Abstract
Objective
To identify the reasons underlying women’s refusal to participate in a pregnancy trial and to identify ways of increasing recruitment.
Design
Mixed methods study using a questionnaire and qualitative interviews.
Sample
A questionnaire asking them to indicate reasons for their decision was completed by 296 pregnant women who declined to participate in one of two trials of nutritional supplementation in a large teaching hospital in the south of England. Qualitative interview data were collected from two samples of pregnant women: 1) 30 women who declined to participate in a trial but completed the questionnaire; and 2) 44 women who participated in a trial.
Results
Most reported reasons in questionnaires by women who declined to participate in a trial were concerns about study requirements, such as not wanting to take study medication, have a bone scan or extra blood tests, or being too busy. Thematic analysis identified differences in self-efficacy and levels of trust in medical research between participants and decliners. Participants believed that the research would cause no harm, while decliners felt they or their unborn child would be at risk. When faced with potential obstacles, participants found ways around them while decliners felt they were insurmountable.
Conclusions
Recruitment methods for pregnancy trials should focus on building women’s trust in the trial and research staff and on enhancing women’s self-efficacy so they feel able to meet trial requirements. Suggestions for building trust include improving visibility of the research team, testimonials from previous participants and advertising study safety and ethical conduct. Self-efficacy can be enhanced by training research staff in empowering styles of communication enabling women to feel heard and supporting them to overcome practical problems associated with participating. These strategies could be implemented relatively easily into pregnancy trial protocols, and their effectiveness tested through their impact on recruitment rates.
Background
It is widely accepted that randomised controlled trials provide the best evidence of the effects of treatments and programmes.(1–3) Despite trial evidence being considered the gold standard, trials face significant challenges in recruiting sufficient numbers of participants, which can lead to unrepresentative samples and jeopardise studies’ external validity.(4, 5) The struggle to reach target sample size can also affect study costs and staff morale when recruitment is slow and studies need to be extended.(5–9) In a review of recruitment rates in 122 clinical trials spanning 18 clinical areas, only 31% reached their target sample size in the intended time.(10) Over half of the reviewed trials (54%) required an extension, and over one third revised their recruitment target over the course of the trial, most to reduce target sample size.
The main barriers to taking part identified in the literature include: treatment preference such as not wanting to change medication, to take a placebo, or take any medication, lack of interest in research, distrust of researchers, additional demands of the study such as extra procedures and appointments which may cause discomfort, and difficulties with travel to the trial site.(2, 11–13) The randomisation aspect of clinical trials seems particularly to cause concern; participants feel they may be missing out on valuable effects of the treatment and dislike the lack of control over their assignment to either group.(14)
In addition to this uncertainty, there are requirements associated with any study which may represent barriers to participation. Interventions may interfere with other commitments such as work, childcare, or domestic duties. Some populations, such as city-dwelling women with low socioeconomic status are challenging to recruit because they can have difficulties with transportation, inflexible work schedules, childcare considerations, unstable housing, difficult personal circumstances, and distrust of medical institutions and research.(15) Though hard to recruit, these are often also the populations who most need support and interventions.(16, 17)
The decision to participate is not only determined by the presence or absence of barriers, but also the perceived benefits of taking part. The most commonly given motivation for deciding to participate is the potential benefit to others.(12, 18, 19) Other reported motivations include: free health services such as scans or imaging(18), trusting and wanting to please the physician(20), and reasons such as endorsement from family members and trust in the medical institution(21).
It has even been suggested that increasingly accessible health care information and consumer empowerment have changed the relationship of the public with research, such that people now evaluate research studies from a consumer perspective.(14, 22) It is argued that problems with recruitment arise because studies are designed primarily to fit theory and budget. To improve recruitment and consequently the validity of clinical trials, research must therefore become more participant-centred.(14) Such research would involve greater input from patients and public, and co-creation of research projects to ensure that participant views and experiences are acknowledged and incorporated into trial design.
Few studies of reasons for participating in clinical trials have involved pregnant women; most involve patients with cancer, cardiovascular or respiratory diseases. Pregnant women are a unique group in that they are considering a second participant, the unborn baby, when making their decision to participate.(23, 24) Clinical trials involving pregnant women therefore face specific enrolment challenges, and pregnancy trials often require large sample sizes to detect significant differences in clinical outcomes for the mother or baby.(6) Recruitment rates for pregnancy trials are consistently low with only around 30% of eligible women typically choosing to participate, and recruitment often slower than expected.(25–27)
Another issue in increasing recruitment to trials is that our understanding about what motivates people to participate or not comes mainly from those who do participate. The reasons women most commonly give for participating in trials whilst pregnant are potential benefit to them and their babies(19, 28–31), contribution to scientific research and improved maternity care(19, 28–30, 32), and the opportunity to receive more than standard care.(32)
The two published studies of non-participants’ views on participating in clinical trials during pregnancy provide some suggestions as to why women might decline. Amongst these are information about the study being conveyed through a letter rather than in person, partner’s opinion of potential risks, a history of pregnancy complications, anxiety about interfering with the normal course of pregnancy, and uncertainty about the safety or effectiveness of the trial.(23, 33) Both these studies were small, however, relied solely on qualitative data and reported the reasons women described without deeper exploration of the issues that might underlie those reasons. A qualitative approach is being used increasingly in obstetrics and gynaecology research to answer questions about experiences of care, develop research methods and inform practice.(34) It is particularly useful in areas where little research exists.(35) This study therefore used mixed methods to answer two research questions:
-
1)
What underlies women’s decisions whether or not to participate in clinical trials during pregnancy?
-
2)
How can we use this knowledge to increase recruitment to clinical trials in pregnancy?
Method
Setting
This study was carried out in a large teaching hospital in the south of England between 2014 and 2016 and recruited from amongst women who had participated or declined to participate in two clinical trials (36, 37). MAternal VItamin D Osteoporosis Study (MAVIDOS) was a randomised controlled trial (RCT) of vitamin D supplementation in pregnancy. The Southampton PRegnancy Intervention for the Next Generation (SPRING) was an RCT of vitamin D plus nurse support for improving women’s diet and body composition. Women were recruited for this study from both trials since SPRING is effectively an extension of MAVIDOS. Both trials required women to take daily capsules, attend two extra ultrasound scan appointments during pregnancy and a DXA scan for the baby just after birth. SPRING also involved a phone call during pregnancy from the research nurse and a home visit one month after birth. The study at hand was given ethics approval by the Southampton and South West Hampshire Research Ethics Committee.
