Abstract
Background/Aims
The seroclearance of hepatitis B virus (HBV) surface antigen (HBsAg) is regarded as a functional cure of chronic hepatitis B (CHB) although it occurs rarely. Recently, several genome-wide association studies (GWASs) revealed various genetic alterations related to the clinical course of HBV infection. However, all of these studies focused on the progression of HBV infection to chronicity and had limited application because of the heterogeneity of HBV genotypes. In the present study, we aimed to determine susceptibility genetic markers for seroclearance of HBsAg in CHB patients with a homogenous viral genotype.
Methods
One hundred patients with CHB who had experienced HBsAg seroclearance before 60 years of age and another 100 with CHB showing high serum levels of HBsAg even after 60 years of age were enrolled. Extreme-phenotype GWAS was conducted using blood samples of participants.
Results
We identified three single nucleotide polymorphisms, rs7944135 (P = 4.17 × 10−6, odds ratio [OR] = 4.16, 95% confidence interval [CI] = 2.27–7.63) at 11q12.1, rs171941 (P = 3.52×10−6, OR = 3.69, 95% CI = 2.13–6.42) at 5q14.1, and rs6462008 (P = 3.40×10−6, OR = 0.34, 95% CI = 0.22–0.54) at 7p15.2 as novel susceptibility loci associated with HBsAg seroclearance in patients with CHB. The flanking genes at these loci including MPEG1, DTX4, MTX3, and HOXA13 were suggested to have functional significance. In addition, through functional analysis, CXCL13 was also presumed to be related.
Conclusions
To the best of our knowledge, this study is the first GWAS regarding the seroclearance of HBsAg in CHB patients. We identify new susceptibility loci for cure of CHB, providing new insights into its pathophysiology.
Introduction
Approximately 686,000 people die each year due to severe and advanced liver diseases such as cirrhosis and cancer induced by chronic hepatitis B (CHB) infection [1–3]. In Korea, public vaccination programs have decreased the incidence of hepatitis B virus (HBV) surface antigen (HBsAg) seropositive patients, but the incidence remains as high as 3% of the entire population because of higher carrier rates among the people over 30 years old who did not undergo universal vaccination and presumed to have been at risk of perinatal (vertical) transmission and horizontal transmission in early childhood [4].
Clinically, the seroclearance of HBsAg is one of the most important goals in the treatment of CHB and is regarded as a safe marker for the discontinuation of antiviral treatment with nucleos(t)ide analogues. HBsAg seroclearance also has a better prognosis than a persistent HBsAg seropositive state [5,6]. Thus, HBsAg seroclearance has been recognized as a marker for permanent remission from HBV infection if there is no pre-existing cirrhosis or viral superinfection and designated as a functional cure in current practice guidelines [7]. However, the rate of HBsAg seroclearance among patients with CHB during clinical management is extremely low worldwide (0.7–1.9% per year)[8–12] and much lower in Korea (0.4% per year)[13], possibly because of differences in prevalent HBV genotype or transmission age [13,14].
Previously, potential clinical variables associated with HBsAg seroclearance in patients with CHB have been suggested, including old age and a low viral load, as determined by low serum HBV DNA or HBsAg levels [11,15–18]. However, these findings appear to just reflect the natural corollary of rapid viral elimination in patients prone to attain earlier seroclearance of HBsAg. Meanwhile, recent genome-wide association studies (GWASs) have identified multiple genomic loci associated with the risk of chronic HBV infection, including human leukocyte antigen (HLA) loci and non-HLA loci [19–21]. However, the findings of previous GWASs require careful interpretation because most of these studies involved subjects with an uncertain history of HBV exposure or an unidentified age of infection [21–24]. The age of the subject at the time of infection is the most important factor that determines the natural course following HBV infection [25]. The majority of the HBsAg negative subjects in previous studies probably had recovered from acute HBV infections during adulthood, whereas the HBsAg carriers recruited were most likely to have been infected with HBV during the perinatal period or preschool age since the studies were performed in endemic areas of HBV. Accordingly, it is uncertain whether the genetic markers disclosed in previous GWASs reflect the genetic mechanisms involved in chronicization of acute HBV infection or those involved in remission of long-term chronic HBV infection. Considering this ambiguity in previous studies, in the present study, we aimed to search for relevant genetic factors focusing on viral clearance in CHB patients. Accordingly, only subjects who had a well-documented history of chronic HBV infection were included and those who had undergone spontaneous remission from an acute HBV infection were excluded from the study.
Clinical studies have indicated that an older age (≥ 60 vs. 30–39 years old, P = 0.007, odds ratio [OR] = 6.07) and a lower HBsAg titer (< 10 vs. ≥ 1000 IU/mL, P <0.001, OR = 13.2) are significantly associated with HBsAg seroclearance in patients with CHB [15,17]. In this study, therefore, an extreme-phenotype GWAS analysis was performed by exploring single nucleotide polymorphisms between patients with CHB who achieved HBsAg seroclearance before 60 years of age and those who exhibited very high serum levels of HBsAg (≥1000 IU/mL) even after the age of 60 years, having low possibility of HBsAg seroclearance in the future. The present study aimed to identify novel associated loci that might expand our knowledge of the genetic mechanism of HBsAg seroclearance in patients with CHB.
Materials and methods
Subjects and ethics
A total of 200 patients who had visited the liver clinic of Korea University Anam Hospital for the follow-up of CHB and who fulfilled the inclusion criteria were enrolled prospectively and consecutively from January 2014 to January 2016. All the patients had been diagnosed as CHB and were regularly followed-up between 2000 and 2014 in our clinic at least twice a year (every 6 months) with routine laboratory tests for serum biochemistry and/or HBV virus markers. Half of them (n = 100) corresponded to the case group and another half (n = 100) to the control group. The patient group was defined as follows: the case group included patients who had experienced HBsAg seroclearance at < 60 years of age and the control group included those who had exhibited a high level (> 1000 IU/mL) of HBsAg at ≥ 60 years of age. All of the study subjects were of Korean ethnicity. Diagnosis of chronic HBV infection was established based on the seropositivity of HBsAg over a 6-month period. The family history of CHB was confirmed through an inquiry or record query process. The seroclearance of HBsAg was defined as the HBsAg level measuring less than the detection limit (0.05 IU/mL), as obtained twice consecutively at least one year apart by the Architect HBsAg QT assay (Abbott Laboratories, Chicago, IL, USA). It was also defined as sero-negative in qualitative HBsAg and anti-HBs assay by commercial methods (COBAS e601, Roche diagnostics, Indianapolis, IN, USA). HBV DNA was measured by COBAS TaqMan® real-time PCR quantification method (Roche Diagnostics) with a detection limit of 20 IU/ml. Hepatitis B e antigen (HBeAg) and anti-HBe were confirmed by immunoradiometric assay (North Institute of Biological Technology, Beijing, China), was used according to the manufacturer’s instruction. None of the participants presented any evidence of concomitant hepatitis C virus, hepatitis D virus, or human immunodeficiency virus infection. All the participants provided written informed consent for participation, the use of their medical data and collection of serum samples for research purpose. This project was approved by the ethics committees at Korea University Anam Hospital (ED13220) and conducted in agreement with the ethical principles of the Declaration of Helsinki.
