Abstract
Knowledge of the host range of a biocontrol agent (BCA) is fundamental. Host range determines the BCA’s economic potential, as well as the possible risk for non-target organisms. Entomopathogenic fungal strains belonging to the genus Beauveria are widely used as BCA, but our knowledge of their physiological host range is only partial. The aim of this study was to improve our understanding of the physiological host range of three Beauveria strains belonging to two species, B. hoplocheli and B. bassiana. We performed laboratory mortality bioassays to assess their pathogenicity and virulence against nine insect pests, belonging to three orders: Lepidoptera, Coleoptera and Diptera. Mortality rate, mean survival time and mycosis rate were used to estimate virulence. Pathogenicity was assessed as the capacity to cause a disease and induce mortality. Virulence was assessed as the severity of the disease based on mortality rate, mean survival time and mycosis rate. The results of this study revealed significant differences in the physiological host range of the three Beauveria strains tested. The three strains were pathogenic to all Diptera and Lepidoptera species tested. In the case of the Coleoptera, only the B. hoplocheli strain was pathogenic to the white grub Hoplochelus marginalis and only the B. bassiana strains were pathogenic to Alphitobius diaperinus. The B. hoplocheli strain was less virulent on Lepidoptera and Diptera than the two B. bassiana strains. The latter both exhibited very similar virulence patterns. The fact that B. hoplocheli and B. bassiana strains have different host ranges means that they can be used as BCA to target different pests. Impacts on non-target insects across multiple orders cannot be ruled out in the absence of ecological host range studies.
Introduction
Host specificity or host range of an entomopathogenic fungus can be defined as the number and taxonomic diversity of the hosts it can infect [1]. Knowledge of the host range of a biocontrol agent (BCA) is fundamental because host range determines the BCA’s possible risk for the environment [2] and economic potential [3]. These two aspects are somewhat correlated, given that a BCA with a broad host range may be lethal for a wide range of target pests and also potentially for a broad range of non-target species [1, 3]. The ecological host range refers to the range of species that an entomopathogenic fungus infects in field conditions. The physiological host range is the range that the pathogen is able to infect under optimized conditions, determined by laboratory tests [4]. The ecological host range is usually considered to be narrower than the physiological host range and a better estimator of the risk for the environment [4–6]. However, assessing the ecological host range of a BCA is a complex task and can only be achieved once the BCA has been introduced in the environment [4]. Thus, the question of host range is not usually explored fully, which means there are gaps in our knowledge. Generally, the characterization of a BCA’s host range is drawn from our knowledge of the hosts on which the strain was collected in natural conditions and a few laboratory pathogenicity tests [7]. In invertebrate pathology, pathogenicity is defined as the capacity to cause a disease to a given host and virulence is defined as the severity of the disease [8, 9]. Different approaches can be used and combined to estimate the virulence of a pathogen. Dose-mortality experiments determine the median lethal dose or concentration, which causes the death of 50% of the test insects; while single dose time-mortality experiments are used to determine the mean survival time or the median lethal time at which 50% of the test insects have died; and fungal growth on the host cadaver can be checked to ascertain completion of the fungal biological cycle [10]. Reduction of other fitness parameters can also be measured, such as fecundity or offspring survival [4].
Entomopathogenic fungi used as commercial BCA have diverse host ranges. Most are capable of infecting a wide range of hosts, although a few have a narrow host range [11]. Fungi belonging to the Entomophthorales order (Entomophthoromycota phylum) tend to have a narrow ecological and physiological host range limited to a small number of taxonomically related species [12, 13]. They include many obligate biotrophic insect pathogens, like all the species belonging to the Entomophthoraceae family [14]. They are difficult to mass-produce and are used primarily for classical biological control with a view to the permanent establishment of the exotic BCA [15]. In contrast, fungi used in inundative or inoculative strategies, involving the regular release of the BCA, have to be mass-produced. The fungi used in these strategies belong mainly to the Hypocreales order (Ascomycota phylum). They are hemibiotrophic and generally have a broad host range [11]. However, among Hypocreales, differences in host range are often mentioned at the species or strain level.
In the genus Beauveria, B. bassiana (Balsamo) Vuillemin is recognized as a generalist species with a broad ecological host range of more than 700 arthropod species, which covers most orders of the class Insecta [6, 16]. B. brongniartii (Saccardo) Petch is often claimed to have a more restricted host range, infecting mostly Coleoptera [17–19]. However, this fungus species has been reported to infect insects from at least seven orders in the field [18, 20]. For several other species, such as B. vermiconia de Hoog & Rao or B. caledonica Bisset & Widden, the number of strains available in collections is too small to draw conclusions about their host range [21]. To date, the species B. hoplocheli Robène-Soustrade & Nibouche (formerly described as B. brongniartii or B. tenella) has only been isolated in natural conditions from the white grub Hoplochelus marginalis (Fairmaire) (Coleoptera: Scarabaeidae) [22]. It is used as a BCA against this pest in Réunion Island [23]. Despite a few preliminary studies, the physiological host range of B. hoplocheli has not been investigated extensively. In laboratory bioassays, B. hoplocheli exhibited little or no virulence to Melolontha melolontha L. (Coleoptera: Scarabaeidae) [24] and to the mango blossom gall midge Procontarinia mangiferae (Felt) (Diptera: Cecidomyiidae), but was pathogenic to the greater wax moth Galleria mellonella L. (Lepidoptera: Pyralidae) [25].
Many studies have compared the virulence of several strains of Beauveria spp. on a given insect host, especially strains of B. bassiana [26–32]. Few works have studied the physiological host range of Beauveria spp. strains by comparing their pathogenicity and virulence on several insect species. For example, 43 B. bassiana strains collected worldwide exhibited a strong variation in virulence against eight lepidopteran species [33]. Twenty-nine genetically diverse B. bassiana strains were pathogenic to nine insect species from five orders, with significantly different levels of virulence [34].
Few studies demonstrate that Beauveria strains or species may differ in their physiological host range, despite the importance of these differences regarding their use as BCA. Therefore, the aim of this study was to characterize the physiological host range of three Beauveria strains belonging to two species, B. hoplocheli and B. bassiana. We tested their pathogenicity and their virulence against nine insect pests, belonging to three orders: Lepidoptera, Coleoptera and Diptera. Mortality rate, mean survival time and mycosis rate were used as estimators of the virulence in single dose mortality bioassays.
