Abstract
Inhibitors are antibodies directed against haemophilia treatment products which interfere with their function. Factor VIII (FVIII) inhibitors in haemophilia A and factor IX (FIX) inhibitors in haemophilia B are significant clinically when they require a change in a patient’s treatment regimen. Their persistence may increase morbidity and mortality. Multiple laboratory tests are now available for detecting and understanding inhibitors in haemophilia. Inhibitors are traditionally measured by their interference in clotting or chromogenic factor assays. They may also be detected using immunologic assays, such as enzyme-linked immunosorbent assay or fluorescence immunoassay. Anti-FVIII or anti-FIX antibodies of IgG4 subclass best correlate with the presence of functional inhibitors. Improvements in inhibitor measurement have been recently introduced. Preanalytical heat treatment of patient specimens allows testing of patients without delaying treatment. Use of chromogenic and immunologic assays may aid in identification of false-positive results, which are frequent among low-titre inhibitors. Validated reagent substitutions can be used to reduce assay cost. New methods for defining assay positivity and reporting low-titre inhibitors have been suggested. Challenges remain in the areas of quality control, assay standardization, monitoring of patients undergoing immune tolerance induction therapy and testing in the presence of modified and novel treatment products.
Keywords: factor VIII, factor IX, haemophilia, inhibitor
1 | INTRODUCTION
People with haemophilia A (HA) or haemophilia B (HB) may develop inhibitors, which are antibodies that interfere with the function of the factor VIII (FVIII) or factor IX (FIX) products with which they are treated. Such antibodies occur as a natural process when the immune system does not recognize the normal clotting factor used for treatment, because the individual produces no clotting factor or produces only a structurally abnormal protein.1,2 Inhibitors act by combining with the factor and either blocking its action in clotting or removing it from the circulation. Inhibitors, by definition, interfere with or “neutralize” clotting factor activity. Anti-FVIII or anti-FIX antibodies that do not interfere with clotting factor function may also be present; these have been called “non-neutralizing” or “non-inhibitory” antibodies.3-5 Thus, antidrug antibodies occurring in haemophilia may or may not interfere with clotting function, and not all are correctly termed “inhibitors.” FVIII or FIX inhibitors may also occur in individuals without haemophilia as part of an autoimmune process.6
Inhibitors in haemophilia are of clinical significance when they require alteration of the patient’s treatment regimen. The most significant inhibitors show an anamnestic response to factor infusion and require the use of by-passing agents to achieve haemostasis and/or immune tolerance induction (ITI) therapy to eliminate the inhibitor.7,8 Some FIX inhibitors may produce a dangerous anaphylactic response.9,10 Non-anamnestic inhibitors may persist at a low level, allowing treatment with larger doses of factor products. Other inhibitors, termed “transient,” disappear spontaneously within 6 months.11 The long-term presence of an inhibitor increases both morbidity12 and mortality13 in haemophilia patients. At first detection, it may not be possible to determine which kind of inhibitor is present, and close monitoring is necessary to determine prognosis. Because early detection of inhibitors can lead to more successful treatment of those requiring ITI,14 annual inhibitor tests have been recommended for all patients with more frequent testing during early exposures to factor, when risk of developing an inhibitor is greatest.15
Innovation in clinical tests for inhibitors has been slow, with advances occurring only at 20-year intervals. This review will describe the evolution of inhibitor testing, its pitfalls, and the testing issues raised by new treatment products in haemophilia.
2 | FACTOR VIII AND FACTOR IX CHARACTERISTICS
FVIII is a large protein of 2332 amino acids that circulates in complex with von Willebrand factor (VWF). It has a structure consisting of 6 domains, designated A1-A2-B-A3-C1-C2, with an activation peptide between the B and A3 domains.2,16 FVIII is released from VWF and the B-domain is removed upon its activation to FVIIIa, the cofactor that facilitates the junction of activated factor IX (FIXa) and factor X on the phospholipid membrane to produce activated factor X (FXa).16 Conventional treatment products for HA have included recombinant full-length FVIII, recombinant FVIII with the B-domain deleted, plasma-derived purified FVIII and plasma-derived FVIII with VWF. Products have recently come to market that have been modified to increase half-life by PEGylation or by fusing FVIII to the Fc portion of immunoglobulins or to albumin.17 A novel FVIII-mimetic drug, Hemlibra® (emicizumab), which has recently been approved for use in FVIII inhibitor patients in the USA, is a bivalent antibody that substitutes for FVIIIa in the production of FXa.18 It may be used to treat patients with inhibitors, because it does not contain FVIII. Other by-passing agents used to treat patients with inhibitors contain activated clotting factors that by-pass FVIII in the clotting cascade; these include recombinant factor VIIa and activated prothrombin complex concentrates (FEIBA).19
FIX is a vitamin K-dependent protease with a mature protein length of 415 amino acids, organized into a gamma-glutamic acid (GLA) domain, two epidermal growth factor-like (EGF) domains, an activation peptide and a serine protease or catalytic domain. It is activated to FIXa when cleaved by activated factor XI or tissue factor-factor VIIa.20 Its decreased immunogenicity compared to FVIII may be influenced by its smaller size and its homology to factors II, VII and X. Conventional treatment products for HB include recombinant full-length FIX and plasma-derived FIX. Modified longer half-life products include Fc-fusion and albumin-fusion FIX.17
3 | INHIBITOR CHARACTERISTICS
Inhibitors in haemophilia are usually polyclonal2,21 and predominately of IgG class, although other immunoglobulin classes are also seen.22-24 FVIII inhibitors usually show both IgG1 and IgG4 subclasses,5,21,25 although IgG1 antibodies are also found in a significant number of patients without functional inhibitors (Table 1A).26 FIX inhibitors are also predominately of IgG4 subclass, with other subclasses seen less frequently (Table 1B).27-29 Thus, in both haemophilia A and B, functional inhibitors are most strongly correlated with the presence of antibodies of IgG4 subclass. The reaction of FVIII inhibitors is time- and temperature-dependent; they react slowly to inactivate FVIII and are most effective at 37°C.30,31 Time dependence is used to distinguish FVIII inhibitors from lupus anticoagulants (LA) and other non-specific inhibitors of coagulation, which usually do not show increased inhibition over time.32 FIX inhibitors are not time-dependent.33 It has been hypothesized that this unique property of FVIII inhibitors is due to steric hindrance caused by the large size of the FVIII/VWF complex. FVIII and FIX inhibitors do not fix complement, consistent with other IgG4 antibodies, and do not form precipitating complexes in gels34,35 Both FVIII and FIX inhibitors have been shown to form circulating immune complexes with their antigens in haemophilia patients, sometimes masking the presence of the inhibitor in vitro.34,36-38 Immune complexes containing FVIII and FVIII inhibitors may be separated by heating or by acidification.34,39 Heating also appears to separate FIX from FIX inhibitors, although perhaps less effectively.40,41 Clearance of circulating immune complexes, rather than blocking of FVIII or IX activity, may be the mechanism of action of some inhibitors. Such clearance may remove antibodies and prevent the in vitro detection of inhibition under conditions of antigen excess.
TABLE 1. Anti-factor VIII (A) and anti-factor IX (B) immunoglobulins (Ig) by fluorescence immunoassay among haemophilia patients negative or positive by modified Nijmegen-Bethesda assay (NBA) compared to healthy donors (% positive). Adapted from 26, 29.
| (A) Haemophilia A | n | IgG1 | IgG2 | IgG3 | IgG4 | IgM |
|---|---|---|---|---|---|---|
| Healthy Donors | 56 | 5.4 | 5.4 | 1.8 | 1.8 | 7.1 |
| NBA-negative | 369 | 23.3* | 8.9 | 3.0 | 6.0 | 3.3 |
| NBA-positive | 122 | 92.6** | 42.6** | 15.6** | 88.5** | 5.9 |
| (B) Haemophilia B | n | IgG1 | IgG2 | IgG3 | IgG4 | IgA | IgE |
|---|---|---|---|---|---|---|---|
| Healthy Donors | 50 | 4 | 4 | 2 | 2 | 6 | 4 |
| NBA-negative | 25 | 12 | 20* | 0 | 12 | 12 | 12 |
| NBA-positive | 12 | 83** | 83** | 58** | 100** | 67** | 75** |
(A) P = .001
P < .0001.
