Abstract
Traumatic brain injuries (TBI) sustained during peri-adolescent development produce lasting neuro-behavioral changes that render individuals at an increased risk for developing substance abuse disorders. Experimental and clinical evidence of a prolonged period of hypodopaminergia after TBI have been well documented, but the effect of juvenile TBI on dopaminergic dysfunction and its relationship with substance abuse have not been investigated. In order to determine the effect of juvenile brain injury on dopaminergic signaling, female mice were injured at 21 days of age and then beginning seven weeks later were assessed for behavioral sensitization to amphetamine, a drug that increases synaptic dopamine availability. Together with a histological analysis of tyrosine hydroxylase, dopamine transporter, and dopamine D2 receptor expression, our data are indicative of a persistent state of hypodopaminergia well into adulthood after a juvenile TBI. Further, mice that sustained a juvenile TBI exhibited a significantly reduced activation of cFos in the urocortin-positive cells of the Edinger-Westphal nucleus in response to ethanol administration. Taken together, these data provide strong evidence for the vulnerability of juveniles to the development of lasting neuro-behavioral problems following TBI, and indicate a role of injury-induced hypodopaminergia as a risk factor for substance abuse later in life.
Keywords: traumatic brain injury, development, alcohol, dopamine, hypodopaminergia
Introduction
Traumatic brain injury (TBI) is a leading cause of long-term disability and death in the United States, affecting approximately 1.7 million individuals annually. Approximately 500,000 emergency department visits related to TBI each year are made by children under the age of 14 (Faul et al., 2010). While long-term disabilities following severe childhood TBI are well known and studied (Cattelani et al., 1998; Jonsson et al., 2004), fewer studies have been devoted to the lasting effects of mild childhood brain injuries. However, emerging data indicate that adults who endured a mild childhood TBI are less likely to complete their education and obtain employment (Anderson et al., 2009; Corrigan et al., 2013). Moreover, adults who experienced a mild TBI (mTBI) with loss of consciousness between the ages of 0–15 years are more likely to engage in alcohol and substance abuse (Corrigan et al., 2013). Given that substance and alcohol abuse contributes to the development of psychiatric disorders, incarceration, and increases the risk of subsequent brain injuries (Bombardier et al., 2002; Fazel et al., 2006; Regier et al., 1990), there is a pressing need to identify the mechanisms by which mTBI during childhood contributes to long-lasting substance abuse issues. This study was designed to investigate mTBI-induced dysregulation of dopaminergic signaling as a mediator of altered behavioral and physiological responses to drugs of abuse.
Traumatic brain injuries produce persistent alterations in dopaminergic systems. Animal models of adult TBI have reported global disturbances in dopamine and its metabolites (Huang et al., 2014; Kobori et al., 2006; Massucci et al., 2004; Shin and Dixon, 2011), as well as tyrosine hydroxylase (Hutson et al., 2011; Yan et al., 2001; Yan et al., 2007), and dopamine transporter expression (Shimada et al., 2014). However, far less is known about the long-term consequences to dopaminergic signaling following juvenile brain injuries, especially in females. There has been relatively little direct investigation of dopaminergic dysfunction in clinical settings but drugs that enhance dopamine neurotransmission are effective at treating some of the cognitive consequences of TBI (Sami and Faruqui, 2015). Dopaminergic dysfunction is associated with alcohol and substance abuse, as changes in dopamine signaling both result from and contribute to drug and alcohol abuse (Dalley et al., 2007; Schmidt et al., 2001). Indeed, psychiatric disorders that involve dopaminergic alterations have a high rate of comorbidity with substance abuse (Dagher and Robbins, 2009; Heinz, 2002; Schmidt et al., 2001; Sullivan and Rudnik-Levin, 2001), and emerging data indicate TBI as a risk factor for substance and alcohol abuse (Weil et al., 2016a).
In an effort to model the clinical phenomenon of substance abuse following childhood TBI, we recently reported that an mTBI during juvenile development in mice increased alcohol consumption as well as the rewarding properties of alcohol in adulthood (Weil et al., 2016b). Moreover, binge-like levels of alcohol produced a greater degree of neuroinflammation, axonal damage, and learning impairments in adult mice that experienced a juvenile mTBI compared to sham-injured mice (Karelina et al., 2017). Importantly, our reported increase in alcohol preference was evident in female, but not male, mice, indicating that injuries occurring at a critical developmental period render females particularly vulnerable to the long-lasting effects on reward processing and consequences of alcohol abuse. In order to begin to identify the mechanism underlying this phenomenon, we assessed dopaminergic signaling and behavioral sensitization to amphetamine in adult mice that experienced a juvenile mTBI.
Materials and Methods
Animals
Swiss Webster mice were purchased from Charles River (Wilmington, MA) and bred at OSU. Pups were weaned at 21 days of age and were housed in a 14:10 light cycle with ad libitum access to food (Harlan-Teklad #8640) and filtered tap water. All procedures were approved by the OSU Institutional Animal Care and Use Committee and were conducted in accordance with NIH guidelines.
Experimental protocol
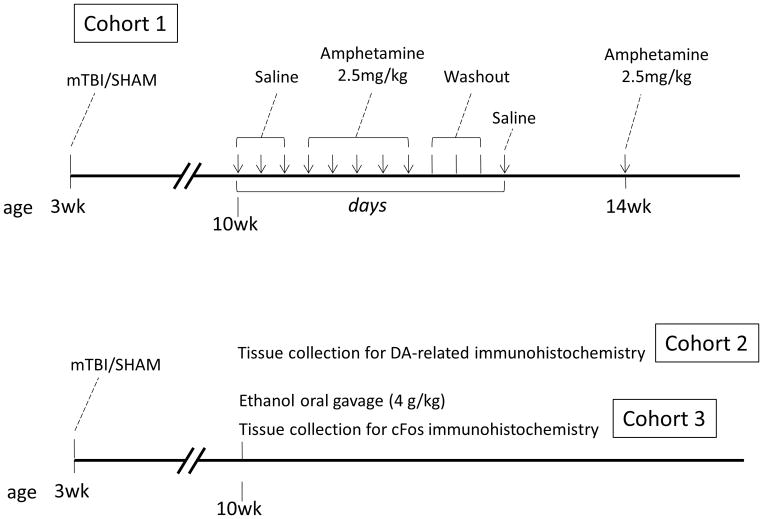
At weaning (21 days of age), female mice underwent TBI or sham injury and were returned to their home cages for a recovery period of 7 weeks. Three cohorts of mice were used in the following experiments as depicted in Figure 1. Cohort 1 was assessed for behavioral sensitization to amphetamine (2.5 mg/kg in saline). Cohort 2 was perfused and brain tissue was collected for immunohistochemical assessment of dopamine-related markers (n = 7–9/group for cohorts 1 and 2). Cohort 3 was administered a single dose of ethanol (4 g/kg of 50% ethanol solution) or vehicle via oral gavage and brain tissue was collected 90 minutes later for immunohistochemical detection of immediate early gene expression (n = 6–7/group for cohort 3).
