Abstract
Posterior urethral injuries typically arise in the context of a pelvic fracture. Retrograde urethrography is the preferred diagnostic test in trauma patients with pelvic fracture where a posterior urethral rupture is suspected. Pelvic fractures however preclude the adequate positioning of the patient on the X-ray table on admission and computed tomography scan with intravenous contrast and delayed films generally performed first. Suprapubic bladder catheter placement under ultrasound guidance should be performed whenever a posterior urethral disruption is suspected. Early diagnosis and proper acute management decrease the associated complications, such as strictures, urinary incontinence and erectile dysfunction. The correct and appropriate initial treatment of associated urethral rupture is critical to the proper healing of the injury. Placing of a suprapubic cystostomy on admission and delayed anastomotic urethroplasty after 3–6 months continues to be the gold standard of treatment. In this paper, we provide a comprehensive review of the literature with a special emphasis on the various treatments available: Open or endoscopic primary realignment, immediate or delayed urethroplasty after suprapubic cystostomy, and delayed optical urethrotomy.
Keywords: Posterior urethral injuries, Distractions defects, Pelvic fracture, Realignment, Urethroplasty, Optical urethrotomy
1. Introduction
1.1. Etiological and anatomical considerations
The male urethra is divided into an anterior and a posterior portion through the urogenital diaphragm. The posterior urethra comprises the prostatic urethra and the membranous urethra.
Posterior urethral injuries frequently arise in the context of a pelvic fracture, typically after injuries due to traffic collisions, being crushed, or falling. Overall, the posterior urethra in men is affected in 3.5%–19% of pelvic fractures. The posterior urethra in women is rarely affected (0%–6%), except by contusions or lacerations by bone fragments [1].
Classically it was thought that in cases of deceleration or crushing, the forces that fracture the bones of the pelvis are transmitted to the prostatomembranous junction, causing an alteration between the anterior urethra and the prostatic apex [2]. Studies performed in cadaver by Mouraviev and Santucci [3] have demonstrated that in the majority of cases the urethral lesionsa rise distal to the external urinary sphincter.
The possibility of an anastomotic reconstruction of a posterior urethral rupture assuring a good urinary continence depends on the sphincter integrity at the bladder neck, as both the internal sphincter at the bladder neck and the external sphincter in the membranous urethra are capable of independently ensuring urinary continence [4].
For the correct treatment of pelvic fractures, the surgeon should have a clear concept of pelvic stability. The degree of pelvic instability depends on the existence of dislocation and displacement of the sacroiliac joint as well as the fracture of the pubic rami.
Pelvic fractures can be divided into two main groups: stable fractures and unstable fractures. In a stable pelvic fracture, the urethral injury can occur when the four pelvic rami are broken (butterfly fracture) (Fig. 1) and the bone fragment is displaced in a posterior direction with the prostate, which is attached to the pubic bone. This displacement is capable of shearing the membranous urethra, affecting, in most cases, the external sphincter.
Figure 1.
Butterfly fracture.
Unstable pelvic fractures that affect the anterior pelvic girdle and the sacroiliac joint, ilium, or sacrum can cause injuries of the posterior urethra due to bone fragments or, more commonly, as a result of the distortion of the pelvic bones during trauma. This bone displacement causes lateral movements that displace the membranous urethra and puboprostatic ligaments in opposite directions.
Unstable diametric pelvic fractures or bilateral fractures of the ischiopubic rami (known as butterfly fractures) are the most likely to cause posterior urethral injuries, as is common in the case of butterfly fractures with diastasis of the sacroiliac joint (Table 1) [1], [5].
Table 1.
Probability of urethral injury by type of fracture.
| Type of fracture | Probability |
|---|---|
| Single ramus | 0.64 |
| Ipsilateral rami | 0.76 |
| Malgaigne (ramus and ipsilateral sacroiliac) | 3.40 |
| Butterfly | 3.85 |
| Butterfly and sacroiliac | 24.02 |
Posterior urethral injuries may vary from a simple mucosal laceration (25%) to a partial (25%) or complete (50%) rupture. The most severe injuries occur as a result of prostate-urethral displacement, secondary progressive fibrosis, and separation of the urethral ends [1].
Urethral injuries alone are not considered to be a vital emergency, with the exception of their frequent association with pelvic fractures and the effects of such fractures on other organs, which occurs in 27% of cases. Initially, the management of associated injuries is often more important than the urethral injury [6].
1.2. Clinical and diagnostic considerations
The first step in the initial management of urethral trauma should be to stabilise the patient and evaluate any associated injuries, especially those that are life-threatening.
The presence of haematuria, blood at the urethral meatus, or urethrorrhagia is associated with urethral injuries. In these cases, acute urinary retention, perineal haematoma, or swelling by extravasation of urine is common [5], [7].
Retrograde urethrography is considered the diagnostic test of choice for the evaluation of posterior urethral injuries, facilitating their proper subsequent management. Pelvic fractures however preclude the adequate positioning of the patient on the X-ray table on admission and computed tomography scan with intravenous contrast and delayed films is generally performed first. Suprapubic bladder catheter placement under ultrasound guidance should be performed whenever a posterior urethral disruption is suspected. Catheter placement permits urine diversion and allows the physician to perform a combined urethrography (anterograde and retrograde) at a later point in time, which can help to determine the location, severity, and extent of the injury (Fig. 2). Nevertheless, if the posterior urethra is not visualised correctly, an MRI scan of the posterior urethra or anterograde endoscopy through the suprapubic route can be performed at a later stage in order to plan urethral reconstruction [5], [7].