(B) P = .0375
P < .0001.
FVIII inhibitors are directed at limited epitopes of the large FVIII molecule, most often against the A2, C1 and C2 domains.2,42,43 Individual FVIII inhibitors show different affinities for FVIII44 and different kinetics of interaction with it.45-48 They are classified as having Type 1 kinetics, if they can be completely neutralized by added FVIII, or Type 2 kinetics, if residual FVIII activity remains in the presence of large amounts of inhibitor. Type 1 and Type 2 inhibitors react differently in inhibitor tests, as described below. Affinity differences in FVIII antibodies have been demonstrated primarily in immunologic assays, and it is unclear how they may affect functional inhibitor assays and whether the affinity and kinetic differences observed in vitro reflect differences in inhibitor action in vivo.
FIX inhibitors are most often directed against the GLA or protease domains.49 Their kinetics have been only minimally studied.
4 | INHIBITOR MEASUREMENT METHODS
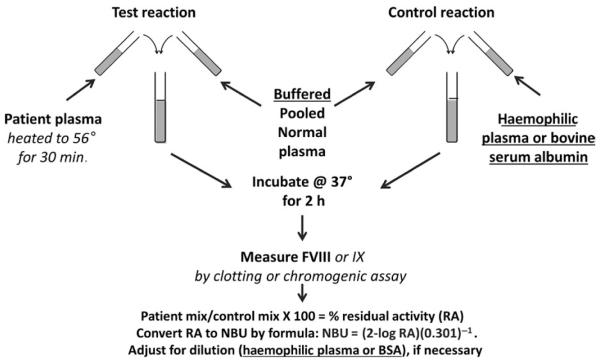
Functional assays for inhibitors are based on the principle of comparison of the activity remaining in a test mixture containing patient plasma and a source of FVIII or FIX with the activity remaining in a control mixture of a diluent and the factor source, when both mixtures are prepared and incubated in parallel (Figure 1). For FVIII inhibitors, a defined time of incubation is used; for FIX inhibitors, incubation is not required. The FVIII or FIX source is usually normal pooled plasma (NPP). Treatment products have sometimes been used; however, recombinant FVIII products lack the VWF present in vivo and do not produce results equivalent to plasma.50 Porcine FVIII has been substituted as the FVIII source to detect inhibitors that cross-react with that product.51 The amount of factor activity remaining in the patient mixture divided by the activity of the control mixture, multiplied by 100, gives the % residual activity (RA), which is converted to arbitrary units of inhibitor. Published inhibitor methods have differed in their definitions of an inhibitor unit, as well as in the time of incubation, reagent composition and method of endpoint determination. Three methods of endpoint determination have been used for FVIII inhibitor tests: one-stage clotting, two-stage clotting and two-stage chromogenic. For FIX inhibitors, one-stage clotting is most commonly used. Strong inhibitors must be diluted prior to testing, introducing the additional variables of diluent and dilution scheme.
FIGURE 1.
Schematic of the CDC Nijmegen-Bethesda assay based on the 1975 Bethesda assay52 with Nijmegen modifications53 underlined and CDC-validated modifications40 in italics
4.1 | Clot-based functional assays
FVIII inhibitor measurement was first described in 1959 by Biggs and Bidwell using the two-stage clotting assay for FVIII, with an incubation time of one hour and one inhibitor unit defined as the amount destroying 75% of the FVIII activity.30 In 1975, a group of investigators meeting in Bethesda, Maryland,52 standardized the inhibitor test used in the United States, defining one Bethesda unit (BU) as the amount of inhibitor in 1 millilitre (mL) of patient plasma that would destroy 50% of the FVIII activity of an equal amount of NPP in 2 hours at 37°C (Figure 1). This was quantitated using a 1-stage clotting assay to measure FVIII activity in a mixture of patient plasma plus NPP and a control mixture of imidazole buffer plus NPP. A graph of log % RA vs dilution was provided for BU calculation. This “Bethesda assay” (BA) was gradually adopted internationally, and methods using the two-stage FVIII assay became less popular. This method was largely unchanged until 1995 when Verbruggen and colleagues53 suggested 2 modifications: (i) buffering of the NPP with imidazole, and (ii) use of factor VIII-deficient plasma (FVIIIDP) rather than buffer in the control mixture and for dilution of higher titre inhibitor plasmas. These changes, underlined in Figure 1, were designed to stabilize the protein concentration and the pH of the mixtures during incubation. Following the observation that it reduced low-titre positive results compared to the BA in 877 specimens, this method, called the Nijmegen modification or the Nijmegen-Bethesda assay (NBA), was endorsed by the International Society on Thrombosis and Haemostasis (ISTH) and became the “gold standard” for inhibitor testing.54 In 2012, CDC investigators validated a previously proposed modification to the NBA using preanalytical heat treatment (PHT) of patient plasma to remove factor, which allows accurate testing of patients recently treated or on prophylaxis or ITI with conventional FVIII or FIX products.40 This CDC-modified NBA (CDC-NBA), performed as shown in Figure 1, has been used to test for both FVIII and IX inhibitors.
4.2 | Chromogenic functional assays
Inhibitor tests using factor assays that have the endpoint of clot formation have certain limitations. The formation of a fibrin clot relies on many variables and may be inhibited by unfractionated heparin (UFH) from central lines or ports,55 LA56 and non-specific inhibitors of coagulation common in children.57 It is often difficult to distinguish this inhibition of clotting from a true factor inhibitor. Because tests for FVIII using chromogenic substrates have a more specific endpoint, cleavage of factor Xa, their use in inhibitor testing was proposed by Blanco et al.58 Chromogenic factor assays also have the advantage of increased precision.59 A chromogenic Bethesda assay (CBA), identical to the CDC-NBA except for use of a chromogenic factor assay to measure the FVIII endpoint, has been described.60 When 1005 specimens were tested with both the CDC-NBA and the CBA, 0.3% of 883 NBA-negative specimens, 54% of 80 positive specimens with 0.5–1.9 Nijmegen-Bethesda units (NBU) and 100% of 42 specimens with ≥2.0 NBU were also positive with the CBA. A fluorescence immunoassay (FLI) recognizing IgG and IgM anti-FVIII antibodies was positive in 50 (98%) of 51 CBA-positive specimens but in only 84 (82%) of 103 NBA-positive specimens (P = .004), suggesting that the CBA is more indicative of the presence of specific anti-FVIII antibodies. The CDC-NBA, CBA and FLI generally agreed on negative specimens and specimens with ≥2.0 NBU; however, among specimens with 0.5–1.9 NBU, 26% did not react in the CBA or demonstrate anti-FVIII antibodies, suggesting that the inhibition observed was not FVIII-specific and that the results were false-positive tests. Some had evidence of non-time-dependent inhibition. The correlation between CDC-NBA and CBA results for specimens with ≥2.0 NBU was excellent (r = .98, P < .0001). Use of FIX chromogenic assays for inhibitor testing has been limited. No commercial reagents for chromogenic FIX measurement have been approved for clinical use by the US Food and Drug Administration (FDA). A FIX CBA has been used to determine that a haemophilia B patient had a LA rather than a specific FIX inhibitor.61 Although often perceived as being more expensive than clotting tests, chromogenic tests used in inhibitor assays may decrease the number of false-positive results and thus the need for additional testing, as well as patient follow-up. Modification of chromogenic test kits through changes in packaging or validation of the ability to freeze reagents can produce assay costs that are equal to or less than the cost of clotting reagents.62
4.3 | Antibody detection assays
Methods developed to detect antibodies to FVIII and FIX include the enzyme-linked immunosorbent assay (ELISA),63–65 immunoprecipitation assay (IP),66 fluorescence immunoassay (FLI)60,67,68 and surface plasmon resonance assay.69 Because they measure both inhibitory and non-inhibitory antibodies, they are not equivalent to and cannot be substituted for functional inhibitor assays. They may be used to screen specimens for those requiring inhibitor assays65 or to confirm the presence of specific antibodies.60,70,71 They are much more sensitive than functional assays, with the FLI capable of detecting FVIII inhibitors diluted to 0.03 NBU.60 An ELISA measuring IgG antibody binding to FVIII immobilized on a plastic surface is commercially available.65 The FLI measures antibody binding to FVIII or FIX immobilized on fluorescent beads.26,29,67,68 Its multiplex capability allows the immunoglobulin subclass profile of the anti-FVIII or anti-FIX to be assessed in one test. As noted above, the presence of a functional inhibitor of FVIII correlates with anti-FVIII antibodies of IgG4 subclass.5,21,64,72 IgG1 antibodies are present in both inhibitor-negative and inhibitor-positive patients; preliminary evidence suggests that they appear earlier and can be predictive of subsequent inhibitor development in HA,26 as well as remaining present when high-titre inhibitors decline.72 The commercial anti-FVIII ELISA has also detected antibodies in some non-haemophilic patients with LA,73 although the IgG subclass was not reported. FIX inhibitors have similarly been linked to the presence of anti-FIX antibodies of IgG4 subclass with ELISA28,49 and FLI.29
