Abstract
Objective
To estimate the prevalence of anal HPV infection, genotype distribution, intraepithelial neoplasia (AIN) and correlates in a cohort of HIV-infected patients attending at Sexually Transmitted Infections (STI) clinic in Brazil.
Study design
A descriptive analysis was performed which includes, demographic, behavioral and clinical data. Anal specimens from HIV-positive men and women were collected during a regular visit and they were used for cytology and histopathology tests, as well as for HPV molecular identification.
Results
A total of 223 patients (143 females and 80 males) were enrolled in the study and, HPV was identified in 68.6% of the sample. The frequency of HR-HPV, HPV16/18 and multiple HPV infection were similar in both groups. The upstream regulatory region (URR) sequencing was carried out in 38 samples identified as HPV16-positive, and European variants were the most frequent (69.2%), followed by Africans (25.6%) and Asiatic-Americans (5.1%). Having more than 20 sexual partners was associated with multiple HPV infection (p = 0.000) while, anal sex and the first intercourse before 15 years of age was a risk factor for any HPV infection (p = 0.001). Being MSM (men who have sex with men) was a risk factor for any HPV and multiple infections (p = 0.002). The CD4 count >500 cells/mm3 was a protective factor for the HPV16/18 (p = 0.048) and multiple infections (p = 0.023), and the undetectable viral load and HAART treatment were both protective for any HPV (p = 0.010), HR-HPV (p = 0.091) and multiple infections (p = 0.006). Abnormal anoscopy was found in 23.7% (53/223) of the total number of patients, and this was significantly associated with all types of investigated HPV infections (p<0.0001).
Conclusions
In this study, anal HPV infection was common among young HIV-positive men and women, particularly in MSM. Anal cancer screening in patients at risk, such as those who are HIV-positive, and mainly those with anal HPV infection and a history of STI, will increase the likelihood of detecting anal intraepithelial neoplasia.
Introduction
Human papillomavirus (HPV) is a common sexually transmitted infection (STI)that can be categorized into two groups, low risk (LR-HPV) and high risk (HR-HPV) with respect to their risk of progression to malignancy [1]. Latent papillomaviruses are detectable only through the demonstration of HPV DNA in clinically and histologically normal skin and mucosa [2]. Most sexually active individuals will acquire at least one genotype of anogenital HPV infection at some time during their lifetime [3], but there are some risk and behavioral factors that could increase the infection frequency or persistence of the virus [4]. HR-HPV types are more disposed to progress to malign lesions, and some of them have variants that differ in biological and epidemiological patterns [5]. Furthermore, several co-infections, such as multiple HR-HPV or HIV co-infection could have a role in the higher probability of the progression of lesions [6].
Several studies identified a fluctuating HPV prevalence around the world, which could be due to some population characteristics, such as age and behaviors or to the sensitivity of the HPV DNA tests [7,8,9]. HR-HPV is associated with 100% of the cervical cancer cases and more than 80% of anal cancer [10,11,12]. The rate of anal cancer has been increasing over the years in patients at higher risk for persistence of HPV virus such as women with previous cervical lesions and in patients with some grade of immunosuppression, as transplanted and HIV positive [13,14,15,16].
The role of HPV in cervical lesions is well documented, but data of anal intraepithelial neoplasia is gaining importance more recently, in both men and women. In men, HPV infection has been strongly associated with anal cancer with approximately 88% of the anal squamous cell cancers occurring annually worldwide [17]. The prevalence of anal HPV infection is higher than cervical HPV infection in women; the same occurs for anal intraepithelial neoplasia (AIN), where the reported prevalence is 23–86% in HIV-positive and 5–22% in HIV-negative women [4,17,18]. Similarities in tumor biology have been demonstrated by anal and cervical cancer. Thus the implementation of an anal cancer screening program could bring a similar success as the cervical cancer program [19,20]. To date, there are no uniform screening guidelines for anal cancer, but some tools could be used in populations at higher risk for anal cancer development, such as a digital anorectal exam, anal Pap cytology and high-resolution anoscopy (HRA) [21].
The relationship between HIV and malignancies has been described, being significantly associated with anal cancer when comparing the general population [10]. Although there are no randomized experiments that demonstrate the effectiveness of this strategy, the anal cancer screening has been discussed and encouraged in groups that are considered to be at risk [22], however, this procedure is not regularly performed during a clinical routine in Brazil. As the higher prevalence and higher risk of complications of HPV infection in HIV-infected patients are well known [23], the screening would be an important preventative tool. Consequently, the goal of this study was to estimate the prevalence of anal HPV infection, HPV genotype distribution and correlates with anal HPV infection and anal intraepithelial neoplasia in HIV-seropositive patients of both genders.
Materials and methods
This is a cross-sectional study of HIV-positive men and women (18–69 years of age) attending a public STI/HIV screening and treatment center in Vitória, Brazil, from March 2013 to February 2016. The Ethics Committee of Universidade Federal do Espírito Santo (UFES), Brazil approved this study. Participants signed a written informed consent before being included in the study. They were invited to answer an interview about socio-demographic, behavioral and clinical data and performing an anal examination, including sample collection.
Interview data
A 20-minutes face-to-face interview was conducted with the use of a standardized questionnaire. Enrolled patients answered the interview questions, which included demographic (age, schooling, marital status, family income); behavioral (tobacco use, alcohol and illicit drug use, age at first sexual intercourse, number of sex partners, types of sexual activity, frequency of condom use in the last year and STI history) and clinical data (use of highly active antiretroviral therapy (HAART), CD4 counts and viral load).