Figure 1. Experimental timeline.
All experimental animals underwent an mTBI or sham injury at weaning and were assessed beginning seven weeks following injury. Cohort 1 was assessed for behavioral sensitization to amphetamine over a two-week period in adulthood. Arrows on the time line indicate days of open field testing. Cohort 2 was euthanized seven weeks following injury for immunohistochemical detection of TBI-induced disruption of dopaminergic markers. At the same time point, cohort 3 was treated with a single dose of ethanol or water then euthanized for immunohistochemical detection of immediate early gene activation in response to ethanol.
Traumatic brain injury
A single mild traumatic brain injury was conducted on 21-day-old mice as previously reported (Karelina et al., 2017; Weil et al., 2016b). Mice were anesthetized with 1.5% inhaled isoflurane and secured into a stereotaxic frame (David Kopf). The skull surface was exposed and a 2 mm diameter impactor tip (Leica) was placed onto the skull surface (−1 mm AP, −1 mm ML relative to bregma). The tip was then retracted and driven into the skull to a depth of 1 mm at a rate of 3 m/s (with a dwell time of 30 msec). The skin was sutured and animals were returned to their home cages. The sham procedure was performed identically except the impactor tip was placed on the skull surface then immediately retracted.
Behavioral testing
Behavioral sensitization to amphetamine was assessed via an open field assay. Locomotor activity in the open field was assessed in a 60 cm3 Plexiglas chamber that was enclosed in a sound and light attenuating cabinet. Total movement during each 60-minute session was tracked via infrared beams and data were generated using the PAS software package (San Diego Instruments). Baseline locomotor activity was determined over a period of three consecutive days during which mice were injected intraperitoneally (IP) with saline immediately before placement into the open field. Beginning the following day, all mice were treated IP with 2.5 mg/kg amphetamine (Sigma) dissolved in saline and immediately placed in the open field chamber. Behavioral sensitization to amphetamine was assessed over a period of five consecutive days during which mice were treated daily with amphetamine and tested in the open field chamber. Mice were weighed daily to account for changes in body mass for dosing, and amphetamine was made fresh every day. After the final day of sensitization, the mice underwent a washout period of three days during which they were unmanipulated, followed by a single dose of IP saline and open field testing. Two weeks later, the mice were injected one final time with 2.5 mg/kg amphetamine and re-tested in the open field. For data processing, locomotor activity for each animal was normalized to its baseline and plotted as percent change from baseline.
Tissue processing and immunohistochemistry
All mice in cohorts 2 and 3 were overdosed with sodium pentobarbital (200 mg/kg) and transcardially perfused with 4% paraformaldehyde. Forty micron sections were sliced coronally on a cryostat throughout the forebrain and collected for free-floating immunohistochemistry. Sections were washed with 0.1M phosphate buffered saline, quenched in 0.3% hydrogen peroxide, and incubated in primary antibody overnight (tyrosine hydroxylase (TH) 1:2000 AB_390204; dopamine transporter (DAT) 1:2000 AB_1586991; dopamine receptor D2 (D2R) 1:1000 AB_2094980). The following day, tissue was washed, incubated with a goat anti-rabbit secondary antibody (AB_357235) then developed via the ABC-DAB method (Vector Laboratories). For double-labeling, tissue was incubated overnight with cFos antibody (1:1000 AB_2106783), developed the following day using DAB, washed and re-incubated overnight with a primary antibody against urocortin 1 (UCN 1:1000, developed by Dr. Wylie Vale). UCN staining was developed using NovaRED (Vector Laboratories).
Photomicrographs of all histology were captured at a 20X magnification through both hemispheres. Analysis was conducted using FIJI software (Schindelin et al., 2012). Dopamine-related histological images in the striatum and ventral tegmental area (VTA) were quantified by determining the optical density of staining in each hemisphere. Optical density in the striatum was obtained in a 0.04mm2 region of interest centered at approximately 1.1mm AP, 1.5mm ML, and 3mm DV relative to bregma. Optical density in the VTA was assessed within a manually outlined region of the medial portion of the VTA, approximately −3.08mm AP, 0.5mm ML, and 4.25 DV relative to bregma. In order to convert pixel values, optical density values were calibrated using a set of gray scale standards. Images were assessed bilaterally for two adjacent brain slices and data averaged across hemispheres and slices within an animal (with the exception of the D2R histology which is reported as ipsilateral staining as percent of contralateral). cFos and UCN expression was quantified by cell counts in the Edinger-Westphal nucleus (EW). All UCN-positive cells (reddish brown in color) in the EW were assessed at approximately −3.4 mm AP, 3.5 mm DV relative to bregma along the midline. Co-labeled cFos-positive cells (dark purple/black in color) were assessed in the same region and data are presented as percent cFos-positive UCN cells.
Statistical analysis
Statistical analysis was conducted using SPSS version 24 (IBM Corp.). Behavioral data were analyzed via repeated measures ANOVA as well as between-group comparisons on specific days using one-way ANOVA. Histological data in the first two cohorts were analyzed via a one-way ANOVA. The cFos/UCN data were assessed via a two-way ANOVA (injury X oral gavage condition). Data are presented as significant for p values less than 0.05. A significant overall ANOVA value was followed up by a Least Significant Difference post-hoc analysis.