Materials and methods
Beauveria strains and spore suspensions
Two strains of B. bassiana (I-2960, I-2961) and one strain of B. hoplocheli (B507), were obtained from Arysta LifeScience. The strains were stored at -80°C using Microbank cryovials (Pro-Lab Diagnostics, Richmond Hill, Canada). Cultures were grown from the cryovial stored strains to prepare spore suspensions for the tests. All cultures were grown at 25°C on potato dextrose agar (PDA) medium until sporulation was observed (three to four weeks). Spore suspensions were prepared by scraping the surface of sporulated cultures and suspending conidia in a sterile solution of 0.05% TWEEN® 80 (Sigma-Aldrich, St. Louis, MO, USA). Conidia suspensions were adjusted to 106 or 108 conidia mL-1 using a Malassez hemocytometer. To determine conidia viability and the number of conidia per milliter, 100 μL of the conidia suspension was plated onto PDA, incubated at 25°C and the colony forming units were counted after five days.
Insects
The pathogenicity of the three Beauveria spp. strains was evaluated on nine insect species belonging to three orders: Diptera, Coleoptera and Lepidoptera (Table 1). We performed pathogenicity tests on five fruit flies found in Réunion: the peach fruit fly, Bactrocera zonata (Saunders), the Mediterranean fruit fly, Ceratitis capitata (Wiedemann), the Mascarene fruit fly, C. catoiri (Guérin-Méneville) endemic in Réunion, the Indian Ocean cucumber fly, Dacus demmerezi (Bezzi) and the melon fly, Zeugodacus cucurbitae (Coquillett). Tests were also carried out on the oriental fruit fly, Bactrocera dorsalis (Hendel), an invasive species not recorded in Réunion at the time of the study, but present in most islands in the western region of the Indian Ocean, including Comoros [35]. Fruit fly strains of B. zonata, C. capitata and C. catoirii were reared on artificial diets [36] in our laboratory for 138, 17 and 157 generations, respectively. D. demmerezi and Z. cucurbitae were reared on zucchini for 22 and 64 generations, respectively. Sugarcane white grub H. marginalis larvae were collected in the field with permission of the owner from plots of sugarcane (Saint-Benoît, Réunion; 20°59'41.05" S, 55°41'16.63" E) and thyme (Petite-Ile, Réunion; 21°20'3.57" S, 55°33'22.13" E). H. marginalis larvae were kept in the laboratory in clean soil, fed with pieces of carrot and quarantined for 20 days to ensure that they were free from entomopathogenic fungal infections. The lesser mealworm, Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae) strain was collected from a poultry farm with permission of the owner (Saint-André, Réunion; 20°56'52.0" S, 55°39'41.2" E). A. diaperinus larvae were maintained on wood shavings and fed with poultry feed pellets until treatments. Larvae of G. mellonella came from a strain reared in our laboratory on an artificial diet adapted from Meyling [37].
Table 1. Insects used in bioassays.
| Species | Common name | Order | Family | Stage | No. insects per treatment | Conidia suspension (conidia.mL-1) |
|---|---|---|---|---|---|---|
| Bactrocera dorsalis | Oriental fruit fly | Diptera | Tephritidae | Adult | 90 | 106 |
| Bactrocera zonata | Peach fruit fly | Diptera | Tephritidae | Adult | 90 | 106 |
| Ceratitis capitata | Mediterranean fruit fly | Diptera | Tephritidae | Adult | 90 | 106 |
| Ceratitis catoirii | Mascarene fruit fly | Diptera | Tephritidae | Adult | 90 | 106 |
| Dacus demmerezi | Indian Ocean cucurbit fly | Diptera | Tephritidae | Adult | 90 | 106 |
| Zeugodacus cucurbitae | Melon fly | Diptera | Tephritidae | Adult | 150 | 106 |
| Alphitobius diaperinus | Lesser mealworm | Coleoptera | Tenebrionidae | Larva | 90 | 108 |
| Hoplochelus marginalis | Sugarcane white grub | Coleoptera | Scarabaeidae | Larva (3rd instar) | 90 | 108 |
| Galleria mellonella | Greater wax moth | Lepidoptera | Pyralidae | Larva | 390 | 106 and 108 |
Bioassays
Bioassays were conducted on two insect species at a time, using G. mellonella for all bioassays. Each bioassay compared four modalities: the three Beauveria strains B507, I-2960 and I-2961, and an untreated control. Each modality was carried out on 30 insects. For fruit flies, 12 to 14-day-old adults were used, with 15 males and 15 females. Strain B507 was not tested on C. catoirii. Each bioassay was repeated three times. The experiments were conducted from May 2015 to August 2016 in the CIRAD laboratory (Réunion). Since B. dorsalis was not recorded in Réunion at the time of the study, bioassays for this species were conducted in the INRAPE laboratory (Comoros).
Insect contamination was realized by dipping the insects in the conidia suspension for 10 seconds. Adult fruit flies were anaesthetized with CO2 prior to treatment and dipped in a suspension at 106 conidia mL-1. This concentration is in the range of the LC50 (lethal concentration required to kill 50% of the target insects) of several B. bassiana strains tested on fruit flies [38–40]. Preliminary bioassays showed that using 106 conidia mL-1, the mortality was similar to control for A. diaperinus and H. marginalis larvae for all strains tested. Therefore, the insects were treated with suspensions at 108 conidia mL-1. Untreated control fruit flies were anaesthetized with CO2 and then dipped in a 0.05% TWEEN® 80 solution like all other control insects.
After treatment, all insects were kept separately in 125 mL plastic containers (flies and white grubs), and 30 mL plastic containers (G. mellonella and A. diaperinus larvae). Adult flies were fed three times a week with a liquid diet containing a 10:1 mix of sucrose and yeast enzymatic hydrolysate (MP Biomedicals, Solon, OH, USA). After dipping, G. mellonella larvae were fed with a 400 mg piece of beeswax and A. diaperinus larvae were fed with a few poultry feed pellets. H. marginalis larvae were kept in sterilized peat and fed once a week with slices of organically grown carrots.
Insect mortality was recorded daily for 30 days for the fruit flies and every other day for the other species. Insects were considered dead if they were unable to produce coordinated movements or showed no response when touched. Cadavers were surface-sterilized in 70% ethanol for five seconds, rinsed in sterile distilled water for five seconds and placed on a sterilized filter paper, moistened with 200 μL sterile distilled water, in a 55 mm vented Petri dish to stimulate external fungal growth. Development of mycosis was checked 10 days after death.