Anal specimen collection
Sample collection was undertaken using a cytobrush inserted 4.0 cm into the anal canal and performing a spiral motion to seize samples from the entire circumference of the anal canal. The samples were spread onto a microscope slide and stained using the Papanicolaou method. The classification of cytological findings was undertaken according to the Bethesda system of cervical cytology [24]. The specimens were fixed in a 10% formalin buffered solution and sent to the laboratories of the Department of Pathology of the University Hospital for processing and analysis. Two pathologists analyzed all the cytological and anal biopsy samples.
DNA extraction / HPV detection and typing
Anal samples were obtained using cytobrush and placed in 2 ml tube containing TE buffer (10mM Tris-HCl; 1mM EDTA; pH 8.0) and store at -70°C. The DNA was isolated using a QIAamp DNA Mini Kit™ kit (QIAGEN INC, Valencia, California. The USA) according to the manufacturer's instructions. The HPV DNA was detected by amplification with PGMY09/11 primers [25], and the positive samples were genotyped by Restriction Fragment Length Polymorphism (RFLP) [26] and by Reverse Line Blot (RLB) [27]. A fragment of the upstream regulatory region (URR) of all HPV16 positive samples was sequenced and aligned together with HPV16 reference sequences of each sublineage in order to identify the HPV variants [28].
High-resolution anoscopy (HRA)
Subjects with any grade of anal cytological abnormality or anal HR-HPV were referred for examination using high-resolution anoscopy (HRA). The examination was performed with a colposcope, and a gynecologist with expertise in colposcopy performed all HRA procedures. A cotton ball with 3% acetic acid was used while anal mucosa was observed and the anoscope was moving out of the anal canal. The results of HRA were considered positive or negative for aceto-white lesions, dots, mosaics, atypical vessels following the Consensus terminology of 2016 IANS International Guidelines for Practice Standards in the Detection of Anal Cancer Precursors [29]. Punch anal biopsy was performed on all suspicious areas (visible lesions) after application of acetic acid.
Statistical analysis
Frequency distribution and descriptive statistics were used to describe the study variables. Estimated prevalence and 95% confidence interval for any HPV type, HR-HPV, HPV16/18 and multiple HPV infection were calculated. Descriptive statistics were calculated in order to describe the most prevalent genotypes in the study. Chi-square analysis or Fisher's exact test were used to evaluate differences in categorical outcome measures. Socio-demographic characteristics were stratified into two groups by gender. Data were coded and stored anonymously in a database. The statistical software SPSS v. 20.0 (Statistical Package for the Social Sciences, IBM, Chicago, USA) was used for the analyses.
Results
Anal samples were collected from 223 HIV-seropositive patients, being 80 men and 143 women, and the median age was 40.0 years (SD = 11.20). Women and men presented the same average with respect to age, the majority were employed (69%) and had completed a secondary education (57.4%) (Table 1). A total of 64.6% reported previous STI and the most common was the Condyloma acuminatum (22.9%), followed by syphilis (16.6%), and 27.7% had more than one STI. The HPV test was positive in 68.6% (153/223) of patients, 71.3% (57/80) among men, and 67.1% (96/143) among women.
Table 1. Socio-demographic characteristics of a sample of patients attending an STI clinic in Vitoria, Brazil (n = 223).
| Variable | Total (%) | Woman | Man | p-value |
|---|---|---|---|---|
| Age (years) | 40.6 ±11.2 | 41.0 ±10.4 | 40.0 ± 12.6 | 0.060 |
| Education level | < 0.0001 | |||
| Less than five years | 29 (13.9) | 24 (17.4) | 05 (7.0) | |
| Primary education | 60 (28.7) | 47 (34.1) | 13 (18.3) | |
| Secondary education | 86 (41.1) | 57 (41.3) | 29 (40.8) | |
| Higher education | 34 (16.3) | 10 (7.2) | 24 (33.8) | |
| Employed | 0.001 | |||
| No | 48 (22.5) | 42 (30.2) | 06 (8.1) | |
| Yes | 147 (69.0) | 86 (61.9) | 61 (82.4) | |
| Retired | 18 (08.5) | 11 (07.9) | 07 (09.5) | |
| Marital status | < 0.001 | |||
| Single | 87 (41.2) | 40 (28.8) | 47 (65.3) | |
| Married | 97 (46.0) | 76 (54.7) | 21 (21.6) | |
| Separated | 13 (06.2) | 10 (07.2) | 03 (04.2) | |
| Widow | 14 (06.6) | 13 (09.4) | 01 (07.1) | |
| Self-reported STI | 0.015 | |||
| Yes | 144 (67.9) | 87 (62.6) | 57 (78.1) | |
| No | 68 (32.1) | 52 (37.4) | 16 (21.9) | |
| Body mass index | 0.060 | |||
| Under weight | 21 (10.2) | 16 (12.4) | 05 (6.5) | |
| Eutrophic | 86 (41.7) | 48 (37.2) | 38 (49.4) | |
| Over weight | 46 (36.9) | 46 (35.7) | 30 (39.0) | |
| Obese | 23 (11.2) | 19 (14.7) | 04 (05.2) | |
| HIV diagnosed (years) | 0.003 | |||
| < 5 | 93 (41.7) | 47 (32.9) | 46 (57.5) | |
| 11 to 10 | 62 (27.8) | 43 (30.0) | 19 (23.8) | |
| 11 to 20 | 65 (29.1) | 51 (35.7) | 14 (17.5) | |
| > 20 | 03 (01.3) | 02 (01.4) | 01 (01.2) | |
| Cytology abnormal | 53 (25.5) | 26 (19.4) | 27 (36.5) | 0.007 |
| AIN (biopsy) | 29 (13.0) | 15 (10.5) | 14 (17.5) | 0.101 |
| HPV + | 153 (68.6) | 96 (67.1) | 57 (71.3) | 0.315 |
Regarding cytological abnormalities in anal specimens, ASC-US (Atypical Squamous Cells of Undetermined Significance), LSIL (Low-grade squamous intraepithelial lesion) and HSIL (High-grade squamous intraepithelial lesion) were found in 20.6%, 2.2% and 0.8% of the total samples, respectively. Classifying by gender, abnormal results were significantly more common in males than in females (p = 0.008), and the abnormalities also were also associated with being HPV positive in the male group (p = 0.038). Thirty-three participants were undergoing biopsies with a colposcopic examination of the anal canal. Twenty-nine (18.3%) participants were diagnosed with anal intraepithelial neoplasia (AIN), twenty cases of LSIL, two cases of HSIL and one case of the anal adenocarcinoma. All the cases of HSIL were related to genotypes of HPV 16 or 18 in HIV-positive women.
