Abstract
BACKGROUND
Ovarian cancer is frequently diagnosed at an advanced stage and 70% of patients experience recurrence months to years from initial diagnosis. The expression of paraneoplastic antigens can result in the occurrence of onconeural autoantibodies in ovarian cancer that may be associated with neurological disorders that are clinically manifested in patients before diagnosis of ovarian cancer. These paraneoplastic antigens can serve as excellent biomarkers not only for early detection but also for monitoring ovarian cancer recurrence.
OBJECTIVE
To assess the immunoreactivity of our previous 3 biomarkers along with 3 paraneoplastic antigens, HARS, Ro52 and CDR2 for the evaluation of their sensitivity in predicting recurrence before the clinical relapse of the ovarian cancer.
METHODS
Western blot immunoassays were performed to assess the immunoreactivity of 6 antigens with 21 recurrent ovarian cancer patients.
RESULTS
The results indicated that antibodies to HARS, Ro52, CDR2 and 5H6 antigens predicted ovarian cancer recurrence 5.03 months before the clinical or symptomatic relapse in 21 ovarian cancer patients with a sensitivity of 90.5% when CA125 levels were below the standard cutoff (35 U/ml).
CONCLUSION
Our study suggests that appearance of onconeural antibodies prior to the rise in CA125 during post treatment surveillance can be a useful diagnostic to predict ovarian cancer recurrence.
Keywords: Paraneoplastic syndromes, Paraneoplastic antigens, Humoral immune response, Onconeural autoantibodies, Serological screening, Immunoreactivity
1. Introduction
Routine disease monitoring of ovarian cancer patients is generally recommended by gynecologic oncologists after the completion of primary surgery and first-line chemotherapeutic treatments. The clinical symptoms of recurrence are determined by measuring the level of serum CA125, one of the most extensively used tumor biomarkers in standard clinical practice for disease surveillance. In a randomized trial performed by Rustin et al. it was shown that ovarian cancer patients who had increased CA125 level before the clinical recurrence followed by chemotherapy treatments did not have a survival benefit compared to the other arm of ovarian cancer patients who received chemotherapy based on clinical evidences of ovarian cancer recurrence [25]. Conversely, a recent study has shown that ovarian cancer patients at risk of recurrence may benefit from early initiation of treatments. Guo et al. reported that when setting the CA125 threshold to 10U/ml instead of 35U/ml, distant recurrent lesions located in spleen, liver and pelvic region were detected in 3 postoperative epithelial ovarian carcinoma patients who had CA125 values 14.5U/ml, 13.5U/ml and 20.9U/ml respectively. In all of these patients, recurrent lesions were detected 2–3 months prior to clinical recurrence and all the patients underwent second cytoreductive surgery. After the surgery, their CA125 values were less than 10U/ml and the patients were in good health. Thus, early treatments were shown to be necessary when there is a risk of recurrence involved [14]. Their study may not be in agreement with the randomized trial by Rustin et al. where only chemotherapy was considered as an early treatment and the impact of second-line cytoreductive surgery was not taken into consideration. Also, patients who participated in that trial were not treated with new salvage chemotherapy regimens that might have improved prognosis [13,22]. Another study reported by Yang et al. showed that in a study population of 152 ovarian cancer patients, the average elevation of CA125 level was 116.28 U/ml at the time of clinical recurrence and the average time that elapsed from the rise in CA125 to the time when recurrent lesions were detected by physical or radiologic examinations was 122 days. The sensitivity and specificity of detecting early ovarian cancer recurrence using CA125 tumor marker alone with a threshold of 35U/ml was 67.39% and 86.79% respectively [28]. Despite its utility in ovarian cancer diagnosis and disease monitoring, CA125 has its limitations. A rise in CA125 to 1,000 IU/ml has been observed in many benign gynecological conditions, such as, intramural leiomyoma, adenexal cystic mass, and ovarian endometrioma [12]. Other studies have documented normalization of CA125 in 50% of patients with ovarian cancer with microscopic disease at the second-look laparotomy [4]. Therefore, there is a dearth of sensitive biomarkers that can predict ovarian cancer recurrence with a sufficient lead time prior to the rise in CA125 during cancer surveillance, so that the patients can benefit from an early therapeutic intervention capable of prolonging the disease-free interval and improve overall survival.
Numerous studies have shown the role of tumor autoantibodies as biomarkers for ovarian cancer diagnosis and its recurrence. These autoantibodies to tumor associated antigens (TAAs) arise due to the generation of humoral immune response before evidence of clinical symptoms in cancer patients [5,7,8]. Our previous study indicated that a 3 biomarker panel, one being a peptide epitope from a known paraneoplastic antigen, predicted ovarian cancer recurrence at a median lead time of 9.07 months with 94.7% sensitivity, 86.7% specificity, and 93.3% accuracy, in a cohort of ovarian cancer patients where normalization of CA125 had occurred after the surgery and completion of chemotherapy [5]. Paraneoplastic antigens can elicit a humoral immune response in cancer patients as these antigens are expressed in the cells of nervous system and tumor [24]. The appearance of these onconeural antibodies in ovarian cancer patients leads to the development of various neurological disorders called paraneoplastic syndromes, particularly dermatomyositis or polymyositis [1,2,15,21]. The diagnosis of ovarian cancer can be preceded by the occurrence of dermatomyositis or polymyositis. Marie et al. reviewed the medical data to evaluate the clinical outcome of 89 patients who had antisynthetase syndrome (ASS) associated with Jo-1 antibodies that target HARS antigen. Concurrent occurrence of Ro52 antibodies was also observed in 36 out of 89 patients. It was reported that 7/36 (19.4%) had colon, breast, ovarian, or esophageal cancers and 28/36 (77%) had interstitial lung disease with poorer prognosis [17]. Other studies have shown that patients with ovarian cancer in association with paraneoplastic cerebellar degeneration harbor Yo antibodies directed against CDR2 antigen that is expressed in tumor cells and Purkinje cells [21]. The frequency of appearance of Yo antibodies in patients with paraneoplastic cerebellar degeneration associated with ovarian cancer and breast cancer was found to be 13/557 (2.3 %) and 4/253 (1.6%) respectively. The diagnosis of 2/13 ovarian cancer patients was preceded by the appearance of paraneoplastic cerebellar degeneration [21]. These onconeural antibodies can occur in the absence of paraneoplastic symptoms leading to their diagnostic utility in asymptomatic subjects. Although the clinical implication of these onconeural antibodies as biomarkers for early diagnosis of ovarian cancer has been reported in many case studies, the usefulness of these antibodies has yet to be evaluated in monitoring disease status in ovarian cancer patients after cytoreductive surgery and chemotherapy treatments. In the present study we evaluated the role of a panel of 3 recombinant paraneoplastic antigens, HARS, CDR2 and Ro52 in combination with 3 of our previous biomarkers in predicting recurrence in new and independent cohort of ovarian cancer patient population in which most of the patients had no elevation in CA125 level months before their clinical recurrence. Our results indicate that autoantibodies to HARS, Ro52, CDR2 and 5H6 antigens predicted ovarian cancer recurrence 5.03 months before the clinical or symptomatic relapse in 21 ovarian cancer patients with a sensitivity of 90.5% when CA125 levels were below the standard cutoff (35 U/ml).
2. Materials and Methods
2.1. Patient Population
Patients diagnosed and treated for late stage serous epithelial ovarian cancer at Karmanos Cancer Institute, St John Health System (Detroit, MI), or Oakwood Hospital (Dearborn, MI) were entered on to the study at the time of their diagnosis. Study participation included collection of serial blood samples starting at or near the time of surgery and continuing for every six months up to five years, concurrent with clinical lab draws. Medical records were reviewed to determine CA125 levels, disease status, chemotherapy status, disease-free interval (DFI) and time to recurrence (TTR) over a multi-year period. Serial serum samples were collected between 2003 and 2014. All participants provided written informed consent. Study procedures were approved by the Wayne State University, St. Johns Health Systems, and Oakwood Hospital Institutional Review Boards.
2.2. Specimen Collection and Processing
Samples were collected and processed using the procedure as described earlier [5]. The demographics of patients in the training set were also described in earlier studies [5]. For each patient in the test set we selected 3 samples; 1) the baseline blood sample (collected at time of diagnosis), 2) the blood sample collected approximately 3–15 months before the clinical recurrence, ideally with normal CA125 and no evidence of disease, and 3) the sample collected as close as possible to clinical recurrence (Supplementary Table S2). The disease status of 3 sequential serum samples correlated to EOD-NED-EOD but the second sample was usually taken while still in chemo so the NED was not actually a true remission, but a response to the chemotherapy. EODs were determined by clinical/imaging data, or elevated CA125 level, or both. Future studies will include more frequent collection of interval samples to increase the pool of samples fitting the ideal profile.
2.3. Cloning of recombinant antigen into bacterial expression vector
All the previous phage bearing tumor antigens such as 4B7, 4H4, 5H6, and T7 1-2a (empty phage capsid protein used as negative control protein) as well as 2 paraneoplastic antigens such as Ro52 and CDR2 were first PCR amplified using different forward primers (containing 5′ restriction site followed by His tag and T7 tag at the N terminus) and reverse primers (containing 3′ stop codon followed by restriction site at the C terminus) using cDNA templates (Supplementary Table S1). For phage antigens, the cDNA templates were obtained from ovarian tumor T7 phage cDNA libraries and for paraneoplastic antigens, cDNAs were prepared from different ovarian cancer cell lines. The PCR products were column purified (Qiagen, Germantown, MD), restriction digested, column purified again and successively ligated to pET-21b bacterial expression vector by following manufacturer’s protocol (EMD Millipore Corporation, San Diego, CA). The ligated DNA was then transformed into BL21-DE3 strain and several colonies were picked and sequenced. Positive colonies bearing the respective genes were further employed for in vivo production of recombinant His and T7-tagged proteins in Bl21-DE3 bacterial strain. All cDNA clones were DNA sequence verified by standard techniques.