Single-nucleotide polymorphism (SNP) genotyping
Two hundred samples were genotyped using a HumanOmni2.5–8 BeadChip (Illumina Inc., San Diego, CA, USA), which includes 2,372,784 SNP loci. Samples were processed according to the Illumina Infinium-II assay manual. SNP quality control for all sets of samples was applied as follows: SNPs were excluded if they (i) were not located on autosomal chromosomes, or had (ii) a call rate of <0.95 in both cases and controls, (iii) a minor allele frequency of <0.01, or (iv) significant deviation from the Hardy–Weinberg equilibrium of (P < 1.0 × 10−5) in controls. Similarly, all the samples were identified with a genotyping rate > 96% and a potential genetic relatedness based on pair–wise identity by state. The remaining 1,365,088 SNPs were used in subsequent analyses for association.
GWAS
The associations between genotypes and chronic HBV clearance were assessed in an additive model by logistic regression with covariate adjustment for sex using PLINK v 1.07 (http://zzz.bwh.harvard.edu/plink). The quantile–quantile plot was generated using R (3.3.2) (http://www.r-project.org/) to evaluate the overall significance of the genome-wide associations and the potential impact of population stratification. The impact of population stratification was also evaluated by calculating the genomic control inflation factor.
The proxy SNPs with regional recombination rates were assessed using LocusZoom (http://locuszoom.org/) and the 1000 Genomes Project data. Haplotype blocks were generated from the Haploview software (http://www.broadinstitute.org/haploview). A haplotype association test was performed using the hap-logistic option in PLINK. A binomial multivariate logistic regression analysis was performed with using R. P-values ≤ 0.05 were considered significant.
Gene ontology analysis was performed on the gene sets harboring the identified SNPs, using DAVID (https://david.ncifcrf.gov). The pathway analysis was performed on the gene sets harboring the identified SNPs, using i-GSEA4GWAS-v2 [26]. Regarding the other options, default values preset in DAVID and i-GSEA4GWAS were employed.
Results
Clinical characteristics of participants
To determine novel loci conferring susceptibility to functional cure from chronic HBV infection, a primary GWAS screen was performed with the aforementioned 200 chronic carriers of HBsAg, who were divided into case (n = 100) and control (n = 100) groups according to the absence or a high level of HBsAg in serum at ≥ 60 years of age. The clinical characteristics of the two groups are summarized in Table 1. The case group was younger than the control group at the time of initial diagnosis of CHB, the initial visit to our clinic, and when GWAS was performed (mean age 60.6 ± 9.3 vs. 69.3 ± 5.4 years, P <0.001), while showing longer duration of total follow-up. The case group included significantly more male subjects (71%) than the control group (45%) (odds ratio [OR] = 0.37, P <0.001). At initial visit, the subjects in the control group exhibited higher levels of serum aminotransferase and were mostly seropositive for HBeAg compared to those in the case group. Thus, more number of patients received antiviral treatment in the control group than in the case group (84% vs 31%, P <0.001), using oral nucleos(t)ide analogues or interferon (IFN), or both. The duration of oral nucleos(t)ide analogue treatment did not differ between the control and case groups (7.3±3.7 vs 7.7±4.0 years). However, at the time of GWAS, 13 subjects in the control group were still seropositive for HBeAg in spite of their old age, while all the subjects in the case group were seronegative for HBeAg, and 66 of them showed anti-HBs seropositivity. The familial history of chronic HBV infection in the mother or siblings was obvious in 151 of 200 patients with the comparable incidences between the case and control groups (70% vs 81%); only 3 insisted on the definite absence of CHB in their family, and for the remaining patients, familial history was uncertain. The comparison of clinical features between the subjects with and without confirmed family history are shown in S1 Table.
Table 1. Clinical features of participants in the present study.
| Case (n = 100) HBsAg (-) at 60 years old |
Control (n = 100) HBsAg >1000IU/mL at 60 years old |
P-value | ||
|---|---|---|---|---|
| Male, n | 71 | 45 | < 0.001a | |
| Age at initial diagnosis, years | 36.0±11.3 | 47.0±13.0 | < 0.001b | |
| Age at 1st visit, years | 43.9±11.4 | 59.9±7.7 | < 0.001b | |
| Age at GWAS, years | 60.6±9.3 | 69.3±5.4 | <0.001b | |
| Follow-up duration, years | 16.7±8.1 | 9.5±5.8 | <0.001b | |
| Age at HBsAg seroclearance, years | 54.7±8.9 | - | - | |
| ALT at 1st visit, IU/L | 36.0 (17–73) | 49 (25–104) | 0.032c | |
| ALT at GWAS, IU/L | 18.0 (13–27) | 22.0 (17–29) | 0.012c | |
| HBeAg (+) at 1st visit, n | 30 | 52 | 0.002a | |
| HBeAg (+) at GWAS, n | 0 | 13 | - | |
| Anti-HBe (+) at 1st visit, n | 70 | 42 | < 0.001a | |
| Anti-HBe (+) at GWAS, n | 90 | 60 | < 0.001a | |
| HBsAg (+) at 1st visit, n | 100 | 100 | - | |
| HBsAg (+) at GWAS, n | 0 | 100 | - | |
| Anti-HBs (+) at GWAS, n | 66 | 0 | 0.218a | |
| HBV DNA < 20 IU/mL at GWAS, n | 94 | 75 | <0.001a | |
| CHB in mother or siblings, n | ||||
| Present | 70 | 81 | 0.071 | |
| Absent | 3 | 0 | ||
| Uncertain | 27 | 19 | ||
| Treatment type: | Non-treated, n | 69 | 16 | < 0.001a |
| IFN only, n | 7 | 0 | ||
| NA only, n | 22 | 81 | ||
| IFN & NA, n | 2 | 3 | ||
| Age of treatment start, years | 43.1±11.6 | 61.3±6.6 | <0.001b | |
| NA treatment period, years | 7.3±3.7 | 7.7±4.0 | 0.639b | |
Data presented as number, mean ± standard deviation, or median (IQR). CHB, chronic hepatitis B; GWAS, genome-wide association study; ALT, alanine aminotransferase (normal range 5–40 U/L); HBeAg, hepatitis B e antigen; IFN, interferon (including PEGylated-interferon); NA, nucleos(t)ide analogues.
a, Chi- square test
b, Student's t-test
c, Mann-Whitney U test.