Figure 2.
Posterior urethra fracture. Combined urethrography.
2. Treatment of posterior urethral injuries
A key concept is the distinction between posterior urethral stricture and posterior urethral rupture secondary to pelvic fracture, as the surgical management of each is distinctly different. In urethral stricture, the urethral spongy tissue is continuous; in posterior urethral rupture, a separation exists between the apex of the prostate or the membranous urethra and the bulbous urethra, with no continuity of the urethra. This separation is caused by the retraction of the urethral ends after rupture; the space between both ends is filled by fibrous tissue resulting from haematoma organisation and urine leakage associated with the rupture [8].
2.1. Partial rupture
Most partial posterior urethral injuries can be treated conservatively by placing a urethral catheter or a suprapubic cystostomy catheter. These injuries can heal successfully without developing fibrosis or important obstructions. An urethrography is advisable every 2 weeks until the injury is completely healed. The presence of secondary stricture can be treated by urethral dilatation or internal optical urethrotomy if the stricture is short and exhibits little fibrosis. Otherwise, end-to-end anastomosis is recommended [9].
2.2. Complete rupture
Treatment options available for complete rupture include primary realignment, immediate urethroplasty, delayed urethroplasty, and delayed internal urethrotomy.
There is controversy between the different authors about the use of primary realignment or placing a suprapubic cystostomy and performing a delayed urethroplasty.
The success rates described in the literature are variable according to the authors and in this review we have tried to collect the most representative series and review those publications comparing both procedures.
Most authors consider surgery to be successful if no further procedures, including self-catheterization, were necessary and no stricture recurrence was noted at follow-up cystoscopy. However, in some series the authors did not mention the criteria to define the success of surgery.
2.2.1. Primary realignment
This technique can be performed via a suprapubic approach (open realignment) or by endoscopic techniques. In posterior urethral trauma with associated rectal or bladder injuries, an immediate surgical exploration with open urethral realignment is advisable. Bladder neck injuries exhibit an increased risk of incontinence and osteomyelitis, and most of the cases directly produced by bone fragments. Rectal injuries are associated with a high rate of infection and fistula formation; as a result, immediate surgical intervention is recommended to evacuate the haematoma and perform a discharge colostomy [5], [7].
The overall condition of the patient, along with the severity of the patient's associated injuries are the most prominent factors when opting for primary endoscopic realignment. If the patient exhibits no fractures in the lower extremities that would limit their placement in the lithotomy position and does not exhibit brain damage or other contraindications for anaesthesia, a primary endoscopic realignment in the first 2 weeks after the injury could be considered.
The benefits of primary realignment are as follows [5], [7], [10]:
-
•
There is a lower incidence of stricture than when performing only suprapubic diversion.
-
•
In the event that a secondary stricture appears, the stricture can be treated endoscopically or managed with dilatation.
-
•
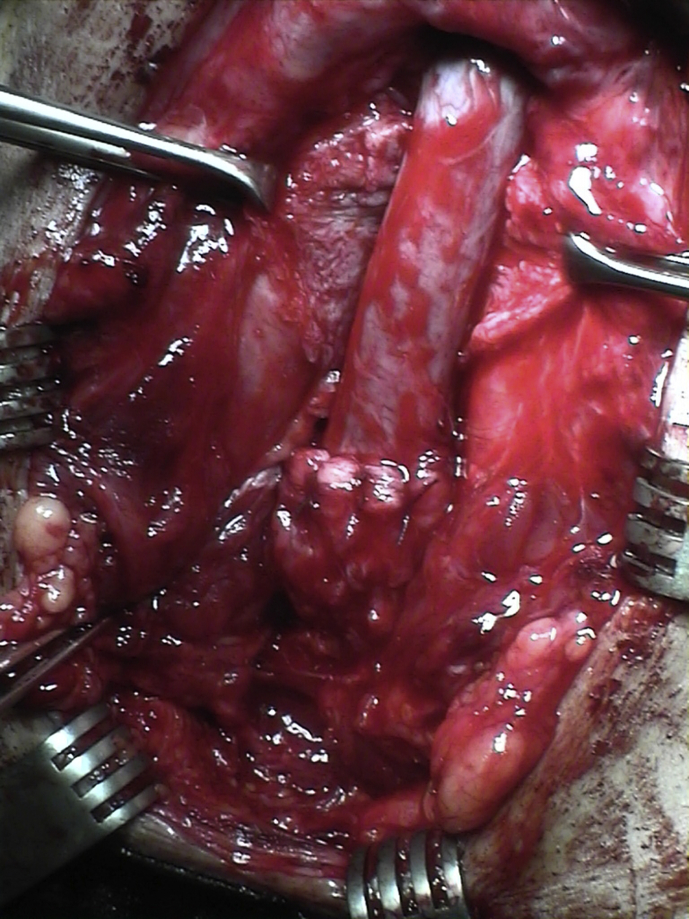
If urethroplasty is later required, the procedure is more easily performed because the urethra and prostate are already aligned (Figure 3, Figure 4).
Figure 3.
Bulbar urethra stricture after primary realignment.
Figure 4.
Bulbar urethra stricture after primary realignment. End-to-end urethroplasty.