5 | RECENT ASSAY MODIFICATIONS
5.1 | Preanalytical heat treatment
Patients undergoing prophylactic or ITI therapy or being treated for a bleeding episode may have FVIII or FIX circulating continuously. Failure to account for this factor in performance of the BA or NBA may produce a false-negative test, unless a relatively high-titre inhibitor is present, by causing RA to be 100% or higher leading to an NBU of 0. This is sometimes accounted for by adding half of the patient’s factor level to the activity of the control mixture before calculating the RA.74 It has not been documented, however, that this modification allows detection of low-titre inhibitors, which may be complexed with the infused factor.34,36 Allain and Frommel39 showed that heating of patient plasmas to 56° for 30 minutes separated antigen-antibody complexes and denatured FVIII. Other investigators have used the same procedure, which is a standard antibody preparation step in immunology, to study postinfusion specimens.75–77 Verbruggen et al76 and others70 have proposed heating plasma to 58° for 90 minutes. This more vigorous process can be demonstrated to significantly reduce antibody levels, as shown in Table 2.78 It also adds significantly to the time for performance of inhibitor tests. Heating to 56° for 30 minutes, followed by centrifugation, has been shown to reduce both FVIII activity and FVIII antigen to <1 unit/decilitre and to result in changes in NBA results.40 Among 202 specimens with negative results before heating, 3% showed a change to positive after heating, including 1% of those from patients with no history of inhibitor and 17% of those with previous positive inhibitors. The value of PHT of specimens in revealing low-titre FVIII inhibitors is greater in patients undergoing ITI79 and those with acquired haemophilia.70 The latter study showed a 10-fold increase in detection of acquired inhibitors after heating; however, using the more vigorous heating method, it also documented some loss of antibodies measured by ELISA. Millner et al41 have documented that FIX inhibitors cannot be accurately measured in the presence of residual FIX. They also showed that heat inactivation or a heat/cold modification improved inhibitor detection with a glycoPEGylated recombinant FIX product, N9-GP. While PHT is successful at eliminating conventional infused FVIII and FIX products, validation of this step with each novel product introduced is required. The bivalent antibody treatment product, Hemlibra® (emicizumab), is not destroyed by heating, but inhibitors can be measured in the presence of the product by CBA.80
TABLE 2. Per cent (%) of baseline anti-factor VIII IgG4 remaining after preanalytical heat treatment of plasma from haemophilia patients with given Nijmegen-Bethesda units (NBU), measured by fluorescence immunoassay in median fluorescence intensity (MFI) 78.
| Baseline IgG4 (MFI) |
% Remaining at 56°C After |
% Remaining at 58°C After |
||||
|---|---|---|---|---|---|---|
| Specimens | NBU | 30′ | 60′ | 30′ | 60′ | |
| Patient A | 1.5 | 169 | 104 | 78 | 76 | 47 |
| Patient B | 1.6 | 1919 | 117 | 102 | 83 | 61 |
| Patient C | 19.3 | 12 596 | 91 | 92 | 100 | 75 |
| Patient D | 1.6 | 16 563 | 91 | 96 | 95 | ND |
| Patient E | 1.1 | 303 | 120 | 147 | 93 | 93 |
| Patient F | 1.1 | 1208 | 115 | 100 | 76 | 65 |
| Patient G | 0.5 | 17 194 | 80 | 93 | 72 | 39 |
| Patient H | 5.4 | 22 156 | 92 | 94 | 88 | 79 |
| Mean | 101 | 100 | 85* | 66* | ||
| Range | 80–120 | 78–147 | 72–93 | 39–93 | ||
Significantly different from original level in paired t test at P < .05; ND, no data.
5.2 | Identification of false-positive results
The finding that more than 25% of specimens with inhibitor titres of 0.5–1.9 NBU lack anti-FVIII antibodies has led to the suggestion that inhibitor results in that range be confirmed with more specific tests.60,71 Testing with the CBA confirms that the inhibition of clotting observed is specific to FVIII and not the result of a LA, UFH contamination or a non-specific inhibitor.58,59 The absence of specific anti-FVIII or anti-FIX antibodies in immunologic tests makes a true inhibitor unlikely. Antibodies are present, however, in some patients who lack a functional inhibitor. Additional steps useful in characterizing very low-titre inhibitors are repeating the inhibitor test to detect laboratory error and testing for LA. Haemophilia patients may have concurrent FVIII inhibitors and LA.58,60 The protocol for the U.S. national inhibitor surveillance programme81 treats positive inhibitor results below 2.0 NBU for FVIII and below 1.0 NBU for FIX as “grey zone” results, requiring repeating the test and performance of CBA for FVIII and antibody tests for FVIII and FIX for confirmation before reporting.
5.3 | Reagent substitutions
Adoption of the NBA has met barriers in some clinical laboratories, due to the increased cost of FVIIIDP over buffer as diluent and the lack of commercial availability of appropriate reagents. Some reagent substitutions have been evaluated. A small study found that substitution of immunodepleted FVIIIDP from two manufacturers for naturally deficient FVIIIDP in the control mixture produced significant differences in inhibitor titres; the authors suggested that this might be due to either lack of VWF or the presence of preparatory antibodies in the FVIIIDP.82 Chemically depleted FVIIIDP was also found to be problematic. The key ISTH study of the NBA, however, used immunodepleted FVIIIDP, also recommending that chemically depleted FVIIIDP not be used due to the possible presence of FVIII fragments.54 A multi-laboratory study demonstrated differences, primarily when the immunodepleted FVIIIDP was used to dilute specimens.83 Because non-plasma diluents have been successfully substituted for FVIIIDP, it seems unlikely that lack of VWF is the key variable. The presence of contaminating antibodies in some FVIIIDP preparations seems more likely. A conclusive study comparing multiple sources of FVIIIDP has not been conducted. This reagent, however, is a potential source of inter-laboratory variability and may not be necessary if non-plasma reagents can be substituted.
Substitution of 4% bovine serum albumin (BSA) for FVIIIDP in the NBA as a cheaper and more uniform option was originally reported to be satisfactory in a small number of specimens.84 This was confirmed by Kershaw et al85 in a larger study. Use of imidazole buffer alone, as in the original BA, has also been supported.83 The efficacy of different diluents in the CDC-NBA was evaluated in 326 specimens, using 4% BSA, imidazole-buffered BSA or imidazole buffer alone. All could be successfully substituted for FVIIIDP in the control mixture, with 4% BSA providing sensitivity of 97% and specificity of 99% compared to FVIIIDP.86 Non-plasma diluents provided greater stability of FVIII activity during incubation than did FVIIIDP (Figure 2).86 A similar stability comparison showed that HEPES-buffered and unbuffered NPP were inferior to imidazole-buffered NPP at maintaining both FVIII and pH stability.86 Comparison of the many commercial buffered NPP reagents has not been reported. Any reagent substitution used in the NBA or the BA must be validated; it cannot be assumed that reagents suitable for other purposes can withstand the 2 hours at 37°C required for these assays. Also, substitutions may alter assay performance: use of BSA required that the threshold for positivity of the CDC-NBA be raised from ≥0.5 to ≥0.6 NBU to achieve the same results, as discussed below.86
FIGURE 2.
