Abstract
The risks to Arctic species from oil releases is a global concern, but their sensitivity to chemically dispersed oil has not been assessed using a curated and standardized dataset from spiked declining tests, which are exposures representative of surface oil spills. Species sensitivity to dispersed oil was determined by their position within species sensitivity distributions (SSDs) using three measures of hydrocarbon toxicity: total petroleum hydrocarbons (TPH), polycyclic aromatic hydrocarbon (PAHs), and naphthalenes. Comparisons of SSDs with Arctic/sub-Arctic versus non-Arctic species, and across SSDs of compositionally similar oils, showed that Arctic and non-Arctic species have comparable sensitivities even with the variability introduced by combining data across studies and oils. Regardless of hydrocarbon measure, hazard concentrations across SSDs were protective of sensitive Arctic species. While the sensitivities of Arctic species to oil exposures resemble those of commonly tested species, PAH-based toxicity data are needed for a greater species diversity including sensitive Arctic species.
Keywords: Oil products, Arctic, Species sensitivity, Acute toxicity, Spiked declining exposures, Chemical dispersant
Graphical abstract
1. Introduction
Increasing periods of open water in the Arctic have expanded shipping activities and opportunities for oil and gas exploration and production, resulting in greater potential for oil spills (Corbett et al., 2010; Gautier et al., 2009; Nevalainen et al., 2017; Noble et al., 2013). Exposure of Artic species in the aquatic environment to petroleum hydrocarbons may be compounded by slower hydrocarbon degradation (Brakstad and Bonaunet, 2006; Venosa and Holder, 2007) and volatilization of toxic fractions at low Arctic temperatures (Perkins et al., 2005), and oil dynamics in and under sea ice (Brandvik and Faksness, 2009; Payne et al., 1991; Seelye, 1979). Additionally, because of the limited complexity of Arctic food webs (e.g., five trophic levels; Borgå et al., 2004; Bradstreet and Cross, 1982; Hobson and Welch, 1992; Welch et al., 1992), impacts on key species such as Arctic cod Boreogadus saida and lower trophic level invertebrates may result in disruptions of energy transfer to higher trophic level vertebrates. Thus, understanding the sensitivity of Arctic species to oil products and other hazardous materials is of high scientific and ecological importance.
The relative sensitivity of Arctic aquatic species compared to temperate species to both physically and chemically dispersed oil has been a significant area of uncertainty because limited toxicity data generally exist for these species (Camus et al., 2015; Chapman and Riddle, 2005; de Hoop et al., 2011; Gardiner et al., 2013). Arctic species have unique biochemical and physiological adaptations that could alter sensitivity to contaminants including: 1) lower metabolic rates that contribute to slower contaminant uptake and delayed toxicological effects; 2) larger lipid content, and thus greater bioaccumulation potential; and 3) physiological adaptations including the presence of blood antifreeze peptides that may alter their sensitivity to petroleum hydrocarbons (Borgå et al., 2004; Chapman and Riddle, 2005; Clarke, 1980; Clarke and Johnston, 1999). Although region-specific toxicity data are ideal (Aurand and Coelho, 2005), there are practical challenges in conducting toxicity tests with Arctic species under controlled laboratory conditions. Challenges include limited seasonal availability of test organisms, and logistical constraints associated with both, culturing tests species and conducting exposures under Arctic conditions. Thus, there are substantial benefits to assessing if previous research on a broader array of species from different regions could be used as surrogates for Arctic species (Barron and Ka’aihue, 2003). Consequently, a re-evaluation of aquatic toxicity data with emphasis on Arctic species would provide further information useful in environmental decision making.
Despite the general recognition that polycyclic aromatic hydrocarbons (PAHs) and heterocyclic compounds are the major determinant of oil toxicity (e.g., Barron et al., 1999; NRC, 2005), most published studies on whole oil products have expressed toxicity as total petroleum hydrocarbon (TPH) concentrations (Bejarano et al., 2014). TPH-based toxicity for a diversity of fish and aquatic invertebrates has been used as a consistent metric for evaluating the relative sensitivity of aquatic species to oil products (e.g., Barron et al., 2013; Bejarano et al., 2014; de Hoop et al., 2011). Toxicity studies of individual hydrocarbon compounds, chemical dispersant-only tests, and the relatively limited studies on physically and chemically dispersed oil products using key pelagic Arctic species have provided insights into their relative sensitivity (de Hoop et al., 2011; Gardiner et al., 2013; Hansen et al., 2014; Hansen et al., 2011; Olsen et al., 2013a; Olsen et al., 2011), and have facilitated comparative assessments based mostly on constant exposures (Camus et al., 2015; de Hoop et al., 2011; Olsen et al., 2011). However, previous comparisons have not included toxicity data of chemically dispersed oil products or have focused solely on spiked declining oil exposures intended to represent typical conditions following surface oil spills. This has implications on the understanding of the relative impacts of chemically dispersed oil and the sensitivity of Arctic species.
The objective of this research was to reassess the acute sensitivity of Arctic and non-Arctic species to oil products using a comprehensive, highly curated and standardized dataset. Data collection focused on tests performed under standardized methods with chemically and physically dispersed oil using three metrics of petroleum hydrocarbon exposure: TPH, total PAHs, and parent naphthalene as a surrogate for water soluble PAHs. To minimize variation due to oil dosing method, only data for spiked declining oil exposures (Fuller et al., 2004; Gardiner et al., 2013) were used in these analyses. This type of research is important to understand if assumptions about the relative sensitivity of aquatic test species hold for species in the Arctic, and to determine if the relative species sensitivity from constant exposures (de Hoop et al., 2011) also apply to declining exposures. Furthermore, the outcomes of these analyses provide information critical to spill response and planning in the Arctic (e.g., derivation of thresholds of concern), including assessments on the relative risks associated with the use of chemical dispersants. Comparisons of relative species sensitivity may also provide further insights on how spill response actions in temperate waters would inform related actions in the Arctic.