Open realignment exhibits a high incidence of erectile dysfunction and incontinence when compared with delayed repair [10]. Webster et al. [10] reviewed 301 patients in 15 clinical series who underwent a primary realignment and compared their rates of incontinence, impotence, and stricture with a group of 236 patients in five clinical series in which cystostomy and delayed repair were indicated. Impotence occurred in 44% of patients undergoing primary realignment compared with 11% of patients treated by delayed methods. The incontinence rate was also higher after primary realignment (20% vs. 2%). All patients (100%) who underwent cystostomy catheter placement exhibited urethral strictures, compared with 64% of patients after primary realignment. However, in most of the patients who initially underwent bladder drainage, delayed anastomosis was later performed with success rates exceeding 90% [10], [11], [12]. An extensive review by Koraitim [13] of articles published in the English language over the past 50 years reached similar conclusions.
It should be noted that the clinical series of primary realignment reviewed by Webster and Koraitim [10], [13] consisted of numerous and various techniques of realignment that may explain the poor results of this analysis compared with other clinical series [14]. Recent publications suggest that the incidence of impotence and incontinence are more related to those injuries resulting from trauma than to the type of treatment. Elliot and Barrett [14] reviewed a clinical series of 57 patients who underwent primary endoscopic realignment; with a mean follow-up of 10.5 years, 21% of patients exhibited some degree of erectile dysfunction, 3.7% exhibited moderate stress incontinence, and 68% exhibited stricture after primary alignment. The incidence of impotence and incontinence after primary realignment has also been reviewed by Kotkin and Koch [15]. The authors reviewed 20 cases of patients with similar posterior urethral injuries treated with immediate realignment or suprapubic urinary diversion and concluded that continence was preserved in 83% and 80% of the patients, respectively, whereas sexual potency was preserved in 73% and 70%, respectively. Furthermore, Mouraviev et al. [16] retrospectively compared primary realignment (n = 57) to suprapubic diversion (n = 39) and concluded that the stricture rate was lower in cases of primary realignment (49% vs. 100%). Additionally, Mouraviev et al. [16] observed lower rates of urinary incontinence (17.7% vs. 24.9%) and erectile dysfunction (33.6% vs. 42.1%) in cases of primary realignment.
The great variety of techniques used in the primary realignment makes the comparison with deferred procedures unclear. Among the primary realignment techniques are the following [5]:
-
•
Simple urethral catheterisation.
-
•
Endoscopic realignment using a rigid and/or flexible cystoscope under fluoroscopic guidance.
-
•
Minimally invasive realignment using attachable or magnetic catheters.
-
•
Open realignment with evacuation of pelvic haematoma and dissection of the prostatic apex, either with or without prostate-urethral anastomosis.
-
•
Open realignment with catheter traction or placement of perineal traction sutures to move the prostate back to its anatomical position.
Table 2 presents the clinical series in which a primary realignment was performed with minimal traction showing more favourable results [14], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26].
Table 2.
Results of primary realignment in complete posterior urethral rupture [14], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26].
| Clinical series | No. of patients | Follow-up, months (range) | Erectile dysfunction, n (%) | Incontinence n (%) | Stricture ratean (%) |
|---|---|---|---|---|---|
| Gibson, 1974 [17] | 35b | NA | 12 (34) | 1 (3) | 26 (74) |
| Crassweller et al., 1977 [18] | 38 | 24–240 | 19/42 (45) | NA | 12 (32) |
| Follis et al., 1992 [19] | 20 | 42 (1–360) | 4 (20) | 2 (10) | 12 (60) |
| El-Abd, 1995 [20] | 44 | NA | 35 (79) | 0 | 44 (100) |
| Elliott and Barret, 1997 [14] | 53 | 126 (1–120) | 11 (21) | 2 (4) | 36 (68) |
| Porter et al., 1997 [21] | 10 | 10.9 (2–31) | 1/7 (14) | 0 | 5 (50) |
| Tahan et al., 1999 [22] | 13 | 29 | 3 (23) | 0 | 5 (39) |
| Asci et al., 1999 [23] | 20 | 39 (19–78) | 4 (20) | 2 (10) | 9 (45) |
| Moudouni et al., 2001 [24] | 23 | 68 (18–155) | 4/29 (14) | 0 | 16 (70) |
| Mouraviev et al., 2005 [16] | 57 | 105 (12–264) | 19 (34) | 10 (18) | 28 (49) |
| Leddy et al., 2012 [25] | 19 | 40 (10–80) | 4/18 (22.2) | 0 | 15 (78.9) |
| Johnsen et al., 2015 [26] | 27 | 40 (1–152) | 21 (78) | 2 (9) | 17 (63) |
NA: Not available.
Stricture requiring internal urethrotomy, open urethroplasty, or more than one dilation.
Five patients with partial rupture.
A comprehensive review of the literature suggests that primary realignment, both open and endoscopic, is associated with impotence rate of approximately 35%, incontinence rates of 2.9%, and incidence of stricture in 62% of cases [5].
Despite the potential benefits of primary realignment, some authors stated that most patients treated by primary endoscopic realignment have their acute injury turned into an unstable chronic disease stated that usually requires daily self dilatation, regular office dilatations or repeated endoscopic procedures. The repeated manipulations can complicate the urethroplasty and delay the definitive treatment [27], [28], [29].
2.2.2. Immediate open urethroplasty
This technique is rarely indicated, as inflammation and haematoma present in the acute phase hinder the proper assessment of the damage and the visualisation of structures and dissection planes [6]. The rates of incontinence, impotence, and stricture are higher than those described for other techniques (21%, 56%, and 49%, respectively) [10], [13], [30], [31].
