Abstract
Cell detachment from the extracellular matrix triggers anoikis. Disseminated tumor cells must adapt to survive matrix deprivation, while still retaining the ability to attach at secondary sites and reinitiate cell division. In this study, we elucidate mechanisms that enable reversible matrix attachment by breast cancer cells. Matrix deprival triggered AMPK activity and concomitantly inhibited AKT activity by upregulating the Akt phosphatase PHLPP2. The resultant pAMPKhigh/pAktlow state was critical for cell survival in suspension, as PHLPP2 silencing also increased anoikis while impairing autophagy and metastasis. In contrast, matrix reattachment led to Akt-mediated AMPK inactivation via PP2C-α-mediated restoration of the pAkthigh/pAMPKlow state. Clinical specimens of primary and metastatic breast cancer displayed an Akt-associated gene expression signature, whereas circulating breast tumor cells displayed an elevated AMPK-dependent gene expression signature. Our work establishes a double-negative feedback loop between Akt and AMPK to control the switch between matrix-attached and matrix-detached states needed to coordinate cell growth and survival during metastasis.
Significance
These findings reveal a molecular switch that regulates cancer cell survival during metastatic dissemination, with the potential to identify targets to prevent metastasis in breast cancer.
Introduction
Metastasis accounts for the vast majority of cancer-associated deaths. The metastatic process involves detachment of cells from the primary site of tumor initiation, entry into the blood stream or the lymphatics, exit from the circulation and reattachment at distant sites to spawn metastatic growth (1). Integrins mediate cell adhesion to the extracellular matrix that provides growth and survival signals (2), whereas matrix deprivation leads to programmed cell death termed "anoikis" (3). Therefore, detached tumor cells must develop resistance to anoikis, while retaining the ability to reattach and grow at a distal site to spawn a successful metastasis. Yet, little is known about cellular signaling pathways that coordinate cell growth and stress-survival signals during the attachment–detachment cascade of metastatic colonization.
The serine/threonine protein kinase Akt (also known as PKB) regulates several cellular processes, including proliferation, survival, and metabolism, and plays a major role in tumor progression (4). Akt is recruited to the plasma membrane by binding to PIP3 and is subsequently phosphorylated by PDK1 and mTOR complex 2 (mTORC2) at T308 and S473, respectively, leading to its full activation. Conversely, Akt signaling is attenuated by dephosphorylation of these sites by protein phosphatase 2A (PP2A) and pleckstrin homology domain leucine-rich repeat protein phosphatases (PHLPP 1 and 2; ref. 5). Upon activation by growth factor signaling, Akt promotes anabolic processes including lipid biosynthesis and protein translation, thus driving cell growth and proliferation.
In contrast, the AMP-activated protein kinase (AMPK) is activated under metabolically stressed conditions and brings about cellular homeostasis by switching on energy-generating catabolic processes like fatty acid oxidation and glycolysis, while inhibiting energy-consuming anabolic pathways including carbohydrate, lipid, and protein biosynthesis (6–8). AMPK is a heterotrimeric protein consisting of α, β, and γ subunits (encoded by α1, α2; β1, β2; and γ1, γ2, γ3). It is allosterically activated by AMP and positively regulated by phosphorylation of T172 residue by upstream kinases LKB1 and CaMKKβ, while negatively regulated by dephosphorylation (9, 10). Although considered a tumor suppressor owing to its growth retarding effects, recent studies have identified context specific protumorigenic roles for AMPK by promoting cell survival under glucose deprivation and hypoxia stress (11, 12).
Under matrix-deprivation stress, Akt activation is sufficient for anoikis resistance in immortalized MDCK cells (13). ErbB2-overexpressing breast cancer cells show increased dependence on Akt for anchorage-independent growth (14). In contrast, pharmacologic inhibition of the PI3K/Akt pathway failed to render T-47D breast cancer cells sensitive to anoikis (15). Thus, the role of Akt in anoikis resistance remains to be fully understood. On the other hand, recent work from our laboratory and that of others has shown matrix deprivation-triggered activation of AMPK and its critical role in anoikis resistance in breast cancer cells (16–18). Thus, independent studies have implicated Akt and AMPK in anoikis resistance, although they have opposing effects on cellular growth and metabolism.
Synergistic and antagonistic relationship between Akt and AMPK has been documented under different cellular contexts; however, little is known about their interplay in maintaining the adherent versus detached states of cells. Intriguingly, we show here that detachment-triggered AMPK concomitantly represses Akt activity. We identify a novel AMPK-mediated PHLPP2 upregulation that inactivates Akt to promote AMPK-induced autophagy and that inhibits anoikis in suspension. Finally, we show that matrix reattachment triggers Akt activity, which in turn represses AMPK through PP2C-α. Our data, thus, identify a novel, reciprocal, inhibitory relationship between AMPK and Akt that regulates adaptation to matrix detachment.
Materials and Methods
Primary cells and culture conditions
Primary breast tissues (cancer and adjacent normal) obtained from the Kidwai Memorial Institute of Oncology (KMIO), Bangalore, as per IRB and in compliance with ethical guidelines of KMIO and the Indian Institute of Science (IISc), were processed into single cells and cultured as described previously (16, 19) in serum-free media containing 10 ng/mL hEGF, 1 µg/mL hydrocortisone, 10 µg/mL insulin, 4 ng/mL heparin and B27. Single cells were seeded in regular TC plates for adherent culture or in ultralow attachment plates (Corning Inc.) for mammosphere culture (16).
Cell lines and cell culture conditions
Breast cancer cell lines MDA-MB-231, MCF7, BT474 (from ATCC in 2016, and validated by STR analysis); A549 and H460 (lung), LN229 (glioma), Hep3B (liver), and HeLa (cervical) cancer cell lines (obtained as kind gifts) were cultured in DMEM (Sigma-Aldrich) supplemented with 10% FBS containing penicillin and streptomycin, at 37°C and 5% CO2. Cell lines were used for experiments within 8 passages after thawing. For short-term suspension cultures of 8 hours and 10 minutes, cancer cells were seeded on dishes coated with 1 mg/mL poly-2-hydroxyethyl-methacrylate (Poly-HEMA; Sigma-Aldrich) dissolved in absolute ethanol. For 48 hours of suspension culture, cells were seeded on dishes coated with 1% noble agar (Sigma-Aldrich). Long-term anchorage-independent (AI) colony formation assay was undertaken by admixing 1 × 105 cancer cells either with a slurry of 1.5% methyl cellulose or in 0.3% soft agar, and layered over 0.6% noble agar. AI colonies from methyl cellulose were harvested for immunoblotting after 7 days or counted after 15 days from 15 random fields of 10× magnification in each 35-mm dish.
Plasmids, transfection, and generation of stable cell lines
GFP-HA-Akt-T308D S473D (#39536; originally submitted by Dr. Julian Downward), referred to as GFP Akt DD in figures, and myc AMPKα2 K45R (#15992; originally submitted by Dr. Morris Birnbaum), referred to as dominant-negative (DN) AMPK, were procured from Addgene. HA myr-Akt and GFP CA CaMKK were provided by Dr. Joseph Testa and Dr. Grahame D. Hardie, respectively, as kind gifts. shRNAs against PHLPP2 (RHS4531-EG23035) and the corresponding control nontargeting shRNA in pGIPZ vector (NT); and inducible shRNA against AMPKα2 (V2THS_57674) and the corresponding control empty pTRIPZ vector (EV) were procured from Dharmacon. Lipofectamine (Invitrogen) was used to transfect plasmid DNA into cells.
MDA-MB-231 cells stably expressing GFP-HA-Akt-T308D S473D were generated by transfection followed by FACS-based sorting for GFP-expressing cells; cells stably expressing HA myr-Akt were generated by cotransfecting a puromycin resistance plasmid at a 10:1 ratio followed by selection with puromycin (0.5 µg/mL) treatment. MDA-MB-231 cells stably expressing specific shRNAs were generated by selection with puromycin followed by sorting cells for high GFP (in case of plasmids in pGIPZ vector) or high RFP (in case of plasmids in pTRIPZ vector) expression.
siRNA oligos against Akt (targeting both isoforms Akt1 and Akt2 [6211 and 6510]) were purchased from Cell Signaling Technology and transfected using oligofectamine (Invitrogen).
Pharmacologic compounds
Pharmacologic compounds used in cell culture include the AMPK inhibitor 6-[4-(2-piperidin-1-ylethoxy-phenyl)]-3-pyridin-4-yl-pyrrazolo [1, 5-a]-pyrimidine (compound C; Cat. No. 171260; 10 µmol/L; referred to as CC in figures), PI3K/Akt inhibitor LY294002 (Cat. No. 440202; 20 µmol/L; referred to as LY in figures), Akt inhibitor Akti VIII (Cat. No. 124018; 10 µmol/L), and MG132 (Cat. No. 474790; 10 µmol/L) from Calbiochem (Merck), AMPK activator A-769662 (100 µmol/L; referred to as A76 in figures) from the University of Dundee, Scotland, cycloheximide (Cat. No. C7698; 0.1 mg/mL; referred to as CHX) and lysosomal inhibitor chloroquine (Cat. No. C6628; 50 µmol/L; referred to as CQ in figures) from Sigma-Aldrich. Dimethyl sulfoxide (DMSO) was used as vehicle control for all compounds except cycloheximide, which was dissolved in water. In experiments carried out in suspension, adherent cells were pretreated with the respective chemicals for 2 hours prior to being subjected to suspension in the continued presence of the chemicals.
