over template biopsy and this may be explained by the added value of mpMRI in detecting significant disease, although the advantage is much lower than would be expected given the reported sensitivity of mpMRI for clinically significant disease [9]. The overall upclassification rate of 26.3% is consistent with the literature. If we believe this to be the actual percentage of patients with clinically significant disease in this cohort then the detection rate of mpMRI of clinically significant disease in our series (4 out of 5 patients, 80%) is consistent as well with what has been reported by other authors [9]. Analysing PIRADS 5 lesions, the overall predictive value for cancer (100%) and for significant disease (66.7%) comes close to results published by Turkbey et al. [8] and Le et al. [9].
The design of the trial, which used the same prostate for both biopsy techniques, is a unique strength of the trial and serves as the best situation possible of a matched-control study. For the best outcome of an MRI-targeted biopsy, a few assumptions have to be made [1]: That the accuracy of MRI in reporting all foci of significant disease within the prostate is the first step [2]; That the MRI detected lesion can be accurately represented and located in the real-time ultrasound model of the prostate; Lastly [3], that the biopsy device is able to precisely target the intended location of the suspicious lesion in the prostate. Only when we can ensure fidelity in all three criteria can we approximate the best outcome for targeted biopsies. Being cognisant of these pitfalls, the authors had sought to reduce the margin of error in each step [21]. For this study, a single dedicated experienced radiologist in the area of mpMRI of the prostate was enlisted. This ensured consistency and accuracy of the reporting of the MRI prostate. The prior reported margin of error using the present robotic biopsy device is 1 mm [11]. The blinding of the surgeon to the MRI findings and mapping of the lesions by the radiologist as well as the protocol of performing the template biopsies before targeted biopsies is a strength of our study and limits the observer bias. Performing the template biopsy before the targeted biopsy ensures complete blinding of the operator as the needle tracks of a prior targeted biopsy might allude to the location of the possible lesion and hence influence the surgeon's planning of the template saturation biopsy locations.
One of the major limitations of the study is the use of cognitive fusion for the targeted biopsies. This introduces operator dependent errors and variability, which would ultimately affect the outcome of the targeted biopsies, possibly explaining the low detection rate in this study. However, the usage of the 24-sector template grid of the prostate modelled after the 27 grid template approved in the START recommendations [10] and standardisation of the reporting of MRI using PIRADS score reduced the inter-operator variability in the cognitive fusion process and ensured consistency during targeted biopsy, data analysis and histopathological correlation. In experienced hands, cognitive or visual estimated targeting has an accuracy of up to 80% and is not inferior to software fusion [25], [28]. Second, we did not have final histology for all patients, which would be the best audit of the targeted biopsies in terms of grade and location. Hence the detection rate of clinically significant disease for each modality is at best a conservative estimate given that not every patient had subsequent radical prostatectomy for whole mount histological correlation. Similarly it was impossible to elicit the true sensitivity and specificity of our mpMRI reporting. Thirdly, performing template biopsy before targeted biopsy may affect the accuracy of the targeted biopsy as the prostate is expected to swell and deform to a certain degree after biopsy of the whole gland, this may affect the subsequent planning of the targeted cores. However, the reverse was not ideal as the needle tracks of the targeted cores seen on ultrasound may allude to the possible location of the lesion, thereby potentially introducing operator bias during template saturation biopsy. Fourthly, the performance of the mpMRI soon after TRUS biopsy may introduce artefacts due to post-biopsy haemorrhage and thus may reduce the accuracy of the MRI. Lastly, the limited number of patients prevents conclusive statements to be made however for a proof of concept study, the authors feel that the sample size is adequate. In addition, in the course of conducting the study, there have been technological advancements made to the robotic biopsy platform, which allowed software-assisted fusion of MRI and ultrasound prostate models, this provided the capability for MRI/US fusion targeted biopsy (in contrast to cognitive targeting) and steered our practice away from cognitive targeting.
The results of this study suggest current over-reliance on risk-stratification by the conventional TRUS prostate biopsy and propose a novel protocol for re-classifying patients prior to AS. The superiority of MRI-targeted biopsy in detecting clinically significant disease (22.2% vs. 15.8% of patients, 5.9% vs. 1.6% per core biopsy) is attributed in part to the ability of mpMRI to detect suspicious lesions as well as to the capability of the biopsy platform to target that lesion in a ultrasound model of the prostate. These results suggest that MRTB, approach and guidance platform notwithstanding, offers a more efficient method of biopsy. Our results are remarkably similar to the study by Pepe et al. [29] who also studied saturation transperineal biopsy and MRI/US fusion targeted biopsy in men with low-risk prostate cancer enrolled in AS protocol. In their study, they have shown that mpMRI/US targeted biopsy improves saturation biopsy detection rate for clinically significant prostate cancer in men enrolled in AS protocols and that the false negative rate of mpMRI for small but significant prostate cancer is equal about to 30% of cases [29]. In a larger study looking at the role of mpMRI-targeted and systematic prostate biopsies in men on active surveillance, Recabal et al. [30] reported an overall upgrading rate of 35% and a false negative rate of mpMRI-targeted biopsy of 10%–17% which comes close to the 20% in our series. The fact that clinically significant disease can still be missed by MRTB, though this may be explained by our use of cognitive rather than software fusion, suggests that investigators perhaps should not be so quick to dismiss a concurrent systematic biopsy at the time of risk-stratification. We seek to address this issue by examining the efficacy of software image fusion in our biopsy platform as a planned extension of this study.