2. Methods
Acute toxicity data (median lethal concentrations, LC50) for aquatic marine species from Arctic and non-Arctic regions were obtained from multiple sources (Anderson et al., 2009; Anderson et al., 1974; Aurand and Coelho, 2005; Bragin et al., 1994; Bragin and Clark, 1995; Clark et al., 2001; Fuller et al., 2004; Gardiner et al., 2013; Goodbody-Gringley et al., 2013; Hansen et al., 2011; Lin et al., 2009; Liu, 2003; Neff et al., 2000; Nordtug et al., 2011; Pace et al., 1995; Perkins et al., 2003, 2005; Rhoton, 2000; Rice et al., 1979; Riebel and Percy, 1990; Singer et al., 1996; Singer et al., 2001; Singer et al., 1998). Criteria for data inclusion were as follows: test performed with chemically dispersible fresh light and medium oil products (API gravity 31.3–44 and 24.8–30.6, respectively); aqueous exposure media prepared by physical (water accommodated fraction, WAF; and moderate energy WAF or MEWAF) or chemically enhanced oil dispersion (chemically enhanced water accommodated fraction, CEWAF); CEWAF prepared with Corexit 9500 or Corexit 9527; tests performed under spiked declining exposures as these are intended to represent typical exposure conditions that may occur following surface oil spills; LC50 values reported on the basis of measured aqueous exposures of TPH (aromatic and aliphatic hydrocarbons [C9–C44]), parent and alkylated homologue PAHs and/or parent naphthalene; and LC50 values reported without qualifiers. In cases where PAH concentrations were not explicitly reported (i.e., Aurand and Coelho, 2005), concentrations were estimated as a proportion of the PAH concentrations in the whole WAF solution. Each record was evaluated and duplicates reported by the same author across several sources removed from the final dataset. In all cases, verification and standardization of currently accepted scientific names was made by querying the world register of marine species (WoRMS Editorial Board, 2015), and designation of Arctic species made based on the Arctic register of marine species (Sirenko et al., 2015).
TPH, PAH and parent naphthalene species sensitivity distributions (SSDs) and their associated 5th percentile hazard concentrations (HC5), or concentrations assumed to be protective of 95% of the species on the SSD, were derived using the methodology detailed elsewhere (Bejarano and Farr, 2013). Briefly, toxicity values were fitted to a log-normal distribution function and randomly re-sampled 2,000 times to derive the SSD mean response and HC5 estimates with associated 95% confidence intervals (95% CI). Only SSDs that passed goodness of fit tests (α= 0.01) (the Anderson–Darling for SSDs with >7 species, and the Kolmogorov–Smirnov test statistics) were included in these analyses (Bejarano and Farr, 2013). Comparisons between pairs of SSDs were made via the log-likelihood (chi-square statistic) (Piegorsch and Bailer, 1997) by fitting individual (e.g., PAH and TPH SSDs) and pooled (e.g., PAH plus TPH SSD) datasets to a log-normal curve, followed by statistical comparisons of these resulting curves.
3. Results
Toxicity data for 35 marine species were included in these analyses, with a total of 8 Arctic species, 2 sub-Arctic species and 25 non-Arctic species, with most data being for crustaceans and fish. Calanoid copepods (Calanus glacialis and C. finmarchicus) and sculpin (Myoxocephalus sp., M. polyacanthocephalus) comprised the majority of the data for Arctic species, followed by Arctic cod (Boreogadus saida), Kelp shrimp (Eualus suckleyi), Dolly varden (Salvelinus malma), and a mysid shrimp (Mysis oculata). While non-Arctic species included a variety of temperate and tropical species, nearly 60% of all records were for standard test species commonly used in toxicity testing (i.e., mysid shrimp- Americamysis bahia, inland silverside- Menidia beryllina). There were 26 oil products in the dataset including Arctic North Slope (ANS), Prudhoe Bay (PB), Cook Inlet, Adriatic, Campbell, Harriett, Kuwait, North Sea, Norman-Wells, Norwegian Sea, Venezuelan, and Wonnich crude oil, as well as No. 2 fuel and Bunker C residual oil products, but not all three petroleum hydrocarbon metrics were available for each oil. Since in most cases there were insufficient data to generate SSDs for individual oil products, assessments were based on SSDs that combined oil products with similar physical/chemical properties (e.g., viscosity, hydrocarbon ranges).
3.1 Species Sensitivities by Region
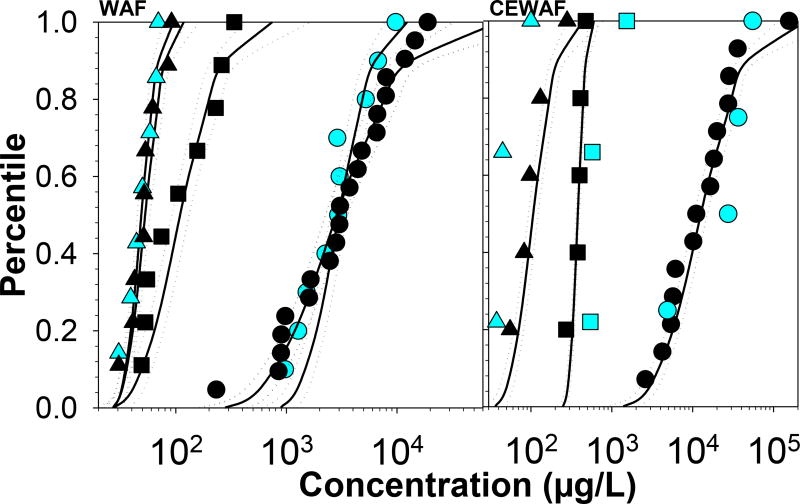
A first analysis included the comparison of SSDs developed for cold-water species (Arctic/sub-Arctic; combined) and non-Arctic species from WAF (including MEWAF) and CEWAF data. Given data limitations for cold-water species, SSDs were developed by combining data from all oil products. SSDs from WAF data for cold-water species and non-Arctic species, showed a high degree of overlap when data were available for the same petroleum hydrocarbon metric (TPH and parent naphthalene) (Figure 1). While SSDs for these two groups of species were not statistically significantly different for parent naphthalene (Chi-square statistic; p>0.05), these were different for TPHs (p<0.03) possibly because of a greater species diversity and the presence of a sensitive species (Montastraea faveolata; 5th percentile occupied on the SSD) within the dataset for non-Arctic species. Consistently HC5 estimates were comparable between these two groups of species for parent naphthalene, but much larger for cold-water species for TPHs (Table 1).