Immunoblotting and immunoprecipitation
For immunoblotting, whole-cell lysates were prepared using lysis buffer containing 50 mmol/L Tris, 50 mmol/L sodium fluoride, 5 mmol/L sodium pyrophosphate, 1 mmol/L EDTA, 1 mmol/L EGTA, 1% Triton X-100, 0.2 mg/mL DTT, 0.2 mg/mL benzamidine, and protease inhibitor (Roche) on ice. Protein concentration was estimated using Bradford method, equal quantity of protein (30–50 µg) per lane was resolved by SDS-PAGE after boiling with sample buffer for 3 minutes at 100°C. Proteins were transferred to PVDF membrane and probed with appropriate antibodies. The membrane was incubated overnight with primary antibody at 4°C followed by washes in TBST, and incubated for 2 hours with HRP-conjugated secondary antibody at room temperature. Chemiluminescence (using ECL substrate from Thermo Fisher Scientific) was used to visualize protein bands. The membranes were stripped using 1 mol/L Tris buffer (pH 6.8) containing 2% sodium dodecyl sulfate and 0.7% β-mercaptoethanol and then used for repeated probing with subsequent antibodies, following washes and blocking.
Multipanel blots were assembled by reprobing the same blot for successive antibodies or by running the same lysate multiple times; α-tubulin served as loading control for each run. Representative immunoblots show data consistent with minimally three independent experiments. Densitometric analyses of Western blots were performed using the Multigauge V2.3 software. Relative protein levels were quantified by normalizing to loading control. Primary antibodies used in the study were against pAMPKαT172, pACCS79, pAktS473, pAktT308, pPRAS40T246, total AMPKα (that recognizes both AMPKα 1 and 2 isoforms), AMPKα2, ACC, Akt, PRAS40, PP2C-α, myc tag, HA tag, cleaved caspase-3, LC3B, GFP, Ubiquitin (Ub), IgG (Cell Signaling Technology), α Tubulin (Calbiochem), PHLPP2 (Abcam), PP2A-Aα/β, and PPM1E (Santa Cruz Biotechnology). HRP-conjugated anti-mouse and anti-rabbit antibodies were obtained from Jackson ImmunoResearch Laboratories. For immunoprecipitation experiments, cells were lysed in buffer containing 20 mmol/L Tris (pH 8), 137 mmol/L NaCl, 10% glycerol, and 1% Nonidet P-40, supplemented with protease inhibitors, sodium fluoride, and sodium orthovanadate. Cellular protein (1 mg) was incubated with one of control IgG, anti-PP2A-Aα/β, anti-PPM1E, or anti-PP2C-α antibody and 15 µL of protein-A sepharose beads for 12 hours at 4°C on end-on rocker. The immune complexes were precipitated by centrifugation at 1300 rpm for 5 minutes at 4°C. The precipitates were washed with Nonidet P-40 lysis buffer supplemented with 1 mol/L NaCl. Immune complexes were resuspended in 50 µL of sample buffer and analyzed by immunoblotting.
Caspase-3 activity assay
Caspase-3 activity was measured by using a CaspGLOW Red Active caspase-3 activity kit from Bio Vision (K-193) as per the manufacturer's instructions. Briefly, 1 × 106 cells were stained with 1 µL of Red-DEVD-FMK for 30 minutes at 37°C and 5% CO2. Cells were washed thrice using wash buffer and analysis was done using BD FACS-CantoII (Becton & Dickinson) equipped with a 488-nm Coherent Sapphire Solid State laser. Red (564–606 nm) fluorescence emission from 104 cells was measured after illumination with blue (488 nm) excitation light. Data were analyzed using Summit software V5.2.1.12465.
Acridine orange assay for autophagy
Autophagy is characterized by formation of acidic vesicular organelles (AVO). To detect AVOs, acridine orange (AO) was used. Acidic compartment causes accumulation of AO, which gives bright red fluorescence upon excitation by 488-nm laser. Measurement of red fluorescence is proportional to increase in AVOs (20).
Cells (2 × 105) were subjected to suspension culture for 48 hours, after which they were trypsinized, counted, and 1 × 105 cells were stained with acridine orange (1 µg/mL) for 15 minutes at 37°C in DMEM + 10% FBS. Post staining, cells were washed thrice in PBS. The cells were analyzed in BD FACS CantoII (Becton & Dickinson) equipped with a blue (488 nm) Coherent Sapphire Solid State laser. Red (564–606 nm) fluorescence emission from 104 cells was measured. Data were analyzed using Summit V5.2.1.12465 software.
Phosphatase activity assay
For phosphatase activity assays, cell lysate was prepared in phosphatase activity buffer (20 mmol/L imidazole–HCl, pH 7.0, 2 mmol/L EDTA, 2 mmol/L EGTA, and protease inhibitor cocktail). PP2A-Aα/β, PPM1E, and PP2C-α activities were measured using Ser/Thr phosphatase assay kit (Cat. No. 17-127; Millipore) according to the manufacturer's protocol. Briefly, 500 µg of total protein lysate prepared from cells under attached (Att), suspension (Sus), and reattached (Re-Att) conditions was independently immunoprecipitated with antibodies against PP2A-Aα/β, PPM1E, or PP2C-α and the activity of the phosphatases was estimated colorimetrically by measuring the released phosphate from threonine phosphopeptide (K-R-pT-I-R-R) with Malachite Green Phosphate detection solution (absorbance measured at 640 nm).
Microarray and data analysis
MDA-MB-231 cells cultured in attached or suspension conditions for 24 hours, and MDA-MB-231 cells stably expressing shAMPKα2, shPHLPP2, and GFP Akt DD cultured in suspension were harvested for microarray experiments. Total cellular RNA was isolated using an RNeasy minikit (Cat No.74104; Qiagen) according to the manufacturer's protocol. The RNA samples were labeled with Cy3 using an Agilent's Quick-Amp labeling Kit (Cat. No. 5190-0442) and subjected to hybridization on Agilent's In situ Hybridization Kit (Cat No.5188-5242). For analysis, a list of signature genes was taken from AmiGO Gene Ontology Consortium (http://amigo.geneontology.org/amigo), Profiler PCR Array list Qiagen, and further validated by KEGG (http://www.genome.jp/kegg/pathway.html) or PubMed (https://www.ncbi.nlm.nih.gov/pubmed/). Heat map of individual and combined data sets was generated using an online tool Morpheus (https://software.broadinstitute.org/morpheus/#). Unsupervised clustering was performed to obtain function based gene expression signature. Genes of interest are color coded; "red" in the heat map represents upregulated genes, whereas "green" represents downregulated genes.
Microarray raw data for primary breast tumor, circulating tumor cells (CTC), and metastases were taken from Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/), with GEO IDs GSE43837, GSE99394, and GSE56493. These raw data files were processed and expression values were calculated in log2 scale. Further, the data were normalized using Z-score. Microarray for GSE43837 set was performed on GPL1352 [U133_X3P] Affymetrix Human X3P Array platform, GSE99394 set was performed on [HTA-2_0] Affymetrix Human Transcriptome Array 2.0 [transcript (gene) version], and GSE56493 set was performed on [U133_X3P] Affymetrix Human X3P ArrayRosetta/Merck Human RSTA Custom Affymetrix 2.0 microarray [HuRSTA-2a520709] platform. Official gene symbols for the corresponding probes were retrieved using DAVID (https://david.ncifcrf.gov/summary.jsp) and expression array platform. Heat maps of combined datasets were generated using Morpheus (https://software.broadinstitute.org/morpheus/#). Semisupervised clustering was performed to obtain function-based gene expression signatures. Box plots were plotted using GraphPad Prism 5.0 software.
Metastasis assay in mice
All animal experiments were reviewed and approved by the Institutional Animal Ethics committee of IISc, Bangalore. Cells (2 × 106) were resuspended in 50 µL DMEM and injected into the lateral tail vein of 5-week-old female NOD-SCID mice. Mice were sacrificed after 2 months of injection, and lungs and liver were dissected to check for metastatic nodules.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 5.0 software using Student t test. All data are presented as mean ± SEM, where *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Results
Matrix deprivation leads to Akt inactivation concomitant to AMPK activation
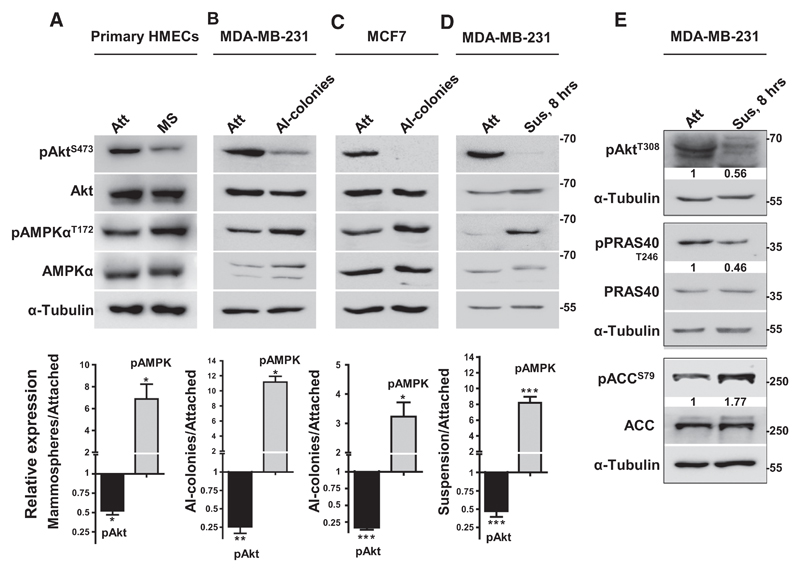
To begin to understand the interplay between Akt and AMPK during the attachment–detachment cascade of metastasis, we first investigated their relative activities under these conditions in breast cells. For this, we measured phosphorylation of Akt at S473 (pAktS473) and phosphorylation of AMPKα at T172 (pAMPKαT172) as surrogate measures of their activities (5, 21), respectively. We recently reported AMPK activation in mammospheres formed by normal HMECs and spheroids formed by breast cancer cells (16). Therefore, we first investigated the status of Akt activity in these 3-dimensional spheroids. Interestingly, concomitant with increase in the levels of pAMPKαT172, our study revealed a significant reduction in the levels of pAktS473 in anchorage-independent spheroids generated by HMECs (Fig. 1A), breast cancer cell lines MDA-MB-231 and MCF7 (Figs. 1B and C), and primary breast cancer-derived cells (Supplementary Fig. S1A), compared with their respective adherently growing cultures. The levels of total Akt and total AMPKα proteins remained unchanged between these two conditions in all the cell types.