The questionnaire
The objective of the questionnaire was to capture the prevalence of reasons for declining to participate in the SPRING trial. Women were approached to participate as they attended for their nuchal translucency scan approximately 12 weeks pregnant. If they declined, they were invited to complete a questionnaire (available on request from the first author) indicating their reasons from this list: I don’t want to take pills during my pregnancy; I don’t want my baby or me to have a bone density scan; I feel too unwell; I don’t like blood tests; the research sounds too complicated; I am too busy; they will ask too many personal questions; I didn’t really understand what I was being asked to do; there is no point in taking part if I end up not getting the vitamin D; I don’t want to take part in any research; there is no point in taking part if I can just get Vitamin D at the chemists; and other reasons accompanied by space for free text. These response options were determined empirically: items were generated from self-reported reasons women gave upon declining to participate in the trials that were recorded in the trial recruitment logs before this study commenced, the items were further developed with feedback from an expert panel. The questionnaire also recorded standard demographic information and invited women to leave contact details if they were prepared to be interviewed about their reasons for deciding not to participate. Data were analysed to provide frequencies of reasons for declining.
The interview
The objective of the interviews were to explore in more depth the influences on women’s decisions of whether or not to participate in a trial, and to compare the perceptions of women who took part with those who declined. Semi-structured interviews (38–40) were carried out with two samples of women recruited using purposeful random sampling(41): one group who had declined to participate in a trial but who completed the questionnaires, and another group who had agreed to participate in one of the two trials described above. This form of interviewing allowed focusing the topic according to an interview guide in order to explore the research questions, but also offered flexibility in follow-up strategies and freedom for the participant to explore anything they felt was relevant around that topic.(39) Comparison of these data allowed differences and similarities in women’s experiences, perceptions and feelings to be identified. Sufficient participants from each trial were interviewed for data saturation to be reached.(42) Women who did participate in a trial, and also provided interview data, are subsequently described as ‘participants’. They were interviewed within six months of completing the trial. Those who declined to participate in a trial and who provided questionnaire data, and the subset of these women who were interviewed, are described as ‘decliners’.
Participants
Participants who had completed the trial were sent invitations to participate in the interview study. Those who agreed to participate were interviewed face-to-face in their own homes and gave written consent for this to be audio-recorded. Interviews were guided by a semi-structured discussion guide (available on request), allowing interviewees to explore why they took part in the trial, their experiences of taking part and improvements for future trials.
Decliners
Women who completed questionnaires and left contact details were contacted by telephone and e-mail to arrange a time for a telephone interview, which was found to be the most convenient approach. Decliners gave audio-recording consent and the interviewer completed an interview consent form on their behalf over the telephone. Interviews were guided by a semi-structured discussion guide (available on request); the questions allowed interviewees to explore relevant aspects of their decision not to participate, why other pregnant women might not participate in clinical trials and what might be done to improve recruitment.
Analysis
Thematic Analysis
Audio-recordings were transcribed verbatim by a member of the research team and another researcher compared the transcript to the recordings to ensure accuracy. These were identified using participant numbers only. An initial coding frame was developed by the research team (TR, WL, SS) based on themes arising from the analyses of the first three participant transcripts, using inductive coding and a constant comparative approach (43, 44). This approach is in line with a relativist ontological and subjective epistemic position, rooted in the belief that reality is always interpreted to a particular frame of reference and based on personal experience and insight. The coding frame was refined through double-coding of 20 transcripts including both participants and decliners. Once a comprehensive coding frame was agreed, each transcript was coded by a single researcher. Regular meetings were held by the coding team to raise and discuss any queries with the coding process. Themes and sub-themes were compiled together with verbatim quotations.
Rigour
Interviews were conducted by highly-trained and experienced qualitative researchers. A subset of five participant transcripts, comprising 2216 lines of text, were double-coded and analysed for inter-rater reliability; discrepancies arose in 134 lines, equating to a 94% level of agreement. Differences in coding were discussed, resolved and used in further development of the coding frame.
Results
Sample Characteristics
Characteristics of women in this study are presented in Table 1. Interviews were conducted with women who took part in MAVIDOS (n=14) or SPRING (n=30) (participants). Of the 478 women who declined to participate in SPRING between February 2015 and November 2016, 189 women completed the questionnaire but did not wish to be contacted for an interview. An additional 101 women completed the questionnaire and provided contact details for a telephone interview (decliners). Of these, 30 were successfully reached and interviewed. The characteristics of the decliners who were not interviewed were comparable to those who agreed to be contacted (Table 1). The mean age and the age of leaving education of the MAVIDOS interviewees were slightly higher than for SPRING participants and decliners. Approximately half of participants and decliners had no other children than the index pregnancy. We have no information on the 188 women who declined to participate in SPRING or to complete the questionnaire, so cannot compare them with those who completed the questionnaire.
Table 1. Characteristics of the women (n = 334).
| Group | Mean age of participants in years (SD) | Level of education N (%) |
Age when left education Mean (SD) |
No. of other children N (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| None | GCSE | Further Education | Higher Education | 0 | 1 | 2 | 3+ | |||
|
MAVIDOS participants (n=14) |
35.99 (5.76) | 0 | 1 (7.14) | 3 (21.43) | 10 (71.43) | 21.21 (1.81) | 5 (35.7) | 7 (50) | 2 (14.3) | 0 |
|
SPRING participants (n=30) |
32.33 (5.52) | 0 | 8 (26.7) | 11 (36.7) | 11 (36.7) | 19.37 (3.31) | 14 (46.7) | 10 (33.3) | 6 (20) | 0 |
|
DECLINERS interviewed (n=30) |
32.84 (4.80) | 2 (6.7) | 4 (13.3) | 8 (26.7) | 16 (53.3) | 19.70 (3.15) | 14 (46.7) | 11 (36.7) | 2 (6.7) | 3 (9.9) |
|
DECLINERS not interviewed but filled in questionnaire (n=260*) |
29.12 (4.84) | 7 (2.7) | 64 (24.6) | 82 (31.6) | 97 (37.3) | 19.29 (3.05) | 106 (40.8) | 104 (40) | 29 (11.2) | 13 (5.2) |
| n = 259 | n = 250 | n = 252 | n = 252 | |||||||
Missing data was only found for decliners who filled in the questionnaire but were not interviewed, these are indicated by valid n in the data columns.