In the absence of injuries requiring urgent action, the management of posterior urethral injuries is performed when the patient is stabilised, typically within the first 10–14 days. The purpose of this technique is to prevent the excessive separation of the urethral ends in complex fractures with a large prostate displacement rather than to prevent secondary stricture; if secondary stricture occurs, the technique ensures the possibility of an easy solution [27]. The pelvic haematoma can be evacuated during the procedure to allow the descent of the prostate and bladder. The urethral continuity can be restored endoscopically or through an abdominal or perineal approach [27]. One-stage perineal anastomosis exhibits stricture rates of 20% [32], significantly worse than delayed urethroplasty.
2.2.3. Delayed urethroplasty
In most cases of traumatic posterior urethral rupture that are treated by delayed techniques, the defect between the prostatic and bulbar urethra is relatively short. Hence, the cases can be treated relatively simply through a perineal approach and by performing end-to-end anastomosis, provided that the haematoma and associated fibrosis are not extensive and that the bladder neck is competent. The progressive perineal approach is typically performed 3–6 months after the injury.
The keys to the success of the surgery include the complete resection of fibrous tissue, the eversion of the mucosa of the bulbar and prostatic ends, and an anastomosis without tension [33], [34].
To perform the anastomosis, the urethra is sectioned first at the level of the rupture or stricture. Subsequently, the sectioned urethra is freed up to the penoscrotal angle, and the urethral ends are spatulated. With this manoeuvre, a defect of 2–2.5 cm can be restored, allowing an end-to-end anastomosis without tension in cases in which there is short gap between the urethral ends (5). This technique has the advantage that at the time of the procedure, the associated injuries and pelvic haematoma have already been resolved, allowing the placement of the patient in the lithotomy position with no problems. The only drawback is that the patient must rely on a suprapubic catheter until the urethroplasty is performed.
When the separation between the prostatic and bulbar urethra is greater than 2–3 cm, there are a number of manoeuvres that must be performed sequentially to achieve sufficient mobility of the anterior urethra prior to overcoming defects of up to 8 cm [35]. Such manoeuvres are as follows: Proximal and midline separation of the corpora cavernosa (Fig. 5), inferior pubectomy, and transposition of the urethra above the corpora cavernosa. In addition to its use as an initial treatment for posterior urethral ruptures, the progressive perineal approach can also be used successfully after failure of other techniques. Webster et al. [36] published their results in 75 patients who used the progressive perineal approach. They found that the greater the distance between the urethral ends, the greater number of manoeuvres had to be performed. However, the success rate was very similar (96%), regardless of the number of manoeuvres performed. Similarly, in a series of 301 patients subjected to deferred urethroplasty, Fu et al. [37] reported a stricture rate of 12.6% and a 12% incidence of de novo erectile dysfunction after surgery. The success rate for the different manoeuvres was 89.3% for simple perineal anastomosis without ancillary procedures (103 patients), 86.5% for perineal anastomosis with separation of the corporeal body (89 patients), 84.2% for perineal anastomosis with inferior pubectomy (95 patients) and 85.7% for perineal anastomosis with urethral rerouting (14 patients).
Figure 5.
Separation of the corpora cavernosa.
Various circumstances may determine and limit the success of primary or secondary perineal anastomotic urethroplasty. Overall, these circumstances are present in less than 5% of cases [5], [29], [37], [38], [39], [40], [41]:
-
•
Defects larger than 7–8 cm: The interposition of a flap of penile or scrotal skin must be evaluated in these cases.
-
•
Urinary fistulas: These cases might require a combined abdominoperineal approach to ensure proper closure.
-
•
Synchronous distal urethral stricture: The presence of spongiofibrosis in the anterior urethra may compromise the vascularity of the posterior bulbar urethra.
-
•
Urinary incontinence: In posterior urethral ruptures, where the external sphincter is typically affected, continence depends on the internal sphincter at the bladder neck. Bladder neck involvement requires a combined approach to repair the urethra and bladder neck in one or two stages. The most common cause of bladder neck incompetence is its circumferential fixation caused by the haematoma and secondary fibrosis resulting from trauma. In most cases, the meticulous dissection to free the bladder neck and the covering of the bladder neck with a greater omentum flap to eliminate dead space and prevent the formation of new fibrous tissue allows the recovery of the sphincter mechanism.
-
•
Surgery or previous urethral manipulation: Several authors have reported an increase in the failure rate (14%–40%) in patients with a history of dilatation, urethrotomy, realignment, or previous urethroplasty.
Koraitim [30] reviewed the results of several techniques, including his own series of 100 patients and 771 patients from other series. In his report, the primary realignment technique (n = 326) was associated with a 53% stricture rate, a 5% incontinence rate, and a 36% impotence rate. Of the patients in whom primary realignment was effective, 42% required posterior instrumentation to achieve stricture stabilisation, and 33% ultimately required an urethroplasty. The primary suture technique (n = 37) was associated with a 49% stricture rate, a 21% incontinence rate, and a 56% impotence rate. Suprapubic diversion, prior to delayed repair (n = 508), was associated with a 97% stricture rate, a 4% incontinence rate, and a 19% impotence rate. However, the reported restenosis rate after delayed urethroplasty was less than 10% [5], [7], and the reported incidence of impotence after delayed urethroplasty was approximately 5% [5], [7].