Results
Juvenile traumatic brain injury impairs behavioral sensitization to amphetamine in adulthood
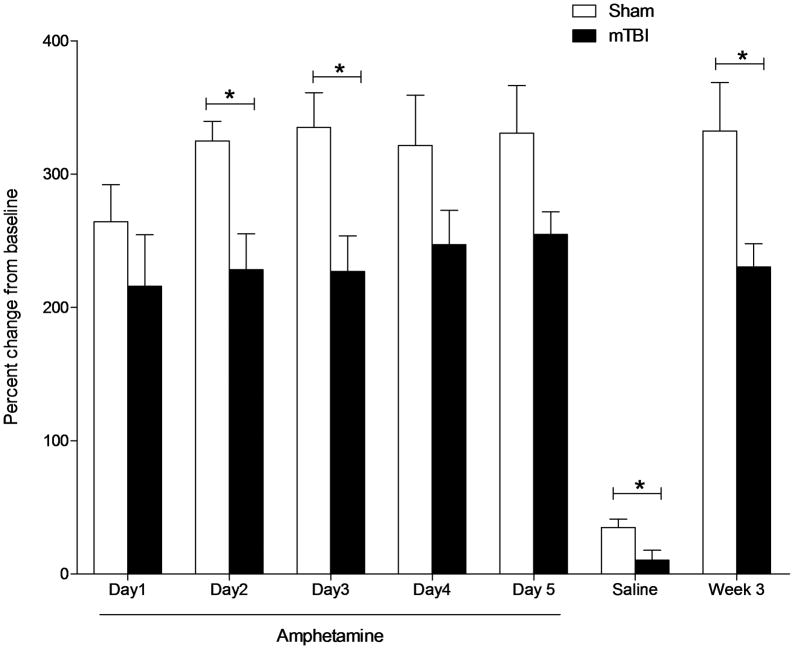
As a readout of the sensitivity of the dopaminergic system, behavioral sensitization to amphetamine was assessed in an open field locomotor assay in adult mice that were injured (or sham-injured) as juveniles. Baseline locomotor activity was similar between sham and mTBI mice (F1,15 = 2.125, p = .167). All mice were assessed over five days in an open field following daily amphetamine injections (Figure 2). All mice exhibited a significant increase in locomotor activity in response to amphetamine relative to saline injections at baseline (at least 200% of baseline). Amphetamine-induced behavioral sensitization was assessed over a 5-day period for each animal relative to its own baseline activity and statistical analysis was performed on relativized data. A repeated measures ANOVA revealed a significant difference in amphetamine-induced locomotor activity between TBI and sham-injured mice (F7,63 = 2.821, p = 0.013). Brain injured mice were significantly less active than sham-injured mice on days two (F1,12 = 10.767, p = 0.007) and three (F1,12 = 8.291, p = 0.015) of testing. Following a three-day washout period, mice were injected with saline and re-tested in the open field. Brain injured mice were again significantly less active than sham-injured mice (F1,12 = 6.084,p = 0.036) indicating a persistent alteration in locomotor activity in the absence of amphetamine in sham, but not TBI mice. After a two-week washout period, mice were re-tested one final time in the open field following a single amphetamine injection. TBI significantly reduced the locomotor response to amphetamine compared to sham injury (F1,11 = 6.394, p = 0.03), indicating that only sham mice exhibit persistent behavioral sensitization to the locomotor effects of amphetamine.
Figure 2. Juvenile mTBI impairs behavioral sensitization to amphetamine in adulthood.
Locomotor activity in an open field is shown as percent of baseline activity. Brain injured mice exhibited a significant reduction in activity following 2.5 mg/kg I.P. amphetamine on days 2 and 3 of treatment compared to sham-injured controls. A persistent alteration in locomotor activity in brain injured mice is evident following a saline injection. Two weeks later, amphetamine treatment revealed persistent behavioral sensitization to the locomotor effects of dopamine agonism in sham, but not mTBI mice.
Juvenile traumatic brain injury disrupts dopaminergic signaling in adulthood
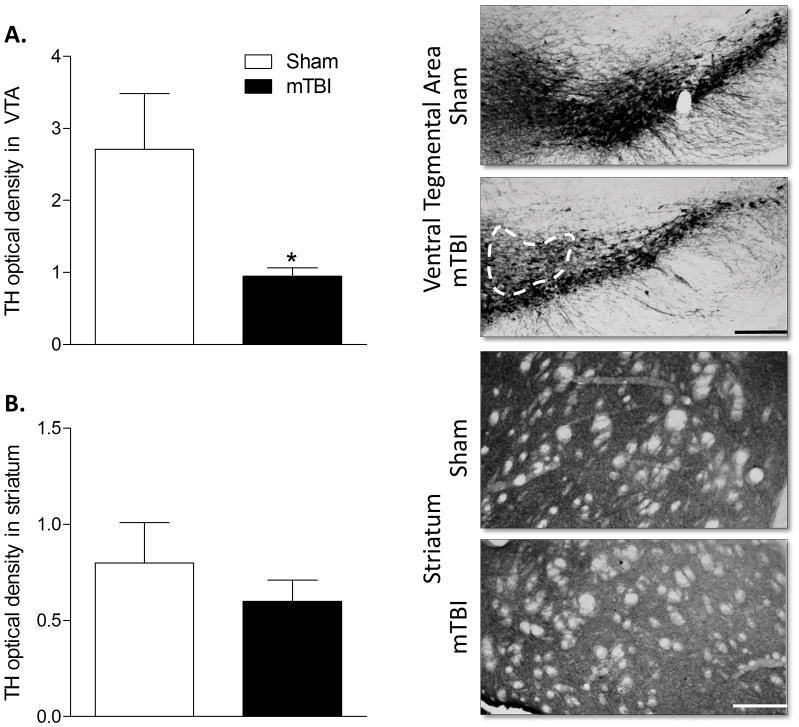
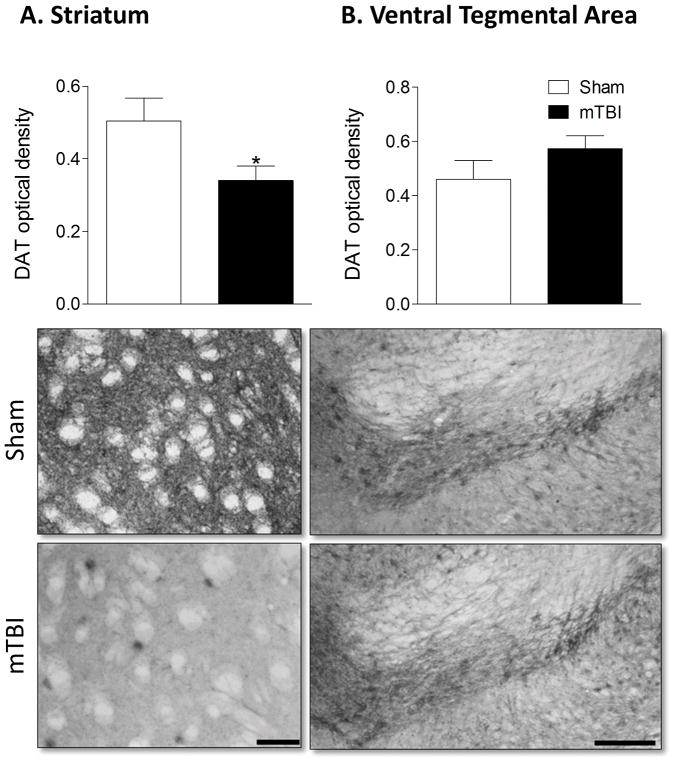
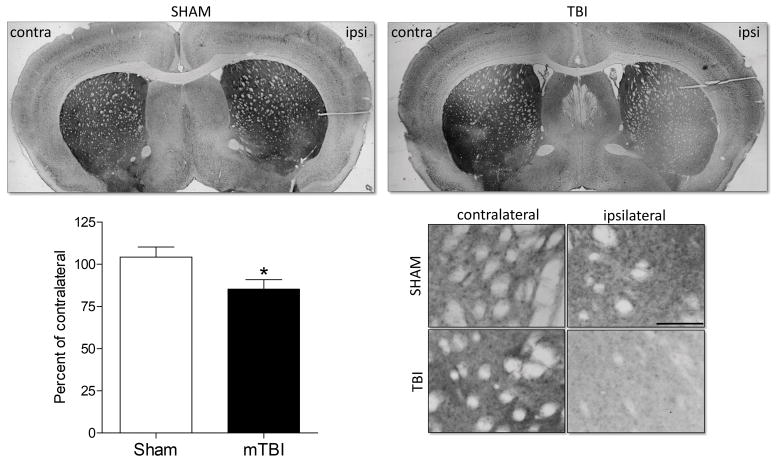
Given the significant reduction in the locomotor response to amphetamine in brain injured mice, we conducted a histological assessment of markers of dopaminergic signaling in the striatum and VTA of TBI and sham-injured mice. Brain injury significantly reduced the bilateral expression of tyrosine hydroxylase (TH) in the VTA ( F1,11 = 5.057, p = 0.048) but did not alter TH expression in the striatum (Figure 3). Bilateral dopamine transporter (DAT) expression was significantly reduced in brain injured mice in the striatum (F1,12 = 5.401, p = 0.04) but did not vary by injury condition in the VTA (Figure 4). Finally, expression of the dopamine receptor D2 (D2R) was significantly reduced in the ipsilateral hemisphere of brain injured mice. The percent of D2R expression relative to the contralateral hemisphere was significantly lower following brain injury (F1,12 = 5.489, p = 0.039) compared to sham injured mice. D2R expression was not altered by brain injury in the VTA.
Figure 3. Juvenile mTBI reduces tyrosine hydroxylase expression in the ventral tegmental area.