Figure 1.
SSDs for TPH (○), PAH (□) and parent naphthalene (△) with data from cold-water species (Arctic and sub-Arctic; blue) and non-Arctic species (back) for water accommodated fractions (WAF, including MEWAF) and chemically enhanced WAF (CEWAF) combining all available data for fresh light (API gravity 31.3–44; 16 oils) and fresh medium (API gravity 24.8–30.6; 4 oils) oil products.
Table 1.
Estimated HC5s and 95% CIs for TPH, PAH and parent naphthalene from several SSDs developed with data from cold-water species (Arctic/sub-Arctic) and non-Arctic species (combined) for water accommodated fractions (WAF, including MEWAF) and chemically enhanced WAF (CEWAF) combining all available data for fresh light (API gravity 31.3–44; 16 oils) and fresh medium (API gravity 24.8–30.6; 4 oils) oil products.
| Exposure media1 |
Species group |
Analytes | HC5 (95%CI) µg/L |
Most sensitive species | SSD Percentile |
|---|---|---|---|---|---|
| WAF+ MEWAF | Cold-water | TPH | 1,259 (988–1,583) | Salvelinus malma | 20th |
| Naphthalene | 32.4 (28.9–36.4) | Boreogadus saida | 14th | ||
| Non-Arctic | TPH | 579 (427–767) | Montastraea faveolata | 5th | |
| PAH | 41.1 (32.1–51.6) | Dendraster excentricus | 11th | ||
| Naphthalene | 32.9 (28.9–37.4) | D. excentricus | 11th | ||
| CEWAF | Non-Arctic | TPH | 2,909 (2,247–3,720) | Crassostrea gigas | 7th |
| PAH | 285 (265–305) | Holmesimysis costata | 20th | ||
| Naphthalene | 50 (41–60.2) | H. costata | 20th |
While there were some CEWAF data for cold-water species for all petroleum hydrocarbon metrics, these datasets were not sufficiently large (≤4 species) to generate SSDs. Despite these limitations, the available data showed similarities between cold-water and non-Arctic species even with the variability introduced by combining data across compositionally different oil products. Regardless of petroleum hydrocarbon metric, HC5 estimates were protective of the most sensitive species on the SSD, though the HC5s based on TPHs from both WAF and CEWAF for non-Arctic species were very close to the percentile occupied by the most sensitive species (5th and 7th percentiles, respectively).
3.2 Species Sensitivities by Oil Type
A second analysis included a comparison of SSDs that combined data for all available aquatic species for fresh oil products that shared similar chemical characteristics. Individual SSDs were developed for TPAH and TPH from WAF (including MEWAF) of light and medium oil products, and CEWAF of medium oil products (Figure 2). There was also sufficient data to generate an SSD for parent naphthalene from WAF with light oil products.
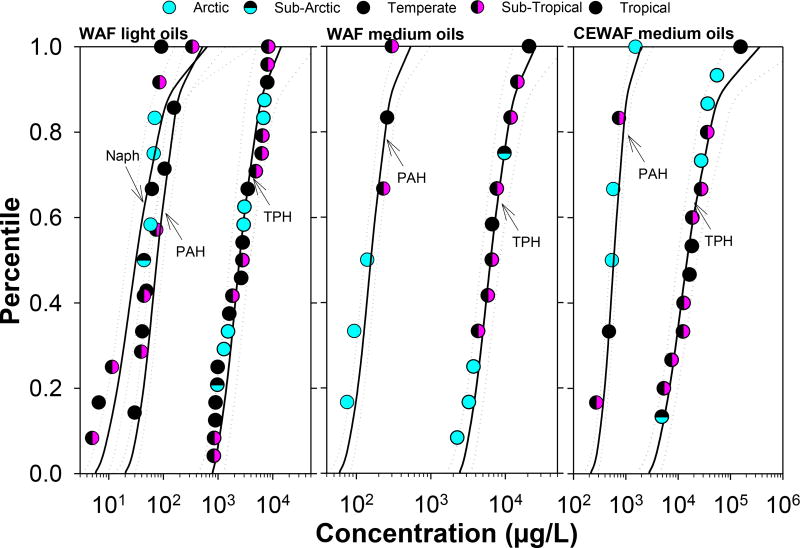
Figure 2.
SSDs developed with data from water accommodated fractions (WAF, including MEWAF) of fresh light oil products (API gravity 31.3–44; 16 oils), and WAF, and chemically enhanced WAF (CEWAF) of fresh medium oil products (API gravity 24.8–30.6; 4 oils).
For each of the WAF and CEWAF treatments, SSDs for TPH were significantly different from SSDs for TPAH and parent naphthalene (p<0.0001). These findings are not surprising given that the PAH fraction in oil is less than 10%. However, there was no significant difference between SSDs for parent naphthalene and PAH from WAF with light oil products (p=0.063), suggesting that parent naphthalene and possibly other low molecular weight PAHs were the drivers of acute toxicity. All SSDs had steep slopes and narrow 95% CI indicating that these data covered a relatively narrow range of concentrations, generally within one order of magnitude between extremes. When toxicity data were available for Arctic species, with the exception of SSDs from WAF with medium oil products, their relative sensitivity fell within the range of toxicity of non-Arctic species across most SSDs, with at least two species being slightly more sensitive than the most sensitive Arctic species on the SSD. In contrast, Arctic species on SSDs from WAF with medium oil products appear to be slightly more sensitive than non-Arctic species, with the most sensitive Arctic species being the Arctic cod (B. saida), followed by sculpin (Myoxocephalus sp.) and a calanoid copepods (C. glacialis). However, aquatic toxicity values for the most sensitive Arctic species were less than one order of magnitude of the least sensitive non-Arctic species.