2.4. Production and purification of recombinant His and T7 tagged proteins
BL21-DE3 bacterial cells bearing clones, pET21b-4B7, pET21b-4H4, pET21b-5H6, pET21b-Ro52, pET21b-CDR2, and pET21b-T71-2a (negative control) were grown overnight in 10 ml LB with 50 μg/ml ampicillin at 37° C. About 4 ml of the overnight culture was added to 400 ml LB with 50 μg/ml ampicillin and was grown at 37° C to OD between 0.4–0.5. After it reached the desired OD, 0.6 mM IPTG was added to induce the production of RNA polymerase that was needed for RNA and subsequent protein synthesis and the culture was grown at 37° C for 3.5 hr. The cells were pelleted at 3,700 rpm for 20 min and the supernatant was discarded. The pellet was frozen at −20°C for at least 30 min and then lysed with BPER lysing buffer (Thermo Fisher Scientific, Grand Island, NY) centrifuged at 15,000 × g and then transferred the supernatant. The pellet containing the inclusion bodies were solubilized in 8M urea because pET21b expression system (EMD Millipore Corporation, San Diego, CA) results in enormous expression of our desired proteins that are found in inclusion bodies that only can be solubilized with 8M urea. The crude His and T7-tagged proteins were purified first using Ni-NTA agarose beads (Thermo Fisher Scientific, Grand Island, NY) following manufacturer’s protocol. Ni-NTA agarose beads binds to His residues that are attached to proteins and results in relatively pure protein. The Ni-NTA purified His-tagged proteins were further purified using agarose beads bound to T7 antibody by following manufacturer’s instruction (EMD Millipore Corporation, San Diego, CA). The second round of purification with T7 antibody bound agarose beads is necessary to remove all bacterial poly-His containing proteins from first round of purification with Ni-NTA beads. Only HARS protein was commercially purchased. This point forward all the recombinant pET21b-antigens will be referred by just their names.
2.5. Immunoscreening of ovarian cancer patient serum samples using purified recombinant antigens
For the purified recombinant Ro52 antigen, 0.06 μg of protein was used because very strong reactivity of Ro52 protein with some ovarian cancer patients was observed in earlier studies and this high intensity of the protein band determined by the Odyssey software was found to be beyond the saturation limit (data not shown). The optimum amount of 0.06 μg for Ro52 antigen was obtained by immunoscreening serum samples obtained from 1 ovarian cancer patient and a patient with benign disease using different microgram amounts of purified Ro52 protein (Supplementary Fig. 1(A–C)). For all other antigens, 1 μg of purified recombinant proteins was used for SDS-PAGE and proteins were transferred onto nitrocellulose membrane. The membrane was blocked in 5% milk in TBST for 1 hr and incubated with ovarian cancer patient’s serum at a dilution of 1:300 for 1 hr at room temperature. The membrane was washed 3 times with TBST followed by incubation with rabbit-anti human secondary antibody conjugated with an IR dye-800 (Rockland Antibodies and Assays, Limerick, PA) at a dilution of 1:5000 for 1 hr at room temperature. After washing the membrane 3 times with TBST, anti-6X His–Tag mouse monoclonal antibody Dylight 680 conjugated (Rockland Antibodies and Assays, Limerick, PA) was added at dilution of 1:10000 and incubated for 1 hr at room temperature. The membrane was scanned at 800 nm and 700 nm separately and the band intensity for each protein was quantitated, normalized to its His-tag using Odyssey software.
2.6. Determination of threshold of each antigen using the training set
Threshold of each antigen was calculated based on the immunoreactivity of T71-2a protein (negative control) with the all the ovarian cancer patients (5 recurrent and 5 non-recurrent) in the training set. The median (Median T71-2a) and standard deviation (STDEV) of the normalized signal intensity values representing the immunoreactivity of T71-2a protein with 5 recurrent patients (serum samples were obtained at months to 1 year before the clinical recurrence) and the 5 non-recurrent patients (serum samples were obtained at approximately 1 year from ovarian cancer diagnosis) in the training set was calculated as shown as Table 2. The threshold for each antigen was chosen in such a way to achieve higher percent specificity against 5 non-recurrent ovarian cancer patients in the training set as shown in Table 2 that also listed the sensitivity of each antigen that reacted with 5 recurrent ovarian cancer patients. For moderate to weakly reactive antigens such as HARS, 4B7, 4H4, 5H6, a threshold of 0.03 (Median T71-2a + 1.3*STDEV) was used. For strongly reactive antigens, such as Ro52 and CDR2 antigens, a threshold of 0.17 (Median T71-2a + 9*STDEV) was used. These thresholds were next applied to determine the sensitivity of each antigen that can predict recurrence in 21 independent ovarian cancer patients in the test set. Although T71-2a, the negative control protein showed 20% sensitivity and 100% specificity in the training set (Table 2), it only revealed 4.8% (1/21) sensitivity in the test set (Table 4). The antigens that showed sensitivity >10% in the test set were only selected for further analyses.
Table 3A.
Association of immunoreactivity of Ro52, CDR2 and HARS antigens with the recurrence status of 21 ovarian cancer patients
| Sample ID | RecurInterval (T2) | Disease status at T2 | CA125 value at T2 | Ro52
|
Western blot figure number | |
|---|---|---|---|---|---|---|
| Comparison of level of Ro52 immunoreactivity at T2 and T1 BC represents immunoreactivity value below cutoff at T2 |
Association of Ro52 immunoreactivity at T2 with recurrence status (Asso-R) | |||||
|
| ||||||
| P178 | −4.20 | In chemo, NED | 203 | Immunoreactivity value at T1 was almost same as at T2 | Asso-R | Fig. 1B (a,b, lane 5) |
| P265 | −4.87 | In chemo, NED | 54 | BC | None | Fig. 1A (a,b, lane 5) |
| P295 | −5.77 | post chemo, NED | 18 | BC | None | |
| P283 | −13.60 | In chemo, NED | 22 | BC | None | |
| P300 | −3.73 | post chemo, NED | 12 | Immunoreactivity value at T2 was 2.2 fold higher than at T1 | Asso-R | |
| P326* | −4.30 | In chemo, stable disease | 41 | Immunoreactivity value at T2 was 51.5 fold higher than at T1 | Asso-R | Fig. 1E (a,b, lane 5) |
| P336 | −10.00 | post chemo, NED | 20 | BC | None | |
| P341 | −7.10 | In chemo, NED | 37 | BC | None | Fig. 1H (a,b, lane 5) |
| P342 | −3.30 | In chemo, NED | 5 | BC | None | |
| P356* | −9.07 | In chemo, toleratIng treatment | 11 | BC at T2; immunoreactivity value at T1 was missing | None | |
| P367* | −4.33 | In chemo, toleratIng treatment | 29 | Immunoreactivity value at T2 was 1.4 fold higher than at T1 | Asso-R | Fig. 1F (a,b, lane 5) |
| P370 | −2.63 | In chemo, NED | 13 | Immunoreactivity value at T2 dropped by 2 fold | Asso-R | Fig. 1D (a,b, lane 5) |
| P386 | −17.27 | In chemo, Not specified | 16 | Immunoreactivity value at T2 was 1.9 fold higher than at T1 | Asso-R | |
| P392 | −5.93 | post chemo, NED | 9 | BC | None | |
| P376 | −9.93 | post chemo, NED | 7 | Immunoreactivity value at T2 was 5.5 fold higher than at T1 | Asso-R | |
| P378 | −12.37 | In chemo, NED | 9 | The value of IMR at T2 significantly dropped by 21.6 fold | Asso-R | |
| P393* | −5.03 | In chemo, EOD | 26 | BC | None | Fig. 1J (a,b, lane 5) |
| P398* | −10.30 | In chemo, EOD | 24 | Immunoreactivity value at T1 was almost same as at T2 | Asso-R | Fig. 1I (a,b, lane 5) |
| P400 | −4.50 | In chemo, NED | 6 | BC | None | |
| P410 | −3.23 | post chemo, NED | 24 | Immunoreactivity value at T2 was 1.6 fold higher than at T1 | Asso-R | Fig. 1C (a,b, lane 5) |
| P413 | −15.53 | In chemo, NED | 8 | Immunoreactivity value at T2 significantly dropped by 20.9 fold | Asso-R | Fig. 1G (a,b, lane 5) |
| Sample ID | RecurInterval (T2) | Disease status at T2 | CA125 value at T2 | CDR2
|
Western blot figure number | |
|---|---|---|---|---|---|---|
| Comparison of level of CDR2 immunoreactivity at T2 and T1 BC represents immunoreactivity value below cutoff at T2 |
Association of CDR2 immunoreactivity at T2 with recurrence status (Asso-R) | |||||
|
| ||||||
| P178 | −4.20 | In chemo, NED | 203 | BC | None | Fig. 1B (a,b, lane 6) |
| P265 | −4.87 | In chemo, NED | 54 | Immunoreactivity value at T2 was 2.4 fold higher than at T1 | Asso-R | Fig. 1A (a,b, lane 6) |
| P295 | −5.77 | post chemo, NED | 18 | Immunoreactivity value at T1 was almost same as at T2 | Asso-R | |
| P283 | −13.60 | In chemo, NED | 22 | Immunoreactivity value at T1 was almost same as at T2 | Asso-R | |
| P300 | −3.73 | post chemo, NED | 12 | Immunoreactivity value at T1 was almost same as at T2 | Asso-R | |
| P326* | −4.30 | In chemo, stable disease | 41 | Immunoreactivity value at T2 dropped by 1.5 fold | Asso-R | Fig. 1E (a,b, lane 6) |
| P336 | −10.00 | post chemo, NED | 20 | BC | None | |
| P341 | −7.10 | In chemo, NED | 37 | Immunoreactivity value at T2 was 1.7 fold higher than at T1 | Asso-R | Fig. 1H (a,b, lane 6) |
| P342 | −3.30 | In chemo, NED | 5 | BC | None | |
| P356* | −9.07 | In chemo, toleratIng treatment | 11 | BC | None | |
| P367* | −4.33 | In chemo, toleratIng treatment | 29 | Immunoreactivity value at T1 was almost same as at T2 | Asso-R | Fig. 1F (a,b, lane 6) |