Figure 1.
Matrix deprivation promotes Akt inactivation concomitant with AMPK activation. Representative immunoblots of the following cell lysates were probed for specified proteins. A, Freshly isolated HMECs cultured in attached condition (Att) or as floating mammospheres (MS) in ultralow-attachment plates for 7 days. B–C, MDA-MB-231 (B) and MCF7 (C) cells cultured in attached condition (Att) or as anchorage-independent spheroids (AI-spheroids) in methylcellulose for 7 days. D–E, MDA-MB-231 cells cultured in attached condition (Att) or subjected to suspension (Sus) for 8 hours on poly-HEMA-coated plates. Graphs represent densitometric quantification of immunoblots; error bars, mean ± SEM; n = 3.
Consistent with mammospheres, subjecting MDA-MB-231 cells to matrix detachment for 8 hours also resulted in a significant decrease in pAktS473 levels concomitant with increase in pAMPKαT172 levels (Fig. 1D). Further, phosphorylation of Akt at T308, which contributes to full Akt activation, was also reduced in suspension (Fig. 1E). In keeping with their active phosphorylation status, we also observed a decrease in the phosphorylation of PRAS40, an Akt substrate (22), and an increase in the phosphorylation of ACC, an AMPK substrate (Fig. 1E; Supplementary Fig. S1B; ref. 23). We obtained similar results as early as 10 minutes of suspension culture (Supplementary Fig. S1B). In vitro kinase assays further substantiated the reduction in Akt activity and increase in AMPK activity in suspension (Supplementary Fig. S1C). We also observed elevated pAMPKαT172 and reduced pAktS473 levels in several other matrix-deprived cancer cell lines of different epithelial origin (Supplementary Fig. S1D). Together, these data revealed reciprocal regulation of AMPK and Akt activities between matrix-attached and matrix-detached conditions, resulting in a pAkthigh/pAMPKlow state in adherently growing cells, whereas a pAMPKhigh/pAktlow state in matrix-detached cells.
Akt repression in suspension is vital for anoikis resistance
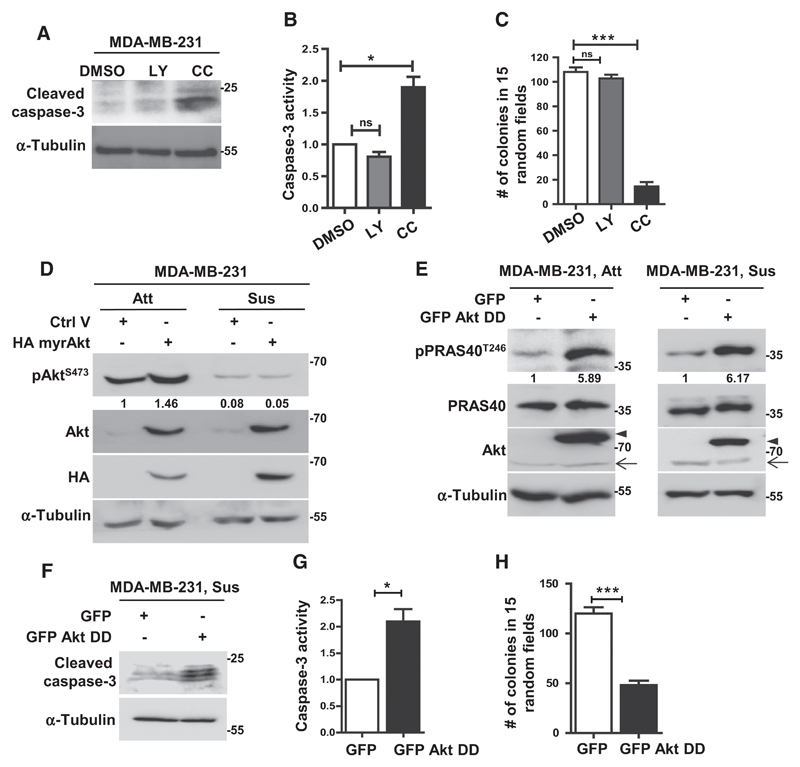
Intrigued by the reduction in Akt activity in detached breast cancer cells, contrary to its generally accepted role in anoikis resistance, we examined the relative functional roles of Akt and AMPK in detached cells. Treatment of MDA-MB-231 cells with the PI3K inhibitor LY294002 led to a decrease in pAktS473 levels (Supplementary Fig. S2A) while treatment with AMPK inhibitor compound C (24) led to a decrease in pACCS79 levels (Supplementary Fig. S2B), confirming the efficacies of these pharmacologic agents. Interestingly, inhibition of AMPK, but not Akt, increased anoikis as revealed by elevated levels of cleaved caspase-3 as well as increase in caspase-3 enzymatic activity in compound C treated cells (Fig. 2A and B). We obtained similar results in MCF7 breast cancer cells also (Supplementary Fig. S2C). Consistent with these observations, we found that inhibition of AMPK, but not Akt, abrogated anchorage-independent colony formation in MDA-MB-231, MCF7, and BT474 cells (Fig. 2C; Supplementary Fig. S2D and S2E). Interestingly, our observations in breast cancer cell lines also held true in HMEC-derived mammospheres, wherein again inhibition or knockdown of Akt failed to affect mammosphere formation (Supplementary Fig. S2F and S2G).
Figure 2.
Enforced Akt activation promotes anoikis. A–C, MDA-MB-231 cells treated with DMSO, LY294002 (LY), or compound C (CC) were subjected to 48 hours of suspension and harvested for immunoblotting (A) and caspase-3 activity assay (B); n = 3. Treated cells were subjected to colony formation in methylcellulose for 15 days (C); n = 4. Error bars, mean ± SEM. D and E, Immunoblot analyses of MDA-MB-231 cells stably expressing control empty vector or HA myr-Akt (D), and control vector (expressing GFP) or GFP-tagged HA-Akt-T308D S473D (GFP Akt DD; E) cultured in adherent (Att) or suspension (Sus) condition; n = 3. F–H, After 48 hours of suspension, MDA-MB-231 cells stably expressing control vector (expressing GFP) or GFP Akt DD were subjected to immunoblotting for cleaved caspase-3 (F) and caspase-3 activity assay (G); n = 3. Cells were subjected to colony formation for 15 days (H); error bars, mean ± SEM of two experiments with three dishes each.
Following this, we sought if it is necessary to maintain reduced Akt activity for stress survival in suspension. To address this, we evaluated the effects of forced Akt activation on anoikis by stably expressing a constitutively active HA myr-Akt construct in MDA-MB-231 cells. Detection with antibodies against HA and total Akt confirmed exogenous protein expression (Fig. 2D). As expected, we observed increase in the levels of pAktS473 in these cells in adherent condition (Fig. 2D). To our surprise, however, when these cells were subjected to matrix deprivation, we failed to detect elevated pAktS473 levels (Fig. 2D). We obtained similar results in MCF7 cells transiently transfected with HA myr-Akt (Supplementary Fig. S2H), suggesting that myr-Akt is still susceptible to negative regulation under matrix deprivation.
One major means of negative regulation of Akt is by dephosphorylation (5). To circumvent this, we used constitutively active, GFP-tagged phosphomimetic HA-Akt-T308D S473D construct (25), which is refractory to the action of phosphatases, and confirmed exogenous expression (Fig. 2E). Further, its expression led to elevated Akt activity in adherent as well as matrix-deprived cells, as gauged by increased levels of pPRAS40T246, a downstream substrate of Akt (Fig. 2E). When these cells were subjected to suspension for 48 hours, to our surprise, we observed increased anoikis (Fig. 2F and G). Consistent with these data, overexpression of GFP-HA-Akt-T308D S473D also impaired anchorage-independent colony formation (Fig. 2H). We obtained similar results in MCF7 cells transiently transfected with GFP-HA-Akt-T308D S473D construct (Supplementary Fig. S2I and S2J), suggesting that under the stress of matrix deprivation, hyperactivation of Akt might be detrimental to cell survival.