Research question 1: What underlies women’s decisions to participate in clinical trials during pregnancy?
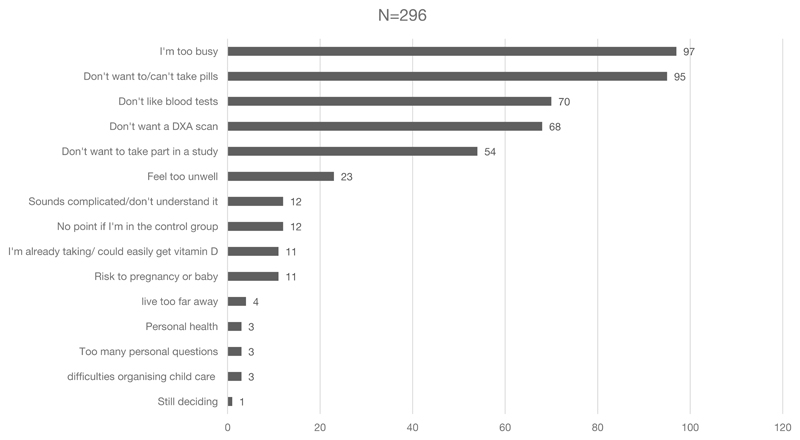
Reasons for not taking part in the SPRING trial given by the 296 decliner questionnaires are depicted in Figure 1. The most common reasons were concerns about study requirements, such as not wanting to take the study medication, have a bone scan or extra blood tests, or being too busy. Free text responses were coded to the existing items, where appropriate, by two researchers. New descriptors were created for the responses that did not align with any existing items.
Figure 1. Women’s reasons for declining to take part in a clinical trial during pregnancy.
Interviews with participants and decliners produced four main themes which addressed Research Question 1: 1) what women brought to the study, including prior knowledge and experiences, social influences, attitudes and feelings; 2) women’s experiences of the recruitment process, including impressions of the study team and facilities, and perceptions of study requirements and benefits; 3) barriers to taking part and thoughts about what might prevent others from taking part; and 4) overarching concerns, including perception of risk to their own or their baby’s health, and any information-seeking they undertook for reassurance. Differences between participants and decliners are explored below by theme and verbatim quotes used for illustration. Differences are summarised in Table 2. Participants are denoted by the letter “P” and decliners by “D”.
Table 2. Comparison of participants’ and decliners’ responses.
| Participants | Decliners |
|---|---|
| Prior knowledge of the importance of substantive topic | Little prior knowledge |
| Previous experience of research by self or others | Little if any previous experience of research |
| Confidence in decision-making | Referral to others for help with decision |
| Experienced the research environment positively (attention, reassurance, comfort) | Experienced the research environment negatively (fear, pain) |
| Focus on benefits | Focus on inconvenience and harm |
| Confidence that they could work around the demands | Anxiety that time demands wouldn’t be managed |
| Saw research demands as reasonable/manageable | Saw research demands as unreasonable/unmanageable |
| Saw risks as minimal | Saw risks as too great |
1. What women brought to the study
Prior knowledge and experience
Women who agreed to participate in MAVIDOS or SPRING tended to have some prior knowledge of the study topic and understanding of the possible consequences of vitamin D deficiency. Decliners on the other hand tended to have little prior knowledge.
Participants tended to have previous experience of research. Some were in professions that provided insight into research, others had either participated themselves, or knew people who had participated in research trials before. Conversely, decliners tended to have little previous experience of research. They described themselves as not being particularly interested in research or trials, although recognising their general worth. The decliner’s accounts reflected this ambivalence.
I had one friend who’d done a similar study a couple of years before… She’d found it brilliant, and really helpful. I think an almost identical study. (P12243) I don’t think about these things very much… I’m not particularly academic myself…I think research is vital, of course it’s vital… but, is it something that is close to my heart? No. (D13011)
Confidence in decision-making
Participants appeared generally confident about making the decision to participate. If they consulted anyone, it tended to be their partners. Women who declined to participate were more likely to seek other people’s opinions and appeared less confident in their own ability to decide.
It was my decision at the end of the day… if [husband] was anti it, it would be different, I suppose, as it’s his child as well. But with regards to other members of my family and friends, all I would have said was it is my decision and I’m doing it. (P4081) My mum and dad because they’re my parents and my partner, if it’s to do with the baby, then 100% because you know it’s his child as well. If it was just purely myself, I would still consult those same people. (D12591)
2. Women’s experience of the recruitment process
Impressions of the study team and facilities
Participants tended to be positive about their first experiences of the study team and the research facility scanning suite. They described enjoying the benefits of the personal attention that taking part in the trial afforded them. Decliners tended to experience the research environment in a negative way. Some described the recruitment process alarming, thinking there was something was wrong with their baby.
Definitely a lot more personalised … this was kind of like a selected thing. And felt a bit more private - felt a little bit more special I suppose in a way. (P14042) One of the ladies took us through to a research room… she went through it all with us… although it’s terrifying because I thought she was going to take me in and say something was wrong with the scan. (D12617)
Both participants and decliners described making a quick decision about whether or not to participate. Participants described having an immediate positive response, whereas decliners tended to report an immediate negative response.
3. Barriers to taking part
Attitudes
Participants focused on the benefits of the study. They seemed to feel the trial did not make unreasonable demands on them or their time and saw it as a benefit to them and their unborn baby. Few decliners identified the benefits of the study and seemed more concerned about the difficulties of meeting study requirements.
I suppose at a selfish level, it’s always, ‘what do I gain from this?’ and certainly additional scans throughout your first pregnancy was a big appeal… I don’t think it was anything too radical to look at vitamin D. (P12229) She said I have to go for blood tests and things, and I don’t do needles, full stop… And that was that… I just can’t… Like, I’m covered in tattoos, but needles, injections, no. (D12656)
Practical considerations
Both participants and decliners described busy lives in which they would have to make arrangements for childcare and taking extra time off work to come to study appointments. Despite the fact that both experienced practical barriers to taking part, participants appeared more confident about accommodating the study requirements, whereas decliners perceived having less flexible working conditions, and expressed anxiety that they would not manage the demands of work and the study.