| P370 | −2.63 | In chemo, NED | 13 | Immunoreactivity value at T2 dropped by 1.6 fold | Asso-R | Fig. 1D (a,b, lane 6) |
| P386 | −17.27 | In chemo, Not specified | 16 | Immunoreactivity value at T2 was 1.4 fold higher than at T1 | Asso-R | |
| P392 | −5.93 | post chemo, NED | 9 | Immunoreactivity value at T2 dropped by 2 fold | Asso-R | |
| P376 | −9.93 | post chemo, NED | 7 | Immunoreactivity value at T1 was almost same as at T2 | Asso-R | |
| P378 | −12.37 | In chemo, NED | 9 | BC | None | |
| P393* | −5.03 | In chemo, EOD | 26 | Immunoreactivity value at T2 dropped by 1.4 fold | Asso-R | Fig. 1J (a,b, lane 6) |
| P398* | −10.30 | In chemo, EOD | 24 | BC | None | Fig. 1I (a,b, lane 6) |
| P400 | −4.50 | In chemo, NED | 6 | Immunoreactivity value at T1 was almost same as at T2 | Asso-R | |
| P410 | −3.23 | post chemo, NED | 24 | Immunoreactivity value at T1 was almost same as at T2 | Asso-R | Fig. 1C (a,b, lane 6) |
| P413 | −15.53 | In chemo, NED | 8 | Immunoreactivity value at T1 was almost same as at T2 | Asso-R | Fig. 1G (a,b, lane 6) |
| Sample ID | RecurInterval (T2) | Disease status at T2 | CA125 value at T2 | HARS
|
Western blot figure number | |
|---|---|---|---|---|---|---|
| Comparison of level of HARS immunoreactivity at T2 and T1 BC represents immunoreactivity value below cutoff at T2 |
Association of HARS immunoreactivity at T2 with recurrence status (Asso-R) | |||||
|
| ||||||
| P178 | −4.20 | In chemo, NED | 203 | Immunoreactivity value at T1 was almost same as at T2 | Asso-R | Fig. 1B (a,b, lane 1) |
| P265 | −4.87 | In chemo, NED | 54 | Immunoreactivity value at T2 was 2.2 fold higher than at T1 | Asso-R | Fig. 1A (a,b, lane 1) |
| P295 | −5.77 | post chemo, NED | 18 | Immunoreactivity value at T1 was almost same as at T2 | Asso-R | |
| P283 | −13.60 | In chemo, NED | 22 | Immunoreactivity value at T1 was almost same as at T2 | Asso-R | |
| P300 | −3.73 | post chemo, NED | 12 | Immunoreactivity value at T1 was almost same as at T2 | Asso-R | |
| P326* | −4.30 | In chemo, stable disease | 41 | Immunoreactivity value at T1 was almost same as at T2 | Asso-R | Fig. 1E (a,b, lane 1) |
| P336 | −10.00 | post chemo, NED | 20 | BC | None | |
| P341 | −7.10 | In chemo, NED | 37 | Immunoreactivity value at T2 was dropped by 1.4 fold at T2 | Asso-R | Fig. 1H (a,b, lane 1) |
| P342 | −3.30 | In chemo, NED | 5 | BC | None | |
| P356* | −9.07 | In chemo, toleratIng treatment | 11 | BC | None | |
| P367* | −4.33 | In chemo, toleratIng treatment | 29 | Immunoreactivity value at T2 was 2 fold higher than at T1 | Asso-R | Fig. 1F (a,b, lane 1) |
| P370 | −2.63 | In chemo, NED | 13 | Immunoreactivity value at T1 was almost same as at T2 | Asso-R | Fig. 1D (a,b, lane 1) |
| P386 | −17.27 | In chemo, Not specified | 16 | BC | None | |
| P392 | −5.93 | post chemo, NED | 9 | Immunoreactivity value at T2 dropped by 2.5 fold | Asso-R | |
| P376 | −9.93 | post chemo, NED | 7 | Immunoreactivity value at T2 dropped by 1.5 fold | Asso-R | |
| P378 | −12.37 | In chemo, NED | 9 | BC | None | |
| P393* | −5.03 | In chemo, EOD | 26 | Immunoreactivity value at T1 was almost same as at T2 | Asso-R | Fig. 1J (a,b, lane 1) |
| P398* | −10.30 | In chemo, EOD | 24 | Immunoreactivity value at T1 was almost same as at T2 | Asso-R | Fig. 1I (a,b, lane 1) |
| P400 | −4.50 | In chemo, NED | 6 | BC | None | |
| P410 | −3.23 | post chemo, NED | 24 | Immunoreactivity value at T2 was 1.6 fold higher than at T1 | Asso-R | Fig. 1C (a,b, lane 1) |
| P413 | −15.53 | In chemo, NED | 8 | BC | None | Fig. 1G (a,b, lane 1) |
Note: Ovarian cancer patients with asterisk had evidence of disease months before the clinical recurrence ; NED: No evidence of disease; EOD: Evidence of disease
T1 represents time at ovarian cancer diagnosis; T2 represents time in months before the clinical recurrence
Table 4.
Reactivity of antigens with 5 recurrent and 5 non-recurrent ovarian cancer patients (training set) and an independent recurrent ovarian patient population (test set, n=21)
| Sample-BSID | Sample | RecurInterval (T2) | Reactivity of Antigen Biomarkers | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Training Set | Training Set | CA125 | HARS | 4B7 | 4H4 | 5H6 | Ro52 | CDR2 | T71-2a | ||
|
| |||||||||||
| 674 | P128-Cancer(R)-T2 | −8.70 | 13 | 0.1 | 0.21 | ||||||
|
|
|||||||||||
| 1740 | P135-Cancer(R)-T2 | −16.30 | 11 | 15.15 | 1.75 | ||||||
|
|
|||||||||||
| 1681 | P146-Cancer(R)-T2 | −8.20 | 25 | 0.09 | 0.45 | ||||||
|
|
|||||||||||
| 3905 | P184-Cancer(R)-T2 | −14.30 | 5 | 0.2 | |||||||
|
|
|||||||||||
| 3776 | P175-Cancer(R)-T2 | −9.50 | 18 | 0.09 | 0.13 | 34.5 | 0.3 | 0.06 | |||
|
|
|||||||||||
| 784 | P25-Cancer(NR)-T2 | 12 | 0.1 | ||||||||
|
|
|||||||||||
| 832 | P164-Cancer(NR)-T2 | 28 | |||||||||
|
|
|||||||||||
| 4012 | P189-Cancer(NR)-T2 | 6 | 0.07 | 1.93 | 0.29 | ||||||
|
|
|||||||||||
| 4069 | P206-Cancer(NR)-T2 | 7 | |||||||||
|
|
|||||||||||
| 7428 | P281-Cancer(NR)-T2 | 6 | 0.06 | 0.07 | 0.04 | ||||||
|
| |||||||||||
| Test Set | Test Set | CA125 | HARS | 4B7 | 4H4 | 5H6 | Ro52 | CDR2 | T71-2a* | Number of Antigens reacting with each patient | |
|
| |||||||||||
| 1667 | P178-Cancer(R)-T2 | −4.20 | 203 | 0.04 | 0.04 | 0.04 | 1.65 | 4 | |||
|
|
|||||||||||
| 4694 | P265-Cancer(R)-T2 | −4.87 | 54 | 0.09 | 0.06 | 0.04 | 0.04 | 1.06 | 5 | ||
|
|
|||||||||||
| 7183 | P295-Cancer(R)-T2 | −5.77 | 18 | 0.22 | 0.21 | 2 | |||||
|
|
|||||||||||
| 5110 | P283 -Cancer(R)-T2 | −13.60 | 22 | 0.05 | 1.82 | 2 | |||||
|
|
|||||||||||
| 11499 | P300-Cancer(R)-T2 | −3.73 | 12 | 0.05 | 0.21 | 0.34 | 3 | ||||
|
|
|||||||||||
| 12898 | P326*-Cancer(R)-T2 | −4.30 | 41 | 0.04 | 1.49 | 0.81 | 3 | ||||
|
|
|||||||||||
| 12912 | P336-Cancer(R)-T2 | −10.00 | 20 | 0 | |||||||
|
|
|||||||||||
| 12897 | P341-Cancer(R)-T2 | −7.10 | 37 | 0.04 | 1.45 | 2 | |||||
|
|
|||||||||||
| 12863 | P342-Cancer(R)-T2 | −3.30 | 5 | 0.04 | 1 | ||||||
|
|
|||||||||||
| 12920 | P356*-Cancer(R)-T2 | −9.07 | 11 | 0 | |||||||
|
|
|||||||||||
| 14698 | P367*-Cancer(R)-T2 | −4.33 | 29 | 1.34 | 4.93 | 0.37 | 3 | ||||
|
|
|||||||||||
| 14697 | P370-Cancer(R)-T2 | −2.63 | 13 | 0.11 | 10.24 | 0.32 | 3 | ||||
|
|
|||||||||||
| 15178 | P386-Cancer(R)-T2 | −17.27 | 16 | 0.25 | 0.5 | 2 | |||||
|
|
|||||||||||
| 15256 | P392-Cancer(R)-T2 | −5.93 | 9 | 0.06 | 0.38 | 2 | |||||
|
|
|||||||||||
| 15264 | P376-Cancer(R)-T2 | −9.93 | 7 | 0.04 | 0.38 | 0.51 | 3 | ||||
|
|
|||||||||||
| 15180 | P378-Cancer(R)-T2 | −12.37 | 9 | 0.22 | 1 | ||||||
|
|
|||||||||||
| 15259 | P393*-Cancer(R)-T2 | −5.03 | 26 | 0.09 | 0.55 | 2 | |||||
|
|
|||||||||||
| 15266 | P398*-Cancer(R)-T2 | −10.30 | 24 | 0.04 | 1.14 | 2 | |||||
|
|
|||||||||||
| 15274 | P400-Cancer(R)-T2 | −4.50 | 6 | 0.21 | 1 | ||||||
|
|
|||||||||||
| 15776 | P410-Cancer(R)-T2 | −3.23 | 24 | 0.07 | 0.2 | 0.2 | 0.12 | 20.21 | 0.36 | 0.06 | 6 |
|
|
|||||||||||
| 15770 | P413-Cancer(R)-T2 | −15.53 | 8 | 0.37 | 1.05 | 2 | |||||
Note: The sero reactivity of the recombinant antigen biomarkers revealed 3 different range of affinity toward serum IgGs such as strong (>10 fold above the cut off, dark blue shade); moderate ((2–10) above the cutoff, medium blue shade) and weak (< 2 fold above the cutoff, light blue shade)
Ovarian cancer patients with asterisk had evidence of disease months before the clinical recurrence ; NED: No evidence of disease; EOD: Evidence of disease.
R represents recurrent ovarian cancer patients; NR represents non-recurrent ovarian cancer patients
T2 represents time in months before the clinical recurrence
Cutoff for HARS, 4B7, 4H4, 5H6: 0.03
Cutoff for Ro52 and CDR2 : 0.17
For calculation of cutoffs, please see Materials and Methods, Table 2
T7 1-2a has been dropped out in the Test set because it reacted with 4.8% of the sample population in the test set, so it was never considered in further analyses in the test set. The antigens that showed sensitivity >10% in the test set were only selected for further analyses.
3. Results
Our goal is to predict recurrence prior to the biochemical (CA125 level) or clinical/radiologic evidence of recurrence so that re-initiation of therapy can maximize the chances of improving overall survival in ovarian cancer patients. To this end we have been utilizing tumor autoantibody biomarkers in ovarian cancer patients diagnosed with late stage serous adenocarcinoma. After subtractive biopanning with sera from ovarian cancer patients and healthy controls we employed protein microarrays using phage lysates of single phage bearing cDNA clone to identify cDNA clones of antigens that specifically reacted with sera from ovarian cancer patients [7]. We found that these clones were good biomarkers for both early detection [7] and recurrence [5] of ovarian cancer. In addition, the antigen clones were frequently homologous to known paraneoplastic antigens and we propose that autoantibodies to these paraneoplastic antigens occur in asymptomatic cancer patients and can be used for diagnostic purposes. After cloning, bacterial expression and purification of the most informative antigen biomarkers, we performed a serological immunoscreening using western blotting to evaluate the sensitivity of these recombinant proteins to predict recurrence prior to the rise in CA125 level (cutoff 35U/ml) or radiologic indication of clinical recurrence in an independent retrospective cohort of ovarian cancer study population.