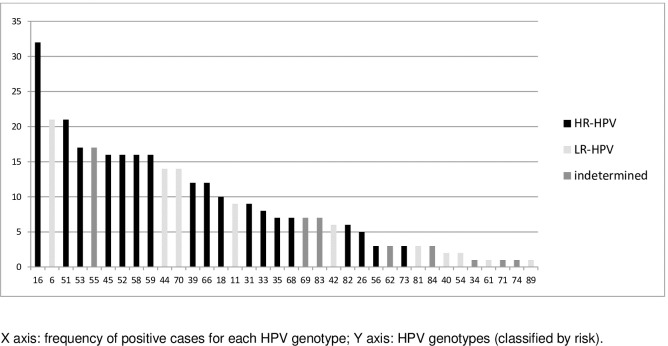
HR-HPV was found in 50.7% (113/223) of the total samples and 74% (113/153) of sample when considering only the HPV-positive cases. HPV16/18 cases were very frequent (40.7%; 46/113), in 32% of HR-HPV positive cases in men and 67.4% in women. The frequency of other HPV genotypes is shown (Fig 1). The URR sequencing was successfully carried out in 38 samples identified as HPV16-positive, and European variants were the most frequent (69.2%), with similar frequency in both groups, followed by African (25.6%) and Asiatic-Americans (5.1%).
Fig 1. Distribution of HPV genotypes in HIV patients attending a STI clinic in Vitoria, Brazil (n = 223).
Behavioral data and their association with HPV are shown in (Table 2). Being younger than 35 years of age was associated to any HPV type (p = 0.002) and multiple HPV infection (p = 0.001); having more than 20 sexual partners was associated with multiple HPV infection (p = 0.000); anal sex was associated with the presence of any HPV (p = 0.001). Reporting of a first intercourse before 15 years of age was linked to any HPV (p = 0.000) and HR-HPV (p = 0.004). Being MSM (Men who have sex with men) was a risk factor for any HPV (p = 0.004), HPV16/18 (p = 0.041) and multiple infections (p = 0.003).
Table 2. Behavioral aspects of HIV patients attending an STI clinic in Vitoria, Brazil (n = 223).
| Variable | Any HPV (n = 153) | HR-HPV (n = 113) | HPV16/18 (n = 46) | Multiple HPV types (n = 85) |
|---|---|---|---|---|
| Age | ||||
| < 25 years | 12 (92.3) | 09 (69.2) | 04 (30.8) | 06 (54.5) |
| 26–35 years | 42 (80.8) | 32 (61.5) | 12 (23.1) | 29 (55.8) |
| 36–50 years | 79 (63.2) | 60 (48.0) | 25 (20.0) | 41 (33.1) |
| > 50 years | 20 (60.6) | 12 (36.4) | 05 (15.2) | 09 (27.3 |
| X2 | 9.640 | 10.731 | 1.642 | 11.033 |
| p-value | 0.022 | 0.097 | 0.650 | 0.012 |
| Number of Partners | ||||
| < 5 partners | 34 (57.6) | 27 (45.8) | 14 (23.7) | 16 (27.1) |
| 6–20 partners | 59 (70.2) | 39 (45.9) | 13 (15.3) | 27 (32.1) |
| 21–50 partners | 32 (82.1) | 24 (61.5) | 07 (17.9) | 22 (57.9) |
| > 50 partners | 28 (70.0) | 23 (57.5) | 12 (30.0) | 20 (51.3) |
| X2 | 6.637 | 7.274 | 4.140 | 13.370 |
| p-value | 0.013 | 0.014 | 0.058 | 0.000 |
| Wearing condoms | ||||
| Never | 31 (75.6) | 28 (68.3) | 13 (31.7) | 15 (36.6) |
| Sometimes | 42 (66.7) | 28 (44.4) | 05 (7.9) | 29 (46.0) |
| Always | 80 (67.2) | 57 (47,9) | 28 (23.5) | 41 (35.3) |
| X2 | 1.149 | 7.039 | 9.884 | 2.056 |
| p-value | 0.563 | 0.134 | 0.070 | 0.358 |
| Anal sex | ||||
| Yes | 122 (74.8) | 92 (56.4) | 39 (23.9) | 71 (44.1) |
| No | 31 (51.7) | 21 (35.0) | 07 (11.7) | 14 (23.7) |
| X2 | 10.942 | 8.247 | 4.026 | 7.557 |
| p-value | 0.001 | 0.016 | 0.045 | 0.006 |
| First sexual intercourse before 15 years of age | ||||
| Yes | 60 (84.5) | 47 (66.2) | 14 (19.7) | 33 (47.8) |
| No | 93 (61.2) | 66 (43.4) | 32 (21.1) | 52 (34.4) |
| X2 | 12.223 | 11.569 | 0.053 | 3.581 |
| p-value | <0.0001 | 0.003 | 0.819 | 0.058 |
| Drug use | ||||
| Yes | 46 (68.7) | 36 (53.7) | 15 (22.4) | 33 (49.3) |
| No | 107 (68.6) | 77 (49.4) | 31 (19.9) | 52 (34.0) |
| X2 | 0.00 | 3.183 | 0.181 | 4.581 |
| p-value | 0.992 | 0.204 | 0.670 | 0.320 |
| Smoking | ||||
| Yes | 30 (66.7) | 25 (55.6) | 11 (24.4) | 21 (46.7) |
| No | 123 (69.1) | 88 (49.4) | 35 (19.7) | 64 (36.6) |
| X2 | 0.084 | 0.608 | 0.502 | 1.539 |
| p-value | 0.450 | 0.738 | 0.479 | 0.215 |
| Sexual identity | ||||
| Woman | 96 (67.1) | 70 (49.0) | 31 (21.7) | 49 (34.5) |
| MSM | 48 (81.4) | 37 (62.7) | 15 (25.4) | 32 (56.1) |
| Straight man | 09 (42.9) | 06 (28.6) | 0 (0) | 04 (19.0) |
| X2 | 11.062 | 8.005 | 6.383 | 11.786 |
| p-value | 0.004 | 0.091 | 0.041 | 0.003 |
n: number of people within the population; Any HPV: Any HPV; HR-HPV: high-risk HPV; HPV 16/18: HPV 16 or 18; Multiple HPV: more than one type of HPV in the same person; MSM: men who have sex with men.