“[The study team] always tell you that they book in advance, when you’re going to be seen. It was always easy if I could let work know, ‘I’ve got an appointment at this time,’ or… Where my shifts are actually pretty stable… then I just worked it around them.” (P12332) I have a long way to travel, and I work, and it’s difficult… my job’s quite full on, it’s quite hard to get out… my boss is really understanding with normal appointments, but to take extra appointments they wouldn’t be so understanding. (D13011)
4. Overarching concerns
Perception of risk
Most participants described feeling confident that the risks involved in the study were minimal. Where they had concerns, they asked questions and reported feeling reassured by the study team. Conversely, most decliners described experiencing the risks of the study as too great, having concerns about side-effects of the supplement (above recommended dose of Vitamin D), interactions with other supplements they were already taking, and potential harm to the baby.
We only had a question about the DXA scan - on the baby. Which we asked her, and she showed us pictures and everything so that was that… Yeah it was fine, every question was answered. (P12309) I didn’t want to take too much [vitamin D] because I take it every day… I just don’t want to do any experiments while I’m pregnant. If it was only me, that would be fine. I could take it, and that’s absolutely ok. But, while I’m pregnant, we don’t know what effects this could have on the baby. (D12642)
Information-seeking
Participants saw the recruitment interview as an opportunity to ask questions and seek reassurance from the study team. Decliners chose not to raise their concerns or ask questions about the study, though they did talk about wanting more information.
It was then really nice to be able to have the opportunity to speak with one of the team when we were there… I just asked them to explain it again, really. And I think some of the reasoning behind where it sort of comes from, why they were looking at the study. (P12503) They probably would have [answered questions], but I didn’t ask… I think it’s just explaining things more I think. Even though it was self-explanatory, the sort of bits that I’d want explaining weren’t explained. (D12588)
Research question 2: How can we use this knowledge to increase recruitment to clinical trials in pregnancy?
Interviews with participants and decliners produced four suggestions for improving recruitment:
1. Offering incentives and benefits
Both participants and decliners suggested that the study had to offer sufficient benefits to make the inconvenience of taking part worthwhile. Some decliners suggested that additional monetary incentives might improve recruitment.
People need something, don’t they, to encourage them to take part in the study, cause lots of people would think, ‘well what’s the point if there’s no benefit to me?’ So like the extra scans I think (P12480) Of course an extra scan is interesting, it’s not [worth] having to go to all the extra appointments… I don’t know if you can get sponsorship from people… £10 vouchers to people that take part in the survey? (D13011)
As well as the more immediate personal benefits such as extra scans and free scan images, participants suggested emphasising the value of the study to society.
Maybe that saying that, you know,’ think of the next generation’… just a little statement at the front you know, ‘this affects your children’s children’. You know, that we could prevent things if we give the opportunity to research. (P12064)
2. Receiving information from a trusted source
Decliners' suggestions for improving recruitment tended to focus on providing more information about the nature and requirements of the study, as well as ensuring face-to-face contact with a researcher or health care professional involved in the trial.
If you get like… an actual nurse or something, telling them about it, or their midwife, then they’ll be more likely to take part in the trial. (D12636)
Both decliners and participants felt it was important to hear from previous participants about their experiences, and thought this would be a good way to reassure women about the safety of the study as well as highlighting the benefits of participating.
Maybe use some of the feedback that you guys collate… That might help people that are on the fence, they don’t know what to do. Might give them more of an idea one way or another. (P12503) Someone that has already done [the trial]. They’ve already been through it, so they know exactly what it’s like… When you buy something you go on the reviews and look at them. It’s the same sort of thing. (D12588)
3. Advertising earlier
Decliners and participants felt that information could be provided earlier in the pregnancy and be more widely advertised, for example in local GP surgeries or by midwives. Decliners suggested that initial information should be made more accessible and avoid overwhelming potential participants.
I suppose certainly in my GP waiting room, they often have adverts up for all the things like that… so for people to start thinking about it beforehand, maybe catch a few more people, because you’d get them at that earlier stage perhaps. (P4050) It would have been good to have the basic details, like they had in the leaflet already, with the change of the word placebo. Then afterwards, if it’s something you’re interested in, there were extra pages that you could have read through for more detail. (D12591)
4. Providing flexible appointment times
Women also suggested that women's hectic lives could be better accommodated by offering appointments at home, local GP surgeries or offering evening and weekend appointments.
I think it’s probably going to put off people if they have to come into the hospital perhaps. Whereas if it’s a quick home visit, then that’s going to make it more appealing. (P12001) Later times in the day, or weekends. A little bit more flexible, because in my eyes…I’m not doing it for me. I’m doing it as part of the research for the NHS as well. Work around [me] a little bit more. (D12588)
Discussion
Main Findings
This study set out to explore what influences women’s decisions to participate in clinical trials during pregnancy and to identify ways of improving recruitment. We contrasted the experiences of women who took part with those who declined, using data collected from questionnaires and qualitative interviews.
1) What underlies women’s decisions to participate in clinical trials during pregnancy?
Decliners viewed pregnancy trials in terms of risk and inconvenience. Their responses to the questionnaire suggested that the need to take study medication was a significant barrier to participation in the trials, and the interviews suggested this was because they saw the associated risk as being unacceptable. The questionnaire also indicated that they could not spare the time to participate, which the interviewees explained was because they felt they could not reorganise their working lives and childcare to accommodate the demands of the study. Their responses were characterised by a sense of anxiety about the trial.
Conversely, participants trusted that the study was safe for them and their baby, and many described no hesitation in deciding to participate. Where these women had concerns, they felt able to raise and resolve them with the help of the study team. Though they had similar work and childcare commitments to those who declined, they were able to overcome these barriers. They were confident in their own ability to make the decision to participate. What they brought to the study, how they experienced the recruitment process and the practical obstacles they perceived to taking part could all be described as reflections of their belief in their ability to undertake a particular action - their sense of self-efficacy (45).
2) How can we use this knowledge to increase recruitment to clinical trials in pregnancy?
Women’s suggestions for improving recruitment reflected their differing viewpoints. Participants were positive about the study and felt that the various benefits of taking part such as extra scans could be highlighted to potential participants. Decliners on the other hand expressed a desire for more explanation and reassurance from trusted sources. Both participants and decliners highlighted the importance of effective advertisement, testimonials from previous participants, and making it easy and worthwhile to participate through offering flexible appointments and something concrete in return for taking part.