In the literature we can find two main reviews comparing primary realignment (open or endoscopic) vs. delayed urethroplasty [42], [43]. The conclusions of both reviews are similar: There is no difference incontinence and impotence rates between the two techniques. Primary realignment has significantly lower rate of urethral stricture. However, the long-term success rate of both procedures, in expert hands, is similar. Primary realignment appears to decrease the risk of urethral stenosis by approximately 30% with a number of 2.76 patients that needed to be treated to prevent one stenosis. The price to be paid by 70% of patients treated with primary realignment is to undergo repeated endoscopic procedures or dilatations before needing to be cured with a definitive anastomotic urethroplasty in a referral center.
2.2.4. Repair after failure of a delayed urethroplasty
Stricture cases secondary to delayed bulboprostatic anastomosis are typically diagnosed in the first 6 months after surgery. If the calibre of the anastomosis is normal in this period, further development of stricture is unlikely [32].
The principles for repair are the same as those in the initial procedure. Conducting an end-to-end anastomosis through a progressive perineal approach is effective in 95% of cases. If the anastomosis cannot be performed, the technique of choice in these cases is substitution urethroplasty in one stage using a penile orscrotalskin island flap. If this procedure is not possible, a two-stage procedure with a scrotal skin flap or a urethroplasty with a split-thickness meshed skin graft should be selected [5].
There are cases in which the entire bulbar urethra has been obliterated by necrosis due to inadequate retrograde blood supply after bulbar urethra transection. In this cases, where there is no bed upon which to perform augmentation urethroplasty, Kulkarni et al. [44] recommended some specific procedures: 1) Preputial tube on a vascular pedicle, 2) place oral mucosa graft on scrotal skin for first stage and mobilize it in a second stage with neovascularity, 3) the Turner Warwick “scrotal drop back”, 4) dorsal buccal mucosa graft with a ventral pedicled preputial flap (in patients who did not have a pubectomy), 5) pedicled preputial or penile skin flap and 6) entero-urethroplasty. Using these techniques the author had published a success rate of 76% in 46 patients.
The indications for a combined abdominoperineal approach include the following: 1) The presence of fistulas at the bladder base, abdominal wall or rectum, 2) the presence of periurethral cavities lined by epithelium, 3) the inability to place the patient in the lithotomy position, and 4) the serious pelvic bone deformities that hinder perineal access to the prostatic apex or correct placing of a retrograde Benique during surgery [5].
Restenosis cases that do not impact urinary flow (calibre greater than 12 Fr) can be monitored or treated by regular dilatations. Internal urethrotomy is typically an option, especially in short and small calibre strictures [5], [40].
2.2.5. Delayed endoscopic urethrotomy
The principles of this procedure were described by Sachse in 1974 [45]. A curved metal catheter is introduced into the urethral end in the anterograde direction through the cystostomy tract. The urethrotome is introduced and visually guided through the urethra, and fibrous tissue is dissected to locate the metal probe. Blandy [46] subsequently described the use of a suprapubic cystoscope to facilitate locating the proximal urethral end, visualising its light and “cutting towards the light”. Currently, C-arm fluoroscopy is used to guide the urethrotome for cutting. After urethrotomy the urethral catheter is kept in place from 1 to 3 weeks, and the suprapubic catheter remains for 2 additional weeks to verify the success of the procedure.
Table 3 presents the results of several clinical series of delayed urethrotomy [20], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58].
Table 3.
Results of optical urethrotomy in posterior urethral ruptures [20], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58].
| Clinical series | No. of patients | Follow-up, months (range) | Repeated urethrotomy, n (%) | Erectile dysfunction, n (%) |
|---|---|---|---|---|
| Gupta and Gill 1986 | 10 | 15.1 (6–24) | 10 (100) | 0 |
| Chiou et al., 1988 | 8 | 43 (12–79) | 7 (88) | 0 |
| Marshall 1989 | 10 | NA | 10 (100) | 0 |
| Barry 1989 | 12 | 22 (1.5–85) | 6 (50) | 0 |
| DeVries and Anderson 1990 | 4 | <4 | 1 (25) | 0 |
| Leonard et al., 1990 | 3 | 31 (13–51) | 1 (33) | 0 |
| Kernohan et al., 1991 | 7 | 35 (21–84) | 7 (100) | 0 |
| Yasuda et al., 1991 | 17 | 44 (12–96) | 7 (41) | 0 |
| Quint &Stanisic 1993 | 10 | 43 (7–108) | 6 (60) | 0 |
| El-Abd 1995 | 284 | NA | 272 (96) | 0 |
| Goel et al., 1997 | 13 | 17.7 (11–24) | 10 (77) | NA |
| Levine and Wessells 2001 | 6 | 60 | 6 (100) | NA |
| Dogra and Nabi 2002a | 61 | 30 (9–44) | 11 (18) | NA |
| total | 445 | 354 (80) |
NA: Not available.
Laser urethrotomy.
Delayed urethrotomy is only indicated for short defects and only in patients who have been previously treated with urethral realignment, in which the bladder neck is competent and the displacement between the prostatic and bulbar urethra is minimal [50]. Although the procedure initially achieves the restoration of the urethral continuity and potency is usually not affected, approximately 80% of patients require subsequent urethral dilatations, new urethrotomy procedures, or stricture resections. Most of these urethrotomy procedures are performed during the first year of follow-up. Other alternatives should be considered after the failure of the initial urethrotomy, as repeating the procedure only provides a temporary improvement. Several authors have reported complications, including the production of false urethral passages and rectal perforations when performing the procedure [7].