HC5 estimates based on TPH and PAH from WAF with light oil products were smaller than those from WAF or CEWAF with medium oil products. These results are consistent with the relatively higher content of lighter hydrocarbon fraction in light oil products, which are known to be acutely toxic (Table 2). Average HC5 estimates for SSDs based on TPH and PAH from medium oil CEWAFs were 1.3 and 3.8 fold greater, respectively, than HC5 from WAF exposures, suggesting that chemical dispersants had a greater impact on the partitioning of PAHs into the aqueous exposure media than they did on the partitioning of TPHs. However, since HC5 estimates between WAF and CEWAF for similar oil products have overlapping confidence intervals for the same petroleum hydrocarbon metric, it is possible that their toxicity is relatively comparable. It is worth noting that regardless of petroleum hydrocarbon metric, HC5 estimates across media preparations were protective of the most sensitive Arctic species on the SSD, even when data from WAF and CEWAF were combined on the same SSD. In only one instance, the HC5 was close to the percentile occupied by an Arctic species (8th percentile), and when present, the mysid shrimp (A. bahia) occupied a similar or lower percentile on the SSD (more sensitive) than the percentile occupied by the most sensitive Arctic species.
Table 2.
Estimated HC5s and 95% CIs for TPH, PAH and parent naphthalene from several SSDs developed with data from water accommodated fractions (WAF, including MEWAF) and chemically enhanced WAF (CEWAF) with light (API gravity 31.3–44; 16 oils), medium (API gravity 24.8–30.6; 4 oils), Alaska North Slope (ANS), Prudhoe Bay (BP) and No. 2 fuel oil products.
| Exposure media1 | Analytes | HC5 (95%CI) µg/L |
Most sensitive Arctic species |
SSD Percentile |
|---|---|---|---|---|
| WAF+MEWAF light oils | TPH | 956 (590–1,472) | Salvelinus malma | 29th |
| PAH | 27.6 (20.1–37.7) | NA | NA | |
| Naphthalene | 8.13 (5.13–12.74) | S. malma | 58th | |
| WAF+MEWAF medium oils | TPH | 2,935 (2,115–3,993) | Boreogadus saida | 8th |
| PAH | 74.9 (58.4–95.4) | B. saida | 17th | |
| CEWAF medium oils | TPH | 3,907 (2,590–5,813) | Myoxocephalus sp. | 73th |
| PAH | 282 (225–350) | Calanus glacialis | 50th | |
| WAF+MEWAF No. 2 fuel oil | TPH | 488 (269–819) | Eualus suckleyi | 30th |
| WAF+MEWAF ANS oil | TPH | 2,501 (1,754–3,459) | B. saida | 13th |
| CEWAF ANS oil | TPH | 4,807 (3,270–6,958) | Myoxocephalus sp. | 75th |
| WAF+MEWAF +CEWAF ANS+PB oils | TPH | 5,600 (4,214–7,386) | B. saida | 25th |
| PAH | 170 (144–199) | Myoxocephalus sp. | 17th | |
| Naphthalene | 33.2 (26.8–40.7) | Myoxocephalus sp. | 17th |
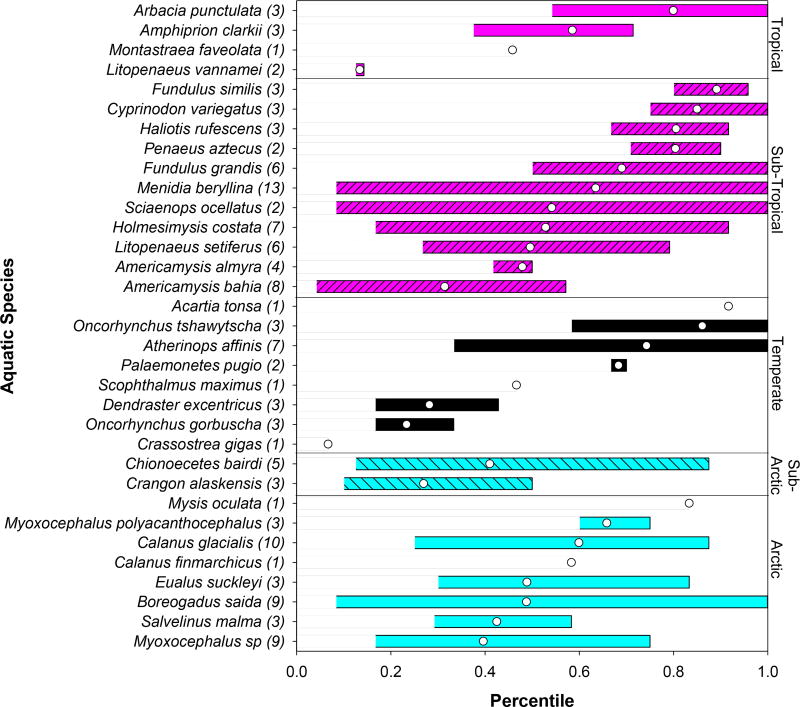
The range of species sensitives was further evaluated based on their relative position across 13 different SSDs. While in a few instances Arctic species were noted to fall either towards the lower or upper ends of SSDs from medium oil products, comparisons showed that the range of percentiles occupied by individual Arctic species was comparable to those of non-Arctic species (Figure 3). Overall, the range of sensitivities of the 8 Arctic species for which toxicity data were available closely resemble those of the most commonly tested temperate/subtropical species, with percentiles of Arctic species within the range of the commonly tested mysid shrimp (A. bahia) and inland silverside (M. beryllina). In 5 of 7 SSDs that included data for the mysid, this species was more sensitive (occupied a lower percentile) than the most sensitive Arctic species. By contrast, in 8 of 11 SSDs that included data for the inland silverside, this species was less sensitive (occupied a higher percentile) than the most sensitive Arctic species. When an Arctic species was more sensitive than these standard tests species, these were generally the Arctic cod (B. saida) or sculpin (Myoxocephalus sp.), which occupied a ≤17th percentile on the SSD. These results suggest that further development of SSDs with data from WAF and CEWAF that include the mysid, may also be protective of Arctic species.
Figure 3.
Range of percentiles occupied by individual species across 13 different SSDs. The extremes of the horizontal bars indicate minimum and maximum percentiles, while the white circle represents the mean percentile. The number in parenthesis indicates the number of SSDs in which individual species were included.