A) Optical density of tyrosine hydroxylase (TH) staining revealed reduced expression in the VTA of brain injured mice, B) but no difference between mTBI and sham mice in the striatum. An asterisk (*) indicates a significant difference, p < 0.05. Representative images of the VTA and striatum are shown with scale bars of 200 μm and 100 μm respectively.
Figure 4. Juvenile mTBI reduces dopamine transporter expression in the striatum.
A) Optical density of dopamine transporter (DAT) staining revealed reduced expression in the striatum of brain injured mice, B) but no difference between mTBI and sham mice in the VTA. An asterisk (*) indicates a significant difference, p < 0.05. Representative images of the striatum and VTA are shown with scale bars of 40 μm and 100 μm respectively.
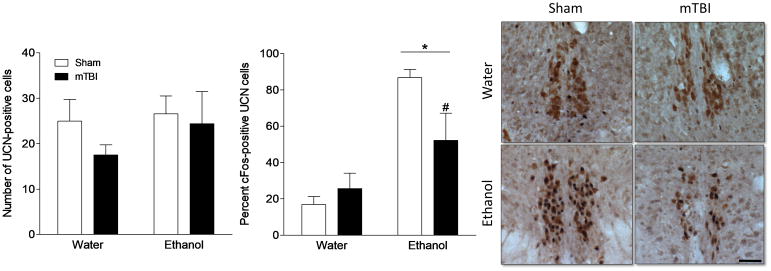
Juvenile brain injury alters activation of urocortin-positive cells of the Edinger-Westphal nucleus following ethanol treatment
In order to determine the impact of TBI-induced hypodopaminergia on the physiological response to alcohol, mice were treated with water or ethanol and assessed for cFos activation in UCN-positive cells of the Edinger-Westphal nucleus (EW). The EW is well-known to be highly sensitive and reactive to both experimentally administered and voluntarily consumed alcohol, and requires dopaminergic signaling to do so (Bachtell et al., 2002; Giardino et al., 2011). The total number of UCN-positive cells was similar across groups and did not differ by surgery (F1,19 = 1.089, p = 0.311) or gavage treatment (F1,19 = .836, p = 0.373). However, treatment with ethanol significantly increased overall cFos expression in the EW (F1,19 = 9.215, p = 0.008) and did so to a greater extent in sham-injured mice. In order to determine the activation status of the ethanol sensitive UCN-positive cells of the EW, we assessed the percent of UCN-positive cells that co-localize with cFos. Ethanol significantly increased the number of UCN/cFos-positive cells regardless of brain injury history (F1,19 = 29.249, p < 0.001), however, a post hoc analysis revealed that following ethanol treatment, brain injured mice (TBI+ethanol) exhibited a significant reduction in cFos expression in UCN cells compared to shams (sham+ethanol).
Discussion
A single mild traumatic brain injury during peri-adolescent development produces long-lasting changes in the brain circuitry that persist well into adulthood. Our data demonstrate a lasting state of hypodopaminergia as well as altered behavioral and physiological responses to drugs of abuse beginning nearly two months following a juvenile mTBI in mice. These findings shed mechanistic light on the substantial vulnerability to the development of drug abuse disorders that is faced by individuals who experience brain injuries, however mild, early in life.
The neurobiological basis of drug abuse and addiction is complex but nearly ubiquitously involves a component of mesolimbic DA signaling whereby dopaminergic cells from the VTA project to regions of the brain’s reward circuitry, including the prefrontal cortex, nucleus accumbens, hippocampus, and amygdala (Pierce and Kumaresan, 2006). The activity of DA within this system is associated with drug reinforcement and addiction (Ikemoto, 2007) as rodents readily self-administer drugs that increase neuronal DA activity and release (Nielsen et al., 1984), but fail to do so following DA depletion via 6-hydroxydopamine (Roberts et al., 1980). Importantly, a tonic reduction of dopaminergic activity, known as hypodopaminergia, renders an individual at a high risk of developing drug abuse problems and is believed to be a driver of drug seeking behaviors (Melis et al., 2005). Indeed, conditions such as Parkinson’s disease and attention deficit hyperactivity disorder, which involve a reduction in dopaminergic production and transmission, are associated with an increased risk of drug abuse (Dagher and Robbins, 2009; Sullivan and Rudnik-Levin, 2001).