3.1. Serological screening of ovarian cancer patients using recombinant protein biomarkers
To determine the threshold of immunoreactivity of each antigen we performed an initial immunoscreening with 5 recurrent and 5 non-recurrent ovarian cancer patients (training set) using 6 biomarkers, namely HARS, 4B7, 4H4, 5H6, Ro52, CDR2, and T7 1-2a (which served as a negative control protein) as described in Materials and Methods section (see reference 5 for patients’ demographics used in the training set) [5], (Table 2). The threshold for each antigen was next applied to evaluate the immunoreactivity of antigens with serum IgGs obtained from 21 ovarian cancer patients at 3 different time points, initially at the time of diagnosis (T1) when the patients had elevated CA125 levels, during the monitoring phase approximately 3–15 months before their clinical recurrence (T2) when most of the patients had their CA125 values within the normal range (<35U/ml) and lastly at the time of recurrence (T3) (discussed in Materials and Methods). Immunoreactivity of 6 antigens was measured by western blot to evaluate the association of immunoreactivity with the recurrence status of ovarian cancer patients months before their clinical recurrence (Table 3(A–B)), Supplementary Table S2 (for patients demographics used in the test set). For data analyses, we focused only on the first 2 time points, T1 and T2 because our goal was to ascertain how early in time an association of immunoreactivity of antigens with recurrence can be made during the surveillance period prior to the rise in CA125 levels.
Table 3B.
Association of immunoreactivity of 4B7, 4H4, and 5H6 antigens with the recurrence status of 21 ovarian cancer patients
| Sample ID | RecurInterval (T2) | Disease status at T2 | CA125 value at T2 | 4B7
|
Western blot figure number | |
|---|---|---|---|---|---|---|
| Comparison of level of 4B7 immunoreactivity at T2 and T1 BC represents immunoreactivity value below cutoff at T2 |
Association of 4B7 immunoreactivity at T2 with recurrence status (Asso-R) | |||||
|
| ||||||
| P178 | −4.20 | In chemo, NED | 203 | Immunoreactivity value at T2 was 2.2 fold higher than at T1 | Asso-R | Fig. 1B (a,b, lane 2) |
| P265 | −4.87 | In chemo, NED | 54 | Immunoreactivity value at T2 was 2 fold higher than at T1 | Asso-R | Fig. 1A (a,b, lane 2) |
| P295 | −5.77 | post chemo, NED | 18 | BC | None | |
| P283 | −13.60 | In chemo, NED | 22 | BC | None | |
| P300 | −3.73 | post chemo, NED | 12 | BC | None | |
| P326* | −4.30 | In chemo, stable disease | 41 | BC | None | Fig. 1E (a,b, lane 2) |
| P336 | −10.00 | post chemo, NED | 20 | BC | None | |
| P341 | −7.10 | In chemo, NED | 37 | BC | None | Fig. 1H (a,b, lane 2) |
| P342 | −3.30 | In chemo, NED | 5 | BC | None | |
| P356* | −9.07 | In chemo, toleratIng treatment | 11 | BC | None | |
| P367* | −4.33 | In chemo, toleratIng treatment | 29 | BC | None | Fig. 1F (a,b, lane 2) |
| P370 | −2.63 | In chemo, NED | 13 | BC | None | Fig. 1D (a,b, lane 2) |
| P386 | −17.27 | In chemo, Not specified | 16 | BC | None | |
| P392 | −5.93 | post chemo, NED | 9 | BC | None | |
| P376 | −9.93 | post chemo, NED | 7 | BC | None | |
| P378 | −12.37 | In chemo, NED | 9 | BC | None | |
| P393* | −5.03 | In chemo, EOD | 26 | BC | None | Fig. 1J (a,b, lane 2) |
| P398* | −10.30 | In chemo, EOD | 24 | BC | None | Fig. 1I (a,b, lane 2) |
| P400 | −4.50 | In chemo, NED | 6 | BC | None | |
| P410 | −3.23 | post chemo, NED | 24 | Immunoreactivity value at T2 was 1.4 fold higher than at T1 | Asso-R | Fig. 1C (a,b, lane 2) |
| P413 | −15.53 | In chemo, NED | 8 | BC | None | Fig. 1G (a,b, lane 2) |
| Sample ID | RecurInterval (T2) | Disease status at T2 | CA125 value at T2 | 4H4
|
Western blot figure number | |
|---|---|---|---|---|---|---|
| Comparison of level of 4H4 immunoreactivity at T2 and T1 BC represents immunoreactivity value below cutoff at T2 |
Association of 4H4 immunoreactivity at T2 with recurrence status (Asso-R) | |||||
|
| ||||||
| P178 | −4.20 | In chemo, NED | 203 | Immunoreactivity value at T2 was 5.6 fold higher than at T1 | Asso-R | Fig. 1B (a,b, lane 3) |
| P265 | −4.87 | In chemo, NED | 54 | Immunoreactivity value at T2 was 1.7 fold higher than at T1 | Asso-R | Fig. 1A (a,b, lane 3) |
| P295 | −5.77 | post chemo, NED | 18 | BC | None | |
| P283 | −13.60 | In chemo, NED | 22 | BC | None | |
| P300 | −3.73 | post chemo, NED | 12 | BC | None | |
| P326* | −4.30 | In chemo, stable disease | 41 | BC | None | Fig. 1E (a,b, lane 3) |
| P336 | −10.00 | post chemo, NED | 20 | BC | None | |
| P341 | −7.10 | In chemo, NED | 37 | BC | None | Fig. 1H (a,b, lane 3) |
| P342 | −3.30 | In chemo, NED | 5 | BC | None | |
| P356* | −9.07 | In chemo, toleratIng treatment | 11 | BC | None | |
| P367* | −4.33 | In chemo, toleratIng treatment | 29 | BC | None | Fig. 1F (a,b, lane 3) |
| P370 | −2.63 | In chemo, NED | 13 | BC | None | Fig. 1D (a,b, lane 3) |
| P386 | −17.27 | In chemo, Not specified | 16 | BC | None | |
| P392 | −5.93 | post chemo, NED | 9 | BC | None | |
| P376 | −9.93 | post chemo, NED | 7 | BC | None | |
| P378 | −12.37 | In chemo, NED | 9 | BC | None | |
| P393* | −5.03 | In chemo, EOD | 26 | BC | None | Fig. 1J (a,b, lane 3) |
| P398* | −10.30 | In chemo, EOD | 24 | BC | None | Fig. 1I (a,b, lane 3) |
| P400 | −4.50 | In chemo, NED | 6 | BC | None | |
| P410 | −3.23 | post chemo, NED | 24 | Immunoreactivity value at T1 was almost same as at T2 | Asso-R | Fig. 1C (a,b, lane 3) |
| P413 | −15.53 | In chemo, NED | 8 | BC | None | Fig. 1G (a,b, lane 3) |
| Sample ID | RecurInterval (T2) | Disease status at T2 | CA125 value at T2 | 5H6
|
Western blot figure number | |
|---|---|---|---|---|---|---|
| Comparison of level of 5H6 immunoreactivity at T2 and T1 BC represents immunoreactivity value below cutoff at T2 |
Association of 5H6 immunoreactivity at T2 with recurrence status (Asso-R) | |||||
|
| ||||||
| P178 | −4.20 | In chemo, NED | 203 | BC | None | Fig. 1B (a,b, lane 4) |
| P265 | −4.87 | In chemo, NED | 54 | Immunoreactivity value at T2 was 1.5 fold higher than at T1 | Asso-R | Fig. 1A (a,b, lane 4) |
| P295 | −5.77 | post chemo, NED | 18 | BC | None | |
| P283 | −13.60 | In chemo, NED | 22 | BC | None | |
| P300 | −3.73 | post chemo, NED | 12 | BC | None | |
| P326* | −4.30 | In chemo, stable disease | 41 | BC | None | Fig. 1E (a,b, lane 4) |
| P336 | −10.00 | post chemo, NED | 20 | BC | None | |
| P341 | −7.10 | In chemo, NED | 37 | BC | None | Fig. 1H (a,b, lane 4) |
| P342 | −3.30 | In chemo, NED | 5 | Immunoreactivity value at T1 was almost same as at T2 | Asso-R | |
| P356* | −9.07 | In chemo, toleratIng treatment | 11 | BC | None | |