AMPK-mediated stabilization of PHLPP2 promotes Akt inactivation in suspension
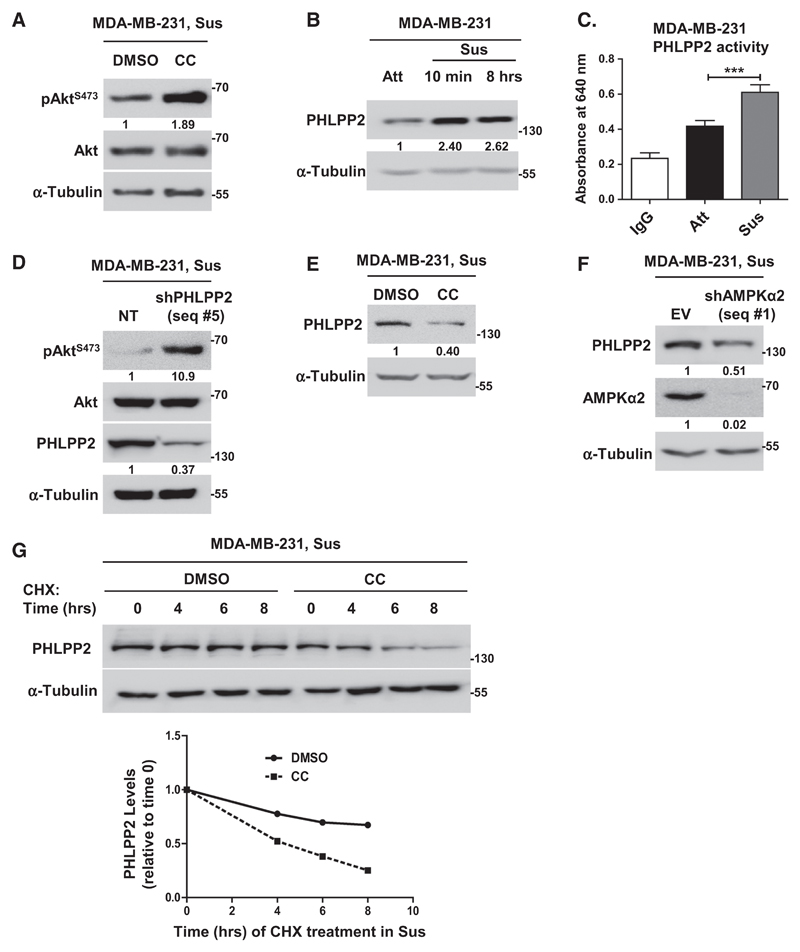
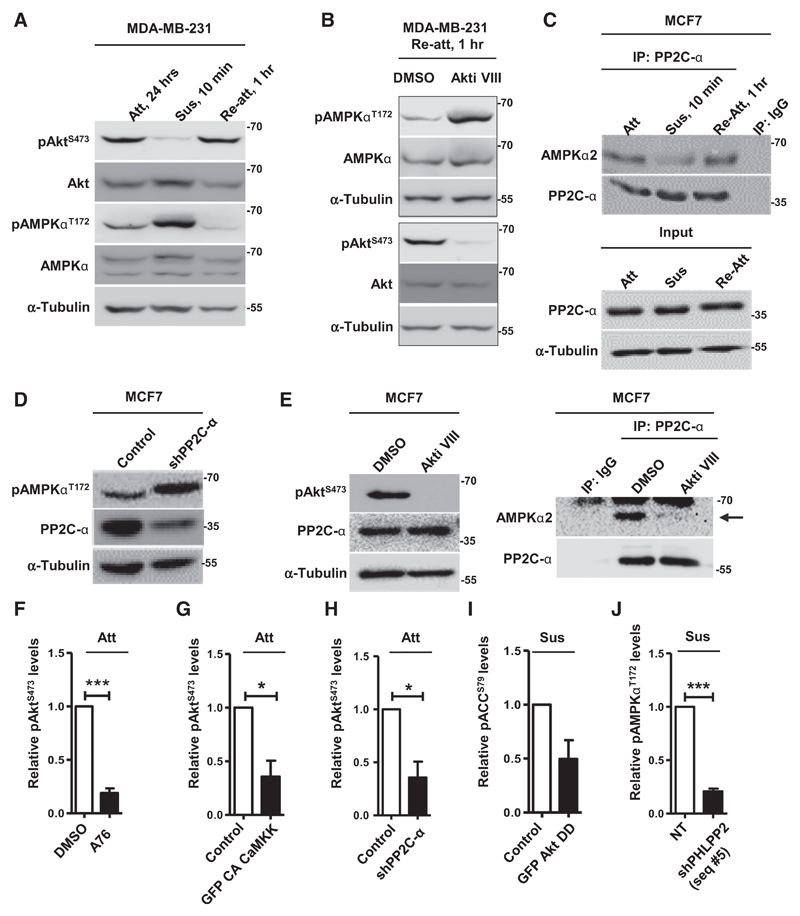
We next investigated the underlying mechanisms of Akt-dephosphorylation in suspension. Because matrix deprivation led to Akt dephosphorylation concomitant with AMPK activation, we investigated if AMPK might be directly involved in this process. Interestingly, inhibition of AMPK using compound C led to elevated pAktS473 levels in MDA-MB-231 (Fig. 3A), MCF7 (Supplementary Fig. S3A), and BT474 (Supplementary Fig. S3B) cells. Further, overexpression of DN AMPK (Supplementary Fig. S3C) or depletion of AMPK using shRNA (Supplementary Fig. S3D) also led to elevated pAktS473 levels in suspension, together revealing a direct role for AMPK in Akt dephosphorylation in matrix-deprived cells.
Figure 3.
AMPK promotes Akt dephosphorylation in suspension via PHLPP. A and B, Representative immunoblots of MDA-MB-231 cells treated with DMSO or compound C (CC) and subjected to suspension (Sus) for 8 hours (n = 5; A), and cells grown in adherent (Att) or suspension (Sus) condition for 10 minutes and 8 hours (n = 3; B). C, Phosphatase assay performed with immunoprecipitated PHLPP2; IgG was used as control; n = 4. Error bars, mean ± SEM. D–G, Representative immunoblots of MDA-MB-231 cells harvested under conditions detailed below. D, Cells stably expressing nontargeting shRNA (NT) or shPHLPP2 (seq #5) and subjected to suspension (Sus) for 8 hours; n = 4. E, Cells treated with DMSO or compound C (CC) were subjected to suspension (Sus) for 8 hours; n = 3. F, Adherent cells stably expressing pTRIPZ empty vector (EV) or shAMPKα2 (seq #1) were induced with doxycycline for 48 hours, followed by suspension for 8 hours; n = 3. G, Adherent cells pretreated with DMSO or AMPK inhibitor (CC) were treated with cycloheximide (CHX) for 20 minutes, followed by suspension culture (Sus) for indicated time points; n = 3. Graph represents quantification of PHLPP2. All values represent densitometric analyses of Western blots to quantify relative levels of specified proteins.
Our observation that matrix deprivation led to Akt dephosphorylation in cells stably overexpressing myr-Akt, but not in cells stably overexpressing the double phosphomimetic Akt DD mutant (Figs. 2D and E), suggested the possible involvement of phosphatases in this process. We, therefore, investigated the roles of the Akt phosphatases PP2A and PHLPP. Inhibition of PP2A with okadaic acid failed to restore Akt phosphorylation in matrix-detached cells (Supplementary Fig. S3E). Interestingly, we observed a significant increase in the protein levels (Fig. 3B) as well as activity (Fig. 3C) of PHLPP2, which has specificity for Akt1 (26), the major Akt isoform expressed by breast cancer cells (27), in matrix-deprived MDA-MB-231 cells. Further, knockdown of PHLPP2 with two independent shRNA sequences led to significant increase in the levels of pAktS473 in suspension (Figs. 3D; Supplementary Fig. S3F). In addition, shPHLPP2 cells also showed remarkable increase in the levels of pAktT308 in suspension (Supplementary Fig. S3G). These data suggested a role for PHLPP2 in Akt inactivation in suspension.
Thereafter, we gauged if AMPK is involved in the observed upregulation of PHLPP2. In the presence of AMPK inhibitor compound C (Fig. 3E), knockdown of AMPK (Fig. 3F) or overexpression of DN AMPK (Supplementary Fig. S3H), we observed reduced levels of PHLPP2 in suspension in MDA-MB-231 cells. Inhibition of AMPK in matrix-deprived MCF7 cells also led to a decrease in PHLPP2 levels, in parallel with an increase in pAktS473 levels (Supplementary Fig. S3A). Also, we observed decreased levels of PHLPP2 upon overexpression of DN AMPK in adherent MDA-MB-231 cells (Supplementary Fig. S3I) and in AMPKα–/– MEFs (Supplementary Fig. S3J). Consistent with these observations, pharmacologic activation of AMPK with A-769662 (Supplementary Fig. S3K), as well as genetic approach involving constitutively active CaMKK (Supplementary Fig. S3L), led to an increase in the levels of PHLPP2 in adherent cells. These data thus identified a novel positive regulation of PHLPP2 by AMPK.
We investigated potential mechanisms underlying AMPK-mediated upregulation of PHLPP2 in suspension. RT-PCR analyses revealed no significant change in the transcript levels of PHLPP2 between matrix-attached and detached cells (both in the presence and absence of AMPK inhibitor; Supplementary Fig. S4Ai). Further, we investigated the levels of miR-205, which has been reported to regulate PHLPP2 levels (28). Quantitative PCR analyses failed to detect significant change in the levels of miR-205 between adherent and matrix-deprived conditions in MDA-MB-231 and MCF7 cells (Supplementary Fig. S4Aii). Further, inhibition of AMPK also did not alter the levels of miR-205 in matrix-deprived cells (Supplementary Fig. S4Aii). Taken together, these observations were suggestive of possible posttranscriptional regulation of PHLPP2 by AMPK. A cycloheximide chase assay revealed a more rapid decrease in the protein levels of PHLPP2 in the presence of AMPK inhibitor (Fig. 3G). We obtained similar data with cells expressing shAMPKα2 (Supplementary Fig. S4B), suggesting that AMPK might promote PHLPP2 protein stabilization. However, MG132 failed to restore protein levels of PHLPP2 under AMPK inhibited condition (Supplementary Fig. S4C). Also, ubiquitination of PHLPP2 did not change between attached and matrix-deprived cells (Supplementary Fig. S4D), suggesting proteasome-independent mechanism of PHLPP2 stabilization. Interestingly, lysosomal inhibitors restored PHLPP2 protein levels under AMPK inhibited condition (Supplementary Fig. S4E), suggesting that AMPK might mediate PHLPP2 stability in detached cells by regulating lysosomal degradation.