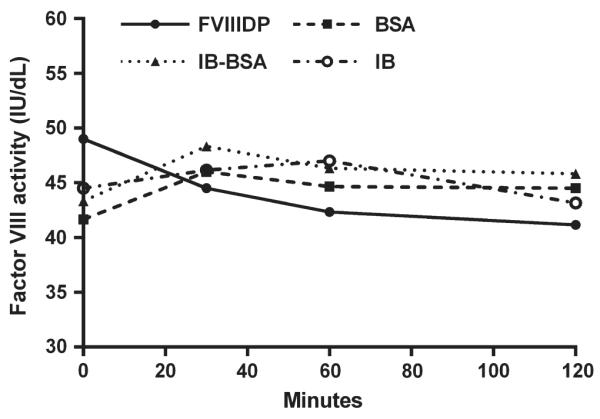
Stability of factor VIII activity in imidazole-buffered normal pooled plasma in a 1:1 mix with factor VIII-deficient plasma (FVIIIDP), 4% bovine serum albumin (BSA), imidazole-buffered BSA (IB-BSA) or imidazole buffer (IB) during 120-minute incubation at 37°C. Mean of duplicate determinations on triplicate specimens. IU/dL = International Units per decilitre86
5.4 | Definition of assay positivity
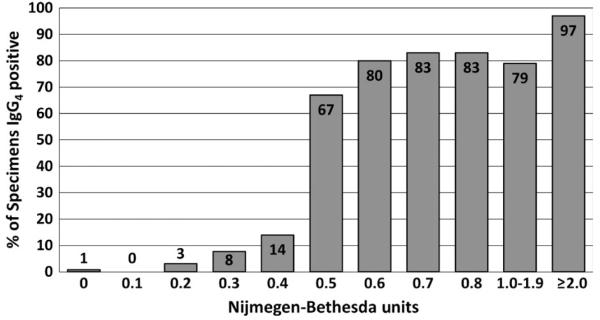
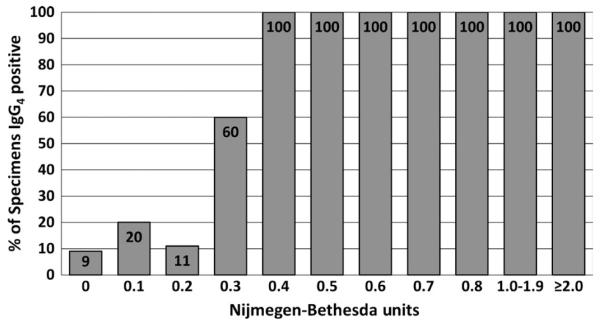
The Scientific and Standardization Committee of the ISTH has recommended that a FVIII inhibitor test by NBA be considered positive if the results are ≥0.6 NBU.11 The definitive paper validating the NBA, however, used ≥0.5 NBU.54 Analysis suggests that the threshold for positivity for these, as for other clinical tests, is method-specific. Using the CDC-NBA, results on over 600 specimens from patients with negative or positive history of an inhibitor suggested that a threshold of ≥0.5 NBU was appropriate for that method.40 Comparison of results of the CDC-NBA with the presence of anti-FVIII IgG4 antibodies showed that the presence of such antibodies increased from 14% at 0.4 NBU to 67% at 0.5 NBU, confirming that threshold (Figure 3).87 For FIX inhibitor tests using the CDC-NBA, a threshold of ≥0.3 NBU was established using more than 200 specimens.40 Comparison of the results with the presence of IgG4 anti-FIX antibodies (Figure 4) confirmed the validity of that threshold, with 60% of specimens at 0.3 NBU and 100% of specimens ≥0.4 NBU having IgG4 anti-FIX antibodies. Tests for specific antibodies are useful in setting thresholds, and their use in confirming antibody presence in the assays’ “grey zones” can reduce false-positive results. In both HA and HB, antibodies are present in some patients with NBU below these limits. They may represent developing inhibitors which have not yet reached the detection threshold of the NBA, antibodies which do not inhibit well in vitro but may have an in vivo effect, or low-affinity antibodies with no clinical significance.
FIGURE 3.
Per cent (%) of haemophilia A specimens positive for anti-factor VIII IgG4 antibodies at various levels of inhibitor measured in Nijmegen-Bethesda units by the CDC Nijmegen-Bethesda assay 87
FIGURE 4.
Per cent (%) of haemophilia B specimens positive for anti-factor IX IgG4 antibodies at various levels of inhibitor measured in Nijmegen-Bethesda units by the CDC Nijmegen-Bethesda assay
5.5 | Inhibitor calculations
Most automated analysers cannot be programmed to calculate %RA and NBU; however, simple statistical programs in database software or on calculators can be used for that purpose to assure that results are calculated correctly and avoid errors in graph reading. The formulas used are as follows: %RA = (factor level of patient + NPP mix/factor level of diluent + NPP mix) × 100, and NBU = (2-log %RA)/0.301.52 If testing of the undiluted patient plasma gives 25%-100% RA, then the original result is used. If it produces a result of <25% RA (>2.0 NBU), dilution of the patient plasma is required to achieve a %RA between 25% and 75% for accurate quantitation. NBU is then multiplied by the dilution factor to calculate a final NBU for reporting (Table 3). When dilutions are carried out, the dilution curve of %RA should be examined. Even very low-titre inhibitors show a dilution effect, while negative inhibitors show similar %RA at all dilutions (Figure 5C). Inhibitors with Type 1 kinetics are expected to show a negative slope within that range, and multiplication of the calculated NBU by the dilution factors produces approximately equivalent results for each dilution (Table 3). Multiplication by a large dilution factor, however, magnifies small errors, and even acceptable variation in the performance of the factor assays can result in differences between dilutions and between different assays when multiplied by 10 or higher. Inhibitors with Type 2 kinetics show similar %RA at multiple dilutions, which can result in progressively higher inhibitor results with multiplication by dilution factors (Table 3). It is usually recommended to report the dilution having RA closest to 50%; however, some laboratories prefer to use the first dilution falling below 75% RA to avoid error introduced by use of larger multiplication factors. For monitoring change in titre over time, it may be most useful to follow the same dilution in each subsequent assay to detect rise or fall in the NBU.
TABLE 3. Examples of calculation of Nijmegen-Bethesda units (NBU) in assays of factor VIII (FVIII) inhibitors with Type I and Type II kinetics.
| Predilution | Patient Mix U/dL FVIIIa | Control Mix U/dL FVIIIa | % Residual Activityb | Calculated NBUc | Total NBUd |
|---|---|---|---|---|---|
| Type 1 inhibitor | |||||
| Undiluted | 19 | 45 | 42.2 | 1.24 | 1.2 |
| 1:2 | 30 | 45 | 66.7 | 0.58 | 1.2 |
| 1:4 | 35 | 45 | 77.8 | 0.36 | 1.4 |
| Type 2 Inhibitor | |||||
| Undiluted | 27 | 45 | 60.0 | 0.74 | 0.7 |
| 1:2 | 27 | 45 | 60.0 | 0.74 | 1.5 |
| 1:4 | 27 | 45 | 60.0 | 0.74 | 3.0 |
Units per decilitre of FVIII activity.
Patient mix units per decilitre FVIII activity/control mix units per decilitre FVIII activity × 100.
NBU read from graph or calculated as NBU = (2-log %residual activity)(0.301)−1.
Calculated NBU × predilution factor.
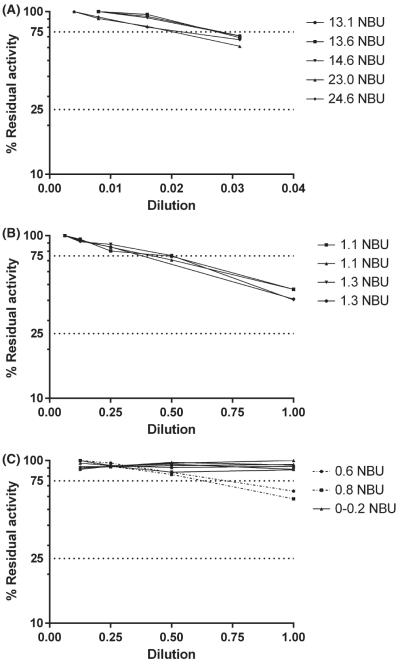
FIGURE 5.