Strengths and Limitations
Participants were recruited from the women invited to participate in the trials over an extended period of time, which ensured that their experiences of the recruitment process were not limited to a specific point in time. The decliners may not represent all those who declined to participate in the trial. The decliners in this study nevertheless represent a valuable sample of women largely overlooked in the existing literature on trial recruitment. They offer the necessary evidence-base to inform the development of future pregnancy trials. A methodological issue with the study is that participants were interviewed face-to-face but decliners over the phone. Participants had established relationships with the research team and were willing to give time to a face-to-face interview, suggesting that they had therefore already invested heavily and successfully in the study which may have biased their responses. They were willing, however, to share their views of negative experiences such as parking difficulties and issues with swallowing the large study vitamin capsules, indicating that their responses were balanced. Decliners had refused to participate in a trial so their participation in this study was made as easy as possible. Asking for face-to-face interviews was deemed unlikely to be successful so telephone interviews were offered. This difference in interview method may have made a difference to the depth of information received from the two groups of interviewees. Finally, the interpretation of the interview data presented in this paper is only one of many possible. A rigorous approach to data collection and analysis was taken, however, to ensure that the interpretation was a fair representation of interviewees’ views and to make data analysis transparent and accountable, including double-coding of selections of the interview transcripts. The convergence of the interpretation presented here with other research suggests that our findings may be transferable to other populations and settings.
Interpretation
What seems fundamental in determining whether or not women participate in the trials is their level of trust in medical research. Many women’s first response to the invitation to participate in a trial appeared to be an instinctive one, based largely on how much they trusted the trial to do them no harm. One indicator of this was the speed with which women made the decision to participate or to decline. This is in line with previous research where pregnant women made independent decisions to participate when they perceived no threat to the baby, but where they perceived potential harm, they were more inclined to seek input from their partner and others (32).
For participants, their trust in research allowed them to be curious about the study and their higher self-efficacy appeared to enable them to be resourceful in overcoming practical barriers. Decliners appeared to have lower levels of trust, less interest in medical research, and lower self-efficacy.
This is not to say that women’s lack of trust in clinical research is irrational or unfounded… examples of disastrous trials. Something about the obvious importance of protecting the baby, pregnancy being particularly susceptible.
The only two other qualitative studies that have interviewed women who refused to participate in clinical trials in pregnancy concluded that negative feelings towards the trial, either because it might do harm or for practical reasons, are major barriers to participation (23, 24). Women in these studies spoke about the potential risks of taking part, and anxiety about ‘meddling’ with the natural progress of their pregnancies. Our larger dataset broadly confirms the findings of these previous studies, even though the latter involved women with high-risk pregnancies which may have affected their perceptions of risk and their motivations to participate in trials. By looking beyond women’s initial reasons for not taking part and comparing the perceptions of participants and decliners, it appears that women’s lack of trust in medical research lies at the heart of their decision not to participate in a trial while pregnant.
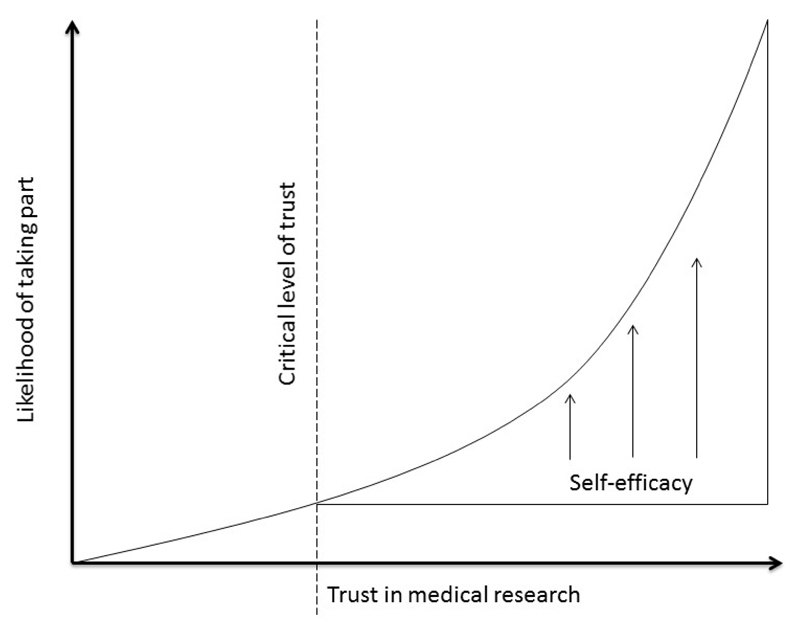
Figure 2 is a representation of the hypothesised relationship between women’s level of trust in medical research, their self-efficacy and their likelihood of participation in a clinical trial. It suggests that a critical level of trust may be required before women perceive there to be value in addressing practical issues. Their ability to overcome practical obstacles to taking part will depend, at that point, on their level of self-efficacy.
Figure 2. Hypothetical model of the relationship between level of trust in medical research, likelihood of participation and the role of self-efficacy in women approached to take part in trials in pregnancy.
Level of trust can also be seen to underlie women’s responses to the extra medical attention they would receive as part of the trial. Rengerink and colleagues found that this extra attention was a motivator for participants (23). In our study, extra attention was for some a reason to participate and for others a reason to decline, depending on whether they felt it would add to the risks associated with the trial and the burden placed on their time. Similarly, women’s level of trust may make them willing or not to find ways to accommodate extra hospital visits required by the trial. Other studies have identified practical difficulties of taking part in trials to be a major reason for declining (23, 24). In our study, both groups of women had similar practical barriers to participation, but women who took part were willing and able to find ways of overcoming these barriers, where for decliners, these barriers were insurmountable. This suggests that it may not be the existence of practical issues per se that stops women taking part in trials as much as their belief in the value of taking part and their self-efficacy in overcoming their issues.
So how can we use this knowledge to increase recruitment to clinical trials in pregnancy?
These findings suggest that to increase recruitment to trials in pregnancy we have to build trust in medical research. It implies that only once women have a level of trust that the research will cause them and their unborn babies no harm, will removing the practical obstacles to participation have an effect on their decision to participate.
Building trust
Women’s decisions to take part or not need to be respected, and we are not advocating persuading women who are not happy to take part. We are instead advocating openly exploring women’s concerns with them and creating a safe space for them to ask questions and to seek reassurance where it is possible to reassure them.
In a positive and trusting psychological state individuals are willing to give up control and accept vulnerability because they feel that the other person is on their side.(46–48) The implication for pregnancy trials is that if women feel that the research team are looking out for them, they are more likely to take the risks they may perceive to be associated with a trial.