GWAS profiling of patients with CHB showing the presence or absence of HBsAg seroclearance
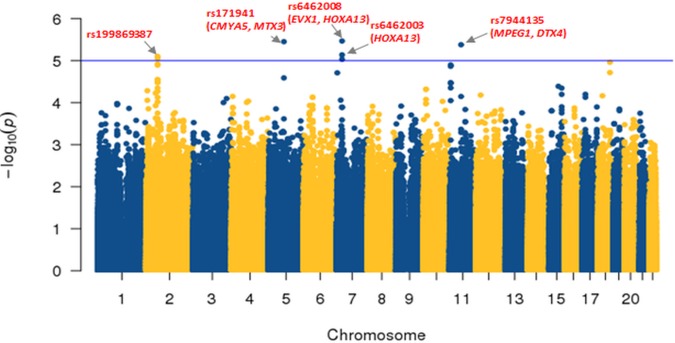
Logistic association analyses were conducted between the SNPs and the seroclearance of HBsAg in patients with CHB. Quantile–quantile plot analysis showed a low genomic inflation factor (λ = 0.893), indicating that the association results were not influenced by population stratification (S1 Fig). Of the 1,365,088 SNP probes, we identified 52 SNPs associated with the status of HBsAg seroclearance with cutoff P-value of 10−4, correcting the effects of gender (S2 Table). The three SNPs (rs6462008, rs171941, and rs7944135) were found as the most significant markers (OR = 0.34, 3.69, and 4.16; P = 3.40×10–6, 3.52×10–6, and 4.17×10–6, respectively), and reside at chromosomes 7p15.2, 5q14.1, and 11q12.1, respectively (Fig 1).
Fig 1. Chromosomal distribution of the single-nucleotide polymorphisms (SNPs) associated with the seroclearance of hepatitis B surface antigen (HBsAg) in patients with chronic hepatitis B (CHB).
The Manhattan plot shows the chromosomal distribution of the–log10 (P value) of SNPs. The threshold cutoff for the significant SNPs (P <10−5) is shown with horizontal line.
Next, to address the effects of the allelic changes of SNPs on gene functions, we examined the flanking genes residing at the regions within 150 kb up- and down-stream from each significantly associated SNP locus. The rs6462008 is located near even-skipped homeobox 1 (EVX1) and homeobox A13 (HOXA13) on chromosome 7p15.2 (P = 3.40×10–6, OR = 0.34, 95% CI = 0.37–0.42). The rs171941 is located near cardiomyopathy associated 5 (CMYA5) and metaxin 3 (MTX3) on chromosome 5q14.1 (P = 3.52×10–6, OR = 3.69, 95% CI = 2.13–6.42). The rs7944135 is located near the macrophage expressed 1 (MPEG1) and deltex E3 ubiquitin ligase 4 (DTX4) on chromosome 11q12.1 (P = 4.17×10–6, OR = 4.16, 95% CI = 2.27–7.63).
In addition, we examined SNPs previously reported to be associated with chronic HBV infection. Twenty-four SNPs were obtained from the literatures and examined. Most of those SNPs were not significant in our data set (S3 Table). Only rs1419881 and rs10484569 showed marginal significance (P = 0.024 and 0.035).
Identification of the regions of interest (ROIs) for the significant SNPs
Next, we determined the ROIs that had at least one SNP with P<5.0×10−6 and more than two susceptibility-associated SNPs (P < 5.0×10−4) among the surrounding SNPs within 50 kb. This analysis revealed three ROIs based on the three most significantly associated SNPs (P < 5.0×10−6) at 7p15.2 (ROI1), 5q14.1 (ROI2), and 11q12.1 (ROI3) (Fig 2). Among these, only ROI3 was valid due to its high linkage disequilibrium (LD) (r2 >0.8) compared with other two ROIs (r2 <0.5).
Fig 2. Three regions of interest (ROIs) for susceptibility loci of HBsAg seroclearance.
Three ROIs at 7p15.2, 5q14.1, and 11q12.1 are shown using LocusZoom (top). For the SNPs in the ROI, −log10 (P value) are plotted, and the significant SNPs are indicated. The section of the ROI is indicated with yellow bars. The recombination rates expressed in centimorgans (cM) per Mb (NCBI Build GRCh37) are also shown. The linkage disequilibrium values of the SNPs in the ROIs plotted using Haploview are shown (bottom). D′-based LD maps are shown for the two different data sets of our Korean cohort (KR) and the public data of the Beijing Han Chinese and Tokyo Japanese cohorts form 1000 Genomes Project data. The D′ scores for each paired SNP are indicated using different colors. As the LD increases, the color of the diamond becomes closer to red. The results show that both cohorts have significant LD among the SNPs within ROI. ROI1 and 2 was excluded for further analyses due to low LD (r2 < 0.5).
The rs7944135 at ROI3 was located in an intergenic region at 11q12.1. This ROI included loci for rs3944255 (P = 7.11×10−5) in an intergenic region and rs5029315 (P = 4.29×10−4) within the 3’ untranslated region of MPEG1 (Table 2). Other representative SNPs, i.e., rs7113936 and rs656163 (P < 10−3) were also included.
Table 2. ROI3 enriched with significant SNPs for HBsAg seroclearance in CHB patients.
| SNP | Allele | Position | Genea | MAF | OR | 95% CI | P-value | ||
|---|---|---|---|---|---|---|---|---|---|
| Case | Control | ||||||||
| ROI3 | rs 5029315 |
T | Chr11: 58977768 |
MPEG1 | 0.349 | 0.2 | 2.46 | 1.49–4.06 | 4.29×10−4 |
|
rs 7944135 |
A |
Chr11: 59020987 |
DTX4, MPEG1 | 0.31 | 0.125 | 4.16 | 2.27–7.63 | 4.17×10−6 | |
| rs 3944255 |
T | Chr11: 59030285 |
DTX4, MPEG1 | 0.29 | 0.14 | 3.16 | 1.79–5.58 | 7.11×10−5 | |
ROI, region of interest; MAF, minor allele frequency; OR, odds ratio; CI, confidence interval.
a: The flanking genes were located ± 150 kb of each SNP.
ROI3 was located within high LD blocks (r2 = 0.75) showing a strong LD (Fig 2). Moreover, ROI3 showed a high LD in an independent cohort study from the Asian population (Beijing Han Chinese and Tokyo Japanese) in phase 3 of the 1000 Genomes Project [27]. Thus, we suggest that the SNPs in this ROI are highly linked with one another, are heritable, and have a functional significance.