| P367* | −4.33 | In chemo, toleratIng treatment | 29 | BC | None | Fig. 1F (a,b, lane 4) |
| P370 | −2.63 | In chemo, NED | 13 | BC | None | Fig. 1D (a,b, lane 4) |
| P386 | −17.27 | In chemo, Not specified | 16 | BC | None | |
| P392 | −5.93 | post chemo, NED | 9 | BC | None | |
| P376 | −9.93 | post chemo, NED | 7 | BC | None | |
| P378 | −12.37 | In chemo, NED | 9 | BC | None | |
| P393* | −5.03 | In chemo, EOD | 26 | BC | None | Fig. 1J (a,b, lane 4) |
| P398* | −10.30 | In chemo, EOD | 24 | BC | None | Fig. 1I (a,b, lane 4) |
| P400 | −4.50 | In chemo, NED | 6 | BC | None | |
| P410 | −3.23 | post chemo, NED | 24 | Immunoreactivity value at T1 was almost same as at T2 | Asso-R | Fig. 1C (a,b, lane 4) |
| P413 | −15.53 | In chemo, NED | 8 | BC | None | Fig. 1G (a,b, lane 4) |
Note: Ovarian cancer patients with asterisk had evidence of disease months before the clinical recurrence ; NED: No evidence of disease; EOD: Evidence of disease. T1 represents time at ovarian cancer diagnosis; T2 represents time in months before the clinical recurrence
3.1.1. Association of immunoreactivity of antigens with recurrence status of ovarian cancer patients having stable disease during monitoring phase
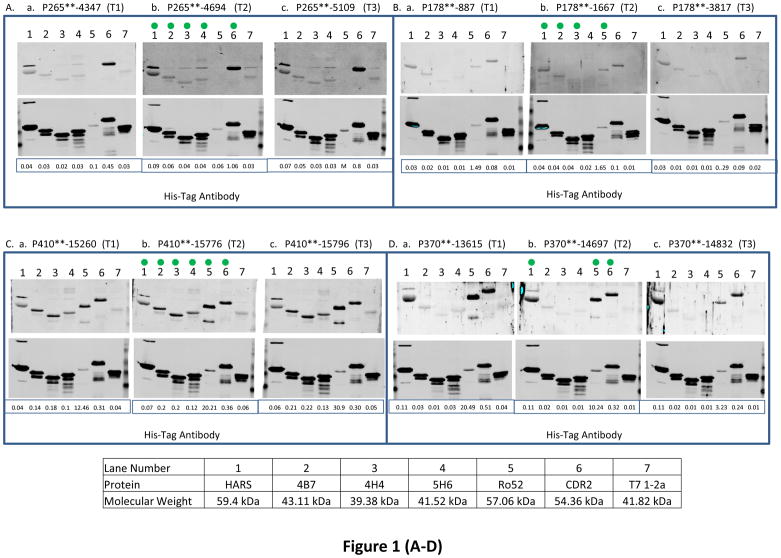
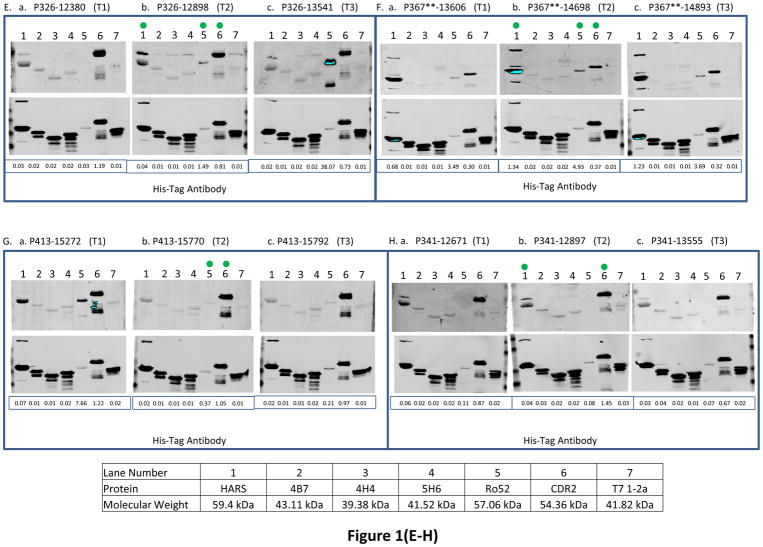
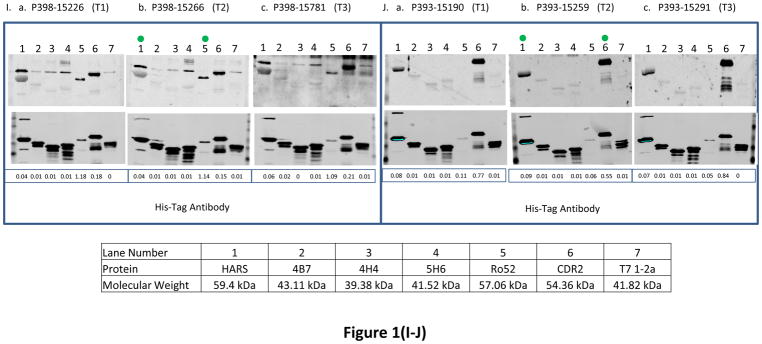
We observed that immunoreactivity of Ro52, CDR2 and HARS antigens was most strongly associated with the recurrence status of 3/5, 3/5 and 4/5 ovarian cancer patients respectively (patients with asterix shown in Table 3A) who had stable disease or under treatment at time T2 when their CA125 values were below or very close to the standard cutoff (35U/ml). In contrast, the immunoreactivity values of 4B7, 4H4 and 5H6 were below cutoff for those patients. The reactivity of Ro52 antigen with ovarian cancer patient P326 was increased by 51.5 fold (fold change is calculated by dividing the normalized signal intensity of the antigen reactivity with the patient’s serum IgG by the normalized signal intensity of the reactivity of His-tag at the N terminus of the antigen with anti-His-tag antibody) in contrast to immunoreactivity values of CDR2 or HARS that dropped by 1.5 fold or remained the same at 4.3 months when her CA125 value was only 41 U/ml before the clinical recurrence compared to the sero-reactivity at the time of diagnosis ((Fig. 1E, a, b, lanes 5, 6, and 1), Table 3A). For patient P367, the immunoreactivity of Ro52 and HARS was increased by 1.4 and 2 fold during the monitoring phases at 4.3 months (CA125 29U/ml) before the clinical recurrence compared to the their immunoreactivity at diagnosis ((Fig. 1F, a, b, lanes 5 and 1), Table 3A). The immunoreactivity of CDR2 antigen with P367 at times T1 and T2 remained almost the same, but the immunoreactivity value was 2.2 fold above the cutoff at T2. Although the patient P398 showed no increase in the serum reactivity with Ro52 protein at 10.3 months (CA125 level 24U/ml) before the clinical recurrence over the time at diagnosis, the immunoreactivity of Ro52 was significantly higher, 6.7 fold above the cutoff at T2 ((Fig. 1I, a, b, lane 5), (Table 3A)). However, the immunoreactivity of HARS and CDR2 antigens with the patient P398 showed weaker to reactivity below cutoff (Fig. 1I, a, b, lanes 1 and 6), (Table 3A). The high reactivity of Ro52 with the patient P398 could be associated with the presence of residual tumor tissues after her first sub-optimal debulking, indicating that a high anti-Ro52 titer is needed for the equilibrium state when tumor cells remain in a dormant state before they develop into a highly progressive phenotype [26]. Although the reactivity of HARS with the patient P393 remained almost the same at T1 and T2, the immunoreactivity of HARS was about 3 fold above its cutoff at T2 in contrast to Ro52 immunoreactivity that was below its cutoff at T2. The immunoreactivity of CDR2 antigen with the same patient P393 dropped by 1.4 fold at T2 (Fig. 1J, a, b, lanes 1, 5 and 6), (Table 3A). CDR2 expression is upregulated in ovarian tumors [10], so there is a possibility of sequestration by antigen blocking of newly synthesized Yo antibodies by circulating CDR2 protein. This can occur by the process of shedding, secretion of tumor antigens or antigens released due to apoptotic cell death as revealed by the proteomic analyses of 3 ovarian cancer cell lines by Faca et al. [11]. These shed antigens can enter into circulation and can bind to their respective antibodies.
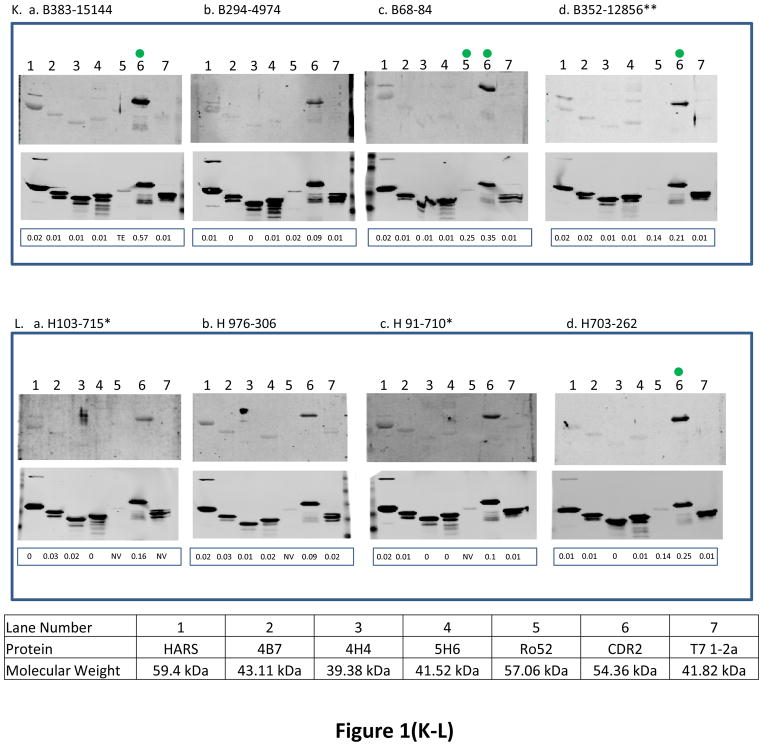
Fig. 1. The reactivity of antigens with serum samples obtained from ovarian cancer patients at 3 different time points and women with other benign diseases and healthy women.
BL21-DE3 bacterial cells bearing individual antigen clone were grown and cell lysate was prepared. One μg of cell lysate for all antigens except Ro52 for which 0.06 μg of protein was loaded and SDS-PAGE followed by transferring protein onto nitrocellulose membrane were performed. The membrane was immunoscreened with patient’s serum IgG (see Material and Methods) and protein band intensity for each protein was quantified using Odyssey software. For patients with double asterisks (**) in panels (A–J), the western blot images were scanned at 6.5 for 800 nm wavelength for better visual purposes but the normalized signal intensity for each antigen listed in those images were obtained from the image scanned at intensity 7.5 for 800 nm wavelength. “M” means missing value for panels (A–J). In each panel, a, b and c represent images of immunoreactivity of antigens with the serum samples obtained from one ovarian cancer patient at different time points (T1: the baseline blood sample collected at time of diagnosis; T2: the blood sample collected approximately 3–15 months before the clinical recurrence, ideally with normal CA125 and no evidence of disease; T3: the sample collected as close as possible to clinical recurrence). In panels (K–L), some samples have (*) beside their names and for those samples images were scanned at intensity 6.5 and quantified data were also obtained from the same images scanned at intensity 6.5 because of technical problems. “TE” means technical error and “NV” means negative value for panels (K–L). Panels (A–J) represent immunoreactivity of antigens with ovarian cancer patients, and panels (K–L) represent immunoreactivity of antigens with benign and healthy women. The green dot on the antigen that is shown on the western blot image at time T2 shows that the normalized signal intensity for that particular antigen is above its cutoff. Underneath each western blot images in a panel, the normalized signal intensity value of each protein band is shown.