Emphasise the role of Patient centred research and co-creation.
In situations of mistrust women may behave in two ways. The first may be to test the motives of the researchers and the truth of what they say about the risks associated with the trial.(46) The second would be to challenge the research team to respond in ways that benefit the women at some cost to the study (49). The first demands complete transparency from the research team about the risks involved, and the second could be overcome by meeting the challenge by, for example, covering expenses incurred by participating, providing child care during appointments, or flexible appointment times.
Discuss that it is important that trials make adjustments where ever possible to meet women’s needs and ameliorate practical barriers to taking part.
Trustworthiness judgements are also based on an initial assessment of available information about a person's moral character, such as prior knowledge or their facial features at first encounter (50–52). Including pictures of research team members and a positive message about them in outward-facing study materials may help form trustworthiness associations (53). Staff pictures and short written profiles could be displayed in waiting rooms. Women in our study suggested that trust could also be built by highlighting past success, patient safety, and participant satisfaction in study materials. Displaying testimonials from previous participants in the research centre would help reassure anxious potential trial recruits. Additionally, trustworthiness could be improved through making an online ‘Trial advisor’ guide available that contained reviews, recommendations and testimonials from previous trial participants. The guide could be advertised on hospital or research websites, and introduced in all leaflets and by research staff at recruitment.
The recruitment interview represents a critical event in the process of instilling trust. Patients trust their doctors more when they feel listened to and that their problems are taken seriously.(54) Information about the trial and the safety and benefits of participating are not sufficient to build trust. Potential participants need also to feel that their concerns are heard and taken seriously by the recruitment team which demands a particular style of communication be adopted by the research team.
Increasing self-efficacy
Our data suggest that supporting women’s self-efficacy, thereby enhancing problem-solving, might further increase their likelihood of agreeing to participate in a trial. Experiences that a) promote reflection on one’s interests, goals and abilities; b) facilitate observing others and hearing about their experiences of success; c) provide positive affirmation and encouragement; and d) promote positive mood through emotional support, can all strengthen self-efficacy (55, 56).
Pregnant women’s agenda is absolutely important and needs to be respected. Emphasise that we advocate creating a research environment that does not dismiss the pregnant women’s needs and concerns, and allows them to voice these so that research teams might better meet them. Clarify the point that clinical trials have a responsibility to the best of their ability meet the needs of their target sample in order to make it safer, easier and more acceptable to take part.
Making previous participants’ testimonials publicly available would enable women to hear about others’ experiences and make clinical trials accountable for providing a safe and acceptable experience for their participants. Increasing self-efficacy could also be achieved through training recruitment staff in an empowering approach to communication. Healthy Conversation Skills (HCS) training is one option which offers a set of accessible, theory-based skills for any health and social care practitioners (57, 58). Through a process of listening, reflecting and goal-setting women may be empowered to identify their issues and explore ways of overcoming them that suit them. Empowering people to problem-solve has been shown to increase self-efficacy (59). Training research staff to use these skills at recruitment would not only raise potential participants’ self-efficacy, but may also increase their level of trust, since they would feel listened to and that their issues were taken seriously. (49)
Conclusions and implications for intervention
Recruitment methods for trials during pregnancy need to focus on building trust in the trial and the research team. Once this trust is built there needs to be an additional focus on strengthening women’s self-efficacy to accommodate the demands of the trial. Mechanisms proposed to achieve this can be implemented relatively easily and there are plans to test their effectiveness in increasing recruitment to existing pregnancy trials. Methods of building trust in research will benefit the whole research community and highlight the need to communicate with the public more effectively about research and its importance.
Funding
The data collection for this study was funded by a grant from the National Institute for Health Research (NIHR) Southampton Biomedical Research Centre. The trials on which this work draws were supported by grants from the Medical Research Council, British Heart Foundation, Arthritis Research UK, Danone Nutricia Early Life Nutrition, National Institute for Health Research (NIHR) Southampton Biomedical Research Centre, University of Southampton and University Hospital Southampton NHS Foundation Trust, NIHR Musculoskeletal Biomedical Research Unit, University of Oxford and the European Union's Seventh Framework Programme (FP7/2007-2013), projects Early Nutrition and ODIN under grant agreements numbers 289346 and 613977.
Footnotes
Disclosure statement:
JB, MB, NCH and TR have received funding from Danone Nutricia Early Life Nutrition. Members of HI’s team have received funding from Nestec and Abbott Nutrition. CC reports personal fees, consultancy, lecture fees, and honoraria from Alliance for Better Bone Health, Amgen, Eli Lilly, GlaxoSmithKline, Medtronic, Merck, Novartis, Pfizer, Roche, Servier, and Takeda, outside the submitted work. NCH reports personal fees, consultancy, lecture fees, and honoraria from Alliance for Better Bone Health, AMGen, MSD, Eli Lilly, Servier, Shire, Consilient Healthcare, and Internis Pharma, outside the submitted work. WL reports consultancy and lecture fees from Danone Nutricia, outside the submitted work.
All other authors have no conflicts of interest to declare.