Impairments in catecholamine systems broadly, and dopamine function specifically have been documented in TBI. Dopamine disruption in TBI manifests as cell loss in the substantia nigra (Hutson et al., 2011; van Bregt et al., 2012), reduced dopamine release (Wagner et al., 2005), and a compensatory reduction in DAT expression (Wagner et al., 2005; Yan et al., 2002). The resulting state of hypodopaminergia is implicated as a key player in cognitive disability and remains an important clinical target of pharmacotherapies to improve symptoms of impaired learning and memory, attention, and behavioral deficits (for review see Bales et al., 2009). It is surprising, therefore, that although TBI is increasingly understood to be an independent risk factor for substance abuse disorders, little effort has been made to link the development of drug abuse problems with the systemic dysfunction of dopamine and reward processing in TBI survivors. The possibility exists that the greater risk of substance abuse in the TBI population reflects, at least in part, a self-medication strategy (Weil et al., 2016a). This, from the patient’s perspective, would be used to reduce anxiety or depressive states and increase reward-related signaling (Bolton et al., 2009; Koob 2013; Graham and Cardon 2008). Additionally, cognitive deficits often present in TBI survivors may reduce the ability to weigh the long-term costs of substance use against the immediate psychological gains, rendering patients potentially susceptible to developing substance use disorders. Thus, any insight into the role of reward circuitry signaling disruption in TBI patients contributes to the development of treatments targeting potential substance abuse in this vulnerable patient population.
Amphetamine increases extracellular DA concentrations by inhibiting its uptake by DAT and promoting the vesicular release of DA into the cytoplasm (Calipari and Ferris, 2013). Repeated administration of amphetamine, in animals, produces two distinct changes in locomotor activity. First, amphetamine directly increases locomotor activity and then, following repeated administration, can induce persistent behavioral change characterized by an increase in the locomotor response even in the absence of amphetamine administration. In the current study, although all mice increased locomotor behavior in response to amphetamine administration, those mice that had experienced a juvenile mTBI failed to demonstrate behavioral sensitization. Reduced immediate locomotor response to amphetamine and attenuated behavioral sensitization have both been associated with dysfunction in the mesolimbic dopamine system or blockade of dopamine receptors (Gold et al., 1988; Kelly and Iversen, 1976; Vezina and Stewart, 1989). Thus the behavioral outcome in these animals is in line with our observation of a persistent state of hypodopaminergia characterized by reduced TH, DAT, and D2R expression throughout the VTA and striatum. Moreover, our data are consistent with published reports on changes in TH and DAT expression following TBI (Hutson et al., 2011; van Bregt et al., 2012; Wagner et al., 2005; Yan et al., 2002); however, we present the first evidence of a persistent alteration in these systems from a juvenile injury lasting into adulthood. Less is known about the expression of D2R following TBI. One report indicates reduced striatal D2R binding in human TBI patients (Donnemiller et al., 2000) and another indicated a marginal increase in D2R binding (Wagner et al., 2014), however both studies only examined patients who were injured as adults. A similar reduction in D2R expression to what we report has also been documented in neonatal hypoxia-ischemia models (Cantagrel et al., 2001; Yang et al., 2007). Because ischemic complications are a common consequence of TBI (Wagner et al., 2004), it is possible that our observed reduction in D2R expression is the direct result of TBI-induced hypoxia/ischemia.
The specific mechanisms that produce alterations in dopamine signaling following TBI are incompletely understood. There is likely some direct damage to the cell bodies and projections of dopaminergic cells but that is unlikely to fully mediate this phenomenon. Importantly, the ascending dopaminergic system undergoes significant functional and anatomical plasticity during late childhood and early adolescence. This is characterized by changing tonic and stimulus evoked firing, alterations in synthetic machinery, transporter expression and receptor distribution (Galvan, 2010; Wahlstrom et al., 2010). This period of rapid neurodevelopment coincides temporally with vulnerability to substance abuse (Guerri and Pascual, 2010). Early experience with drugs of abuse is a key risk factor for the development of substance abuse disorders and has been shown experimentally to acutely activate and chronically modulate the long-term function of the dopamine system (Guerri and Pascual, 2010; Pascual et al., 2009). Similarly, TBI initially induces an upregulation of dopamine signaling that later resolves into a long-term hypodopaminergic state (Hutson et al., 2011; Massucci et al., 2004; Shimada et al., 2014; van Bregt et al., 2012; Yan et al., 2002). The possibility exists, therefore, that injuries that occur during this period of rapid neurological development and plasticity in the ascending dopamine systems interfere with normal homeostatic processes and thus persistently dysregulate this system. Additionally, ongoing inflammation, a nearly ubiquitous finding in traumatically injured brains, also serves to inhibit dopamine signaling and is thus also a potential mediator of hypodopaminergia (Carvey et al., 2003; Iravani et al., 2002).
Our finding of a persistent state of hypodopaminergia after juvenile mTBI is particularly important in the context of increased rates of substance abuse in the brain injured population. Alcohol abuse after TBI is a major impediment to recovery and increases the risk of sustaining a subsequent injury (for review see Weil et al., 2016a). Using the same model of juvenile mTBI as reported here, we have previously reported increased alcohol self-administration in adult mice after early life brain injury (Weil et al., 2016b). Moreover, binge-like levels of alcohol consumption significantly exacerbate TBI-associated pathophysiology, even if the drinking occurs months after the mTBI (Karelina et al., 2017). Here, we have begun to uncover a potential mechanism for increased alcohol preference via TBI-induced hypodopaminergia. We report that hypodopaminergia is associated with an altered physiological response to ethanol in the highly ethanol-sensitive Edinger-Westphal nucleus. Specifically, while sham-injured animals exhibited a high sensitivity of the EW in response to ethanol, EW activation of brain injured mice was considerably reduced. Dopamine is critical for the activation of the EW by ethanol, as dopamine antagonism blocks ethanol induction of cFos in the EW (Bachtell et al., 2002). This is consistent with our findings of attenuated dopaminergic activity following TBI leading to reduced activation by alcohol or amphetamine. Critically, reduced physiological and behavioral responsivity to drugs of abuse are key risk factors for the development of substance abuse disorders (Morean and Corbin, 2010; Türker et al., 1998).