HPV infection and HIV data are shown in (Table 3). CD4 count >500cells/mmᶟ was a protective factor for HPV16/18 (p = 0.048) and multiple infections (p = 0.023); the undetectable viral load was protective for any HPV (p = 0.046), HR-HPV (p = 0.003) and multiple infections (p<0.001), and not being in a program of HAART treatment was a risk factor for any HPV (p = 0.010) and multiple infections (p = 0.006). Abnormal anoscopy was found in 23.7% of the total patients (53/223), and it was significantly associated with all types of investigated HPV infections.
Table 3. Prevalence and correlates associated with anal HPV infection in HIV patients attending an STI clinic in Vitoria, Brazil (n = 223).
| Variable | Any HPV (n = 153) | HR-HPV (n = 113) | HPV16/18 (n = 46) | Multiple HPV types (n = 85) |
|---|---|---|---|---|
| HIV time | ||||
| Less than 5 years | 69 (75.0) | 52 (56.5) | 20 (21.7) | 42 (46.7) |
| 5–10 years | 36 (57.1) | 25 (39.7) | 08 (12.7) | 17 (27.0) |
| 11–20 years | 45 (70.8) | 35 (53.8) | 17 (26.8) | 24(37.5) |
| Over 20 years | 03 (100.0) | 01 (33.3) | 01 (33.3) | 02 (66.7) |
| X2 | 6.975 | 7.349 | 3.997 | 7.085 |
| p-value | 0.073 | 0.060 | 0.262 | 0.069 |
| Undetectable viral load | ||||
| Yes | 86 (64.2) | 56 (41.8) | 20 (14.9) | 36 (27.1) |
| No | 64 (77.1) | 54 (65.1) | 23 (27.7) | 47 (58.0) |
| X2 | 3.998 | 11.396 | 5.273 | 20.320 |
| p-value | 0.046 | 0.003 | 0.220 | <0.0001 |
| CD4 count (cells/mmᶟ) | ||||
| < 200 | 12 (80.0) | 10 (66.7) | 06 (40.0) | 10 (66.7) |
| 200 a 349 | 23 (76.7) | 16 (53.3) | 09 (30.0) | 13 (44.8) |
| 350 a 500 | 30 (76.9) | 25 (64.1) | 04 (10.3) | 18 (47.4) |
| > 500 | 85 (62.5) | 59 (43.4) | 25 (18.4) | 43 (31.9) |
| X2 | 6.314 | 11.577 | 12.278 | 11.479 |
| p-value | 0.277 | 0.314 | 0.031 | 0.043 |
| HAART | ||||
| Yes | 117 (64.6) | 85(47.0) | 36 (19.9) | 61 (34.1) |
| No | 35 (85.4) | 27 (65.0) | 09 (22.0) | 23 (57.5) |
| X2 | 6.651 | 4.785 | 0.880 | 7.585 |
| p-value | 0.010 | 0.091 | 0.767 | 0.006 |
| Previous STI | ||||
| Yes | 100 (69.4) | 73 (50.7) | 35 (24.3) | 59 (41.8) |
| No | 44 (64,7) | 39 (50.0) | 10 (14.7) | 19 (27.9) |
| X2 | 0.811 | 0.113 | 2.546 | 3.791 |
| p-value | 0.368 | 0.945 | 0.111 | 0.052 |
| Abnormal anoscopy | ||||
| Yes | 32 (97.0) | 23(69.7) | 14 (42.4) | 19 (57.6) |
| No | 57 (45.2) | 42 (33.3) | 14 (11.1) | 29 (23.0) |
| X2 | 28.399 | 14.595 | 17.673 | 14.820 |
| p-value | <0.0001 | 0.0001 | <0.0001 | 0.0001 |
| Abnormal anal cytology | ||||
| Yes | 41 (77.4) | 32 (60.4) | 18 (34.0) | 28 (54.9) |
| No | 103 (66.5) | 72 (46.5) | 35 (14.8) | 51 (33.1) |
| X2 | 2.206 | 3.202 | 9.127 | 7.677 |
| p-value | 0.138 | 0.202 | 0.003 | 0.006 |
| AIDS-defining illness | ||||
| Yes | 38 (76.0) | 32 (64.0) | 10 (20.9) | 21 (42.0) |
| No | 115 (66.9) | 81 (47.1) | 36 (16.7) | 64 (37.0) |
| X2 | 1.511 | 5.054 | 0.200 | 0.277 |
| p-value | 0.219 | 0.080 | 0.886 | 0.599 |
n: number of people within the population; Any HPV: Any type of HPV; HR-HPV: high-risk HPV; HPV 16/18: HPV 16 or 18; Multiple HPV: more than one type of HPV in the same person; HAART: highly active antiretroviral therapy, CD4: CD4 + T lymphocytes rate per microliter blood.