Linearity of dilution curves of specimens with various Nijmegen-Bethesda units (NBU) 87
The original publication on the BA recommended that positive specimens be read from the curve with RA between 25% and 75% and stated that results with 75%-100% RA might require “more sensitive methods” for the detection of low-titre inhibitors.52This has been interpreted to mean that inhibitors in that range cannot be accurately quantitated.88 Using today’s assays (Figure 5), it can be demonstrated that when known FVIII inhibitors are diluted, the curves are linear and have a negative slope between 75 and 100% RA, suggesting that inhibitor titres in this range (<0.4 NBU) can be quantitated.87 These curves, however, do not cross the Y-axis at 0 NBU, indicating that the titre cannot be accurately detected below a limit, which for the CDC-NBA has been calculated to be 0.2 NBU. This was consistent with the finding that inhibitor titres in healthy individuals have a mean + 3 standard deviations of 0.17, indicating a limit of detection (LOD) of 0.2 NBU.87 It is most accurate to report quantitative results from assays only for inhibitors equal to or greater than the LOD and to report lower results as less than the LOD. Inhibitors in the range between the LOD and the threshold for positivity, however, may be reported and in some cases, appear to be clinically significant.87
6 | QUALITY CONTROL
Most published methods do not address the issue of quality control for inhibitor assays. Inter-laboratory variation has been documented to be large by proficiency testing and external quality assessment programs worldwide. Coefficients of variation (CVs) as high as 50% and false-positive rates up to 32% have been seen on distributed specimens.88,89 It is likely that differences in methods and reagents contribute to this variability among well-qualified laboratories.88 Among North American laboratories surveyed by the North American Specialized Coagulation Laboratory Association, 20% reported using the Nijmegen method, 10% the Bethesda assay and 70% a combination of components best described as a hybrid assay.88,90 Many reported using multiple vendors for reagents such as NPP. Careful internal quality control, as used for other clinical tests, must be applied to inhibitor tests as well and has been shown to produce acceptable intra-laboratory reproducibility.40
Analytical variables playing a role in inter-laboratory variability include differences in key reagents, such as NPP, FVIIIDP and diluent; dilutions used for strong inhibitors91; incubation method; and controls. There have been only a few studies validating individual commercial reagents for suitability in inhibitor assays, discussed above. There has been no evidence that use of different instruments or reagents for clotting factor measurement as the endpoint determination for inhibitor assays influences results with conventional products. Comparison of 3 APTT reagents with a modified FIX product found no effect on the inhibitor results, when a heating step was used.41 If products are adequately removed by PHT, then only the added NPP will be measured in the test, and reagent differences should not affect the results. Factor measurement for inhibitor assays should be performed using a calibrator related to an international standard for FVIII and FIX, to produce consistency of results over time and allow use of published reference ranges.
Attempts to establish an international standard for inhibitor assays have been unsuccessful. To provide two levels of controls, use of a known inhibitor plasma diluted in FVIIIDP to a level of 1 NBU as a positive control and monitoring of the FVIII activity of the control mixture as the negative control has been suggested.40 Using the CDC-NBA, the CV of the negative control was found to be 9.8% (n = 117) and CV of the positive control to be 10.3% (n = 114). Commercial control reagents are also available, usually prepared from monoclonal antibodies. Each assay should be evaluated to determine that both controls are within an acceptable range before reporting results. Careful adherence to a standard method and use of controls to assess assay validity can minimize intra-laboratory variability. The problem of inter-laboratory variability can only be addressed by the adoption of evidence-based uniform methods and validated reagents across laboratories.
7 | PREANALYTICAL ISSUES
Preanalytical variables important for inhibitor assays include presence in the specimen of UFH or other contaminating substances, LA, non-specific inhibitors of coagulation and infused or endogenous factor.92 Appropriate clinical information must be collected to assess for these variables, including whether the specimen was collected from a port or central line flushed with UFH and any treatment product that might be present in the specimen. If PHT is used, it is not considered necessary to ask the patient to refrain from treatment to “wash out” the factor prior to testing with conventional treatment products.40,79 Information on the effects of by-passing agents and longer-acting products on inhibitor assays is incomplete and is discussed below. If UFH is present, the specimen may be treated to remove it prior to testing, or a CBA can be used.55 Presence of other anticoagulant drugs could also influence test results.
8 | MONITORING OF IMMUNE TOLERANCE INDUCTION (ITI) THERAPY
Because the NBA is a relatively insensitive test for inhibitors, detecting levels only to 0.2-0.3 NBU,86,87 modifications have been proposed to increase assay sensitivity for monitoring for inhibitor eradication during ITI therapy. Dardikh et al93 developed a method using concentrated patient plasma, a ratio of patient plasma to NPP of 3:1 and a chromogenic endpoint and reported a LOD of 0.03 NBU, similar to that of the FLI.60 It allowed identification of the presence of inhibitors after successful ITI which reportedly reduced half-life and recovery, although the number of patients studied was small (n = 7). Immunologic methods may be more sensitive and specific for following ITI. Anti-FVIII IgG and/or IgM antibodies measured by FLI were absent in 15 of 15 patients with successful ITI, 0 of 5 patients with failed ITI and 7 of 18 patients with ongoing ITI.26 Other investigators found IgG4 anti-FVIII antibodies to disappear after successful ITI in 23 patients, although other antibody subclasses remained.94
9 | MODIFIED AND NOVEL TREATMENT PRODUCTS
Most methods for inhibitor measurement have been designed and validated for conventional treatment products: recombinant and plasma-derived FVIII and FIX with little structural modification. Recently, altered recombinant products have been introduced with longer half-lives to allow fewer infusions. In addition, novel treatment modalities not containing FVIII or FIX have entered practice. Information on inhibitor testing in patients treated with modified products is limited. Because of their long half-lives and patient-specific pharmacokinetics,17,95 the process of “wash out” of factor prior to inhibitor testing becomes even more challenging with these products.
While measurement of postinfusion factors levels with some products is influenced by the APTT reagent used, that is not expected to be the case for inhibitor assays using PHT, as removal of treatment products with PHT leaves only the added NPP to be measured, not the product or patient’s factor. That appears to be the best course of action for laboratories in dealing with patients on multiple products; however, validation for each product that the PHT method used adequately removes both functional and immunologic reactivity is required. Few such studies have been reported, some using spiked specimens which may not be equivalent to patient plasmas. To date, the most significant problem noted has been with the FVIII-mimetic Hemlibra® (emicizumab). That drug, which is an antibody and cannot be removed by PHT, interferes with inhibitor measurement in clot-based assays; however, because it does not react with bovine factors, a bovine chromogenic assay can be used.80 An example of results on a patient receiving Hemlibra® during clinical trials is shown in Table 4 with both assay types.
TABLE 4. Example of factor VIII (FVIII) inhibitor measurement on a haemophilia A patient specimen containing Hemlibra® (emicizumab, ACE910) using the Nijmegen-Bethesda assay and the chromogenic Bethesda assay.
| Predilution | Patient Mix U/dL FVIIIa |
Control Mix U/dL FVIIIa |
% Residual Activityb |
Calculated Unitsc | Total Unitsd | |
|---|---|---|---|---|---|---|
| Nijmegen-Bethesda Assay |
Undiluted | 116 | 37 | 313.5 | 0 NBU | IS |
| Chromogenic Bethesda Assay |
Undiluted | 23 | 37 | 62.2 | 0.69 CBU | 0.7 CBU |
IS, interfering substance.
Units per decilitre of FVIII activity.
Patient mix units per decilitre FVIII activity/control mix units per decilitre FVIII activity × 100.
Nijmegen-Bethesda units (NBU) or chromogenic Bethesda units (CBU) calculated as NBU or CBU = (2-log %residual activity) (0.301)−1.
Calculated NBU × predilution factor.
10 | INTERPRETATION OF INHIBITOR TEST RESULTS
Laboratory demonstration of inhibition or presence of antibodies does not always indicate a clinically significant condition. Inhibitor diagnosis is based on laboratory findings, response to therapy, and pharmacokinetic studies. The ISTH and UK guidelines state that <66% recovery of factor 10–15 minutes after infusion indicates a clinically significant inhibitor.11,15 Because low-titre results in clot-based assays may represent false-positive tests or a transient inhibitor, both follow-up testing and clinical observation are required to determine the patient’s prognosis. False-positive inhibitors have influenced the results of clinical trials.71 Conversely, pharmacokinetic studies are also recommended for those with poor response to therapy and negative inhibitor titres.15 It is not clear that all antibodies that can combine with factor and remove it from the circulation will be detected in vitro as inhibitors.