In addition, we further sought to determine whether the haplotype construction using the SNPs in the ROI can improve the statistical significance of the associations with the SNPs. As shown in Table 3, we found that the haplotypes of “T-A” (rs5029315–rs7944135) in ROI3 showed more significant associations (P = 3.45×10−6, OR = 4.22) than the individual SNPs (rs5029315, P = 4.29×10−4; rs7944135, P = 4.17×10−6; rs3944255, P = 7.11×10−5).
Table 3. Haplotype analysis in the ROI for HBsAg seroclearance in patients with CHB.
| CHR | BP1 | BP2 | SNP1 | SNP2 | SNP3 | Haplotype | OR | P-value |
|---|---|---|---|---|---|---|---|---|
| 11 | 58977768 | 59020987 | rs5029315 | rs7944135 | TA | 4.22 | 3.45×10−6 | |
| 11 | 58977768 | 59030285 | rs5029315 | rs7944135 | rs3944255 | TAT | 4.48 | 4.74×10−6 |
| 11 | 59020987 | 59030285 | rs7944135 | rs3944255 | AT | 4.46 | 4.77×10−6 |
The three most significantly associated SNPs within ROI (rs5029315, rs7944135, and rs3944255).
ROI, region of interest; CHB, chronic hepatitis B; CHR, chromosome; BP, base pair; SNP, single nucleotide polymorphism; OR, odds ratio.
Functional association of the significant SNPs
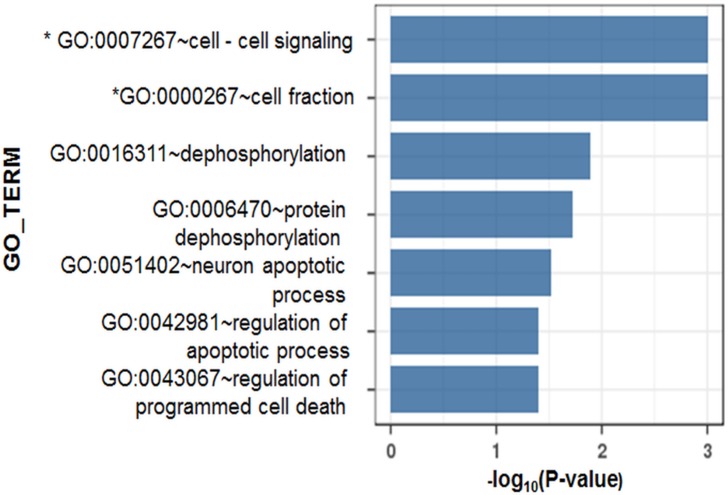
Next, we performed gene ontology analysis to determine whether the associated SNPs exhibit a functional enrichment. By inputting the identities of the genes harboring significant SNPs with P < 5×10−4 to the web-based software DAVID and i-GSEA4GWAS, we found significant functional enrichment in seven gene ontologies (Fig 3). Of these, in particular, the pathways of cell-cell signaling (P < 0.001, false discovery rate [FDR] = 0.028) and cell fraction (P < 0.001, FDR = 0.059) had highly enriched associations with the identified susceptibility-associated SNPs with an FDR cutoff < 0.2. Among the genes assigned to the cell-cell signaling gene ontology term in this study, C-X-C-chemokine ligand 13 (CXCL13) had the most significant flanking SNPs (rs11931577, P = 9.85×10−5, OR 0.27, 95% CI = 0.14–0.52). These genes have been noted to play important roles in the process of HBV infection.
Fig 3. Gene-set enrichment study of the significantly associated SNPs.
The functional enrichment among the flanking genes of identified susceptibility-associated SNPs (P < 5.0×10−4) was estimated by gene ontology and pathway analyses implemented using DAVID and i-GSEA4GWAS softwares, respectively. The significance of the susceptibility-associated SNPs was estimated with a cutoff of P < 5.0×10−4. * P-values obtained from the pathway analysis are indicated.
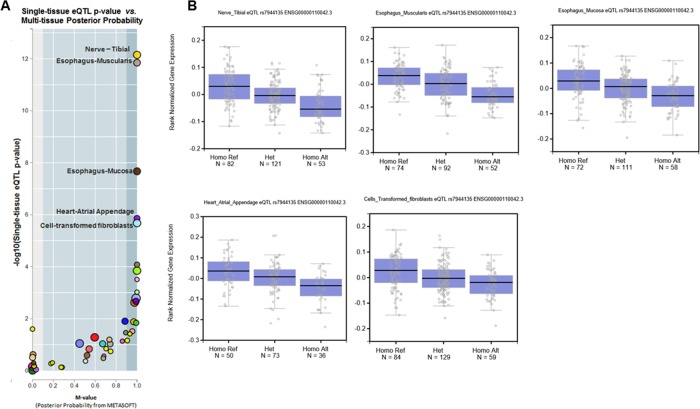
To further elaborate the functional significance of the SNPs, we performed a cis-expression quantitative trait loci (QTL) analysis to evaluate the associations of the SNPs with the expression levels of cis-mapped genes. This analysis is based on the correlation between genotype and tissue-specific RNA levels of the target gene. When we examined rs7944135 using the web-based QTL analysis tool GTEX portal (http://www.gtexportal.org), we found that the polymorphism of rs7944135 was significantly associated with the gene expression of the flanking gene DTX4 in at least five cells or tissue types (nerve, esophagus mucosa and muscularis, fibroblast, and heart, P < 1.0×10−5; Fig 4). Along with GG, GA, and AA genotypes of rs7944135, the DTX4 expression significantly reduced. Those genotypes were identified in 46/46/8 in case group and 75/25/0 in control group. This result may support the functional relevance of rs7944135 and its flanking gene DTX4 in the HBsAg seroclearance of patients with CHB.
Fig 4. Genotype-tissue expression analysis of rs7944135.
A. A cis-expression quantitative trait locus analysis and multi-tissue posterior probability for rs7944135 and DTX4 are shown using the GTEx portal database. The five tissues with the highest statistical significance in the cis-expression quantitative trait locus analysis (P-value < 10−5) are indicated. This means that the expression level of DTX4 significantly differs according to the allele of rs7944135 in those five tissues. B. The graphs for expression level of DTX4 according to the genotypes of rs7944135 in five tissues. With the alteration to A, minor allele, of rs7944135, that was associated with acquisition of HBsAg seroclearance, the expression of DTX4 is decreased.