3.1.2. Association of immunoreactivity of antigens with recurrence status of ovarian cancer patients having no evidence of disease during monitoring phase
The immunoreactivity of Ro52, CDR2, HARS, 4B7, 4H4, and 5H6 antigens showed association of recurrence with 8/16, 12/16, 10/16, 3/16, 3/16 and 3/16 ovarian cancer patients respectively who had no evidence of disease (NED), except one patient whose disease was not specified, at a median lead time of 5.85 months before the clinical recurrence at time T2 when most of the patients had CA125 levels below the standard cutoff (35 U/ml), with the exception of only one patient P178 who had a high CA125 value 203 U/ml at time T2 (Table 3(A–B)). For Ro52, HARS, 4B7 antigens, reactivity increased by 1.6, 1.6 and 1.4 fold with the patient P410, however, the immunoreactivity values of CDR2, 4H4 and 5H6 remained the same at T1 and T2 (individual immunoreactivity values of CDR2, 4H4 and 5H6 were 2, 6.6 and 4 fold higher than their cutoffs at time T2) during the monitoring phases at 3.23 months (CA125 24U/ml) before the clinical recurrence compared to their time at diagnosis (Fig. 1C, a, b, lanes 5, 1, 2, 6, 3, and 4). The immunoreactivity of CDR2 and Ro52 antigens with the patient P370 dropped by 1.6 and 2 fold at recurrence interval of 2.63 months (CA125 13U/ml). However, immunoreactivity of HARS remained the same at T1 and T2 (individual immunoreactivity was 3.6 fold higher that its cutoff at T2) ((Fig. 1D, a, b, lanes 6, 5 and 1), Table 3A). The drop in immunoreactivity of CDR2 and Ro52 (individual signal intensity values for both the antigens at time T2 were still 1.9 and 60 fold above cutoff) for P370 who had very short DFI 2.63 months could be related to the aggressive tumor growth that overpowered immune surveillance. Studies have indicated that tumor cells secrete immunosuppressive factors like IL-10, PEG2, TGFβ that suppress humoral immune effector cells [26]. Tumor cells inhibit the expression of major histocompatibility complex I and upregulate the expression of inhibitory ligands such as PD-L1 resulting in inhibition of T cell signaling pathways [19]. The patient P413 showed a decline in reactivity with HARS (immunoreactivity at T2 was below cutoff) and Ro52 revealed a 20.9 fold decrease in immunoreactivity (Ro52 immunoreactivity at T2 was 2.2 fold above the cutoff) at 15.5 months (CA125 8U/ml) compared to the reactivity values at times when the patients were diagnosed (Fig. 1G, a, b, lanes 1 and 5). Although immunoreactivity of CDR2 with P413 remained same at T1 and T2, the immunoreactivity was 6.2 fold higher than its cutoff at T2 ((Fig. 1G, lanes 1, 5, and 6), Table 3A). Patient P413 responded well to first-line chemotherapy as indicated by her CA125 value 8U/ml after undergoing optimal debulking that resulted in little to no microscopic residual tumor tissues during monitoring phase which can result in very low expression of Ro52 with concurrent reduction in the anti-Ro52 antibody titer. Titers of paraneoplastic antibodies have been shown to drop and even disappear with remission of the disease and concurrent reappearance of the antibodies takes place when the disease recurs [20]. Both patients P265 and P341 showed an increase in CDR2 immunoreactivity by 2.4 and 1.7 fold and HARS immunoreactivity was increased by 2.2 and decreased by 1.4 fold at recurrence intervals of 4.87 months (CA125 level 54U/ml) and 7.1 months (CA125 37 U/ml) before the radiologic evidence of recurrence compared to the values at their diagnosis times. In contrast, the immunoreactivity of Ro52 remained below cutoff for both the patients at time T2 ((Fig. 1A, 1H, a, b, lanes 6, 1 and 5), Table 3A). The patient P265 reacted with 4B7, 4H4 and 5H6 antigens with a fold increase in reactivity of 2, 1.7 and 1.5 at 4.87 months (CA125 level 54U/ml) before the clinical recurrence compared to the time at diagnosis ((Fig. 1A, a, b, lanes 2, 3 and 4), Table 3B). The patient P178 reacted with 4B7 and 4H4 antigens and the fold increase in reactivity was 2.2 and 5.6 at 4.2 months before the clinical confirmation of recurrence compared to diagnosis time, however, the immunoreactivity of CDR2 and 5H6 at time T2 remained below the cutoff (Fig. 1B, a, b lanes 2, 3, 6 and 4). The immunoreactivity of Ro52 and HARS with the patient P178 remained same at T1 and T2, but only the immunoreactivity of Ro52 was 9.7 fold higher than the cutoff at time T2 (Fig. 1B, a, b, lanes 5, and 1).
3.1.3. Serological screening of antigens using healthy women and women with benign gynecological diseases
The 6 recombinant biomarkers were also tested for their immunoreactivity with the serum IgGs obtained from few healthy women and women with benign gynecological disease (they all had ovarian cysts/Benign Cystic Ovarian Neoplasms) (Fig. 1(K–L)). As the analyses of immunoreactivity of antigens with all the benign and healthy women is generally performed to achieve a higher specificity for the early diagnosis of ovarian cancer and not for predicting recurrence in a cohort of patients who are under surveillance during monitoring phase, only few western blot images of sero-reactivity of 6 antigens with benign and healthy women were shown for the present study. Only CDR2 antigen exhibited strong reactivity with a patient with benign disease, B383 (3.4 fold above cutoff) and with other benign and healthy women, the reactivity was in the range of 1.2 to 2 fold above the CDR2 cutoff. The frequency of CDR2 antigen reactivity with healthy and benign samples was higher more often than the rest of the 5 antigens.
The above results indicated that out of 6 recombinant antigens employed to assess their sero-reactivity with serum IgGs obtained from 21 ovarian cancer patients, 3 antigens, Ro52, CDR2 and HARS showed high frequency and strong reactivity, and the remaining 3 antigens, 4B7, 4H4 and 5H6 showed low frequency and moderate reactivity during the monitoring phase when most of the patients had CA125 levels above the standard cutoff (35U/ml).
3.2. Determination of sensitivity of antigens based on their serological immunoreactivity with ovarian cancer patients for prediction of recurrence before the clinical relapse
The serologic reactivity of all the 6 recombinant antigens with serum IgGs obtained from 5 recurrent and 5 non-recurrent ovarian cancer patients (training set), and 21 recurrent ovarian cancer patients (test set) at time T2 before the clinical recurrence is shown in Table 4. The sensitivity of 6 antigens (single or in combination) to predict recurrence before the clinical recurrence in 21 ovarian cancer patients (test set) was determined.
3.2.1. Determination of sensitivity using one antigen at a time
Analyses of western blot immunoassays revealed that individually, Ro52, CDR2, HARS, 4B7, 4H4, and 5H6 antigens resulted in sensitivities of 52.4% (11/21), 71.4% (15/21), 66.7% (14/21), 14.3% (3/21), 14.3% (3/21) and 14.3% (3/21) respectively ((Tables (4–5)).
3.2.2. Determination of sensitivity using a combination of any 2 antigens at a time
High sensitivities were observed for a combination of any 2 antigens, for example, 86% (18/21) for Ro52 and CDR2 antigens, 81% (17/21) for CDR2 and HARS antigens, 81% (17/21) for Ro52 and HARS respectively. Among the other combinations of antigens in a panel of 2 that resulted in moderate sensitivities were 62% (13/21) for 5H6 and Ro52 antigens, 76% (16/21) for each of the combinations of 4B7 and CDR2, 4H4 and CDR2, and 5H6 and CDR2 antigens, and 71.4% (15/21) for 5H6 and HARS antigens respectively (Table 5).
Table 5.
Sensitivity of 6 antigens (in combinations of 1, 2 or 3 antigen panels) to predict recurrence prior to clinical recurrence in 21 ovarian cancer patients
| Antigen (Single or in combination of 2 or 3 antigen panels) | Sensitivity of antigen panels to predict recurrence in 21 ovarian cancer patients |
|---|---|
| Ro52 | 52.4% (11/21) |
| CDR2 | 71.4% (15/21) |
| HARS | 66.7% (14/21) |
| 4B7 | 14.3% (3/21) |
| 4H4 | 14.3% (3/21) |
| 5H6 | 14.3% (3/21) |
| Ro52+CDR2 | 86% (18/21) |
| CDR2+HARS | 81% (17/21) |
| Ro52+HARS | 81% (17/21) |
| Ro52+5H6 | 62% (13/21) |
| CDR2+4B7 or CDR2+4H4 or CDR2+5H6 | 76% (16/21) |
| HARS+5H6 | 71.4% (15/21) |
| Ro52+CDR2+5H6 * | 90.5% (19/21) |
| HARS+CDR2+5H6 or Ro52+CDR2+HARS | 86% (18/21) |
| CDR2+4B7+5H6 or CDR2+4H4+5H6 | 80.9% (17/21) |
| Ro52+4B7+5H6 or Ro52+4H4+5H6 | 61.9% (13/21) |
Note:
Combination of 3 antigens that resulted in highest sensitivity
3.2.3. Determination of sensitivity using a combination of any 3 antigens at a time
High sensitivities were observed for a combination of any 3 antigens, for example, 90.5% (19/21) for Ro52, CDR2 and 5H6 antigens (Fig. 2A), 86% (18/21) for HARS, CDR2 and 5H6 (Table 5), 86% (18/21) for Ro52, CDR2 and HARS (Fig. 2B), 80.9% (17/21) for CDR2, 4B7 and 5H6 or CDR2, 4H4 and 5H6 respectively (Table 5). Among the other panel of 3 antigens, moderate sensitivities were observed for 61.9% (13/21) for the combinations, Ro52, 4B7 and 5H6, or Ro52, 4H4 and 5H6 (Table 5).
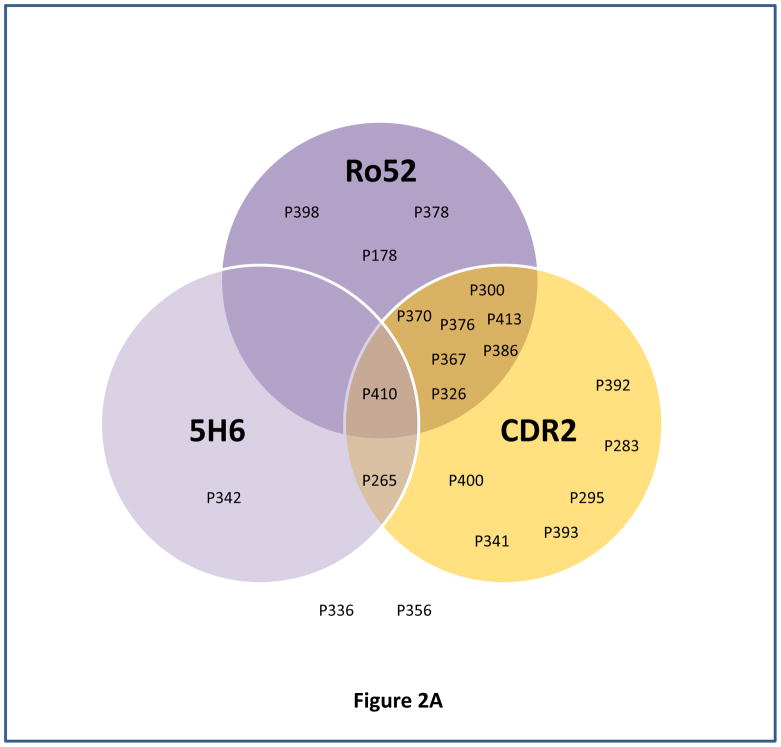
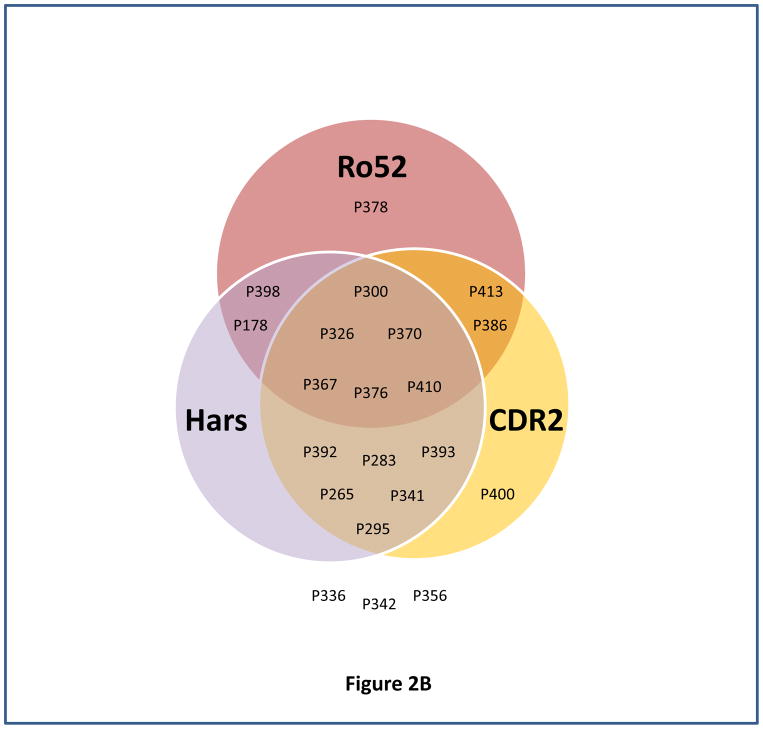
Fig. 2. Determination of sensitivity of different combination of antigens for predicting ovarian cancer recurrence using venn diagram.