The intraurethral prosthesis is not recommended for patients with stricture after pelvic injuries because fibrous tissue tends to grow within the lumen of the prosthesis [59], [60].
3. Conclusion
Retrograde urethrography is the preferred diagnostic test in trauma patients with pelvic fracture where a posterior urethral rupture is suspected. If the patient's condition is permissible, conducting a primary endoscopic realignment should be considered. Patients who develop urethral stricture after the realignment must be treated with end to end urethroplasty. Placing a suprapubic cystostomy catheter and performing delayed urethroplasty 3–6 months later is the standard treatment in the majority of patients and ensure excellent functional results.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Second Military Medical University.
References
- 1.Koraitim M.M., Marzouk M.E., Atta M.A., Orabi S.S. Risk factors and mechanism of urethral injury in pelvic fractures. Br J Urol. 1996;77:876–880. doi: 10.1046/j.1464-410x.1996.01119.x. [DOI] [PubMed] [Google Scholar]
- 2.Dixon C.M., Hricak H., McAninch J.W. Magnetic resonance imaging of traumatic posterior urethral defects and pelvic crush injuries. J Urol. 1992;148:1162–1165. doi: 10.1016/s0022-5347(17)36849-0. [DOI] [PubMed] [Google Scholar]
- 3.Mouraviev V.B., Santucci R.A. Cadaveric anatomy of pelvic fracture urethral distraction: most injuries are distal to the external urinary sphinter. J Urol. 2005;173:869–872. doi: 10.1097/01.ju.0000152252.48176.69. [DOI] [PubMed] [Google Scholar]
- 4.Turner-Warwick R. Observations on the treatment of traumatic urethral injuries and the value of the fenestrated urethral catheter. Br J Surg. 1973;60:775–781. doi: 10.1002/bjs.1800601007. [DOI] [PubMed] [Google Scholar]
- 5.Djakovic N., Plas E., Martínez-Piñeiro L., Lynch Th, Mor Y., Santucci R.A. 2012. EAU Guidelines Urological Trauma.http://uroweb.org/wp-content/uploads/EAU-Guidelines-Urological_Trauma-2012.pdf [Google Scholar]
- 6.Chapple C.R., Png D. Contemporary management of urethral trauma and the post-traumatic stricture. Curr Opin Urol. 1999;9:253–260. doi: 10.1097/00042307-199905000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Martínez-Piñeiro L., Ríos E., de La Peña J.J. Tratado de urología [Compendium of urology] Prous Science; Barcelona: 2006. Traumatismos de uretra y genitales [Urethral and genital injuries] pp. 1615–1639. In: En Jimenez Cruz FJ y Rioja LA [In Jimenez Cruz FJ and Rioja LA], editors. [Google Scholar]
- 8.Martínez-Piñeiro J.A., Carcamo P., García-Matres M.J., Martínez-Piñeiro L., Iglesias J.R., Rodriguez-Ledesma J.M. Excision and anastomotic repair for urethral stricture disease: experience with 150 cases. Eur Urol. 1997;32:433–441. [PubMed] [Google Scholar]
- 9.Venn S.N., Mundy A.R. Immediate management of major trauma to the urinary tract. Eur Urol. 1998;33:1–8. [Google Scholar]
- 10.Webster G.D., Mathes G.L., Selli C. Prostatomembranous urethral injuries. A review of the literature and a rational approach to their management. J Urol. 1983;130:898–902. doi: 10.1016/s0022-5347(17)51561-x. [DOI] [PubMed] [Google Scholar]
- 11.McAninch J.W. Pubectomy in repair of membranous urethral stricture. Urol Clin Norh Am. 1989;16:297–302. [PubMed] [Google Scholar]
- 12.Webster G.D. Perineal repair of membranous urethral stricture. Urol Clin North Am. 1989;16:303–315. [PubMed] [Google Scholar]
- 13.Koraitim M.M. Pelvic fracture urethral injuries: the unresolved controversy. J Urol. 1999;161:1433–1441. [PubMed] [Google Scholar]
- 14.Elliott D.S., Barret D.M. Long-term follow-up and evaluation of primary realignment of posterior urethral disruptions. J Urol. 1997;157:814–816. [PubMed] [Google Scholar]
- 15.Kotkin L., Koch M.O. Impotence and incontinence after immediate realignment of posterior urethral trauma: results of injury or management? J Urol. 1996;155:1600–1603. [PubMed] [Google Scholar]
- 16.Mouraviev V.B., Coburn M., Santucci R.A. The treatment of posterior urethral disruption associated with pelvic fractures: comparative experience of early realignment versus delayed urethroplasty. J Urol. 2005;173:873–876. doi: 10.1097/01.ju.0000152145.33215.36. [DOI] [PubMed] [Google Scholar]
- 17.Gibson G.R. Urological management and complications of fractured pelvis and ruptures urethra. J Urol. 1974;111:353–355. doi: 10.1016/s0022-5347(17)59964-4. [DOI] [PubMed] [Google Scholar]
- 18.Crassweller P.O., Farrow G.A., Robson C.J., Russell J.L., Colapinto V. Traumatic rupture of the supramembranous urethra. J Urol. 1977;118:770–771. doi: 10.1016/s0022-5347(17)58188-4. [DOI] [PubMed] [Google Scholar]