Data analysis
The aim of the first analysis was to compare the effect of the treatments on two variables used to estimate virulence: mortality rate and mycosis rate. The mycosis rate was calculated as the percentage of cadavers showing external fungal growth out of the total number of tested insects. The experimental design allowed us to compare four treatments on two insect species in each bioassay. The presence of G. mellonella in each bioassay allowed an estimation of the bioassay effect as a fixed replication effect and the design was thus analysed as a classical factorial design. As two spore suspensions were used at 106 or 108 conidia.mL-1, we conducted separate analyses on the bioassays using each of the two doses. The analysis of the mycosis rate for bioassays using a 108 conidia.mL-1 could not be performed due to missing data for G. mellonella. We conducted the analysis with a generalized linear model using a binomial distribution and a logit link with SAS GLIMMIX procedure [41]. The model included insect species, treatments, insect species x treatment interaction, bioassays and bioassays x treatments interaction as fixed factors. To solve some convergence issues, maximum likelihood with Laplace approximation was preferred to the default pseudo-likelihood technique. The insects used in the experiments had a different life span and different developmental stages (adult or larva). Therefore, the mean survival time for control insects varied depending on the insect species. In order to take this factor into account and to ensure that mortality rates were not too high in controls, we analysed the mortality rates when the mortality rate for the control reached 0.2. The insect species x treatments interaction was significant (P < 0.05) for both suspensions at 106 and 108 conidia.mL-1. Consequently, we carried out between treatment comparisons with each insect species separately. To do so, we used a generalized linear model with a binomial distribution with the SAS GENMOD procedure, using treatment and bioassays as fixed effects. Pairwise between treatment differences were tested using a likelihood ratio test.
The second analysis focused on survival curves. We used the Kaplan-Meier estimator, a non-parametric statistic, to compare the effects of the three Beauveria strains on insect survival within an insect species. Survival curves were modelled using the SAS LIFETEST procedure and a log-rank test was performed to detect significant differences between treatments. Since multiple pairwise comparisons of strains increase the overall type 1 error, Sidak’s correction was applied to adjust the significance thresholds in order to yield an experiment-wise P-value of 0.05. The strains’ virulence was estimated by the mean survival time computed using the SAS LIFETEST procedure.
Results
Analysis of mortality rate and Kaplan-Meier survival curves
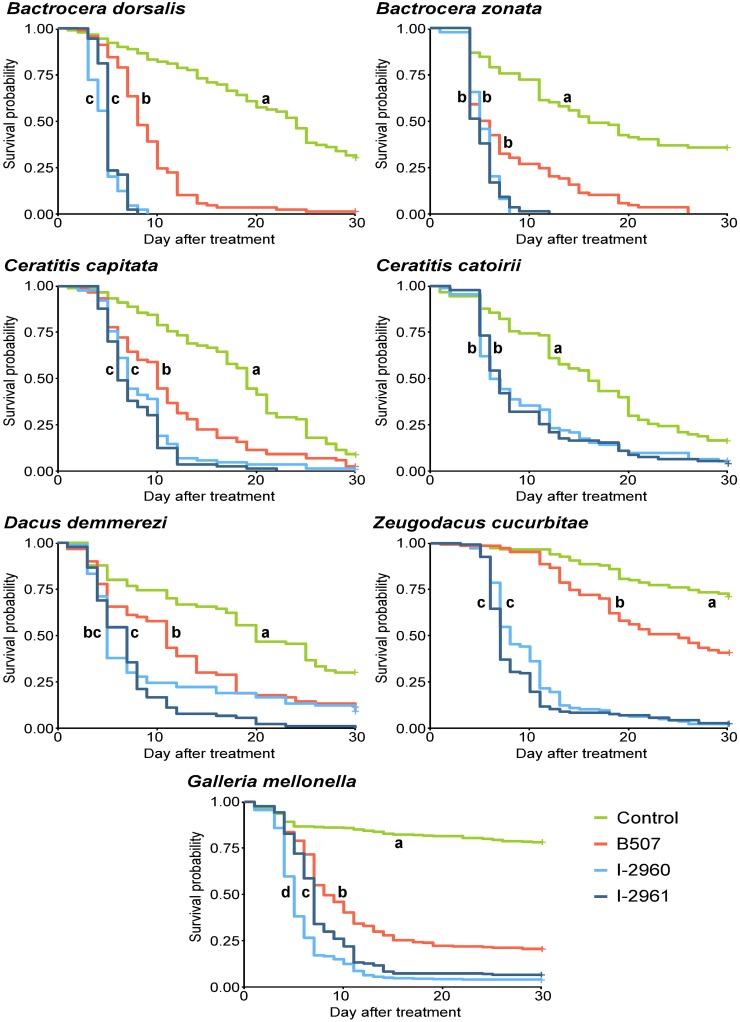
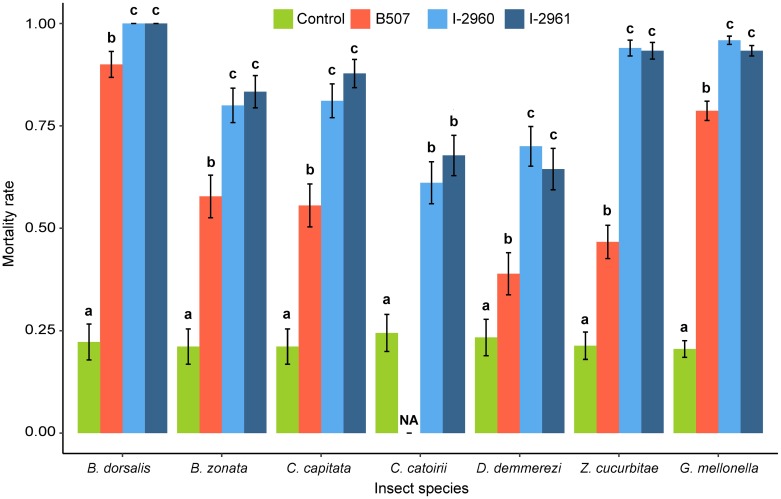
The analysis of mortality rates revealed that the effects of treatment (F = 36.76; DF = 3, 81; P < 0.0001), insect species (F = 8.62; DF = 5, 81; P < 0.0001), as well as the interaction insect species x treatment (F = 3.55; DF = 17, 81; P < 0.0001) were highly significant for the six fruit fly species and G. mellonella treated with 106 conidia.mL-1. The analysis of mortality rates also revealed that the effects of the treatment (F = 53.28; DF = 3, 25; P < 0.0001), the species (F = 45.69; DF = 2, 25; P < 0.0001) and the insect species x treatment interaction (F = 29.00; DF = 6, 25; P < 0.0001) were highly significant for the species H. marginalis, A. diaperinus and G. mellonella treated with 108 conidia.mL-1. The highly significant interaction between insect species and treatment shows that the differences between treatments depend on the insect species. Consequently, to compare the treatments, the insect mortality rate and survival were analysed independently for each species. The three Beauveria strains used at 106 conidia.mL-1 were pathogenic to all the fruit fly species tested and to G. mellonella larvae as shown by the mortality rate and Kaplan-Meier survival curves, which differed significantly from the controls, irrespective of the Beauveria strain used (Figs 1 and 2). The mortality rate and the Kaplan-Meier survival analysis revealed differences in virulence between the Beauveria strains. B. bassiana strains I-2960 and I-2961 were significantly more virulent than the B. hoplocheli strain B507 for all fruit flies tested using the Kaplan-Meier survival analysis (except for B. zonata) and mortality rates (Figs 1 and 2). This result is also illustrated by the mean survival times estimated from the Kaplan-Meier survival analysis (S1 Table). Strains I-2960 and I-2961 exhibited a very similar virulence pattern for the different fruit flies (Figs 1 and 2 and S1 Table). The mortality rate and the Kaplan-Meier survival analysis of G. mellonella treated with 106 conidia.mL-1 showed that B507 was significantly less virulent than B. bassiana strains (Figs 1 and 2). The Kaplan-Meier survival analysis showed that I-2961 was significantly less virulent than I-2960 at 106 conidia.mL-1 (Fig 1).