Venn diagrams represented the immunoreactivity of each antigen (above its cutoff) with ovarian cancer patient’s serum sample obtained at time T2 (the blood sample collected approximately 3–15 months before the clinical recurrence, ideally with normal CA125 and no evidence of disease). Panels (A–B) represent venn diagram of different combination of antigens, Ro52, CDR2 and 5H6 (A), and Ro52, HARS, and CDR2 (B) used for determining sensitivity for predicting ovarian cancer recurrence.
Our results indicate that a panel of 3 antigens, Ro52, CDR2 and 5H6 resulted in 90.5% sensitivity in predicting recurrence in 21 ovarian cancer patients at a median lead time of 5.03 months before the clinical relapse when CA125 levels were within the normal range (<35U/ml). Although addition of HARS into that panel did not improve the sensitivity, it will be considered in the biomarker panel because first, it showed high frequency and strong reactivity with the ovarian cancer patients serum samples, second, it belongs to paraneoplastic antigen family and one of our previous antigen 4B7 showed peptide homology with HARS, and third, tumor microenvironment shows different levels of immunological suppression that is associated with varying levels of antibody response for different paraneoplastic antigens that in many cases complement each other. Out of 2 patients, P336, and P356, who appeared not to recur by these criteria, patient P336 had the third longest DFI of 15.1 months, there is a possibility of low antigen expression due to very low tumor burden that can result in low titers of antibodies within undetectable range.
4. Discussion
Studies have shown that early onset of some paraneoplastic neurological symptoms is generally associated with the occurrence of onconeural antibodies that can serve as a diagnostic tool for a suspicion of ovarian cancer in asymptomatic high-risk patients carrying BRCA1/2 mutations [6,29]. Very few studies have shown the utility of these onconeural antibodies for disease monitoring in cancer patients. One study reported that antibodies to paraneoplastic antigen Ma2 showed a high sensitivity, specificity and accuracy (AUC between 0.734 and 0.816) to predict early recurrence in 124 patients who had small intestine neuroendocrine tumors (SI-NETs) [9].
In our present study, we assessed the immunoreactivity of 6 recombinant antigens with serum IgGs obtained from 21 ovarian cancer patients to predict recurrence at various times prior to clinical/radiologic evidence when the level of CA125 was below the normal range (35 U/ml). Three paraneoplastic antigens, Ro52, CDR2 and HARS showed strong immunoreactivity association and the other 3 antigens, 4B7, 4H4 and 5H6 exhibited moderate immunoreactivity association with the recurrence status of the 21 ovarian cancer patients, majority of which showed no elevation of CA125 (standard cutoff 35U/ml) (Table (3A–3B)). Out of those 3 recombinant antigens, only 4B7 showed amino acid homology with the known paraneoplastic antigens, Histidyl t-RNA synthetase or HARS. Despite the homology to the C-terminal region with HARS protein, a similar frequency of reactivity was not observed between the recombinant 4B7 peptide and the full length HARS protein. A similar discordance was observed in most patients diagnosed with idiopathic myositis using epitope mapping of HARS protein which showed that 3 epitopes located at the N terminal region were mostly the reactive peptide segments [18]. As dermatomyositis is often associated with the occurrence of ovarian cancer, several processes that cause epitope spreading resulting in broadening of anti-HARS specificity can also occur during the course of development of ovarian cancer [18]. Although 4B7, 4H4 and 5H6 antigens encoded short peptides, exhibited low frequency and weak to moderate serological reactivity toward the ovarian cancer patients, these antigens still hold potential as biomarkers to monitor disease better than CA125 because of their high specificities against the 5 non-recurrent patients in the training set (Table 2). Those previous 3 biomarkers, 4B7, 4H4, and 5H6 were T7 phage encoded peptides and in our previous study their immunoreactivity was assessed by robotically printing the individual phage lysates on nitrocellulose membranes that were immunoscreened against patients’ sera. Therefore, the immunoreactivities of these antigens assessed by the current study could not be compared with that of our previous ovarian cancer recurrence study because in the current study we used purified recombinant proteins in western blot immunoassay in contrast to the previous study in which T7 phage lysates of the individual phage clones were employed for immunoscreening on protein microarrays.
The paraneoplastic antigen Ro52 is an E3 ubiquitin ligase and elevated levels of circulating anti-Ro52 antibodies have been shown to cause autoimmunity in patients with Sjögren’s syndrome and systemic lupus erythematosus (SLE) [3]. Mechanistically Ro52 causes inflammation by the process of ubiquitination of interferon regulatory factors [3]. The paraneoplastic antigen CDR2 has been shown to be expressed in Purkinje cells, testis and ovarian cancer [27]. There is an association of onconeural anti-Yo antibodies (targets CDR2 antigen) with ovarian cancer patients who developed paraneoplastic cerebellar degeneration before cancer diagnosis [21]. CDR2 is a cell cycle regulated protein that is highly expressed during mitosis in tumor cells. CDR2 interacts with c-Myc protein that can enhance gene transcription [23]. The occurrence of anti-Jo-1 antibodies targeting HARS antigen has been associated with myositis, a paraneoplastic neurological disorder that causes inflammation and weakness in muscles. Twenty-five percent of patients who are diagnosed with polymyositis or dermatomyositis harbor anti-Jo-1 antibodies [16]. Reports indicated that concurrent appearance of Jo-1 and Ro52 antibodies in patients diagnosed with antisynthetase syndrome (ASS) was associated with elevated risk of breast, ovarian, and esophagus cancers [17]. Our study employed serial ovarian cancer serum samples that were not used in our discovery of these biomarkers. However, a limitation of our present study is that the training set population which was used to set the threshold of each antigen to achieve high specificity was comprised of only 5 recurrent and 5 non-recurrent ovarian cancer patients. The reasons for using a smaller size of patient population in the training set for the determination of threshold of each antigen were first, in the present study, we wanted to reevaluate the strength of immunoreactivity of the previous biomarkers, 4B7, 4H4, 5H6 [5] on the western blot platform to predict ovarian cancer recurrence prior to its clinical recurrence in patients who showed no elevation in CA125 level above its clinical threshold (35 U/ml) which led us to use the same training set as before; second, for our previous study, we were able to accrue few non-recurrent ovarian cancer patients (because the disease often recurs) who met patients’ accrual criteria and those non-recurrent ovarian cancer patients were split into training and test sets which made the size of the training set smaller than expected [5]; third, in our earlier immunoassay based studies, the same patient population of 5 recurrent and 5 non-recurrent ovarian cancer patients (current training set) as well as serum samples obtained from patients with paraneoplastic syndrome were immunoscreened with known paraneoplastic antigens to determine the immunoreactivity of those paraneoplastic antigens using paraneoplastic myositis line blots (EuroImmun, Morris Plains, New Jersey) and Paraneoplastic Antigen line blots (Ravo Diagnostika, Freiburg, Germany). Similar immunoreactivity of HARS, Ro52 and CDR2 antigens with both recurrent and non-recurrent ovarian cancer patients in the training set was observed both on western blot (current study) and paraneoplastic antigen line blots (previous study) (data not shown). The immunoscreening analyses provided insights into usefulness of using true paraneoplastic protein antigens for early diagnosis and recurrence of ovarian cancer (data not shown). Therefore, our present was enhanced by using the same ovarian cancer patient population in the training set to choose the threshold of each antigen that was applied to an independent test set patient population for the evaluation of their potential as biomarkers for prediction of ovarian cancer recurrence with a longer lead time than CA125. We propose to use a large independent population of recurrent and non-recurrent ovarian cancer patients in the training set for future validation studies. In addition, the test set study population did not have non-recurrent ovarian cancer patients. As our present study was a prospective-retrospective pilot study based on finding the utility of 6 biomarkers for prediction of ovarian cancer recurrence in patients prior to clinical recurrence, we needed an independent patient population in the test set who had CA125 levels below its threshold (35 U/ml) during the monitoring phase before clinical recurrence. The present study only focused on determining the sensitivity of the immunoassay used for prediction of ovarian cancer recurrence. If we had included non-recurrent ovarian cancer patients, we could have measured specificity along with the sensitivity of the immunoassay. Therefore, in the study we included this as one of the limitations of the study. We propose to include both recurrent and non-recurrent ovarian cancer patients for the future validation studies.
5. Conclusion
In conclusion, a combination of 4 antigens, Ro52, CDR2, HARS and 5H6 in a panel, showed a sensitivity of 90.5 % in a western blot-based immunoassay for early prediction of recurrence in 21 ovarian cancer patients during the surveillance period when most of these patients had normal levels of CA125 level (cutoff 35U/ml). The median lead time of prediction of recurrence was 5.03 months which was better than CA125. We propose that paraneoplastic autoantibodies occur in asymptomatic cancer patients and can be used for early detection of cancer. Our goal for a future study is to evaluate the potential utility of these 6 markers in combination with other tumor associated antigens that have been shown to be overexpressed in late stage serous adenocarcinoma with concurrent elicitation of humoral immune response in ovarian cancer patients, to develop a panel of biomarkers that can predict the recurrence during the monitoring phase prior to clinical recurrence when the level of CA125 remains below the standard cutoff (35U/ml). Early prediction of recurrence before the cancer progresses to more aggressive phenotype can provide patients some time to be treated with conventional chemotherapy regimen to prevent recurrence of ovarian cancer.
Supplementary Material
Table 2.