Finally, it is important to note that this dataset was derived using female mice, as only female mice exhibit an increased preference for ethanol in this TBI model (Weil et al., 2016b). Given that females are largely excluded from both clinical and experimental TBI research, these data shed light on an underdeveloped area of research into sex-differences underlying TBI outcomes in general, and post-traumatic drinking in particular. As the rate of TBI incidence continues to increase in girls and women (Comstock et al., 2015; Covassin et al., 2003; Lincoln et al., 2011), it is becoming apparent that women may also suffer worse outcomes than men (Farace and Alves, 2000; Farin et al., 2003). Further, the consequences of AUD are significantly more severe in women, putting women at a greater risk for alcohol-related physical illness, cognitive impairment and reproductive problems (Ceylan-Isik et al., 2010; White et al., 2015). Although further research is necessary in order to directly compare the sex-differences in changes to the reward circuitry following TBI, the finding of a lasting dysfunction in the dopamine system in our model confirms a susceptibility in females to TBI-induced alterations related to the development of substance abuse problems.
Taken together, these data contribute to our growing understanding of how early life injuries can produce lifelong cognitive and behavioral impairments (Ajao et al., 2012; Scott et al., 2015; Weil et al., 2016b). Given that children make up a large proportion of TBI-related emergency room visits (Faul et al., 2010), there is now an critical need to identify and manage the mechanisms that drive high rates of substance abuse in individuals who were brain injured as adolescents or younger. Finally, there is a substantial body of evidence that substance abuse after TBI interferes with effective rehabilitation and has serious negative consequences for behavioral recovery, occupational success, physical health and the risk of additional injuries. Targeting dysfunction in reward systems and substance abuse could therefore significantly improve long-term outcomes in this vulnerable population.
Figure 5. Juvenile mTBI reduces dopamine receptor D2 expression in the striatum.
Dopamine receptor D2 (D2R) density is shown in the ipsilateral striatum as percent of the contralateral striatum density. Brain injured mice exhibit a significant reduction in D2R staining in the injured (ipsilateral) hemisphere. An asterisk (*) indicates a significant difference, p < 0.05. Shown are representative coronal sections through the striatum, boxed areas through the striatum are enlarged and shown below, scale bar = 100 μm.
Figure 6. Juvenile mTBI reduces cFos expression in urocortin-positive cells of the Edinger Westphal nucleus.
Shown are percent of UCN-positive cells (light brown staining) in the Edinger Westphal nucleus that co-express cFos (black staining) following a single administration of water or ethanol (4g/kg). Ethanol increased cFos expression in both sham and mTBI mice but brain injury significantly reduced the percent of activated UCNcells. An asterisk (*) indicates a significant difference between ethanol and water treatment, a pound sign (#) indicates a significant difference from the sham+ethanol group, p < 0.05. Scale bar = 50μm.
Acknowledgments
The authors are grateful to undergraduate research assistants Joseph Abraham, Samuel Nicholson, Timothy ED Corrigan, McKenna Guilds, Lauren Martin, Ruhee Patel, and Katarina Schneiderman. We would also like to thank Dr. Howard Gu and Dr. Georgia Bishop for assistance and guidance throughout this project.
Funding Sources
This work was supported by the Huron Foundation and the Ohio State University Wexner Medical Center Neuroscience Program. Additional support for behavioral experiments was provided by the National Institutes of Health (NINDS NS045758).
Abbreviations
- D2R
dopamine D2 receptor
- DAT
dopamine transporter
- mTBI
mild traumatic brain injury
- TH
tyrosine hydroxylase
- UCN
urocortin
- VTA
ventral tegmental area
References
- Ajao DO, Pop V, Kamper JE, Adami A, Rudobeck E, Huang L, Vlkolinsky R, Hartman RE, Ashwal S, Obenaus A, Badaut J. Traumatic brain injury in young rats leads to progressive behavioral deficits coincident with altered tissue properties in adulthood. J Neurotrauma. 2012;29:2060–2074. doi: 10.1089/neu.2011.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson V, Brown S, Newitt H, Hoile H. Educational, vocational, psychosocial, and quality-of-life outcomes for adult survivors of childhood traumatic brain injury. J Head Trauma Rehabil. 2009;24:303–312. doi: 10.1097/HTR.0b013e3181ada830. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Tsivkovskaia NO, Ryabinin AE. Alcohol-induced c-Fos expression in the Edinger-Westphal nucleus: pharmacological and signal transduction mechanisms. J Pharmacol Exp Ther. 2002;302:516–524. doi: 10.1124/jpet.102.036046. [DOI] [PubMed] [Google Scholar]
- Bales JW, Wagner AK, Kline AE, Dixon CE. Persistent cognitive dysfunction after traumatic brain injury: A dopamine hypothesis. Neurosci Biobehav Rev. 2009;33:981–1003. doi: 10.1016/j.neubiorev.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombardier CH, Rimmele CT, Zintel H. The magnitude and correlates of alcohol and drug use before traumatic brain injury. Arch Phys Med Rehabil. 2002;83:1765–1773. doi: 10.1053/apmr.2002.36085. [DOI] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ. Amphetamine mechanisms and actions at the dopamine terminal revisited. J Neurosci. 2013;33:8923–8925. doi: 10.1523/JNEUROSCI.1033-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantagrel S, Gressens P, Bodard S, Suc AL, Laugier J, Guilloteau D, Chalon S. mRNA D(2) dopaminergic receptor expression after hypoxia-ischemia in rat immature brain. Biol Neonate. 2001;80:68–73. doi: 10.1159/000047123. [DOI] [PubMed] [Google Scholar]
- Carvey PM, Chang Q, Lipton JW, Ling Z. Prenatal exposure to the bacteriotoxin lipopolysaccharide leads to long-term losses of dopamine neurons in offspring: a potential, new model of Parkinson’s disease. Front Biosci. 2003;8:s826–837. doi: 10.2741/1158. [DOI] [PubMed] [Google Scholar]