Fig 1. Kaplan-Meier survival curves for six fruit fly species and Galleria mellonella treated with Beauveria hoplocheli strain B507, B. bassiana strains I-2960 and I-2961 using 106 conidia.mL-1 suspensions.
Different letters indicate significant differences between treatments within an insect species (log-rank test, P < 0.05 after Sidak’s correction). Crosses indicate censored data.
Fig 2. Mortality rate of six fruit fly species and Galleria mellonella treated with Beauveria hoplocheli strain B507, B. bassiana strains I-2960 and I-2961 using 106 conidia.mL-1 suspensions.
Data presented are means ± SEM, with three replicates of 30 insects for each treatment and each species. Mortality rates were calculated at the time when the mortality rate of the control reached 0.2. For each insect species, a generalized linear model was fitted and pairwise between treatment differences were tested using a likelihood ratio test. Different letters indicate significant differences between treatments (P < 0.05).
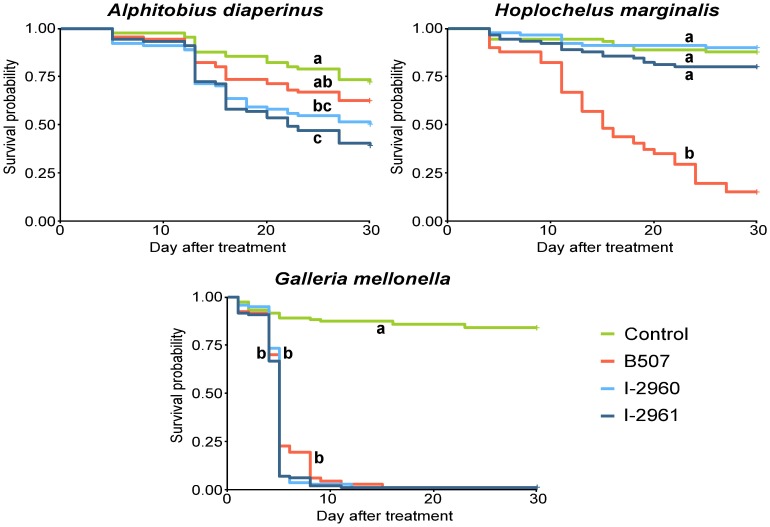
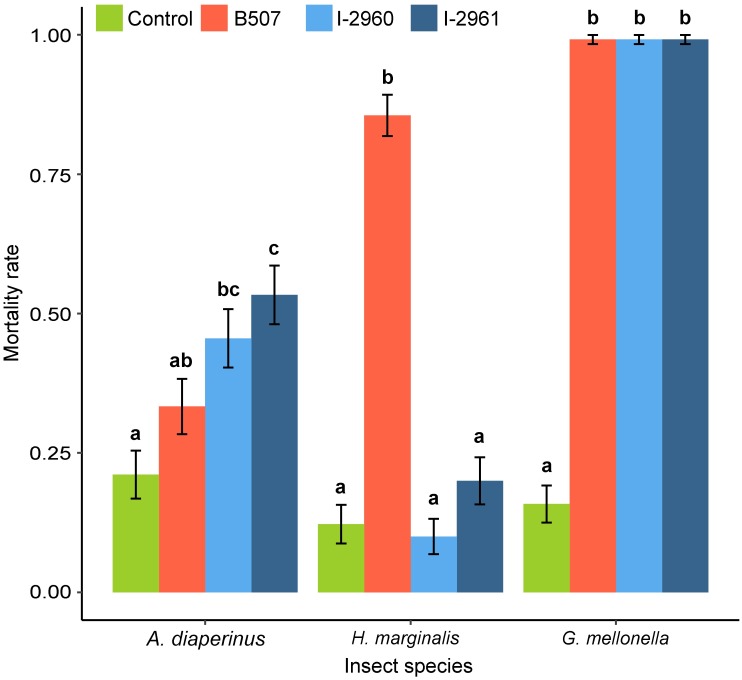
The three strains demonstrated a similar high level of virulence on G. mellonella at 108 conidia.mL-1 (Figs 3 and 4 and S1 Table). B. hoplocheli strain B507 was the only strain pathogenic to the white grub H. marginalis at the tested dose of 108 conidia.mL-1. The mortality rate and the Kaplan-Meier survival curves of H. marginalis larvae treated with the two B. bassiana strains were not significantly different from the control (Figs 3 and 4). The two B. bassiana strains were pathogenic to A. diaperinus larvae, resulting in mortality rates and Kaplan-Meier survival curves that were significantly different from the control (Figs 3 and 4). When A. diaperinus larvae were treated with strain B507, the mortality rate and the Kaplan-Meier survival curve were not significantly different from the control (Figs 3 and 4).
Fig 3. Kaplan-Meier survival curves for Alphitobius diaperinus, Hoplochelus marginalis and Galleria mellonella treated with Beauveria hoplocheli strain B507 and B. bassiana strains I-2960 and I-2961 using 108 conidia.mL-1 suspensions.
Different letters indicate significant differences between treatments within an insect species (log-rank test, P < 0.05 after Sidak’s correction). Crosses indicate censored data.
Fig 4. Mortality rate of Alphitobius diaperinus, Hoplochelus marginalis and Galleria mellonella treated with Beauveria hoplocheli strain B507, B. bassiana strains I-2960 and I-2961 using 108 conidia.mL-1 suspensions.
Data presented are means ± SEM, with three replicates of 30 insects for each treatment and each species. Mortality rates were calculated at the time when the mortality rate of the control reached 0.2. For each insect species, a generalized linear model was fitted and pairwise between treatment differences were tested using a likelihood ratio test. Different letters indicate significant differences between treatments (P < 0.05).