Subgroup GWAS regarding HBsAg seroclearance
We performed subgroup analysis to investigate the SNPs associated with HBsAg seroclearance in the subjects who had received antiviral treatment, and further sought the SNPs associated with HBsAg seroconversion, i.e. acquisition of anti-HBs within the case group who had accomplished HBsAg seroclearance. The results are presented in S4 Table. The top 30 SNPs associated with HBsAg seroclearance, except rs171941, rs2153442, and rs4748035, did not overlap between the whole study population and the subpopulation given antiviral treatment, although most of the associated SNPs in each group still maintained statistical significance (P<0.05) in the other group. However, the SNPs associated with the HBsAg seroconversion were completely distinct from those associated with HBsAg seroclearance.
Discussion
Almost all healthy adults who are not immunosuppressed will rapidly recover from HBV infection following a short-term acute phase. Those rare people who progress to chronicity with adulthood HBV infection might have some genetic defects in the process of clearing HBV. On the other hand, people infected with HBV in infancy or early childhood almost always progress to chronic infections probably because of immature immunity against HBV in that age [25], and recovery from HBV infection in these patients is very rare. Thus, genetic factors associated with HBsAg seroclearance in CHB patients who had been infected in childhood are likely to differ from those determining the chronicization of adulthood HBV infections. In this respect, it is somewhat difficult to define what previous GWASs really meant by their results, since those studies did not exactly clarify the onset time of HBV infection for both patients with and without HBsAg. In all probability, however, the findings of previous GWASs mainly appear to represent genetic markers associated with the chronicization of adult HBV infection rather than viral remission in CHB patients infected in early childhood, for most of previous GWASs were conducted in East Asia, and almost all the participants assigned in the control group without HBsAg are presumed to have recovered from acute phase of adult-onset HBV infection.
To the best of our knowledge, the present study is the first GWAS that attempted to elucidate the genetic factors associated with recovery from chronic HBV infection. To overcome the limitation of having a relatively small number of participants, we performed extreme phenotype GWAS, employing the two groups of patients at opposite poles in HBsAg serodynamics; the subjects that experienced HBsAg seroclearance at < 60 years of age and those who exhibited high serum levels of HBsAg even at ≥ 60 years of age. Furthermore, all the subjects had been confirmed to have HBV infection for a long duration. In addition, this study has the strength of excluding impacts according to heterogeneous HBV genotypes because almost all CHB cases in Korea are infected by HBV genotype C [14].
Most clinical characteristics of the case and control groups in the present study appear to reflect the phenomena occurring as the consequence of different natural history of viral infection, rather than the ones causally related. The serum level of HBV DNA was higher, and anti-HBe seropositivity was lower in subjects showing persistently high level of HBsAg (the control group) compared with those subjects showing HBsAg seroclearance (the case group). HBsAg carriers, who had shown abnormal serum aminotransferase levels during the initial visit, required antiviral treatment in the majority of cases because of persisting viremia and signs of hepatic inflammation. Notably, the treatment periods were not significantly different between the case and control groups, suggesting that antiviral agents did not significantly influence HBsAg seroclearance.
Many studies indicated that male subjects are more susceptible to aggravation of chronic hepatitis B than female subjects because of their sex hormones [28,29], which might suggest strong immune response in males. In similar context, the male predominance in HBsAg-serocleared patients in the present study might suggest the potential impact of gender on HBV elimination in CHB patients in the endemic region of vertical HBV transmission. Comparable findings have been reported in two cohorts in Taiwan and Korea [5,30].
Apart from gender, some SNPs (50 SNPs with P-value of <10−4) were independently and potentially associated with HBsAg seroclearance in multivariate analysis. Furthermore, the three most significantly associated SNPs (P <5.0×10−6) and one SNP elicited from web-based functional analysis in the present study have not been revealed to be associated with chronicity of HBV infection in previous GWAS. Although the flanking genes near these susceptibility loci were not reported to be specifically associated with the seroclearance of HBsAg, several studies have shown their associations with HBV infection.
HOXA13, which is a flanking gene of rs6462008 and rs6462003 (the 1st and 4th most significant SNPs), is related to the control of cell proliferation, and its expression is increased in hepatocellular carcinoma and liver cirrhosis [31]. Moreover, it was reported to be associated with interferon (IFN) expression [32]. Its sequential action through bone morphogenetic protein 2 (BMP2), JunB, B-cell chronic lymphocytic leukemia/lymphoma 3 (BCL3), and interferon regulatory factor 1 (IRF1) ultimately leads to an increase in the expression of IFN-β or interleukin-1β. This pathway represents an alternative mechanism for the induction of inflammatory and apoptotic genes in the absence of the IFN-α/β receptor during viral infection. CHB might occur during an immunosuppressed state that is mediated by several actions of HBV protein X, such as, down-regulation of type I IFN receptor and the inhibition of extracellular IFN-α-mediated signal transduction [33]. Thus, the inflammatory pathway via HOXA13, an alternative mechanism functioning in the absence of type I IFN receptor, may play a crucial role in the elimination of HBV in patients with CHB.
MTX3, the flanking gene of rs171941, encodes a mitochondrial outer membrane protein. This molecule may regulate the permeability of the mitochondrial membrane, and its deficiency may allow cells to resist tumor necrosis factor-induced apoptosis [34]. Because HBV protein X also perturbs mitophagy and apoptosis signals to promote the survival of HBV, a change in the expression of MTX3 may influence the medical course of CHB.
MPEG1 and DTX4, the flanking genes of rs7944135, appear to be biologically relevant because their functions have been associated with viral clearance through the activation of the IFN pathway. MPEG1 was originally identified as a potential marker of mature macrophages [35], and is one of the interferon-induced genes that inhibit HBV replication in transgenic mouse hepatocytes [36]. Taken together, we suggest that it is biologically plausible that MPEG1 could influence the susceptibility to HBsAg seroclearance.