5. Conclusion
A combination of template saturation biopsy and MRI-targeted biopsy upgrades a quarter of our patients presumed to be low-risk by initial TRUS prostate biopsy. MRTB detects 80% of these patients with higher percentage core positivity. In patients with PIRADS 5 score, MRTB detects all Gleason score ≥7 cancers. Our data suggest that a combination of MRTB and template saturation biopsy may optimally risk-classify this group of “low-risk” patients diagnosed on initial conventional TRUS prostate biopsy and a larger study is needed to confirm this.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Second Military Medical University.
References
- 1.Klotz L., Vesprini D., Sethukavalan P., Jethava V., Zhang L., Jain S. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33:272–277. doi: 10.1200/JCO.2014.55.1192. [DOI] [PubMed] [Google Scholar]
- 2.Tosoian J.J., Mamawala M., Epstein J.I., Landis P., Wolf S., Trock B.J. Intermediate and longer-term outcomes from a prospective active-surveillance program for favorable-risk prostate cancer. J Clin Oncol. 2015;33:3379–3385. doi: 10.1200/JCO.2015.62.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilt T.J., Brawer M.K., Jones K.M., Barry M.J., Aronson W.J., Fox S. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203–213. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dall'Era M.A., Albertsen P.C., Bangma C., Carroll P.R., Carter H.B., Cooperberg M.R. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol. 2012;62:976–983. doi: 10.1016/j.eururo.2012.05.072. [DOI] [PubMed] [Google Scholar]
- 5.D'Amico A.V., Whittington R., Malkowicz S.B., Schultz D., Blank K., Broderick G.A. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 6.Fu Q., Moul J.W., Banez L.L., Sun L., Mouraviev V., Xie D. Association between percentage of tumor involvement and Gleason score upgrading in low-risk prostate cancer. Med Oncol. 2012;29:3339–3344. doi: 10.1007/s12032-012-0270-4. [DOI] [PubMed] [Google Scholar]
- 7.Tay K.J., Mendez M., Moul J.W., Polascik T.J. Active surveillance for prostate cancer: can we modernize contemporary protocols to improve patient selection and outcomes in the focal therapy era? Curr Opin Urol. 2015;25:185–190. doi: 10.1097/MOU.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 8.Turkbey B., Mani H., Shah V., Rastinehad A.R., Bernardo M., Pohida T. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol. 2011;186:1818–1824. doi: 10.1016/j.juro.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le J.D., Tan N., Shkolyar E., Lu D.Y., Kwan L., Marks L.S. Multifocality and prostate cancer detection by multiparametric magnetic resonance imaging: correlation with whole-mount histopathology. Eur Urol. 2015;67:569–576. doi: 10.1016/j.eururo.2014.08.079. [DOI] [PubMed] [Google Scholar]
- 10.Moore C.M., Kasivisvanathan V., Eggener S., Emberton M., Futterer J.J., Gill I.S. Standards of reporting for MRI-targeted biopsy studies (START) of the prostate: recommendations from an International Working Group. Eur Urol. 2013;64:544–552. doi: 10.1016/j.eururo.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 11.Ho H., Yuen J.S., Mohan P., Lim E.W., Cheng C.W. Robotic transperineal prostate biopsy: pilot clinical study. Urology. 2011;78:1203–1208. doi: 10.1016/j.urology.2011.07.1389. [DOI] [PubMed] [Google Scholar]
- 12.Ho H.S., Mohan P., Lim E.D., Li D.L., Yuen J.S., Ng W.S. Robotic ultrasound-guided prostate intervention device: system description and results from phantom studies. Int J Med Robot. 2009;5:51–58. doi: 10.1002/rcs.232. [DOI] [PubMed] [Google Scholar]
- 13.Rothke M., Blondin D., Schlemmer H.P., Franiel T. PI-RADS classification: structured reporting for MRI of the prostate. Rofo. 2013;185:253–261. doi: 10.1055/s-0032-1330270. [Article in German] [DOI] [PubMed] [Google Scholar]
- 14.Barentsz J.O., Richenberg J., Clements R., Choyke P., Verma S., Villeirs G. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746–757. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mufarrij P., Sankin A., Godoy G., Lepor H. Pathologic outcomes of candidates for active surveillance undergoing radical prostatectomy. Urology. 2010;76:689–692. doi: 10.1016/j.urology.2009.12.075. [DOI] [PubMed] [Google Scholar]