4. Discussion
In the current study, comparisons of the relative sensitivity of Arctic and non-Arctic species to oil products were made using SSDs. While SSDs have been widely accepted in the field of environmental toxicology, concerns regarding their utility are generally associated with the potential lack of ecological relevance (e.g., reviewed in Posthuma et al., 2002). However, in the absence of alternate approaches, SSDs are useful in supporting environmental decisions if these are developed with high-quality data and based on reasonable assumptions. As a result, SSDs are used and recommended in both regulatory and risk assessment applications (e.g., Posthuma et al., 2002; Suter II, 2016).
Results from the current study showed that: 1) there was a high degree of similarity between cold-water and non-Arctic species resulting in comparable HC5 estimates; 2) the relative sensitivity of Arctic species was within the range of non-Arctic species across most SSDs; and 3) HC5 estimates across media preparations were protective even of the most sensitive Arctic species. Some of these results are consistent with previous data interpretations concluding that there may not be significant regional differences in the sensitivity of aquatic species to a variety of compounds (Camus et al., 2015; Chapman and Riddle, 2005; de Hoop et al., 2011; Olsen et al., 2011), at least based on short acute exposures and existing toxicological information. In pure compound testing, Arctic and temperate fish and invertebrates did not differ in their sensitivity to naphthalene (de Hoop et al., 2011), 2-methyl-naphthalene (Olsen et al., 2011) or the dispersant Corexit 9500A (Hansen et al., 2014). Likewise, SSDs developed by de Hoop et al. (2011) for constant exposures to whole oil mixtures expressed as TPH, found a maximum three-fold difference in sensitivity between species from polar regions and species from other areas, but these differences were not statistically significant. Similar analyses using acute and chronic toxicity data from constant exposures to artificially produced water (a mixture of PAHs, alkylphenols and organic acids) also found that HC values for Arctic and temperate species were comparable (Camus et al., 2015).
While related comparative studies are available, there has been little emphasis on assessments that include toxicity data of chemically dispersed oil, and evaluations have not focused solely on spiked declining oil exposures intended to represent conditions typical of surface oil spills. In the current study, evaluations were made using curated data only from spiked declining oil exposures, with assessments based on several metrics of petroleum hydrocarbon exposure including PAHs, which have been recommended for hazard assessments (Bejarano et al., 2014). Overall, the range of percentiles occupied by individual Arctic species across several SSDs was comparable to those of non-Arctic species, and closely resembled the ranges occupied by common standard test species. Arctic species had similar or slightly lower sensitivities than the mysid (A. bahia), with Arctic cod (B. saida) or sculpin (Myoxocephalus sp.) being among the most sensitive Arctic species, falling in some cases below the 20th percentile of SSDs. In comparison, previous studies with naphthalene showed greater sensitivity of Arctic cod than temperate fish species (de Hoop et al., 2011), while Gardiner et al., (2013) did not find differences in sensitivities between Arctic cod, and three temperate fish species (A. affinis, M. beryllina, and F. grandis) and other invertebrate species (e.g. copepods, mysids or crabs). In the current study, Calanoid copepods were moderately sensitive to dispersed oil products as their position on SSDs was generally at or above the 50th percentile (7 of 9 SSDs). However, toxicity data has shown slightly higher sensitivity for C. finmarchicus (3,267± 1,548 µg/L; n=6) than for C. glacialis (6,180± 2,491 µg/L; n=3) (Gardiner et al., 2013; Hansen et al., 2011; Nordtug et al., 2011), which could be attributed to larger lipid contents in C. glacialis (Hansen et al., 2013; Hjorth and Nielsen, 2011; Jensen et al., 2008). Thus, understanding the biological traits that drive differences in relative species sensitivities continues to be a research priority.
The eight Arctic species included in the current study are ecologically important as they play key roles in the Arctic food web (e.g., Nahrgang et al., 2016; Nordtug et al., 2011). However, since limited oil toxicity data are currently available for these species, additional tests under various experimental conditions are needed at least for the most sensitive species. In the current study, juvenile Arctic cod was among the most sensitive Arctic species, yet, earlier life stages are likely to be even more sensitive. For example, eggs of Arctic cod exposed to low concentrations (<10 µg/L) of petroleum hydrocarbons in WAF from a light oil showed developmental effects manifested in larvae, including spine malformation, yolk sac alterations and reduced length (Nahrgang et al., 2016). Given current knowledge gaps, further toxicity studies could include spiked declining exposures with single hydrocarbons, and physically and chemically dispersed oil, reporting acute and chronic endpoints from an extended observation period, and when possible, including different life stages of the same species. Longer observation periods are critically important as Arctic species are known to exhibit increase response times to petroleum hydrocarbon exposures possibly related to their adaptations to low temperatures (Gardiner et al., 2013; Hansen et al., 2013; Olsen et al., 2011). Although not the focus of the current study, two additional and important research needs regarding exposures to petroleum hydrocarbons include: 1) understanding how unique characteristics of Arctic species (i.e., lower metabolic rates, larger lipid content, the presence of blood antifreeze peptides, etc.) influence their vulnerability; and 2) determining impacts on populations of key Arctic species and their implications on resilience and recovery, and ecological processes (i.e., trophic level impacts). In addition to these recommendations, there are several practical challenges in conducting toxicity tests under Arctic conditions requiring further consideration. The Arctic marine environment is extremely complex, with seasonally varying salinity, temperature, ice cover and incident solar radiation that can affect exposure pathways, hydrocarbon toxicity, and the sensitivity of aquatic organisms to petroleum hydrocarbon exposures (Nevalainen et al., 2017). Because of these complexities, standardized laboratory oil dosing and WAF preparations may not be representative of hydrocarbon partitioning and composition in the Arctic environment. Furthermore, toxicity data collected under prescribed test conditions may not represent the range of Arctic species sensitivity under varying or suboptimal salinity and temperature regimes (Barron and Ka’aihue, 2003). Phototoxicity is also known to be an important determinant of petroleum toxicity, but risks to Arctic species are largely unknown (Barron and Ka'aihue, 2001). Thus, additional research on several key areas is recommended to more completely define the sensitivity of Arctic species, and in particular on the contributing factors that influence oil toxicity in the Arctic environment. Data from such studies are important in informing both modelling efforts on the impacts of oil spills (Olsen et al., 2013b) and oil spill response and planning in the Arctic (NRC, 2014), as well as allowing further and more detailed comparisons of toxicological responses between Arctic and non-Arctic species.