Analysis of mycosis rate
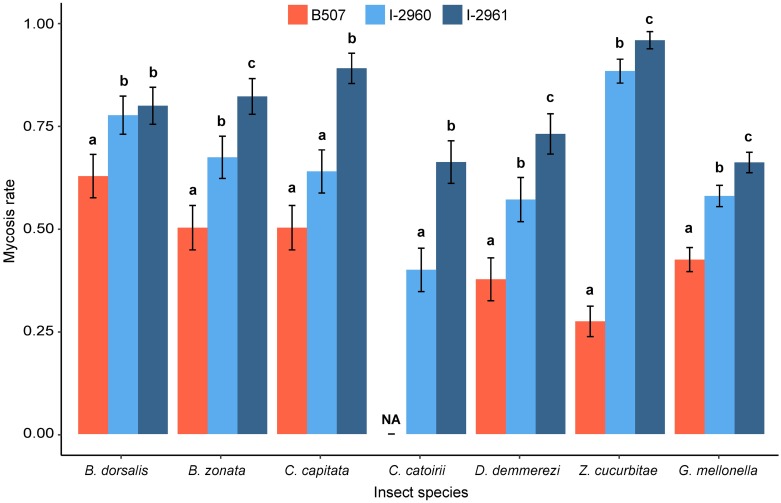
The analysis of the mycosis rate revealed that the treatments (F = 31.60; DF = 2, 55; P < 0.0001), and the insect species x treatment interaction (F = 2.08; DF = 11, 55; P = 0.038) had a significant effect for the six fruit fly species and G. mellonella treated with 106 conidia.mL-1, while the insect species’ effect was not significant (F = 0.66; DF = 5, 55; P = 0.66). No mycosis was recorded on the control insects. The mycosis rate induced by the B. hoplocheli strain B507 was significantly lower than the rates induced by the two B. bassiana strains for four out of five of the fruit flies tested and for G. mellonella (Fig 5). The B. bassiana strain I-2961 induced significantly higher mycosis rates than strain I-2960 for all fruit flies tested (except B. dorsalis) and G. mellonella, although the mortality rates caused by both strains were similar (Figs 1, 2 and 5). In the case of H. marginalis, the B. hoplocheli strain caused a mycosis rate of 0.27 ± 0.05 for a mortality rate of 0.86 ± 0.04. None of the white grubs that died after treatment with strain I-2960 developed external fungal growth. For strain I-2961, the mycosis rate was 0.04 ± 0.02, which was significantly different from the control (Chi2 = 5.66; DF = 1; P = 0.0174). No mycosis was recorded on A. diaperinus larvae, irrespective of the Beauveria strain considered.
Fig 5. Mycosis rate of six fruit fly species and Galleria mellonella treated with Beauveria hoplocheli strain B507 and B. bassiana strains I-2760 and I-2761 using 106 conidia.mL-1 suspensions.
Data presented are means ± SEM, with three replicates of 30 insects for each treatment and each species. Mycosis rates were calculated using the percentage of cadavers showing external fungal growth out of the total number of tested insects. For each insect species, a generalized linear model was fitted and pairwise between treatment differences were tested using a likelihood ratio test. Different letters indicate significant differences between treatments (P < 0.05).
Discussion
We demonstrated that there are significant differences in the physiological host range of the three Beauveria strains tested. The B. bassiana strains and the B. hoplocheli strain express different pathogenicity patterns across several insects belonging to the Coleoptera order. B. bassiana strains killed A. diaperinus, but were not pathogenic to H. marginalis, although they are both beetle larval stages. These findings confirmed the results of previous studies, which showed that the strain I-2960 was not pathogenic to H. marginalis [24, 42]. Few studies have reported an absence of pathogenicity for B. bassiana strains. It is interesting to note that the work by Maurer et al. [43] shows that several B. bassiana strains that were isolated from insects other than Ostrinia nubilalis, were not pathogenic to this species. Strain I-2961 did not cause a significant increase in H. marginalis mortality rate, although it produced mycosis on a few individuals. It is possible that the fungus was able to complete its biological cycle after overcoming the insect’s defences. The B. hoplocheli strain B507 was pathogenic to H. marginalis, but was not pathogenic to A. diaperinus. Neuvéglise et al. [24] also found that several B. hoplocheli strains were not pathogenic to M. melolontha, a beetle belonging to the Melolonthinae subfamily, the same subfamily as H. marginalis. These results confirm that, to date, B. hoplocheli is the only available BCA for controlling H. marginalis in sugarcane fields.
The B. hoplocheli strain, B507, was pathogenic to the tested fruit flies and to the greater wax moth, but was less virulent than the two B. bassiana strains. Such differences in virulence have been observed at the inter- and the intra-species level for Beauveria. At the inter-species level, Goble et al. [44] showed that B. brongniartii isolates were less effective against the Asian long-horned beetle than other Hypocreales fungal species, including Beauveria asiatica. Differences in virulence have also been reported at the intra-species level [33, 34, 45]. Differences in virulence observed among the Beauveria strains could be linked to conidial attachment on the cuticle, germination, as well as strategies to evade the host’s immune system [46]. In addition, virulence is affected by factors including cuticle-degrading enzymes or toxic proteins, which are produced by the fungus [31, 47]. The main difference between the two B. bassiana strains was their potential to cause mycosis. Strain I-2961 sporulated significantly more on fruit fly cadavers than I-2960. The two B. bassiana strains were pathogenic to A. diaperinus larvae, but no mycosis was observed. Fungal development on host cadavers is a crucial parameter for BCA selection because the effectiveness of insect population control depends on the fungus’ capacity to complete its biological cycle and transmission to other insects [48]. Many factors might affect the sporulation on cadavers, including temperature, humidity, conidia number and insect age [49, 50].
Using laboratory bioassays to characterize their physiological host range, we demonstrated that B. hoplocheli strain B507 and the two B. bassiana strains I-2961 and I-2960 can infect a wide range of insects belonging to three different orders. Strain I-2961 and, to a lesser extent, strain I-2960, were also known pathogens of the Coleoptera Rhynchophorus ferrugineus [51, 52]. Strain I-2960 showed pathogenicity toward the lepidopteran pests Ostrinia nubilalis, Paysandisia archon and Thaumetopoea pityocampa [53, 54]. This broad host range means that both B. bassiana strains have great potential to control diverse pests. As yet, the B. hoplocheli strain B507 has only been used to control the white grub H. marginalis, but we have shown that this species is not specific to Coleoptera and can infect Diptera and Lepidoptera. Hu et al. [55] suggested that speciation in the Metarhizium genus was closely related to host specificity, with an evolutionary route going from specialists to generalists, via intermediate host range species. Further pathogenicity and genomic studies are required to determine whether such a speciation pattern exists in Beauveria. However, as in the Metarhizium genus, it seems that most Beauveria species have a broad host range, which is probably linked to ecological fitness [55].