- 16.Serefoglu E.C., Altinova S., Ugras N.S., Akincioglu E., Asil E., Balbay M.D. How reliable is 12-core prostate biopsy procedure in the detection of prostate cancer? Can Urol Assoc J. 2013;7:E293–E298. doi: 10.5489/cuaj.11224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rabbani F., Stroumbakis N., Kava B.R., Cookson M.S., Fair W.R. Incidence and clinical significance of false-negative sextant prostate biopsies. J Urol. 1998;159:1247–1250. [PubMed] [Google Scholar]
- 18.Pepe P., Garufi A., Priolo G., Pennisi M. Transperineal versus transrectal MRI/TRUS fusion targeted biopsy: detection rate of clinically significant prostate cancer. Clin Genitourin Cancer. 2017;15:e33–e36. doi: 10.1016/j.clgc.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Kuru T.H., Wadhwa K., Chang R.T., Echeverria L.M., Roethke M., Polson A. Definitions of terms, processes and a minimum dataset for transperineal prostate biopsies: a standardization approach of the Ginsburg Study Group for Enhanced Prostate Diagnostics. BJU Int. 2013;112:568–577. doi: 10.1111/bju.12132. [DOI] [PubMed] [Google Scholar]
- 20.Sivaraman A., Sanchez-Salas R., Barret E., Ahallal Y., Rozet F., Galiano M. Transperineal template-guided mapping biopsy of the prostate. Int J Urol. 2015;22:146–151. doi: 10.1111/iju.12660. [DOI] [PubMed] [Google Scholar]
- 21.Valerio M., Anele C., Charman S.C., van der Meulen J., Freeman A., Jameson C. Transperineal template prostate-mapping biopsies: an evaluation of different protocols in the detection of clinically significant prostate cancer. BJU Int. 2016;118:384–390. doi: 10.1111/bju.13306. [DOI] [PubMed] [Google Scholar]
- 22.Kirkham A.P., Emberton M., Allen C. How good is MRI at detecting and characterising cancer within the prostate? Eur Urol. 2006;50:1163–1174. doi: 10.1016/j.eururo.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 23.Villers A., Lemaitre L., Haffner J., Puech P. Current status of MRI for the diagnosis, staging and prognosis of prostate cancer: implications for focal therapy and active surveillance. Curr Opin Urol. 2009;19:274–282. doi: 10.1097/MOU.0b013e328329a2ed. [DOI] [PubMed] [Google Scholar]
- 24.Dianat S.S., Carter H.B., Macura K.J. Magnetic resonance-guided prostate biopsy. Magn Reson Imaging Clin N Am. 2015;23:621–631. doi: 10.1016/j.mric.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Wysock J.S., Rosenkrantz A.B., Huang W.C., Stifelman M.D., Lepor H., Deng F.M. A prospective, blinded comparison of magnetic resonance (MR) imaging-ultrasound fusion and visual estimation in the performance of MR-targeted prostate biopsy: the PROFUS trial. Eur Urol. 2014;66:343–351. doi: 10.1016/j.eururo.2013.10.048. [DOI] [PubMed] [Google Scholar]
- 26.Siddiqui M.M., Rais-Bahrami S., Truong H., Stamatakis L., Vourganti S., Nix J. Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol. 2013;64:713–719. doi: 10.1016/j.eururo.2013.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonn G.A., Chang E., Natarajan S., Margolis D.J., Macairan M., Lieu P. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol. 2014;65:809–815. doi: 10.1016/j.eururo.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cerantola Y., Haberer E., Torres J., Alameldin M., Aronson S., Levental M. Accuracy of cognitive MRI-targeted biopsy in hitting prostate cancer-positive regions of interest. World J Urol. 2016;34:75–82. doi: 10.1007/s00345-015-1588-2. [DOI] [PubMed] [Google Scholar]
- 29.Pepe P., Garufi A., Priolo G., Pennisi M. Can MRI/TRUS fusion targeted biopsy replace saturation prostate biopsy in the re-evaluation of men in active surveillance? World J Urol. 2016;34:1249–1253. doi: 10.1007/s00345-015-1749-3. [DOI] [PubMed] [Google Scholar]
- 30.Recabal P., Assel M., Sjoberg D.D., Lee D., Laudone V.P., Touijer K. The efficacy of multiparametric magnetic resonance imaging and magnetic resonance imaging targeted biopsy in risk classification for patients with prostate cancer on active surveillance. J Urol. 2016;196:374–381. doi: 10.1016/j.juro.2016.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]