The growing body of literature from comparative studies collectively support the premise that toxicity data from spiked declining and constant exposures on non-Arctic aquatic species could be used as surrogate for Arctic species, or to supplement datasets with Arctic species resulting in greater taxonomic and functional diversity. Assessments based on larger and more robust datasets would likely have lower uncertainty and may offer greater protection of the most sensitive species. These larger datasets could aid in risk based evaluations of physically and chemically-dispersed oil in the Arctic and in other cold-water regions.
Highlights.
Open waters in the Arctic have expanded opportunities for oil exploration
Species sensitivity was assessed using a curated dataset of spiked declining oil exposures
Arctic cod and sculpin are among the most sensitive Arctic species to oil exposures
Artic and non-Arctic aquatic species have comparable sensitivities to oil exposures
Hazard concentrations are protective of the most sensitive Arctic species
Acknowledgments
Special thanks to M. Smit (Shell Global) for his review of an earlier version of this manuscript. The views expressed in the present study are those of the authors and do not necessarily reflect the views or policies of the USEPA and the USACE. This publication does not constitute an endorsement of any commercial product.
Funding: Partial financial support of this research was received by J.Q. Word under a Joint Industry Program including Shell Exploration and Production Company, ExxonMobil Upstream Research Company, Statoil Petroleum ASA, ConocoPhillips Company, and BP.
References
- Anderson BS, Arenella-Parkerson D, Phillips BM, Tjeerdema RS, Crane D. Preliminary investigation of the effects of dispersed Prudhoe Bay Crude Oil on developing topsmelt embryos, Atherinops affinis. Environmental Pollution. 2009;157:1058–1061. doi: 10.1016/j.envpol.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Anderson JW, Neff JM, Cox BA, Tatem HE, Hightower GM. Characteristics of dispersions and water-soluble extracts of crude and refined oils and their toxicity to estuarine crustaceans and fish. Marine Biology. 1974;27:75–88. [Google Scholar]
- Aurand D, Coelho G. Technical Report. EM&A Inc.; Lusby, MD: 2005. Cooperative aquatic toxicity testing of dispersed oil and the “Chemical response to Oil Spills: Ecological Effects Research Forum (CROSERF)”; p. 79. [Google Scholar]
- Barron M, Podrabsky T, Ogle S, Ricker R. Are aromatic hydrocarbons the primary determinant of petroleum toxicity to aquatic organisms? Aquatic Toxicology. 1999;46:253–268. [Google Scholar]
- Barron MG, Hemmer MJ, Jackson CR. Development of aquatic toxicity benchmarks for oil products using species sensitivity distributions. Integrated Environmental Assessment and Management. 2013;9:610–615. doi: 10.1002/ieam.1420. [DOI] [PubMed] [Google Scholar]
- Barron MG, Ka'aihue L. Potential for photoenhanced toxicity of spilled oil in Prince William Sound and Gulf of Alaska waters. Marine Pollution Bulletin. 2001;43:86–92. doi: 10.1016/s0025-326x(01)00037-6. [DOI] [PubMed] [Google Scholar]
- Barron MG, Ka’aihue L. Critical evaluation of CROSERF test methods for oil dispersant toxicity testing under subarctic conditions. Marine Pollution Bulletin. 2003;46:1191–1199. doi: 10.1016/S0025-326X(03)00125-5. [DOI] [PubMed] [Google Scholar]
- Bejarano AC, Clark JR, Coelho GM. Issues and challenges with oil toxicity data and implications for their use in decision making: A quantitative review. Environmental Toxicology and Chemistry. 2014;33:732–742. doi: 10.1002/etc.2501. [DOI] [PubMed] [Google Scholar]
- Bejarano AC, Farr JK. Development of short, acute exposure hazard estimates: A tool for assessing the effects of chemical spills in aquatic environments. Environmental Toxicology and Chemistry. 2013;32:1918–1927. doi: 10.1002/etc.2255. [DOI] [PubMed] [Google Scholar]
- Borgå K, Fisk AT, Hoekstra PF, Muir DC. Biological and chemical factors of importance in the bioaccumulation and trophic transfer of persistent organochlorine contaminants in Arctic marine food webs. Environmental Toxicology and Chemistry. 2004;23:2367–2385. doi: 10.1897/03-518. [DOI] [PubMed] [Google Scholar]
- Bradstreet MS, Cross WE. Trophic relationships at high Arctic ice edges. Arctic. 1982:1–12. [Google Scholar]
- Bragin G, Clark J, Pace C. MSRC Technical Report Series 94-015. Marine Spill Response Corporation; Washington, DC: 1994. Comparison of physically and chemically dispersed crude oil toxicity to both regional and national test species under continuous and spiked exposure scenarios; pp. 1–45. [Google Scholar]
- Bragin GE, Clark JR. MSRC Technical Report Series. Marine Spill Response Corporation; Washington, D.C.: 1995. Chemically dispersed crude oils: toxicity to regional and national test species under constant and spiked exposures; pp. 1–44. [Google Scholar]
- Brakstad OG, Bonaunet K. Biodegradation of petroleum hydrocarbons in seawater at low temperatures (0–5 C) and bacterial communities associated with degradation. Biodegradation. 2006;17:71–82. doi: 10.1007/s10532-005-3342-8. [DOI] [PubMed] [Google Scholar]
- Brandvik PJ, Faksness L-G. Weathering processes in Arctic oil spills: Meso-scale experiments with different ice conditions. Cold Regions Science and Technology. 2009;55:160–166. [Google Scholar]
- Camus L, Brooks S, Geraudie P, Hjorth M, Nahrgang J, Olsen G, Smit M. Comparison of produced water toxicity to Arctic and temperate species. Ecotoxicology and Environmental Safety. 2015;113:248–258. doi: 10.1016/j.ecoenv.2014.12.007. [DOI] [PubMed] [Google Scholar]