References
- 1.Abel U, Koch A. The role of randomization in clinical studies: myths and beliefs. J Clin Epidemiol. 1999;52(6):487–97. doi: 10.1016/s0895-4356(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 2.Gul RB, Ali PA. Clinical trials: the challenge of recruitment and retention of participants. Journal of Clinical Nursing. 2010;19(1–2):227–33. doi: 10.1111/j.1365-2702.2009.03041.x. [DOI] [PubMed] [Google Scholar]
- 3.Rick J, Graffy J, Knapp P, Small N, Collier DJ, Eldridge S, et al. Systematic techniques for assisting recruitment to trials (START): study protocol for embedded, randomized controlled trials. Trials. 2014;15(1):407. doi: 10.1186/1745-6215-15-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher L, Hessler D, Naranjo D, Polonsky W. AASAP: A program to increase recruitment and retention in clinical trials. Patient Education and Counseling. 2012;86(3):372–7. doi: 10.1016/j.pec.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Treweek S, Mitchell E, Pitkethly M, Cook J, Kjeldstrøm M, Taskila T, et al. Strategies to improve recruitment to randomised controlled trials. Cochrane Database Syst Rev. 2010;4(4) doi: 10.1002/14651858.MR000013.pub5. [DOI] [PubMed] [Google Scholar]
- 6.Tooher RL, Middleton PF, Crowther CA. A thematic analysis of factors influencing recruitment to maternal and perinatal trials. BMC pregnancy and childbirth. 2008;8(1):36. doi: 10.1186/1471-2393-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rengerink KO, Opmeer BC, Logtenberg SL, Hooft L, Bloemenkamp KW, Haak MC, et al. Improving participation of patients in clinical trials-rationale and design of IMPACT. BMC medical research methodology. 2010;10(1):85. doi: 10.1186/1471-2288-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams CM, Maher CG, Hancock MJ, McAuley JH, Lin C-WC, Latimer J. Recruitment rate for a clinical trial was associated with particular operational procedures and clinician characteristics. Journal of clinical epidemiology. 2014;67(2):169–75. doi: 10.1016/j.jclinepi.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 9.McDonald AM, Knight RC, Campbell MK, Entwistle VA, Grant AM, Cook JA, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials. 2006;7(1):9. doi: 10.1186/1745-6215-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell MK, Snowdon C, Francis D, Elbourne DR, McDonald AM, Knight RC, et al. Recruitment to randomised trials: strategies for trial enrolment and participation study. The STEPS study. Health technology assessment. 2007;11(48):1–123. doi: 10.3310/hta11480. [DOI] [PubMed] [Google Scholar]
- 11.Coday M, Boutin-Foster C, Sher TG, Tennant J, Greaney ML, Saunders SD, et al. Strategies for retaining study participants in behavioral intervention trials: retention experiences of the NIH Behavior Change Consortium. Annals of Behavioral Medicine. 2005;29(2):55–65. doi: 10.1207/s15324796abm2902s_9. [DOI] [PubMed] [Google Scholar]
- 12.Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: a systematic review. Journal of clinical epidemiology. 1999;52(12):1143–56. doi: 10.1016/s0895-4356(99)00141-9. [DOI] [PubMed] [Google Scholar]
- 13.Stead M, Eadie D, Gordon D, Angus K. “Hello, hello—it’s English I speak!”: a qualitative exploration of patients’ understanding of the science of clinical trials. Journal of Medical Ethics. 2005;31(11):664–9. doi: 10.1136/jme.2004.011064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gross D, Fogg L. Clinical trials in the 21st century: The case for participant-centered research¶. Research in nursing & health. 2001;24(6):530–9. doi: 10.1002/nur.10010. [DOI] [PubMed] [Google Scholar]
- 15.El-Khorazaty MN, Johnson AA, Kiely M, El-Mohandes AA, Subramanian S, Laryea HA, et al. Recruitment and retention of low-income minority women in a behavioral intervention to reduce smoking, depression, and intimate partner violence during pregnancy. BMC public health. 2007;7(1):233. doi: 10.1186/1471-2458-7-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baird J, Jarman M, Lawrence W, Black C, Davies J, Tinati T, et al. The effect of a behaviour change intervention on the diets and physical activity levels of women attending Sure Start Children’s Centres: results from a complex public health intervention. BMJ Open. 2014;4(7) doi: 10.1136/bmjopen-2014-005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawrence W, Keyte J, Tinati T, Haslam C, Baird J, Margetts B, et al. A mixed-methods investigation to explore how women living in disadvantaged areas might be supported to improve their diets. Journal of Health Psychology. 2012;17(6):785–98. doi: 10.1177/1359105311425271. [DOI] [PubMed] [Google Scholar]
- 18.Hollada J, Marfori W, Tognolini A, Speier W, Ristow L, Ruehm SG. Successful patient recruitment in CT imaging clinical trials: what factors influence patient participation? Academic radiology. 2014;21(1):52–7. doi: 10.1016/j.acra.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 19.Meshaka R, Jeffares S, Sadrudin F, Huisman N, Saravanan P. Why do pregnant women participate in research? A patient participation investigation using Q-Methodology. Health Expectations : An International Journal of Public Participation in Health Care and Health Policy. 2017;20(2):188–97. doi: 10.1111/hex.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenkins V, Fallowfield L. Reasons for accepting or declining to participate in randomized clinical trials for cancer therapy. British Journal of Cancer. 2000;82(11):1783. doi: 10.1054/bjoc.2000.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ECRI. Patients' Reasons for Participation in Clinical Trials and Effect of Trial Participation of Patient Outcomes. 2002;(74):1–38. [Google Scholar]
- 22.Austin E. Flying Double-blind: Would You Be Willing To Risk Your Health For Science? Chicago Tribune. 2000:16–23. [Google Scholar]
- 23.Rengerink KO, Logtenberg S, Hooft L, Bossuyt PM, Mol BW. Pregnant womens’ concerns when invited to a randomized trial: a qualitative case control study. BMC pregnancy and childbirth. 2015;15(1):207. doi: 10.1186/s12884-015-0641-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohanna K, Tunna K. Withholding consent to participate in clinical trials: decisions of pregnant women. BJOG: An International Journal of Obstetrics & Gynaecology. 1999;106(9):892–7. doi: 10.1111/j.1471-0528.1999.tb08426.x. [DOI] [PubMed] [Google Scholar]
- 25.Poston L, Briley AL, Barr S, Bell R, Croker H, Coxon K, et al. Developing a complex intervention for diet and activity behaviour change in obese pregnant women (the UPBEAT trial); assessment of behavioural change and process evaluation in a pilot randomised controlled trial. BMC pregnancy and childbirth. 2013;13(1):148. doi: 10.1186/1471-2393-13-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poston L. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre randomised controlled trial. Lancet Diabetes Endocrinol. 2015;3 doi: 10.1016/S2213-8587(15)00227-2. [DOI] [PubMed] [Google Scholar]