The comparison of behavioral aspects of anal cytology results are shown. (Table 4). Fifty-three (25.5%) participants were diagnosed with abnormal cytology.
Table 4. Comparison of behavioral aspects by anal cytology results in HIV patients attending an STI clinic in Vitoria, Brazil (n = 223).
| Variable | Abnormal cytology (n = 53) | Normal cytology (n = 155) |
|---|---|---|
| Number of partners | n (%) | n (%) |
| < 20 | 29 (21.2) | 107 (75.9) |
| > 20 | 24 (33.8) | 47 (66.2) |
| X2 | 3.814 | 3.814 |
| p-value | 0.051 | 0.051 |
| Wearing condom | ||
| Never | 07 (20.0) | 31 (81.6) |
| Sometimes | 13 (24.5) | 46 (78.05) |
| Always | 33 (34.0) | 78 (70.3) |
| X2 | 2.422 | 2.422 |
| p-value | 0.298 | 0.298 |
| Anal sex | ||
| Yes | 45 (21.6) | 108 (51.9) |
| No | 8 (4.0) | 47 (22.6) |
| X2 | 4.709 | 4.709 |
| p-value | 0.030 | 0.030 |
| First intercourse <15 years of age | ||
| Yes | 20 (29.4) | 48 (70.6) |
| No | 33 (23.6) | 107 (76.4) |
| X2 | 0.822 | 0.822 |
| p-value | 0.365 | 0.365 |
| Use of drugs | ||
| Yes | 24 (38.1) | 39 (61.9) |
| No | 29 (20.0) | 116 (80.0) |
| X2 | 7.573 | 7.573 |
| p-value | 0.006 | 0.006 |
| Smoking | ||
| Yes | 14 (26.4) | 29 (55.6) |
| No | 39 (23.6) | 126 (48.0) |
| X2 | 1.430 | 1.596 |
| p-value | 0.232 | 0.206 |
| Sexual identity | ||
| Woman | 26 (19.4) | 108 (80.6) |
| MSM | 24 (43.6) | 31 (56.4) |
| Straight men | 03 (15.8) | 16 (84.2) |
| X2 | 13.094 | 13.094 |
| p-value | 0.001 | 0.001 |
MSM: men who have sex with men.
Discussion
This study is the first epidemiological study conducted in Espírito Santo state, Brazil, that reports the prevalence rates, the distribution of HPV genotypes and the factors associated with anal HPV infection in HIV patients. The prevalence of anal HPV infection was frequent in this study (68.6%), and it was similar in both genders, even when considering HR-HPV and HPV16/18 infections. In the study population, MSM presented a significantly higher prevalence of HPV, as did having more than 20 partners. This result is similar to results of other studies that were conducted worldwide [7,8], and it points out the role of risk behavior in STI infections. Also, in this study, a first sexual intercourse before 15 years of age has a strong correlation with the HR-HPV infection.
Regarding the HPV genotypes, the tests used were able to identify 39 different types of HPV. The most frequent HR-HPV types were 16, 51 and 52, and the most frequent LR-HPV types were 6, 53 and 70. Some of the types have a low prevalence in the general population but present higher frequency in HIV population [8,30]. The HPV16 was the genotype that was more frequently found in our samples, and together with the HPV18 was significantly associated with abnormal anoscopy and abnormal anal cytology. We expect that following the HPV vaccination in young people, after a period of a few years, there should be a decrease the intraepithelial lesions caused by HPV 16 within a general population, this should also be true for cases of also HIV-seropositive [14; 31].
The results of a global review concluded that 84% of the invasive anal cancer cases contain HPV DNA, most individuals (87% of those who were HPV-positive) were positive for HPV16, and fewer (6%) were positive for HPV18 [32]. In a meta-analysis study, HPV was very common in the anal canal, and the causative agent of most anal cancer, of which HPV16 was detected in about one third (35%) of the HIV-positive men but only about one in eight (13%) of HIV-negative men [23]. The association of HPV16 with carcinoma could be explained by its elevate persistence rate which is higher than other HR-HPV types [33,34], and some HPV16 variants also showed a reduced clearance frequency [35,36]. Our study showed a higher frequency of HPV16 European variants and no association between non-European variants and AIN; the predominance of European variants in this study implies a lower risk for the development of HSIL as previously described in the body of literature covering our region [37]. Investigating HIV-negative patients in the same geographic area [38], found a consistent association between HPV16 non-European variants and high-grade cervical intraepithelial lesions [8]. This fact raises the relevance in studying the relation between variants and anal lesions since this relation is well documented for cervical malignancy [35,36,37].
In our study several socio-demographic characteristics had significant differences between men and women; higher education was more frequent in men; the more recent HIV diagnoses (less than 5 years) was more frequent in men, even with an average age over 40 years. Regarding clinical variables, HIV viral load and low CD4 counts were significantly associated with HR-HPV and multiple infections, which could raise the risk of persistent infection and consequently, of malignant development. Our result showed that HAART therapy was protective for multiple HPV infection, and it was previously demonstrated that HR-HPV prevalence was lower in patients in the long-term application of HAART [39, 40]. However, the incidence of HPV-induced lesions in the anal canal are still high in patients on a program of HAART [41], and the frequency of anal cancer was no different in the pre-HAART and HAART eras [42]. These results could suggest that the immunological restoration by medication was not enough for the clearance of HPV-persistent infection.