The immune system of host detects intruding pathogens including HBV by Toll-like receptors (TLRs) and retinoic acid-inducible gene I (RIG-I), and triggers immune reactions such as conjugation of TNF receptor-associated factor 3 (TRAF3) and non-canonical IkB kinases (IKKs), including TANK-binding kinase 1 (TBK1) and IKKi. The complex phosphorylates interferon regulatory factor (IRF), resulting in increased activity of the type I interferons and restricted activity of virus. In chronic HBV infection, HBV protein X suppresses this antiviral pathway [37]. Against this suppression, GS-9620, an oral agonist of TLR-7, has been proposed as a candidate for a new antiviral drug for CHB, as recent studies reported the antiviral effect of this drug [38,39]. DTX4 is also involved with this pathway. It induces TBK1 ubiquitination and degradation through conjugation with nucleotide-binding domain, leucine rich containing family, pyrin domain containing 4 (NLRP4) and thereby restricts the induction of type I IFN signaling [40]. Thus, DTX4 expression can suppress virus-triggered IFN signaling [37,41,42]. Congruently, our cis-expression QTL analyses demonstrated a functional association between rs7944135 and the expression of DTX4 using the GTEX database. Presence of the minor allele of rs7944135 that was associated with acquisition of HBsAg seroclearance, lead to decrease in DTX4 expression. Therefore, these results indicate that the allele alteration of rs7944135 could change the expression of DTX4 and subsequently influence sustenance of HBV and maintenance of serum titer of HBsAg. However, the association was not confirmed in the liver tissue due to the smaller number of samples. Nonetheless, that association is expected to be reproduced for the liver tissue in the future studies because the significant associations were noted for many tissues. Regarding the linkage disequilibrium analyses, to the rs7944135, haplotypes with rs5029315 had a lower P-value and a higher OR than those with rs7944135 alone although its P-value was lower than that of rs6462008 alone. Nevertheless, the haplotype of rs7944135 with rs5029315 may contribute an additional benefit for the prediction of HBsAg seroclearance in patients with CHB.
CXCL13, which is the flanking gene of rs11931577 in the cell-cell signaling pathway, functions to attract B lymphocytes and promote the migration and aggregation. It is expressed in an age-dependent manner in mouse hepatic macrophages and plays an integral role in facilitating an effective immune response against HBV [43]. A high level of CXCL13 contributes to the control of the viral load during treatment with nucleos(t)ide analogues [44]. Its action may also contribute to achieving HBsAg seroclearance.
In addition, subgroup analysis revealed some SNPs that were associated with HBsAg seroclearance in the subjects who received antiviral treatment. In particular, rs171941 was highly associated with HBsAg seroclearance not only in the whole study population but also in the subgroup that received antiviral treatment, suggesting its important association with HBsAg serodynamics during antiviral treatment. Also, many SNPs were found significantly associated with the acquisition of anti-HBs within the subjects who attained HBsAg seroclearance. This indicates that some additional genetic factors might be related to the appearance of anti-HBs after the occurrence of HBsAg seroclearance. However, we did not conduct a further bioinformatics data search for those SNPs, considering the small number of subjects in the subgroups.
In summary, the present study is the first GWAS to investigate the genetic predisposition associated with the functional cure in patients with CHB. Our GWAS analysis revealed three novel susceptibility loci for HBsAg seroclearance in a Korean CHB population, including rs6462008 at 7p15.2, rs171941 at 5q14.1 and rs7944135 at 11q12.1. These SNPs are located adjacent to the HOXA13, MTX3, MPEG1 and DTX4 genes, respectively. In particular, rs7944135 showed a functional relevance with DTX4 gene expression in web-based bioinformatics analysis. Moreover, another SNP rs11931577 is located near CXCL13 gene, and modulates the immune response against HBV infection. Further investigations are required for the validation of significant SNPs and the identification of the associations between those SNPs and the functions of flanking genes.
The results of the present study might be helpful for predicting the clinical outcomes of patients with CHB and elucidating the mechanism of viral elimination in chronic HBV infection. In addition, the SNPs and their flanking genes that are suggested to be associated with HBsAg seroclearance might act as targets for new pharmaceutical CHB treatments and represent clinical evidence for considering the discontinuation of antiviral treatment in patients with CHB.
Supporting information
The distributions of observed p-values did not deviate from the null distribution, which excluded systematic bias due to bad genotyping or population structure. The y-axis is the observed -log10(p) values; the x-axis is the expected -log10(p) values. The genomic control inflation factor (λ) is 0.893.
(PPTX)
Data were presented as number (%), mean ± standard deviation, or median (IQR). ALT, alanine aminotransferase (normal range 5–40 U/L); HBeAg, hepatitis B e antigen; IFN, interferon (including PEGylated-interferon); NA, nucleos(t)ide analogues.
(DOCX)
The significant SNPs (p < 1.0ⅹ10−4) are presented. SNP, single nucleotide polymorphism; rsID, reference SNP identity; Chr, chromosome; A1, minor allele in the entire cohort; A2, major allele; OR, odds ratio; CI, confidence interval. a: The flanking genes were located ± 150 kb of each SNP. The odds ratio for the minor allele in an additive model. P-value was obtained by logistic regression test for the minor allele additive model.
(DOCX)
SNP, single nucleotide polymorphism; GWAS, genome-wide association study; rsID, reference SNP identity; A1, minor allele; A2, major allele; chr, chromosome; OR, odds ratio; CI, confidence interval; PMID, pubmed identity. The odds ratio for the minor allele in a additive model. P-value was obtained by logistic regression test for the minor allele additive model.
(DOCX)
The most significant SNPs (up to 30 SNPs) are presented according to each outcome with P-values of other outcomes, as well. SNP, single nucleotide polymorphism; rsID, reference SNP identity; Chr, chromosome. a: The flanking genes were located ± 150 kb of each SNP. The odds ratio for the minor allele in an additive model. P-value was obtained by logistic regression test for the minor allele additive model.
(XLSX)
Acknowledgments
We thank Macrogen (www.macrogen.co.kr) for technical support, and Editage (www.editage.co.kr) for the English language review.
Abbreviations
- CHB
chronic hepatitis B
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- GWAS
genome-wide association study
- HLA
human leukocyte antigen
- SNP
single-nucleotide polymorphism
- IFN
interferon
Data Availability
All relevant data are available from figshare: https://figshare.com/articles/gtReport_txt/6614975 (DOI: 10.6084/m9.figshare.6614975).