PHLPP2 knockdown inhibits autophagy and promotes anoikis
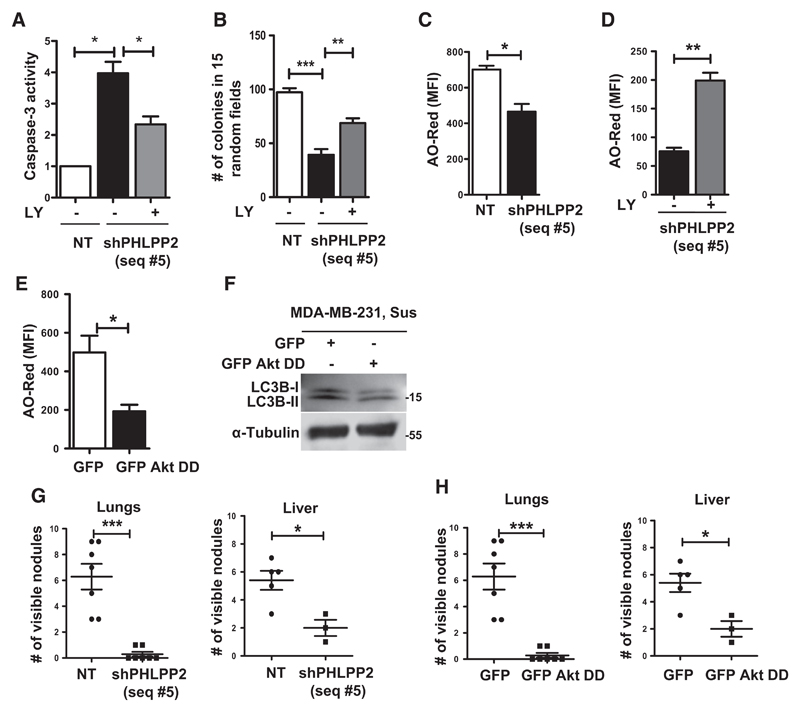
To further understand the biological significance of AMPK-mediated Akt inactivation through PHLPP2, we investigated the effects of PHLPP2 knockdown in anoikis and anchorage-independent colony formation. When subjected to suspension for 48 hours, we observed increased levels of cleaved caspase-3 (Supplementary Fig. S5A) as well as elevated caspase-3 enzyme activity (Fig. 4A) in shPHLPP2 (seq #5) cells as compared with control cells expressing nontargeting (NT) shRNA. Similar observations were made with an independent shPHLPP2 (seq #3) expressing cells (Supplementary Fig. S5B) as well as in MCF7 cells (Supplementary Figs. S5C and S5D). Consistent with this, PHLPP2 knockdown cells formed significantly reduced number of colonies in methylcellulose (Figs. 4B; Supplementary Fig. S5E and S5F). Moreover, the effects of PHLPP2 depletion on anoikis and anchorage-independent growth were partially reversed in the presence of LY294002 (Fig. 4A and B; Supplementary Fig. S5A), suggesting that PHLPP2 depletion promotes anoikis and impairs anchorage-independent growth, at least in part, through Akt activation.
Figure 4.
Downregulation of PHLPP2 promotes anoikis and impairs autophagy and metastasis. A–D, MDA-MB-231 cells stably expressing nontargeting shRNA (NT) or shPHLPP2 (seq #5) were subjected to 48 hours of suspension in the presence of DMSO or LY294002 (LY) and harvested for caspase-3 activity assay (A), subjected to soft-agar colony formation (B), and AO assay (C and D); n = 3. E and F, MDA-MB-231 cells stably expressing control GFP vector or GFP-tagged HA-Akt-T308D S473D (GFP Akt DD) were subjected to suspension (Sus) for 48 hours and harvested for AO assay (E) and immunoblotting (F); n = 3. G and H, Graphs represent number of lung (left) and liver (right) nodules following tail-vein injection of cells described below; each dot represents number of nodules in a single mouse: G, Nontargeting shRNA (NT) or shPHLPP2 (seq #5). H, Control GFP vector or GFP-tagged HA-Akt-T308D S473D (GFP Akt DD). Error bars, mean ± SEM in all experiments.
Recent studies have revealed the induction of autophagy as a critical survival strategy for anoikis resistance (29). Because Akt inhibits autophagy (30) and PHLPP2 knockdown cells showed Akt activation, we investigated if impairment of autophagy is responsible for anoikis in these cells. Compared with control NT cells, we observed reduced AO-Red fluorescence (Fig. 4C) and decreased levels of LC3B-II (Supplementary Fig. S5A) in MDA-MB-231 shPHLPP2 cells. We obtained similar results in MCF7 cells transiently transfected with shPHLPP2 construct (Supplementary Fig. S5G), indicating reduced autophagy upon PHLPP2 depletion. Furthermore, treatment with LY294002 restored autophagy induction in matrix-deprived shPHLPP2 cells (Fig. 4D; Supplementary Fig. S5A), suggesting that elevated Akt activity in these cells might be one possible reason for reduced autophagy. Consistent with this notion, we observed that MDA-MB-231 cells stably expressing GFP-HA-Akt-T308D S473D also displayed reduced AO-Red fluorescence and decreased levels of LC3B-II, indicating reduced autophagy (Fig. 4E and F). Thus, our observations suggest that AMPK-mediated suppression of Akt activity possibly facilitates anoikis resistance through facilitating the induction of autophagy.
Because anoikis resistance contributes to metastatic potential of cancer cells, we investigated the effects of PHLPP2 knockdown or overexpression of phosphomimetic Akt on metastasis. Tail-vein injections in nude mice revealed that MDA-MB-231 cells stably expressing shPHLPP2 (Fig. 4G; Supplementary Fig. S5H) or Akt-T308D S473D (Fig. 4H; Supplementary Fig. S5I) were impaired in their metastatic potential, thus corroborating our observations of increased anoikis and decreased anchorage-independent growth.
Adhesion-dependent double-negative feedback loop between AMPK and Akt
Our data thus far revealed that detachment-triggered AMPK leads to Akt inactivation, which is critical for stress survival under detachment. We reasoned that after exiting the circulation and following attachment at the new site, proliferative signals are critical for secondary tumor growth, which might require reactivation of Akt and inactivation of AMPK. Therefore, we investigated the status of AMPK and Akt signaling in cells subjected to reattachment following matrix detachment. Reattachment of MDA-MB-231 (Fig. 5A) and MCF7 (Supplementary Fig. S6A) cells following matrix deprivation quickly led to restoration of pAktS473 levels comparable with originally adherent cells. Concomitant with reattachment, we observed rapid dephosphorylation of AMPK, as early as 1 hour of reattachment, in both MDA-MB-231 (Fig. 5A) and MCF7 (Supplementary Fig. S6A) cells. Further, reattachment of cells in the presence of Akt inhibitor impaired the attachment-triggered dephosphorylation of AMPK in both MDA-MB-231 (Fig. 5B) and MCF7 (Supplementary Fig. S6B) cells, suggesting a direct role for Akt in AMPK dephosphorylation following adhesion.
Figure 5.
Matrix reattachment leads to Akt-dependent repression of AMPK activity. A–E, Representative immunoblots of cells harvested under conditions detailed below. A, MDA-MB-231 cells cultured in attachment (Att), suspension for 10 minutes (Sus), or allowed to reattach (Re-att); n = 3. B, MDA-MB-231 cells subjected to 10 minutes of suspension (Sus) were allowed to reattach (Re-att) in the presence of DMSO or Akt inhibitor; n = 3. C, Lysates of MCF7 cells cultured in conditions of attachment (Att), suspension (Sus), and reattachment (Re-att) were immunoprecipitated (IP) with control IgG or anti–PP2C-α antibodies and analyzed by immunoblotting. The input represents 2% of the whole-cell lysate used for each immunoprecipitation; n = 3. D, Adherent MCF7 cells transfected with vector control (pLKO.1) or shPP2C-α; n = 3. E, Lysates of adherent MCF7 cells treated with DMSO or Akt inhibitor were immunoprecipitated and analyzed as described in C; n = 3. F–J, Graphs represent densitometric quantification of immunoblots (Supplementary Figs. S7A-S7E) for relative phospho-protein levels from cells harvested under conditions detailed below: F, Adherent MDA-MB-231 cells treated with DMSO or AMPK activator (A76; also see Supplementary Fig. S7A); n = 3. G, Adherent MDA-MB-231 cells transfected with control vector pEGFP or GFP-tagged constitutively active CaMKK (GFP CA CaMKK; also see Supplementary Fig. S7B); n = 3. H, Adherent MCF7 cells transfected with vector control (pLKO.1) or shPP2C-α (also see Supplementary Fig. S7C); n = 2. I, MCF7 cells transfected with GFP or GFP-HA-Akt-T308D S473D and subjected to suspension (8 hours; also see Supplementary Fig. S7D); n = 2. J, MDA-MB-231 cells stably expressing nontargeting shRNA (NT) or shPHLPP2 (seq #5) and subjected to suspension (48 hours; also see Supplementary Fig. S7E); n = 3. Error bars, mean ± SEM.
We sought to investigate the role of phosphatases in the adhesion-mediated dephosphorylation of AMPK. We did not detect any differences in the levels or activities of PP2A-Aα/β, PPM1E, and the monomeric phosphatase PP2C-α, that have been identified as cellular AMPK phosphatases, between adhered and detached conditions (Supplementary Fig. S6C and S6D). In immunoprecipitation experiments using individual phosphatase-specific antibodies, we observed no change in the interaction between AMPK and PP2A-Aα/β or PPM1E between adherent and detached cells (Supplementary Fig. S6E and S6F). Interestingly, we observed a noticeable increase in the interaction between AMPK and PP2C-α under adhesion compared with detachment (Fig. 5C). A reverse pulldown using AMPKα2-specific antibodies further confirmed these results (Supplementary Fig. S6G). Consistent with this, PP2C-α knockdown led to increased pAMPKαT172 levels in adherent cells (Fig. 5D). Further, inhibition of Akt, while it did not cause a change in PP2C-α activity (Supplementary Fig. S6H), impaired the interaction between AMPK and PP2C-α in adherent cells (Fig. 5E), together suggesting an Akt-dependent, PP2C-α–mediated dephosphorylation of AMPK in adhesion.