When these entomopathogenic fungal strains are used as BCA, their broad host range could be a concern in terms of their impact on non-target species. The ecological host range should be considered, as it is not unusual that hosts infected in the laboratory have never been found infected in the field [4]. Hypocreales fungi, such as Beauveria spp., are facultative insect pathogens capable of saprophytic and endophytic life stages. Therefore, soil characteristics, abiotic factors, plant species, as well as agricultural practices can have a great impact on their persistence and activity [56–58]. There is some evidence that B. bassiana strains may be adapted to a habitat type rather than to a particular host [59]. When choosing a suitable BCA, it is important to study the physiological host range, combined with an assessment of the impact that environmental conditions have on the fungal strain’s development. An evaluation of the persistence and distribution of the introduced biocontrol agent B. hoplocheli throughout Réunion is currently underway. This research will help shed light on the factors influencing its effectiveness and impact.
Supporting information
Kaplan-Meier analysis was used to estimate the mean survival time for each Beauveria strain in each bioassay (three replicates of 30 insects).
(DOCX)
Acknowledgments
The authors would like to thank M. Hoarau, S. Nouhou and H. Telismart for their technical help with the laboratory work, S. Glénac, J. Payet, R. Tibère, C. Lallemand and J. Hascoat for collecting and rearing the insects. Special thanks are also owed to E. Gozé for his advice on statistical analysis.
Data Availability
Raw data is available in Rohrlich, Clara; Merle, Isabelle; Mze Hassani, Issa; Verger Manon; Zuin Michel; Besse Samantha; Robène Isabelle; Nibouche Samuel; Costet Laurent, 2018, "Raw data for Rohrlich et al. 2018 PLOS ONE Variation in physiological host range in three strains of two species of the entomopathogenic fungus Beauveria", doi:10.18167/DVN1/CBONRN. All other relevant data are within the paper and its Supporting Information file.
Funding Statement
This work was co-funded by 1) the European Agricultural Fund for Rural Development (https://ec.europa.eu/agriculture/rural-development-2014-2020_en), by the Conseil Départemental de La Réunion (http://www.cg974.fr/), by the Conseil Régional de La Réunion (https://www.regionreunion.com) and by the Centre de Coopération Internationale en Recherche Agronomique pour le Développement (https://www.cirad.fr/); 2) the French Ministère de l’Agriculture et de l’Alimentation (http://agriculture.gouv.fr/), the French Ministère de la Transition Ecologique et Solidaire (https://www.ecologique-solidaire.gouv.fr/) and received financial support from the Office National de l’Eau et des Milieux Aquatiques (http://www.onema.fr/) as part of the "Pour et Sur le Plan Ecophyto 2" AttractMyFly project; 3) the ANRT (Association Nationale de la Recherche et de la Technologie) through the CIFRE Ph.D grant N°2014/1446 of RC; 4) the Arysta LifeScience Group. The role of the Arysta LifeScience Group was limited. to the choice of the Beauveria strains studied and to providing support in the form of salaries for authors CR, SB and MZ; it did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section. All other funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This work was carried out on the Plant Protection Platform which is co-financed by the Groupe d’Intérêt Scientifique "Infrastructures en Biologie, Santé et Agronomie" (http://www.ibisa.net).
References
- 1.Brodeur J. Host specificity in biological control: insights from opportunistic pathogens. Evol Appl. 2012;5(5):470–80. doi: 10.1111/j.1752-4571.2012.00273.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Lenteren JC, Bale J, Bigler F, Hokkanen HMT, Loomans AJM. Assessing risks of releasing exotic biological control agents of arthropod pests. Annu Rev Entomol. 2006;51:609–34. doi: 10.1146/annurev.ento.51.110104.151129 [DOI] [PubMed] [Google Scholar]
- 3.Glare T, Caradus J, Gelernter W, Jackson T, Keyhani N, Köhl J, et al. Have biopesticides come of age? Trends Biotechnol. 2012;30(5):250–8. doi: 10.1016/j.tibtech.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 4.Hajek AE, Goettel MS. Guidelines for evaluating effects of entomopathogens on non-target organisms In: Lacey LA, Kaya HK, editors. Field Manual of Techniques in Invertebrate Pathology. Dordrecht: Springer Netherlands; 2007. p. 816–33. [Google Scholar]
- 5.Fargues J, Remaudiere G. Considerations on the specificity of entomopathogenic fungi. Mycopathologia. 1977;62(1):31–7. [Google Scholar]
- 6.Goettel M, Poprawski T, Vandenberg J, Li Z, Roberts D. Safety to nontarget invertebrates of fungal biocontrol agents In: Laird M, Lacey L, Davidson E, editors. Safety of microbial insecticides: CRC Press; 1990. p. 209–29. [Google Scholar]
- 7.Van Driesche R, Hoddle M. Non-target effects of insect biocontrol agents and trends in host specificity since 1985. CAB Reviews. 2016;11(44):1–66. [Google Scholar]
- 8.Shapiro-Ilan DI, Fuxa JR, Lacey LA, Onstad DW, Kaya HK. Definitions of pathogenicity and virulence in invertebrate pathology. J Invertebr Pathol. 2005;88(1):1–7. doi: 10.1016/j.jip.2004.10.003 [DOI] [PubMed] [Google Scholar]
- 9.Onstad D, Fuxa J, Humber R, Oestergaard J, Shapiro-Ilan D, Gouli V, et al. An abridged glossary of terms used in invertebrate pathology 2006. http://www.sipweb.org/resources/glossary.html. [Google Scholar]
- 10.Inglis GD, Enkerli J, Goettel MS. Laboratory techniques used for entomopathogenic fungi: Hypocreales In: Lacey LA, editor. Manual of techniques in invertebrate pathology. 2nd ed London, UK: Academic Press; 2012. p. 189–253. [Google Scholar]
- 11.Faria MRd Wraight SP. Mycoinsecticides and mycoacaricides: a comprehensive list with worldwide coverage and international classification of formulation types. Biol Control. 2007;43(3):237–56. [Google Scholar]
- 12.Pell JK, Eilenberg J, Hajek AE, Steinkraus DC. Biology, ecology and pest management potential of Entomophthorales In: Butt TM, Jackson C, Magan N, editors. Fungi as biocontrol agents: progress, problems and potential. Wallingford, UK: CAB International; 2001. p. 71–153. [Google Scholar]
- 13.Jensen AB, Thomsen L, Eilenberg J. Value of host range, morphological, and genetic characteristics within the Entomophthora muscae species complex. Mycol Res. 2006;110(8):941–50. [DOI] [PubMed] [Google Scholar]
- 14.Gryganskyi A, Humber R, Smith M, Hodge K, Huang B, Voigt K, et al. Phylogenetic lineages in Entomophthoromycota. Persoonia. 2013;30:94–105. doi: 10.3767/003158513X666330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hajek AE, Gardescu S, Delalibera Jr. I. Classical biological control of insects and mites: a worldwide catalogue of pathogen and nematode introductions. Morgantown, WV: FHTET, USDA Forest Service; 2016. 57 p.