Funding Statement
This work was funded in full by the National Research Foundation of Korea (NRF; https://www.nrf.re.kr) funded by the Korea government (MSIP), grant number NRF-2012R1A5A2048183. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48(2): 335–352. doi: 10.1016/j.jhep.2007.11.011 [DOI] [PubMed] [Google Scholar]
- 2.Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012; 30(12): 2212–2219. doi: 10.1016/j.vaccine.2011.12.116 [DOI] [PubMed] [Google Scholar]
- 3.Trepo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014; 384(9959): 2053–2063. doi: 10.1016/S0140-6736(14)60220-8 [DOI] [PubMed] [Google Scholar]
- 4.Cho EJ, Kim SE, Suk KT, An J, Jeong SW, Chung WJ, et al. Current status and strategies for hepatitis B control in Korea. Clin Mol Hepatol. 2017;23(3): 205–211. doi: 10.3350/cmh.2017.0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu CM, Liaw YF. HBsAg seroclearance in asymptomatic carriers of high endemic areas: appreciably high rates during a long-term follow-up. Hepatology. 2007;45(5): 1187–1192. doi: 10.1002/hep.21612 [DOI] [PubMed] [Google Scholar]
- 6.Chen YC, Sheen IS, Chu CM, Liaw YF. Prognosis following spontaneous HBsAg seroclearance in chronic hepatitis B patients with or without concurrent infection. Gastroenterology. 2002;123(4): 1084–1089. [DOI] [PubMed] [Google Scholar]
- 7.EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2): 370–398. doi: 10.1016/j.jhep.2017.03.021 [DOI] [PubMed] [Google Scholar]
- 8.Brunetto MR, Giarin MM, Oliveri F, Chiaberge E, Baldi M, Alfarano A, et al. Wild-type and e antigen-minus hepatitis B viruses and course of chronic hepatitis. Proc Natl Acad Sci U S A. 1991;88(10): 4186–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liaw YF, Sheen IS, Chen TJ, Chu CM, Pao CC. Incidence, determinants and significance of delayed clearance of serum HBsAg in chronic hepatitis B virus infection: a prospective study. Hepatology. 1991;13(4): 627–631. [PubMed] [Google Scholar]
- 10.Kato Y, Nakao K, Hamasaki K, Kato H, Nakata K, Kusumoto Y, et al. Spontaneous loss of hepatitis B surface antigen in chronic carriers, based on a long-term follow-up study in Goto Islands, Japan. J Gastroenterol. 2000;35(3): 201–205. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira SC, Chacha SG, Souza FF, Teixeira AC, Santana RC, Villanova MG, et al. Factors associated with spontaneous HBsAg clearance in chronic hepatitis B patients followed at a university hospital. Ann Hepatol. 2014;13(6): 762–770. [PubMed] [Google Scholar]
- 12.Chu CM, Liaw YF. Hepatitis B surface antigen seroclearance during chronic HBV infection. Antivir Ther. 2010;15(2): 133–143. doi: 10.3851/IMP1497 [DOI] [PubMed] [Google Scholar]
- 13.Nam SW, Jung JJ, Bae SH, Choi JY, Yoon SK, Cho SH, et al. Clinical outcomes of delayed clearance of serum HBsAG in patients with chronic HBV infection. Korean J Intern Med. 2007;22(2): 73–76. doi: 10.3904/kjim.2007.22.2.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bae SH, Yoon SK, Jang JW, Kim CW, Nam SW, Choi JY, et al. Hepatitis B virus genotype C prevails among chronic carriers of the virus in Korea. J Korean Med Sci. 2005;20(5): 816–820. doi: 10.3346/jkms.2005.20.5.816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Yang HI, Lee MH, Lu SN, Jen CL, Wang LY, et al. Incidence and determinants of spontaneous hepatitis B surface antigen seroclearance: a community-based follow-up study. Gastroenterology. 2010;139(2): 474–482. doi: 10.1053/j.gastro.2010.04.048 [DOI] [PubMed] [Google Scholar]
- 16.Tseng TC, Liu CJ, Su TH, Wang CC, Chen CL, Chen PJ, et al. Serum hepatitis B surface antigen levels predict surface antigen loss in hepatitis B e antigen seroconverters. Gastroenterology. 2011;141(2): 517–525, 25.e1-2. doi: 10.1053/j.gastro.2011.04.046 [DOI] [PubMed] [Google Scholar]
- 17.Tseng TC, Liu CJ, Yang HC, Su TH, Wang CC, Chen CL, et al. Determinants of spontaneous surface antigen loss in hepatitis B e antigen-negative patients with a low viral load. Hepatology. 2012;55(1): 68–76. doi: 10.1002/hep.24615 [DOI] [PubMed] [Google Scholar]
- 18.Tseng TC, Kao JH. Clinical utility of quantitative HBsAg in natural history and nucleos(t)ide analogue treatment of chronic hepatitis B: new trick of old dog. J Gastroenterol. 2013;48(1): 13–21. doi: 10.1007/s00535-012-0668-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Z, Liu Y, Zhai X, Dai J, Jin G, Wang L, et al. New loci associated with chronic hepatitis B virus infection in Han Chinese. Nat Genet. 2013;45(12): 1499–1503. doi: 10.1038/ng.2809 [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Si L, Zhai Y, Hu Y, Hu Z, Bei JX, et al. Genome-wide association study identifies 8p21.3 associated with persistent hepatitis B virus infection among Chinese. Nat Commun. 2016;7: 11664 doi: 10.1038/ncomms11664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim YJ, Kim HY, Lee JH, Yu SJ, Yoon JH, Lee HS, et al. A genome-wide association study identified new variants associated with the risk of chronic hepatitis B. Hum Mol Genet. 2013;22(20): 4233–4238. doi: 10.1093/hmg/ddt266 [DOI] [PubMed] [Google Scholar]
- 22.Nishida N, Sawai H, Matsuura K, Sugiyama M, Ahn SH, Park JY, et al. Genome-wide association study confirming association of HLA-DP with protection against chronic hepatitis B and viral clearance in Japanese and Korean. PLoS One. 2012;7(6): e39175 doi: 10.1371/journal.pone.0039175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishida N, Sawai H, Kashiwase K, Minami M, Sugiyama M, Seto WK, et al. New susceptibility and resistance HLA-DP alleles to HBV-related diseases identified by a trans-ethnic association study in Asia. PLoS One. 2014;9(2): e86449 doi: 10.1371/journal.pone.0086449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang KH, Lin CL, Hsu CW, Lai MW, Chien RN, Yeh CT. UGT2B28 genomic variation is associated with hepatitis B e-antigen seroconversion in response to antiviral therapy. Sci Rep. 2016;6: 34088 doi: 10.1038/srep34088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertoletti A, Hong M. Age-Dependent Immune Events during HBV Infection from Birth to Adulthood: An Alternative Interpretation. Front Immunol. 2014;5: 441 doi: 10.3389/fimmu.2014.00441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang K, Chang S, Guo L, Wang J. I-GSEA4GWAS v2: a web server for functional analysis of SNPs in trait-associated pathways identified from genome-wide association study. Protein Cell. 2015;6(3): 221–224. doi: 10.1007/s13238-014-0114-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genomes Project C, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. 2015;526(7571): 68–74. doi: 10.1038/nature15393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macek Jilkova Z, Decaens T, Marlu A, Marche H, Jouvin-Marche E, Marche PN. Sex Differences in Spontaneous Degranulation Activity of Intrahepatic Natural Killer Cells during Chronic Hepatitis B: Association with Estradiol Levels. Mediators Inflamm. 2017;2017: 3214917 doi: 10.1155/2017/3214917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang SH, Chen PJ, Yeh SH. Gender disparity in chronic hepatitis B: Mechanisms of sex hormones. J Gastroenterol Hepatol. 2015;30(8): 1237–1245. doi: 10.1111/jgh.12934 [DOI] [PubMed] [Google Scholar]