Thus, our data showed that while matrix detachment-triggered AMPK activation inhibits Akt, attachment-triggered Akt activation inhibits AMPK. These data are suggestive of a double-negative feedback loop between these two kinases in matrix-adhered versus matrix-detached states of cells. To further confirm this, we tested the effect of forced activation of AMPK on pAktS473 levels in adherent conditions (where normally AMPK activity is low) and forced activation of Akt on pAMPKαT172 levels in suspension (where normally Akt activity is low). Activation of AMPK in adherent conditions, mediated by AMPK activator A-769662 (Fig. 5F; Supplementary Fig. S7A), overexpression of constitutively active AMPK-upstream kinase CaMKK (Fig. 5G; Supplementary Fig. S7B), or knockdown of PP2C-α (Fig. 5H; Supplementary Fig. S7C), promoted Akt inactivation. On the other hand, forced activation of Akt in suspension, as observed in cells stably expressing phosphomimetic Akt-T308D S473D (Fig. 2E) or shPHLPP2 (Fig. 3D), promoted AMPK inactivation (Fig. 5I and J; Supplementary Fig. S7D and S7E). Collectively, these data identify a novel double-negative feedback loop between the two cellular kinases Akt and AMPK, which maintains an attachment-triggered pAkthigh/pAMPKlow state in adherent cells while maintaining a detachment-triggered pAMPKhigh/pAktlow state in matrix-deprived cells.
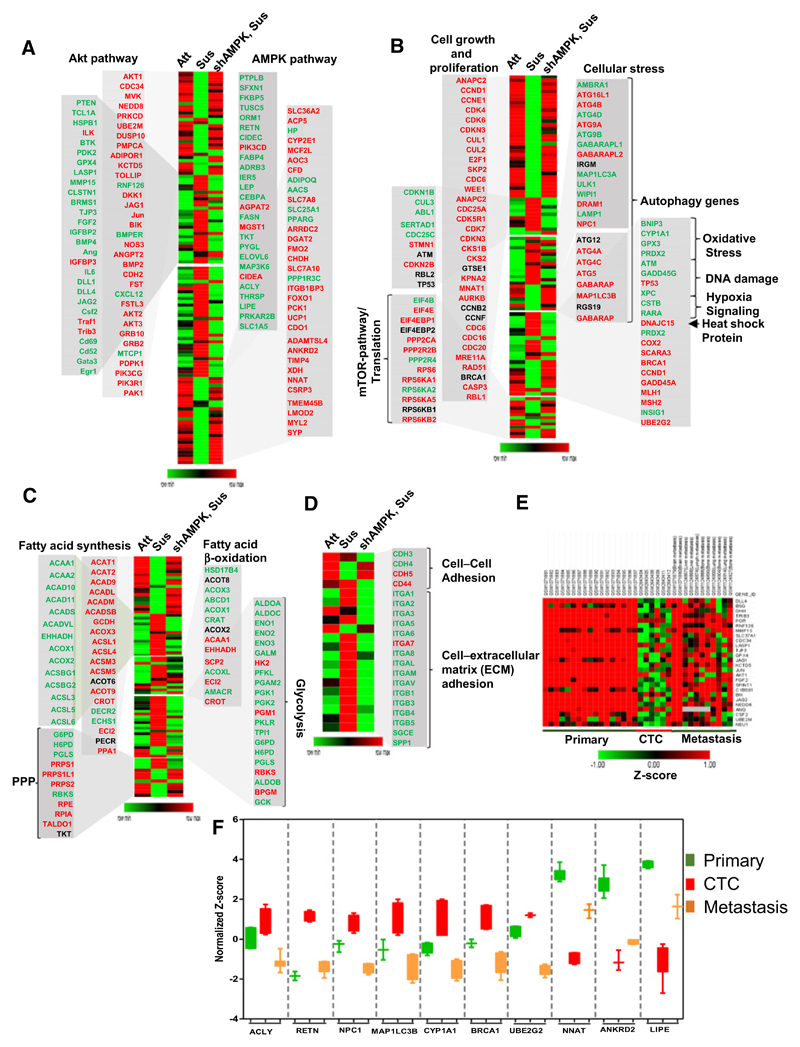
To further corroborate the double-negative cross-talk between AMPK and Akt a microarray-based gene expression analysis was performed between adherent and detached MDA-MB-231 cells. Consistent with higher levels of pAkt in adherent cells, we observed elevated Akt gene signature, including upregulation of AKT1, CDC34, NEDD8, PRKCD, and DUSP10 (31) in adherent condition. Further, in keeping with a role for Akt signaling in anabolic pathways, we observed elevated expression of genes involved in fatty acid synthesis (such as ACAA1 and 2, ACAD 10 and 11, ACOX 1 and 2, ACSBG 1 and 2, and ACSL 3, 5, and 6) and pentose phosphate pathway (such as G6PD, H6PD, PGLS, PRPS1, and PRPS1L1; ref. 32). Additionally, we observed elevated expression of genes involved in the mTOR pathway and protein synthesis (such as EIF4B, HIF1A, RPS6, EIF4EBP1, EIF4EBP2, PPP2CA, RPS6, and IKBKB; ref. 31) and in cell growth and proliferation (such as ANAPC2, CDK4, CDK6, CDKN3, CUL1, E2F1, SKP2, CDC6, and WEE1; ref. 33) in adherent cells (Fig. 6A and B).
Figure 6.
Microarray-based expression profiling of genes regulated in matrix-attached versus matrix-detached states of cells. A–D, Heat map depicting unsupervised clustering of gene expression profiles from MDA-MB-231 cells cultured in adherent condition (Att), suspension for 24 hours (Sus), and expressing shAMPKα2. Genes of interest are color coded. Red, highly expressed genes; green, downregulated genes. The zoomed section of the heat map represents genes involved in Akt and AMPK pathways (A), cell growth, proliferation, autophagy, and cellular stress conditions (B), metabolic processes (C), and cell–cell and cell–ECM adhesions (D). E, Heat map depicting semisupervised clustering of genes involved in Akt signaling across primary breast tumor, CTCs, and metastases. F, Box plots show distribution of normalized (Z-score) gene expression of AMPK-dependent genes from microarray data available for primary tumor (GSE43837), CTCs (GSE99394), and metastases of breast cancer patients (GSE43837 and GSE56493).
Similarly, AMPK activation in matrix-detached cells was supported by the upregulation of AMPK gene signature, including PTPLB, SFXN1, FKBP5, TUSC5, ORM1, CEBP1, MAP3K6, and PRKAR2B (Fig. 6A; refs. 18 and 34). Additionally, genes involved in catabolic processes including fatty acid oxidation (such as HSD17B4, ACOT8, ACOX3, ABCD1M, and ACOX1; ref. 32), and glycolysis (such as ALDOA, ALDOC, ENO1,2,3, GALM, HK2, G6PD, and ALDOB; ref. 32), and genes involved in stress-responsive pathways including autophagy, oxidative stress, and hypoxia (such as ATG4, ATG9, GABARAPL1, IRGM, MAP1LC3A, and ULK1; ref. 35) were elevated in detached MDA-MB-231 cells (Fig. 6C).
Moreover, AMPK knockdown reversed the expression of several of these genes in matrix-detached cells (Fig. 6A–C), revealing their AMPK dependency. We also observed AMPK-dependent altered expression of integrins that are known to be involved in anoikis resistance and contribute to metastasis (36) in detached cells (Fig. 6D). In addition, microarray data analyses of matrix-detached PHLPP2-knockdown cells and GFP-HA-Akt-T308D S473D-expressing cells also exhibited upregulation of the Akt pathway and apoptotic genes while showing downregulation of autophagy-related genes (Supplementary Fig. S7F–S7H), thus supporting our experimental data. Collectively, these data supported the concept of double-negative feedback loop between Akt and AMPK in matrix-adhered versus detached states of cells.
In order to understand the functional relevance of the double-negative feedback between Akt and AMPK in breast cancer progression, we examined publicly available microarray data from the GEO of patient-derived primary breast tumors (GSE43837), circulating tumor cells (GSE99394), and metastatic lesions at different organs (GSE43837 and GSE56493). Similar to adherently growing MDA-MB-231 cells (Fig. 6 A–D), heat map generated from cDNA microarrays of patient samples revealed an Akt pathway associated gene expression pattern in primary and metastatic lesions (Fig. 6E) that was suppressed in CTCs. In contrast, and similar to detached MDA-MB-231 cells (Fig. 6B), we observed AMPK-dependent gene expression, including those of stress signaling and autophagy, in patient-derived CTC microarray data that were suppressed in primary and metastatic lesions (Fig. 6F). For example, box plot analysis showed AMPK-dependent upregulation of ACLY, RETN (18), and autophagy-related genes NPC1, LC3B, CYP1A1, BRCA1, and UBE2G2 (37), while revealing downregulation of NNAT, ANKRD2, and LIPE (Fig. 6F; ref. 18) in CTCs.