The prevalence of abnormal anal cytology (25.5%) shows that it is a common finding in HIV-infected patients. Repeated anal cytology testing and referral for HRA could increase the likelihood of AIN diagnosis. The highest positivity of abnormal anal cytology in our study occurred in the MSM population (43.6%), wich is similar to the rate of 53.6% described in a Spanish cohort of MSM that was HIV-infected [43]. Our study found one case of anal adenocarcinoma and two cases of AIN II in HIV positive women; however, because the disease prevalence is much higher in HIV-infected MSM compared with women, it is necessary to undertaken further studies to define the best screening tool for HIV positive women. We had a high concordance of HRA with positive biopsies (90.6%), showing that HRA is an important test for increasing the diagnosis of AIN, a similar result to the study by Gimenez in Manaus in Amazonas state, Brazil, which found a high sensitivity of the HRA, 90.0% [27]. In our study, 18.3% of HRA were positive for 159 examinations executed, a higher rate of positivity in MSM (41.4%). In general, AIN prevalence rates range from 10.4% to 68.0% among asymptomatic patients (28–30). The gold standard diagnostic for AIN is histopathology. For now, it is possible to note that anal cytology may be a screening test for AIN and cancer in an HIV population.
We found some study limitations, for example, missing data regarding additional tests such as anal cytology due to unsatisfactory samples, although all patients underwent anal cytology. Some patients refused to undergo anoscopy due to complaining of pain or bleeding. We did not have an additional blinded pathologist to perform confirmation of the results. Due to the low frequency of reporting some risk factors in the samples, the number of subjects studied was sufficient enough to find a statistical association between some independent variables and anal HPV infection. The possibility of having been response bias cannot be ruled out due to the general tendency to give socially acceptable answers. However, the limitations do not diminish the importance of this study in providing visibility to a neglected population during preventive actions in assisting HIV-infected individuals.
Some aspects of both genital cancers (cervical and anal) are shared, as the HPV infection and persistence as a risk factor, HPV16 as the main associated genotype and progression to carcinoma after the emergence of the previous intraepithelial lesions.
Since the HR-HPV prevalence and the frequency of the related cancers are strictly associated and have a higher risk in HIV positive individuals, we believe the introduction of HPV DNA testing in this population could have an important role in stratifying the risk of HIV-seropositive patients, as has been proposed by other researchers in the literature [44].
Conclusions
These results reinforce the need to establish an anal cancer screening on high-risk patients, such as HIV-positive women and men. The anus and cervix susceptibility to HPV infection and these HPV-induced malignancies might be explained by the same embryological origins. Anal and cervical cancers have similar tumor biology; hence a similar cancer screening program would appear to be reasonable, considering the success of cervical cancer screening programs around the world [45]. A co-test using anal cytology and HPV genotyping may be considered a useful tool, especially considering the increase in sensitivity when both tests are used [46]. Patients at risk of anal cancer, such as those who are HIV-positive, especially with the lower CD4 count, and MSM, would benefit with an earlier diagnosis if we considered the identification of HR-HPV infection before the emergence of the abnormal cells [47].
Currently, the Centre for Disease Control and Prevention (CDC) in the USA recommends that high-risk populations be vaccinated using the quadrivalent or nonavalent vaccines [22]. In Brazil, their recommendations for vaccination against HPV infection include immunocompromised, MSM and HIV patients in a broad age range [23]. This proposal shows the concern regarding HPV infections and consequent development of lesions within the risk groups, hence we believe that anal screening, improved by molecular tests, in the HIV population will be the next step toward reducing HPV-related morbidity and mortality [48].
Supporting information
(DOCX)
(SAV)
Acknowledgments
We thank the manager and the patients of the center of reference in STI/AIDS, which allowed us to follow up on this project.
Data Availability
My data are all contained within the paper and/or Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Muñoz N, Castellsagué X, de González AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine. 2006. August 31;24 Suppl 3: S3/1-10. Epub 2006 Jun 23. Review. [DOI] [PubMed] [Google Scholar]
- 2.Cubie HA. Diseases associated with human papillomavirus infection. Virology. 2013; 445(1–2):21–34. doi: 10.1016/j.virol.2013.06.007 [DOI] [PubMed] [Google Scholar]
- 3.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Vol. 100 B. Human papillomaviruses.IARC MonogrEvalCarcinog Risks Hum. 2012; 100B:255–296. Available: http://monographs.iarc.france\ENG\Monographsvol100b-11pdf. Accessed January 13, 2016. [Google Scholar]
- 4.Moscicki AB, Darragh TM, Berry-Lawhorn JM, Roberts JM, Khan MJ, Boardman LA, et al. Screening for Anal Cancer in Women.J Low Genit Tract Dis. 2015. July;19(3 Suppl 1): S27–42. doi: 10.1097/LGT.0000000000000117 Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xi LF, Critchlow CW, Wheeler CM, Koutsky LA, Galloway DA, Kuypers J, et al. Risk of anal carcinoma in situ in relation to human papillomavirus type 16 variants.Cancer Res. 1998. September 1; 58(17):3839–44. [PubMed] [Google Scholar]