- Cattelani R, Lombardi F, Brianti R, Mazzucchi A. Traumatic brain injury in childhood: intellectual, behavioural and social outcome into adulthood. Brain Injury. 1998;12:283–296. doi: 10.1080/026990598122584. [DOI] [PubMed] [Google Scholar]
- Ceylan-Isik AF, McBride SM, Ren J. Sex difference in alcoholism: who is at a greater risk for development of alcoholic complication? Life Sci. 2010;87:133–138. doi: 10.1016/j.lfs.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comstock RD, Currie DW, Pierpoint LA, Grubenhoff JA, Fields SK. An Evidence-Based Discussion of Heading the Ball and Concussions in High School Soccer. JAMA Pediatr. 2015;169:830–837. doi: 10.1001/jamapediatrics.2015.1062. [DOI] [PubMed] [Google Scholar]
- Corrigan JD, Bogner J, Mellick D, Bushnik T, Dams-O’Connor K, Hammond FM, Hart T, Kolakowsky-Hayner S. Prior history of traumatic brain injury among persons in the Traumatic Brain Injury Model Systems National Database. Archives of Physical Medicine and Rehabilitation. 2013;94:1940–1950. doi: 10.1016/j.apmr.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covassin T, Swanik CB, Sachs ML. Sex Differences and the Incidence of Concussions Among Collegiate Athletes. J Athl Train. 2003;38:238–244. [PMC free article] [PubMed] [Google Scholar]
- Dagher A, Robbins TW. Personality, addiction, dopamine: insights from Parkinson’s disease. Neuron. 2009;61:502–510. doi: 10.1016/j.neuron.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Lääne K, Peña Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnemiller E, Brenneis C, Wissel J, Scherfler C, Poewe W, Riccabona G, Wenning GK. Impaired dopaminergic neurotransmission in patients with traumatic brain injury: a SPECT study using 123I-beta-CIT and 123I-IBZM. Eur J Nucl Med. 2000;27:1410–1414. doi: 10.1007/s002590000308. [DOI] [PubMed] [Google Scholar]
- Farace E, Alves WM. Do women fare worse: a metaanalysis of gender differences in traumatic brain injury outcome. J Neurosurg. 2000;93:539–545. doi: 10.3171/jns.2000.93.4.0539. [DOI] [PubMed] [Google Scholar]
- Farin A, Deutsch R, Biegon A, Marshall LF. Sex-related differences in patients with severe head injury: greater susceptibility to brain swelling in female patients 50 years of age and younger. J Neurosurg. 2003;98:32–36. doi: 10.3171/jns.2003.98.1.0032. [DOI] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald M, Coronado V. N.C.f.I.P.a.C, editor . Traumatic brain injury in the United States: Emergency department visits, hospitalizations and deaths 2002–2006. Atlanta (GA): Centers for Disease Control and Prevention; 2010. [Google Scholar]
- Fazel S, Bains P, Doll H. Substance abuse and dependence in prisoners: a systematic review. Addiction. 2006;101:181–191. doi: 10.1111/j.1360-0443.2006.01316.x. [DOI] [PubMed] [Google Scholar]
- Galvan A. Adolescent development of the reward system. Front Hum Neurosci. 2010;4:6. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino WJ, Cocking DL, Kaur S, Cunningham CL, Ryabinin AE. Urocortin-1 within the centrally-projecting Edinger-Westphal nucleus is critical for ethanol preference. PLoS One. 2011;6:e26997. doi: 10.1371/journal.pone.0026997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold LH, Swerdlow NR, Koob GF. The role of mesolimbic dopamine in conditioned locomotion produced by amphetamine. Behav Neurosci. 1988;102:544–552. doi: 10.1037//0735-7044.102.4.544. [DOI] [PubMed] [Google Scholar]
- Guerri C, Pascual M. Mechanisms involved in the neurotoxic, cognitive, and neurobehavioral effects of alcohol consumption during adolescence. Alcohol. 2010;44:15–26. doi: 10.1016/j.alcohol.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Heinz A. Dopaminergic dysfunction in alcoholism and schizophrenia--psychopathological and behavioral correlates. Eur Psychiatry. 2002;17:9–16. doi: 10.1016/s0924-9338(02)00628-4. [DOI] [PubMed] [Google Scholar]
- Huang EY, Tsai TH, Kuo TT, Tsai JJ, Tsui PF, Chou YC, Ma HI, Chiang YH, Chen YH. Remote effects on the striatal dopamine system after fluid percussion injury. Behav Brain Res. 2014;267:156–172. doi: 10.1016/j.bbr.2014.03.033. [DOI] [PubMed] [Google Scholar]
- Hutson CB, Lazo CR, Mortazavi F, Giza CC, Hovda D, Chesselet MF. Traumatic brain injury in adult rats causes progressive nigrostriatal dopaminergic cell loss and enhanced vulnerability to the pesticide paraquat. J Neurotrauma. 2011;28:1783–1801. doi: 10.1089/neu.2010.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iravani MM, Kashefi K, Mander P, Rose S, Jenner P. Involvement of inducible nitric oxide synthase in inflammation-induced dopaminergic neurodegeneration. Neuroscience. 2002;110:49–58. doi: 10.1016/s0306-4522(01)00562-0. [DOI] [PubMed] [Google Scholar]
- Jonsson CA, Horneman G, Emanuelson I. Neuropsychological progress during 14 years after severe traumatic brain injury in childhood and adolescence. Brain Injury. 2004;18:921–934. doi: 10.1080/02699050410001671900. [DOI] [PubMed] [Google Scholar]
- Karelina K, Gaier KR, Prabhu M, Wenger V, Corrigan TE, Weil ZM. Binge ethanol in adulthood exacerbates negative outcomes following juvenile traumatic brain injury. Brain Behav Immun. 2017;60:304–311. doi: 10.1016/j.bbi.2016.11.009. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Iversen SD. Selective 6OHDA-induced destruction of mesolimbic dopamine neurons: abolition of psychostimulant-induced locomotor activity in rats. Eur J Pharmacol. 1976;40:45–56. doi: 10.1016/0014-2999(76)90352-6. [DOI] [PubMed] [Google Scholar]
- Kobori N, Clifton GL, Dash PK. Enhanced catecholamine synthesis in the prefrontal cortex after traumatic brain injury: implications for prefrontal dysfunction. J Neurotrauma. 2006;23:1094–1102. doi: 10.1089/neu.2006.23.1094. [DOI] [PubMed] [Google Scholar]
- Lincoln AE, Caswell SV, Almquist JL, Dunn RE, Norris JB, Hinton RY. Trends in concussion incidence in high school sports: a prospective 11-year study. Am J Sports Med. 2011;39:958–963. doi: 10.1177/0363546510392326. [DOI] [PubMed] [Google Scholar]
- Massucci JL, Kline AE, Ma X, Zafonte RD, Dixon CE. Time dependent alterations in dopamine tissue levels and metabolism after experimental traumatic brain injury in rats. Neurosci Lett. 2004;372:127–131. doi: 10.1016/j.neulet.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Melis M, Spiga S, Diana M. The dopamine hypothesis of drug addiction: hypodopaminergic state. Int Rev Neurobiol. 2005;63:101–154. doi: 10.1016/S0074-7742(05)63005-X. [DOI] [PubMed] [Google Scholar]