Discussion
Once tumors metastasize to a distal site, they are mostly fatal to the patient due to lack of strategies currently to treat metastasis. Therefore, understanding the molecular mechanisms that contribute to cancer metastasis can advance treatment approach. Tumor cells grow adherently both in primary and secondary tumor sites, but they need to overcome the stress of matrix deprivation during transit through the circulation. Yet, what maintains the states of adhesion, characterized by cell growth and proliferation, versus the state of matrix-detachment, characterized by stress survival, remains poorly understood. In this study, we show that AMPK activation triggered by matrix deprivation leads to a concomitant inactivation of Akt by stabilizing PHLPP2 in breast cancer cells. Knockdown of PHLPP2 or constitutive activation of Akt increased anoikis while impairing autophagy, thus inhibiting anchorage-independent growth and metastasis, and highlighting the importance of suppression of Akt activity for surviving the stress of matrix deprivation. We further demonstrate that matrix reattachment-triggered Akt activation concomitantly promotes AMPK inactivation through yet another phosphatase PP2C-α. Our data, thus, reveal for the first time a reversible, double-negative feedback loop between AMPK and Akt between matrix-adhered versus detached states that can coordinate the intracellular signaling pathways involved in cell growth and stress survival during cancer progression.
Akt signaling represents a widely accepted prosurvival pathway that is known to aid anoikis resistance and tumor progression (38). Surprisingly, we observed Akt repression concomitant with AMPK activation in a large array of matrix-deprived epithelial cancer cells (of lung, glial, cervix, liver origin) including breast cancer cell lines MDA-MB-231 and MCF7. This prompted us to investigate if suppression of Akt activity in suspension is required for cell survival. To address this, we enforced Akt activation in matrix-deprived cells using constitutively active Akt constructs. Interestingly, even though both myr-Akt and the phosphomimetic mutant Akt-T308D S473D led to elevated Akt activity in adherent cells, we detected increased Akt activity in suspension only with the expression of Akt-T308D S473D, but not with myr-Akt, an oft-used construct throughout literature. Based on our findings that matrix deprivation upregulates the Akt phosphatase PHLPP2, we predict that myristoylated, membrane-tethered forms of Akt are still susceptible to negative regulation in suspension by phosphatases. Interestingly, expression of Akt-T308D S473D, which is refractory to dephosphorylation at the key activating phosphorylation sites, promoted anoikis, suggesting that high Akt activity might be detrimental to cancer cell survival in suspension. This is in agreement with yet another report that showed elevated Akt activation leads to cell death due to increase in reactive oxygen species (39).
Our data revealed AMPK-dependent upregulation of PHLPP2 in suspension. However, we failed to detect changes in transcript levels of PHLPP2, suggesting possible posttranscriptional regulation. Although PHLPP2 has been shown to be targeted for ubiquitin-mediated degradation (40), we failed to see changes in its ubiquitination or effect of inhibition of proteasomal degradation. Instead, our data suggested a possible role for lysosomal degradation in regulating PHLPP2 protein levels. Interestingly, a recent paper has demonstrated a role for PHLPP1 and Akt in the regulation of chaperone-mediated autophagy that regulates protein degradation (41). However, a selective chaperone-dependent targeting of PHLPP2 for lysosomal degradation remains to be explored. Yet another possible mechanism of PHLPP2 upregulation could involve rapid protein synthesis as we observed elevated PHLPP2 levels within 10 minutes of suspension. Because AMPK activation is known to inhibit cap-dependent translation in suspension (18), this might possibly involve stress-associated alternate modes of translation.
Reduced PHLPP2 protein expression in colon cancer and pancreatic ductal adenocarcinoma patient samples, and antitumorigenic effects of its overexpression in colon and pancreatic cancer cell lines (42, 43) have largely supported tumor-suppressive functions for PHLPP2. In contrast, our results showed that depletion of PHLPP2 rendered breast cancer cells anoikis-sensitive and impaired metastasis. In support of this, a recent study using PTEN/TP53-mutant prostate cancer mice showed that myc drives proliferation and metastasis through activation of PHLPP2 and suppression of the Akt pathway (44). Together, these findings begin to highlight novel, context-specific tumorigenic functions for PHLPP2.
We report here an AMPK-mediated suppression of Akt activity in matrix-deprived cell survival. We speculate that such a negative regulation of Akt by AMPK might be favorable to matrix-deprived cells because this can shift cellular signaling from energy-consuming/anabolic processes (mediated by Akt activation) to energy producing/catabolic processes (mediated by AMPK activation), thus restoring energy homeostasis. This is consistent with a recent finding revealing AMPK-mediated inhibition of mTOR and suppression of protein synthesis in anoikis resistance (18). Further, the catabolic process of autophagy, which is also known to regulate energy balance, plays a key role in anoikis-resistance (29). Interestingly, Akt activation has been shown to inhibit autophagy (30). In keeping with this, our present data showed that PHLPP2 knockdown or enforced Akt activation in matrix-deprived cells inhibited autophagy and increased anoikis. Thus, our data suggest that AMPK-mediated Akt inactivation might additionally contribute to anoikis resistance by promoting autophagy.
Matrix attachment leads to Akt activation through integrin signaling (45), while we have identified elevated calcium and ROS levels as triggers for detachment-induced AMPK activation (46). We show here that reattachment to the matrix restores Akt activity leading to AMPK inactivation through increased interaction between AMPK and its phosphatase, PP2C-α. While PP2C-α is predominantly nuclear localized (47), AMPK is reported to show nuclear localization and cytoplasmic redistribution through Ran-dependent import and CRM1-mediated export pathways (48). Interestingly, Akt has been shown to regulate RanBP and CRM1-dependent nuclear-cytoplasmic shuttling of proteins (49). Thus, one possible way by which Akt could regulate the interaction between AMPK and PP2C-α might involve Akt-dependent redistribution of AMPK.
These findings, thus, identify a novel, and reversible, double-negative feedback loop between AMPK and Akt between matrix-attached and matrix-deprived conditions. Comparison of microarray gene expression data of primary tumors, circulating tumor cells, and metastatic lesions further suggested that such a reciprocal regulation might exist in metastatic breast cancers. We propose that such a reversible regulation might help rapid switching between pAkthigh/pAMPKlow state in adhesion that favors cell growth, to pAMPKhigh/pAktlow state that allows adaptation to matrix-detachment stress (Fig. 7), yet retaining the ability to restore growth following proper attachment at the secondary site. Disrupting this loop using AMPK or PHLPP2 inhibitors might provide novel therapeutic strategies to restrict metastatic cancer spread.
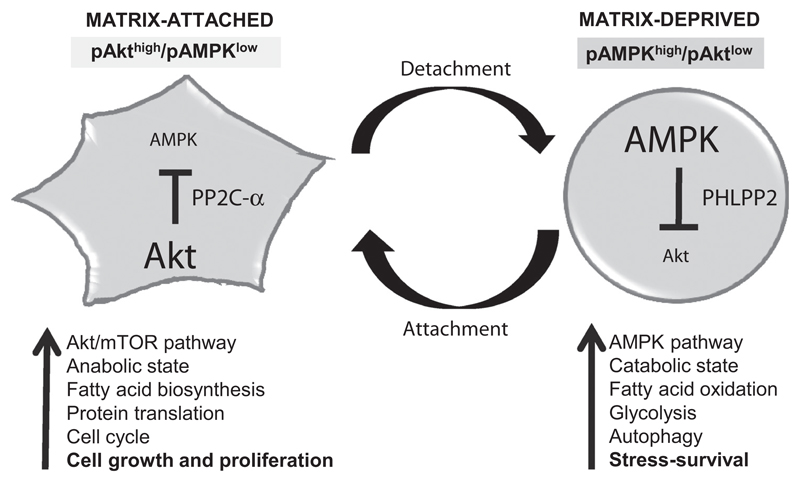
Figure 7.
A model for double-negative feedback loop between two cellular kinases AMPK and Akt. We show that detachment-triggered AMPK concomitantly inactivates Akt through the phosphatase PHLPP2, resulting in a pAMPKhigh/pAktlow catabolic state that facilitates stress-survival in matrix-deprived cells. In contrast, adhesion-triggered Akt keeps AMPK under check by the phosphatase PP2C-α, maintaining a pAkthigh/pAMPKlow anabolic state, which is conducive for cell growth and proliferation. Thus, between attachment and detachment, AMPK and Akt constitute a reversible double-negative feedback loop, maintaining stable pAkthigh/pAMPKlow and pAMPKhigh/pAktlow states, yet retaining the ability to switch between these two cellular states.
Supplementary Material
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Acknowledgments
This work was supported by the Wellcome Trust/DBT India Alliance Fellowship (grant number 500112-Z-09-Z) awarded to A. Rangarajan. Grants from DBT-IISc partnership program and support from DST-FIST and UGC, Government of India, to the Department of MRDG are also acknowledged.