- 27.Kinnunen TI, Aittasalo M, Koponen P, Ojala K, Mansikkamäki K, Weiderpass E, et al. Feasibility of a controlled trial aiming to prevent excessive pregnancy-related weight gain in primary health care. BMC pregnancy and childbirth. 2008;8(1):37. doi: 10.1186/1471-2393-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodger MA, Makropoulos D, Walker M, Keely E, Karovitch A, Wells PS. Participation of pregnant women in clinical trials: will they participate and why? American journal of perinatology. 2003;20(02):069–76. doi: 10.1055/s-2003-38318. [DOI] [PubMed] [Google Scholar]
- 29.Smyth RM, Jacoby A, Elbourne D. Deciding to join a perinatal randomised controlled trial: Experiences and views of pregnant women enroled in the Magpie Trial. Midwifery. 2012;28(4):e538–e45. doi: 10.1016/j.midw.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Lyerly AD, Namey EE, Gray B, Swamy G, Faden RR. Women's views about participating in research while pregnant. IRB. 2012;34(4):1. [PubMed] [Google Scholar]
- 31.Kenyon S, Dixon-Woods M, Jackson C, Windridge K, Pitchforth E. Participating in a trial in a critical situation: a qualitative study in pregnancy. Quality and Safety in Health Care. 2006;15(2):98–101. doi: 10.1136/qshc.2005.015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baker L, Lavender T, Tincello D. Factors that influence women's decisions about whether to participate in research: an exploratory study. Birth. 2005;32(1):60–6. doi: 10.1111/j.0730-7659.2005.00346.x. [DOI] [PubMed] [Google Scholar]
- 33.Mohanna K, Tunna K. Withholding consent to participate in clinical trials: decisions of pregnant women. British journal of obstetrics and gynaecology. 1999;106(9):892–7. doi: 10.1111/j.1471-0528.1999.tb08426.x. [DOI] [PubMed] [Google Scholar]
- 34.Pope C, Campbell R. Qualitative research in obstetrics and gynaecology. BJOG: An International Journal of Obstetrics & Gynaecology. 2001;108(3):233–7. doi: 10.1111/j.1471-0528.2001.00077.x. [DOI] [PubMed] [Google Scholar]
- 35.Doshani A, Pitchforth E, Mayne C, Tincello DG. The value of qualitative research in urogynaecology. BJOG: An International Journal of Obstetrics & Gynaecology. 2009;116(1):3–6. doi: 10.1111/j.1471-0528.2008.01924.x. [DOI] [PubMed] [Google Scholar]
- 36.Cooper C. Maternal gestational vitamin D supplementation and offspring bone health (MAVIDOS): a multicentre, double-blind, randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2016;4 doi: 10.1016/S2213-8587(16)00044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baird J, Barker M, Harvey NC, Lawrence W, Vogel C, Jarman M, et al. Southampton PRegnancy Intervention for the Next Generation (SPRING): protocol for a randomised controlled trial. Trials. 2016;17(1):493. doi: 10.1186/s13063-016-1603-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Creswell JW, Poth CN. Qualitative inquiry and research design: Choosing among five approaches. Sage publications; 2017. [Google Scholar]
- 39.Jenner B, Flick U, von Kardoff E, Steinke I. A companion to qualitative research. Sage; 2004. [Google Scholar]
- 40.Braun V, Clarke V. Successful qualitative research: A practical guide for beginners. Sage; 2013. [Google Scholar]
- 41.Kuzel AJ. Sampling in qualitative inquiry. 1992.
- 42.Fusch PI, Ness LR. Are we there yet? Data saturation in qualitative research. The Qualitative Report. 2015;20(9):1408. [Google Scholar]
- 43.Swift J, Tischler V. Qualitative research in nutrition and dietetics: getting started. Journal of Human Nutrition and Dietetics. 2010;23(6):559–66. doi: 10.1111/j.1365-277X.2010.01116.x. [DOI] [PubMed] [Google Scholar]
- 44.Boyatzis RE. Transforming qualitative information: Thematic analysis and code development. Sage; 1998. [Google Scholar]
- 45.Bandura A. Self-efficacy: The exercise of control. Macmillan; 1997. [Google Scholar]
- 46.Schul Y, Peri N. Influences of Distrust (and Trust) on Decision Making. Social Cognition. 2015;33(5):414–35. [Google Scholar]
- 47.Colquitt JA, Scott BA, LePine JA. Trust, trustworthiness, and trust propensity: a meta-analytic test of their unique relationships with risk taking and job performance. Journal of applied psychology. 2007;92(4):909. doi: 10.1037/0021-9010.92.4.909. [DOI] [PubMed] [Google Scholar]
- 48.Kim PH, Dirks KT, Cooper CD, Ferrin DL. When more blame is better than less: The implications of internal vs. external attributions for the repair of trust after a competence-vs. integrity-based trust violation. Organizational Behavior and Human Decision Processes. 2006;99(1):49–65. [Google Scholar]
- 49.Simpson JA. Psychological foundations of trust. Current directions in psychological science. 2007;16(5):264–8. [Google Scholar]
- 50.Chang LJ, Doll BB, van ’t Wout M, Frank MJ, Sanfey AG. Seeing is believing: Trustworthiness as a dynamic belief. Cognitive Psychology. 2010;61(2):87–105. doi: 10.1016/j.cogpsych.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 51.Winston JS, Strange BA, O'Doherty J, Dolan RJ. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nature neuroscience. 2002;5(3):277–83. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- 52.Delgado MR, Frank RH, Phelps EA. Perceptions of moral character modulate the neural systems of reward during the trust game. Nature neuroscience. 2005;8(11):1611–8. doi: 10.1038/nn1575. [DOI] [PubMed] [Google Scholar]
- 53.Falvello V, Vinson M, Ferrari C, Todorov A. The Robustness of Learning about the Trustworthiness of Other People. Social Cognition. 2015;33(5):368–86. [Google Scholar]
- 54.Croker JE, Swancutt DR, Roberts MJ, Abel GA, Roland M, Campbell JL. Factors affecting patients’ trust and confidence in GPs: evidence from the English national GP patient survey. BMJ Open. 2013;3(5) doi: 10.1136/bmjopen-2013-002762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bandura A. Health promotion from the perspective of social cognitive theory. Psychology and health. 1998;13(4):623–49. [Google Scholar]
- 56.Reddan G. Improving Exercise Science students’ self-efficacy in making positive career decisions
- 57.Barker M, Baird J, Lawrence W, Jarman M, Black C, Barnard K, et al. The Southampton Initiative for Health: a complex intervention to improve the diets and increase the physical activity levels of women from disadvantaged communities. Journal of health psychology. 2010 doi: 10.1177/1359105310371397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lawrence W, Black C, Tinati T, Cradock S, Begum R, Jarman M, et al. Making every contact count: Longitudinal evaluation of the impact of training in behaviour change on the work of health and social care practitioners. Journal of Health Psychology. 2016;21(2):138–51. doi: 10.1177/1359105314523304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abraham C, Gardner B. What psychological and behaviour changes are initiated by ‘expert patient’ training and what training techniques are most helpful? Psychology & Health. 2009;24(10):1153–65. doi: 10.1080/08870440802521110. [DOI] [PubMed] [Google Scholar]