- 19.Follis W.H., Koch M.O., McDougal W.S. Immediate management of prostatomembranous urethral disruptions. J Urol. 1992;147:1259–1262. doi: 10.1016/s0022-5347(17)37534-1. [DOI] [PubMed] [Google Scholar]
- 20.El-Abd S. Endoscopic treatment of posttraumatic urethral obliteration: experience in 396 patients. J Urol. 1995;153:67–71. doi: 10.1097/00005392-199501000-00025. [DOI] [PubMed] [Google Scholar]
- 21.Porter J.R., Takayama T.K., Defalco A.J. Traumatic posterior urethral injury and early realigment using magnetic urethral catheters. J Urol. 1997;158:425–430. [PubMed] [Google Scholar]
- 22.Tahan H., Randrianantenaina A., Michel F. Treatment of complete rupture of the posterior urethra by endoscopic treatment. Prog Urol. 1999;9:489–495. [PubMed] [Google Scholar]
- 23.Asci R., Sarikaya S., Buyukalpelli R., Saylik A., Yilmaz A.F., Yildiz S. Voiding and sexual dysfunctions after pelvic fracture urethra injuries treated with either initial cystostomy and delayed urethroplasty or immediate primary urethral realignment. Scand J Urol Nephrol. 1999;33:228–233. doi: 10.1080/003655999750015826. [DOI] [PubMed] [Google Scholar]
- 24.Moudouni S.M., Patard J.J., Manuta A., Guiraud P., Lobel B., Guille F. Early endoscopic realignment of post-traumatic posterior urethral disruption. Urology. 2001;57:628–632. doi: 10.1016/s0090-4295(00)01068-2. [DOI] [PubMed] [Google Scholar]
- 25.Leddy L.S., Vanni A.J., Wessells H., Voelzke B.B. Outcomes of endoscopic realignment of pelvic fracture associated urethral injuries at a level 1 trauma center. J Urol. 2012;188:174–178. doi: 10.1016/j.juro.2012.02.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnsen N.V., Dmochowski R.R., Mock S., Reynolds W.S., Milam D.F., Kaufman M.R. Primary endoscopic realignment of urethral disruption injuries- a doubled-edged sword? J Urol. 2015;194:1022–1026. doi: 10.1016/j.juro.2015.03.112. [DOI] [PubMed] [Google Scholar]
- 27.Tausch T.J., Morey A.F. The case against primary endoscopic realignment of pelvic fracture urethral injuries. Arab J Urol. 2015;13:13–16. doi: 10.1016/j.aju.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tausch T.J., Morey A.F., Scott F., Simhan J. Unintended negative consequences of primary endoscopic realignment for men with pelvic fracture urethral injuries. J Urol. 2014;192:1720–1724. doi: 10.1016/j.juro.2014.06.069. [DOI] [PubMed] [Google Scholar]
- 29.Rios E., Martinez-Piñeiro L. Words of wisdom. Re: primary endoscopic realignment of urethral disruption injuries—a double-Edged Sword? Eur Urol. 2016;69:536–537. doi: 10.1016/j.eururo.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 30.Koraitim M.M. Pelvic fracture urethral injuries: evaluation of various methods of management. J Urol. 1996;156:1288–1291. doi: 10.1016/s0022-5347(01)65571-x. [DOI] [PubMed] [Google Scholar]
- 31.Mundy A.R. The role of delayed primary repair in the acute management of pelvic fracture injuries to the urethra. Br J Urol. 1991;68:273–276. doi: 10.1111/j.1464-410x.1991.tb15322.x. [DOI] [PubMed] [Google Scholar]
- 32.Mundy A.R. Urethroplasty for posterior urethral strictures. Br J Urol. 1996;78:243–247. doi: 10.1046/j.1464-410x.1996.11617.x. [DOI] [PubMed] [Google Scholar]
- 33.Cooperberg M.R., McAnnich J.W., Alsikafi N.F., Elliot S.P. Urethral reconstruction for traumatic posterior urethral disruption: outcomes of a 25-year experience. J Urol. 2007;178:2006–2010. doi: 10.1016/j.juro.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 34.Koraitim M. On the art of anastomotic posterior urethroplasty: a 27-year experience. J Urol. 2005;173:135–139. doi: 10.1097/01.ju.0000146683.31101.ff. [DOI] [PubMed] [Google Scholar]
- 35.Webster G.D., Ramon J. Repair of pelvic fracture posterior urethral defects using an elaborated perineal approach. J Urol. 1991;145:744–748. doi: 10.1016/s0022-5347(17)38442-2. [DOI] [PubMed] [Google Scholar]
- 36.Webster G.D., Peterson A.C. Simple perineal and elaborated perineal posterior urethroplasty. Arab J Urol. 2015;13:17–23. doi: 10.1016/j.aju.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu Q., Zhang J., Sa Y., Jin S., Xu Y. Transperineal bulboprostatic anastomosis in patients with simple traumatic posterior urethral stricture: a retrospective study from a referral urethral centre. Urology. 2009;74:1132–1136. doi: 10.1016/j.urology.2009.05.078. [DOI] [PubMed] [Google Scholar]
- 38.Culty T., Boccon-Gibod L. Anastomotic urethroplaty for posttraumatic urethral stricture: previous urethral manipulation has a negative impact on the final autcome. J Urol. 2007;177:1374–1377. doi: 10.1016/j.juro.2006.11.092. [DOI] [PubMed] [Google Scholar]