The authors thank Drs. M. Vijaya Kumar and Rekha V. Kumar for their help in procuring primary tissue samples at KMIO; Drs Benoit Viollet for AMPK DKO cells and Deepak Saini for shRNA of phosphatases; Sukrutha Reddy, Nehanjali Dwivedi, and Sunaina Rao for help with immunoblotting; and Srikanth S. Manda, Institute of Bioinformatics, for analysis of microarray data. The authors acknowledge the Central Animal Facility and FACS facility at IISc.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors' Contributions
Conception and design: M. Saha, S. Kumar, S.K. Hindupur, A. Rangarajan
Development of methodology: M. Saha, S. Kumar, S. Bukhari, S.A. Balaji, S.K. Hindupur
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): M. Saha, S. Kumar, S.A. Balaji
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): M. Saha, S. Kumar, S. Bukhari, P. Kumar, S.K. Hindupur, A. Rangarajan
Writing, review, and/or revision of the manuscript: M. Saha, S. Kumar, S. Bukhari, P. Kumar, S.K. Hindupur, A. Rangarajan
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): M. Saha, A. Rangarajan
Study supervision: A. Rangarajan
References
- 1.Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449–58. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 2.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–33. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 3.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase–AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 5.Franke T. PI3K/Akt: getting it right matters. Oncogene. 2008;27:6473–88. doi: 10.1038/onc.2008.313. [DOI] [PubMed] [Google Scholar]
- 6.Hardie DG. AMP-activated protein kinase: a cellular energy sensor with a key role in metabolic disorders and in cancer. Biochem Soc Transact. 2011;39:1–13. doi: 10.1042/BST0390001. [DOI] [PubMed] [Google Scholar]
- 7.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–23. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardie DG, Schaffer BE, Brunet A. AMPK: an energy-sensing pathway with multiple inputs and outputs. Trends Cell Biol. 2016;26:190–201. doi: 10.1016/j.tcb.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carling D, Sanders M, Woods A. The regulation of AMP-activated protein kinase by upstream kinases. Int J Obesity. 2008;32:S55–9. doi: 10.1038/ijo.2008.124. [DOI] [PubMed] [Google Scholar]
- 10.Viollet B, Horman S, Leclerc J, Lantier L, Foretz M, Billaud M, Giri S, et al. AMPK inhibition in health and disease. Crit Rev Bioch Mol Biol. 2010;45:276–95. doi: 10.3109/10409238.2010.488215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeon S-M, Hay N. The double-edged sword of AMPK signaling in cancer and its therapeutic implications. Arch Pharm Res. 2015;38:346–57. doi: 10.1007/s12272-015-0549-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zadra G, Batista JL, Loda M. Dissecting the dual role of AMPK in cancer: from experimental to human studies. Mol Cancer Res. 2015;13:1059–72. doi: 10.1158/1541-7786.MCR-15-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khwaja A, Rodriguez-Viciana P, Wennström S, Warne PH, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–93. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hermanto U, Zong CS, Lu-Hai W. ErbB2-overexpressing human mammary carcinoma cells display an increased requirement for the phosphatidylinositol 3-kinase signaling pathway in anchorage-independent growth. Oncogene. 2001;20:7551. doi: 10.1038/sj.onc.1204964. [DOI] [PubMed] [Google Scholar]
- 15.Eckert LB, Repasky GA, Ulkü AS, McFall A, Zhou H, Sartor CI, et al. Involvement of Ras activation in human breast cancer cell signaling, invasion, and anoikis. Cancer Res. 2004;64:4585–92. doi: 10.1158/0008-5472.CAN-04-0396. [DOI] [PubMed] [Google Scholar]
- 16.Hindupur SK, Balaji SA, Saxena M, Pandey S, Sravan GS, Heda N, et al. Identification of a novel AMPK-PEA15 axis in the anoikis-resistant growth of mammary cells. Breast Cancer Res. 2014;16:420. doi: 10.1186/s13058-014-0420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeon S-M, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661–5. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng T, Leprivier G, Robertson MD, Chow C, Martin MJ, Laderoute KR, et al. The AMPK stress response pathway mediates anoikis resistance through inhibition of mTOR and suppression of protein synthesis. Cell Death Differ. 2012;19:501–10. doi: 10.1038/cdd.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dey D, Saxena M, Paranjape AN, Krishnan V, Giraddi R, Kumar MV, et al. Phenotypic and functional characterization of human mammary stem/progenitor cells in long term culture. PloS One. 2009;4:e5329. doi: 10.1371/journal.pone.0005329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 2004;11:448–57. doi: 10.1038/sj.cdd.4401359. [DOI] [PubMed] [Google Scholar]
- 21.Hardie DG. AMPK: positive and negative regulation, and its role in whole-body energy homeostasis. Curr Opin Cell Biol. 2015;33:1–7. doi: 10.1016/j.ceb.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Kovacina KS, Park GY, Bae SS, Guzzetta AW, Schaefer E, Birnbaum MJ, et al. Identification of a proline-rich Akt substrate as a 14-3-3 binding partner. J Biol Chem. 2003;278:10189–94. doi: 10.1074/jbc.M210837200. [DOI] [PubMed] [Google Scholar]
- 23.Davies SP, Sim AT, Hardie DG. Location and function of three sites phosphorylated on rat acetylCoA carboxylase by the AMPactivated protein kinase. FEBS J. 1990;187:183–90. doi: 10.1111/j.1432-1033.1990.tb15293.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aoki M, Batista O, Bellacosa A, Tsichlis P, Vogt PK. The akt kinase: molecular determinants of oncogenicity. Proc Nat Acad Sci. 1998;95:14950–55. doi: 10.1073/pnas.95.25.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25:917–31. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 27.Stål O, Pérez-Tenorio G, Åkerberg L, Olsson B, Nordenskjöld B, Skoog L, et al. Akt kinases in breast cancer and the results of adjuvant therapy. Breast Cancer Res. 2003;5:R37. doi: 10.1186/bcr569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai J, Fang L, Huang Y, Li R, Yuan J, Yang Y, et al. miR-205 targets PTEN and PHLPP2 to augment AKT signaling and drive malignant phenotypes in non-small cell lung cancer. Cancer Res. 2013;73:5402–15. doi: 10.1158/0008-5472.CAN-13-0297. [DOI] [PubMed] [Google Scholar]
- 29.Fung C, Lock R, Gao S, Salas E, Debnath J. Induction of autophagy during extracellular matrix detachment promotes cell survival. Mol Biol Cell. 2008;19:797–806. doi: 10.1091/mbc.E07-10-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noguchi M, Hirata N, Suizu F. The links between AKT and two intracellular proteolytic cascades: ubiquitination and autophagy. Biochim Biophys Acta. 2014;1846:342–52. doi: 10.1016/j.bbcan.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Creighton C. A gene transcription signature of the Akt/mTOR pathway in clinical breast tumors. Oncogene. 2007;26:4648–55. doi: 10.1038/sj.onc.1210245. [DOI] [PubMed] [Google Scholar]
- 32.LeBleu VS, O'Connell JT, Gonzalez Herrera KN, Wikman H, Pantel K, Haigis MC, et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16:992–1003. doi: 10.1038/ncb3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nurse P. A long twentieth century of the cell cycle and beyond. Cell. 2000;100:71–8. doi: 10.1016/s0092-8674(00)81684-0. [DOI] [PubMed] [Google Scholar]
- 34.Zhou J, Huang W, Tao R, Ibaragi S, Lan F, Ido Y, et al. Inactivation of AMPK alters gene expression and promotes growth of prostate cancer cells. Oncogene. 2009;28:1993–2002. doi: 10.1038/onc.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo JY, White E. Cold Spring Harbor symposia on quantitative biology. Cold Spring Harbor Laboratory Press; 2017. Autophagy, metabolism, and cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vachon PH. Integrin signaling, cell survival, and anoikis: distinctions, differences, and differentiation. J Sig Transduct. 2011;2011 doi: 10.1155/2011/738137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ouimet M, Koster S, Sakowski E, Ramkhelawon B, van Solingen C, Oldebeken S, et al. Mycobacterium tuberculosis induces the miR-33 locus to reprogram autophagy and host lipid metabolism. Nat Immunol. 2016;17:677–86. doi: 10.1038/ni.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheung M, Testa JR. Diverse mechanisms of AKT pathway activation in human malignancy. Curr Cancer Drug Targets. 2013;13:234–44. doi: 10.2174/1568009611313030002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nogueira V, Park Y, Chen CC, Xu PZ, Chen ML, Tonic I, et al. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell. 2008;14:458–70. doi: 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warfel NA, Newton AC. Pleckstrin homology domain leucine-rich repeat protein phosphatase (PHLPP): a new player in cell signaling. J Biol Chem. 2012;287:3610–6. doi: 10.1074/jbc.R111.318675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arias E, Koga H, Diaz A, Mocholi E, Patel B, Cuervo AM. Lysosomal mTORC2/PHLPP1/Akt regulate chaperone-mediated autophagy. Mol Cell. 2015;59:270–84. doi: 10.1016/j.molcel.2015.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J, Weiss HL, Rychahou P, Jackson LN, Evers BM, Gao T. Loss of PHLPP expression in colon cancer: role in proliferation and tumorigenesis. Oncogene. 2009;28:994–1004. doi: 10.1038/onc.2008.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qiao M, Wang Y, Xu X, Lu J, Dong Y, Tao W, et al. Mst1 is an interacting protein that mediates PHLPPs' induced apoptosis. Mol Cell. 2010;38:512–23. doi: 10.1016/j.molcel.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 44.Nowak DG, Cho H, Herzka T, Watrud K, DeMarco DV, Wang VM, et al. MYC drives Pten/Trp53-deficient proliferation and metastasis due to IL6 secretion and AKT suppression via PHLPP2. Cancer Discov. 2015;5:636–51. doi: 10.1158/2159-8290.CD-14-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.King TD, Song L, Jope RS. AMP-activated protein kinase (AMPK) activating agents cause dephosphorylation of Akt and glycogen synthase kinase-3. Biochem Pharmacol. 2006;71:1637–47. doi: 10.1016/j.bcp.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sundararaman A, Amirtham U, Rangarajan A. Calcium-oxidant signaling network regulates AMP-activated protein kinase (AMPK) activation upon matrix deprivation. J Biol Chem. 2016;291:14410–29. doi: 10.1074/jbc.M116.731257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin X, Duan X, Liang YY, Su Y, Wrighton KH, Long J, et al. PPM1A functions as a smad phosphatase to terminate TGFbeta signaling. Cell. 2016;166:1597. doi: 10.1016/j.cell.2006.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kazgan N, Williams T, Forsberg LJ, Brenman JE. Identification of a nuclear export signal in the catalytic subunit of AMP-activated protein kinase. Mol Biol Cell. 2010;21:3433–42. doi: 10.1091/mbc.E10-04-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoon SO, Shin S, Liu Y, Ballif BA, Woo MS, Gygi SP, et al. Ran-binding protein 3 phosphorylation links the Ras and PI3-kinase pathways to nucleocytoplasmic transport. Mol Cell. 2008;29:362–75. doi: 10.1016/j.molcel.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.