- Chapman PM, Riddle MJ. Toxic effects of contaminants in polar marine environments. Environmental Science and Technology. 2005;39:200A–206A. doi: 10.1021/es0532537. [DOI] [PubMed] [Google Scholar]
- Clark JR, Bragin GE, Febbo R, Letinski DJ. Proceedings of the 2001 International Oil spill Conference. American Petroleum Institute; Tampa, FL, USA: 2001. Toxicity of physically and chemically dispersed oils under continuous and environmentally realistic exposure conditions: Applicability to dispersant use decisions in spill response planning; pp. 1249–1255. [Google Scholar]
- Clarke A. A reappraisal of the concept of metabolic cold adaptation in polar marine invertebrates. Biological Journal of the Linnean Society. 1980;14:77–92. [Google Scholar]
- Clarke A, Johnston NM. Scaling of metabolic rate with body mass and temperature in teleost fish. Journal of Animal Ecology. 1999;68:893–905. [Google Scholar]
- Corbett J, Lack D, Winebrake J, Harder S, Silberman J, Gold M. Arctic shipping emissions inventories and future scenarios. Atmospheric Chemistry and Physics. 2010;10:9689–9704. [Google Scholar]
- de Hoop L, Schipper AM, Leuven RSEW, Huijbregts MAJ, Olsen GH, Smit MGD, Hendriks AJ. Sensitivity of polar and temperate marine organisms to oil components. Environmental Science and Technology. 2011;45:9017–9023. doi: 10.1021/es202296a. [DOI] [PubMed] [Google Scholar]
- Fuller C, Bonner J, Page C, Ernest A, McDonald T, McDonald S. Comparative toxicity of oil, dispersant, and oil plus dispersant to several marine species. Environmental Toxicology and Chemistry. 2004;23:2941–2949. doi: 10.1897/03-548.1. [DOI] [PubMed] [Google Scholar]
- Gardiner WW, Word JQ, Word JD, Perkins RA, McFarlin KM, Hester BW, Word LS, Ray CM. The acute toxicity of chemically and physically dispersed crude oil to key Arctic species under Arctic conditions during the open water season. Environmental Toxicology and Chemistry. 2013;32:2284–2300. doi: 10.1002/etc.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier DL, Bird KJ, Charpentier RR, Grantz A, Houseknecht DW, Klett TR, Moore TE, Pitman JK, Schenk CJ, Schuenemeyer JH. Assessment of undiscovered oil and gas in the Arctic. Science. 2009;324:1175–1179. doi: 10.1126/science.1169467. [DOI] [PubMed] [Google Scholar]
- Goodbody-Gringley G, Wetzel DL, Gillon D, Pulster E, Miller A, Ritchie KB. Toxicity of Deepwater Horizon source oil and thechemical dispersant, Corexit® 9500, to coral larvae. PLoS ONE. 2013;8:e45574. doi: 10.1371/journal.pone.0045574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen BH, Altin D, Bonaunet K, Øverjordet IB. Acute toxicity of eight oil spill response chemicals to temperate, boreal, and Arctic species. Journal of Toxicology and Environmental Health. 2014;Part A 77:495–505. doi: 10.1080/15287394.2014.886544. [DOI] [PubMed] [Google Scholar]
- Hansen BH, Altin D, Øverjordet IB, Jager T, Nordtug T. Acute exposure of water soluble fractions of marine diesel on Arctic Calanus glacialis and boreal Calanus finmarchicus: Effects on survival and biomarker response. Science of the Total Environment. 2013;449:276–284. doi: 10.1016/j.scitotenv.2013.01.020. [DOI] [PubMed] [Google Scholar]
- Hansen BH, Altin D, Rørvik SF, Øverjordet IB, Olsen AJ, Nordtug T. Comparative study on acute effects of water accommodated fractions of an artificially weathered crude oil on Calanus finmarchicus and Calanus glacialis (Crustacea: Copepoda) Science of The Total Environment. 2011;409:704–709. doi: 10.1016/j.scitotenv.2010.10.035. [DOI] [PubMed] [Google Scholar]
- Hjorth M, Nielsen TG. Oil exposure in a warmer Arctic: potential impacts on key zooplankton species. Marine Biology. 2011;158:1339–1347. [Google Scholar]
- Hobson K, Welch H. Determination of trophic relationships within a high Arctic marine food web using δ13C and δ15 N analysis. Marine Ecology Progress Series. 1992;84:9–18. [Google Scholar]
- Jensen MH, Nielsen TG, Dahllöf I. Effects of pyrene on grazing and reproduction of Calanus finmarchicus and Calanus glacialis from Disko Bay, West Greenland. Aquatic Toxicology. 2008;87:99–107. doi: 10.1016/j.aquatox.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Lin CY, Anderson BS, Phillips BM, Peng AC, Clark S, Voorhees J, Wu H-DI, Martin MJ, McCall J, Todd CR, Hsieh F, Crane D, Viant MR, Sowby ML, Tjeerdema RS. Characterization of the metabolic actions of crude versus dispersed oil in salmon smolts via NMR-based metabolomics. Aquatic Toxicology. 2009;95:230–238. doi: 10.1016/j.aquatox.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Liu B. The School of Renewable Natural Resources. Louisiana State University; Baton Rouge, LA, USA: 2003. Toxicity of South Louisiana Crude Oil, Alaskan North Slope Crude Oil and Dispersant Corexit 9500 to Gulf Killifish, White Shrimp and Eastern Oyster. [Google Scholar]
- Nahrgang J, Dubourg P, Frantzen M, Storch D, Dahlke F, Meador JP. Early life stages of an Arctic keystone species (Boreogadus saida) show high sensitivity to a water-soluble fraction of crude oil. Environmental Pollution. 2016;218:605–614. doi: 10.1016/j.envpol.2016.07.044. [DOI] [PubMed] [Google Scholar]
- Neff JM, Ostazeski S, Gardiner W, Stejskal I. Effects of weathering on the toxicity of three offshore Australian crude oils and a diesel fuel to marine animals. Environmental Toxicology and Chemistry. 2000;19:1809–1821. [Google Scholar]
- Nevalainen M, Helle I, Vanhatalo J. Preparing for the unprecedented—Towards quantitative oil risk assessment in the Arctic marine areas. Marine Pollution Bulletin. 2017;114:90–101. doi: 10.1016/j.marpolbul.2016.08.064. [DOI] [PubMed] [Google Scholar]