11 | CONCLUSIONS
Multiple laboratory tests are now available for detecting and understanding inhibitors in haemophilia. More accurate chromogenic tests have advantages over traditional clot-based tests. Measurement of specific anti-FVIII or anti-FIX antibodies may be the logical first step in screening for inhibitors, with only antibody-positive specimens receiving functional testing. This could decrease the number of inhibitor tests required, and thus their burden, for clinical laboratories, as well as providing more rapid results. Addition of immunologic tests to the clinical repertoire could bring about a paradigm change in inhibitor care by providing a better picture of the immune response.
ACKNOWLEDGMENTS
The author wishes to thank her collaborators in the Laboratory Team of the Division of Blood Disorders, including S. Jean Platt, Anne S. Rice, Dorothy Ellingsen, Jennifer Driggers, Amanda B. Payne, Brian Boylan, and Christopher J. Bean.
Footnotes
DISCLOSURES
The author states that she has no interests which might be perceived as posing a conflict or bias. The findings and conclusions in this report are those of the author and do not necessarily represent the views of the Centers for Disease Control and Prevention.
REFERENCES
- 1. Hoyer LW Why do so many haemophilia A patients develop an inhibitor? Br J Haematol 1995. 90 498–501 [DOI] [PubMed] [Google Scholar]
- 2. Lollar P Pathogenic antibodies to coagulation factors. Part one: Factor VIII and Factor IX J Thromb Haemost 2004. 2 1082–1095 [DOI] [PubMed] [Google Scholar]
- 3. Gilles JGG Arnout J Vermylen J Saint-Remy JMR Anti-factor VIII antibodies of hemophiliac patients are frequently directed towards nonfunctional determinants and do not exhibit isotypic restriction Blood 1993. 82 2452–2461 [PubMed] [Google Scholar]
- 4. Lebreton A Lapalud P Chambost H et al. Prevalence and epitope specificity of non-neutralising antibodies in a large cohort of haemophilia A patients without inhibitors Thromb Haemost 2011. 105 954–961 [DOI] [PubMed] [Google Scholar]
- 5. Whelan SFJ Hofbauer CJ Horling FM et al. Distinct characteristics of antibody responses against factor VIII in healthy individuals and in different cohorts of hemophilia A patients Blood 2013. 121 1039–1048 [DOI] [PubMed] [Google Scholar]
- 6. Franchini M Mannucci PM Acquired haemophilia A: A 2013 update Thromb Haemost 2013. 110 1114–1120 [DOI] [PubMed] [Google Scholar]
- 7. Makris M Hay CRM Gringeri A D’Oiron R How I treat inhibitors in haemophilia Haemophilia 2012. 18 Suppl. 4 48–53 [DOI] [PubMed] [Google Scholar]
- 8. Kempton CL Meeks SL Toward optimal therapy for inhibitors in hemophilia Blood 2014. 124 3365–3372 [DOI] [PubMed] [Google Scholar]
- 9. Warrier I Ewenstein BM Koerper MA et al. Factor IX inhibitors and anaphylaxis in haemophilia B Haemophilia 1996. 2 259–260 [DOI] [PubMed] [Google Scholar]
- 10. Chitlur M Warrier I Rajpurkar M Lusher JM Inhibitors in factor IX deficiency a report of the ISTH-SSC international FIX inhibitor registry (1997-2006) Haemophilia 2009. 15 1027–1031 [DOI] [PubMed] [Google Scholar]
- 11. Blanchette VS Key NS Ljung LR et al. Definitions in hemophilia: communication from the SSC of the ISTH J Thromb Haemost 2014. 12 1935–1939 [DOI] [PubMed] [Google Scholar]
- 12. Monahan PE Baker JR Riske B Soucie JM Physical functioning in boys with hemophilia in the U.S. Am J Prev Med 2011. 41 S360–S368 [DOI] [PubMed] [Google Scholar]
- 13. Walsh CE Soucie JM Miller CH the United States Hemophilia Treatment Centers Network Impact of inhibitors on hemophilia A mortality in the United States Am J Hematol 2015. 90 400–405 [DOI] [PubMed] [Google Scholar]
- 14. DiMichele DM Kroner BL The North American immune tolerance registry: practices, outcomes, outcome predictors Thromb Haemost 2002. 87 52–57 [PubMed] [Google Scholar]
- 15. Collins PW Chalmers E Hart DP et al. Diagnosis and treatment of factor VIII and IX inhibitors in congenital haemophilia: Br J Haematol 2013. 160 153–170 4th [DOI] [PubMed] [Google Scholar]
- 16. Pipe SW Functional roles of the factor VIII B domain Haemophilia 2009. 15 1187–1196 [DOI] [PubMed] [Google Scholar]
- 17. Powell JS Longer-acting clotting factor concentrates for hemophilia J Thromb Haemost 2015. 13 Suppl 1 S167–S175 [DOI] [PubMed] [Google Scholar]
- 18. Shima M Hanabusa H Taki M et al. Factor VIII-mimetic function of humanized bispecific antibody in hemophilia A N Engl J Med 2016. 374 2044–2053 [DOI] [PubMed] [Google Scholar]
- 19. Franchini M Coppola A Tagliaferri A Lippi G FEIBA versus NovoSeven in hemophilia patients with inhibitors Semin Thromb Haemost 2013. 39 772–778 [DOI] [PubMed] [Google Scholar]
- 20. Thompson AR Structure, function, and molecular defects of factor IX Blood 1986. 67 565–572 [PubMed] [Google Scholar]
- 21. Hultin MB London FS Shapiro SS Yount WJ Heterogeneity of factor VIII antibodies: Further immunochemical and biologic studies Blood 1977. 49 807–817 [PubMed] [Google Scholar]
- 22. Feinstein DI Rapaport SI Chong MNY Immunologic characterization of 12 factor VIII inhibitors Blood 1969. 34 85–90 [PubMed] [Google Scholar]
- 23. Carroll RR Panush RS Kitchens CS Spontaneous disappearance of an IgA anti-factor IX inhibitor in a child with Christmas disease Am J Hematol 1984. 17 321–325 [DOI] [PubMed] [Google Scholar]
- 24. Carmona E Aznar JA Jorquera JI Villanueva MJ Sánchez-Cuenca JM Detection of two different anti-Factor VIII/von Willebrand factor antibodies of the IgA class in a hemophilic patient with a polyclonal Factor VIII inhibitor of the IgG class Thromb Res 1991. 63 73–84 [DOI] [PubMed] [Google Scholar]
- 25. Fulcher CA de Graaf Mahoney S Zimmerman TS FVIII inhibitor IgG subclass and FVIII polypeptide specificity determined by immunoblotting Blood 1987. 69 1475–1480 [PubMed] [Google Scholar]
- 26. Boylan B Rice AS Dunn AL et al. Characterization of the anti-factor VIII immunoglobulin profile in patients with hemophilia A by use of a fluorescence-based immunoassay J Thromb Haemost 2015. 13 47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ørstavik KH Miller CH IgG subclass identification of inhibitors to factor IX in haemophilia B patients Br J Haematol 1988. 68 451–454 [DOI] [PubMed] [Google Scholar]
- 28. Sawamoto Y Shima M Yamamoto M et al. Measurement of anti-factor IX IgG subclasses in haemophilia B patients who developed inhibitors with episodes of allergic reactions to factor IX concentrates Thromb Res 1996. 83 279–286 [DOI] [PubMed] [Google Scholar]
- 29. Boylan B Rice AS Neff AT et al. Survey of the anti-factor IX immunoglobulin profiles in patients with hemophilia B using a fluorescence-based immunoassay J Thromb Haemost 2016. 14 1931–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Biggs R Bidwell E A method for the study of antihaemophilic globulin inhibitors with reference to six cases Br J Haematol 1959. 5 379–395 [DOI] [PubMed] [Google Scholar]
- 31. Breckenridge RT Ratnoff OD Studies on the nature of the circulating anticoagulant directed against antihemophilic factor: with notes on an assay for anthemophilic factor Blood 1962. 20 137–149 [PubMed] [Google Scholar]
- 32. Tiede A Werwitzke S Scharf RE Laboratory diagnosis of acquired hemophilia A: Limitations, consequences, and challenges Semin Thromb Haemost 2014. 40 803–811 [DOI] [PubMed] [Google Scholar]