- 6.Reusser NM, Downing C, Guidry J, Tyring SK. HPV Carcinomas in Immunocompromised Patients. J Clin Med. 2015. January 29;4(2):260–81. doi: 10.3390/jcm4020260 Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blas MM, Brown B, Menacho L, Alva IE, Silva-Santisteban A, Carcamo C.HPV Prevalence in Multiple Anatomical Sites among Men Who Have Sex with Men in Peru. PLoS One. 2015. October 5; 10(10): e0139524 doi: 10.1371/journal.pone.0139524 eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colón-López V, Ortiz AP, Del Toro-Mejías L, Clatts MC, Palefsky JM. Epidemiology of anal HPV Infection in High-Risk Men Attending a Sexually Transmitted Infection Clinic in Puerto Rico.PLoS One. 2014. January 6;9(1):e83209 doi: 10.1371/journal.pone.0083209 eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palefsky JM, Holly EA, Ralston ML, et al. Prevalence and risk factors for anal human papillomavirus infection in human immunodeficiency virus (HIV)-positive and high-risk HIV-negative women. J Infect Dis 2001; 183:383–91. doi: 10.1086/318071 [DOI] [PubMed] [Google Scholar]
- 10.Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators.Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007. May 10;356(19):1928–43. doi: 10.1056/NEJMoa061760 . [DOI] [PubMed] [Google Scholar]
- 11.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, et al. A review of human carcinogens—Part B: biological agents. Lancet Oncol. 2009. April;10(4):321–2. [DOI] [PubMed] [Google Scholar]
- 12.De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis.Int J Cancer. 2009. April 1;124(7):1626–36. doi: 10.1002/ijc.24116 [DOI] [PubMed] [Google Scholar]
- 13.Daling JR, Madeleine MM, Johnson LG, Schwartz SM, Shera KA, Wurscher MA, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004; 101(2):270–280. doi: 10.1002/cncr.20365 [DOI] [PubMed] [Google Scholar]
- 14.Edgren G, Sparén P. Risk of anogenital cancer after diagnosis of cervical intraepithelial neoplasia: a prospective population-based study. Lancet Oncol. 2007. April;8(4):311–6. doi: 10.1016/S1470-2045(07)70043-8 [DOI] [PubMed] [Google Scholar]
- 15.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007. July 7;370(9581):59–67. Review. doi: 10.1016/S0140-6736(07)61050-2 [DOI] [PubMed] [Google Scholar]
- 16.D'Souza G, Cook RL, Ostrow D, Johnson-Hill LM, Wiley D, Silvestre T. Anal cancer screening behaviors and intentions in men who have sex with men. J Gen Intern Med. 2008. September;23(9):1452–7. doi: 10.1007/s11606-008-0698-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shvetsov YB, Hernandez BY, McDuffie K, Wilkens LR, Zhu X, Ning L, et al. Duration and clearance of anal human papillomavirus (HPV) infection among women: the Hawaii HPV cohort study. Clin Infect Dis. 2009; 48(5):536–546. doi: 10.1086/596758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stier EA.; Sebring MC; Mendez AE; Ba FS; Trimble DD; Chiao EY. Prevalence of anal human papillomavirus infection and anal HPV-related disorders in women: a systematic review. American Journal of Obstetrics and Gynecology, September 2015, Vol.213(3), pp.278–309 ScienceDirect (Elsevier B.V.) doi: 10.1016/j.ajog.2015.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Oortmarssen GJ, Habbema JD. Duration of preclinical cervical cancer and reduction in incidence of invasive cancer following negative pap smears. Int JEpidemiol. 1995. April;24(2):300–7. [DOI] [PubMed] [Google Scholar]
- 20.Roberts JR, Siekas LL, Kaz AM. Anal intraepithelial neoplasia: A review of diagnosis and management. World J Gastrointest Oncol. 2017. February 15;9(2):50–61. doi: 10.4251/wjgo.v9.i2.50 Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sudenga SL, Nyitray AG, Torres BN, Silva R, Villa L, Lazcano-Ponce E, et al. Comparison of anal HPV natural history among men by country of residence: Brazil, Mexico, and the United States. J Infect 2017. July;75(1):35–47. doi: 10.1016/j.jinf.2017.03.010Epub 2017 Mar 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Health Quality Ontario. Anal dysplasia screening: an evidence-based analysis. Ont Health Technol Assess Ser. 2007;7(4):1–43. Epub 2007 Jun 1. PubMed PMID:23074504; PubMed Central PMCID: PMC3377578. [PMC free article] [PubMed] [Google Scholar]
- 23.Machalek DA, Poynten M, Jin F, Fairley CK, Farnsworth A, Garland SM, et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol. 2012;13(5):487–500. doi: 10.1016/S1470-2045(12)70080-3 Review. [DOI] [PubMed] [Google Scholar]
- 24.Apgar BS, Zoschnick L, Wright TC Jr. The 2001 Bethesda System terminology. Am Fam Physician. 2003. November 15;68(10):1992–8. Review [PubMed] [Google Scholar]
- 25.Gravitt PE, Peyton CL, Alessi TQ, Wheeler CM, Coutlée F, Hildesheim A, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000; 38(1): 357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernard HU, Chan SY, Manos MM, Ong CK, Villa LL, Delius H, et al. Identification and assessment of known and novel human papillomaviruses by polymerase chain reaction amplification, restriction fragment length polymorphisms, nucleotide sequence, and phylogenetic algorithms. J Infect Dis. 1994. November;170(5):1077–85. Erratum in: J Infect Dis 1996; 173(2):516. [DOI] [PubMed] [Google Scholar]
- 27.Estrade C, Menoud PA, Nardelli-Haefliger D, Sahli R. Validation of a low-cost human papillomavirus genotyping assay based on PGMY PCR and reverse blotting hybridization with reusable membranes. J ClinMicrobiol. 2011; 49(10):3474–81. doi: 10.1128/JCM.05039-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Z, Schiffman M, Herrero R, DeSalle R, Anastos K, et al. Evolution and Taxonomic Classification of Human Papillomavirus 16 (HPV16)-Related Variant Genomes: HPV31, HPV33, HPV35, HPV52, HPV58 and HPV67. PLoS ONE 2011; 6: e20183 doi: 10.1371/journal.pone.0020183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hillman RJ, Cuming T, Darragh T, Nathan M, Berry-Lawthorn M, Goldstone S, Law C, Palefsky J, Barroso LF, Stier EA, Bouchard C, Almada J, Jay N. 2016. IANS International Guidelines for Practice Standards in the Detection of Anal Cancer Precursors. J Low Genit Tract Dis. 2016 Oct;20(4):283–91. doi: 10.1097/LGT.0000000000000256 [DOI] [PubMed] [Google Scholar]