- Noble B, Ketilson S, Aitken A, Poelzer G. Strategic environmental assessment opportunities and risks for Arctic offshore energy planning and development. Marine Policy. 2013;39:296–302. [Google Scholar]
- Nordtug T, Olsen AJ, Altin D, Overrein I, Storøy W, Hansen BH, De Laender F. Oil droplets do not affect assimilation and survival probability of first feeding larvae of North-East Arctic cod. Science of The Total Environment. 2011;412–413:148–153. doi: 10.1016/j.scitotenv.2011.10.021. [DOI] [PubMed] [Google Scholar]
- NRC. Oil Spill Dispersants: Efficacy and Effects. The National Academies Press; Washington DC: 2005. p. 377. [Google Scholar]
- NRC. Responding to Oil Spills in the U.S. Arctic Marine Environment. The National Academies Press; Washington, DC: 2014. p. 210. [Google Scholar]
- Olsen AJ, Nordtug T, Altin D, Lervik M, Hansen BH. Effects of dispersed oil on reproduction in the cold water copepod Calanus finmarchicus (Gunnerus) Environmental Toxicology and Chemistry. 2013a;32:2045–2055. doi: 10.1002/etc.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen G, Klok C, Hendriks AJ, Geraudie P, De Hoop L, De Laender F, Farmen E, Grøsvik BE, Hansen BH, Hjorth M. Toxicity data for modeling impacts of oil components in an Arctic ecosystem. Marine Environmental Research. 2013b;90:9–17. doi: 10.1016/j.marenvres.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Olsen GH, Smit MG, Carroll J, Jæger I, Smith T, Camus L. Arctic versus temperate comparison of risk assessment metrics for 2-methyl-naphthalene. Marine Environmental Research. 2011;72:179–187. doi: 10.1016/j.marenvres.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Pace CB, Clark JR, Bragin GE. Proceedings of the 1995 International Oil Spill Conference. American Petroleum Institute; Washington, DC: 1995. Comparing crude oil toxicity under standard and environmentally realistic exposures; pp. 1003–1004. [Google Scholar]
- Payne JR, Mcnabb GD, Jr, Clayton JR., Jr Oil-weathering behavior in Arctic environments. Polar Research. 1991;10:631–662. [Google Scholar]
- Perkins RA, Rhoton S, Behr-Andres C. Toxicity of dispersed and undispersed, fresh and weathered oil to larvae of a cold-water species, Tanner crab (C. bairdi), and standard warm-water test species. Cold Regions Science and Technology. 2003;36:129–140. [Google Scholar]
- Perkins RA, Rhoton S, Behr-Andres C. Comparative marine toxicity testing: A cold-water species and standard warm-water test species exposed to crude oil and dispersant. Cold Regions Science and Technology. 2005;42:226–236. [Google Scholar]
- Piegorsch WW, Bailer AJ. Statistics for Environmental Biology and Toxicology. Chapman & Hall; London, UK: 1997. [Google Scholar]
- Posthuma L, Suter GW, Traas TP. Species Sensitivity Distributions in Ecotoxicology. Lewis Publisher; Boca Raton, FL: 2002. [Google Scholar]
- Rhoton S. Acute Toxicity of the Oil Dispersant Corexit 9500, and Fresh and Weathered Alaska North Slope Crude Oil to the Alaskan Tanner Crab (C. bairdi), Two Standard Test Species, and V. fischeri (Microtox Assay) Alaska Department of Environmental Conservation. Institute of Northern Engineering, University of Alaska; Fairbanks, AK, USA: 2000. [Google Scholar]
- Rice SD, Moles A, Taylor TL, Karinen JF. International Oil Spill Conference. American Petroleum Institute; 1979. Sensitivity of 39 Alaskan marine species to Cook Inlet crude oil and No. 2 fuel oil; pp. 549–554. [Google Scholar]
- Riebel P, Percy J. Acute toxicity of petroleum hydrocarbons to the Arctic shallow-water mysid, Mysis oculata (Fabricius) Sarsia. 1990;75:223–232. [Google Scholar]
- Seelye M. A field study of brine drainage and oil entrainment in first-year sea ice. Journal of Glaciology. 1979;22:473–502. [Google Scholar]
- Singer M, George S, Jacobson S, Weetman L, Tjeerdema R, Blondina G, Sowby M, Aurand D. Evaluation of the aquatic effects of crude oil, dispersants, and their mixtures, Arctic and marine oil spill program technical seminar. Ministry of Supply and Services; Canada, Alberta, Canada: 1996. pp. 497–514. [Google Scholar]
- Singer MM, Aurand DV, Coelho GM, Bragin GE, Clark JR, Jacobson S, Sowby M, Tjeerdema R. Making, measuring, and using water-accommodated fractions of petroleum for toxicity testing; Proceedings of the 2001 International Oil Spill Conference; 2001. pp. 1269–1274. [Google Scholar]
- Singer MM, George S, Lee I, Jacobson S, Weetman LL, Blondina G, Tjeerdema RS, Aurand D, Sowby ML. Effects of dispersant treatment on the acute aquatic toxicity of petroleum hydrocarbons. Archives of Environmental Contamination and Toxicology. 1998;34:177–187. doi: 10.1007/s002449900302. [DOI] [PubMed] [Google Scholar]
- Sirenko B, Clarke C, Hopcroft R, Huettmann F, Bluhm B, Gradinger R. [on 2015-06-17];The Arctic register of marine species (ARMS) compiled by the Arctic Ocean Diversity (ArcOD) project. 2015 Accessed at http://www.marinespecies.org/arms.
- Suter GW., II . Organism-level extrapolation methods. In: Suter GW II, editor. Ecological risk assessment. Second. CRC press; Boca Raton, FL: 2016. pp. 357–381. [Google Scholar]
- Venosa A, Holder E. Biodegradability of dispersed crude oil at two different temperatures. Marine Pollution Bulletin. 2007;54:545–553. doi: 10.1016/j.marpolbul.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Welch HE, Bergmann MA, Siferd TD, Martin KA, Curtis MF, Crawford RE, Conover RJ, Hop H. Energy flow through the marine ecosystem of the Lancaster Sound region, Arctic Canada. Arctic. 1992:343–357. [Google Scholar]
- WoRMS Editorial Board. World Register of Marine Species. 2015 Available from http://www.marinespecies.org.