- 33. Roberts HR Gross GP Webster WP Dejanov II Penick GD Acquired inhibitors of plasma factor IX A study of their induction, properties and neutralization. Am J Med Sci 1966. 251 43–50 [DOI] [PubMed] [Google Scholar]
- 34. Lazarchick J Hoyer LW The properties of immune complexes formed by human antibodies to factor VIII J Clin Invest 1977. 60 1070–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ørstavik KH Nilsson IM A study of acquired inhibitors of factor IX by means of precipitating rabbit antisera against factor IX Thromb Res 1978. 12 863–874 [DOI] [PubMed] [Google Scholar]
- 36. Goodnight SH Britell CW Wuepper KD Osterud B Circulating factor IX antigen-inhibitor complexes in hemophilia B− following infusion of a factor IX concentrate Blood 1979. 53 93–103 [PubMed] [Google Scholar]
- 37. Miller CH Orstavik KH Hilgartner MW Characterization of an occult inhibitor to factor IX in a haemophilia B patient Br J Haematol 1985. 61 329–338 [DOI] [PubMed] [Google Scholar]
- 38. Nilsson IM Berntorp E Zettervall O Dahlbäck B Noncoagulation inhibitory factor VIII antibodies after induction of tolerance to factor VIII in hemophilia A patients Blood 1990. 75 378–383 [PubMed] [Google Scholar]
- 39. Allain JP Frommel D Antibodies to factor VIII. I. Variations in stability of antigen-antibody complexes in hemophilia A Blood 1973. 42 437–444 [PubMed] [Google Scholar]
- 40. Miller CH Platt SJ Rice AS Kelly F Soucie JM the Hemophilia Inhibitor Research Study I Validation of Nijmegen-Bethesda assay modifications to allow inhibitor measurement during replacement therapy and facilitate inhibitor surveillance J Thromb Haemost 2012. 10 1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Millner AH Tiefenbacher S Robinson M Boesen HT A variation of the Nijmegen-Bethesda assay using heat or a novel heat/cold pre-treatment for the detection of FIX inhibitors in the presence of residual FIX activity Int J Lab Hematol 2016. 38 639–647 [DOI] [PubMed] [Google Scholar]
- 42. Scandella D de Graaf Mahoney S Mattingly M Roeder D Timmons L Fulcher CA Epitope mapping of human factor VIII inhibitor antibodies by deletion analysis of factor VIII fragments expressed in Escherichia coli Proc Natl Acad Sci USA 1988. 85 6152–6156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kahle J Orlowski A Stichel D et al. Frequency and epitope specificity of anti–factor VIII C1 domain antibodies in acquired and congenital hemophilia A Blood 2017. 130 808–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hofbauer CJ Whelan SFJ Hirschler M et al. Affinity of FVIII-specific antibodies reveals major differences between neutralizing and non-neutralizing antibodies in humans Blood 2015. 125 1180–1188 [DOI] [PubMed] [Google Scholar]
- 45. Biggs R Bidwell E Macfarlane RG The mode of action and aetiology of antihaemophilic globulin inhibitors Le Sang 1959. 30 340–351 [PubMed] [Google Scholar]
- 46. Biggs R Austen DEG Denson KWE Rizza CR Borrett R The mode of action of antibodies which destroy factor VIII. I. Antibodies which have second-order concentration graphs Br J Haematol 1972a. 23 125–135 [DOI] [PubMed] [Google Scholar]
- 47. Biggs R Austen DEG Denson KWE Borrett R Rizza CR The mode of action of antibodies which destroy factor VIII. II. Antibodies which give complex concentration graphs Br J Haematol 1972b. 23 137–155 [DOI] [PubMed] [Google Scholar]
- 48. Gawryl MS Hoyer LW Inactivation of factor VIII coagulant activity by two different types of human antibodies Blood 1982. 60 1103–1109 [PubMed] [Google Scholar]
- 49. Christophe OD Lenting PJ Cherel G et al. Functional mapping of anti-factor IX inhibitors developed in patients with severe hemophilia B Blood 2001. 98 1416–1423 [DOI] [PubMed] [Google Scholar]
- 50. Bravo MI Da Rocha-Souto B Grancha S Jorquera JI Native plasma-derived FVIII/VWF complex has lower sensitivity to FVIII inhibitors than the combination of isolated FVIII and VWF proteins. Impact on Bethesda assay titration of FVIII inhibitors Haemophilia 2014. 20 905–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kempton CL Abshire TC Deveras RA et al. Pharmacokinetics and safety of OBI-1, a recombinant B domain-deleted porcine factor VIII, in subjects with haemophilia A Haemophilia 2012. 18 798–804 [DOI] [PubMed] [Google Scholar]
- 52. Kasper CK Aledort L Aronson D et al. A more uniform measurement of factor VIII inhibitors Thromb Diath Haemorrh 1975. 34 869–872 [PubMed] [Google Scholar]
- 53. Verbruggen B Novakova I Wessels H Boezeman J van den Berg M Mauser-Bunschoten E The Nijmegen modification of the Bethesda assay for factor VIII: C inhibitors: improved specificity and reliability Thromb Haemost 1995. 73 247–251 [PubMed] [Google Scholar]
- 54. Giles AR Verbruggen B Rivard GE Teitel J Walker I A detailed comparison of the performance of the standard versus the Nijmegen modification of the Bethesda assay in detecting factor VIII: C inhibitors in the haemophilia A population of Canada. Association of Hemophilia Centre Directors of Canada. Factor VIII/IX Subcommittee of Scientific and Standardization Committee of International Society on Thrombosis and Haemostasis Thromb Haemost 1998. 79 872–875 [PubMed] [Google Scholar]
- 55. Manco-Johnson MJ Nuss R Jacobson LJ Heparin neutralization is essential for accurate measurement of factor VIII activity and inhibitor assays in blood samples drawn from implanted venous access devices J Lab Clin Med 2000. 136 74–79 [DOI] [PubMed] [Google Scholar]
- 56. Blanco AN Cardozo MA Candela M Santarelli MT Bianco RP Lazzari MA Anti-factor VIII inhibitors and lupus anticoagulants in haemophilia A patients Thromb Haemost 1997. 77 656–659 [PubMed] [Google Scholar]
- 57. Shaw PH Reynolds S Gunawardena S Krishnamurti L Ritchey AK The prevalence of bleeding disorders among healthy pediatric patients with abnormal preprocedural coagulation studies J Pediatr Hematol Oncol 2008. 30 135–141 [DOI] [PubMed] [Google Scholar]
- 58. Blanco AN Peirano AA Grosso SH Gennari LC Pérez Bianco R Lazzari MA A chromogenic substrate method for detecting and titrating anti-factor VIII antibodies in the presence of lupus anticoagulant Haematologica 2002. 87 271–278 [PubMed] [Google Scholar]
- 59. Peyvandi F Oldenburg J Friedman KD A critical appraisal of one-stage and chromogenic assays of factor VIII activity J Thromb Haemost 2016. 14 248–261 [DOI] [PubMed] [Google Scholar]
- 60. Miller CH Rice AS Boylan B et al. Comparison of clot-based, chromogenic and fluorescence assays for measurement of factor VIII inhibitors in the US Hemophilia Inhibitor Research Study J Thromb Haemost 2013. 11 1300–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Barnbrock A Heller C Königs C Budde U Schwabe D Lupus anticoagulants associated inhibitor against factor IX in a young patient with haemophilia B Haemophilia 2016. 22 e437–e439 [DOI] [PubMed] [Google Scholar]
- 62. Kitchen S Blakemore J Friedman KD et al. A computer-based model to assess costs associated with the use of factor VIII and factor IX one-stage and chromogenic activity assays J Thromb Haemost 2016. 14 757–764 [DOI] [PubMed] [Google Scholar]
- 63. Regnault V Stoltz JF Quantitation of factor VIII antibodies by an enzyme-linked immunoassay method Blood 1994. 83 1155–1156 [PubMed] [Google Scholar]