- 16.Feng M, Poprawski T, Khachatourians GG. Production, formulation and application of the entomopathogenic fungus Beauveria bassiana for insect control: current status. Biocontrol Sci Technol. 1994;4(1):3–34. [Google Scholar]
- 17.Shah PA, Pell JK. Entomopathogenic fungi as biological control agents. Appl Microbiol Biotechnol. 2003;61(5–6):413–23. doi: 10.1007/s00253-003-1240-8 [DOI] [PubMed] [Google Scholar]
- 18.Vestergaard S, Cherry A, Keller S, Goettel M. Safety of hyphomycete fungi as microbial control agents In: Hokkanen HMT, Hajek AE, editors. Environmental impacts of microbial insecticides: need and methods for risk assessment. Progress in Biological Control. Dordrecht, Netherlands: Springer; 2003. p. 35–62. [Google Scholar]
- 19.Imoulan A, Hussain M, Kirk PM, El Meziane A, Yao Y-J. Entomopathogenic fungus Beauveria: Host specificity, ecology and significance of morpho-molecular characterization in accurate taxonomic classification. J Asia Pac Entomol. 2017;20:1204–12. [Google Scholar]
- 20.Leatherdale D. The arthropod hosts of entomogenous fungi in Britain. BioControl. 1970;15(4):419–35. [Google Scholar]
- 21.Rehner SA, Minnis AM, Sung GH, Luangsa-ard JJ, Devotto L, Humber RA. Phylogeny and systematics of the anamorphic, entomopathogenic genus Beauveria. Mycologia. 2011;103(5):1055–73. doi: 10.3852/10-302 [DOI] [PubMed] [Google Scholar]
- 22.Robène-Soustrade I, Jouen E, Pastou D, Payet-Hoareau M, Goble TA, Linderme D, et al. Description and phylogenetic placement of Beauveria hoplocheli sp. nov. used in the biological control of the sugarcane white grub, Hoplochelus marginalis, in Reunion Island. Mycologia. 2015;107(6):1221–32. doi: 10.3852/14-344 [DOI] [PubMed] [Google Scholar]
- 23.Vercambre B, Goebel O, Riba G, Marchal M, Neuvéglise C, Ferron P. Success in biological control of a soil pest, Hoplochelus marginalis, in Reunion island: choice of a suitable fungus VIth International Colloquium on Invertebrate Pathology and Microbial Control; 1993. December 7–9; Montpellier, France: Society for Invertebrate Pathology; 1994. p. 283–8. [Google Scholar]
- 24.Neuvéglise C, Brygoo Y, Riba G. 28s rDNA group-I introns: a powerful tool for identifying strains of Beauveria brongniartii. Mol Ecol. 1997;6(4):373–81. [DOI] [PubMed] [Google Scholar]
- 25.Bricca ES, Nibouche S, Delatte H, Normand F, Amouroux P. Test of the pathogenicity of two commercial Beauveria strains on third-instar larvae of the mango blossom gall midge, Procontarinia mangiferae (Felt)(Diptera: Cecidomyiidae). Fruits. 2014;69(3):189–94. [Google Scholar]
- 26.Quesada-Moraga E, Vey A. Intra-specific variation in virulence and in vitro production of macromolecular toxins active against locust among Beauveria bassiana strains and effects of in vivo and in vitro passage on these factors. Biocontrol Sci Technol. 2003;13(3):323–40. [Google Scholar]
- 27.Quesada-Moraga E, Landa B, Muñoz-Ledesma J, Jiménez-Diáz R, Santiago-Alvarez C. Endophytic colonisation of opium poppy, Papaver somniferum, by an entomopathogenic Beauveria bassiana strain. Mycopathologia. 2006;161(5):323–9. doi: 10.1007/s11046-006-0014-0 [DOI] [PubMed] [Google Scholar]
- 28.Valero-Jiménez CA, Debets AJ, van Kan JA, Schoustra SE, Takken W, Zwaan BJ, et al. Natural variation in virulence of the entomopathogenic fungus Beauveria bassiana against malaria mosquitoes. Malar J. 2014;13(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carneiro AA, Gomes EA, Guimarães CT, Fernandes FT, Carneiro NP, Cruz I. Molecular characterization and pathogenicity of isolates of Beauveria spp. to fall armyworm. Pesq Agropec Bras. 2008;43(4):513–20. [Google Scholar]
- 30.Talaei-Hassanloui R, Kharazi-Pakdel A, Goettel M, Mozaffari J. Variation in virulence of Beauveria bassiana isolates and its relatedness to some morphological characteristics. Biocontrol Sci Technol. 2006;16(5):525–34. [Google Scholar]
- 31.Zare M, Talaei-Hassanloui R, Fotouhifar K-B. Relatedness of proteolytic potency and virulence in entomopathogenic fungus Beauveria bassiana isolates. J Crop Prot. 2014;3(4):425–34. [Google Scholar]
- 32.Todorova S, Côté J-C, Martel P, Coderre D. Heterogeneity of two Beauveria bassiana strains revealed by biochemical tests, protein profiles and bio-assays of Leptinotarsa decemlineata (Col.: Chrysomelidae) and Coleomegilla macultata lengi (Col.: Coccinellidae) larvae. Entomophaga. 1994;39(2):159–69. [Google Scholar]
- 33.Wraight SP, Ramos ME, Avery PB, Jaronski ST, Vandenberg JD. Comparative virulence of Beauveria bassiana isolates against lepidopteran pests of vegetable crops. J Invertebr Pathol. 2010;103(3):186–99. doi: 10.1016/j.jip.2010.01.001 [DOI] [PubMed] [Google Scholar]
- 34.Uma Devi K, Padmavathi J, Uma Maheswara Rao C, Khan AAP, Mohan MC. A study of host specificity in the entomopathogenic fungus Beauveria bassiana (Hypocreales, Clavicipitaceae). Biocontrol Sci Technol. 2008;18(10):975–89. [Google Scholar]
- 35.Mze Hassani I, Raveloson-Ravaomanarivo LH, Delatte H, Chiroleu F, Allibert A, Nouhou S, et al. Invasion by Bactrocera dorsalis and niche partitioning among tephritid species in Comoros. Bull Entomol Res. 2016;106(6):749–58. doi: 10.1017/S0007485316000456 [DOI] [PubMed] [Google Scholar]
- 36.Duyck PF, Quilici S. Survival and development of different life stages of three Ceratitis spp. (Diptera: Tephritidae) reared at five constant temperatures. Bull Entomol Res. 2002;92(6):461–9. [DOI] [PubMed] [Google Scholar]
- 37.Meyling NV. Methods for isolation of entomopathogenic fungi from the soil environment. Laboratory manual. University of Copenhagen; 2007.