- Morean ME, Corbin WR. Subjective response to alcohol: a critical review of the literature. Alcohol Clin Exp Res. 2010;34:385–395. doi: 10.1111/j.1530-0277.2009.01103.x. [DOI] [PubMed] [Google Scholar]
- Nielsen JA, Duda NJ, Mokler DJ, Moore KE. Self-administration of central stimulants by rats: a comparison of the effects of d-amphetamine, methylphenidate and McNeil 4612. Pharmacol Biochem Behav. 1984;20:227–232. doi: 10.1016/0091-3057(84)90247-8. [DOI] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse: Results from the epidemiologic catchment area (eca) study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Roberts DC, Koob GF, Klonoff P, Fibiger HC. Extinction and recovery of cocaine self-administration following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1980;12:781–787. doi: 10.1016/0091-3057(80)90166-5. [DOI] [PubMed] [Google Scholar]
- Sami MB, Faruqui R. The effectiveness of dopamine agonists for treatment of neuropsychiatric symptoms post brain injury and stroke. Acta Neuropsychiatr. 2015;27:317–326. doi: 10.1017/neu.2015.17. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K, Nolte-Zenker B, Patzer J, Bauer M, Schmidt LG, Heinz A. Psychopathological correlates of reduced dopamine receptor sensitivity in depression, schizophrenia, and opiate and alcohol dependence. Pharmacopsychiatry. 2001;34:66–72. doi: 10.1055/s-2001-15184. [DOI] [PubMed] [Google Scholar]
- Scott C, McKinlay A, McLellan T, Britt E, Grace R, MacFarlane M. A comparison of adult outcomes for males compared to females following pediatric traumatic brain injury. Neuropsychology. 2015;29:501–508. doi: 10.1037/neu0000074. [DOI] [PubMed] [Google Scholar]
- Shimada R, Abe K, Furutani R, Kibayashi K. Changes in dopamine transporter expression in the midbrain following traumatic brain injury: an immunohistochemical and in situ hybridization study in a mouse model. Neurol Res. 2014;36:239–246. doi: 10.1179/1743132813Y.0000000289. [DOI] [PubMed] [Google Scholar]
- Shin SS, Dixon CE. Oral fish oil restores striatal dopamine release after traumatic brain injury. Neurosci Lett. 2011;496:168–171. doi: 10.1016/j.neulet.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MA, Rudnik-Levin F. Attention deficit/hyperactivity disorder and substance abuse. Diagnostic and therapeutic considerations. Ann N Y Acad Sci. 2001;931:251–270. doi: 10.1111/j.1749-6632.2001.tb05783.x. [DOI] [PubMed] [Google Scholar]
- Türker T, Sodmann R, Goebel U, Jatzke S, Knapp M, Lesch KP, Schuster R, Schütz H, Weiler G, Stöber G. High ethanol tolerance in young adults is associated with the low-activity variant of the promoter of the human serotonin transporter gene. Neurosci Lett. 1998;248:147–150. doi: 10.1016/s0304-3940(98)00347-4. [DOI] [PubMed] [Google Scholar]
- van Bregt DR, Thomas TC, Hinzman JM, Cao T, Liu M, Bing G, Gerhardt GA, Pauly JR, Lifshitz J. Substantia nigra vulnerability after a single moderate diffuse brain injury in the rat. Exp Neurol. 2012;234:8–19. doi: 10.1016/j.expneurol.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P, Stewart J. The effect of dopamine receptor blockade on the development of sensitization to the locomotor activating effects of amphetamine and morphine. Brain Res. 1989;499:108–120. doi: 10.1016/0006-8993(89)91140-2. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Bayir H, Ren D, Puccio A, Zafonte RD, Kochanek PM. Relationships between cerebrospinal fluid markers of excitotoxicity, ischemia, and oxidative damage after severe TBI: the impact of gender, age, and hypothermia. J Neurotrauma. 2004;21:125–136. doi: 10.1089/089771504322778596. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Scanlon JM, Becker CR, Ritter AC, Niyonkuru C, Dixon CE, Conley YP, Price JC. The influence of genetic variants on striatal dopamine transporter and D2 receptor binding after TBI. J Cereb Blood Flow Metab. 2014;34:1328–1339. doi: 10.1038/jcbfm.2014.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AK, Sokoloski JE, Ren D, Chen X, Khan AS, Zafonte RD, Michael AC, Dixon CE. Controlled cortical impact injury affects dopaminergic transmission in the rat striatum. J Neurochem. 2005;95:457–465. doi: 10.1111/j.1471-4159.2005.03382.x. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Luciana M. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neurosci Biobehav Rev. 2010;34:631–648. doi: 10.1016/j.neubiorev.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil ZM, Corrigan JD, Karelina K. Alcohol abuse after traumatic brain injury: Experimental and clinical evidence. Neurosci Biobehav Rev. 2016a;62:89–99. doi: 10.1016/j.neubiorev.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Weil ZM, Karelina K, Gaier KR, Corrigan TE, Corrigan JD. Juvenile Traumatic Brain Injury Increases Alcohol Consumption and Reward in Female Mice. J Neurotrauma. 2016b;33:895–903. doi: 10.1089/neu.2015.3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A, Castle IJ, Chen CM, Shirley M, Roach D, Hingson R. Converging Patterns of Alcohol Use and Related Outcomes Among Females and Males in the United States, 2002 to 2012. Alcohol Clin Exp Res. 2015;39:1712–1726. doi: 10.1111/acer.12815. [DOI] [PubMed] [Google Scholar]
- Yan HQ, Kline AE, Ma X, Hooghe-Peters EL, Marion DW, Dixon CE. Tyrosine hydroxylase, but not dopamine beta-hydroxylase, is increased in rat frontal cortex after traumatic brain injury. Neuroreport. 2001;12:2323–2327. doi: 10.1097/00001756-200108080-00009. [DOI] [PubMed] [Google Scholar]
- Yan HQ, Kline AE, Ma X, Li Y, Dixon CE. Traumatic brain injury reduces dopamine transporter protein expression in the rat frontal cortex. Neuroreport. 2002;13:1899–1901. doi: 10.1097/00001756-200210280-00013. [DOI] [PubMed] [Google Scholar]
- Yan HQ, Ma X, Chen X, Li Y, Shao L, Dixon CE. Delayed increase of tyrosine hydroxylase expression in rat nigrostriatal system after traumatic brain injury. Brain Res. 2007;1134:171–179. doi: 10.1016/j.brainres.2006.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZJ, Torbey M, Li X, Bernardy J, Golden WC, Martin LJ, Koehler RC. Dopamine receptor modulation of hypoxic-ischemic neuronal injury in striatum of newborn piglets. J Cereb Blood Flow Metab. 2007;27:1339–1351. doi: 10.1038/sj.jcbfm.9600440. [DOI] [PMC free article] [PubMed] [Google Scholar]