- 64. Gautier P Sultan Y Parquet-Gernez A Meriane F Guerois C Derlon A Detection and IgG subclass analysis of antibodies to factor VIII in multitransfused haemophiliacs and healthy individuals Haemophilia 1996. 2 88–94 [DOI] [PubMed] [Google Scholar]
- 65. Sahud MA Pratt KP Zhukov O Qu K Thompson AR ELISA system for detection of immune responses to FVIII: A study of 246 samples and correlation with the Bethesda assay Haemophilia 2007. 13 317–322 [DOI] [PubMed] [Google Scholar]
- 66. Klinge J Auerswald G Budde U et al. Detection of all anti-factor VIII antibodies in haemophilia A patients by the Bethesda assay and a more sensitive immunoprecipitation assay Haemophilia 2001. 7 26–32 [DOI] [PubMed] [Google Scholar]
- 67. Lavigne-Lissalde G Tarrade C Lapalud P et al. Simultaneous detection and epitope mapping of anti-factor VIII antibodies Thromb Haemost 2008. 99 1090–1096 [DOI] [PubMed] [Google Scholar]
- 68. Krudysz-Amblo J Parhami-Seren B Butenas S et al. Quantitation of anti-factor VIII antibodies in human plasma Blood 2009. 113 2587–2594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lewis KB, Hughes RJ, Epstein MS. et al. Phenotypes of allo- and autoimmune antibody responses to FVIII characterized by surface plasmon resonance. PLoS ONE. 2013;8:e61120. doi: 10.1371/journal.pone.0061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Batty P Platton S Bowles L Pasi KJ Hart DP Pre-analytical heat treatment and a FVIII ELISA improve Factor VIII antibody detection in acquired haemophilia A Br J Haematol 2014. 166 953–956 [DOI] [PubMed] [Google Scholar]
- 71. Auerswald G Kurnik K Aledort LM et al. The EPIC study: a lesson to learn Haemophilia 2015. 21 622–628 [DOI] [PubMed] [Google Scholar]
- 72. Montalvão SAL Tucunduva AC Siqueira LH Sambo ALA Medina SS Ozelo MC A longitudinal evaluation of anti-FVIII antibodies demonstrated IgG4 subclass is mainly correlated with high-titre inhibitor in haemophilia A patients Haemophilia 2015. 21 686–692 [DOI] [PubMed] [Google Scholar]
- 73. Sahud M Zhukov O Mo K Popov J Dlott J False-positive results in ELISA-based anti FVIII antibody assay may occur with lupus anticoagulant and phospholipid antibodies Haemophilia 2012. 18 777–781 [DOI] [PubMed] [Google Scholar]
- 74.Kitchen S, McCraw A, Echenagucia M. Diagnosis of Hemophilia and Other Bleeding Disorders: A Laboratory Manual. 2nd World Federation of Hemophilia; Montreal: 2010. [Google Scholar]
- 75. Kershaw G Jayakodi D Dunkley S Laboratory identification of factor inhibitors: The perspective of a large tertiary hemophilia center Semin Thromb Haemost 2009. 35 760–768 [DOI] [PubMed] [Google Scholar]
- 76. Verbruggen B Diagnosis and quantification of factor VIII inhibitors Haemophilia 2010. 16 20–24 [DOI] [PubMed] [Google Scholar]
- 77. Gatti L Mannucci PM Use of porcine factor VIII in the management of seventeen patients with factor VIII antibodies Thromb Haemost 1984. 51 379–384 [PubMed] [Google Scholar]
- 78.Boylan B, Miller CH. Effects of preanalytical heat treatment in factor VIII inhibitor assays on detection of factor VIII antibody levels. Haemophilia. 2018 doi: 10.1111/hae.13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. de Lima Montalvão SA Tucunduva AC de Almeida Sambo AL De Paula EV de Souza Medina S Ozelo MC Heat treatment of samples improve the performance of the Nijmegen-Bethesda assay in hemophilia A patients undergoing immune tolerance induction Thromb Res 2015. 136 1280–1284 [DOI] [PubMed] [Google Scholar]
- 80. Adamkewicz JI Schmitt C Asikanius E et al. Factor VIII (FVIII) inhibitor testing using a validated chromogenic Bethesda assay (CBA) in HAVEN 1 (BH29884), a phase 3 trial of emicizumab in persons with hemophilia A (PwHA) with inhibitors RPTH 2017. 1 Suppl 1 724–725 [Google Scholar]
- 81. Soucie JM Miller CH Kelly FM Oakley M Brown DL Kucab P A public health approach to the prevention of inhibitors in hemophilia Am J Prev Med 2014. 47 669–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Verbruggen B Giles A Samis J Verbeek K Mensink E Novákovà I The type of factor VIII deficient plasma used influences the performance of the Nijmegen modification of the Bethesda assay for factor VIII inhibitors Thromb Haemost 2001. 86 1435–1439 [PubMed] [Google Scholar]
- 83. Pouplard C Desconclois C Sobas F Aillaud MF Ternisien C Caron C Does the presence of von Willebrand factor in FVIII-deficient plasma influences the measurement of FVIII inhibitor titres in haemophilia A patients? Int J Lab Hematol 2015. 37 125–132 [DOI] [PubMed] [Google Scholar]
- 84. Verbruggen B Van Heerde W Novákovà I Lillicrap D Giles A A 4% solution of bovine serum albumin may be used in place of factor VIII: C deficient plasma in the control sample in the Nijmegen modification of the Bethesda factor VIII: C inhibitor assay Thromb Haemost 2002. 88 362–364 [PubMed] [Google Scholar]
- 85. Kershaw GW Chen LS Jayakodi D Dunkley SM Validation of 4% albumin as a diluent in the Bethesda Assay for FVIII inhibitors Thromb Res 2013. 132 735–741 [DOI] [PubMed] [Google Scholar]
- 86.Miller CH, Payne AB, Driggers J, Ellingsen D, Boylan B, Bean CJ. Reagent substitutions in the Centers for Disease Control and Prevention Nijmegen-Bethesda assay for factor VIII inhibitors. Haemophilia. 2018 doi: 10.1111/hae13434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Miller CH Boylan B Shapiro AD Lentz SR Wicklund BM the Hemophilia Inhibitor Research Study I Limit of detection and threshold for positivity of the Centers for Disease Control and Prevention assay for factor VIII inhibitors J Thromb Haemost 2017b. 15 1971–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Peerschke EIB Castellone DD Ledford-Kraemer M Van Cott EM Meijer P the NASCOLA Proficiency Testing Committee Laboratory assessment of factor VIII inhibitor titer: the North American Specialized Coagulation Laboratory Association experience Am J Clin Pathol 2009. 131 552–558 [DOI] [PubMed] [Google Scholar]
- 89. Favaloro EJ Verbruggen B Miller CH Laboratory testing for factor inhibitors Haemophilia 2014. 20 Suppl 4 94–98 [DOI] [PubMed] [Google Scholar]
- 90.Pruthi RK, Plumhoff EA, Nichols WL, Meijer P, Van Cott EM. Quality of factor VIII inhibitor testing in North American Specialized Coagulation Laboratories. Am J Hematol. 2014;89:E27. doi: 10.1111/ijlh.12359. [DOI] [PubMed] [Google Scholar]
- 91. Verbruggen B Dardikh M Polenewen R Van Duren C Meijer P The factor VIII inhibitor assays can be standardized: Results of a workshop J Thromb Haemost 2011. 9 2003–2008 [DOI] [PubMed] [Google Scholar]
- 92. Adcock DM Mammen J Nair SC de Lima Montalvão SA Quality laboratory issues in bleeding disorders Haemophilia 2016. 22 84–89 [DOI] [PubMed] [Google Scholar]
- 93. Dardikh M Albert T Masereeuw R et al. Low-titre inhibitors, undetectable by the Nijmegen assay, reduce factor VIII half-life after immune tolerance induction J Thromb Haemost 2012. 10 706–708 [DOI] [PubMed] [Google Scholar]
- 94. Van Helden PMW Van Den Berg HM Gouw SC et al. IgG subclasses of anti-FVIII antibodies during immune tolerance induction in patients with hemophilia A Br J Haematol 2008. 142 644–652 [DOI] [PubMed] [Google Scholar]
- 95. Collins P Chalmers E Chowdary P et al. The use of enhanced half-life coagulation factor concentrates in routine clinical practice: guidance from UKHCDO Haemophilia 2016. 22 487–498 [DOI] [PubMed] [Google Scholar]