- 38.Ekesi S, Maniania N, Lux S. Mortality in three African tephritid fruit fly puparia and adults caused by the entomopathogenic fungi, Metarhizium anisopliae and Beauveria bassiana. Biocontrol Sci Technol. 2002;12(1):7–17. [Google Scholar]
- 39.Quesada-Moraga E, Ruiz-García A, Santiago-Álvarez C. Laboratory Evaluation of Entomopathogenic Fungi Beauveria bassiana and Metarhizium anisopliae Against Puparia and Adults of Ceratitis capitata (Diptera: Tephritidae). J Econ Entomol. 2006;99(6):1955–66. [DOI] [PubMed] [Google Scholar]
- 40.De la Rosa W, Lopez F, Liedo P. Beauveria bassiana as a pathogen of the Mexican fruit fly (Diptera: Tephritidae) under laboratory conditions. J Econ Entomol. 2002;95(1):36–43. [DOI] [PubMed] [Google Scholar]
- 41.SAS Institute. SAS/STAT. 9.3 ed. Cary, NC, USA: SAS Institute Inc.; 2010.
- 42.Neuvéglise C, Brygoo Y, Vercambre B, Riba G. Comparative analysis of molecular and biological characteristics of strains of Beauveria brongniartii isolated from insects. Mycol Res. 1994;98(3):322–8. [Google Scholar]
- 43.Maurer P, Couteaudier Y, Girard P, Bridge P, Riba G. Genetic diversity of Beauveria bassiana and relatedness to host insect range. Mycol Res. 1997;101(2):159–64. [Google Scholar]
- 44.Goble TA, Rehner SA, Long SJ, Gardescu S, Hajek AE. Comparing virulence of North American Beauveria brongniartii and commercial pathogenic fungi against Asian longhorned beetles. Biol Control. 2014;72:91–7. [Google Scholar]
- 45.Keller S, Schweizer C, Shah P. Differential susceptibility of two Melolontha populations to infections by the fungus Beauveria brongniartii. Biocontrol Sci Technol. 1999;9(3):441–6. [Google Scholar]
- 46.Lu H-L, St. Leger R. Insect immunity to entomopathogenic fungi. Adv Genet. 2016;94:251–85. doi: 10.1016/bs.adgen.2015.11.002 [DOI] [PubMed] [Google Scholar]
- 47.Ortiz-Urquiza A, Riveiro-Miranda L, Santiago-Alvarez C, Quesada-Moraga E. Insect-toxic secreted proteins and virulence of the entomopathogenic fungus Beauveria bassiana. J Invertebr Pathol. 2010;105(3):270–8. doi: 10.1016/j.jip.2010.07.003 [DOI] [PubMed] [Google Scholar]
- 48.Hajek AE, St. Leger RJ. Interactions between fungal pathogens and insect hosts. Annu Rev Entomol. 1994;39(1):293–322. [Google Scholar]
- 49.Fargues J, Luz C. Effects of fluctuating moisture and temperature regimes on sporulation of Beauveria bassiana on cadavers of Rhodnius prolixus. Biocontrol Sci Technol. 1998;8(3):323–34. [Google Scholar]
- 50.Tefera T, Pringle K. Effect of exposure method to Beauveria bassiana and conidia concentration on mortality, mycosis, and sporulation in cadavers of Chilo partellus (Lepidoptera: Pyralidae). J Invertebr Pathol. 2003;84(2):90–5. [DOI] [PubMed] [Google Scholar]
- 51.European Food Safety Authority. Peer review of the pesticide risk assessment of the active substance Beauveria bassiana strain NPP111B005. EFSA Journal. 2015;13(10):4264. [Google Scholar]
- 52.European Food Safety Authority. Peer review of the pesticide risk assessment of the active substance Beauveria bassiana strain 147. EFSA Journal. 2015;13(10):4261. [Google Scholar]
- 53.Besse S, Bonhomme A, Panchaud K. Preventive and curative efficacy of Ostrinil®, a Beauveria bassiana spores based microgranule formulation, against the Castniid palm borer Paysandisia archon (Burmeister, 1880). 3rd Annual Biocontrol Industry Meeting; 2008 Oct 20–21; Lucerne, Switzerland.
- 54.Bonnet C, Martin J, Mazet R, Correard M, Besse S. Beauveria bassiana (Bals.-Criv) Vuillemin: an entomopathogen to reduce the expansion of the pine processionary plants transported by container. 3e Conférence sur l’entretien des Zones Non Agricoles; 2013 Oct 15–17; Toulouse, France: Association Française de Protection des Plantes (AFPP); 2013. p. 209–13. French.
- 55.Hu X, Xiao G, Zheng P, Shang Y, Su Y, Zhang X, et al. Trajectory and genomic determinants of fungal-pathogen speciation and host adaptation. Proc Natl Acad Sci U S A. 2014;111(47):16796–801. doi: 10.1073/pnas.1412662111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quesada-Moraga E, Navas-Cortés JA, Maranhao EA, Ortiz-Urquiza A, Santiago-Álvarez C. Factors affecting the occurrence and distribution of entomopathogenic fungi in natural and cultivated soils. Mycol Res. 2007;111(8):947–66. [DOI] [PubMed] [Google Scholar]
- 57.Meyling NV, Lubeck M, Buckley EP, Eilenberg J, Rehner SA. Community composition, host range and genetic structure of the fungal entomopathogen Beauveria in adjoining agricultural and seminatural habitats. Mol Ecol. 2009;18(6):1282–93. doi: 10.1111/j.1365-294X.2009.04095.x [DOI] [PubMed] [Google Scholar]
- 58.Moonjely S, Barelli L, Bidochka MJ. Insect pathogenic fungi as endophytes. Adv Genet. 2016;94:107–35. doi: 10.1016/bs.adgen.2015.12.004 [DOI] [PubMed] [Google Scholar]
- 59.Bidochka MJ, Menzies FV, Kamp AM. Genetic groups of the insect-pathogenic fungus Beauveria bassiana are associated with habitat and thermal growth preferences. Arch Microbiol. 2002;178(6):531–7. doi: 10.1007/s00203-002-0490-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kaplan-Meier analysis was used to estimate the mean survival time for each Beauveria strain in each bioassay (three replicates of 30 insects).
(DOCX)
Data Availability Statement
Raw data is available in Rohrlich, Clara; Merle, Isabelle; Mze Hassani, Issa; Verger Manon; Zuin Michel; Besse Samantha; Robène Isabelle; Nibouche Samuel; Costet Laurent, 2018, "Raw data for Rohrlich et al. 2018 PLOS ONE Variation in physiological host range in three strains of two species of the entomopathogenic fungus Beauveria", doi:10.18167/DVN1/CBONRN. All other relevant data are within the paper and its Supporting Information file.