- 30.Nagata N, Watanabe K, Nishijima T, Tadokoro K, Watanabe K, Shimbo T, et al. Prevalence of Anal Human Papillomavirus Infection and Risk Factors among HIV-positive Patients in Tokyo, Japan. PLoS One. 2015. September 14;10(9): e0137434 doi: 10.1371/journal.pone.0137434 eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaturvedi AK, Engels EA, Gilbert ES, Chen BE, Storm H, Lynch CF, et al. Second cancers among 104,760 survivors of cervical cancer: evaluation of long-term risk. J Natl Cancer Inst. 2007:7;99(21):1634–43. [DOI] [PubMed] [Google Scholar]
- 32.Hoots BE, Palefsky JM, Pimenta JM, Smith JS. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer. 2009. May 15;124(10):2375–83. doi: 10.1002/ijc.24215 Review. [DOI] [PubMed] [Google Scholar]
- 33.Moscicki A-B, Ma Y, Farhat S, Jay J, Hanson E, Benningfield S, et al. Natural history of anal human papillomavirus infection in heterosexual women and risks associated with persistence. Clin Infect Dis. 2014;58(6):804–811. doi: 10.1093/cid/cit947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor S, Bunge E, Bakker M, Castellsagué X. The incidence, clearance and persistence of non-cervical human papillomavirus infections: a systematic review of the literature. BMC Infect Dis. 2016. June 14; 16:293 doi: 10.1186/s12879-016-1633-9 Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xi LF, Kiviat NB, Hildesheim A, Galloway DA, Wheeler CM, Ho J, Koutsky LA.Human papillomavirus type 16 and 18 variants: race-related distribution and persistence. J Natl Cancer Inst. 2006. August 2;98(15):1045–52. [DOI] [PubMed] [Google Scholar]
- 36.Burk RD, Harari A, Chen Z. Human papillomavirus genome variants. Virology.2013. October;445(1–2):232–43. doi: 10.1016/j.virol.2013.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volpini LPB, Boldrini NAT, de Freitas LB, Miranda AE, Spano LC. The high prevalence of HPV and HPV16 European variants in cervical and anal samples of HIV-seropositive women with normal Pap test results. PLoS One. 2017, 20;12(4):e0176422 doi: 10.1371/journal.pone.0176422 eCollection 2017. Erratum in: PLoS One. 2017 May 18;12 (5):e0178357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freitas LB, Chen Z, Muqui EF, Boldrini NA, Miranda AE, Spano LC, Burk RD.Human papillomavirus 16 non-European variants are preferentially associated with high-grade cervical lesions.PLoS One. 2014. July 1;9(7): e100746 doi: 10.1371/journal.pone.0100746 eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minkoff H, Zhong Y, Burk RD, Palefsky JM, Xue X, Watts DH, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis. 2010. March;201(5):681–90. doi: 10.1086/650467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goeieman BJ, Firnhaber CS, Jong E, Michelow P, Kegorilwe P, Swarts A, et al. Prevalence of anal HPV and Anal Dysplasia in HIV-Infected Women from Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2017. July1; 75(3): e59e64 doi: 10.1097/QAI.0000000000001300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palefsky JM. Antiretroviral therapy and anal cancer: the good, the bad, and the unknown. Sex Transm Dis. 2012. July;39(7):501–3. doi: 10.1097/OLQ.0b013e31825f7921 [DOI] [PubMed] [Google Scholar]
- 42.Piketty C, Selinger-Leneman H, Grabar S, Duvivier C, Bonmarchand M, Abramowitz L, et al. Marked increase in the incidence of invasive anal cancer among HIV-infected patients despite treatment with combination antiretroviral therapy. AIDS. 2008. June 19;22(10):1203–11. doi: 10.1097/QAD.0b013e3283023f78 [DOI] [PubMed] [Google Scholar]
- 43.Torres M, González C, del Romero J, Viciana P, Ocampo A, Rodríguez-Fortúnez P, et al. Anal human papillomavirus genotype distribution in HIV-infected men who have sex with men by geographical origin, age, andcytological status in a Spanish cohort.JClinMicrobiol. 2013. Nov;51(11):3512–20. doi: 10.1128/JCM.01405-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iribarren Díaz M, Ocampo Hermida A, González-Carreró Fojón J, Longueira Suárez R, Rivera Gallego A, Casal Núñez E, et al. Preliminary results of a screening program for anal cancer and its precursors for HIV-infected men who have sex with men in Vigo-Spain. Rev Esp Enferm Dig. 2017; 109(4):242–249. doi: 10.17235/reed.2017.4274/2016 [DOI] [PubMed] [Google Scholar]
- 45.Yu JB, Gross CP, Wilson LD, Smith BD. NCI SEER public-use data: applications and limitations in oncology research. Oncology (Williston Park). 2009; 23(3):288–95. Review. [PubMed] [Google Scholar]
- 46.Schiffman M, Burk RD, Boyle S, Raine-Bennett T, Katki HA, Gage JC, et al. A study of genotyping for management of human papillomavirus-positive, cytology-negative cervical screening results. J Clin Microbiol. 2015. January;53(1):52–9. doi: 10.1128/JCM.02116-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hidalgo-Tenorio C, Gil-Anguita C, Ramírez-Taboada J, Esquivias J, López-Ruz MA, Balgahata OM, et al. Risk factors for infection by oncogenic human papillomaviruses in HIV-positive MSM patients in the ART era (2010–2016). Medicine (Baltimore). 2017. September;96(39): e8109 doi: 10.1097/MD.0000000000008109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burd EM, Dean CL. Human Papillomavirus. Microbiol Spectr. 2016. August;4(4). doi: 10.1128/microbiolspec.DMIH2-0001-2015 Review. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(SAV)
Data Availability Statement
My data are all contained within the paper and/or Supporting Information files.