- 39.Koraitim M. Failed posterior urethroplasty: lessons learned. Urology. 2003;62:719–722. doi: 10.1016/s0090-4295(03)00573-9. [DOI] [PubMed] [Google Scholar]
- 40.Koraitim M. Unsuccessful outcomes after posterior urethroplasty: definition, diagnosis and treatment. Urology. 2012;79:1168–1174. doi: 10.1016/j.urology.2011.11.078. [DOI] [PubMed] [Google Scholar]
- 41.Lumen N., Hoebeke P., de Troyer B., Yssebaert B., Oesterlink W. Perineal anastomotic urethroplasty for posttraumatic urethral stricture with or without previous urethral manipulations: a review of 61 cases with long-term follow-up. J Urol. 2008;170:1196–1200. doi: 10.1016/j.juro.2008.10.170. [DOI] [PubMed] [Google Scholar]
- 42.Warner J.N., Santucci R.A. The management of the acute setting of pelvic fracture urethral injury (realignment vs suprapubic cystostomy alone) Arab J Urol. 2015;13:7–12. doi: 10.1016/j.aju.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barret K., Braga L.H., Farrokhyar F., Davies T.O. Primary realignment vs suprapubic cystostomy for the management of pelvic fracture-associated urethral injuries: a systematic review and meta-analysis. Urology. 2014;83:924–929. doi: 10.1016/j.urology.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 44.Kulkarni S.B., Joshi P.M., Hunter C., Surana S., Shahrour W., Alhajeri F. Complex posteiror urethral injury. Arab J Urol. 2015;13:43–52. doi: 10.1016/j.aju.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sachse H. Treatment of urethral stricture: trnasurethral slit in view using sharp section. Fortschr Med. 1974;92:12–15. [PubMed] [Google Scholar]
- 46.Blandy J.P. Urethral stricture. Postgrad Med. 1980;56:383–418. doi: 10.1136/pgmj.56.656.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gupta N.P., Gill I.S. Core-through optical internal urethrotomy in management of impassable traumatic posterior urethral strictures. J Urol. 1986;136:1018–1021. doi: 10.1016/s0022-5347(17)45193-7. [DOI] [PubMed] [Google Scholar]
- 48.Chiou R.K., Gonzalez R., Ortlip S., Fraley E.E. Endoscopic treatment of posterior urethral obliteration: long-term follow-up and comparison with transpubic urethroplasty. J Urol. 1988;140:508–511. doi: 10.1016/s0022-5347(17)41704-6. [DOI] [PubMed] [Google Scholar]
- 49.Marshall F.F. Endoscopic reconstruction of traumatic urethral transections. Urol Clin North Am. 1989;16:313–318. [PubMed] [Google Scholar]
- 50.Barry J.M. Visual urethrotomy in the management of the obliterated membranous urethra. Urol Clin North Am. 1989;16:319–324. [PubMed] [Google Scholar]
- 51.DeVries C.R., Anderson R.U. Endoscopic urethroplasty: an improved technique. J Urol. 1990;143:1225–1226. doi: 10.1016/s0022-5347(17)40232-1. [DOI] [PubMed] [Google Scholar]
- 52.Leonard M.P., Emtaje J., Perez R., Morales A. Endoscopic management of urethral stricture: cut-to-the-light procedure. Urology. 1990;35:117–120. doi: 10.1016/0090-4295(90)80056-s. [DOI] [PubMed] [Google Scholar]
- 53.Kernohan R.M., Anwar K.K., Johnston S.R. Complete urethral stricture of the membranous urethra: a different perspective. Br J Urol. 1991;65:51–54. doi: 10.1111/j.1464-410x.1990.tb14661.x. [DOI] [PubMed] [Google Scholar]
- 54.Yasuda T., Yamanishi T., Isaka S., Okano T., Masai M., Shimazaki J. Endoscopic re-establishment of membranous urethral disruption. J Urol. 1991;145:977–999. doi: 10.1016/s0022-5347(17)38505-1. [DOI] [PubMed] [Google Scholar]
- 55.Quint J.H., Stanisic T.H. Above and below delayed endoscopic treatment of traumatic posterior urethral disruption. J Urol. 1993;149:484–487. doi: 10.1016/s0022-5347(17)36124-4. [DOI] [PubMed] [Google Scholar]
- 56.Goel M.C., Kumar M., Kapoor R. Endoscopic management of traumatic posterior urethral stricture: early results and follow-up. J Urol. 1997;157:95–97. [PubMed] [Google Scholar]
- 57.Levine J., Wessells H. Comparison of open and endoscopic treatment of posttraumatic posterior urethral strictures. World J Surg. 2001;25:1597–1601. doi: 10.1007/s00268-001-0156-7. [DOI] [PubMed] [Google Scholar]
- 58.Dogra P.N., Nabi G. Core-through urethrotomy using the neodynium yag laser for obliterative urethral strictures after traumatic urethral disruption and/or distraction defects: long-term outcome. J Urol. 2002;167:543–546. doi: 10.1016/S0022-5347(01)69082-7. [DOI] [PubMed] [Google Scholar]
- 59.Jordan G.H. Management of membranous urethral distraction injuries via the perineal approach. In: McAninch J.W., editor. Traumatic and reconstructive urology. WB Saunders; Philadelphia: 1996. [Google Scholar]
- 60.Baert L., Verhamme L., Van Poppel H., Vandeursen H., Baert J. Long-term consequences of urethral stents. J Urol. 1993;150:853–855. doi: 10.1016/s0022-5347(17)35631-8. [DOI] [PubMed] [Google Scholar]