- 30.Park YM, Lee SG. Clinical features of HBsAg seroclearance in hepatitis B virus carriers in South Korea: A retrospective longitudinal study. World J Gastroenterol. 2016;22(44): 9836–9843. doi: 10.3748/wjg.v22.i44.9836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quagliata L, Matter MS, Piscuoglio S, Arabi L, Ruiz C, Procino A, et al. Long noncoding RNA HOTTIP/HOXA13 expression is associated with disease progression and predicts outcome in hepatocellular carcinoma patients. Hepatology. 2014;59(3): 911–923. doi: 10.1002/hep.26740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodman AG, Zeng H, Proll SC, Peng X, Cilloniz C, Carter VS, et al. The alpha/beta interferon receptor provides protection against influenza virus replication but is dispensable for inflammatory response signaling. J Virol. 2010;84(4): 2027–2037. doi: 10.1128/JVI.01595-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho IR, Oh M, Koh SS, Malilas W, Srisuttee R, Jhun BH, et al. Hepatitis B virus X protein inhibits extracellular IFN-alpha-mediated signal transduction by downregulation of type I IFN receptor. Int J Mol Med. 2012;29(4): 581–586. doi: 10.3892/ijmm.2012.879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ono K, Wang X, Kim SO, Armstrong LC, Bornstein P, Han J. Metaxin deficiency alters mitochondrial membrane permeability and leads to resistance to TNF-induced cell killing. Protein Cell. 2010;1(2): 161–173. doi: 10.1007/s13238-010-0017-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spilsbury K, O'Mara MA, Wu WM, Rowe PB, Symonds G, Takayama Y. Isolation of a novel macrophage-specific gene by differential cDNA analysis. Blood. 1995;85(6): 1620–1629. [PubMed] [Google Scholar]
- 36.Wieland SF, Vega RG, Muller R, Evans CF, Hilbush B, Guidotti LG, et al. Searching for Interferon-Induced Genes That Inhibit Hepatitis B Virus Replication in Transgenic Mouse Hepatocytes. Journal of Virology. 2003;77(2): 1227–1236. doi: 10.1128/JVI.77.2.1227-1236.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Li Y, Mao A, Li C, Li Y, Tien P. Hepatitis B virus X protein suppresses virus-triggered IRF3 activation and IFN-beta induction by disrupting the VISA-associated complex. Cell Mol Immunol. 2010;7(5): 341–348. doi: 10.1038/cmi.2010.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niu C, Li L, Daffis S, Lucifora J, Bonnin M, Maadadi S, et al. Toll-like receptor 7 agonist GS-9620 induces prolonged inhibition of HBV via a type I interferon-dependent mechanism. J Hepatol. 2018;68(5): 922–931. doi: 10.1016/j.jhep.2017.12.007 [DOI] [PubMed] [Google Scholar]
- 39.Li L, Barry V, Daffis S, Niu C, Huntzicker E, French DM, et al. Anti-HBV response to toll-like receptor 7 agonist GS-9620 is associated with intrahepatic aggregates of T cells and B cells. J Hepatol. 2018;68(5): 912–921. doi: 10.1016/j.jhep.2017.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cui J, Li Y, Zhu L, Liu D, Songyang Z, Wang HY, et al. NLRP4 negatively regulates type I interferon signaling by targeting the kinase TBK1 for degradation via the ubiquitin ligase DTX4. Nat Immunol. 2012;13(4): 387–395. doi: 10.1038/ni.2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang J, Tang H. Mechanism of inhibiting type I interferon induction by hepatitis B virus X protein. Protein Cell. 2010;1(12): 1106–1117. doi: 10.1007/s13238-010-0141-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei C, Ni C, Song T, Liu Y, Yang X, Zheng Z, et al. The hepatitis B virus X protein disrupts innate immunity by downregulating mitochondrial antiviral signaling protein. J Immunol. 2010;185(2): 1158–1168. doi: 10.4049/jimmunol.0903874 [DOI] [PubMed] [Google Scholar]
- 43.Publicover J, Gaggar A, Nishimura S, Van Horn CM, Goodsell A, Muench MO, et al. Age-dependent hepatic lymphoid organization directs successful immunity to hepatitis B. J Clin Invest. 2013;123(9): 3728–3739. doi: 10.1172/JCI68182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu C, Huang X, Werner M, Broering R, Ge J, Li Y, et al. Elevated Expression of Chemokine CXCL13 in Chronic Hepatitis B Patients Links to Immune Control during Antiviral Therapy. Front Immunol. 2017;8: 323 doi: 10.3389/fimmu.2017.00323 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The distributions of observed p-values did not deviate from the null distribution, which excluded systematic bias due to bad genotyping or population structure. The y-axis is the observed -log10(p) values; the x-axis is the expected -log10(p) values. The genomic control inflation factor (λ) is 0.893.
(PPTX)
Data were presented as number (%), mean ± standard deviation, or median (IQR). ALT, alanine aminotransferase (normal range 5–40 U/L); HBeAg, hepatitis B e antigen; IFN, interferon (including PEGylated-interferon); NA, nucleos(t)ide analogues.
(DOCX)
The significant SNPs (p < 1.0ⅹ10−4) are presented. SNP, single nucleotide polymorphism; rsID, reference SNP identity; Chr, chromosome; A1, minor allele in the entire cohort; A2, major allele; OR, odds ratio; CI, confidence interval. a: The flanking genes were located ± 150 kb of each SNP. The odds ratio for the minor allele in an additive model. P-value was obtained by logistic regression test for the minor allele additive model.
(DOCX)
SNP, single nucleotide polymorphism; GWAS, genome-wide association study; rsID, reference SNP identity; A1, minor allele; A2, major allele; chr, chromosome; OR, odds ratio; CI, confidence interval; PMID, pubmed identity. The odds ratio for the minor allele in a additive model. P-value was obtained by logistic regression test for the minor allele additive model.
(DOCX)
The most significant SNPs (up to 30 SNPs) are presented according to each outcome with P-values of other outcomes, as well. SNP, single nucleotide polymorphism; rsID, reference SNP identity; Chr, chromosome. a: The flanking genes were located ± 150 kb of each SNP. The odds ratio for the minor allele in an additive model. P-value was obtained by logistic regression test for the minor allele additive model.
(XLSX)
Data Availability Statement
All relevant data are available from figshare: https://figshare.com/articles/gtReport_txt/6614975 (DOI: 10.6084/m9.figshare.6614975).