Determination of threshold of an antigen that discriminated recurrent ovarian cancer patients (serum was drawn close to 1 year before clinical recurrence) from non-recurrent ovarian cancer patients (serum was drawn close to 1 year after diagnosis) in the training set
| Antigen biomarker | Threshold of antigen | Sensitivity (percent reactivity of an antigen with recurrent ovarian cancer patients) | Specificity (percent non-reactivity of an antigen with non-recurrent ovarian cancer patients |
|---|---|---|---|
| Ro52 | 0.17 | 60% (3/5) | 75% (3/4)* |
| CDR2 | 0.17 | 80% (4/5) | 80% (4/5) |
| HARS | 0.03 | 40% (2/5) | 40% (2/5) |
| 4B7 | 0.03 | 20% (1/5) | 80% (4/5) |
| 4H4 | 0.03 | • | • |
| 5H6 | 0.03 | 20% (1/5) | 80% (4/5) |
| T7 1-2a (negative control) Δ | 0.03 | 20% (1/5) | 100% (5/5) |
Note:
Calculation of threshold for each antigen:
Normalized signal intensity value was calculated by dividing the background corrected signal intensity obtained with patient’s serum by background corrected signal intensity obtained from His-tag antibody
Median of normalized signal intensity value of T7 1-2a (negative control) with 5 recurrent and 5 non-recurrent ovarian cancer patients in the training set was 0.010
Standard Deviation (STDEV) of the normalized signal intensity value of T7 1-2a (negative control) with 5 recurrent and 5 non-recurrent ovarian cancer patients in the training set was 0.0173
Please note that the threshold values were adjusted to 2 places of decimal in excel worksheet (data not shown)
Threshold for HARS, 4B7, 4H4, 5H6:
(Median T7 1-2a + 1.3*STDEV)= (0.010 +1.3× 0.0173) = 0.03 (after adjusted to 2 places of decimal)
Threshold for Ro52, CDR2:
(Median T7 1-2a + 9*STDEV)= (0.010 +9× 0.0173) = 0.17 (after adjusted to 2 places of decimal)
Did not have data for 1 non-recurrent ovarian cancer patient for Ro52 protein
• Using the threshold 0.03, 4H4 did not get selected but it was still used for the test set because training set had small sample size and strict rules could not be applied.
Acknowledgments
This project was supported by The Barbara and Fred Erb Chair in Cancer Genetics and an NIH grant R21 CA187278-0. Laura Hurley was supported by T32 training grant CA009531. The authors would like to thank Mike Saleh for volunteering his time in the laboratory. A special thanks to the patients and healthy volunteers who donated serum and tissue for the study. This work could not be done without their willingness to participate in research.
References
- 1.Albert ML, Darnell JC, Bender A, Francisco LM, Bhardwaj N, Darnell RB. Tumor-specific killer cells in paraneoplastic cerebellar degeneration. Nat Med. 1998;4:1321–1324. doi: 10.1038/3315. [DOI] [PubMed] [Google Scholar]
- 2.Antoine JC, Absi L, Honnorat J, Boulesteix JM, de BT, Vial C, Butler M, De CP, Michel D. Antiamphiphysin antibodies are associated with various paraneoplastic neurological syndromes and tumors. Arch Neurol. 1999;56:172–177. doi: 10.1001/archneur.56.2.172. [DOI] [PubMed] [Google Scholar]
- 3.Aqrawi LA, Kvarnstrom M, Brokstad KA, Jonsson R, Skarstein K, Wahren-Herlenius M. Ductal epithelial expression of Ro52 correlates with inflammation in salivary glands of patients with primary Sjogren’s syndrome. Clin Exp Immunol. 2014;177:244–252. doi: 10.1111/cei.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atack DB, Nisker JA, Allen HH, Tustanoff ER, Levin L. CA 125 surveillance and second-look laparotomy in ovarian carcinoma. Am J Obstet Gynecol. 1986;154:287–289. doi: 10.1016/0002-9378(86)90657-5. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee M, Dyson G, Levin NK, Shah JP, Morris R, Munkarah A, Tainsky MA. Tumor autoantibodies as biomarkers for predicting ovarian cancer recurrence. Cancer Biomark. 2012;11:59–73. doi: 10.3233/CBM-2012-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatterjee M, Hurley LC, Tainsky MA. Paraneoplastic antigens as biomarkers for early diagnosis of ovarian cancer. Gynecol Oncol Rep. 2017;21:37–44. doi: 10.1016/j.gore.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatterjee M, Mohapatra S, Ionan A, Bawa G, Ali-Fehmi R, Wang X, Nowak J, Ye B, Nahhas FA, Lu K, Witkin SS, Fishman D, Munkarah A, Morris R, Levin NK, Shirley NN, Tromp G, Abrams J, Draghici S, Tainsky MA. Diagnostic markers of ovarian cancer by high-throughput antigen cloning and detection on arrays. Cancer Res. 2006;66:1181–1190. doi: 10.1158/0008-5472.CAN-04-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatterjee M, Tainsky MA. Autoantibodies as biomarkers for ovarian cancer. Cancer Biomark. 2010;8:187–201. doi: 10.3233/CBM-2011-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui T, Hurtig M, Elgue G, Li SC, Veronesi G, Essaghir A, Demoulin JB, Pelosi G, Alimohammadi M, Oberg K, Giandomenico V. Paraneoplastic antigen Ma2 autoantibodies as specific blood biomarkers for detection of early recurrence of small intestine neuroendocrine tumors. PLoS One. 2010;5:e16010. doi: 10.1371/journal.pone.0016010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darnell JC, Albert ML, Darnell RB. Cdr2, a target antigen of naturally occuring human tumor immunity, is widely expressed in gynecological tumors. Cancer Res. 2000;60:2136–2139. [PubMed] [Google Scholar]
- 11.Faca VM, Ventura AP, Fitzgibbon MP, Pereira-Faca SR, Pitteri SJ, Green AE, Ireton RC, Zhang Q, Wang H, O’Briant KC, Drescher CW, Schummer M, McIntosh MW, Knudsen BS, Hanash SM. Proteomic analysis of ovarian cancer cells reveals dynamic processes of protein secretion and shedding of extra-cellular domains. PLoS One. 2008;3:e2425. doi: 10.1371/journal.pone.0002425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghaemmaghami F, Karimi ZM, Hamedi B. High levels of CA125 (over 1,000 IU/ml) in patients with gynecologic disease and no malignant conditions: three cases and literature review. Arch Gynecol Obstet. 2007;276:559–561. doi: 10.1007/s00404-007-0381-x. [DOI] [PubMed] [Google Scholar]
- 13.Guarneri V, Barbieri E, Dieci MV, Piacentini F, Conte P. Timing for starting second-line therapy in recurrent ovarian cancer. Expert Rev Anticancer Ther. 2011;11:49–55. doi: 10.1586/era.10.204. [DOI] [PubMed] [Google Scholar]
- 14.Guo N, Peng Z. Does serum CA125 have clinical value for follow-up monitoring of postoperative patients with epithelial ovarian cancer? Results of a 12-year study. J Ovarian Res. 2017;10:14. doi: 10.1186/s13048-017-0310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoftberger R, Sabater L, Velasco F, Ciordia R, Dalmau J, Graus F. Carbonic anhydrase-related protein VIII antibodies and paraneoplastic cerebellar degeneration. Neuropathol Appl Neurobiol. 2014;40:650–653. doi: 10.1111/nan.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howard OM, Dong HF, Yang D, Raben N, Nagaraju K, Rosen A, Casciola-Rosen L, Hartlein M, Kron M, Yang D, Yiadom K, Dwivedi S, Plotz PH, Oppenheim JJ. Histidyl-tRNA synthetase and asparaginyl-tRNA synthetase, autoantigens in myositis, activate chemokine receptors on T lymphocytes and immature dendritic cells. J Exp Med. 2002;196:781–791. doi: 10.1084/jem.20020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marie I, Hatron PY, Dominique S, Cherin P, Mouthon L, Menard JF, Levesque H, Jouen F. Short-term and long-term outcome of anti-Jo1-positive patients with anti-Ro52 antibody. Semin Arthritis Rheum. 2012;41:890–899. doi: 10.1016/j.semarthrit.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Martin A, Shulman MJ, Tsui FW. Epitope studies indicate that histidyl-tRNA synthetase is a stimulating antigen in idiopathic myositis. FASEB J. 1995;9:1226–1233. doi: 10.1096/fasebj.9.12.7672516. [DOI] [PubMed] [Google Scholar]
- 19.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller FW, Twitty SA, Biswas T, Plotz PH. Origin and regulation of a disease-specific autoantibody response. Antigenic epitopes, spectrotype stability, and isotype restriction of anti-Jo-1 autoantibodies. J Clin Invest. 1990;85:468–475. doi: 10.1172/JCI114461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monstad SE, Storstein A, Dorum A, Knudsen A, Lonning PE, Salvesen HB, Aarseth JH, Vedeler CA. Yo antibodies in ovarian and breast cancer patients detected by a sensitive immunoprecipitation technique. Clin Exp Immunol. 2006;144:53–58. doi: 10.1111/j.1365-2249.2006.03031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris RT, Monk BJ. Ovarian cancer: relevant therapy, not timing, is paramount. Lancet. 2010;376:1120–1122. doi: 10.1016/S0140-6736(10)61515-2. [DOI] [PubMed] [Google Scholar]
- 23.O’Donovan KJ, Diedler J, Couture GC, Fak JJ, Darnell RB. The onconeural antigen cdr2 is a novel APC/C target that acts in mitosis to regulate c-myc target genes in mammalian tumor cells. PLoS One. 2010;5:e10045. doi: 10.1371/journal.pone.0010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pelosof LC, Gerber DE. Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clin Proc. 2010;85:838–854. doi: 10.4065/mcp.2010.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rustin GJ, van der Burg ME, Griffin CL, Guthrie D, Lamont A, Jayson GC, Kristensen G, Mediola C, Coens C, Qian W, Parmar MK, Swart AM. Early versus delayed treatment of relapsed ovarian cancer (MRC OV05/EORTC 55955): a randomised trial. Lancet. 2010;376:1155–1163. doi: 10.1016/S0140-6736(10)61268-8. [DOI] [PubMed] [Google Scholar]
- 26.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 27.Totland C, Aarskog NK, Eichler TW, Haugen M, Nostbakken JK, Monstad SE, Salvesen HB, Mork S, Haukanes BI, Vedeler CA. CDR2 antigen and Yo antibodies. Cancer Immunol Immunother. 2011;60:283–289. doi: 10.1007/s00262-010-0943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang ZJ, Zhao BB, Li L. The significance of the change pattern of serum CA125 level for judging prognosis and diagnosing recurrences of epithelial ovarian cancer. J Ovarian Res. 2016;9:57. doi: 10.1186/s13048-016-0266-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Zhu Q, Han SX, Zhou CY, Cai MJ, Dai LP, Zhang JY. Autoimmune response to PARP and BRCA1/BRCA2 in cancer. Oncotarget. 2015;6:11575–11584. doi: 10.18632/oncotarget.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.